Unwrap RAP1’s Mystery at Kinetoplastid Telomeres
Abstract
:1. The Telomere Structure and Telomere Functions
2. Yeast and Mammalian RAP1 Homologs Are Essential for Telomeric Silencing and Suppress Telomere Recombination
2.1. ScRap1 Is a Key Player of Telomeric Silencing in S. cerevisiae
2.2. RAP1 Homologs in Higher Eukaryotes Suppress Telomere Recombination
3. T. brucei RAP1 Ensures VSG Monoallelic Expression and Suppresses Telomere Recombination through Unusual Mechanisms
3.1. Trypanosoma brucei Undergoes Antigenic Variation to Evade the Host’s Immune Response
3.2. Multiple Mechanisms Are Employed to Ensure VSG Monoallelic Expression
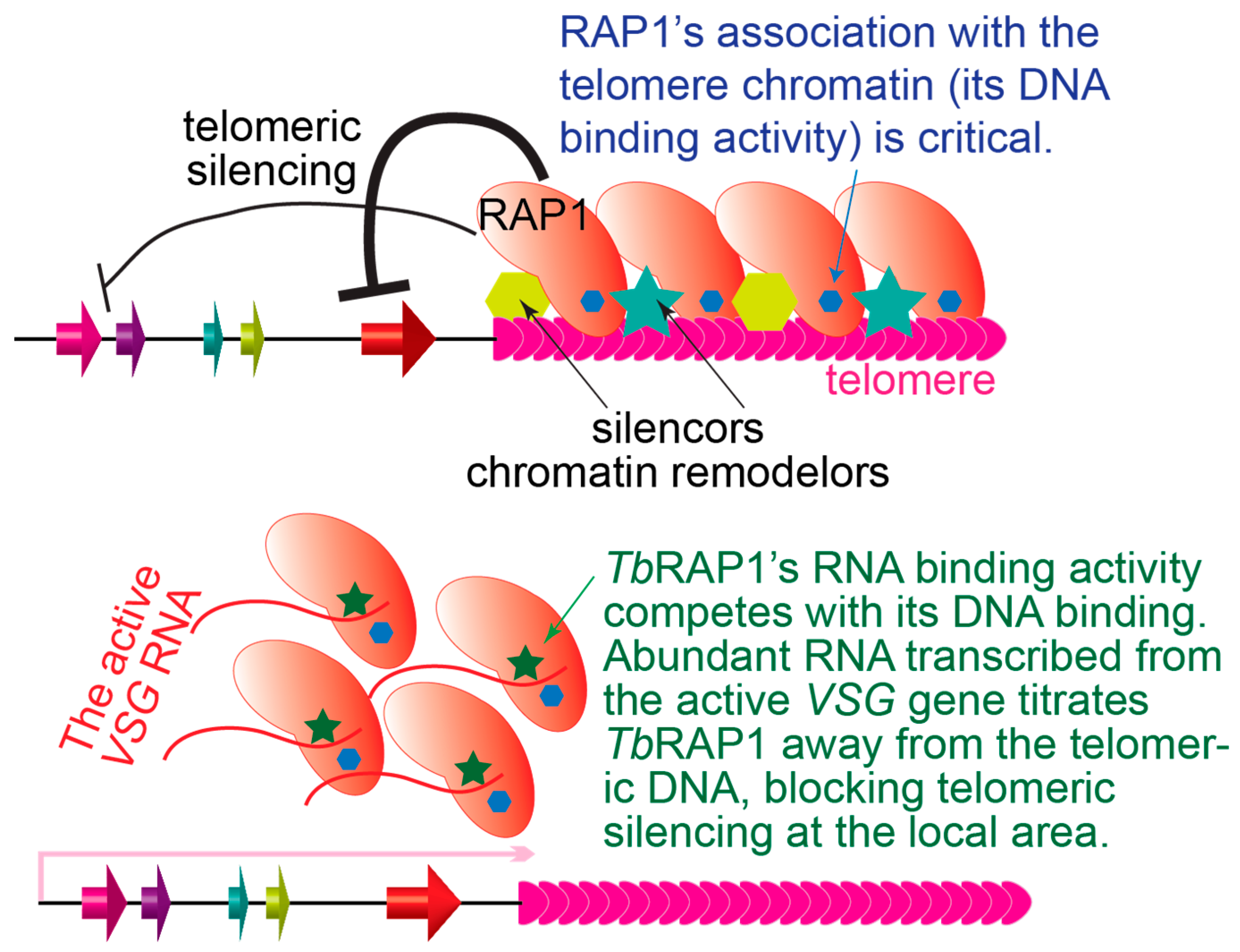
3.3. Competition between TbRAP1’s DNA and RNA Binding Activities Is Essential for VSG Monoallelic Expression
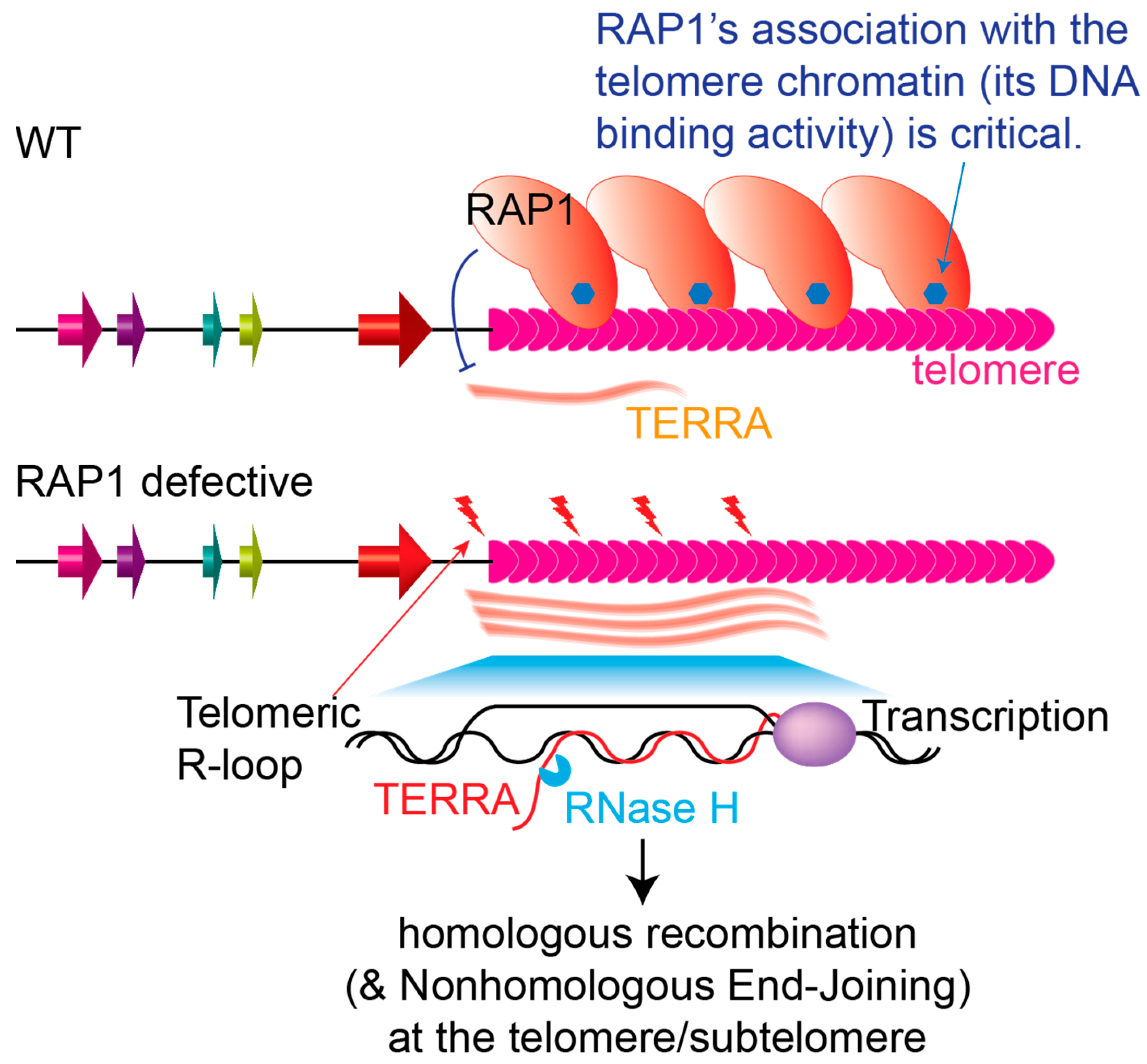
3.4. TbRAP1 Helps Maintain Telomere Stability and Suppresses VSG Switching
4. Discussion
Funding
Conflicts of Interest
References
- Shay, J.W.; Wright, W.E. Telomeres and telomerase: Three decades of progress. Nat. Rev. Genet. 2019, 20, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Lyčka, M.; Bubeník, M.; Závodník, M.; Peska, V.; Fajkus, P.; Demko, M.; Fajkus, J.; Fojtová, M. TeloBase: A community-curated database of telomere sequences across the tree of life. Nucleic Acids Res. 2023, gkad672. [Google Scholar] [CrossRef] [PubMed]
- Podlevsky, J.D.; Bley, C.J.; Omana, R.V.; Qi, X.; Chen, J.J. The telomerase database. Nucleic Acids Res. 2008, 36, D339–D343. [Google Scholar] [CrossRef] [PubMed]
- Bonnell, E.; Pasquier, E.; Wellinger, R.J. Telomere Replication: Solving Multiple End Replication Problems. Front. Cell Dev. Biol. 2021, 9, 668171. [Google Scholar] [CrossRef]
- Wellinger, R.J. In the end, what’s the problem. Mol. Cell 2014, 53, 855–856. [Google Scholar] [CrossRef]
- Munoz-Jordan, J.L.; Cross, G.A.M.; de Lange, T.; Griffith, J.D. t-loops at trypanosome telomeres. EMBO J. 2001, 20, 579–588. [Google Scholar] [CrossRef]
- Griffith, J.D.; Comeau, L.; Rosenfield, S.; Stansel, R.M.; Bianchi, A.; Moss, H.; de Lange, T. Mammalian telomeres end in a large duplex loop. Cell 1999, 97, 503–514. [Google Scholar] [CrossRef]
- Murti, K.G.; Prescott, D.M. Telomeres of polytene chromosomes in a ciliated protozoan terminate in duplex DNA loops. Proc. Natl. Acad. Sci. USA 1999, 96, 14436–14439. [Google Scholar] [CrossRef]
- Nikitina, T.; Woodcock, C.L. Closed chromatin loops at the ends of chromosomes. J. Cell Biol. 2004, 166, 161–165. [Google Scholar] [CrossRef]
- Lu, X.; Liu, L. Genome stability from the perspective of telomere length. Trends Genet. 2023. online ahead of print. [Google Scholar] [CrossRef]
- de Lange, T. How telomeres solve the end-protection problem. Science 2009, 326, 948–952. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, R.J.; Karlseder, J. Telomeres: Protecting chromosomes against genome instability. Nat. Rev. Mol. Cell Biol. 2010, 11, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Ruis, P.; Boulton, S.J. The end protection problem—An unexpected twist in the tail. Genes Dev. 2021, 35, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Casari, E.; Gnugnoli, M.; Rinaldi, C.; Pizzul, P.; Colombo, C.V.; Bonetti, D.; Longhese, M.P. To Fix or Not to Fix: Maintenance of Chromosome Ends Versus Repair of DNA Double-Strand Breaks. Cells 2022, 11, 3224. [Google Scholar] [CrossRef] [PubMed]
- de Lange, T. Shelterin-Mediated Telomere Protection. Annu. Rev. Genet. 2018, 52, 223–247. [Google Scholar] [CrossRef] [PubMed]
- Van Ly, D.; Low, R.R.J.; Frölich, S.; Bartolec, T.K.; Kafer, G.R.; Pickett, H.A.; Gaus, K.; Cesare, A.J. Telomere Loop Dynamics in Chromosome End Protection. Mol. Cell 2018, 71, 510–525.e6. [Google Scholar] [CrossRef] [PubMed]
- de Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef]
- Stewart, J.A.; Chaiken, M.F.; Wang, F.; Price, C.M. Maintaining the end: Roles of telomere proteins in end-protection, telomere replication and length regulation. Mutat. Res. 2012, 730, 12–19. [Google Scholar] [CrossRef]
- Li, B.; Zhao, Y. Regulation of antigenic variation by Trypanosoma brucei telomere proteins depends on their unique DNA binding activities. Pathogens 2021, 10, 967. [Google Scholar] [CrossRef]
- Li, B. Telomere maintenance in African trypanosomes. Front. Mol. Biosci. 2023, 10, 1302557. [Google Scholar] [CrossRef]
- Lim, C.J.; Cech, T.R. Shaping human telomeres: From shelterin and CST complexes to telomeric chromatin organization. Nat. Rev. Mol. Cell Biol. 2021, 22, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Chong, L.; van Steensel, B.; Broccoli, D.; Erdjument-Bromage, H.; Hanish, J.; Tempst, P.; de Lange, T. A human telomeric protein. Science 1995, 270, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Broccoli, D.; Smogorzewska, A.; Chong, L.; de Lange, T. Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nat. Genet. 1997, 17, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Bilaud, T.; Brun, C.; Ancelin, K.; Koering, C.E.; Laroche, T.; Gilson, E. Telomeric localization of TRF2, a novel human telobox protein. Nat. Genet. 1997, 17, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Houghtaling, B.R.; Cuttonaro, L.; Chang, W.; Smith, S. A Dynamic Molecular Link between the Telomere Length Regulator TRF1 and the Chromosome End Protector TRF2. Curr. Biol. 2004, 14, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Safari, A.; O’Connor, M.S.; Chan, D.W.; Laegeler, A.; Qin, J.; Songyang, Z. PTOP interacts with POT1 and regulates its localization to telomeres. Nat. Cell Biol. 2004, 6, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.Z.; Hockemeyer, D.; Krutchinsky, A.N.; Loayza, D.; Hooper, S.M.; Chait, B.T.; de Lange, T. POT1-interacting protein PIP1: A telomere length regulator that recruits POT1 to the TIN2/TRF1 complex. Genes Dev. 2004, 18, 1649–1654. [Google Scholar] [CrossRef]
- Baumann, P.; Podell, E.; Cech, T.R. Human pot1 (protection of telomeres) protein: Cytolocalization, gene structure, and alternative splicing. Mol. Cell. Biol. 2002, 22, 8079–8087. [Google Scholar] [CrossRef]
- Baumann, P.; Cech, T.R. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 2001, 292, 1171–1175. [Google Scholar] [CrossRef]
- Loayza, D.; de Lange, T. POT1 as a terminal transducer of TRF1 telomere length control. Nature 2003, 424, 1013–1018. [Google Scholar] [CrossRef]
- Li, B.; Oestreich, S.; de Lange, T. Identification of human Rap1: Implications for telomere evolution. Cell 2000, 101, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kaminker, P.; Campisi, J. TIN2, a new regulator of telomere length in human cells. Nat. Genet. 1999, 23, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.Z.; Donigian, J.R.; van Overbeek, M.; Loayza, D.; Luo, Y.; Krutchinsky, A.N.; Chait, B.T.; de Lange, T. TIN2 binds TRF1 and TRF2 simultaneously and stabilizes the TRF2 complex on telomeres. J. Biol. Chem. 2004, 279, 47264–47271. [Google Scholar] [CrossRef] [PubMed]
- Wellinger, R.J. The CST complex and telomere maintenance: The exception becomes the rule. Mol. Cell 2009, 36, 168–169. [Google Scholar] [CrossRef] [PubMed]
- Rice, C.; Skordalakes, E. Structure and function of the telomeric CST complex. Comput. Struct. Biotechnol. J. 2016, 14, 161–167. [Google Scholar] [CrossRef]
- Lim, C.J.; Barbour, A.T.; Zaug, A.J.; Goodrich, K.J.; McKay, A.E.; Wuttke, D.S.; Cech, T.R. The structure of human CST reveals a decameric assembly bound to telomeric DNA. Science 2020, 368, 1081–1085. [Google Scholar] [CrossRef]
- Miyake, Y.; Nakamura, M.; Nabetani, A.; Shimamura, S.; Tamura, M.; Yonehara, S.; Saito, M.; Ishikawa, F. RPA-like mammalian Ctc1-Stn1-Ten1 complex binds to single-stranded DNA and protects telomeres independently of the Pot1 pathway. Mol. Cell 2009, 36, 193–206. [Google Scholar] [CrossRef]
- Olson, C.L.; Barbour, A.T.; Wieser, T.A.; Wuttke, D.S. RPA engages telomeric G-quadruplexes more effectively than CST. Nucleic Acids Res. 2023, 51, 5073–5086. [Google Scholar] [CrossRef]
- Barbour, A.T.; Wuttke, D.S. RPA-like single-stranded DNA-binding protein complexes including CST serve as specialized processivity factors for polymerases. Curr. Opin. Struct. Biol. 2023, 81, 102611. [Google Scholar] [CrossRef]
- Wang, S.S.; Zakian, V.A. Sequencing of Saccharomyces telomeres cloned using T4 DNA polymerase reveals two domains. Mol. Cell Biol. 1990, 10, 4415–4419. [Google Scholar]
- Wellinger, R.J.; Zakian, V.A. Everything you ever wanted to know about Saccharomyces cerevisiae telomeres: Beginning to end. Genetics 2012, 191, 1073–1105. [Google Scholar] [CrossRef] [PubMed]
