Abstract
Several clinical trials have been revolutionizing the perioperative treatment of early-stage non-small cell lung cancer (NSCLC). Many of these clinical trials involve cancer immunotherapies with antibody drugs that block the inhibitory immune checkpoints programmed death 1 (PD-1) and its ligand PD-L1. While these new treatments are expected to improve the treatment outcome of NSCLC patients after pulmonary resection, several major clinical questions remain, including the appropriate timing of immunotherapy (neoadjuvant, adjuvant, or both) and the identification of patients who should be treated with neoadjuvant and/or adjuvant immunotherapies, because some early-stage NSCLC patients are cured by surgical resection alone. In addition, immunotherapy may induce immune-related adverse events that will require permanent treatment in some patients. Based on this fact as well, it is desirable to select appropriate patients for neoadjuvant/adjuvant immunotherapies. So far, data from several important trials have been published, with findings demonstrating the efficacy of adjuvant atezolizumab (IMpower010 trial), neoadjuvant nivolumab plus platinum-doublet chemotherapy (CheckMate816 trial), and several perioperative (neoadjuvant plus adjuvant) immunotherapies (AEGEAN, KEYNOTE-671, NADIM II, and Neotorch trials). In addition to these key trials, numerous clinical trials have reported a wealth of data, although most of the above clinical questions have not been completely answered yet. Because there are so many ongoing clinical trials in this field, a comprehensive understanding of the results and/or contents of these trials is necessary to explore answers to the clinical questions above as well as to plan a new clinical trial. In this review, we comprehensively summarize the recent data obtained from clinical trials addressing such questions.
1. Introduction
The clinical application of immune checkpoint inhibitors (ICIs) has revolutionized the treatment of advanced-stage non-small cell lung cancer (NSCLC) and small cell lung cancer. Several antibodies blocking the inhibitory immune checkpoints programmed death 1 (PD-1) and its ligand PD-L1 and other checkpoints such as cytotoxic T-lymphocyte associated antigen-4 (CTLA-4) have been developed. Anti-PD-1/PD-L1 inhibitor monotherapies and combination therapies with cytotoxic chemotherapy (with/without anti-angiogenic agents) and/or anti-CTLA-4 inhibitors are now standard front-line therapies for advanced-stage NSCLC without a targetable driver mutation [1]. One of the important features of ICI-based treatments is the so-called “long tail effect”, which contributes to the curative effects in some advanced-stage NSCLC patients.
After the exciting success of immunotherapies in the advanced-stage setting, the subsequent logical step was the clinical application of ICIs for patients with non-metastatic NSCLC to improve cure rates and prolong overall survival (OS). The PACIFIC trial demonstrated a significant improvement in progression-free survival (PFS) and OS in patients with unresectable clinical stage III NSCLC treated with durvalumab after concurrent chemoradiotherapy, with improvements even in 5-year OS [2]. Four phase III trials (IMpower010 (NCT02486718) [3], PEARLS (NCT02504372) [4], BR31 (NCT02273375), and ANVIL (NCT02595944)) are now evaluating the efficacy and the safety of atezolizumab, pembrolizumab, durvalumab, and nivolumab, respectively, after curative resection for NSCLCs.
In the neoadjuvant setting, several small-scale single-arm studies have reported good treatment efficacies of ICI monotherapies in early-stage NSCLC patients [5]. These results led to the initiation of clinical trials of neoadjuvant ICI monotherapies or combination therapies in early-stage NSCLC. Furthermore, several clinical trials with a perioperative setting (neoadjuvant plus adjuvant ICIs, the so-called sandwich regimens) are also ongoing. Thus, a great number of clinical trials are now underway in this field, and the results of these trials are reported at every major conference. In order to provide the best treatment for the patient in front of us in our daily clinical practice, and in order to build new evidence in the future, it is necessary to have a comprehensive understanding of the results of these trials. Therefore, in this review, we summarize the recent advances in adjuvant, neoadjuvant, and perioperative treatments using ICIs in patients with resectable NSCLC. To collect as much information as possible about the literature and clinical trials on neoadjuvant, adjuvant, and perioperative treatments for NSCLC, we searched PubMed using a combination of the following keywords: “lung cancer”, “NSCLC”, “adjuvant”, “neoadjuvant”, “perioperative treatment”, and “immune checkpoint inhibitors”. Of the articles found using these search terms, all available review articles were checked, and individual clinical trial information was evaluated using its original data and included in the reference list of this paper if they were determined to be pertinent.
Although many clinical trials have been conducted in this field, there is still insufficient biomarker analysis data that are useful for better patient selection. Although some studies reported the results of biomarker analysis such as tumor mutation burden, gene expression data, liquid biopsy, tumor-infiltrating immune cells, and gut microbiota, their usefulness has not yet been fully verified, therefore in this review, we focused on summarizing clinical trial data but not including biomarker studies.
2. Treatment Outcomes of Early-Stage NSCLC before the ICI Era
Radical resection together with lymph node dissection is the standard of care for most NSCLC patients with clinical stage I/II disease and some patients with clinical stage III disease. The clinical stages are mainly determined by enhanced computed tomography (CT) and FDG-PET/CT examination.
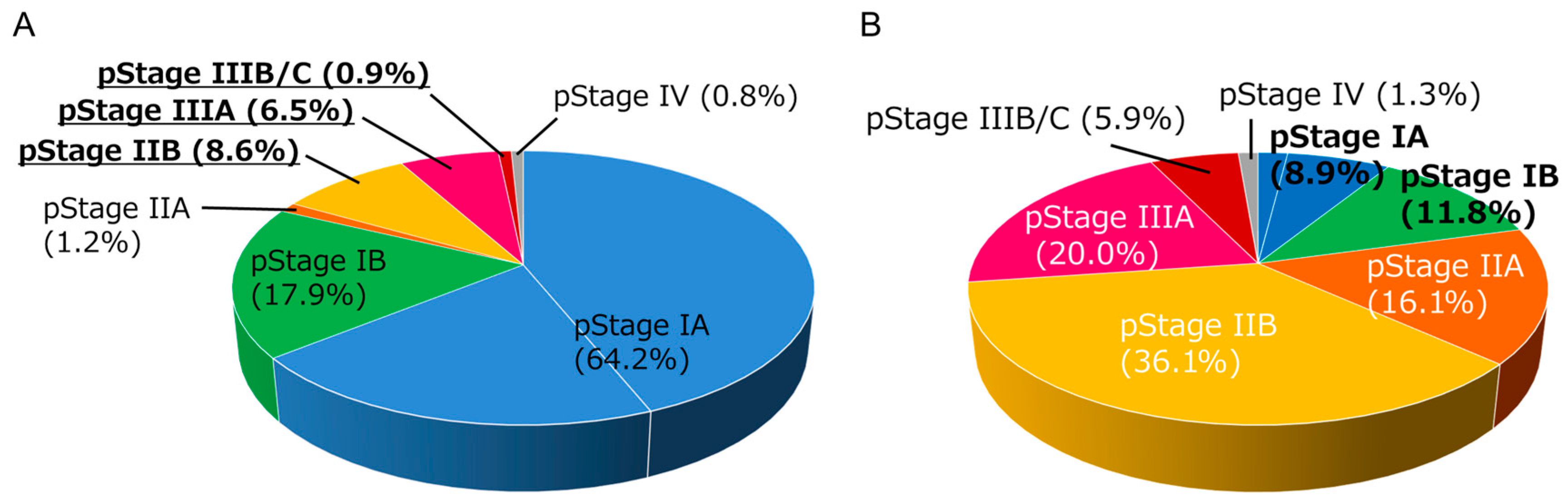
After pulmonary resection, a detailed pathological examination enables a more accurate diagnosis of the spread of tumor cells (pathological stage, pStage). In NSCLC, there is a substantial discrepancy between clinical stage and pathological stage as exemplified in Figure 1A,B. Adjuvant treatment is applied in patients with good performance status in some pStage I patients and in pStage II–III patients. While a recent large-scale registry database analysis reported that approximately 60% of patients with pStage II or III disease received adjuvant treatment in the real-world setting in Japan [6], the primary analysis of this registry database showed that approximately 50% of pStage II patients and 75% of pStage III patients experienced disease recurrence or death within 5 years [7]. Therefore, the clinical application of new drugs in the neoadjuvant and/or adjuvant setting including tyrosine kinase inhibitors such as osimertinib [8] and ICIs, as summarized in this review, has been a research focus.

