GelMA/PEDOT:PSS Composite Conductive Hydrogel-Based Generation and Protection of Cochlear Hair Cells through Multiple Signaling Pathways
Abstract
:1. Introduction
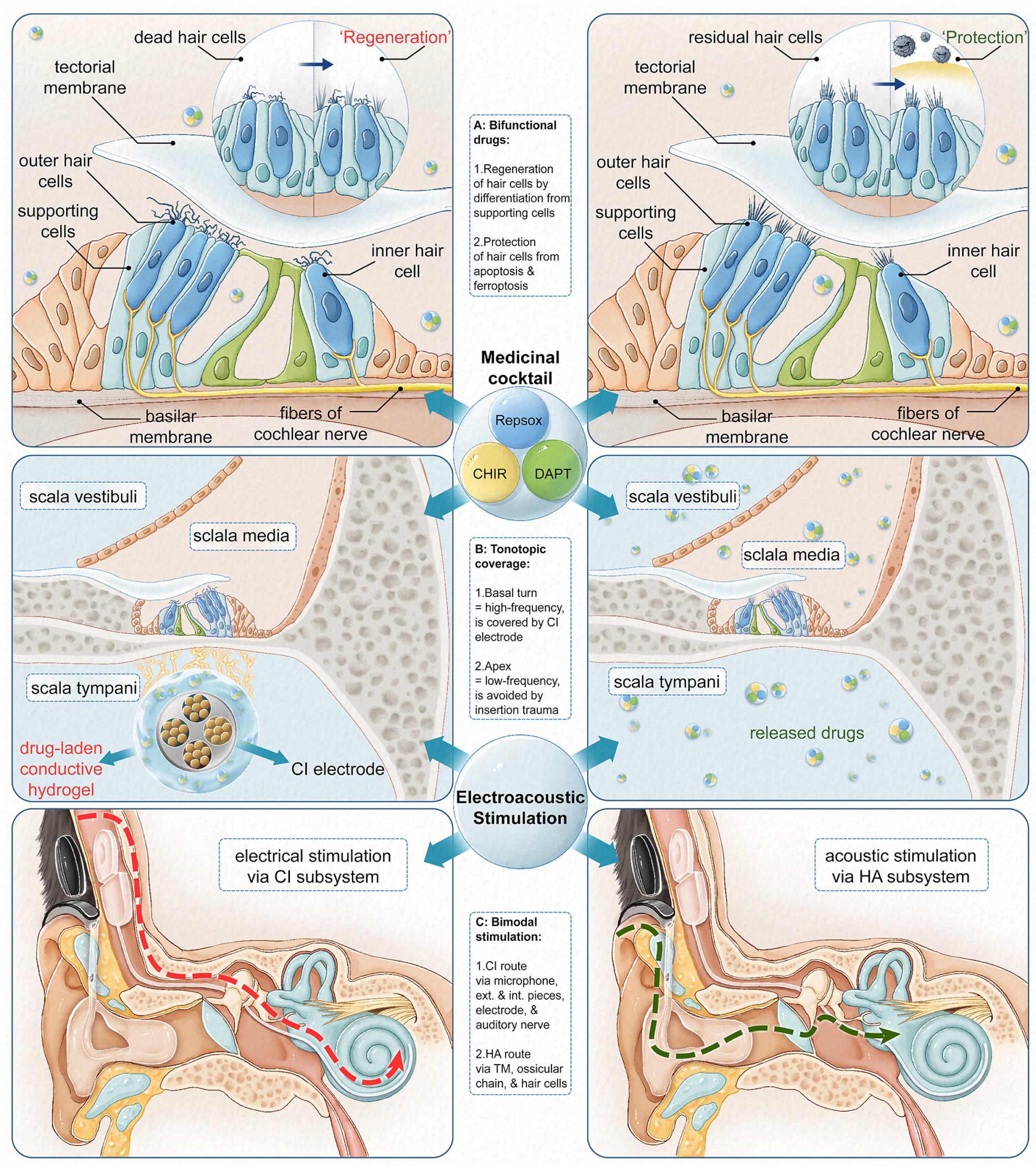
1.1. Cochlear Implantation and Electroacoustic Stimulation
1.2. Pharmacotherapy for Cochlear Hair Cell Generation and Protection
1.3. Inner Ear Drug Delivery Using Biomaterial-Modified Cochlear Implant Electrode
1.4. Conductive HG-Facilitated Generation and Protection of Hair Cells
2. Materials and Methods
2.1. Preparation of the Hydrogel
2.2. Verification of Hydrogel Production
2.3. Mechanical Analysis of the Conductive Hydrogel
2.4. Electrochemical Analysis of the Conductive Hydrogel
2.5. Morphological Analysis of the Conductive Hydrogel
2.6. Calculation of Swelling Ratio of the Conductive Hydrogel
2.7. Degradation and Stability Test of the Conductive Hydrogel
2.8. Drug Loading in and Release from the Conductive Hydrogel
2.9. Analysis of Biocompatibility and Biosafety of the Conductive Hydrogel
2.10. Animals Strains Used for Various Studies
2.11. Separation and Differentiation of Cochlear Supporting Cells
2.12. Immunofluorescent Microscopy for Cochlear Cells in an Organoid
2.13. Cell Culture of HEI-OC1 Cochlear Cells
2.14. Establishment of an Aminoglycoside-Induced Ototoxic Model
2.15. Assessment of Cell Viability of HEI-OC1
2.16. Measurement of Apoptosis in HEI-OC1 Cells
2.17. RNA Isolation and Quantitative Real-Time PCR
2.18. Quantification of Oxidative Stress in HEI-OC1 Cells
2.19. Measurement of Ferroptosis in HEI-OC1
2.20. Transcriptomic Sequencing and Bioinformatic Analysis
2.21. Statistical Analysis
3. Results
3.1. GelMA/PEDOT:PSS Conductive Hydrogel Exhibited Desirable Material Properties
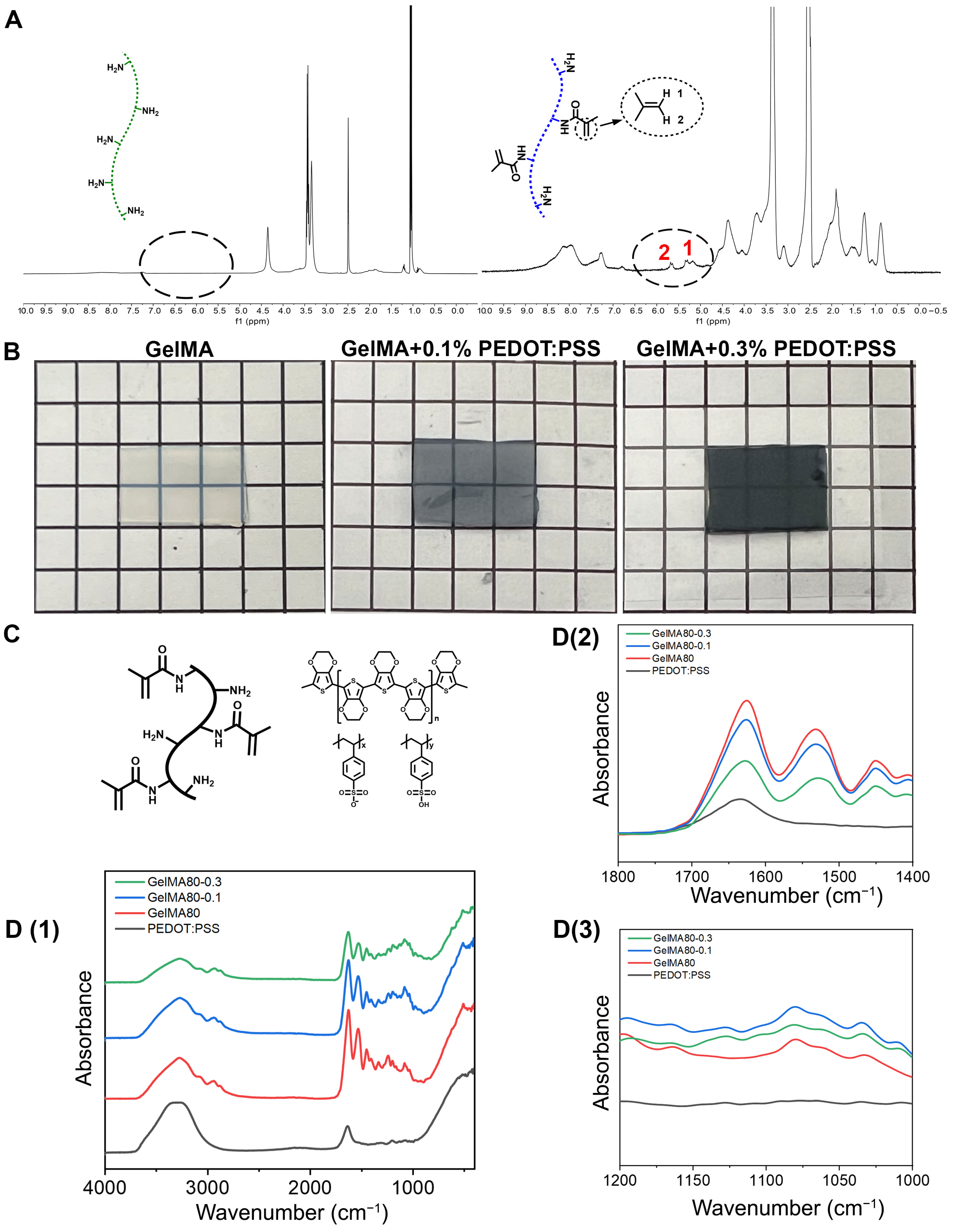
3.1.1. GelMA/PEDOT:PSS Composite Hydrogel Was Successfully Prepared
3.1.2. GelMA/PEDOT:PSS Hydrogel Exhibited Satisfactory Mechanical Features
3.1.3. GelMA/PEDOT:PSS Hydrogel Exhibited Satisfactory Electrochemical Features
3.1.4. GelMA/PEDOT:PSS Hydrogel Displayed a Cell-Friendly 3D Porous Morphology
3.2. Conductive Hydrogel Shows Reasonable Biostability and Biodegradation
3.3. Conductive Hydrogel Demonstrates Remarkable Biocompatibility and Biosafety
3.4. Conductive Hydrogel Supports Sustained Release of Medicinal Cocktail
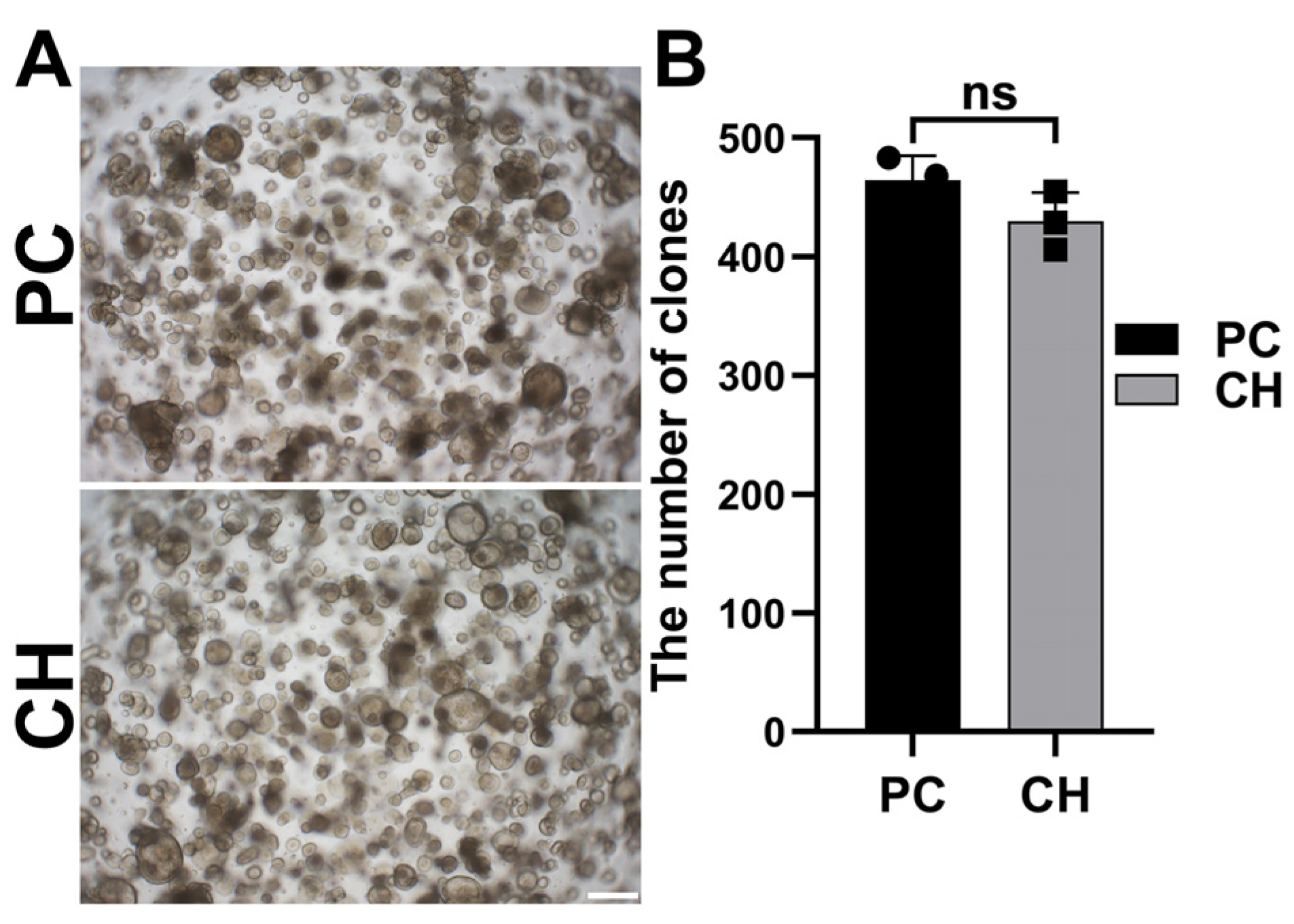
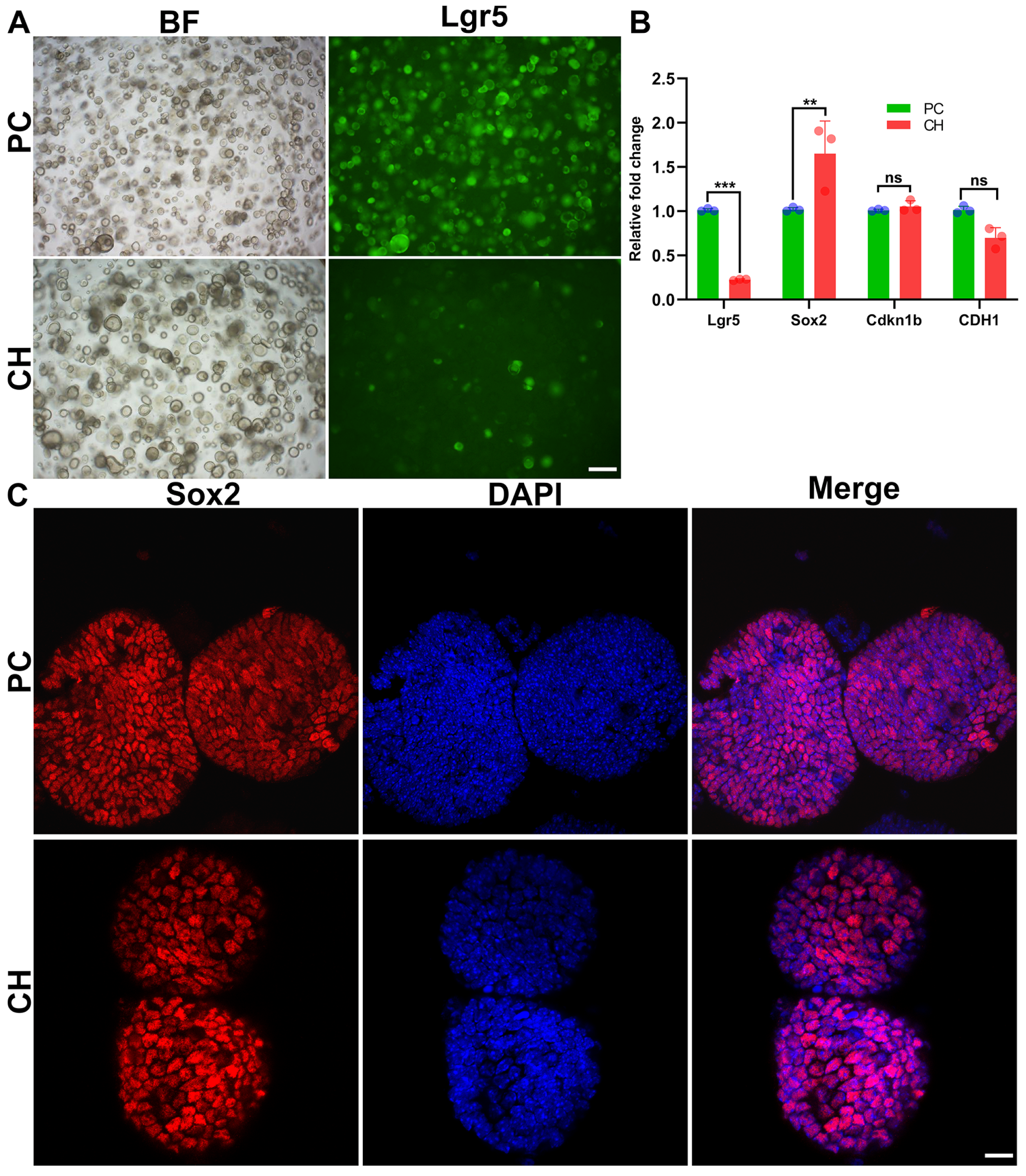
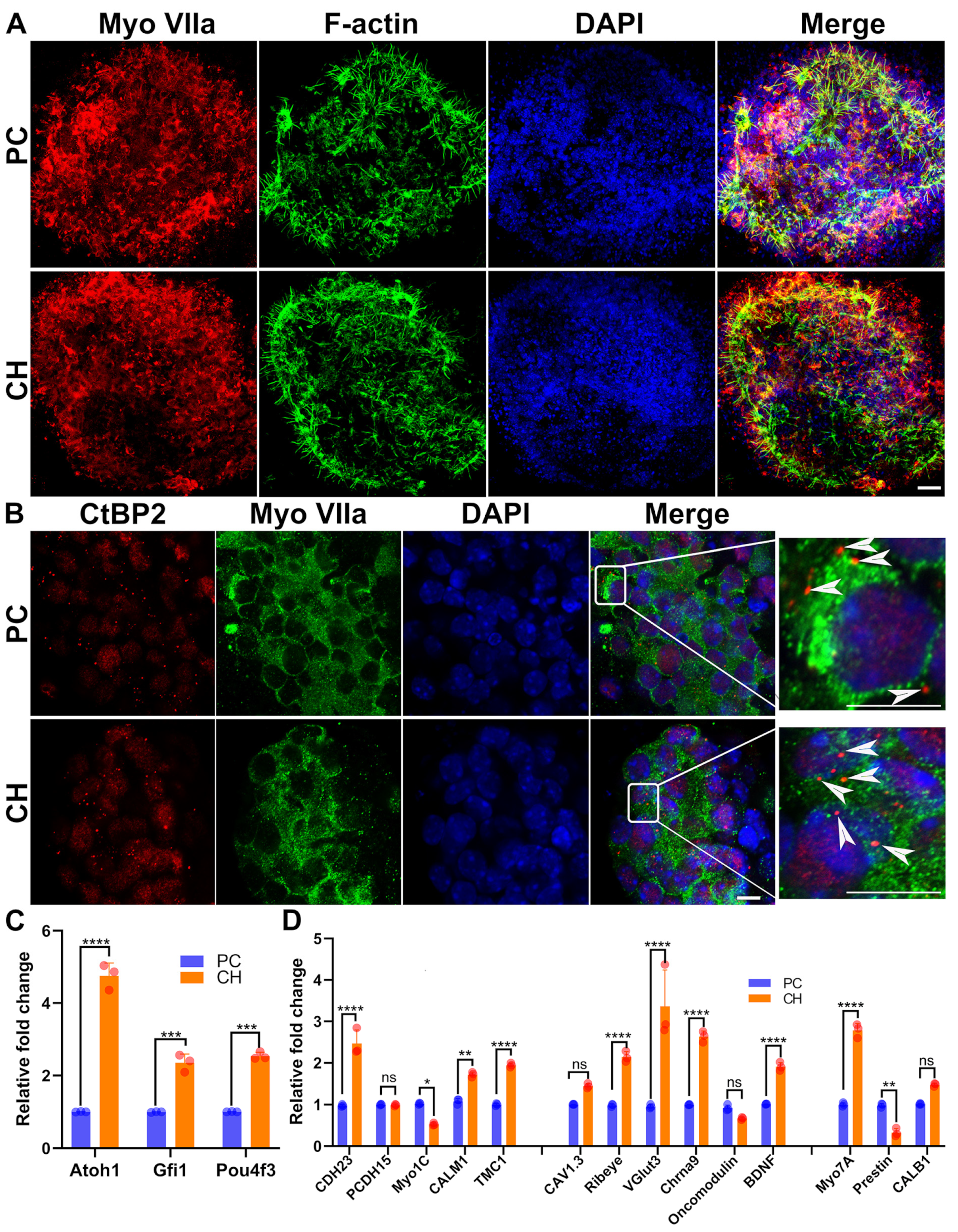
3.5. Conductive Hydrogel Facilitates Cochlear Cell Expansion in Cochlear Organoids
3.6. Drug Cocktail Released by Conductive Hydrogel Promotes the Generation of Hair Cells
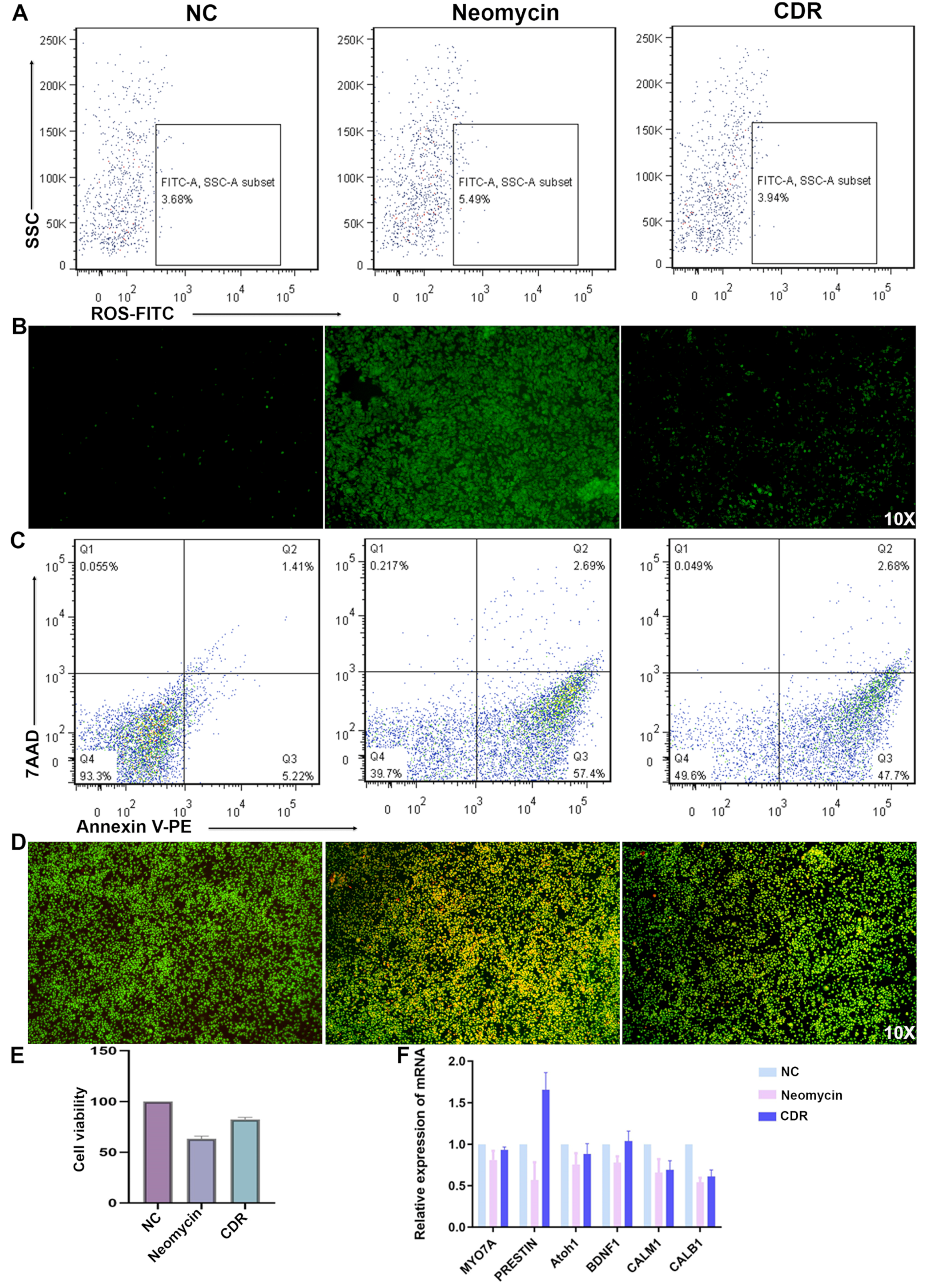
3.7. Drug Cocktail Released by Conductive Hydrogel Protects Hair Cells from Apoptosis
3.8. Drugs Released by Conductive Hydrogel Upregulates Hair Cell Function-Related Genes
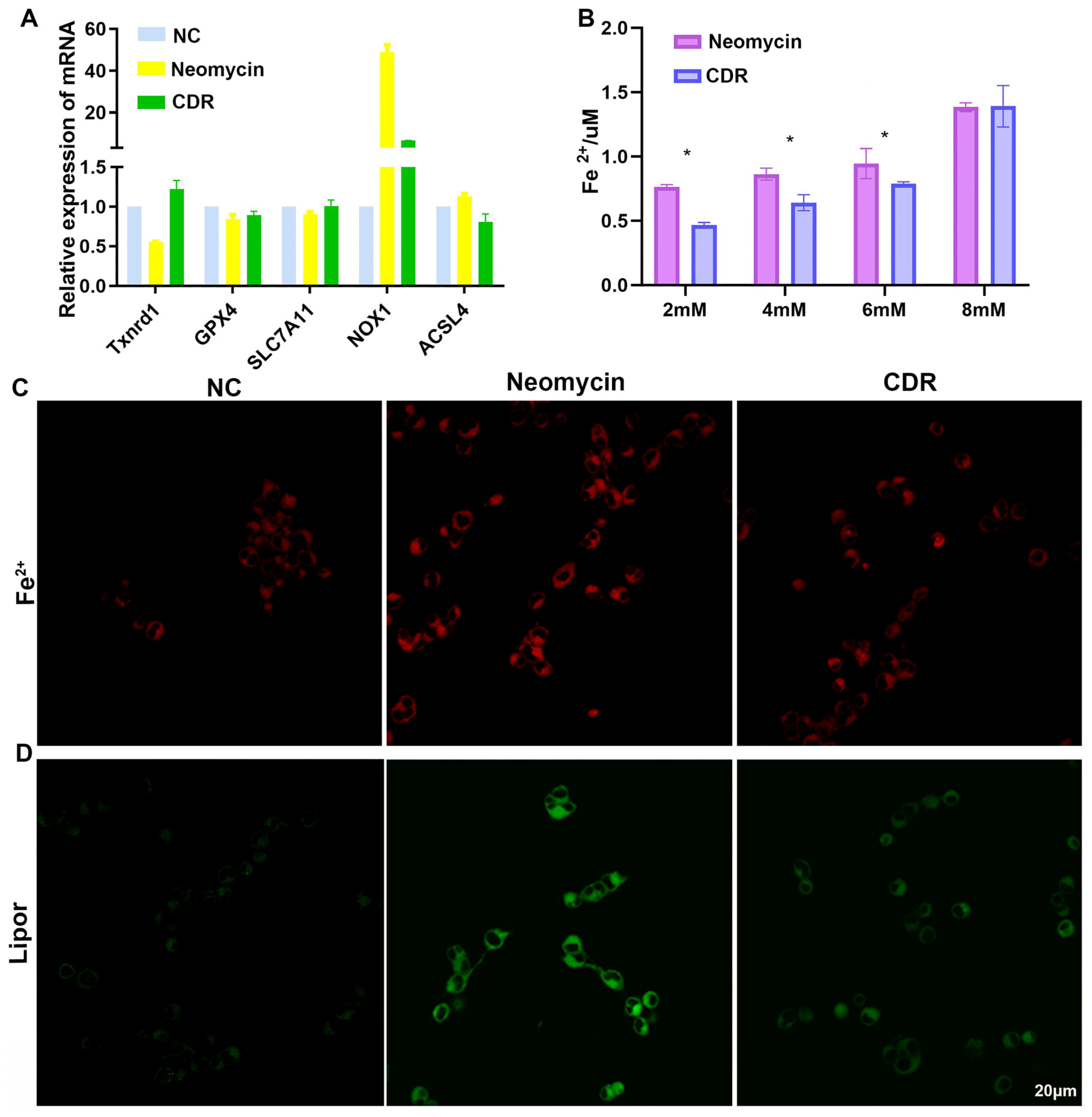
3.9. Drug Cocktail Released by Conductive Hydrogel Protects Hair Cells from Ferroptosis
3.10. Potential Molecular Mechanism Underlying the Hydrogel-Facilitated, Medicinal Cocktail-Induced Otoprotective Effects
4. Discussion
4.1. GelMA/PEDOT:PSS Conductive Hydrogel as a Potentially Suitable Coating for CI Electrode
4.2. GelMA/PEDOT:PSS Conductive Hydrogel as an Adequate Reservoir for Intracochlear Drug Delivery
4.3. Conductive Hydrogel-Released Drug Cocktail Possesses Otoregenerative Potential
4.4. Conductive Hydrogel-Released Drug Cocktail Exerts Otoprotective Effect