- Konig, P.; Rhodes, D. Recognition of telomeric DNA. Trends Biochem. Sci. 1997, 22, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Konig, P.; Giraldo, R.; Chapman, L.; Rhodes, D. The crystal structure of the DNA-binding domain of yeast RAP1 in complex with telomeric DNA. Cell 1996, 85, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Wahlin, J.; Cohn, M. Saccharomyces cerevisiae RAP1 binds to telomeric sequences with spatial flexibility. Nucleic Acids Res. 2000, 28, 2292–2301. [Google Scholar] [CrossRef] [PubMed]
- Ribaud, V.; Ribeyre, C.; Damay, P.; Shore, D. DNA-end capping by the budding yeast transcription factor and subtelomeric binding protein Tbf1. EMBO J. 2012, 31, 138–149. [Google Scholar] [CrossRef]
- Bourns, B.D.; Alexander, M.K.; Smith, A.M.; Zakian, V.A. Sir proteins, Rif proteins, and Cdc13p bind Saccharomyces telomeres in vivo. Mol. Cell. Biol. 1998, 18, 5600–5608. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.R.; Weilbaecher, R.G.; Walterscheid, M.; Lundblad, V. Identification of the single-strand telomeric DNA binding domain of the Saccharomyces cerevisiae Cdc13 protein. Proc. Natl. Acad. Sci. USA 2000, 97, 6457–6462. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wu, Z.; Chen, H.; Zhong, Q.; Shi, S.; Li, G.; Wu, J.; Lei, M. Structural insights into telomere protection and homeostasis regulation by yeast CST complex. Nat. Struct. Mol. Biol. 2020, 27, 752–762. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Challoner, P.B. Identification of a telomeric DNA sequence in Trypanosoma brucei. Cell 1984, 36, 447–457. [Google Scholar] [CrossRef]
- Meyne, J.; Ratliff, R.L.; Moyzis, R.K. Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc. Natl. Acad. Sci. USA 1989, 86, 7049–7053. [Google Scholar] [CrossRef]
- Li, B.; Espinal, A.; Cross, G.A.M. Trypanosome telomeres are protected by a homologue of mammalian TRF2. Mol. Cell. Biol. 2005, 25, 5011–5021. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Figueiredo, L.M.; Espinal, A.; Okubo, E.; Li, B. RAP1 is essential for silencing telomeric variant surface glycoprotein genes in Trypanosoma brucei. Cell 2009, 137, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Jehi, S.E.; Wu, F.; Li, B. Trypanosoma brucei TIF2 suppresses VSG switching by maintaining subtelomere integrity. Cell Res. 2014, 24, 870–885. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, M.A.G.; Tonini, M.L.; Afrin, M.; Li, B. POLIE suppresses telomerase-mediated telomere G-strand extension and helps ensure proper telomere C-strand synthesis in trypanosomes. Nucleic Acids Res. 2022, 50, 2036–2050. [Google Scholar] [CrossRef] [PubMed]
- Reis, H.; Schwebs, M.; Dietz, S.; Janzen, C.J.; Butter, F. TelAP1 links telomere complexes with developmental expression site silencing in African trypanosomes. Nucleic Acids Res. 2018, 46, 2820–2833. [Google Scholar] [CrossRef] [PubMed]
- Olovnikov, A.M. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J. Theor. Biol. 1973, 41, 181–190. [Google Scholar] [CrossRef]
- Ferreira, M.G.; Miller, K.M.; Cooper, J.P. Indecent exposure: When telomeres become uncapped. Mol. Cell 2004, 13, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Pizzul, P.; Rinaldi, C.; Bonetti, D. The multistep path to replicative senescence onset: Zooming on triggering and inhibitory events at telomeric DNA. Front. Cell Dev. Biol. 2023, 11, 1250264. [Google Scholar] [CrossRef]
- Lee, J.W.; Ong, E.B.B. Genomic Instability and Cellular Senescence: Lessons from the Budding Yeast. Front. Cell Dev. Biol. 2020, 8, 619126. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Wang, Z.; Liu, J.P. Roles of Telomere Biology in Cell Senescence, Replicative and Chronological Ageing. Cells 2019, 8, 54. [Google Scholar] [CrossRef]
- Greider, C.W.; Blackburn, E.H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature 1989, 337, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Greider, C.W.; Blackburn, E.H. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell 1987, 51, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Greider, C.W.; Blackburn, E.H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 1985, 43, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, G.E.; Fountain, A.J.; van Roon, A.M.; Rangan, R.; Das, R.; Collins, K.; Nguyen, T.H.D. Structure of human telomerase holoenzyme with bound telomeric DNA. Nature 2021, 593, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; He, Y.; Wang, Y.; Song, H.; Zhou, Z.H.; Feigon, J. Structure of active human telomerase with telomere shelterin protein TPP1. Nature 2022, 604, 578–583. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Feigon, J. Telomerase structural biology comes of age. Curr. Opin. Struct. Biol. 2022, 76, 102446. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.M.; Frydrychova, R.C.; Biessmann, H. Drosophila telomeres: An exception providing new insights. Bioessays 2008, 30, 25–37. [Google Scholar] [CrossRef]
- Pardue, M.L.; DeBaryshe, P.G. Drosophila telomeres: A variation on the telomerase theme. Fly 2008, 2, 101–110. [Google Scholar] [CrossRef]
- Zhang, J.M.; Zou, L. Alternative lengthening of telomeres: From molecular mechanisms to therapeutic outlooks. Cell Biosci. 2020, 10, 30. [Google Scholar] [CrossRef]
- Hou, K.; Yu, Y.; Li, D.; Zhang, Y.; Zhang, K.; Tong, J.; Yang, K.; Jia, S. Alternative Lengthening of Telomeres and Mediated Telomere Synthesis. Cancers 2022, 14, 2194. [Google Scholar] [CrossRef]
- Chen, Q.; Ijpma, A.; Greider, C.W. Two survivor pathways that allow growth in the absence of telomerase are generated by distinct telomere recombination events. Mol. Cell. Biol. 2001, 21, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Lue, N.F.; Yu, E.Y. Telomere recombination pathways: Tales of several unhappy marriages. Curr. Genet. 2017, 63, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Li, B. Keeping balance between genetic stability and plasticity at the telomere and subtelomere of Trypanosoma brucei. Front. Cell Dev. Biol. 2021, 9, 699639. [Google Scholar] [CrossRef] [PubMed]
- Li, B. DNA double-strand breaks and telomeres play important roles in Trypanosoma brucei antigenic variation. Eukaryot. Cell 2015, 14, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Sima, N.; McLaughlin, E.J.; Hutchinson, S.; Glover, L. Escaping the immune system by DNA repair and recombination in African trypanosomes. Open Biol. 2019, 9, 190182. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, R.; Morrison, L.J.; Hall, J.P.J. DNA Recombination Strategies during Antigenic Variation in the African Trypanosome. Microbiol. Spectr. 2015, 3, MDNA3-0016. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, A.; Gilson, E.; Magdinier, F. Telomeric position effect: From the yeast paradigm to human pathologies? Biochimie 2008, 90, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Kim, D.Y.; Kim, W. Regulation of Gene Expression by Telomere Position Effect. Int. J. Mol. Sci. 2021, 22, 12807. [Google Scholar] [CrossRef]
- Elgin, S.C.; Reuter, G. Position-effect variegation; heterochromatin formation, and gene silencing in Drosophila. Cold Spring Harb. Perspect. Biol. 2013, 5, a017780. [Google Scholar] [CrossRef]
- Gottschling, D.E.; Aparicio, O.M.; Billington, B.L.; Zakian, V.A. Position effect at S. cerevisiae telomeres: Reversible repression of pol II transcription. Cell 1990, 63, 751–762. [Google Scholar] [CrossRef]
- Mason, J.M.; Konev, A.Y.; Golubovsky, M.D.; Biessmann, H. Cis- and trans-acting influences on telomeric position effect in Drosophila melanogaster detected with a subterminal transgene. Genetics 2003, 163, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Tennen, R.I.; Bua, D.J.; Wright, W.E.; Chua, K.F. SIRT6 is required for maintenance of telomere position effect in human cells. Nat. Commun. 2011, 2, 433. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Zou, Y.; Shay, J.W.; Wright, W.E. Telomere position effect in human cells. Science 2001, 292, 2075–2077. [Google Scholar] [CrossRef]
- Koering, C.E.; Pollice, A.; Zibella, M.P.; Bauwens, S.; Puisieux, A.; Brunori, M.; Brun, C.; Martins, L.; Sabatier, L.; Pulitzer, J.F.; et al. Human telomeric position effect is determined by chromosomal context and telomeric chromatin integrity. EMBO Rep. 2002, 3, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Robin, J.D.; Ludlow, A.T.; Batten, K.; Magdinier, F.; Stadler, G.; Wagner, K.R.; Shay, J.W.; Wright, W.E. Telomere position effect: Regulation of gene expression with progressive telomere shortening over long distances. Genes Dev. 2014, 28, 2464–2476. [Google Scholar] [CrossRef]