Figure 1.
Distribution of pathological stages in non-small cell lung cancer (NSCLC) patients (n = 1590) who received lobectomy plus mediastinal lymph node dissection in our institution between 2007–2021. (A) Distribution of pathological stages in patients with clinical stage I NSCLC (n = 1285). Note that approximately 16% of patients experienced up-staging at pathological examination. (B) Distribution of pathological stages in patients with clinical stage II NSCLC (n = 305). Note that approximately 20% of patients had experienced down-staging into stage I at pathological examination.
3. Overview of ICIs
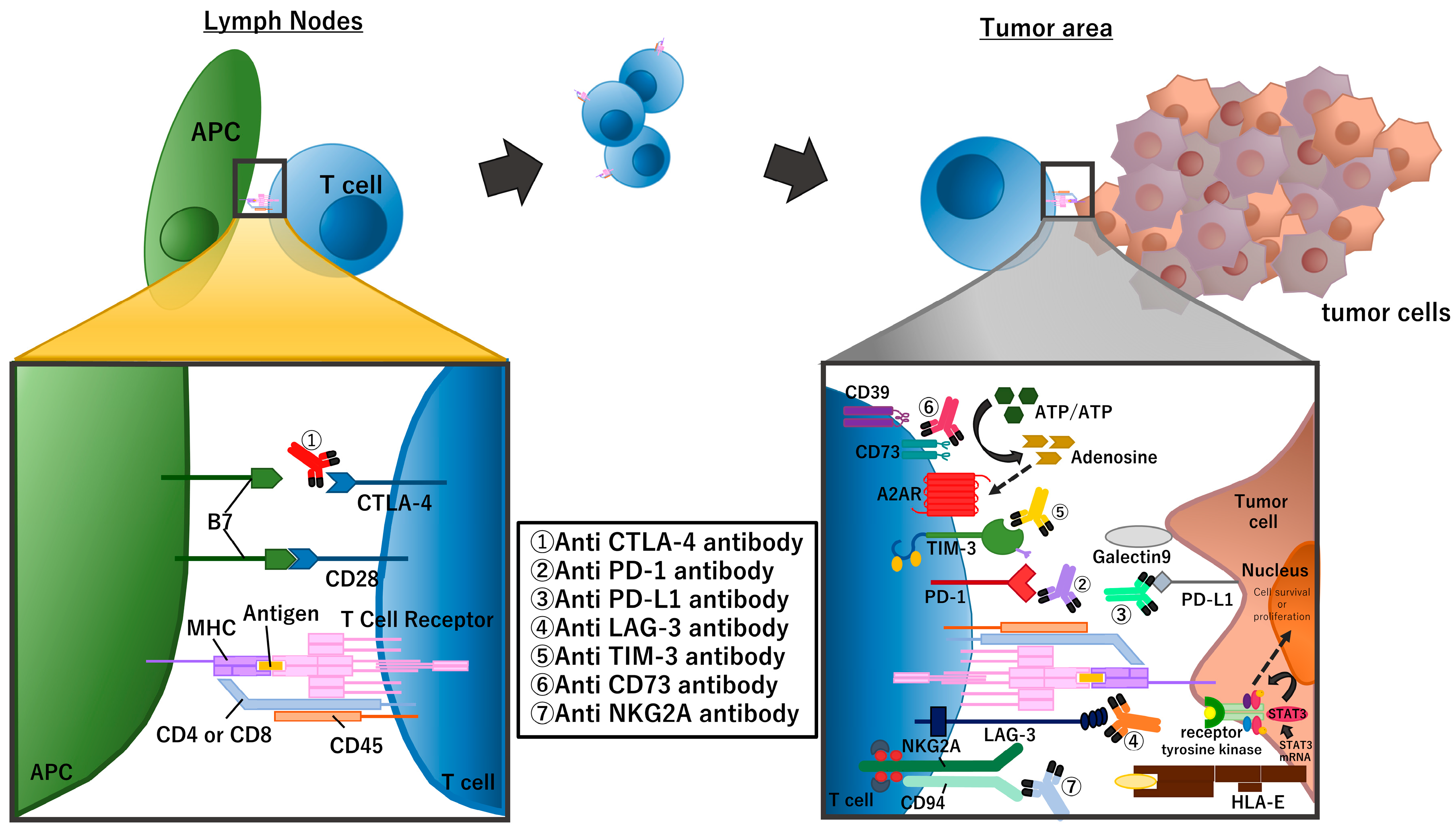
Cytotoxic CD8-positive T cells are the main player in the anti-tumor immune response [9,10]. These T cells are activated only when the T cell receptor (TCR) recognizes the antigen/MHC-1 complexes on the surface of antigen-presenting cells (APC) (first signal) and the CD28 molecule on T cells binds to the B7 molecules (CD80/CD86) on the APC (second signal) at the same time. The CTLA-4 molecule, expressed on T cells, functions as a negative regulator of this T cell activation; CTLA-4 also binds to the B7 molecules on the APC at a higher affinity compared with CD28. Therefore, blockade of the CTLA-4 pathway, for example with anti-CTLA4 antibodies, promotes cytotoxic T cell activation [11].
Activated cytotoxic T cells infiltrate into the tumor area and kill tumor cells by enhancing pore formation in the tumor cell membrane and causing subsequent secretion of death-inducing granules containing granzymes, perforin, cathepsin C, and granulysin. Cytotoxic T cells also promote tumor cell death through Fas-FASL (Fas ligand) interactions. Tumor cell killing only takes a few minutes, and a single cytotoxic T cell can carry out serial or simultaneous killing of multiple tumor cells [12]. Immune cells, including cytotoxic T cells, have self-inhibitory mechanisms involving immune-checkpoint molecules that ensure the appropriate regulation of the immune response. PD-1 is one of the most important immune-checkpoint molecules that is expressed in exhausted cytotoxic T cells. Tumor cells exploit this inhibitory pathway by expressing the PD-1 ligands, PD-L1 or PD-L2, to induce an immunosuppressive state that facilitates tumor cell growth.
Many recent clinical studies of NSCLC in perioperative settings use one of the PD-1 inhibitors (nivolumab, pembrolizumab, cemiplimab, sintilimab, and toripalimab) or PD-L1 inhibitors (atezolizumab, durvalumab, and avelumab). In addition to PD-1/PD-L1, there are many other checkpoint molecules including the aforementioned CTLA-4, lymphocyte-activation gene 3 (LAG-3) [13], T-cell immunoglobulin and mucin domain-3 (TIM-3), and T-cell immunoreceptor with Ig and ITIM domains (TIGIT) and immune suppressive mechanisms including loss of stimulator of interferon genes (STING) expression [14] and the adenosine pathway (Figure 2).
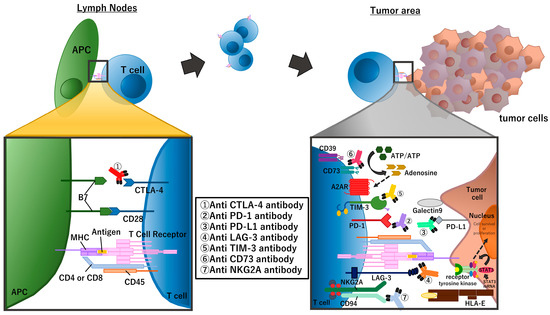
Figure 2.
Summary of immune checkpoint molecules and the inhibitors targeting these molecules. The figure focuses on molecules that are targeted in clinical trials of perioperative treatment for NSCLC.