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chadha, S.; Cieza, A. World Health Organization and Its Initiative for Ear and Hearing Care. Otolaryngol. Clin. N. Am. 2018, 51, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.L. Cochlear Implantation in Adults. N. Engl. J. Med. 2020, 382, 1531–1542. [Google Scholar] [CrossRef]
- Mudry, A.; Mills, M. The early history of the cochlear implant: A retrospective. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Szyfter, W.; Karlik, M.; Sekula, A.; Harris, S.; Gawecki, W. Current indications for cochlear implantation in adults and children. Otolaryngol. Pol. 2019, 73, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Kuhlmey, M.; Kim, A.H. Electroacoustic Stimulation. Otolaryngol. Clin. N. Am. 2019, 52, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Woodson, E.A.; Reiss, L.A.J.; Turner, C.W.; Gfeller, K.; Gantz, B.J. The Hybrid cochlear implant: A review. Adv. Oto-Rhino-Laryngol. 2010, 67, 125–134. [Google Scholar] [CrossRef]
- Irving, S.; Gillespie, L.; Richardson, R.; Rowe, D.; Fallon, J.B.; Wise, A.K. Electroacoustic stimulation: Now and into the future. Biomed Res. Int. 2014, 2014, 350504. [Google Scholar] [CrossRef]
- Perkins, E.; Lee, J.; Manzoor, N.; O’Malley, M.; Bennett, M.; Labadie, R.; Rivas, A.; Haynes, D.; Gifford, R. The Reality of Hearing Preservation in Cochlear Implantation: Who Is Utilizing EAS? Otol. Neurotol. 2021, 42, 832–837. [Google Scholar] [CrossRef]
- Tarabichi, O.; Jensen, M.; Hansen, M.R. Advances in hearing preservation in cochlear implant surgery. Curr. Opin. Otolaryngol. Head Neck Surg. 2021, 29, 385–390. [Google Scholar] [CrossRef]
- O’Leary, S.J.; Monksfield, P.; Kel, G.; Connolly, T.; Souter, M.A.; Chang, A.; Marovic, P.; O’Leary, J.S.; Richardson, R.; Eastwood, H. Relations between cochlear histopathology and hearing loss in experimental cochlear implantation. Hear. Res. 2013, 298, 27–35. [Google Scholar] [CrossRef]
- Gantz, B.J.; Turner, C.; Gfeller, K.E.; Lowder, M.W. Preservation of hearing in cochlear implant surgery: Advantages of combined electrical and acoustical speech processing. Laryngoscope 2005, 115, 796–802. [Google Scholar] [CrossRef]
- Parys, Q.A.; Van Bulck, P.; Loos, E.; Verhaert, N. Inner Ear Pharmacotherapy for Residual Hearing Preservation in Cochlear Implant Surgery: A Systematic Review. Biomolecules 2022, 12, 529. [Google Scholar] [CrossRef]
- McLean, W.J.; Yin, X.; Lu, L.; Lenz, D.R.; McLean, D.; Langer, R.; Karp, J.M.; Edge, A.S.B. Clonal Expansion of Lgr5-Positive Cells from Mammalian Cochlea and High-Purity Generation of Sensory Hair Cells. Cell Rep. 2017, 18, 1917–1929. [Google Scholar] [CrossRef] [PubMed]
- Salt, A.N.; Hirose, K. Communication pathways to and from the inner ear and their contributions to drug delivery. Hear. Res. 2018, 362, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Plontke, S.K.; Biegner, T.; Kammerer, B.; Delabar, U.; Salt, A.N. Dexamethasone concentration gradients along scala tympani after application to the round window membrane. Otol. Neurotol. 2008, 29, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Walshe, P.; Viani, L.; Al-Rubeai, M. Surface biotechnology for refining cochlear implants. Trends Biotechnol. 2013, 31, 678–687. [Google Scholar] [CrossRef]
- Hahn, H.; Salt, A.N.; Biegner, T.; Kammerer, B.; Delabar, U.; Hartsock, J.J.; Plontke, S.K. Dexamethasone levels and base-to-apex concentration gradients in the scala tympani perilymph after intracochlear delivery in the guinea pig. Otol. Neurotol. 2012, 33, 660–665. [Google Scholar] [CrossRef]