- Stadler, G.; Rahimov, F.; King, O.D.; Chen, J.C.; Robin, J.D.; Wagner, K.R.; Shay, J.W.; Emerson, C.P.; Wright, W.E. Telomere position effect regulates DUX4 in human facioscapulohumeral muscular dystrophy. Nat. Struct. Mol. Biol. 2013, 20, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Ludlow, A.T.; Min, J.; Robin, J.D.; Stadler, G.; Mender, I.; Lai, T.P.; Zhang, N.; Wright, W.E.; Shay, J.W. Regulation of the Human Telomerase Gene TERT by Telomere Position Effect-Over Long Distances (TPE-OLD): Implications for Aging and Cancer. PLoS Biol. 2016, 14, e2000016. [Google Scholar] [CrossRef]
- Glover, L.; Alsford, S.; Beattie, C.; Horn, D. Deletion of a trypanosome telomere leads to loss of silencing and progressive loss of terminal DNA in the absence of cell cycle arrest. Nuc. Acids Res. 2007, 35, 872–880. [Google Scholar] [CrossRef]
- Alsford, S.; Kawahara, T.; Isamah, C.; Horn, D. A sirtuin in the African trypanosome is involved in both DNA repair and telomeric gene silencing but is not required for antigenic variation. Mol. Microbiol. 2007, 63, 724–736. [Google Scholar] [CrossRef]
- Glover, L.; Horn, D. Repression of polymerase I-mediated gene expression at Trypanosoma brucei telomeres. EMBO Rep. 2006, 7, 93–99. [Google Scholar] [CrossRef]
- Horn, D.; Cross, G.A.M. Position-dependent and promoter-specific regulation of gene expression in Trypanosoma brucei. EMBO J. 1997, 16, 7422–7431. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Junior, L.H.; Hernandez-Rivas, R.; Ralph, S.A.; Montiel-Condado, D.; Ruvalcaba-Salazar, O.K.; Rojas-Meza, A.P.; Mancio-Silva, L.; Leal-Silvestre, R.J.; Gontijo, A.M.; Shorte, S.; et al. Telomeric heterochromatin propagation and histone acetylation control mutually exclusive expression of antigenic variation genes in malaria parasites. Cell 2005, 121, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Duraisingh, M.T.; Voss, T.S.; Marty, A.J.; Duffy, M.F.; Good, R.T.; Thompson, J.K.; Freitas-Junior, L.H.; Scherf, A.; Crabb, B.S.; Cowman, A.F. Heterochromatin silencing and locus repositioning linked to regulation of virulence genes in Plasmodium falciparum. Cell 2005, 121, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Green, B.; Benoit, N.; Sobel, J.D.; Schatz, M.C.; Wheelan, S.; Cormack, B.P. Cell wall protein variation, break-induced replication, and subtelomere dynamics in Candida glabrata. Mol. Microbiol. 2021, 116, 260–276. [Google Scholar] [CrossRef]
- López-Fuentes, E.; Gutiérrez-Escobedo, G.; Timmermans, B.; Van Dijck, P.; De Las Peñas, A.; Castaño, I. Candida glabrata’s Genome Plasticity Confers a Unique Pattern of Expressed Cell Wall Proteins. J. Fungi 2018, 4, 67. [Google Scholar] [CrossRef]
- De Las Penas, A.; Juarez-Cepeda, J.; Lopez-Fuentes, E.; Briones-Martin-Del-Campo, M.; Gutierrez-Escobedo, G.; Castano, I. Local and regional chromatin silencing in Candida glabrata: Consequences for adhesion and the response to stress. FEMS Yeast Res. 2015, 15, fov056. [Google Scholar] [CrossRef]
- Shore, D.; Nasmyth, K. Purification and cloning of a DNA binding protein from yeast that binds to both silencer and activator elements. Cell 1987, 51, 721–732. [Google Scholar] [CrossRef]
- Lustig, A.J.; Kurtz, S.; Shore, D. Involvement of the silencer and UAS binding protein RAP1 in regulation of telomere length. Science 1990, 250, 549–553. [Google Scholar] [CrossRef]
- Chikashige, Y.; Hiraoka, Y. Telomere binding of the Rap1 protein is required for meiosis in fission yeast. Curr. Biol. 2001, 11, 1618–1623. [Google Scholar] [CrossRef]
- Kanoh, J.; Ishikawa, F. spRap1 and spRif1, recruited to telomeres by Taz1, are essential for telomere function in fission yeast. Curr. Biol. 2001, 11, 1624–1630. [Google Scholar] [CrossRef]
- Cai, Y.; Kandula, V.; Kosuru, R.; Ye, X.; Irwin, M.G.; Xia, Z. Decoding telomere protein Rap1: Its telomeric and nontelomeric functions and potential implications in diabetic cardiomyopathy. Cell Cycle 2017, 16, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Khattar, E.; Tergaonkar, V. Role of Rap1 in DNA damage response: Implications in stem cell homeostasis and cancer. Exp. Hematol. 2020, 90, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Pandya, U.M.; Sandhu, R.; Li, B. Silencing subtelomeric VSGs by Trypanosoma brucei RAP1 at the insect stage involves chromatin structure changes. Nucleic Acids Res. 2013, 41, 7673–7682. [Google Scholar] [CrossRef] [PubMed]
- Nanavaty, V.; Sandhu, R.; Jehi, S.E.; Pandya, U.M.; Li, B. Trypanosoma brucei RAP1 maintains telomere and subtelomere integrity by suppressing TERRA and telomeric RNA:DNA hybrids. Nucleic Acids Res. 2017, 45, 5785–5796. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, A.K.; Afrin, M.; Yang, X.; Saha, A.; Sayeed, S.K.A.; Pan, X.; Ji, Z.; Wong, K.B.; Zhang, M.; Zhao, Y.; et al. The RRM-mediated RNA binding activity in T. brucei RAP1 is essential for VSG monoallelic expression. Nat. Commun. 2023, 14, 1576. [Google Scholar] [CrossRef] [PubMed]
- Afrin, M.; Kishmiri, H.; Sandhu, R.; Rabbani, M.A.G.; Li, B. Trypanosoma brucei RAP1 has essential functional domains that are required for different protein interactions. mSphere 2020, 5, e00027-20. [Google Scholar] [CrossRef] [PubMed]
- Afrin, M.; Gaurav, A.K.; Yang, X.; Pan, X.; Zhao, Y.; Li, B. TbRAP1 has an unusual duplex DNA binding activity required for its telomere localization and VSG silencing. Sci. Adv. 2020, 6, eabc4065. [Google Scholar] [CrossRef]
- Barry, J.D.; McCulloch, R. Antigenic variation in trypanosomes: Enhanced phenotypic variation in a eukaryotic parasite. Adv. Parasitol. 2001, 49, 1–70. [Google Scholar]
- de Lange, T.; Borst, P. Genomic environment of the expression-linked extra copies of genes for surface antigens of Trypanosoma brucei resembles the end of a chromosome. Nature 1982, 299, 451–453. [Google Scholar] [CrossRef]
- Cross, G.A.M.; Kim, H.S.; Wickstead, B. Capturing the variant surface glycoprotein repertoire (the VSGnome) of Trypanosoma brucei Lister 427. Mol. Biochem. Parasitol. 2014, 195, 59–73. [Google Scholar] [CrossRef]
- Hertz-Fowler, C.; Figueiredo, L.M.; Quail, M.A.; Becker, M.; Jackson, A.; Bason, N.; Brooks, K.; Churcher, C.; Fahkro, S.; Goodhead, I.; et al. Telomeric expression sites are highly conserved in Trypanosoma brucei. PLoS ONE 2008, 3, e3527. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.S.M.; Cosentino, R.O.; Förstner, K.U.; Guizetti, J.; Wedel, C.; Kaplan, N.; Janzen, C.J.; Arampatzi, P.; Vogel, J.; Steinbiss, S.; et al. Genome organization and DNA accessibility control antigenic variation in trypanosomes. Nature 2018, 563, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Gerloff, D.L.; Woods, N.T.; Farago, A.A.; Monteiro, A.N. BRCT domains: A little more than kin, and less than kind. FEBS Lett. 2012, 586, 2711–2716. [Google Scholar] [CrossRef] [PubMed]
- Peña-Guerrero, J.; Fernández-Rubio, C.; García-Sosa, A.T.; Nguewa, P.A.; Domains, B.R.T. Functions, and Implications in Disease-New Therapeutic Targets for Innovative Drug Discovery against Infections. Pharmaceutics 2023, 15, 1839. [Google Scholar] [CrossRef] [PubMed]
- Prouse, M.B.; Campbell, M.M. The interaction between MYB proteins and their target DNA binding sites. Biochim. Biophys. Acta 2012, 1819, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Luo, Q.; Li, Y.; Yin, L.; Zhou, N.; Li, X.; Gan, J.; Dong, A. Structural insights into target DNA recognition by R2R3-MYB transcription factors. Nucleic Acids Res. 2020, 48, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Pardo, B.; Marcand, S. Rap1 prevents telomere fusions by nonhomologous end joining. EMBO J. 2005, 24, 3117–3127. [Google Scholar] [CrossRef]
- Marcand, S.; Pardo, B.; Gratias, A.; Cahun, S.; Callebaut, I. Multiple pathways inhibit NHEJ at telomeres. Genes Dev. 2008, 22, 1153–1158. [Google Scholar] [CrossRef]
- Negrini, S.; Ribaud, V.; Bianchi, A.; Shore, D. DNA breaks are masked by multiple Rap1 binding in yeast: Implications for telomere capping and telomerase regulation. Genes Dev. 2007, 21, 292–302. [Google Scholar] [CrossRef]
- Kyrion, G.; Boakye, K.A.; Lustig, A.J. C-terminal truncation of RAP1 results in the deregulation of telomere size, stability, and function in Saccharomyces cerevisiae. Mol. Cell. Biol. 1992, 12, 5159–5173. [Google Scholar]
- Kyrion, G.; Liu, K.; Liu, C.; Lustig, A.J. RAP1 and telomere structure regulate telomere position effects in Saccharomyces cerevisiae. Genes Dev. 1993, 7, 1146–1159. [Google Scholar] [CrossRef] [PubMed]
- Shore, D. RAP1: A protean regulator in yeast. Trends Genet. 1994, 10, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Vignais, M.L.; Huet, J.; Buhler, J.M.; Sentenac, A. Contacts between the factor TUF and RPG sequences. J. Biol. Chem. 1990, 265, 14669–14674. [Google Scholar] [CrossRef] [PubMed]