4. Adjuvant ICI Treatments vs. Neoadjuvant ICI Treatments
In studies examining the efficacy of chemotherapy, both neoadjuvant [15] and adjuvant approaches [16,17] have shown superior efficacy in OS compared with surgery alone (but only by approximately 5%). However, because evidence for adjuvant chemotherapies was established earlier, several neoadjuvant trials using cytotoxic chemotherapies were terminated before maturation. Therefore, adjuvant chemotherapies have been considered the standard of care until the establishment of recent novel adjuvant/neoadjuvant treatments. However, in the new era of ICI treatments, the best strategy for surgical candidates with NSCLC, such as neoadjuvant immunotherapy vs. adjuvant immunotherapy vs. perioperative (neoadjuvant plus adjuvant, the so-called sandwich regimen) immunotherapy, remains unclear.
In an in vivo study of mice with tumors derived from highly metastatic breast cancer cell lines, mice treated with neoadjuvant immunotherapy and surgical resection had longer survival than those treated with upfront surgery followed by adjuvant immunotherapy [18]. While this in vivo observation was supported by the systemic expansion of tumor-specific cytotoxic T cells in peripheral blood and organs after neoadjuvant immunotherapy, whether this phenomenon also occurs in NSCLC patients is unclear. In the clinical setting of patients with resectable stage III or IV melanoma, event-free survival (EFS) was significantly longer for patients who received neoadjuvant pembrolizumab plus adjuvant pembrolizumab than patients who received adjuvant pembrolizumab alone [19]. However, whether this observation is applicable to NSCLC is unclear because the shapes of the EFS curves were quite different from the usual EFS curves after pulmonary resection. Here we summarize the possible advantages and disadvantages of neoadjuvant and adjuvant immunotherapies.
4.1. Advantages and Disadvantages of Neo-Adjuvant Immunotherapies
Theoretically, neoadjuvant immunotherapy will be able to prime a more effective immune reaction compared with adjuvant immunotherapy because of the abundant neoantigen and maintained lymphatic system around the primary tumor. Neoadjuvant immunotherapy also has advantages in terms of offering the earliest treatment against potential micrometastases, and treatments can be provided in patients with good compliance. Additionally, effective neoadjuvant immunotherapy will increase the resectability of the tumor (chance for R0 resection and/or avoidance of pneumonectomy). Furthermore, it is also possible that neoadjuvant treatment will provide some time for patients for tobacco cessation and respiratory rehabilitation. Neoadjuvant immunotherapies may also provide useful tumor samples for investigating drug tolerance mechanisms that have been widely studied for molecular-targeted agents [20,21] but not for immunotherapies.
There are also some disadvantages of neoadjuvant immunotherapy including delayed or missed surgical resection because of disease progression or severe adverse events including immune-related adverse events (irAEs). Neoadjuvant chemotherapy plus immunotherapy resulted in patients who could not receive surgical resection because of various reasons in phase III trials, comprising 15–20% of the overall enrolled patients [22,23,24]. There are also possibilities of severe irAEs after pulmonary resection that should be differentiated from surgical complications. As discussed above, some clinical N1–2 patients at preoperative image examination may have false-positive nodal status. Therefore, neoadjuvant immunotherapy may be an over-treatment for these patients.
4.2. Advantages and Disadvantages of Adjuvant Immunotherapies
As shown in Figure 1, there are substantial discrepancies between the clinical stage and the pathological stage in NSCLC patients. Therefore, adjuvant ICI treatment may have an advantage in that the treatment strategies can be determined on the basis of the most “correct” TNM staging. In the near future, it may be possible to identify the subgroup of patients who will be cured by surgery alone, for example, by circulating tumor DNA (ctDNA) detection [25,26,27]. Such information will lead to the administration of systemic treatments only for patients with a higher risk of recurrence in adjuvant ICI treatment strategies. Additionally, treatment strategies starting from pulmonary resection will ensure patients do not lose the opportunity to undergo surgical resection, the most robust treatment modality for local control of the primary tumor.
Notably, some patients may not be able to receive adjuvant treatment if the performance status is worsened after pulmonary resection partially from post-surgical complications. Studies showed that only 66% of patients received pre-planned adjuvant chemotherapy after pulmonary resection, while almost all patients (97%) received chemotherapy in the neoadjuvant group [28]. Similarly, the adjuvant setting sometimes requires dose reduction compared with the neoadjuvant setting; however, whether the full dose of chemotherapy is more effective when given with immunotherapeutic agents is unclear.
4.3. Necessity of Adjuvant Immunotherapy following Neoadjuvant Immunotherapy plus Surgical Resection
Whether adjuvant immunotherapy will improve patient outcomes after neoadjuvant immunotherapy plus surgical resection also remains unclear. In the CheckMate816 study (neoadjuvant nivolumab plus chemotherapy without adjuvant immunotherapy), patients who achieved a pathological complete response (pCR) showed excellent survival, suggesting that adjuvant immunotherapy may not be necessary for these patients. The potential role of adjuvant immunotherapy in patients who did not achieve pCR will be discussed in later sections.
5. Evidence of Neo-Adjuvant Immunotherapies
Neoadjuvant treatment with ICI includes ICI monotherapy, ICI plus ICI combination, ICI plus chemotherapy combination, and ICI plus chemoradiotherapy combination. Although neoadjuvant treatment using ICI monotherapy has shown some therapeutic efficacy, the most attention has been paid to the ICI plus chemotherapy strategies including perioperative (adjuvant ICI monotherapy in addition to neoadjuvant ICI plus chemotherapy) treatments.
One of the main purposes of neoadjuvant treatment is to reduce the tumor burden (primary tumor and/or metastatic lymph nodes), reduce the difficulty of the surgical procedure, avoid pneumonectomy, and/or improve the likelihood of complete resection. The other important purpose is the elimination of micrometastases by the earliest systemic treatment. In neoadjuvant studies, pCR and MPR data have been reported as some of the important parameters of efficacy. However, it should be noted that important elements for this calculation might be different between studies, e.g., pathological evaluation methods and formulas for calculation (whether the denominator of the equation is the total number of enrolled patients or the number of patients who underwent surgical resection).
5.1. ICI Monotherapies
The first piece of evidence regarding the usefulness of perioperative immunotherapies for NSCLC was reported by Forde PM, et al. in 2017 [5]. Nivolumab was administered only twice before surgery; however, it induced a MPR in 45% (9 of 20) of resected tumors. Responses occurred in both PD-L1-positive and -negative tumors, and a high tumor mutation burden (TMB) was associated with pathological response. The 5-year follow-up data of this study were recently reported and showed that eight of nine (89%) patients with MPR were alive and disease-free. Additionally, pre-treatment tumor PD-L1 positivity (TPS ≥ 1%) was associated with favorable recurrence-free survival (RFS) (HR, 0.36, 95% CI, 0.07–1.85) [29]. However, subsequent studies of neoadjuvant ICI monotherapies, using atezolizumab (with/without adjuvant atezolizumab [30]) or durvalumab [31], reported lower pCR and/or MPR results compared with the CheckMate 159 trial (Table 1). In these trials, surgical resection was performed in 88–93% of patients, and complete resection (R0) was achieved in 76–89% of cases. The pCR rates ranged from 0% to 10%. One study showed that the addition of radiotherapy to ICI monotherapy may improve the pathological response compared with ICI monotherapy alone (Table 1) [32].

Table 1.
Clinical trials of neoadjuvant ICI monotherapies.
