Evaluation of the Mechanisms Involved in the Development of Bladder Toxicity following Exposure to Occupational Bladder Cancer Causative Chemicals Using DNA Adductome Analysis
Abstract
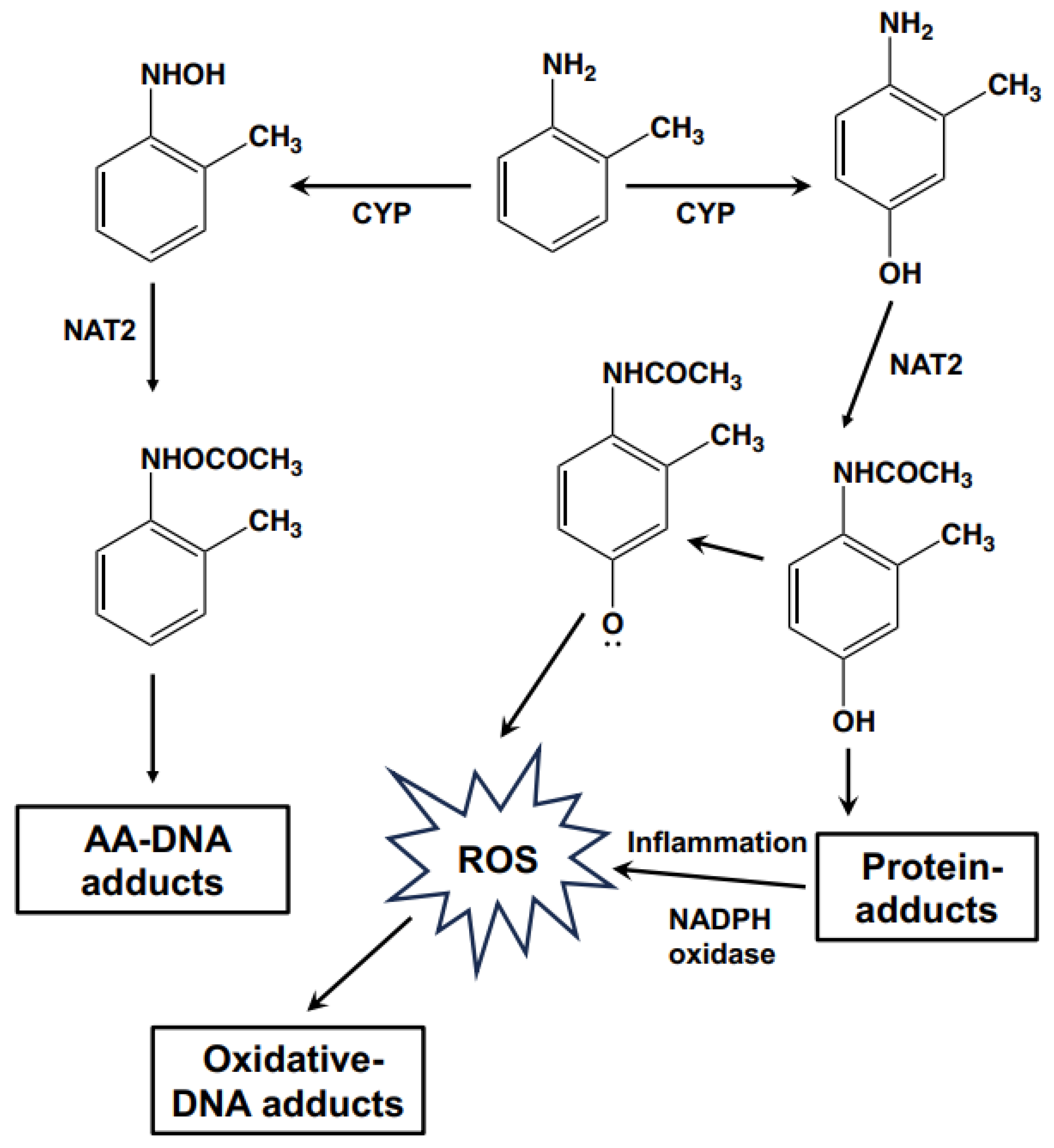
:1. Introduction
2. Materials and Methods
2.1. Test Chemical
2.2. Experimental Animals
2.3. Experimental Design
2.4. Immunohistochemistry and TUNEL Assays
2.5. DNA Adductome Analysis
2.6. Statistics
3. Results
3.1. Experiment 1
3.1.1. Body and Organ Weights and Consumption of Food and Water
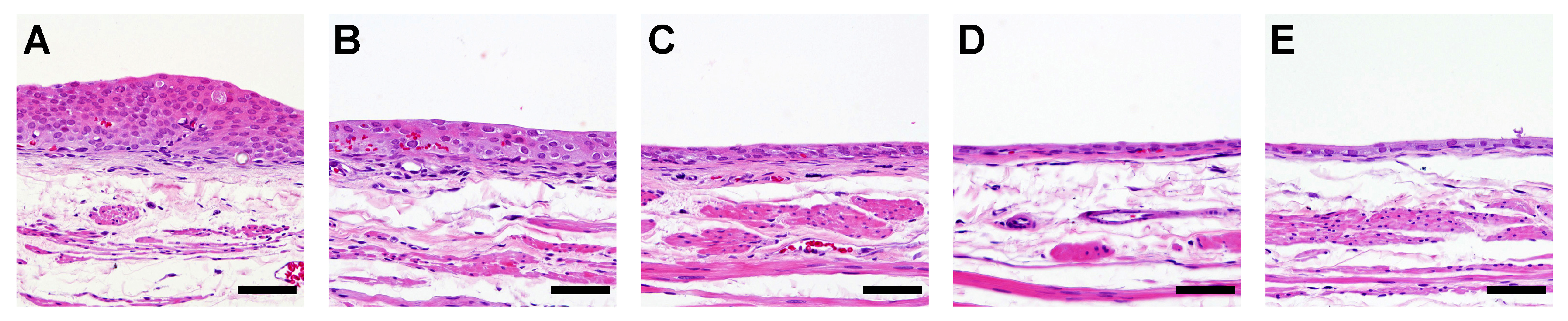
3.1.2. Histological and Immunohistological Analyses of the Urothelium
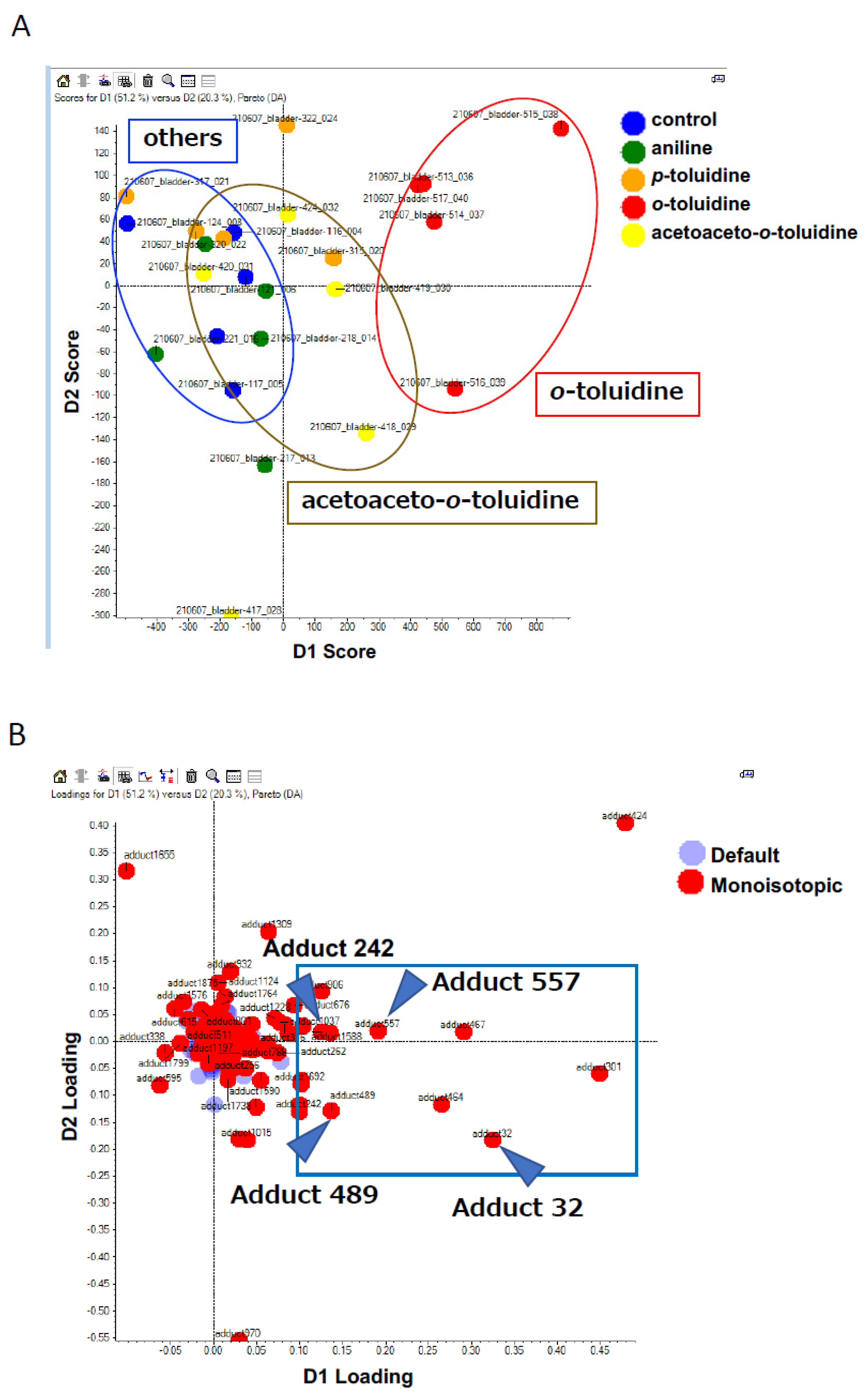
3.1.3. Comprehensive Analysis of DNA Adducts Induced by OTD and AAOT Treatment
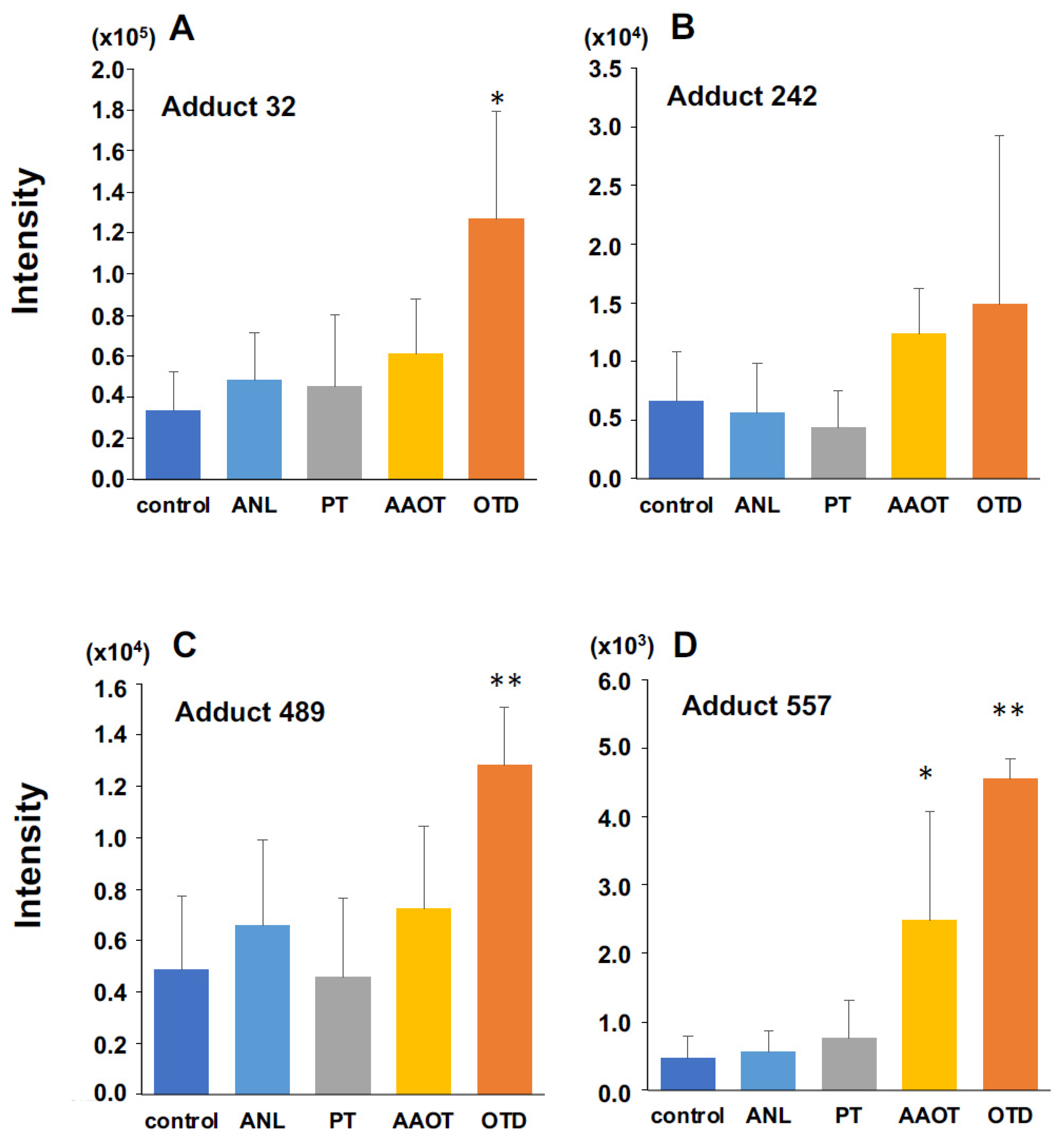
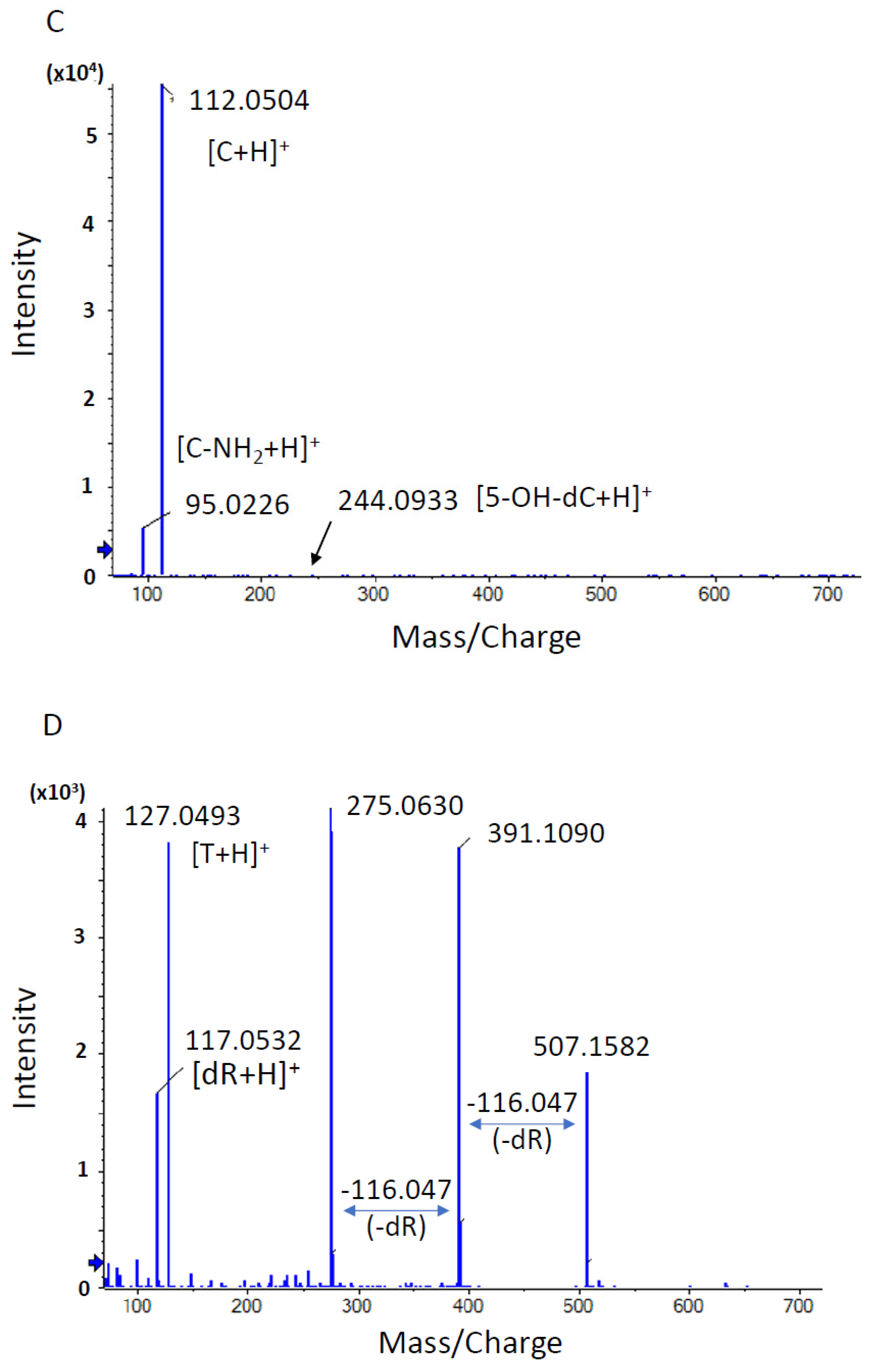
3.1.4. Identification of DNA Adducts Correlated with OTD and AAOT Treatment Using an In-House DNA Adduct Database
3.2. Experiment 2
3.2.1. Body and Organ Weights and Consumption of Food and Water
3.2.2. Histological and Immunohistological Analyses of the Urothelium
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Cumberbatch, M.G.K.; Noon, A.P. Epidemiology, aetiology and screening of bladder cancer. Transl. Androl. Urol. 2019, 8, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Loomis, D.; Guha, N.; Hall, A.L.; Straif, K. Identifying occupational carcinogens: An update from the IARC monographs. Occup. Environ. Med. 2018, 75, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Omae, K.; Takebayashi, T.; Tanaka, S.; Koda, S. An epidemic of bladder cancer: Ten cases of bladder cancer in male Japanese workers exposed to ortho-toluidine. J. Occup. Health 2018, 60, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Yukimatsu, N.; Gi, M.; Okuno, T.; Fujioka, M.; Suzuki, S.; Kakehashi, A.; Yanagiba, Y.; Suda, M.; Koda, S.; Nakatani, T.; et al. Promotion effects of acetoaceto-o-toluidide on N-butyl-N-(4-hydroxybutyl)nitrosamine-induced bladder carcinogenesis in rats. Arch. Toxicol. 2019, 93, 3617–3631. [Google Scholar] [CrossRef] [PubMed]
- IARC. Chemical Agents and Related Occupations; WHO Press: Lyon, France, 2012; p. 562. [Google Scholar]
- Beyerbach, A.; Farmer, P.B.; Sabbioni, G. Synthesis and analysis of DNA adducts of aryl amines. Biomarkers 1996, 1, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.R.; Sabbioni, G. Identification of DNA adducts using HPLC/MS/MS following in vitro and in vivo experiments with arylamines and nitroarenes. Chem. Res. Toxicol. 2003, 16, 1251–1263. [Google Scholar] [CrossRef]
- Guo, J.; Villalta, P.W.; Weight, C.J.; Bonala, R.; Johnson, F.; Rosenquist, T.A.; Turesky, R.J. Targeted and untargeted detection of DNA adducts of aromatic amine carcinogens in human bladder by ultraperformance liquid chromatography-high-resolution mass spectrometry. Chem. Res. Toxicol. 2018, 31, 1382–1397. [Google Scholar] [CrossRef]
- Ishino, K.; Kato, T.; Kato, M.; Shibata, T.; Watanabe, M.; Wakabayashi, K.; Nakagama, H.; Totsuka, Y. Comprehensive DNA adduct analysis reveals pulmonary inflammatory response contributes to genotoxic action of magnetite nanoparticles. Int. J. Mol. Sci. 2015, 16, 3474–3492. [Google Scholar] [CrossRef]
- Stornetta, A.; Villalta, P.W.; Hecht, S.S.; Sturla, S.J.; Balbo, S. Screening for DNA alkylation mono and cross-linked adducts with a comprehensive LC-MS(3) Adductomic approach. Anal. Chem. 2015, 87, 11706–11713. [Google Scholar] [CrossRef]
