IFN-Type-I Response and Systemic Immunity in Rectal Adenocarcinoma Patients Treated with Conventional or Hypofractionated Neoadjuvant Radiotherapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Plasma and Peripheral Blood Mononuclear Cells Collection
2.3. ELISA
2.4. Flow Cytometry
2.5. Cell Line Experiments
2.6. Statistical Analysis
3. Results
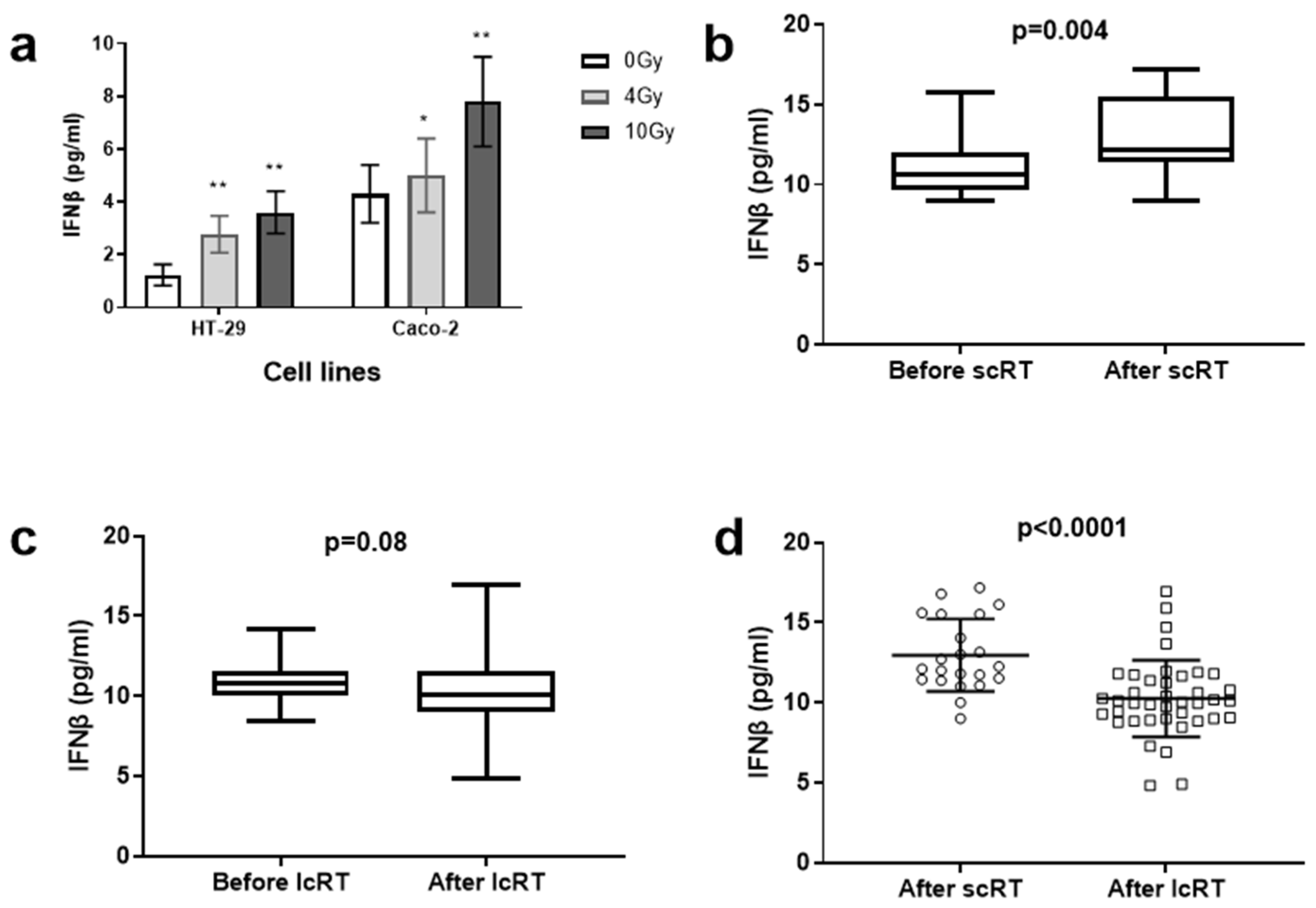
3.1. Cell Line Experiments
3.2. Plasma IFNβ Levels in the Two Cohorts
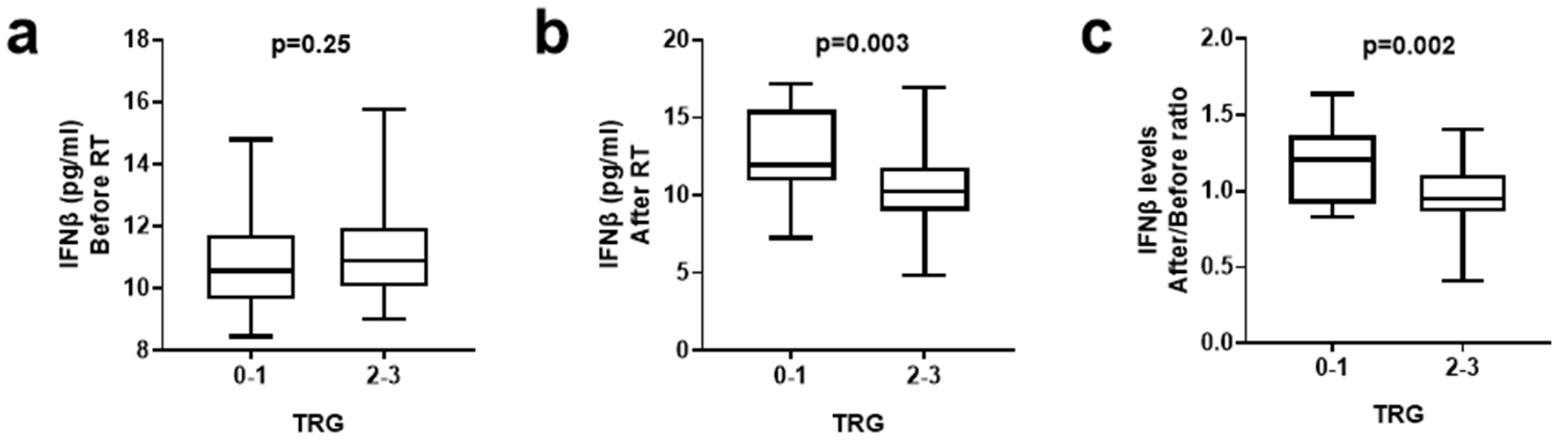
3.3. IFNβ Levels and Tumor Regression Grade
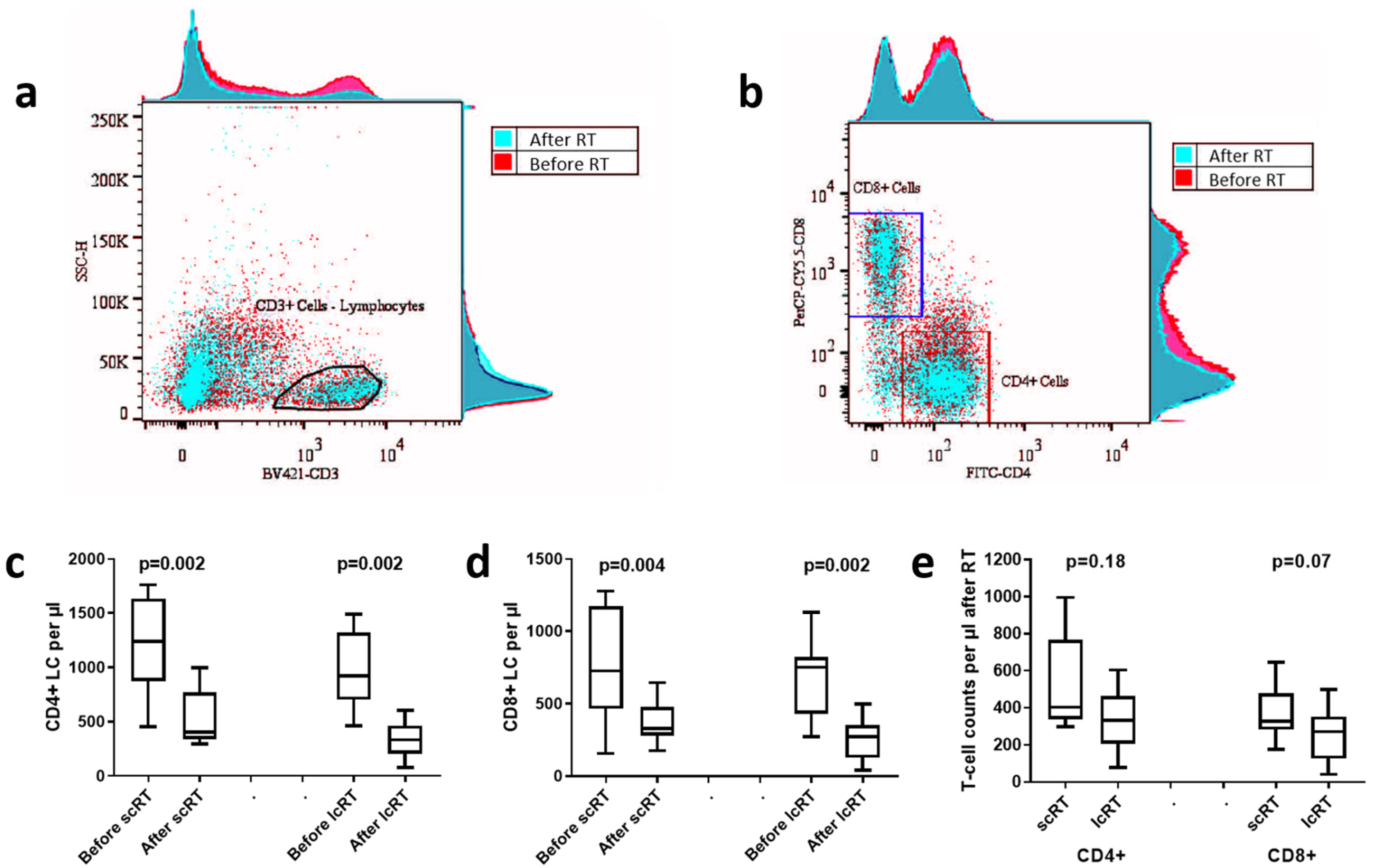
3.4. Lymphocyte Count Analysis
3.5. CD4/CD8 Lymphocyte Subset Analysis
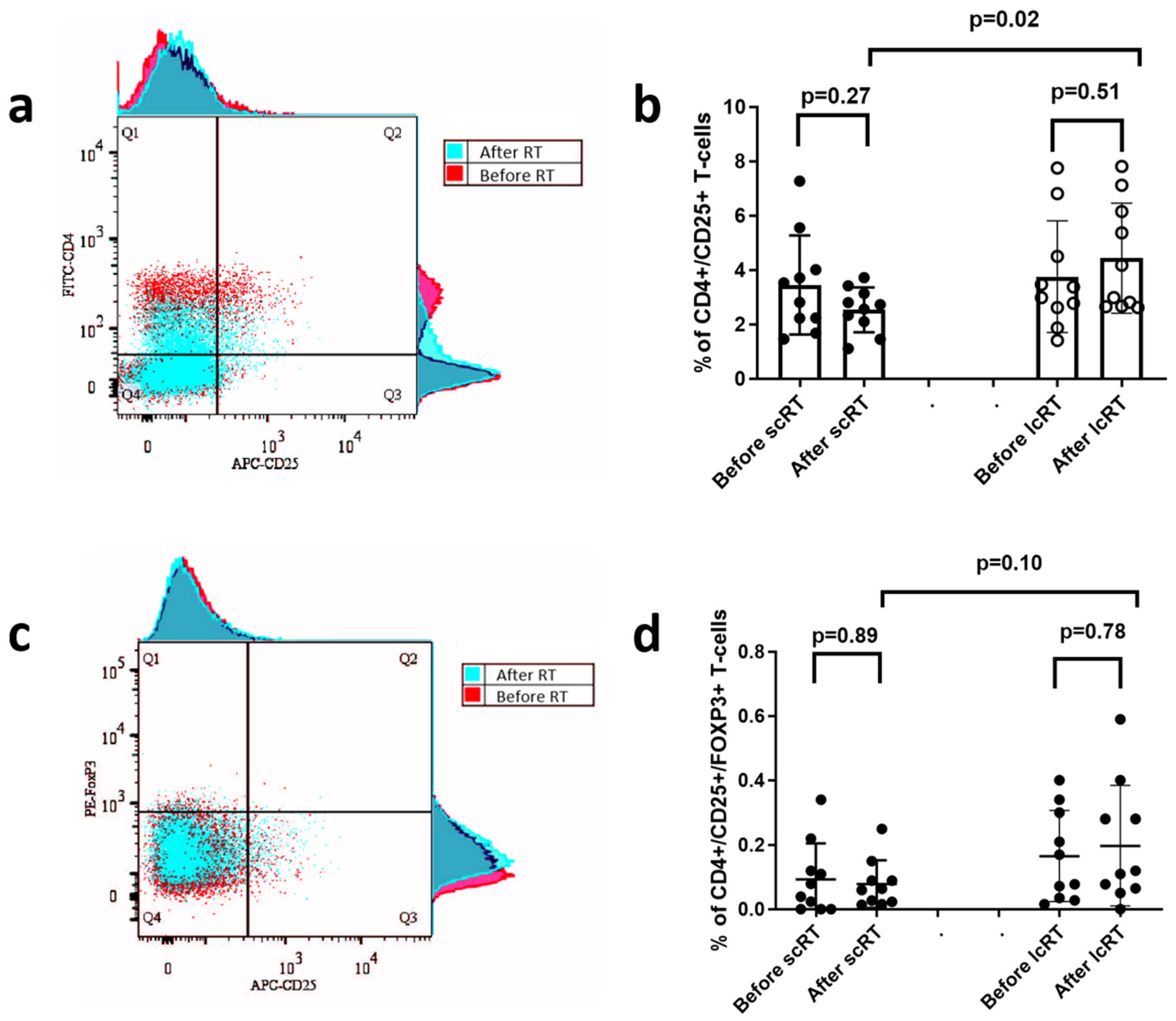
3.6. CD25+ and FOXP3+ Regulatory T-Cell Subset Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koukourakis, I.M.; Koukourakis, M.I. Combining the past and present to advance immuno-radiotherapy of cancer. Int. Rev. Immunol. 2023, 42, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-Year Survival Outcomes from the PACIFIC Trial: Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Theelen, W.; Chen, D.; Verma, V.; Hobbs, B.P.; Peulen, H.M.U.; Aerts, J.; Bahce, I.; Niemeijer, A.L.N.; Chang, J.Y.; de Groot, P.M.; et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Respir. Med. 2021, 9, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liang, H.; Xu, M.; Yang, X.; Burnette, B.; Arina, A.; Li, X.D.; Mauceri, H.; Beckett, M.; Darga, T.; et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 