- Tan, F.; Zhu, Y.; Ma, Z.; Al-Rubeai, M. Recent advances in the implant-based drug delivery in otorhinolaryngology. Acta Biomater. 2020, 108, 46–55. [Google Scholar] [CrossRef]
- Akbar, T.F.; Tondera, C.; Minev, I. Conductive Hydrogels for Bioelectronic Interfaces. In Neural Interface Engineering; Guo, L., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 237–265. [Google Scholar]
- Sagdic, K.; Fernández-Lavado, E.; Mariello, M.; Akouissi, O.; Lacour, S.P. Hydrogels and conductive hydrogels for implantable bioelectronics. Mrs. Bull. 2023, 48, 495–505. [Google Scholar] [CrossRef]
- Gifford, R.H.; Davis, T.J.; Sunderhaus, L.W.; Menapace, C.; Buck, B.; Crosson, J.; O’Neill, L.; Beiter, A.; Segel, P. Combined Electric and Acoustic Stimulation with Hearing Preservation: Effect of Cochlear Implant Low-Frequency Cutoff on Speech Understanding and Perceived Listening Difficulty. Ear Hear. 2017, 38, 539–553. [Google Scholar] [CrossRef]
- Tan, F.; O’Neill, F.; Naciri, M.; Dowling, D.; Al-Rubeai, M. Cellular and transcriptomic analysis of human mesenchymal stem cell response to plasma-activated hydroxyapatite coating. Acta Biomater. 2012, 8, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- Henkelman, S.; Rakhorst, G.; Blanton, J.; van Oeveren, W. Standardization of incubation conditions for hemolysis testing of biomaterials. Mater. Sci. Eng. C—Biomim. Supramol. Syst. 2009, 29, 1650–1654. [Google Scholar] [CrossRef]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Madisen, L.; Zwingman, T.A.; Sunkin, S.M.; Oh, S.W.; Zariwala, H.A.; Gu, H.; Ng, L.L.; Palmiter, R.D.; Hawrylycz, M.J.; Jones, A.R.; et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010, 13, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Lumpkin, E.A.; Collisson, T.; Parab, P.; Omer-Abdalla, A.; Haeberle, H.; Chen, P.; Doetzlhofer, A.; White, P.; Groves, A.; Segil, N.; et al. Math1-driven GFP expression in the developing nervous system of transgenic mice. Gene Expr. Patterns 2003, 3, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Kalinec, G.; Thein, P.; Park, C.; Kalinec, F. HEI-OC1 cells as a model for investigating drug cytotoxicity. Hear. Res. 2016, 335, 105–117. [Google Scholar] [CrossRef]
- Kalinec, G.M.; Park, C.; Thein, P.; Kalinec, F. Working with Auditory HEI-OC1 Cells. J. Vis. Exp. JoVE 2016, 54425. [Google Scholar] [CrossRef]
- Spencer, A.R.; Primbetova, A.; Koppes, A.N.; Koppes, R.A.; Fenniri, H.; Annabi, N. Electroconductive Gelatin Methacryloyl-PEDOT:PSS Composite Hydrogels: Design, Synthesis, and Properties. ACS Biomater. Sci. Eng. 2018, 4, 1558–1567. [Google Scholar] [CrossRef]
- Gao, C.; Li, Y.; Liu, X.; Huang, J.; Zhang, Z. 3D bioprinted conductive spinal cord biomimetic scaffolds for promoting neuronal differentiation of neural stem cells and repairing of spinal cord injury. Chem. Eng. J. 2023, 451, 138788. [Google Scholar] [CrossRef]
- Tang, Y.; Varyambath, A.; Ding, Y.; Chen, B.; Huang, X.; Zhang, Y.; Yu, D.G.; Kim, I.; Song, W. Porous organic polymers for drug delivery: Hierarchical pore structures, variable morphologies, and biological properties. Biomater. Sci. 2022, 10, 5369–5390. [Google Scholar] [CrossRef]