- Totsuka, Y.; Maesako, Y.; Ono, H.; Nagai, M.; Kato, M.; Gi, M.; Wanibuchi, H.; Fukushima, S.; Shiizaki, K.; Nakagama, H. Comprehensive analysis of DNA adducts (DNA adductome analysis) in the liver of rats treated with 1,4-dioxane. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2020, 96, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Totsuka, Y.; Watanabe, M.; Lin, Y. New horizons of DNA adductome for exploring environmental causes of cancer. Cancer Sci. 2021, 112, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Totsuka, Y.; Lin, Y.; He, Y.; Ishino, K.; Sato, H.; Kato, M.; Nagai, M.; Elzawahry, A.; Totoki, Y.; Nakamura, H.; et al. DNA adductome analysis identifies N-Nitrosopiperidine involved in the etiology of esophageal cancer in Cixian, China. Chem. Res. Toxicol. 2019, 32, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Stolk, J.; Hiltermann, T.J.; Dijkman, J.H.; Verhoeven, A.J. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am. J. Respir. Cell. Mol. Biol. 1994, 11, 95–102. [Google Scholar] [CrossRef]
- Suzuki, S.; Arnold, L.L.; Pennington, K.L.; Kakiuchi-Kiyota, S.; Cohen, S.M. Effects of co-administration of dietary sodium arsenite and an NADPH oxidase inhibitor on the rat bladder epithelium. Toxicology 2009, 261, 41–46. [Google Scholar] [CrossRef]
- Suzuki, S.; Cohen, S.M.; Arnold, L.L.; Pennington, K.L.; Gi, M.; Kato, H.; Naiki, T.; Naiki-Ito, A.; Wanibuchi, H.; Takahashi, S. Cell proliferation of rat bladder urothelium induced by nicotine is suppressed by the NADPH oxidase inhibitor, apocynin. Toxicol. Lett. 2021, 336, 32–38. [Google Scholar] [CrossRef]