- Idrissi, F.Z.; Pina, B. Functional divergence between the half-sites of the DNA-binding sequence for the yeast transcriptional regulator Rap1p. Biochem. J. 1999, 341, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Idrissi, F.Z.; Fernández-Larrea, J.B.; Piña, B. Structural and functional heterogeneity of Rap1p complexes with telomeric and UASrpg-like DNA sequences. J. Mol. Biol. 1998, 284, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Matot, B.; Le Bihan, Y.V.; Lescasse, R.; Pérez, J.; Miron, S.; David, G.; Castaing, B.; Weber, P.; Raynal, B.; Zinn-Justin, S.; et al. The orientation of the C-terminal domain of the Saccharomyces cerevisiae Rap1 protein is determined by its binding to DNA. Nucleic Acids Res. 2012, 40, 3197–3207. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.; Peyser, R.; Ho, J.; Babbin, C.; Bohan, N.; Cortes, A.; Erley, J.; Fatima, M.; Flinn, J.; Horwitz, E.; et al. Genome-wide gene expression responses to experimental manipulation of Saccharomyces cerevisiae repressor activator protein 1 (Rap1) expression level. Genomics 2023, 115, 110625. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lustig, A.J. Genetic analysis of Rap1p/Sir3p interactions in telomeric and HML silencing in Saccharomyces cerevisiae. Genetics 1996, 143, 81–93. [Google Scholar] [CrossRef]
- Cockell, M.; Palladino, F.; Laroche, T.; Kyrion, G.; Liu, C.; Lustig, A.J.; Gasser, S.M. The carboxy termini of Sir4 and Rap1 affect Sir3 localization: Evidence for a multicomponent complex required for yeast telomeric silencing. J. Cell Biol. 1995, 129, 909–924. [Google Scholar] [CrossRef]
- Liu, C.; Mao, X.; Lustig, A.J. Mutational analysis defines a C-terminal tail domain of RAP1 essential for telomeric silencing in Saccharomyces cerevisiae. Genetics 1994, 138, 1025–1040. [Google Scholar] [CrossRef]
- Hecht, A.; Strahl-Bolsinger, S.; Grunstein, M. Spreading of transcriptional repressor SIR3 from telomeric heterochromatin. Nature 1996, 383, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Moretti, P.; Freeman, K.; Coodly, L.; Shore, D. Evidence that a complex of SIR proteins interacts with the silencer and telomere-binding protein RAP1. Genes Dev. 1994, 8, 2257–2269. [Google Scholar] [CrossRef] [PubMed]
- Moazed, D.; Kistler, A.; Axelrod, A.; Rine, J.; Johnson, A.D. Silent information regulator protein complexes in Saccharomyces cerevisiae—A sir2/sir4 complex and evidence for a regulatory domain in sir4 that inhibits its interaction with sir3. Proc. Natl. Acad. Sci. USA 1997, 94, 2186–2191. [Google Scholar] [CrossRef] [PubMed]
- Moretti, P.; Shore, D. Multiple interactions in sir protein recruitment by Rap1p at silencers and telomeres in yeast. Mol. Cell. Biol. 2001, 21, 8082–8094. [Google Scholar] [CrossRef]
- Luo, K.; Vega-Palas, M.A.; Grunstein, M. Rap1-Sir4 binding independent of other Sir, yKu, or histone interactions initiates the assembly of telomeric heterochromatin in yeast. Genes Dev. 2002, 16, 1528–1539. [Google Scholar] [CrossRef]
- Feeser, E.A.; Wolberger, C. Structural and functional studies of the Rap1 C-terminus reveal novel separation-of-function mutants. J. Mol. Biol. 2008, 380, 520–531. [Google Scholar] [CrossRef]
- Chen, Y.; Rai, R.; Zhou, Z.R.; Kanoh, J.; Ribeyre, C.; Yang, Y.; Zheng, H.; Damay, P.; Wang, F.; Tsujii, H.; et al. A conserved motif within RAP1 has diversified roles in telomere protection and regulation in different organisms. Nat. Struct. Mol. Biol. 2011, 18, 213–221. [Google Scholar] [CrossRef]
- Buck, S.W.; Shore, D. Action of a RAP1 carboxy-terminal silencing domain reveals an underlying competition between HMR and telomeres in yeast. Genes Dev. 1995, 9, 370–384. [Google Scholar] [CrossRef]
- Straight, A.F.; Shou, W.Y.; Dowd, G.J.; Turck, C.W.; Deshaies, R.J.; Johnson, A.D.; Moazed, D. Net1, a Sir2-associated nucleolar protein required for rDNA silencing and nucleolar integrity. Cell 1999, 97, 245–256. [Google Scholar] [CrossRef]
- Tanny, J.C.; Kirkpatrick, D.S.; Gerber, S.A.; Gygi, S.P.; Moazed, D. Budding yeast silencing complexes and regulation of Sir2 activity by protein-protein interactions. Mol. Cell. Biol. 2004, 24, 6931–6946. [Google Scholar] [CrossRef]
- Rudner, A.D.; Hall, B.E.; Ellenberger, T.; Moazed, D. A nonhistone protein-protein interaction required for assembly of the SIR complex and silent chromatin. Mol. Cell. Biol. 2005, 25, 4514–4528. [Google Scholar] [CrossRef] [PubMed]
- Cubizolles, F.; Martino, F.; Perrod, S.; Gasser, S.M. A homotrimer-heterotrimer switch in Sir2 structure differentiates rDNA and telomeric silencing. Mol. Cell 2006, 21, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Buchberger, J.R.; Onishi, M.; Li, G.; Seebacher, J.; Rudner, A.D.; Gygi, S.P.; Moazed, D. Sir3-nucleosome interactions in spreading of silent chromatin in Saccharomyces cerevisiae. Mol. Cell. Biol. 2008, 28, 6903–6918. [Google Scholar] [CrossRef] [PubMed]
- Strahl-Bolsinger, S.; Hecht, A.; Luo, K.; Grunstein, M. SIR2 and SIR4 interactions differ in core and extended telomeric heterochromatin in yeast. Genes Dev. 1997, 11, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, G.J.; Tanny, J.C.; Rudner, A.D.; Gerber, S.A.; Danaie, S.; Gygi, S.P.; Moazed, D. Steps in assembly of silent chromatin in yeast: Sir3-independent binding of a Sir2/Sir4 complex to silencers and role for Sir2-dependent deacetylation. Mol. Cell. Biol. 2002, 22, 4167–4180. [Google Scholar] [CrossRef]
- Ghidelli, S.; Donze, D.; Dhillon, N.; Kamakaka, R.T. Sir2p exists in two nucleosome-binding complexes with distinct deacetylase activities. EMBO J. 2001, 20, 4522–4535. [Google Scholar] [CrossRef]
- Landry, J.; Sutton, A.; Tafrov, S.T.; Heller, R.C.; Stebbins, J.; Pillus, L.; Sternglanz, R. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc. Natl. Acad. Sci. USA 2000, 97, 5807–5811. [Google Scholar] [CrossRef]
- Hickman, M.A.; Froyd, C.A.; Rusche, L.N. Reinventing heterochromatin in budding yeasts: Sir2 and the origin recognition complex take center stage. Eukaryot. Cell 2011, 10, 1183–1192. [Google Scholar] [CrossRef]
- Bi, X. Heterochromatin structure: Lessons from the budding yeast. IUBMB Life 2014, 66, 657–666. [Google Scholar] [CrossRef]
- Hecht, A.; Laroche, T.; Strahl-Bolsinger, S.; Gasser, S.M.; Grunstein, M. Histone H3 and H4 N-termini interact with SIR3 and SIR4 proteins: A molecular model for the formation of heterochromatin in yeast. Cell 1995, 80, 583–592. [Google Scholar] [CrossRef]
- Sampath, V.; Yuan, P.; Wang, I.X.; Prugar, E.; van Leeuwen, F.; Sternglanz, R. Mutational analysis of the Sir3 BAH domain reveals multiple points of interaction with nucleosomes. Mol. Cell. Biol. 2009, 29, 2532–2545. [Google Scholar] [CrossRef] [PubMed]
- Carmen, A.A.; Milne, L.; Grunstein, M. Acetylation of the yeast histone H4 N-terminus regulates its binding to heterochromatin protein SIR3. J. Biol. Chem. 2001, 19, 19. [Google Scholar] [CrossRef] [PubMed]
- Georgel, P.T.; DeBeer, M.A.P.; Pietz, G.; Fox, C.A.; Hansen, J.C. Sir3-dependent assembly of supramolecular chromatin structures in vitro. Proc. Natl. Acad. Sci. USA 2001, 98, 8584–8589. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.; Utley, R.T.; Lacoste, N.; Tan, S.; Briggs, S.D.; Cote, J. Interplay of chromatin modifiers on a short basic patch of histone H4 tail defines the boundary of telomeric heterochromatin. Mol. Cell 2007, 28, 1002–1014. [Google Scholar] [CrossRef] [PubMed]
- Martino, F.; Kueng, S.; Robinson, P.; Tsai-Pflugfelder, M.; van Leeuwen, F.; Ziegler, M.; Cubizolles, F.; Cockell, M.M.; Rhodes, D.; Gasser, S.M. Reconstitution of yeast silent chromatin: Multiple contact sites and O-AADPR binding load SIR complexes onto nucleosomes in vitro. Mol. Cell 2009, 33, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, N.; Redon, S.; Pfeiffer, V.; Dees, M.; Lingner, J.; Luke, B. Subtelomeric repetitive elements determine TERRA regulation by Rap1/Rif and Rap1/Sir complexes in yeast. EMBO Rep. 2011, 12, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, G.; Van der Ploeg, L.H. Transcription of telomere repeats in protozoa. EMBO J. 1989, 8, 2633–2638. [Google Scholar] [CrossRef]
- Azzalin, C.M.; Reichenbach, P.; Khoriauli, L.; Giulotto, E.; Lingner, J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science 2007, 318, 798–801. [Google Scholar] [CrossRef]
- Schoeftner, S.; Blasco, M.A. Developmentally regulated transcription of mammalian telomeres by DNA-dependent RNA polymerase II. Nat. Cell Biol. 2008, 10, 228–236. [Google Scholar] [CrossRef]