- Alonso, J.M.; Andrade Del Olmo, J.; Perez Gonzalez, R.; Saez-Martinez, V. Injectable Hydrogels: From Laboratory to Industrialization. Polymers 2021, 13, 650. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Patsis, P.A.; Hauser, S.; Voigt, D.; Rothe, R.; Gunther, M.; Cui, M.; Yang, X.; Wieduwild, R.; Eckert, K.; et al. Cytocompatible, Injectable, and Electroconductive Soft Adhesives with Hybrid Covalent/Noncovalent Dynamic Network. Adv. Sci. 2019, 6, 1802077. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Rusznak, Z.; Szucs, G. Spiral ganglion neurones: An overview of morphology, firing behaviour, ionic channels and function. Pflug. Arch. 2009, 457, 1303–1325. [Google Scholar] [CrossRef] [PubMed]
- Bansal, M.; Dravid, A.; Aqrawe, Z.; Montgomery, J.; Wu, Z.; Svirskis, D. Conducting polymer hydrogels for electrically responsive drug delivery. J. Control. Release 2020, 328, 192–209. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.S.; Janda, C.Y.; Chang, J.; Zheng, G.X.Y.; Larkin, K.A.; Luca, V.C.; Chia, L.A.; Mah, A.T.; Han, A.; Terry, J.M.; et al. Non-equivalence of Wnt and R-spondin ligands during Lgr5+ intestinal stem-cell self-renewal. Nature 2017, 545, 238–242. [Google Scholar] [CrossRef]
- Ye, Z.; Su, Z.; Xie, S.; Liu, Y.; Wang, Y.; Xu, X.; Zheng, Y.; Zhao, M.; Jiang, L. Yap-lin28a axis targets let7-Wnt pathway to restore progenitors for initiating regeneration. eLife 2020, 9, e55771. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Odajima, J.; Hunt, S.L.; Dubus, P.; Ortega, S.; Malumbres, M.; Barbacid, M. Cdk2 is dispensable for cell cycle inhibition and tumor suppression mediated by p27(Kip1) and p21(Cip1). Cancer Cell 2005, 7, 591–598. [Google Scholar] [CrossRef]
- Aban, C.E.; Lombardi, A.; Neiman, G.; Biani, M.C.; La Greca, A.; Waisman, A.; Moro, L.N.; Sevlever, G.; Miriuka, S.; Luzzani, C. Downregulation of E-cadherin in pluripotent stem cells triggers partial EMT. Sci. Rep. 2021, 11, 2048. [Google Scholar] [CrossRef]
- Nan, B.; Gu, X.; Huang, X. The Role of the Reactive Oxygen Species Scavenger Agent, Astaxanthin, in the Protection of Cisplatin-Treated Patients against Hearing Loss. Drug Des. Devel. Ther. 2019, 13, 4291–4303. [Google Scholar] [CrossRef]
- Wang, M.; Dong, Y.; Gao, S.; Zhong, Z.; Cheng, C.; Qiang, R.; Zhang, Y.; Shi, X.; Qian, X.; Gao, X.; et al. Hippo/YAP signaling pathway protects against neomycin-induced hair cell damage in the mouse cochlea. Cell. Mol. Life Sci. 2022, 79, 79. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Qian, X.; Hu, M.; Zhang, R.; Liu, N.; Huang, Y.; Yang, J.; Zhang, J.; Bai, H.; Yang, Y.; et al. STYK1 promotes autophagy through enhancing the assembly of autophagy-specific class III phosphatidylinositol 3-kinase complex I. Autophagy 2020, 16, 1786–1806. [Google Scholar] [CrossRef]
- Lai, Y.; Zhang, Z.; Li, J.; Li, W.; Huang, Z.; Zhang, C.; Li, X.; Zhao, J. STYK1/NOK correlates with ferroptosis in non-small cell lung carcinoma. Biochem. Biophys. Res. Commun. 2019, 519, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Elkhoury, K.; Morsink, M.; Sanchez-Gonzalez, L.; Kahn, C.; Tamayol, A.; Arab-Tehrany, E. Biofabrication of natural hydrogels for cardiac, neural, and bone Tissue engineering Applications. Bioact. Mater. 2021, 6, 3904–3923. [Google Scholar] [CrossRef]
- Zhu, T.; Ni, Y.; Biesold, G.M.; Cheng, Y.; Ge, M.; Li, H.; Huang, J.; Lin, Z.; Lai, Y. Recent advances in conductive hydrogels: Classifications, properties, and applications. Chem. Soc. Rev. 2023, 52, 473–509. [Google Scholar] [CrossRef]
- Lin, C.C.; Chiu, T.; Chiou, H.P.; Chang, C.M.; Hsu, C.J.; Wu, H.P. Residual hearing preservation for cochlear implantation surgery. Tzu. Chi. Med. J. 2021, 33, 359–364. [Google Scholar] [CrossRef]