- Suzuki, S.; Shiraga, K.; Sato, S.; Punfa, W.; Naiki-Ito, A.; Yamashita, Y.; Shirai, T.; Takahashi, S. Apocynin, an NADPH oxidase inhibitor, suppresses rat prostate carcinogenesis. Cancer Sci. 2013, 104, 1711–1717. [Google Scholar] [CrossRef]
- Kato, A.; Naiki-Ito, A.; Nakazawa, T.; Hayashi, K.; Naitoh, I.; Miyabe, K.; Shimizu, S.; Kondo, H.; Nishi, Y.; Yoshida, M.; et al. Chemopreventive effect of resveratrol and apocynin on pancreatic carcinogenesis via modulation of nuclear phosphorylated GSK3beta and ERK1/2. Oncotarget 2015, 6, 42963–42975. [Google Scholar] [CrossRef]
- Fuji, S.; Suzuki, S.; Naiki-Ito, A.; Kato, H.; Hayakawa, M.; Yamashita, Y.; Kuno, T.; Takahashi, S. The NADPH oxidase inhibitor apocynin suppresses preneoplastic liver foci of rats. Toxicol. Pathol. 2017, 45, 544–550. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. Bioassay of o-toluidine hydrochloride for possible carcinogenicity. Natl. Cancer Inst. Carcinog. Tech. Rep. Ser. 1979, 153, 1–147. [Google Scholar]
- Frazier, K.S.; Seely, J.C.; Hard, G.C.; Betton, G.; Burnett, R.; Nakatsuji, S.; Nishikawa, A.; Durchfeld-Meyer, B.; Bube, A. Proliferative and nonproliferative lesions of the rat and mouse urinary system. Toxicol. Pathol. 2012, 40, 14S–86S. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, S.; Takasu, S.; Tsukamoto, T.; Mutoh, M.; Masuda, S.; Sugimura, T.; Wakabayashi, K.; Totsuka, Y. Induction of glandular stomach cancers in Helicobacter pylori-infected Mongolian gerbils by 1-nitrosoindole-3-acetonitrile. Int. J. Cancer 2012, 130, 259–266. [Google Scholar] [CrossRef]