5.2. ICI Combinations
In treatment of advanced-stage NSCLC, ICI combination therapies such as nivolumab plus the anti-CTLA-4 antibody ipilimumab showed superior efficacy over chemotherapy irrespective of the PD-L1 status (e.g., CheckMate 227) [35]. Therefore, it is reasonable to test the efficacy of such ICI combination therapies in neoadjuvant settings. However, one pilot trial of neoadjuvant nivolumab plus ipilimumab was terminated early by investigator consensus (after 9 of 15 patients were enrolled) because of toxicity [36]. On the other hand, the NEOSTAR randomized phase II trial successfully compared nivolumab alone with nivolumab plus ipilimumab in the neoadjuvant setting. Nivolumab and nivolumab plus ipilimumab treatments resulted in 22% (5/23) and 38% (8/21) MPR rates, respectively [37]. Additionally, nivolumab plus ipilimumab resulted in a higher pCR rate compared with nivolumab alone (29% vs. 9%, respectively, Table 2) and greater frequencies of effector, tissue-resident memory, and effector memory T cells. In a pilot analysis of microbiota, the abundance of Ruminococcus and Akkermansia spp. was associated with MPR in the nivolumab plus ipilimumab treatment group. The results of the nivolumab plus ipilimumab arm in the CheckMate 816 study (discussed later), although terminated early, may support the evaluation of the potential usefulness of this ICI combination regimen in the neoadjuvant setting.
There are many immune checkpoint molecules on the surface of immune cells in addition to PD-1 and CTLA-4 (Figure 2), and several novel immune checkpoint inhibitors are now being developed as anti-cancer agents [38]. Some of these novel immune checkpoint inhibitors have been tested in the neoadjuvant setting. For example, the NEOpredict-Lung trial, a phase II study, is comparing nivolumab monotherapy vs. nivolumab plus relatlimab, an anti-LAG-3 antibody [39]. The NeoCOAST trial is a phase II study evaluating durvalumab alone and in combination with the novel immunotherapeutic agents oleclumab, an anti-CD73 monoclonal antibody, monalizumab, an anti-NKG2A monoclonal antibody, and dambatilsen, an anti-STAT3 antisense oligonucleic acid [40]. While it was not possible to statistically compare these treatment groups, all combination therapies demonstrated a higher MPR compared with durvalumab monotherapy.

Table 2.
Clinical trials of neoadjuvant ICI combinations.
Table 2.
Clinical trials of neoadjuvant ICI combinations.
| Phase: Trial Name (Registry ID) Study Start Date (Reference) | Target Stage | N | ICI | pCR Rate | MPR Rate | DFS/EFS PFS/RFS | OS |
|---|---|---|---|---|---|---|---|
| pII: NEOSTAR (NCT03158129) June 2017 [37] | I–IIIA | 44 | N vs. N + I | N: 9% N + I: 29% | N: 22% N + I: 38% | RFS Median was not reached | Median was not reached |
| pII: NEOpredict-Lung (NCT04205552) March 2020 [39] | IB–IIIA | 60 | N vs. N + R | pCR or MPR rates N: 28% N + R: 32% | both arms 12 m DFS 91% [95% CI: 78–97%] | both arms 12 m OS 96% [95% CI: 83–99%] | |
| pII: JHU and MSKCC Study (NCT02259621) September 2014 [36] | IB (≥4 cm)–IIIA | 9 | N + I (+two additional doses of N) | 22% | NA | 2y RFS 33% | NA |
ICI: immune checkpoint inhibitor, MPR: major pathological response (tumors with no more than 10% viable tumor cells), pCR: pathological complete response, DFS: disease-free survival, EFS: event-free survival, PFS: progression-free survival, RFS: recurrence-free survival, OS: overall survival, NA: not available, I: ipilimumab, N: nivolumab, R: relatlimab.
5.3. Combination Therapies with ICI plus Cytotoxic Chemotherapies
Greater efficacies of PD-1 or PD-L1 inhibitors plus cytotoxic chemotherapies were reported in several phase I and II trials in the neoadjuvant setting [41,42,43,44], and these findings were further confirmed in the CheckMate816 trial [23]. Theoretically, immunotherapy will have additive effects because cytotoxic drugs will cause increased neoantigen release around the tumors by killing cancer cells. Furthermore, the addition of cytotoxic agents may prevent hyper-progressive disease [45], which may cause inoperability if it occurs in a neoadjuvant setting.
In the phase III Checkmate 816 trial, neoadjuvant nivolumab plus platinum-doublet (CDDP-based/CBDCA-based therapy) reduced the risk of relapse or death by 37% compared with chemotherapy alone (hazard ratio for event-free survival (EFS), 0.63; p = 0.005) [23]. The pCR and MPR rates were significantly better in the nivolumab-plus-chemotherapy group than in the chemotherapy group. In subgroup analyses, patients with cStage IIIA NSCLC (hazard ratio: 0.54, 95% CI 0.37–0.80) or those with high PD-L1 expression (TPS 50% or higher, hazard ratio: 0.24, 95% CI 0.10–0.61) showed better EFS with the addition of nivolumab, while the impact of the addition of nivolumab was not so high in the counterpart subgroups (patients with cStage IB–II NSCLC, hazard ratio: 0.87, 95% CI 0.48–1.56, and those with negative PD-L1 expression, hazard ratio: 0.85, 95% CI 0.54–1.32) (Table 3).

Table 3.
Clinical trials of neoadjuvant ICI plus chemotherapy.
5.4. ICI Sandwich Therapies (Neoadjuvant ICI plus Chemotherapy Followed by Adjuvant ICI Monotherapy)
In many phase III trials using ICI plus cytotoxic chemotherapy as neoadjuvant treatment, postoperative administration of ICI monotherapy is also planned (often called perioperative treatments or sandwich regimens). In the NADIM single-arm phase II trial, the efficacy and safety of this strategy were reported. Additionally, several biomarker analyses using tumor specimens and/or blood samples were performed in this trial to identify patients who will benefit from neoadjuvant ICI treatment or patients who have poor prognosis after neoadjuvant ICI treatment (patients who may need adequate adjuvant treatment).
The initial results of several phase III studies of perioperative ICI treatments, including the AEGEAN trial [46], Neotoarch trial [47], KEYNOTE-671 trial [22], and NADIM II trial (a randomized phase II trial) [48], were recently reported (Table 4). In these trials, neoadjuvant ICI plus chemotherapy significantly improved the pCR and MPR rates and EFS (and OS in some trials) over neoadjuvant chemotherapy plus placebo. Similar to the results in the CheckMate816 trial, a higher proportion of patients achieved pCR after neoadjuvant ICI plus chemotherapy and these patients had excellent EFS. Therefore, an important clinical question remains as to whether adjuvant ICI monotherapy is necessary (or has some role) in patients who do not achieve pCR. In the subgroup analysis in some perioperative ICI trials, the survival curves for the ICI combination group were much better than those for the placebo group among patients who did not achieve pCR (at least compared with similar data from the CheckMate816 trial). Such data may suggest that there are some patients who would benefit from adjuvant ICI monotherapy after neoadjuvant ICI plus chemotherapy and surgical resection. This is an intriguing result because this suggests that a drug that fails to eradicate all cancer cells preoperatively with chemotherapy can eradicate cancer cells by monotherapy postoperatively. This biological paradox and the financial toxicity when adjuvant ICI monotherapy is applied to all patients should be explored in future studies.

Table 4.
Clinical trials on ICI sandwich treatments.
5.5. Combination Therapies with Chemoradiotherapy (CRT)
Several studies have explored the addition of radiotherapy in ICI plus cytotoxic chemotherapies [51,52,53]. Compared with the neoadjuvant trials described above, these trials are mainly focused on patients with more advanced stages (for example, clinical N2 and/or stage III disease only) (Table 5). Radiotherapy is expected to increase the local control of mediastinal lymph node metastases; furthermore, it is expected that the abscopal effect of radiotherapy may promote anti-tumor immune reactions of ICI treatment [54].

Table 5.
Clinical trials on combination therapies with ICI plus chemoradiotherapy.
There is currently insufficient data to support the use of ICI plus chemoradiotherapy over ICI plus chemotherapy because it is likely that treatment-related and/or surgery-related adverse events may increase after neoadjuvant ICI plus chemoradiotherapies. Further study is essential to examine if this combination therapy is useful to reduce the risk of inoperability when used for patients with marginally resectable tumors before treatment.