2014, 41, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, I.M.; Xanthopoulou, E.; Sgouras, T.I.; Kouroupi, M.; Giatromanolaki, A.; Kouloulias, V.; Tiniakos, D.; Zygogianni, A. Preoperative chemoradiotherapy induces multiple pathways related to anti-tumour immunity in rectal cancer. Br. J. Cancer 2023, 129, 1852–1862. [Google Scholar] [CrossRef] [PubMed]
- Formenti, S.C.; Rudqvist, N.P.; Golden, E.; Cooper, B.; Wennerberg, E.; Lhuillier, C.; Vanpouille-Box, C.; Friedman, K.; Ferrari de Andrade, L.; Wucherpfennig, K.W.; et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat. Med. 2018, 24, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Demaria, S.; Guha, C.; Schoenfeld, J.; Morris, Z.; Monjazeb, A.; Sikora, A.; Crittenden, M.; Shiao, S.; Khleif, S.; Gupta, S.; et al. Radiation dose and fraction in immunotherapy: One-size regimen does not fit all settings, so how does one choose? J. Immunother. Cancer 2021, 9, e002038. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Bujko, K.; Wyrwicz, L.; Rutkowski, A.; Malinowska, M.; Pietrzak, L.; Krynski, J.; Michalski, W.; Oledzki, J.; Kusnierz, J.; Zajac, L.; et al. Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: Results of a randomized phase III study. Ann. Oncol. 2016, 27, 834–842. [Google Scholar] [CrossRef]
- Ngan, S.Y.; Burmeister, B.; Fisher, R.J.; Solomon, M.; Goldstein, D.; Joseph, D.; Ackland, S.P.; Schache, D.; McClure, B.; McLachlan, S.A.; et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J. Clin. Oncol. 2012, 30, 3827–3833. [Google Scholar] [CrossRef] [PubMed]