- O’Connell, B.P.; Hunter, J.B.; Wanna, G.B. The importance of electrode location in cochlear implantation. Laryngoscope Investig. Otolaryngol. 2016, 1, 169–174. [Google Scholar] [CrossRef]
- Dhanasingh, A.; Jolly, C. An overview of cochlear implant electrode array designs. Hear. Res. 2017, 356, 93–103. [Google Scholar] [CrossRef]
- Jia, H.; Francois, F.; Bourien, J.; Eybalin, M.; Lloyd, R.V.; Van De Water, T.R.; Puel, J.L.; Venail, F. Prevention of trauma-induced cochlear fibrosis using intracochlear application of anti-inflammatory and antiproliferative drugs. Neuroscience 2016, 316, 261–278. [Google Scholar] [CrossRef]
- Fayad, J.N.; Makarem, A.O.; Linthicum, F.H., Jr. Histopathologic assessment of fibrosis and new bone formation in implanted human temporal bones using 3D reconstruction. Otolaryngol.—Head Neck Surg. 2009, 141, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Ertas, Y.N.; Ozpolat, D.; Karasu, S.N.; Ashammakhi, N. Recent Advances in Cochlear Implant Electrode Array Design Parameters. Micromachines 2022, 13, 1081. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Ng, H.Y.; Lin, Y.H.; Liu, E.W.; Lin, T.J.; Chiu, H.T.; Ho, X.R.; Yang, H.A.; Shie, M.Y. The 3D printed conductive grooved topography hydrogel combined with electrical stimulation for synergistically enhancing wound healing of dermal fibroblast cells. Biomater. Adv. 2022, 142, 213132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Zhang, M.M.; Zhang, R.L.; Liu, H.N.; Chen, H.; Zhang, X.F.; Li, C.; Zeng, Q.; Chen, Y.H.; Huang, G.Z. Conductive GelMA/PEDOT: PSS Hybrid Hydrogel as a Neural Stem Cell Niche for Treating Cerebral Ischemia-Reperfusion Injury. Front. Mater. 2022, 9, 914994. [Google Scholar] [CrossRef]
- Oktay, S.; Alemdar, N. Electrically controlled release of 5-fluorouracil from conductive gelatin methacryloyl-based hydrogels. J. Appl. Polym. Sci. 2019, 136, 46914. [Google Scholar] [CrossRef]
- Liu, H.; Hao, J.; Li, K.S. Current strategies for drug delivery to the inner ear. Acta Pharm. Sin. B 2013, 3, 86–96. [Google Scholar] [CrossRef]
- Li, X.J.; Morgan, C.; Goff, L.A.; Doetzlhofer, A. Follistatin promotes LIN28B-mediated supporting cell reprogramming and hair cell regeneration in the murine cochlea. Sci. Adv. 2022, 8, eabj7651. [Google Scholar] [CrossRef]
- Li, X.J.; Morgan, C.; Nadar-Ponniah, P.T.; Kolanus, W.; Doetzlhofer, A. TRIM71 reactivation enhances the mitotic and hair cell-forming potential of cochlear supporting cells. EMBO Rep. 2023, 24, e56562. [Google Scholar] [CrossRef]
- White, P.M.; Doetzlhofer, A.; Lee, Y.S.; Groves, A.K.; Segil, N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature 2006, 441, 984–987. [Google Scholar] [CrossRef]
- Xu, J.; Tsai, Y.L.; Hsu, S.H. Design Strategies of Conductive Hydrogel for Biomedical Applications. Molecules 2020, 25, 5296. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Lei, L.; Bai, Y.; Qin, X.; Liu, J.; Huang, W.; Lv, Q. Current Understanding of Hydrogel for Drug Release and Tissue Engineering. Gels 2022, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Coco, A.; Epp, S.B.; Fallon, J.B.; Xu, J.; Millard, R.E.; Shepherd, R.K. Does cochlear implantation and electrical stimulation affect residual hair cells and spiral ganglion neurons? Hear. Res. 2007, 225, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, A.N.; Robles, U.A.; Huynh, M.; Nayagam, B.A.; Green, R.A.; Poole-Warren, L.A.; Fallon, J.B.; Shepherd, R.K. Electrochemical and biological performance of chronically stimulated conductive hydrogel electrodes. J. Neural. Eng. 2020, 17, 026018. [Google Scholar] [CrossRef] [PubMed]