- Toyoda, T.; Matsushita, K.; Morikawa, T.; Yamada, T.; Miyoshi, N.; Ogawa, K. Distinct differences in the mechanisms of mucosal damage and gamma-H2AX formation in the rat urinary bladder treated with o-toluidine and o-anisidine. Arch. Toxicol. 2019, 93, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Okuno, T.; Gi, M.; Fujioka, M.; Yukimatu, N.; Kakehashi, A.; Takeuchi, A.; Endo, G.; Endo, Y.; Wanibuchi, H. Acetoaceto-o-toluidide enhances cellular proliferative activity in the urinary bladder of rats. Toxicol. Sci. 2019, 169, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M.; Yahagi, T.; Honda, M.; Seino, Y.; Matsushima, T.; Sugimura, T. Demonstration of mutagenicity of aniline and o-toluidine by norharman. Proc. Jpn. Acad. Ser. B 1977, 53, 34–37. [Google Scholar] [CrossRef]
- English, J.C.; Bhat, V.S.; Ball, G.L.; McLellan, C.J. Establishing a total allowable concentration of o-toluidine in drinking water incorporating early lifestage exposure and susceptibility. Regul. Toxicol. Pharmacol. 2012, 64, 269–284. [Google Scholar] [CrossRef]
- Böhm, F.; Schmid, D.; Denzinger, S.; Wieland, W.F.; Richter, E. DNA adducts of Ortho-toluidine in human bladder. Biomarkers 2011, 16, 120–128. [Google Scholar] [CrossRef]
- Skipper, P.L.; Kim, M.Y.; Sun, H.L.; Wogan, G.N.; Tannenbaum, S.R. Monocyclic aromatic amines as potential human carcinogens: Old is new again. Carcinogenesis 2010, 31, 50–58. [Google Scholar] [CrossRef]
- Chao, M.W.; Erkekoglu, P.; Tseng, C.Y.; Ye, W.; Trudel, L.J.; Skipper, P.L.; Tannenbaum, S.R.; Wogan, G.N. Intracellular generation of ROS by 3,5-dimethylaminophenol: Persistence, cellular response, and impact of molecular toxicity. Toxicol. Sci. 2014, 141, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.W.; Erkekoglu, P.; Tseng, C.Y.; Ye, W.; Trudel, L.J.; Skipper, P.L.; Tannenbaum, S.R.; Wogan, G.N. Protective effects of ascorbic acid against the genetic and epigenetic alterations induced by 3,5-dimethylaminophenol in AA8 cells. J. Appl. Toxicol. 