- Erlandsson, J.; Holm, T.; Pettersson, D.; Berglund, A.; Cedermark, B.; Radu, C.; Johansson, H.; Machado, M.; Hjern, F.; Hallbook, O.; et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): A multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017, 18, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, I.M.; Kouloulias, V.; Tiniakos, D.; Georgakopoulos, I.; Zygogianni, A. Current status of locally advanced rectal cancer therapy and future prospects. Crit. Rev. Oncol. Hematol. 2023, 186, 103992. [Google Scholar] [CrossRef] [PubMed]
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Shen, L.J.; Wan, J.F.; Zhang, H.; Wang, Y.; Wu, X.; Wang, J.W.; Wang, R.J.; Sun, Y.Q.; Tong, T.; et al. [Short-course radiotherapy combined with CAPOX and PD-1 inhibitor for the total neoadjuvant therapy of locally advanced rectal cancer: The preliminary single-center findings of a prospective, multicentre, randomized phase II trial (TORCH)]. Zhonghua Wei Chang. Wai Ke Za Zhi 2023, 26, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Suwinski, R.; Wzietek, I.; Tarnawski, R.; Namysl-Kaletka, A.; Kryj, M.; Chmielarz, A.; Wydmanski, J. Moderately low alpha/beta ratio for rectal cancer may best explain the outcome of three fractionation schedules of preoperative radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, M.I.; Damilakis, J. LQ-based model for biological radiotherapy planning. Med. Dosim. 