- Murdan, S. Electro-responsive drug delivery from hydrogels. J. Control. Release 2003, 92, 1–17. [Google Scholar] [CrossRef]
- Defourny, J.; Mateo Sanchez, S.; Schoonaert, L.; Robberecht, W.; Davy, A.; Nguyen, L.; Malgrange, B. Cochlear supporting cell transdifferentiation and integration into hair cell layers by inhibition of ephrin-B2 signalling. Nat. Commun. 2015, 6, 7017. [Google Scholar] [CrossRef]
- Roccio, M.; Perny, M.; Ealy, M.; Widmer, H.R.; Heller, S.; Senn, P. Molecular characterization and prospective isolation of human fetal cochlear hair cell progenitors. Nat. Commun. 2018, 9, 4027. [Google Scholar] [CrossRef]
- Li, W.; Wu, J.; Yang, J.; Sun, S.; Chai, R.; Chen, Z.Y.; Li, H. Notch inhibition induces mitotically generated hair cells in mammalian cochleae via activating the Wnt pathway. Proc. Natl. Acad. Sci. USA 2015, 112, 166–171. [Google Scholar] [CrossRef]
- Lyra-Leite, D.M.; Gutiérrez-Gutiérrez, O.; Wang, M.M.; Zhou, Y.; Cyganek, L.; Burridge, P.W. A review of protocols for human iPSC culture, cardiac differentiation, subtype-specification, maturation, and direct reprogramming. Star. Protoc. 2022, 3, 101560. [Google Scholar] [CrossRef]
- Uribe-Etxebarria, V.; Pineda, J.R.; García-Gallastegi, P.; Agliano, A.; Unda, F.; Ibarretxe, G. Notch and Wnt Signaling Modulation to Enhance DPSC Stemness and Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 7389. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Karp, J.M. Stomaching Notch. EMBO J. 2015, 34, 2489–2491. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yin, X.; Qin, H.; Zhu, F.; Liu, H.; Yang, W.; Zhang, Q.; Xiang, C.; Hou, P.; Song, Z.; et al. Two supporting factors greatly improve the efficiency of human iPSC generation. Cell Stem Cell 2008, 3, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Mead, B.E.; Safaee, H.; Langer, R.; Karp, J.M.; Levy, O. Engineering Stem Cell Organoids. Cell Stem Cell 2016, 18, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Papoutsakis, E.T. Stem-cell niche based comparative analysis of chemical and nano-mechanical material properties impacting ex vivo expansion and differentiation of hematopoietic and mesenchymal stem cells. Adv. Health Mater. 2013, 2, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Facklam, A.L.; Volpatti, L.R.; Anderson, D.G. Biomaterials for Personalized Cell Therapy. Adv. Mater. 2020, 32, e1902005. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Farin, H.F.; van Es, J.H.; Clevers, H.; Langer, R.; Karp, J.M. Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat. Methods 2014, 11, 106–112. [Google Scholar] [CrossRef]
- Qin, T.; Ho, C.C.; Wang, B.; Hui, C.C.; Sham, M.H. Sufu- and Spop-mediated regulation of Gli2 is essential for the control of mammalian cochlear hair cell differentiation. Proc. Natl. Acad. Sci. USA 2022, 119, e2206571119. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, W.; Laurent, T.; Ding, S. Small molecules, big roles—The chemical manipulation of stem cell fate and somatic cell reprogramming. J. Cell Sci. 2012, 125, 5609–5620. [Google Scholar] [CrossRef]
- LeMasurier, M.; Gillespie, P.G. Hair-cell mechanotransduction and cochlear amplification. Neuron 2005, 48, 403–415. [Google Scholar] [CrossRef]