2015, 35, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, T.; Akagi, J.; Cho, Y.M.; Mizuta, Y.; Onami, S.; Suzuki, I.; Ogawa, K. Detection of γ-H2AX, a biomarker for DNA double-strand breaks, in urinary bladders of N-butyl-N-(4-hydroxybutyl)-nitrosaminetreated rats. J. Toxicol. Pathol. 2013, 26, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Totsuka, Y.; Wakabayashi, K. Biological significance of aminophenyl-β-carboline derivatives formed from co-mutagenic action of β-carbolines and aniline and o-toluidine and its effect on tumorigenesis in humans: A review. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2020, 850–851, 503148. [Google Scholar] [CrossRef]
- Toyoda, T.; Totsuka, Y.; Matsushita, K.; Morikawa, T.; Miyoshi, N.; Wakabayashi, K.; Ogawa, K. γ-H2AX formation in the urinary bladder of rats treated with two norharman derivatives obtained from o-toluidine and aniline. J. Appl. Toxicol. 2018, 38, 537–543. [Google Scholar] [CrossRef]


| Treatment | No. of Rats | Body Weight (g) | Liver (No. = 6) | Consumption | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Absolute (g) | Relative (%) | Food (g/day) | Water (g/day) | |||||||||||||
| Control | 18 | 228.2 | ± | 8.3 | 8.1 | ± | 0.3 | 3.5 | ± | 0.1 | 13.2 | ± | 0.8 | 19.7 | ± | 1.1 |
| ANL | 18 | 214.7 | ± | 6.5 *** | 8.5 | ± | 0.2 | 3.9 | ± | 0.0 *** | 12.3 | ± | 1.5 | 20.2 | ± | 1.6 |
| PT | 18 | 200.2 | ± | 8.0 *** | 11.2 | ± | 0.7 *** | 5.5 | ± | 0.2 *** | 10.7 | ± | 3.4 *** | 18.0 | ± | 3.4 * |
| AAOT | 18 | 207.5 | ± | 10.1 *** | 9.3 | ± | 0.7 ** | 4.4 | ± | 0.2 *** | 12.8 | ± | 1.1 | 19.1 | ± | 1.3 |
| OTD | 18 | 210.2 | ± | 8.2 *** | 8.6 | ± | 0.6 | 4.1 | ± | 0.2 *** | 11.8 | ± | 1.9 * | 19.3 | ± | 2.0 |