- Luke, B.; Panza, A.; Redon, S.; Iglesias, N.; Li, Z.; Lingner, J. The Rat1p 5’ to 3’ exonuclease degrades telomeric repeat-containing RNA and promotes telomere elongation in Saccharomyces cerevisiae. Mol. Cell 2008, 32, 465–477. [Google Scholar] [CrossRef]
- Bah, A.; Wischnewski, H.; Shchepachev, V.; Azzalin, C.M. The telomeric transcriptome of Schizosaccharomyces pombe. Nucleic Acids Res. 2012, 40, 2995–3005. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Nanavaty, V.P.; Li, B. Telomere and subtelomere R-loops and antigenic variation in trypanosomes. J. Mol. Biol. 2019, 432, 4167–4185. [Google Scholar] [CrossRef] [PubMed]
- Bettin, N.; Pegorar, C.O.; Cusanelli, E. The Emerging Roles of TERRA in Telomere Maintenance and Genome Stability. Cells 2019, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Kroupa, M.; Tomasova, K.; Kavec, M.; Skrobanek, P.; Buchler, T.; Kumar, R.; Vodickova, L.; Vodicka, P. TElomeric repeat-containing RNA (TERRA): Physiological functions and relevance in cancer. Front. Oncol. 2022, 12, 913314. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, M.; Chartrand, P. TERRA, a Multifaceted Regulator of Telomerase Activity at Telomeres. J. Mol. Biol. 2020, 432, 4232–4243. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.V.; Feretzaki, M.; Lingner, J. The makings of TERRA R-loops at chromosome ends. Cell Cycle 2021, 20, 1745–1759. [Google Scholar] [CrossRef]
- Crossley, M.P.; Bocek, M.; Cimprich, K.A. R-Loops as Cellular Regulators and Genomic Threats. Mol. Cell 2019, 73, 398–411. [Google Scholar] [CrossRef]
- Hegazy, Y.A.; Fernando, C.M.; Tran, E.J. The balancing act of R-loop biology: The good, the bad, and the ugly. J. Biol. Chem. 2020, 295, 905–913. [Google Scholar] [CrossRef]
- Toubiana, S.; Selig, S. DNA:RNA hybrids at telomeres—When it is better to be out of the (R) loop. FEBS J. 2018, 285, 2552–2566. [Google Scholar] [CrossRef]
- Castano, I.; Pan, S.J.; Zupancic, M.; Hennequin, C.; Dujon, B.; Cormack, B.P. Telomere length control and transcriptional regulation of subtelomeric adhesins in Candida glabrata. Mol. Microbiol. 2005, 55, 1246–1258. [Google Scholar] [CrossRef]
- De Las Penas, A.; Pan, S.J.; Castano, I.; Alder, J.; Cregg, R.; Cormack, B.P. Virulence-related surface glycoproteins in the yeast pathogen Candida glabrata are encoded in subtelomeric clusters and subject to RAP1- and SIR-dependent transcriptional silencing. Genes Dev. 2003, 17, 2245–2258. [Google Scholar] [CrossRef] [PubMed]
- Domergue, R.; Castano, I.; De Las Penas, A.; Zupancic, M.; Lockatell, V.; Hebel, J.R.; Johnson, D.; Cormack, B.P. Nicotinic acid limitation regulates silencing of Candida adhesins during UTI. Science 2005, 308, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Weuts, A.; Voet, T.; Verbeeck, J.; Lambrechts, N.; Wirix, E.; Schoonjans, L.; Danloy, S.; Marynen, P.; Froyen, G. Telomere length homeostasis and telomere position effect on a linear human artificial chromosome are dictated by the genetic background. Nucleic Acids Res. 2012, 40, 11477–11489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Z.; Liu, X.; Wang, S.; Zhang, Y.; He, X.; Sun, S.; Ma, S.; Shyh-Chang, N.; Liu, F.; et al. Telomere-dependent and telomere-independent roles of RAP1 in regulating human stem cell homeostasis. Protein Cell 2019, 10, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Shay, J.W. Long-range telomere regulation of gene expression: Telomere looping and telomere position effect over long distances (TPE-OLD). Differentiation 2018, 99, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sidlauskaite, E.; Le Gall, L.; Mariot, V.; Dumonceaux, J. DUX4 Expression in FSHD Muscles: Focus on Its mRNA Regulation. J. Pers. Med. 2020, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Lemmers, R.J.; van der Maarel, S.M.; van Deutekom, J.C.; van der Wielen, M.J.; Deidda, G.; Dauwerse, H.G.; Hewitt, J.; Hofker, M.; Bakker, E.; Padberg, G.W.; et al. Inter- and intrachromosomal sub-telomeric rearrangements on 4q35: Implications for facioscapulohumeral muscular dystrophy (FSHD) aetiology and diagnosis. Hum. Mol. Genet. 1998, 7, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Robin, J.D.; Ludlow, A.T.; Batten, K.; Gaillard, M.C.; Stadler, G.; Magdinier, F.; Wright, W.E.; Shay, J.W. SORBS2 transcription is activated by telomere position effect-over long distance upon telomere shortening in muscle cells from patients with facioscapulohumeral dystrophy. Genome Res. 2015, 25, 1781–1790. [Google Scholar] [CrossRef]
- Milne, G.T.; Jin, S.; Shannon, K.B.; Weaver, D.T. Mutations in two Ku homologs define a DNA end-joining repair pathway in Saccharomyces cerevisiae. Mol. Cell. Biol. 1996, 16, 4189–4198. [Google Scholar] [CrossRef]
- Teo, S.H.; Jackson, S.P. Identification of Saccharomyces cerevisiae DNA ligase IV: Involvement in DNA double-strand break repair. Embo J. 1997, 16, 4788–4795. [Google Scholar] [CrossRef]
- Wilson, T.E.; Grawunder, U.; Lieber, M.R. Yeast DNA ligase IV mediates non-homologous DNA end joining. Nature 1997, 388, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, G.; Lindahl, T.; Schar, P. Saccharomyces cerevisiae LIF1: A function involved in DNA double-strand break repair related to mammalian XRCC4. Embo. J. 1998, 17, 4188–4198. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Trujillo, K.; Ramos, W.; Sung, P.; Tomkinson, A.E. Promotion of Dnl4-catalyzed DNA end-joining by the Rad50/Mre11/Xrs2 and Hdf1/Hdf2 complexes. Mol. Cell 2001, 8, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Sarthy, J.; Bae, N.S.; Scrafford, J.; Baumann, P. Human RAP1 inhibits non-homologous end joining at telomeres. EMBO J. 2009, 28, 3390–3399. [Google Scholar] [CrossRef] [PubMed]
- Bae, N.S.; Baumann, P. A RAP1/TRF2 complex inhibits nonhomologous end-joining at human telomeric DNA ends. Mol. Cell 2007, 26, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Lototska, L.; Yue, J.X.; Li, J.; Giraud-Panis, M.J.; Songyang, Z.; Royle, N.J.; Liti, G.; Ye, J.; Gilson, E.; Mendez-Bermudez, A. Human RAP1 specifically protects telomeres of senescent cells from DNA damage. EMBO Rep. 2020, 21, e49076. [Google Scholar] [CrossRef]
- Yu, E.Y.; Yen, W.F.; Steinberg-Neifach, O.; Lue, N.F. Rap1 in Candida albicans: An unusual structural organization and a critical function in suppressing telomere recombination. Mol. Cell. Biol. 2010, 30, 1254–1268. [Google Scholar] [CrossRef]
- Malyavko, A.N.; Petrova, O.A.; Zvereva, M.I.; Dontsova, O.A. Functional duplication of Rap1 in methylotrophic yeasts. Sci. Rep. 2019, 9, 7196. [Google Scholar] [CrossRef]
- Sfeir, A.; Kabir, S.; van Overbeek, M.; Celli, G.B.; de Lange, T. Loss of Rap1 induces telomere recombination in the absence of NHEJ or a DNA damage signal. Science 2010, 327, 1657–1661. [Google Scholar] [CrossRef]
- Rai, R.; Chen, Y.; Lei, M.; Chang, S. TRF2-RAP1 is required to protect telomeres from engaging in homologous recombination-mediated deletions and fusions. Nat. Commun. 2016, 7, 10881. [Google Scholar] [CrossRef]
- Mugnier, M.R.; Stebbins, C.E.; Papavasiliou, F.N. Masters of Disguise: Antigenic Variation and the VSG Coat in Trypanosoma brucei. PLoS Pathog. 2016, 12, e1005784. [Google Scholar] [CrossRef]
- Wickstead, B.; Ersfeld, K.; Gull, K. The small chromosomes of Trypanosoma brucei involved in antigenic variation are constructed around repetitive palindromes. Genome Res. 2004, 14, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Cross, G.A.M. Identification, purification and properties of clone-specific glycoprotein antigens constituting the surface coat of Trypanosoma brucei. Parasitology 1975, 71, 393–417. [Google Scholar] [CrossRef] [PubMed]
- Gunzl, A.; Bruderer, T.; Laufer, G.; Schimanski, B.; Tu, L.C.; Chung, H.M.; Lee, P.T.; Lee, M.G. RNA polymerase I transcribes procyclin genes and variant surface glycoprotein gene expression sites in Trypanosoma brucei. Eukaryot. Cell 2003, 2, 542–551. [Google Scholar] [CrossRef]
- Aresta-Branco, F.; Sanches-Vaz, M.; Bento, F.; Rodrigues, J.A.; Figueiredo, L.M. African trypanosomes expressing multiple VSGs are rapidly eliminated by the host immune system. Proc. Natl. Acad. Sci. USA 2019, 116, 20725–20735. [Google Scholar] [CrossRef]
- Myler, P.; Nelson, R.G.; Agabian, N.; Stuart, K. Two mechanisms of expression of a variant antigen gene of Trypanosoma brucei. Nature 1984, 309, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Myler, P.J.; Allison, J.; Agabian, N.; Stuart, K. Antigenic variation in African trypanosomes by gene replacement or activation of alternative telomeres. Cell 1984, 39, 203–211. [Google Scholar] [CrossRef]