1994, 19, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Mace, A.G.; Pai, R.K.; Stocchi, L.; Kalady, M.F. American Joint Committee on Cancer and College of American Pathologists regression grade: A new prognostic factor in rectal cancer. Dis. Colon Rectum 2015, 58, 32–44. [Google Scholar] [CrossRef]
- Fenton, S.E.; Saleiro, D.; Platanias, L.C. Type I and II Interferons in the Anti-Tumor Immune Response. Cancers 2021, 13, 1037. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Giatromanolaki, A. Tumor draining lymph nodes, immune response, and radiotherapy: Towards a revisal of therapeutic principles. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188704. [Google Scholar] [CrossRef] [PubMed]
- Goedegebuure, R.S.A.; Kleibeuker, E.A.; Buffa, F.M.; Castricum, K.C.M.; Haider, S.; Schulkens, I.A.; Ten Kroode, L.; van den Berg, J.; Jacobs, M.; van Berkel, A.M.; et al. Interferon- and STING-independent induction of type I interferon stimulated genes during fractionated irradiation. J. Exp. Clin. Cancer Res. 2021, 40, 161. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.J.; Zangl, L.M.; Hyun, M.; Massoud, E.; Schroeder, K.; Alexandridis, R.A.; Morris, Z.S. ATM inhibition augments type I interferon response and antitumor T-cell immunity when combined with radiation therapy in murine tumor models. J. Immunother. Cancer 2023, 11, e007474. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, M.H.; Carmi, Y.; Reticker-Flynn, N.E.; Kwek, S.S.; Madhireddy, D.; Martins, M.M.; Gherardini, P.F.; Prestwood, T.R.; Chabon, J.; Bendall, S.C.; et al. Systemic Immunity Is Required for Effective Cancer Immunotherapy. Cell 2017, 168, 487–502.e415. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.Y.; Wang, S.L.; Song, Y.W.; Jin, J.; Wang, W.H.; Liu, Y.P.; Ren, H.; Fang, H.; Tang, Y.; Zhao, X.R.; et al. Radiation-Induced Lymphopenia Predicts Poorer Prognosis in Patients with Breast Cancer: A Post Hoc Analysis of a Randomized Controlled Trial of Postmastectomy Hypofractionated Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, S.G. Field size effects on the risk and severity of treatment-induced lymphopenia in patients undergoing radiation therapy for solid tumors. Adv. Radiat. Oncol. 2018, 3, 512–519. [Google Scholar] [CrossRef]
- Gao, R.; Shi, G.P.; Wang, J. Functional Diversities of Regulatory T Cells in the Context of Cancer Immunotherapy. Front. Immunol. 2022, 13, 833667. [Google Scholar] [CrossRef] [PubMed]
- An, N.; Wang, H.; Jia, W.; Jing, W.; Liu, C.; Zhu, H.; Yu, J. The prognostic role of circulating CD8(+) T cell proliferation in patients with untreated extensive stage small cell lung cancer. J. Transl. Med. 2019, 17, 402. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Zu, S.; Lu, Y. Characteristics of circulating adaptive immune cells in patients with colorectal cancer. Sci. Rep. 2022, 12, 18166. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Hu, P.; Zhang, J. The Correlation Between Low-Dose Radiotherapy Area of the Mediastinum and CD8+T Cells and the Efficacy of Radiotherapy for Non-Small Cell Lung Cancer. Cancer Manag. Res. 2024, 16, 23–35. [Google Scholar] [CrossRef]