6. Evidence of Adjuvant Immunotherapies
Unlike neoadjuvant strategies for which pathologic response can be evaluable, the efficacy of adjuvant treatment can only be validated based on DFS data. Therefore, the form of large phase III trials is necessary to demonstrate its statistically significant benefit. To date, results from the IMpower010 and PEARLS trials have been published.
The efficacy of adjuvant ICI monotherapy, after adjuvant platinum-doublet chemotherapy, was reported by the IMpower010 study [3]. This study demonstrated the efficacy of adjuvant atezolizumab for 1 year compared with the best supportive care in patients with pathologic stage II–IIIA NSCLC with PD-L1 TPS ≥ 1% (HR for disease-free survival (DFS): 0.66 [95% CI: 0.50–0.88; p = 0.0039]). The highest improvement in OS was seen in stage II–IIIA patients with tumors that expressed PD-L1 TPS ≥ 50% (HR = 0.43, 95% CI: 0.24–0.78) [56]. An interim analysis of the Phase III PEARLS/KEYNOTE-091 trial evaluating pembrolizumab as adjuvant chemotherapy in patients with pathologic stage IB-IIIA NSCLC was also reported. Postoperative adjuvant pembrolizumab maintained a favorable improvement in DFS at 3 years. The median DFS for the overall population was 53.6 months in the pembrolizumab group and 42.0 months in the placebo group (hazard ratio [HR]: 0.76, 95% confidence interval [CI]: 0.63–0.91, p = 0.0014) [39]. However, the efficacy of adjuvant pembrolizumab was not associated with PD-L1 expression status, and the detailed mechanism of this phenomenon is unclear. There are several ongoing large clinical trials in the adjuvant setting such as ANVIL, BR31, and ALCHEMIST trials (Table 6).

Table 6.
Clinical trials on adjuvant ICI.
7. Implications for Clinical Practice
These neoadjuvant, adjuvant, or perioperative clinical trials have demonstrated dramatic efficacy of ICI therapy over conventional standard treatment for some NSCLC patients. Since the drugs available at this time vary from country to country, we must take into account the results of these clinical trials and provide the best possible treatment for NSCLC patients using the available drug(s). In addition, these treatments have the potential, although not very likely, for unfavorable outcomes (e.g., risk of inoperability after neoadjuvant treatment or risk of developing severe irAE), so treatment strategies should be determined with patients considering their philosophy as well as levels of understanding of the treatment.
Our personal opinions, in situations where several treatment options are considerable, include neoadjuvant ICI treatment (especially ICI plus chemotherapy) would be superior to upfront surgery or CRT followed by surgery in patients with potential systemic disease (e.g., cN2 disease). On the other hand, neoadjuvant CRT, as a powerful tool for local control, remains a useful treatment option when surgical margins will be limited due to direct invasion of the primary tumor, such as tumors invading the chest wall near the vertebral body or superior sulcus tumors. In such cases, we consider that adjuvant atezolizumab would be one of the treatment options after surgical resection.
It should be noted that most of the currently available data have been obtained from patients participating in clinical trials. Therefore, there are insufficient data on the efficacy and safety of neoadjuvant, adjuvant, and perioperative treatment with ICIs in patients who do not meet the eligibility criteria for clinical trials (e.g., patients with autoimmune diseases) but who are considered at high risk of recurrence. Therefore, it is necessary to accumulate data on these patients as real-world data by conducting clinical practice with paying sufficient attention to safety.
8. Future Directions
Definitive evidence has shown the efficacy of neoadjuvant, adjuvant, and perioperative treatments using ICIs to improve patient survival and even exhibit curative effects in some patients. However, many challenges remain to be overcome. The most important and urgent need is to identify biomarkers to select appropriate patients who should receive these immunotherapies. Because of irAEs, some patients will require permanent therapy. Furthermore, some patients may experience lethal irAEs such as myocarditis. Therefore, ideally, immunotherapy must be applied to patients who have a high risk of recurrence and who will receive benefit from the therapy. Here we will summarize the attempts to develop such prognostic and predictive biomarkers.
8.1. Prognostic Biomarkers
The pathological stage, as well as the clinical stage in the case of neoadjuvant treatment, is one of the most important prognostic biomarkers for NSCLC. Therefore, neoadjuvant, adjuvant, and perioperative treatments are defined on the basis of these stages in several guidelines. However, the current TNM staging is determined on the basis of the spread of tumor cells but does not reflect the biology of tumor cells. Therefore, many groups have analyzed and reported the expression status of numerous genes, proteins, or clinicopathological markers as prognostic factors [58,59,60,61]. However, none have been used in clinical practice for the treatment of early-stage NSCLCs.
Recent evidence supports that circulating tumor DNA (ctDNA) detection after pulmonary resection indicates the presence of minimal residual disease (MRD), which is directly related to disease recurrence. Several retrospective studies have observed a high risk of recurrence in patients with positive ctDNA after pulmonary resection [62,63,64]. Some of these studies also reported that adjuvant cytotoxic chemotherapy may have some benefit in patients with positive ctDNA but not in patients without detectable ctDNA [63,64]. However, in the IMpower010 study of adjuvant atezolizumab compared with the best supportive care, both patient groups, those with and without ctDNA, benefited from adjuvant atezolizumab (although survival was better in patients without detectable ctDNA) [65]. Efforts are now being performed to increase the sensitivity of MRD detection to classify patients into two groups: Those with a high risk of recurrence who should receive adjuvant treatment and those who can be cured by surgery alone. However, a strategy using MRD detection is not applicable to deciding on neoadjuvant immunotherapy.
8.2. Predictive Biomarkers
There are several biomarkers to identify patients who may benefit from immunotherapies, including the PD-L1 status of tumor cells (and immune cells in the tumor microenvironment), tumor mutation burden, invasion of CD8-positive cells, and the absence of suppressive immune cells. Among these potential markers, the PD-L1 TPS was reported as a clinically meaningful biomarker to predict the efficacies of adjuvant, neoadjuvant, and/or perioperative anti-PD-1/PD-L1 antibodies, as reported in many trials including IMpower010, CheckMate816, and AEGEAN studies [3,23,24].
On the other hand, many studies did not support the usefulness of TMB as a predictive biomarker in perioperative settings. However, responses were also noted in patients with negative or low PD-L1 expression. Additionally, some patients with high PD-L1 expression also experience disease recurrence. Therefore, the identification of biomarkers that enhance the predictive impact of PD-L1 TPS is highly anticipated. Candidate biomarkers include tumor-infiltrating lymphocytes, immune scores, tertiary lymphoid structure, and gut microbiota.
9. Conclusions
The clinical application of ICIs is now dramatically changing the treatment of early-stage NSCLC patients who are candidates for surgical resection. Many neoadjuvant, adjuvant, and perioperative ICI treatments have shown significant survival benefits in these patients. In addition, ongoing clinical trials will provide more options for perioperative systemic treatments. As a future challenge, we have to collect real-world patients’ data to complement clinical trial data; in addition, prognostic and/or predictive biomarker studies are desirable for optimal individual treatment for resectable NSCLC patients.
Author Contributions
Conceptualization, K.S.; literature search, S.F., K.S. and A.H.; resources, K.S. and Y.T.; writing—original draft preparation, S.F. and K.S.; writing—review and editing, all the authors.; supervision, K.S. and Y.T.; funding acquisition, K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partly supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science [grant number 22K07291 to K.S.].
Institutional Review Board Statement
Figure 1 was created from the clinical database of our department. The research use of clinical data, which have been collected retrospectively, was approved by the institutional review boards (IRB No. 31-068), and the written informed consent from each patient was waived due to its retrospective nature by the IRB.
Informed Consent Statement
Not applicable.
Data Availability Statement
All essential information will be available from the corresponding author upon reasonable request.