| Treatment | No. of Rats | Simple Hyperplasia | Ki67 (%) | γH2AX (%) | TUNEL (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 6 | 0 | 1.7 | ± | 0.4 | 0.6 | ± | 0.2 | 0.6 | ± | 0.4 |
| ANL | 6 | 0 | 2.0 | ± | 0.5 | 0.9 | ± | 0.4 | 0.4 | ± | 0.3 |
| PT | 6 | 1 | 1.7 | ± | 0.4 | 0.7 | ± | 0.3 | 0.4 | ± | 0.2 |
| AAOT | 6 | 5 * | 3.6 | ± | 0.7 *** | 1.9 | ± | 0.7 *** | 0.5 | ± | 0.3 |
| OTD | 6 | 6 ** | 4.9 | ± | 1.3 *** | 2.6 | ± | 0.8 *** | 0.6 | ± | 0.2 |
| Treatment | No. of Rats | Body Weight (g) | Liver (No. = 6) | Consumption | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Absolute (g) | Relative (%) | Food (g/day) | Water (g/day) | |||||||||||||
| OTD | 12 | 215.1 | ± | 5.9 ** | 8.9 | ± | 0.5 | 4.1 | ± | 0.2 *** | 11.5 | ± | 2.0 | 20.1 | ± | 2.0 |
| OTD + APOL | 12 | 214.1 | ± | 8.9 *** | 9.0 | ± | 0.7 | 4.1 | ± | 0.1 *** | 11.8 | ± | 1.7 | 20.5 | ± | 1.8 |
| OTD + APOH | 12 | 213.1 | ± | 6.7 *** | 9.0 | ± | 0.2 | 4.2 | ± | 0.1 *** | 11.7 | ± | 1.9 | 19.1 | ± | 2.0 |
| Control | 6 | 232.0 | ± | 11.3 | 8.5 | ± | 0.5 | 3.7 | ± | 0.1 | 12.9 | ± | 1.4 | 20.1 | ± | 1.0 |
| APOH | 6 | 237.2 | ± | 8.5 | 8.8 | ± | 0.3 | 3.7 | ± | 0.0 | 13.3 | ± | 1.3 | 20.1 | ± | 0.6 |
| Treatment | No. of Rats | Normal | Simple Hyperplasia | Ki67 (%) | γ-H2AX (%) | TUNEL (%) | 8-OHdG (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | |||||||||||||||
| OTD | 12 | 0 | 2 | 10 | 2.1 | ± | 0.7 a | 1.7 | ± | 0.7 a | 0.7 | ± | 0.3 | 1.7 | ± | 0.3 a |
| OTD + APOL | 12 | 0 | 3 | 9 | 1.5 | ± | 0.9 | 1.0 | ± | 0.4 ** | 0.6 | ± | 0.3 | 1.2 | ± | 0.2 *** |
| OTD + APOH | 12 | 1 | 6 | 5 | 0.9 | ± | 0.4 *** | 0.7 | ± | 0.3 *** | 0.5 | ± | 0.2 | 0.9 | ± | 0.2 *** |