- Rudenko, G.; McCulloch, R.; Dirksmulder, A.; Borst, P. Telomere exchange can be an important mechanism of variant surface glycoprotein gene switching in Trypanosoma brucei. Mol. Biochem. Parasitol. 1996, 80, 65–75. [Google Scholar] [CrossRef]
- Horn, D.; McCulloch, R. Molecular mechanisms underlying the control of antigenic variation in African trypanosomes. Curr. Opin. Microbiol. 2010, 13, 700–705. [Google Scholar] [CrossRef]
- Eid, J.; Sollner-Webb, B. Stable integrative transformation of Trypanosoma brucei that occurs exclusively by homologous recombination. Proc. Natl. Acad. Sci. USA 1991, 88, 2118–2121. [Google Scholar] [CrossRef]
- Lee, M.-S.; van der Ploeg, L.H.T. Homologous recombination and stable transfection in the parasitic protozoan Trypanosoma brucei. Science 1990, 250, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.L.; McCulloch, R. Trypanosoma brucei homologous recombination is dependent on substrate length and homology, though displays a differential dependence on mismatch repair as substrate length decreases. Nucleic Acids Res. 2007, 35, 3478–3493. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, R.; Barry, J.D. A role for RAD51 and homologous recombination in Trypanosoma brucei antigenic variation. Genes Dev. 1999, 13, 2875–2888. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Stockdale, C.; Lapsley, C.; Wilkes, J.; McCulloch, R. Interactions among Trypanosoma brucei RAD51 paralogues in DNA repair and antigenic variation. Mol. Microbiol. 2011, 81, 434–456. [Google Scholar] [CrossRef] [PubMed]
- Hartley, C.L.; McCulloch, R. Trypanosoma brucei BRCA2 acts in antigenic variation and has undergone a recent expansion in BRC repeat number that is important during homologous recombination. Mol. Microbiol. 2008, 68, 1237–1251. [Google Scholar] [CrossRef] [PubMed]
- Girasol, M.J.; Krasilnikova, M.; Marques, C.A.; Damasceno, J.D.; Lapsley, C.; Lemgruber, L.; Burchmore, R.; Beraldi, D.; Carruthers, R.; Briggs, E.M.; et al. RAD51-mediated R-loop formation acts to repair transcription-associated DNA breaks driving antigenic variation in Trypanosoma brucei. Proc. Natl. Acad. Sci. USA 2023, 120, e2309306120. [Google Scholar] [CrossRef] [PubMed]
- Burton, P.; McBride, D.J.; Wilkes, J.M.; Barry, J.D.; McCulloch, R. Ku heterodimer-independent end joining in Trypanosoma brucei cell extracts relies upon sequence microhomology. Eukaryot. Cell 2007, 6, 1773–1781. [Google Scholar] [CrossRef]
- Conway, C.; Proudfoot, C.; Burton, P.; Barry, J.D.; McCulloch, R. Two pathways of homologous recombination in Trypanosoma brucei. Mol. Microbiol. 2002, 45, 1687–1700. [Google Scholar] [CrossRef]
- Glover, L.; McCulloch, R.; Horn, D. Sequence homology and microhomology dominate chromosomal double-strand break repair in African trypanosomes. Nucleic Acids Res. 2008, 36, 2608–2618. [Google Scholar] [CrossRef]
- Barbet, A.F.; Kamper, S.M. The importance of mosaic genes to trypanosome survival. Parasitol. Today 1993, 9, 63–66. [Google Scholar] [CrossRef]
- Kamper, S.M.; Barbet, A.F. Surface epitope variation via mosaic gene formation is potential key to long-term survival of Trypanosoma brucei. Mol. Biochem. Parasitol. 1992, 53, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Benmerzouga, I.; Concepcion-Acevedo, J.; Kim, H.S.; Vandoros, A.V.; Cross, G.A.M.; Klingbeil, M.M.; Li, B. Trypanosoma brucei Orc1 is essential for nuclear DNA replication and affects both VSG silencing and VSG switching. Mol. Microbiol. 2013, 87, 196–210. [Google Scholar] [CrossRef]
- Devlin, R.; Marques, C.A.; Paape, D.; Prorocic, M.; Zurita-Leal, A.C.; Campbell, S.J.; Lapsley, C.; Dickens, N.; McCulloch, R. Mapping replication dynamics in Trypanosoma brucei reveals a link with telomere transcription and antigenic variation. Elife 2016, 5, e12765. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Cross, G.A.M. TOPO3alpha influences antigenic variation by monitoring expression-site-associated VSG switching in Trypanosoma brucei. PLoS Pathog. 2010, 6, e1000992. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Cross, G.A.M. Identification of Trypanosoma brucei RMI1/BLAP75 homologue and its roles in antigenic variation. PLoS ONE 2011, 6, e25313. [Google Scholar] [CrossRef] [PubMed]
- Boothroyd, C.E.; Dreesen, O.; Leonova, T.; Ly, K.I.; Figueiredo, L.M.; Cross, G.A.M.; Papavasiliou, F.N. A yeast-endonuclease-generated DNA break induces antigenic switching in Trypanosoma brucei. Nature 2009, 459, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Glover, L.; Alsford, S.; Horn, D. DNA break site at fragile subtelomeres determines probability and mechanism of antigenic variation in African trypanosomes. PLoS Pathog. 2013, 9, e1003260. [Google Scholar] [CrossRef] [PubMed]
- Thivolle, A.; Mehnert, A.K.; Tihon, E.; McLaughlin, E.; Dujeancourt-Henry, A.; Glover, L. DNA double strand break position leads to distinct gene expression changes and regulates VSG switching pathway choice. PLoS Pathog. 2021, 17, e1010038. [Google Scholar] [CrossRef]
- Alsford, S.; Horn, D.; Glover, L. DNA breaks as triggers for antigenic variation in African trypanosomes. Genome Biol. 2009, 10, 223. [Google Scholar] [CrossRef]
- Hovel-Miner, G.A.; Boothroyd, C.E.; Mugnier, M.; Dreesen, O.; Cross, G.A.M.; Papavasiliou, F.N. Telomere length affects the frequency and mechanism of antigenic variation in Trypanosoma brucei. PLoS Pathog. 2012, 8, e1002900. [Google Scholar] [CrossRef]
- Bernards, A.; Michels, P.A.M.; Lincke, C.R.; Borst, P. Growth of chromosome ends in multiplying trypanosomes. Nature 1983, 303, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Jehi, S.E.; Nanavaty, V.; Li, B. Trypanosoma brucei TIF2 and TRF suppress VSG switching using overlapping and independent mechanisms. PLoS ONE 2016, 11, e0156746. [Google Scholar] [CrossRef] [PubMed]
- Jehi, S.E.; Li, X.; Sandhu, R.; Ye, F.; Benmerzouga, I.; Zhang, M.; Zhao, Y.; Li, B. Suppression of subtelomeric VSG switching by Trypanosoma brucei TRF requires its TTAGGG repeat-binding activity. Nucleic Acids Res. 2014, 42, 12899–12911. [Google Scholar] [CrossRef]
- Khamlichi, A.A.; Feil, R. Parallels between Mammalian Mechanisms of Monoallelic Gene Expression. Trends Genet. 2018, 34, 954–971. [Google Scholar] [CrossRef] [PubMed]
- Chess, A. Monoallelic Gene Expression in Mammals. Annu. Rev. Genet. 2016, 50, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Oppikofer, M.; Kueng, S.; Gasser, S.M. SIR-nucleosome interactions: Structure-function relationships in yeast silent chromatin. Gene 2013, 527, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Tarutani, Y.; Takayama, S. Monoallelic gene expression and its mechanisms. Curr. Opin. Plant Biol. 2011, 14, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Massah, S.; Beischlag, T.V.; Prefontaine, G.G. Epigenetic events regulating monoallelic gene expression. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 337–358. [Google Scholar] [CrossRef]
- Gunzl, A.; Kirkham, J.K.; Nguyen, T.N.; Badjatia, N.; Park, S.H. Mono-allelic VSG expression by RNA polymerase I in Trypanosoma brucei: Expression site control from both ends? Gene 2015, 556, 68–73. [Google Scholar] [CrossRef]
- Cestari, I.; Stuart, K. Transcriptional regulation of telomeric expression sites and antigenic variation in trypanosomes. Curr. Genom. 2018, 19, 119–132. [Google Scholar] [CrossRef]
- Faria, J.; Briggs, E.M.; Black, J.A.; McCulloch, R. Emergence and adaptation of the cellular machinery directing antigenic variation in the African trypanosome. Curr. Opin. Microbiol. 2022, 70, 102209. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Gull, K. A pol I transcriptional body associated with VSG mono-allelic expression in Trypanosoma brucei. Nature 2001, 414, 759–763. [Google Scholar] [CrossRef] [PubMed]
- López-Escobar, L.; Hänisch, B.; Halliday, C.; Ishii, M.; Akiyoshi, B.; Dean, S.; Sunter, J.D.; Wheeler, R.J.; Gull, K. Stage-specific transcription activator ESB1 regulates monoallelic antigen expression in Trypanosoma brucei. Nat. Microbiol. 2022, 7, 1280–1290. [Google Scholar] [CrossRef]
- Lopez-Farfan, D.; Bart, J.M.; Rojas-Barros, D.I.; Navarro, M. SUMOylation by the E3 Ligase TbSIZ1/PIAS1 Positively Regulates VSG Expression in Trypanosoma brucei. PLoS Pathog. 2014, 10, e1004545. [Google Scholar] [CrossRef] [PubMed]
- Saura, A.; Iribarren, P.A.; Rojas-Barros, D.; Bart, J.M.; López-Farfán, D.; Andrés-León, E.; Vidal-Cobo, I.; Boehm, C.; Alvarez, V.E.; Field, M.C.; et al. SUMOylated SNF2PH promotes variant surface glycoprotein expression in bloodstream trypanosomes. EMBO Rep. 2019, 20, e48029. [Google Scholar] [CrossRef] [PubMed]
- Field, M.C. Deviating from the norm: Nuclear organisation in trypanosomes. Curr. Opin. Cell Biol. 2023, 85, 102234. [Google Scholar] [CrossRef] [PubMed]