- Qiu, X.; Muller, U. Sensing sound: Cellular specializations and molecular force sensors. Neuron 2022, 110, 3667–3687. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, H.R.; Ahn, S.C. Vesicular glutamate transporter 3 is strongly upregulated in cochlear inner hair cells and spiral ganglion cells of developing circling mice. Neurosci. Lett. 2015, 584, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.X.; Payne, S.; Yang-Hood, A.; Li, S.Z.; Davis, B.; Carlquist, J.; V-Ghaffari, B.; Gantz, J.A.; Kallogjeri, D.; Fitzpatrick, J.A.J.; et al. Vesicular Glutamatergic Transmission in Noise-Induced Loss and Repair of Cochlear Ribbon Synapses. J. Neurosci. 2019, 39, 4434–4447. [Google Scholar] [CrossRef] [PubMed]
- Coate, T.M.; Scott, M.K.; Gurjar, M. Current concepts in cochlear ribbon synapse formation. Synapse 2019, 73, e22087. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.C.; Frank, T.; Khimich, D.; Hoch, G.; Riedel, D.; Chapochnikov, N.M.; Yarin, Y.M.; Harke, B.; Hell, S.W.; Egner, A.; et al. Tuning of synapse number, structure and function in the cochlea. Nat. Neurosci. 2009, 12, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Passaniti, A.; Kleinman, H.K.; Martin, G.R. Matrigel: History/background, uses, and future applications. J. Cell Commun. Signal. 2022, 16, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, M.T.; Crook, C.J.; Ku, H.T. Towards organoid culture without Matrigel. Commun. Biol. 2021, 4, 1387. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, S.; Hu, Y.N.; Chen, X.; Cheng, C.; Fu, X.L.; Zhang, S.S.; Wang, X.L.; Che, Y.W.; Zhang, C.; et al. Ti3C2TxMXene Composite 3D Hydrogel Potentiates mTOR Signaling to Promote the Generation of Functional Hair Cells in Cochlea Organoids. Adv. Sci. 2022, 9, e2203557. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Ferracci, G.; Zheng, J.; Cho, N.J.; Lee, B.H. Gelatin methacryloyl and its hydrogels with an exceptional degree of controllability and batch-to-batch consistency. Sci. Rep. 2019, 9, 6863. [Google Scholar] [CrossRef]
- Abi-Hachem, R.N.; Zine, A.; Van De Water, T.R. The injured cochlea as a target for inflammatory processes, initiation of cell death pathways and application of related otoprotectives strategies. Recent Pat. CNS Drug Discov. 2010, 5, 147–163. [Google Scholar] [CrossRef]
- Wong, A.C.; Ryan, A.F. Mechanisms of sensorineural cell damage, death and survival in the cochlea. Front. Aging Neurosci. 2015, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ye, J.; Kong, W.; Zhang, S.; Zheng, Y. Programmed cell death pathways in hearing loss: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2020, 53, e12915. [Google Scholar] [CrossRef]
- Pecina-Slaus, N. Wnt signal transduction pathway and apoptosis: A review. Cancer Cell Int. 2010, 10, 22. [Google Scholar] [CrossRef]
- Dang, T.P. Notch, apoptosis and cancer. Adv. Exp. Med. Biol. 2012, 727, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Capelo, A. Dual role for TGF-beta1 in apoptosis. Cytokine Growth Factor Rev. 2005, 16, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M.; Pratt, D.A. The chemical basis of ferroptosis. Nat. Chem. Biol. 2019, 15, 1137–1147. [Google Scholar] [CrossRef]
- Zheng, J.; Conrad, M. The Metabolic Underpinnings of Ferroptosis. Cell Metab. 2020, 32, 920–937. [Google Scholar] [CrossRef]
- Mei, H.; Zhao, L.; Li, W.; Zheng, Z.; Tang, D.; Lu, X.; He, Y. Inhibition of ferroptosis protects House Ear Institute-Organ of Corti 1 cells and cochlear hair cells from cisplatin-induced ototoxicity. J. Cell. Mol. Med. 2020, 24, 12065–12081. [Google Scholar] [CrossRef]