| Control | 6 | 6 | 0 | 0 | 0.7 | ± | 0.4 | 0.2 | ± | 0.1 | 0.7 | ± | 0.6 | 0.3 | ± | 0.1 |
| APOH | 6 | 6 | 0 | 0 | 0.6 | ± | 0.4 | 0.3 | ± | 0.1 | 0.6 | ± | 0.6 | 0.3 | ± | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, S.; Gi, M.; Komiya, M.; Obikane, A.; Vachiraarunwong, A.; Fujioka, M.; Kakehashi, A.; Totsuka, Y.; Wanibuchi, H. Evaluation of the Mechanisms Involved in the Development of Bladder Toxicity following Exposure to Occupational Bladder Cancer Causative Chemicals Using DNA Adductome Analysis. Biomolecules 2024, 14, 36. https://doi.org/10.3390/biom14010036
Suzuki S, Gi M, Komiya M, Obikane A, Vachiraarunwong A, Fujioka M, Kakehashi A, Totsuka Y, Wanibuchi H. Evaluation of the Mechanisms Involved in the Development of Bladder Toxicity following Exposure to Occupational Bladder Cancer Causative Chemicals Using DNA Adductome Analysis. Biomolecules. 2024; 14(1):36. https://doi.org/10.3390/biom14010036
Chicago/Turabian StyleSuzuki, Shugo, Min Gi, Masami Komiya, Asuka Obikane, Arpamas Vachiraarunwong, Masaki Fujioka, Anna Kakehashi, Yukari Totsuka, and Hideki Wanibuchi. 2024. "Evaluation of the Mechanisms Involved in the Development of Bladder Toxicity following Exposure to Occupational Bladder Cancer Causative Chemicals Using DNA Adductome Analysis" Biomolecules 14, no. 1: 36. https://doi.org/10.3390/biom14010036
APA StyleSuzuki, S., Gi, M., Komiya, M., Obikane, A., Vachiraarunwong, A., Fujioka, M., Kakehashi, A., Totsuka, Y., & Wanibuchi, H. (2024). Evaluation of the Mechanisms Involved in the Development of Bladder Toxicity following Exposure to Occupational Bladder Cancer Causative Chemicals Using DNA Adductome Analysis. Biomolecules, 14(1), 36. https://doi.org/10.3390/biom14010036