- Berriman, M.; Ghedin, E.; Hertz-Fowler, C.; Blandin, G.; Renauld, H.; Bartholomeu, D.C.; Lennard, N.J.; Caler, E.; Hamlin, N.E.; Haas, B.; et al. The genome of the African trypanosome Trypanosoma brucei. Science 2005, 309, 416–422. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.M.; Myler, P.J.; Blandin, G.; Berriman, M.; Crabtree, J.; Aggarwal, G.; Caler, E.; Renauld, H.; Worthey, E.A.; Hertz-Fowler, C.; et al. Comparative genomics of trypanosomatid parasitic protozoa. Science 2005, 309, 404–409. [Google Scholar] [CrossRef]
- Michaeli, S. Trans-splicing in trypanosomes: Machinery and its impact on the parasite transcriptome. Future Microbiol. 2011, 6, 459–474. [Google Scholar] [CrossRef]
- Gunzl, A. The pre-mRNA splicing machinery of trypanosomes: Complex or simplified? Eukaryot. Cell 2010, 9, 1159–1170. [Google Scholar] [CrossRef]
- Faria, J.; Luzak, V.; Müller, L.S.M.; Brink, B.G.; Hutchinson, S.; Glover, L.; Horn, D.; Siegel, T.N. Spatial integration of transcription and splicing in a dedicated compartment sustains monogenic antigen expression in African trypanosomes. Nat. Microbiol. 2021, 6, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Glover, L.; Hutchinson, S.; Alsford, S.; Horn, D. VEX1 controls the allelic exclusion required for antigenic variation in trypanosomes. Proc. Natl. Acad. Sci. USA 2016, 113, 7225–7230. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.; Glover, L.; Hutchinson, S.; Boehm, C.; Field, M.C.; Horn, D. Monoallelic expression and epigenetic inheritance sustained by a Trypanosoma brucei variant surface glycoprotein exclusion complex. Nat. Commun. 2019, 10, 3023. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Nguyen, B.N.; Lee, J.H.; Panigrahi, A.K.; Gunzl, A. Characterization of a novel class I transcription factor A (CITFA) subunit that is indispensable for transcription by the multifunctional RNA polymerase I of Trypanosoma brucei. Eukaryot. Cell 2012, 11, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Muller, L.S.; Park, S.H.; Siegel, T.N.; Gunzl, A. Promoter occupancy of the basal class I transcription factor A differs strongly between active and silent VSG expression sites in Trypanosoma brucei. Nucleic Acids Res. 2014, 42, 3164–3176. [Google Scholar] [CrossRef] [PubMed]
- Kassem, A.; Pays, E.; Vanhamme, L. Transcription is initiated on silent variant surface glycoprotein expression sites despite monoallelic expression in Trypanosoma brucei. Proc. Natl. Acad. Sci. USA 2014, 111, 8943–8948. [Google Scholar] [CrossRef] [PubMed]
- Vanhamme, L.; Poelvoorde, P.; Pays, A.; Tebabi, P.; Van Xong, H.; Pays, E. Differential RNA elongation controls the variant surface glycoprotein gene expression sites of Trypanosoma brucei. Mol. Microbiol. 2000, 36, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, G. Epigenetics and transcriptional control in African trypanosomes. Essays Biochem. 2010, 48, 201–219. [Google Scholar]
- Stanne, T.M.; Rudenko, G. Active VSG expression sites in Trypanosoma brucei are depleted of nucleosomes. Eukaryot. Cell 2010, 9, 136–147. [Google Scholar] [CrossRef]
- Figueiredo, L.M.; Cross, G.A.M. Nucleosomes are depleted at the VSG expression site transcribed by RNA polymerase I in African trypanosomes. Eukaryot. Cell 2010, 9, 148–154. [Google Scholar] [CrossRef]
- Povelones, M.L.; Gluenz, E.; Dembek, M.; Gull, K.; Rudenko, G. Histone H1 plays a role in heterochromatin formation and VSG expression site silencing in Trypanosoma brucei. PLoS Pathog. 2012, 8, e1003010. [Google Scholar] [CrossRef] [PubMed]
- Alsford, S.; Horn, D. Cell-cycle-regulated control of VSG expression site silencing by histones and histone chaperones ASF1A and CAF-1b in Trypanosoma brucei. Nucleic Acids Res. 2012, 40, 10150–10160. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.; Wand, M.; Foulston, L.; Young, R.; Harley, K.; Terry, S.; Ersfeld, K.; Rudenko, G. A novel ISWI is involved in VSG expression site downregulation in African trypanosomes. EMBO J. 2007, 26, 2400–2410. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, M.S.; Kushwaha, M.; Ersfeld, K.; Fullbrook, A.; Stanne, T.M.; Rudenko, G. NLP is a novel transcription regulator involved in VSG expression site control in Trypanosoma brucei. Nucleic Acids Res. 2011, 39, 2018–2031. [Google Scholar] [CrossRef]
- Stanne, T.M.; Narayanan, M.S.; Ridewood, S.; Ling, A.; Witmer, K.; Kushwaha, M.; Wiesler, S.; Wickstead, B.; Wood, J.; Rudenko, G. Identification of the ISWI chromatin remodeling complex of the early branching eukaryote Trypanosoma brucei. J. Biol. Chem. 2015, 290, 29760. [Google Scholar]
- Denninger, V.; Fullbrook, A.; Bessat, M.; Ersfeld, K.; Rudenko, G. The FACT subunit TbSpt16 is involved in cell cycle specific control of VSG expression sites in Trypanosoma brucei. Mol. Microbiol. 2010, 78, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Denninger, V.; Rudenko, G. FACT plays a major role in histone dynamics affecting VSG expression site control in Trypanosoma brucei. Mol. Microbiol. 2014, 94, 945–962. [Google Scholar] [CrossRef]
- Narayanan, M.S.; Rudenko, G. TDP1 is an HMG chromatin protein facilitating RNA polymerase I transcription in African trypanosomes. Nucleic Acids Res. 2013, 41, 2981–2992. [Google Scholar] [CrossRef]
- Figueiredo, L.M.; Janzen, C.J.; Cross, G.A.M. A histone methyltransferase modulates antigenic variation in African trypanosomes. PLoS Biol. 2008, 6, e161. [Google Scholar] [CrossRef]
- Sandhu, R.; Li, B. Telomerase activity is required for the telomere G-overhang structure in Trypanosoma brucei. Sci. Rep. 2017, 7, 15983. [Google Scholar] [CrossRef]
- Sandhu, R.; Li, B. Examination of the telomere G-overhang structure in Trypanosoma brucei. J. Vis. Exp. 2011, 47, 1959. [Google Scholar]
- Dreesen, O.; Li, B.; Cross, G.A.M. Telomere structure and shortening in telomerase-deficient Trypanosoma brucei. Nucleic Acids Res. 2005, 33, 4536–4543. [Google Scholar] [CrossRef]
- Sandhu, R.; Sanford, S.; Basu, S.; Park, M.; Pandya, U.M.; Li, B.; Chakrabarti, K. A trans-spliced telomerase RNA dictates telomere synthesis in Trypanosoma brucei. Cell Res. 2013, 23, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Kolet, L.; Doniger, T.; Biswas, V.K.; Unger, R.; Tzfati, Y.; Michaeli, S. The Trypanosoma brucei telomerase RNA (TER) homologue binds core proteins of the C/D snoRNA family. FEBS Lett. 2013, 587, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Monroy-Eklund, A.; Klotz, K.; Saha, A.; Davis, J.; Li, B.; Laederach, A.; Chakrabarti, K. In vivo architecture of the telomerase RNA catalytic core in Trypanosoma brucei. Nucleic Acids Res. 2021, 49, 12445–12466. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.Z.; Schwebs, M.; Briggs, E.; Weisert, N.; Reis, H.; Lemgruber, L.; Luko, K.; Wilkes, J.; Butter, F.; McCulloch, R.; et al. Genome maintenance functions of a putative Trypanosoma brucei translesion DNA polymerase include telomere association and a role in antigenic variation. Nucleic Acids Res. 2020, 48, 9660–9680. [Google Scholar] [CrossRef]
- Rudd, S.G.; Glover, L.; Jozwiakowski, S.K.; Horn, D.; Doherty, A.J. PPL2 translesion polymerase is essential for the completion of chromosomal DNA replication in the African trypanosome. Mol. Cell 2013, 52, 554–565. [Google Scholar] [CrossRef]
- Janzen, C.J.; Lander, F.; Dreesen, O.; Cross, G.A.M. Telomere length regulation and transcriptional silencing in KU80-deficient Trypanosoma brucei. Nucleic Acids Res. 2004, 32, 6575–6584. [Google Scholar] [CrossRef]
- Conway, C.; McCulloch, R.; Ginger, M.L.; Robinson, N.P.; Browitt, A.; Barry, J.D. Ku is important for telomere maintenance, but not for differential expression of telomeric VSG genes, in African trypanosomes. J. Biol. Chem. 2002, 277, 21269–21277. [Google Scholar] [CrossRef]
- Saha, A.; Gaurav, A.K.; Pandya, U.M.; Afrin, M.; Sandhu, R.; Nanavaty, V.; Schnur, B.; Li, B. TbTRF suppresses the TERRA level and regulates the cell cycle-dependent TERRA foci number with a TERRA binding activity in its C-terminal Myb domain. Nucleic Acids Res. 2021, 49, 5637–5653. [Google Scholar] [CrossRef]
- Giresi, P.G.; Lieb, J.D. Isolation of active regulatory elements from eukaryotic chromatin using FAIRE (Formaldehyde Assisted Isolation of Regulatory Elements). Methods 2009, 48, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Renauld, H.; Aparicio, O.M.; Zierath, P.D.; Billington, B.L.; Chhablani, S.K.; Gottschling, D.E. Silent domains are assembled continuously from the telomere and are defined by promoter distance and strength, and by SIR3 dosage. Genes Dev. 1993, 7, 1133–1145. [Google Scholar] [CrossRef]