- Jian, B.; Pang, J.; Xiong, H.; Zhang, W.; Zhan, T.; Su, Z.; Lin, H.; Zhang, H.; He, W.; Zheng, Y. Autophagy-dependent ferroptosis contributes to cisplatin-induced hearing loss. Toxicol. Lett. 2021, 350, 249–260. [Google Scholar] [CrossRef]
- Ramkumar, V.; Mukherjea, D.; Dhukhwa, A.; Rybak, L.P. Oxidative Stress and Inflammation Caused by Cisplatin Ototoxicity. Antioxidants 2021, 10, 1919. [Google Scholar] [CrossRef]
- Mason, E.F.; Rathmell, J.C. Cell metabolism: An essential link between cell growth and apoptosis. Biochim. Biophys. Acta 2011, 1813, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.L.; Kornbluth, S. The tangled circuitry of metabolism and apoptosis. Mol. Cell 2013, 49, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Medina, C.B.; Mehrotra, P.; Arandjelovic, S.; Perry, J.S.A.; Guo, Y.; Morioka, S.; Barron, B.; Walk, S.F.; Ghesquiere, B.; Krupnick, A.S.; et al. Metabolites released from apoptotic cells act as tissue messengers. Nature 2020, 580, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liang, C.H.; Huang, B.; Zhuang, X.; Cui, W.; Yang, L.; Yang, Y.; Zhang, Y.; Fu, X.; Zhang, X.; et al. Tryptophan Metabolism Acts as a New Anti-Ferroptotic Pathway to Mediate Tumor Growth. Adv. Sci. 2023, 10, e2204006. [Google Scholar] [CrossRef]
- Yang, J.; Dai, X.; Xu, H.; Tang, Q.; Bi, F. Regulation of Ferroptosis by Amino Acid Metabolism in Cancer. Int. J. Biol. Sci. 2022, 18, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Ruel, J.; Chabbert, C.; Nouvian, R.; Bendris, R.; Eybalin, M.; Leger, C.L.; Bourien, J.; Mersel, M.; Puel, J.L. Salicylate enables cochlear arachidonic-acid-sensitive NMDA receptor responses. J. Neurosci. 2008, 28, 7313–7323. [Google Scholar] [CrossRef] [PubMed]
- Sokolowski, B.H.; Sakai, Y.; Harvey, M.C.; Duzhyy, D.E. Identification and localization of an arachidonic acid-sensitive potassium channel in the cochlea. J. Neurosci. 2004, 24, 6265–6276. [Google Scholar] [CrossRef]
- Heinrich, U.R.; Helling, K. Nitric oxide—A versatile key player in cochlear function and hearing disorders. Nitric Oxide 2012, 27, 106–116. [Google Scholar] [CrossRef]
- Shi, X.; Dai, C.; Nuttall, A.L. Altered expression of inducible nitric oxide synthase (iNOS) in the cochlea. Hear. Res. 2003, 177, 43–52. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, F.; Li, X.; Li, X.; Xu, M.; Shahzad, K.A.; Hou, L. GelMA/PEDOT:PSS Composite Conductive Hydrogel-Based Generation and Protection of Cochlear Hair Cells through Multiple Signaling Pathways. Biomolecules 2024, 14, 95. https://doi.org/10.3390/biom14010095
Tan F, Li X, Li X, Xu M, Shahzad KA, Hou L. GelMA/PEDOT:PSS Composite Conductive Hydrogel-Based Generation and Protection of Cochlear Hair Cells through Multiple Signaling Pathways. Biomolecules. 2024; 14(1):95. https://doi.org/10.3390/biom14010095
Chicago/Turabian StyleTan, Fei, Xuran Li, Xiao Li, Maoxiang Xu, Khawar Ali Shahzad, and Lei Hou. 2024. "GelMA/PEDOT:PSS Composite Conductive Hydrogel-Based Generation and Protection of Cochlear Hair Cells through Multiple Signaling Pathways" Biomolecules 14, no. 1: 95. https://doi.org/10.3390/biom14010095
APA StyleTan, F., Li, X., Li, X., Xu, M., Shahzad, K. A., & Hou, L. (2024). GelMA/PEDOT:PSS Composite Conductive Hydrogel-Based Generation and Protection of Cochlear Hair Cells through Multiple Signaling Pathways. Biomolecules, 14(1), 95. https://doi.org/10.3390/biom14010095