- Novikov, S.N.; Baldueva, I.A.; Zozulya, A.Y.; Emelyanova, N.V.; Girdyuk, D.V.; Arsenyev, A.I.; Alexandrovna, E.; Tyuryaeva, E.I.; Antipov, E.; Girshovich, M.M.; et al. Peripheral blood lymphocyte changes after stereotactic ablative body radiotherapy to lung or liver metastases in patients with oligometastatic cancers. Radiat. Oncol. J. 2023, 41, 23–31. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koukourakis, I.M.; Xanthopoulou, E.; Koukourakis, M.I.; Tiniakos, D.; Kouloulias, V.; Zygogianni, A. IFN-Type-I Response and Systemic Immunity in Rectal Adenocarcinoma Patients Treated with Conventional or Hypofractionated Neoadjuvant Radiotherapy. Biomolecules 2024, 14, 448. https://doi.org/10.3390/biom14040448
Koukourakis IM, Xanthopoulou E, Koukourakis MI, Tiniakos D, Kouloulias V, Zygogianni A. IFN-Type-I Response and Systemic Immunity in Rectal Adenocarcinoma Patients Treated with Conventional or Hypofractionated Neoadjuvant Radiotherapy. Biomolecules. 2024; 14(4):448. https://doi.org/10.3390/biom14040448
Chicago/Turabian StyleKoukourakis, Ioannis M., Erasmia Xanthopoulou, Michael I. Koukourakis, Dina Tiniakos, Vassilis Kouloulias, and Anna Zygogianni. 2024. "IFN-Type-I Response and Systemic Immunity in Rectal Adenocarcinoma Patients Treated with Conventional or Hypofractionated Neoadjuvant Radiotherapy" Biomolecules 14, no. 4: 448. https://doi.org/10.3390/biom14040448