- Konig, P.; Fairall, L.; Rhodes, D. Sequence-specific DNA recognition by the Myb-like domain of the human telomere binding protein TRF1: A model for the protein-DNA complex. Nucleic Acids Res. 1998, 26, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; Rieger, K.J.; Morschhauser, J. Functional analysis of CaRAP1, encoding the Repressor/activator protein 1 of Candida albicans. Gene 2003, 307, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Fujita, I.; Tanaka, M.; Kanoh, J. Identification of the functional domains of the telomere protein Rap1 in Schizosaccharomyces pombe. PLoS ONE 2012, 7, e49151. [Google Scholar] [CrossRef] [PubMed]
- Nett, I.R.; Martin, D.M.; Miranda-Saavedra, D.; Lamont, D.; Barber, J.D.; Mehlert, A.; Ferguson, M.A. The phosphoproteome of bloodstream form Trypanosoma brucei, causative agent of African sleeping sickness. Mol. Cell Proteom. 2009, 8, 1527–1538. [Google Scholar] [CrossRef]
- Urbaniak, M.D.; Martin, D.M.; Ferguson, M.A. Global quantitative SILAC phosphoproteomics reveals differential phosphorylation is widespread between the procyclic and bloodstream form lifecycle stages of Trypanosoma brucei. J. Proteome Res. 2013, 12, 2233–2244. [Google Scholar] [CrossRef]
- Cestari, I.; McLeland-Wieser, H.; Stuart, K. Nuclear phosphatidylinositol 5-phosphatase is essential for allelic exclusion of variant surface glycoprotein genes in trypanosomes. Mol. Cell. Biol. 2019, 39, e00395-18. [Google Scholar] [CrossRef]
- Cestari, I.; Stuart, K. Inositol phosphate pathway controls transcription of telomeric expression sites in trypanosomes. Proc. Natl. Acad. Sci. USA 2015, 112, E2803–E2812. [Google Scholar] [CrossRef]
- Touray, A.O.; Rajesh, R.; Isebe, T.; Sternlieb, T.; Loock, M.; Kutova, O.; Cestari, I. PI(3,4,5)P3 allosteric regulation of repressor activator protein 1 controls antigenic variation in trypanosomes. Elife 2023, 12, RP89331. [Google Scholar] [CrossRef] [PubMed]
- Martinez, P.; Gomez-Lopez, G.; Garcia, F.; Mercken, E.; Mitchell, S.; Flores, J.M.; de Cabo, R.; Blasco, M.A. RAP1 protects from obesity through its extratelomeric role regulating gene expression. Cell Rep. 2013, 3, 2059–2074. [Google Scholar] [CrossRef]
- Yeung, F.; Ramírez, C.M.; Mateos-Gomez, P.A.; Pinzaru, A.; Ceccarini, G.; Kabir, S.; Fernández-Hernando, C.; Sfeir, A. Nontelomeric role for Rap1 in regulating metabolism and protecting against obesity. Cell Rep. 2013, 3, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- Budzak, J.; Jones, R.; Tschudi, C.; Kolev, N.G.; Rudenko, G. An assembly of nuclear bodies associates with the active VSG expression site in African trypanosomes. Nat. Commun. 2022, 13, 101. [Google Scholar] [CrossRef] [PubMed]
- Budzak, J.; Rudenko, G. Pedal to the Metal: Nuclear Splicing Bodies Turbo-Charge VSG mRNA Production in African Trypanosomes. Front. Cell Dev. Biol. 2022, 10, 876701. [Google Scholar] [CrossRef] [PubMed]
- Sheader, K.; Vaughan, S.; Minchin, J.; Hughes, K.; Gull, K.; Rudenko, G. Variant surface glycoprotein RNA interference triggers a precytokinesis cell cycle arrest in African trypanosomes. Proc. Natl. Acad. Sci. USA 2005, 102, 8716–8721. [Google Scholar] [CrossRef]
- Smith, T.K.; Vasileva, N.; Gluenz, E.; Terry, S.; Portman, N.; Kramer, S.; Carrington, M.; Michaeli, S.; Gull, K.; Rudenko, G. Blocking variant surface glycoprotein synthesis in Trypanosoma brucei triggers a general arrest in translation initiation. PLoS ONE 2009, 4, e7532. [Google Scholar] [CrossRef]
- Maris, C.; Dominguez, C.; Allain, F.H. The RNA recognition motif, a plastic RNA-binding platform to regulate post-transcriptional gene expression. FEBS J. 2005, 272, 2118–2131. [Google Scholar] [CrossRef]
- Ridewood, S.; Ooi, C.P.; Hall, B.; Trenaman, A.; Wand, N.V.; Sioutas, G.; Scherwitzl, I.; Rudenko, G. The role of genomic location and flanking 3’UTR in the generation of functional levels of variant surface glycoprotein in Trypanosoma brucei. Mol. Microbiol. 2017, 106, 614–634. [Google Scholar] [CrossRef]
- Munoz-Jordan, J.L.; Davies, K.P.; Cross, G.A.M. Stable expression of mosaic coats of variant surface glycoproteins in Trypanosoma brucei. Science 1996, 272, 1795–1797. [Google Scholar] [CrossRef]
- Batram, C.; Jones, N.G.; Janzen, C.J.; Markert, S.M.; Engstler, M. Expression site attenuation mechanistically links antigenic variation and development in Trypanosoma brucei. Elife 2014, 3, e02324. [Google Scholar] [CrossRef]
- Zimmermann, H.; Subota, I.; Batram, C.; Kramer, S.; Janzen, C.J.; Jones, N.G.; Engstler, M. A quorum sensing-independent path to stumpy development in Trypanosoma brucei. PLoS Pathog. 2017, 13, e1006324. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, L.M.D.; Egler, F.; Arnold, K.; Papavasiliou, N.; Clayton, C.; Erben, E. Functional insights from a surface antigen mRNA-bound proteome. Elife 2021, 10, e68136. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, G.B.; Tinti, M.; Ridgway, M.; Horn, D. Control of Variant Surface Glycoprotein Expression by CFB2 in Trypanosoma brucei and Quantitative Proteomic Connections to Translation and Cytokinesis. mSphere 2022, 7, e0006922. [Google Scholar] [CrossRef] [PubMed]
- Viegas, I.J.; de Macedo, J.P.; Serra, L.; De Niz, M.; Temporão, A.; Pereira, S.S.; Mirza, A.H.; Bergstrom, E.; Rodrigues, J.A.; Aresta-Branco, F.; et al. N6-methyladenosine in poly(A) tails stabilize VSG transcripts. Nature 2022, 604, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Maudlin, I.E.; Kelly, S.; Schwede, A.; Carrington, M. VSG mRNA levels are regulated by the production of functional VSG protein. Mol. Biochem. Parasitol. 2020, 241, 111348. [Google Scholar] [CrossRef] [PubMed]
- Glover, L.; Horn, D. Trypanosomal histone gammaH2A and the DNA damage response. Mol. Biochem. Parasitol. 2012, 183, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Cerritelli, S.M.; Crouch, R.J. Ribonuclease H: The enzymes in eukaryotes. FEBS J. 2009, 276, 1494–1505. [Google Scholar] [CrossRef]
- Briggs, E.; Crouch, K.; Lemgruber, L.; Lapsley, C.; McCulloch, R. Ribonuclease H1-targeted R-loops in surface antigen gene expression sites can direct trypanosome immune evasion. PLoS Genet. 2018, 14, e1007729. [Google Scholar] [CrossRef]
- Khadaroo, B.; Teixeira, M.T.; Luciano, P.; Eckert-Boulet, N.; Germann, S.M.; Simon, M.N.; Gallina, I.; Abdallah, P.; Gilson, E.; Géli, V.; et al. The DNA damage response at eroded telomeres and tethering to the nuclear pore complex. Nat. Cell Biol. 2009, 11, 980–987. [Google Scholar] [CrossRef]
- Kabir, S.; Hockemeyer, D.; de Lange, T. TALEN gene knockouts reveal no requirement for the conserved human shelterin protein Rap1 in telomere protection and length regulation. Cell Rep. 2014, 9, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Martinez, P.; Thanasoula, M.; Carlos, A.R.; Gomez-Lopez, G.; Tejera, A.M.; Schoeftner, S.; Dominguez, O.; Pisano, D.G.; Tarsounas, M.; Blasco, M.A. Mammalian Rap1 controls telomere function and gene expression through binding to telomeric and extratelomeric sites. Nat. Cell Biol. 2010, 12, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.C.K.; Patel, H.; Chia, M.; Moretto, F.; Frith, D.; Snijders, A.P.; van Werven, F.J. Repression of Divergent Noncoding Transcription by a Sequence-Specific Transcription Factor. Mol. Cell 2018, 72, 942–954.e7. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.C.K.; Van Werven, F.J. Transcribe this way: Rap1 confers promoter directionality by repressing divergent transcription. Transcription 2019, 10, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Teo, H.; Ghosh, S.; Luesch, H.; Ghosh, A.; Wong, E.T.; Malik, N.; Orth, A.; de Jesus, P.; Perry, A.S.; Oliver, J.D.; et al. Telomere-independent Rap1 is an IKK adaptor and regulates NF-kappaB-dependent gene expression. Nat. Cell Biol. 2010, 12, 758–767. [Google Scholar] [CrossRef]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef]
- Oksuz, O.; Henninger, J.E.; Warneford-Thomson, R.; Zheng, M.M.; Erb, H.; Vancura, A.; Overholt, K.J.; Hawken, S.W.; Banani, S.F.; Lauman, R.; et al. Transcription factors interact with RNA to regulate genes. Mol. Cell 2023, 83, 2449–2463.e13. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B. Unwrap RAP1’s Mystery at Kinetoplastid Telomeres. Biomolecules 2024, 14, 67. https://doi.org/10.3390/biom14010067
Li B. Unwrap RAP1’s Mystery at Kinetoplastid Telomeres. Biomolecules. 2024; 14(1):67. https://doi.org/10.3390/biom14010067
Chicago/Turabian StyleLi, Bibo. 2024. "Unwrap RAP1’s Mystery at Kinetoplastid Telomeres" Biomolecules 14, no. 1: 67. https://doi.org/10.3390/biom14010067