Conflicts of Interest
K.S. has received research funding from AstraZeneca and honoraria from Chugai Pharmaceuticals, AstraZeneca, and Taiho; he is on the advisory board of AstraZeneca outside of the submitted work. Hamada has received honoraria from AstraZeneca, Chugai Pharmaceuticals, and Ono Pharmaceuticals outside of the submitted work. Y.T. has received research funding from AstraZeneca, Boehringer-Ingelheim, Chugai Pharmaceuticals, Daiichi Sankyo, Japan Blood Products Organization, Medtronic, Otsuka Pharmaceuticals, and Taiho and received an honorarium from AstraZeneca, Bristol Myers Squibb, Chugai Pharmaceuticals, Covidien Japan, CSL Behring, Eli Lilly, Japan Blood Products Organization, Johnson and Johnson, MiRTeL, MSD, Nihon Medi-Physics, Novartis, Ono Pharmaceuticals, Phase 1, Roche, Taiho, and Takeda Pharmaceuticals; he has been on the advisory board of AstraZeneca, Chugai Pharmaceuticals, and Ono Pharmaceuticals outside of the submitted work. All other authors report no conflict of interest.
References
- Singh, N.; Temin, S.; Baker, S., Jr.; Blanchard, E.; Brahmer, J.R.; Celano, P.; Duma, N.; Ellis, P.M.; Elkins, I.B.; Haddad, R.Y.; et al. Therapy for Stage IV Non-Small-Cell Lung Cancer without Driver Alterations: ASCO Living Guideline. J. Clin. Oncol. 2022, 40, 3323–3343. [Google Scholar] [CrossRef] [PubMed]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-Year Survival Outcomes from the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB–IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef]
- O’Brien, M.; Paz-Ares, L.; Marreaud, S.; Dafni, U.; Oselin, K.; Havel, L.; Esteban, E.; Isla, D.; Martinez-Marti, A.; Faehling, M.; et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB–IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): An interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022, 23, 1274–1286. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Chaft, J.E.; Smith, K.N.; Anagnostou, V.; Cottrell, T.R.; Hellmann, M.D.; Zahurak, M.; Yang, S.C.; Jones, D.R.; Broderick, S.; et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N. Engl. J. Med. 2018, 378, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Suda, K.; Mitsudomi, T.; Shintani, Y.; Okami, J.; Ito, H.; Ohtsuka, T.; Toyooka, S.; Mori, T.; Watanabe, S.I.; Asamura, H.; et al. Clinical Impacts of EGFR Mutation Status: Analysis of 5780 Surgically Resected Lung Cancer Cases. Ann. Thorac. Surg. 2021, 111, 269–276. [Google Scholar] [CrossRef]
- Okami, J.; Shintani, Y.; Okumura, M.; Ito, H.; Ohtsuka, T.; Toyooka, S.; Mori, T.; Watanabe, S.I.; Date, H.; Yokoi, K.; et al. Demographics, Safety and Quality, and Prognostic Information in Both the Seventh and Eighth Editions of the TNM Classification in 18,973 Surgical Cases of the Japanese Joint Committee of Lung Cancer Registry Database in 2010. J. Thorac. Oncol. 2019, 14, 212–222. [Google Scholar] [CrossRef]
- Wu, Y.L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.W.; Kato, T.; et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef]
- Kamphorst, A.O.; Wieland, A.; Nasti, T.; Yang, S.; Zhang, R.; Barber, D.L.; Konieczny, B.T.; Daugherty, C.Z.; Koenig, L.; Yu, K.; et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science 2017, 355, 1423–1427. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yu, H.; Rozeboom, L.; Rivard, C.J.; Ellison, K.; Dziadziuszko, R.; Suda, K.; Ren, S.; Wu, C.; Hou, L.; et al. LAG-3 Protein Expression in Non-Small Cell Lung Cancer and Its Relationship with PD-1/PD-L1 and Tumor-Infiltrating Lymphocytes. J. Thorac. Oncol. 2017, 12, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Lohinai, Z.; Dora, D.; Caldwell, C.; Rivard, C.J.; Suda, K.; Yu, H.; Rivalland, G.; Ellison, K.; Rozeboom, L.; Dziadziuszko, R.; et al. Loss of STING expression is prognostic in non-small cell lung cancer. J. Surg. Oncol. 2022, 125, 1042–1052. [Google Scholar] [CrossRef]
- Preoperative chemotherapy for non-small-cell lung cancer: A systematic review and meta-analysis of individual participant data. Lancet 2014, 383, 1561–1571. [CrossRef]
- Douillard, J.Y.; Tribodet, H.; Aubert, D.; Shepherd, F.A.; Rosell, R.; Ding, K.; Veillard, A.S.; Seymour, L.; Le Chevalier, T.; Spiro, S.; et al. Adjuvant cisplatin and vinorelbine for completely resected non-small cell lung cancer: Subgroup analysis of the Lung Adjuvant Cisplatin Evaluation. J. Thorac. Oncol. 2010, 5, 220–228. [Google Scholar] [CrossRef]
- Pignon, J.P.; Tribodet, H.; Scagliotti, G.V.; Douillard, J.Y.; Shepherd, F.A.; Stephens, R.J.; Dunant, A.; Torri, V.; Rosell, R.; Seymour, L.; et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J. Clin. Oncol. 2008, 26, 3552–3559. [Google Scholar] [CrossRef]
- Liu, J.; Blake, S.J.; Yong, M.C.; Harjunpää, H.; Ngiow, S.F.; Takeda, K.; Young, A.; O’Donnell, J.S.; Allen, S.; Smyth, M.J.; et al. Improved Efficacy of Neoadjuvant Compared to Adjuvant Immunotherapy to Eradicate Metastatic Disease. Cancer Discov. 2016, 6, 1382–1399. [Google Scholar] [CrossRef]
- Patel, S.P.; Othus, M.; Chen, Y.; Wright, G.P.; Yost, K.J.; Hyngstrom, J.R.; Hu-Lieskovan, S.; Lao, C.D.; Fecher, L.A.; Truong, T.-G.; et al. Neoadjuvant–Adjuvant or Adjuvant-Only Pembrolizumab in Advanced Melanoma. N. Engl. J. Med. 2023, 388, 813–823. [Google Scholar] [CrossRef]
- Suda, K.; Mitsudomi, T. Drug Tolerance to EGFR Tyrosine Kinase Inhibitors in Lung Cancers with EGFR Mutations. Cells 2021, 10, 1590. [Google Scholar] [CrossRef]
- Suda, K.; Bunn, P.A., Jr.; Rivard, C.J.; Mitsudomi, T.; Hirsch, F.R. Primary Double-Strike Therapy for Cancers to Overcome EGFR Kinase Inhibitor Resistance: Proposal from the Bench. J. Thorac. Oncol. 2017, 12, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Wakelee, H.; Liberman, M.; Kato, T.; Tsuboi, M.; Lee, S.-H.; Gao, S.; Chen, K.-N.; Dooms, C.; Majem, M.; Eigendorff, E.; et al. Perioperative Pembrolizumab for Early-Stage Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Heymach, J.V.; Harpole, D.; Mitsudomi, T.; Taube, J.M.; Galffy, G.; Hochmair, M.; Winder, T.; Zukov, R.; Garbaos, G.; Gao, S.; et al. Abstract CT005: AEGEAN: A phase 3 trial of neoadjuvant durvalumab + chemotherapy followed by adjuvant durvalumab in patients with resectable NSCLC. Cancer Res. 2023, 83, CT005. [Google Scholar] [CrossRef]
- Ohara, S.; Suda, K.; Sakai, K.; Nishino, M.; Chiba, M.; Shimoji, M.; Takemoto, T.; Fujino, T.; Koga, T.; Hamada, A.; et al. Prognostic implications of preoperative versus postoperative circulating tumor DNA in surgically resected lung cancer patients: A pilot study. Transl. Lung Cancer Res. 2020, 9, 1915–1923. [Google Scholar] [CrossRef]
- Abbosh, C.; Frankell, A.M.; Harrison, T.; Kisistok, J.; Garnett, A.; Johnson, L.; Veeriah, S.; Moreau, M.; Chesh, A.; Chaunzwa, T.L.; et al. Tracking early lung cancer metastatic dissemination in TRACERx using ctDNA. Nature 2023, 616, 553–562. [Google Scholar] [CrossRef]
- Gale, D.; Heider, K.; Ruiz-Valdepenas, A.; Hackinger, S.; Perry, M.; Marsico, G.; Rundell, V.; Wulff, J.; Sharma, G.; Knock, H.; et al. Residual ctDNA after treatment predicts early relapse in patients with early-stage non-small cell lung cancer. Ann. Oncol. 2022, 33, 500–510. [Google Scholar] [CrossRef]
- Felip, E.; Rosell, R.; Maestre, J.A.; Rodríguez-Paniagua, J.M.; Morán, T.; Astudillo, J.; Alonso, G.; Borro, J.M.; González-Larriba, J.L.; Torres, A.; et al. Preoperative Chemotherapy Plus Surgery versus Surgery Plus Adjuvant Chemotherapy versus Surgery Alone in Early-Stage Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2010, 28, 3138–3145. [Google Scholar] [CrossRef]
- Rosner, S.; Reuss, J.E.; Zahurak, M.; Zhang, J.; Zeng, Z.; Taube, J.; Anagnostou, V.; Smith, K.N.; Riemer, J.; Illei, P.B.; et al. Five-Year Clinical Outcomes after Neoadjuvant Nivolumab in Resectable Non–Small Cell Lung Cancer. Clin. Cancer Res. 2023, 29, 705–710. [Google Scholar] [CrossRef]
- Chaft, J.E.; Oezkan, F.; Kris, M.G.; Bunn, P.A.; Wistuba, I.I.; Kwiatkowski, D.J.; Owen, D.H.; Tang, Y.; Johnson, B.E.; Lee, J.M.; et al. Neoadjuvant atezolizumab for resectable non-small cell lung cancer: An open-label, single-arm phase II trial. Nat. Med. 2022, 28, 2155–2161. [Google Scholar] [CrossRef]
- Wislez, M.; Mazieres, J.; Lavole, A.; Zalcman, G.; Carre, O.; Egenod, T.; Caliandro, R.; Dubos-Arvis, C.; Jeannin, G.; Molinier, O.; et al. Neoadjuvant durvalumab for resectable non-small-cell lung cancer (NSCLC): Results from a multicenter study (IFCT-1601 IONESCO). J. ImmunoTherapy Cancer 2022, 10, e005636. [Google Scholar] [CrossRef] [PubMed]
- Altorki, N.K.; McGraw, T.E.; Borczuk, A.C.; Saxena, A.; Port, J.L.; Stiles, B.M.; Lee, B.E.; Sanfilippo, N.J.; Scheff, R.J.; Pua, B.B.; et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: A single-centre, randomised phase 2 trial. Lancet Oncol. 2021, 22, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Besse, B.; Adam, J.; Cozic, N.; Chaput-Gras, N.; Planchard, D.; Mezquita, L.; Masip, J.R.; Lavaud, P.; Naltet, C.; Gazzah, A.; et al. 1215O—SC Neoadjuvant atezolizumab (A) for resectable non-small cell lung cancer (NSCLC): Results from the phase II PRINCEPS trial. Ann. Oncol. 2020, 31, S794–S795. [Google Scholar] [CrossRef]
- Kagimoto, A.; Tsutani, Y.; Mimae, T.; Miyata, Y.; Ikeda, N.; Ito, H.; Maniwa, Y.; Suzuki, K.; Tsuboi, M.; Yoshimura, K.; et al. Preoperative nivolumab to evaluate pathological response in patients with stage I non-small cell lung cancer: A study protocol of phase II trial (POTENTIAL). BMJ Open 2021, 11, e043234. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Reuss, J.E.; Anagnostou, V.; Cottrell, T.R.; Smith, K.N.; Verde, F.; Zahurak, M.; Lanis, M.; Murray, J.C.; Chan, H.Y.; McCarthy, C.; et al. Neoadjuvant nivolumab plus ipilimumab in resectable non-small cell lung cancer. J. ImmunoTherapy Cancer 2020, 8, e001282. [Google Scholar] [CrossRef]
- Cascone, T.; William, W.N., Jr.; Weissferdt, A.; Leung, C.H.; Lin, H.Y.; Pataer, A.; Godoy, M.C.B.; Carter, B.W.; Federico, L.; Reuben, A.; et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: The phase 2 randomized NEOSTAR trial. Nat. Med. 2021, 27, 504–514. [Google Scholar] [CrossRef]
- Mamdani, H.; Matosevic, S.; Khalid, A.B.; Durm, G.; Jalal, S.I. Immunotherapy in Lung Cancer: Current Landscape and Future Directions. Front. Immunol. 2022, 13, 823618. [Google Scholar] [CrossRef]
- Schuler, M.H.H.; Cuppens, K.; Ploenes, T.; Vanbockrijck, M.; Wiesweg, M.; Darwiche, K.; Schramm, A.; Maes, B.; Hegedus, B.; Schildhaus, H.U.; et al. LBA37 A randomized, multicentric phase II study of preoperative nivolumab plus relatlimab or nivolumab in patients with resectable non-small cell lung cancer (NEOpredict-Lung). Ann. Oncol. 2022, 33, S1404. [Google Scholar] [CrossRef]
- Cascone, T.; García-Campelo, R.; Spicer, J.; Weder, W.; Daniel, D.; Spigel, D.; Hussein, M.; Mazieres, J.; Oliveira, J.; Yau, E.; et al. Abstract CT011: NeoCOAST: Open-label, randomized, phase 2, multidrug platform study of neoadjuvant durvalumab alone or combined with novel agents in patients (pts) with resectable, early-stage non-small-cell lung cancer (NSCLC). Cancer Res. 2022, 82, CT011. [Google Scholar] [CrossRef]
- Shu, C.A.; Gainor, J.F.; Awad, M.M.; Chiuzan, C.; Grigg, C.M.; Pabani, A.; Garofano, R.F.; Stoopler, M.B.; Cheng, S.K.; White, A.; et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Zinner, R.; Axelrod, R.; Solomides, C.C.; Cowan, S.; Leiby, B.; Bhatia, A.K.; Sundermeyer, M.L.; Hooper, D.C.; Harshyne, L.; Lu-Yao, G.L.; et al. Neoadjuvant nivolumab (N) plus cisplatin (C)/pemetrexed (P) or cisplatin /gemcitabine (G) in resectable NSCLC. J. Clin. Oncol. 2020, 38, 9051. [Google Scholar] [CrossRef]
- Rothschild, S.I.; Zippelius, A.; Eboulet, E.I.; Prince, S.S.; Betticher, D.; Bettini, A.; Früh, M.; Joerger, M.; Lardinois, D.; Gelpke, H.; et al. SAKK 16/14: Durvalumab in Addition to Neoadjuvant Chemotherapy in Patients with Stage IIIA(N2) Non–Small-Cell Lung Cancer—A Multicenter Single-Arm Phase II Trial. J. Clin. Oncol. 2021, 39, 2872–2880. [Google Scholar] [CrossRef] [PubMed]
- Provencio, M.; Nadal, E.; Insa, A.; García-Campelo, M.R.; Casal-Rubio, J.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; De Castro Carpeño, J.; et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 1413–1422. [Google Scholar] [CrossRef]
- Suda, K. The ABCs of preventing hyperprogressive disease after immunotherapy: Awareness, biomarkers, and combination. J. Thorac. Dis. 2019, 11, S347–S351. [Google Scholar] [CrossRef] [PubMed]
- Heymach, J.V.; Mitsudomi, T.; Harpole, D.; Aperghis, M.; Jones, S.; Mann, H.; Fouad, T.M.; Reck, M. Design and Rationale for a Phase III, Double-Blind, Placebo-Controlled Study of Neoadjuvant Durvalumab + Chemotherapy Followed by Adjuvant Durvalumab for the Treatment of Patients With Resectable Stages II and III non-small-cell Lung Cancer: The AEGEAN Trial. Clin. Lung Cancer 2022, 23, e247–e251. [Google Scholar]
- Wu, L.; Zhang, W.; Zhang, P.; Wang, W.; Fang, W.; Xing, W.; Chen, Q.; Mei, J.; Yang, L.; Tan, L.; et al. Perioperative toripalimab + platinum-doublet chemotherapy vs chemotherapy in resectable stage II/III non-small cell lung cancer (NSCLC): Interim event-free survival (EFS) analysis of the phase III Neotorch study. J. Clin. Oncol. 2023, 41, 425126. [Google Scholar]
- Provencio, M.; Nadal, E.; González-Larriba, J.L.; Martinez-Marti, A.; Bernabe, R.; Bosch-Barrera, J.; Casal-Rubio, J.; Calvo, V.; Insa, A.; Ponce, S.; et al. Perioperative nivolumab and chemotherapy in stage III non–small-cell lung cancer. N. Engl. J. Med. 2023, 389, 504–513. [Google Scholar] [CrossRef]
- Rizvi, N.; Gandara, D.; Solomon, B.; Kim, A.; Brunelli, A.; Sun, S.; Gitlitz, B.; Tajima, K.; Lin, W.; Sandler, A.; et al. P2.17–27 IMpower030: Phase III Study Evaluating Neoadjuvant Treatment of Resectable Stage II-IIIB NSCLC with Atezolizumab + Chemotherapy. J. Thorac. Oncol. 2018, 13, S863. [Google Scholar] [CrossRef]
- Cascone, T.; Provencio, M.; Sepesi, B.; Lu, S.; Aanur, N.; Li, S.; Spicer, J. Checkmate 77T: A phase III trial of neoadjuvant nivolumab (NIVO) plus chemotherapy (chemo) followed by adjuvant nivo in resectable early-stage NSCLC. J. Clin. Oncol. 2020, 38, TPS9076. [Google Scholar] [CrossRef]
- Hamada, A.; Soh, J.; Hata, A.; Nakamatsu, K.; Shimokawa, M.; Yatabe, Y.; Oizumi, H.; Tsuboi, M.; Horinouchi, H.; Yoshino, I.; et al. Phase II Study of Neoadjuvant Concurrent Chemo-immuno-radiation Therapy Followed by Surgery and Adjuvant Immunotherapy for Resectable Stage IIIA-B (Discrete N2) Non–small-cell Lung Cancer: SQUAT trial (WJOG 12119L). Clin. Lung Cancer 2021, 22, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cho, J.; Lee, C.; Lee, C.Y.; Lee, J.G.; Kim, D.J.; Shim, H.S.; Lim, S.M.; Kim, H.R.; Cho, B.C.; et al. Phase Ib study of neoadjuvant concurrent chemoradiation plus durvalumab followed by surgery and adjuvant durvalumab for resectable stage III NSCLC. J. Clin. Oncol. 2023, 41, 8556. [Google Scholar] [CrossRef]
- Lemmon, C.A.; Videtic, G.M.M.; Murthy, S.; Stephans, K.L.; Shapiro, M.; Ahmad, U.; Raymond, D.; Velcheti, V.; Bribriesco, A.; Jia, X.; et al. A Phase 1 Study of Concurrent Neoadjuvant Pembrolizumab Plus Chemoradiation Followed by Consolidation Pembrolizumab in Patients With Resectable Stage IIIA NSCLC. JTO Clin. Res. Rep. 2022, 3, 100359. [Google Scholar] [CrossRef] [PubMed]
- Nobler, M.P. The abscopal effect in malignant lymphoma and its relationship to lymphocyte circulation. Radiology 1969, 93, 410–412. [Google Scholar] [CrossRef]
- Idris, B. 950O—Ipilimumab plus nivolumab and chemoradiotherapy followed by surgery in patients with resectable and borderline resectable lung cancer: The INCREASE trial. Ann. Oncol. 2022, 33, S982. [Google Scholar]
- Felip, E.; Altorki, N.; Zhou, C.; Vallieres, E.; Martínez-Marti, A.; Rittmeyer, A.; Chella, A.; Reck, M.; Goloborodko, O.; Huang, M.; et al. Overall survival with adjuvant atezolizumab after chemotherapy in resected stage II-IIIA non-small cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Ann. Oncol. 2023, in press. [Google Scholar] [CrossRef]
- Sands, J.M.; Mandrekar, S.J.; Kozono, D.; Oxnard, G.R.; Hillman, S.L.; Wigle, D.A.; Govindan, R.; Carlisle, J.; Gray, J.; Salama, J.K.; et al. Integration of immunotherapy into adjuvant therapy for resected non-small-cell lung cancer: ALCHEMIST chemo-IO (ACCIO). Immunotherapy 2021, 13, 727–734. [Google Scholar] [CrossRef]
- Ohara, S.; Suda, K.; Tomizawa, K.; Takemoto, T.; Fujino, T.; Hamada, A.; Koga, T.; Nishino, M.; Chiba, M.; Sato, K.; et al. Prognostic value of plasma fibrinogen and d-dimer levels in patients with surgically resected non-small cell lung cancer. Surg. Today 2020, 50, 1427–1433. [Google Scholar] [CrossRef]
- Buccheri, G.; Torchio, P.; Ferrigno, D. Plasma levels of D-dimer in lung carcinoma: Clinical and prognostic significance. Cancer 2003, 97, 3044–3052. [Google Scholar] [CrossRef]
- Garinet, S.; Wang, P.; Mansuet-Lupo, A.; Fournel, L.; Wislez, M.; Blons, H. Updated Prognostic Factors in Localized NSCLC. Cancers 2022, 14, 1400. [Google Scholar] [CrossRef]
- Suda, K.; Ohara, S.; Fujino, T.; Hamada, A.; Chiba, M.; Shimoji, M.; Takemoto, T.; Soh, J.; Mitsudomi, T. Frequent EGFR Mutations and Better Prognosis in Positron Emission Tomography-Negative, Solid-Type Lung Cancer. Clin. Lung Cancer 2022, 23, e60–e68. [Google Scholar] [CrossRef] [PubMed]
- Moding, E.J.; Nabet, B.Y.; Alizadeh, A.A.; Diehn, M. Detecting Liquid Remnants of Solid Tumors: Circulating Tumor DNA Minimal Residual Disease. Cancer Discov. 2021, 11, 2968–2986. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Guo, W.; Zhang, F.; Lv, F.; Ji, Y.; Peng, Y.; Chen, X.; Bao, H.; Xu, Y.; Shao, Y.; et al. Dynamic recurrence risk and adjuvant chemotherapy benefit prediction by ctDNA in resected NSCLC. Nat. Commun. 2021, 12, 6770. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Mei, J.; Kang, R.; Deng, S.; Chen, Y.; Yang, Y.; Feng, G.; Deng, Y.; Gan, F.; Lin, Y.; et al. Perioperative ctDNA-Based Molecular Residual Disease Detection for Non-Small Cell Lung Cancer: A Prospective Multicenter Cohort Study (LUNGCA-1). Clin. Cancer Res. 2022, 28, 3308–3317. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Das Thakur, M.; Srivastava, M.K.; Zou, W.; Xu, H.; Ballinger, M.; Felip, E.; Wakelee, H.; Altorki, N.K.; Reck, M.; et al. 2O IMpower010: Biomarkers of disease-free survival (DFS) in a phase III study of atezolizumab (atezo) vs best supportive care (BSC) after adjuvant chemotherapy in stage IB-IIIA NSCLC. Ann. Oncol. 2021, 32, S1374. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).