Novel Organic–Inorganic Nanocomposite Hybrids Based on Bioactive Glass Nanoparticles and Their Enhanced Osteoinductive Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of nBGs
2.2. Synthesis of Hybrid Materials
2.3. Material Characterization
2.3.1. nBG Characterization
2.3.2. Hybrid Material Characterization
2.4. In Vitro Bioactivity Assays
2.5. Hybrid Degradation Test
2.6. Cell Culture
2.6.1. Cytocompatibility Assays
2.6.2. Cell Differentiation Assays
3. Results and Discussion
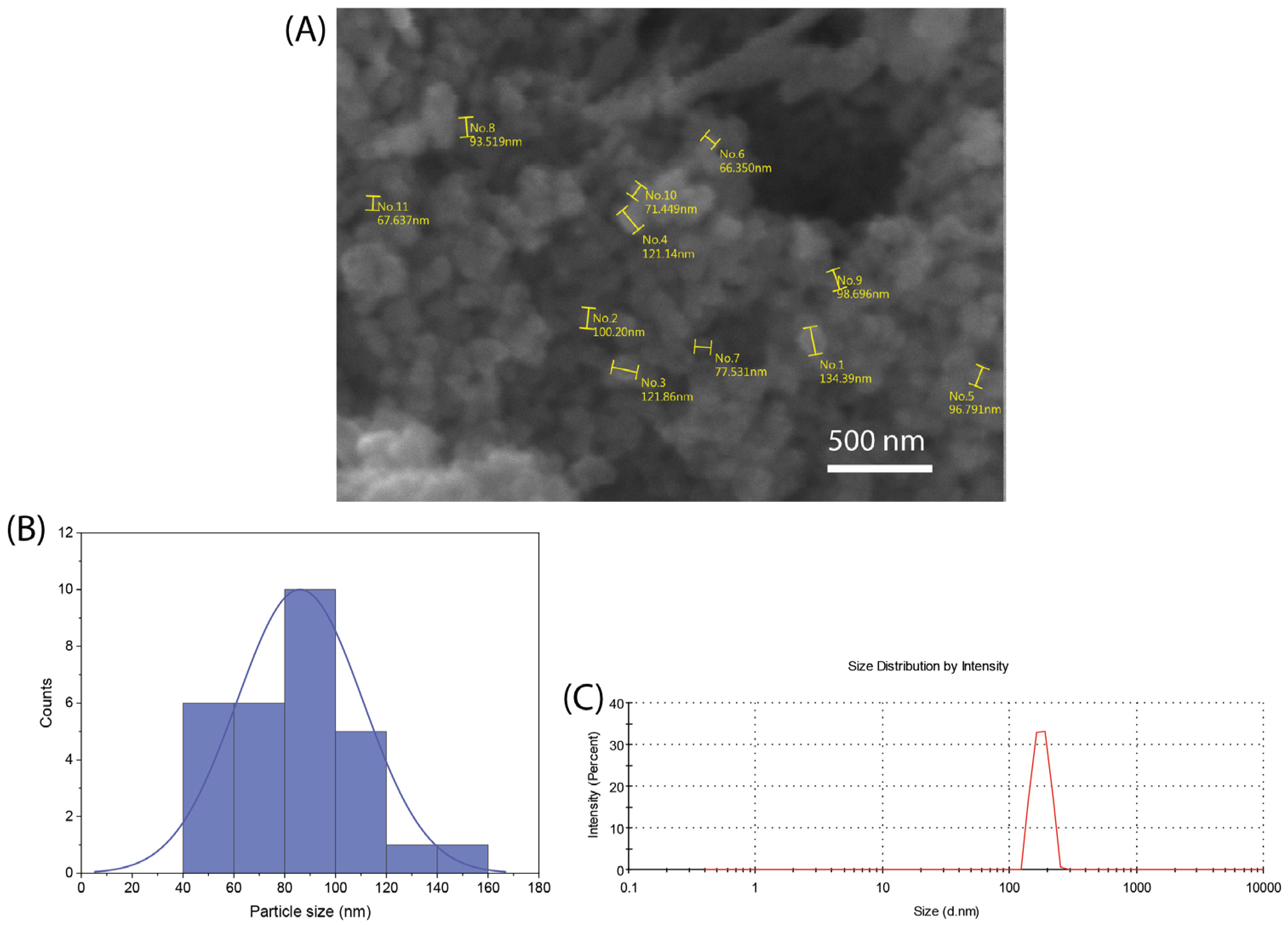
3.1. nBG Characterization
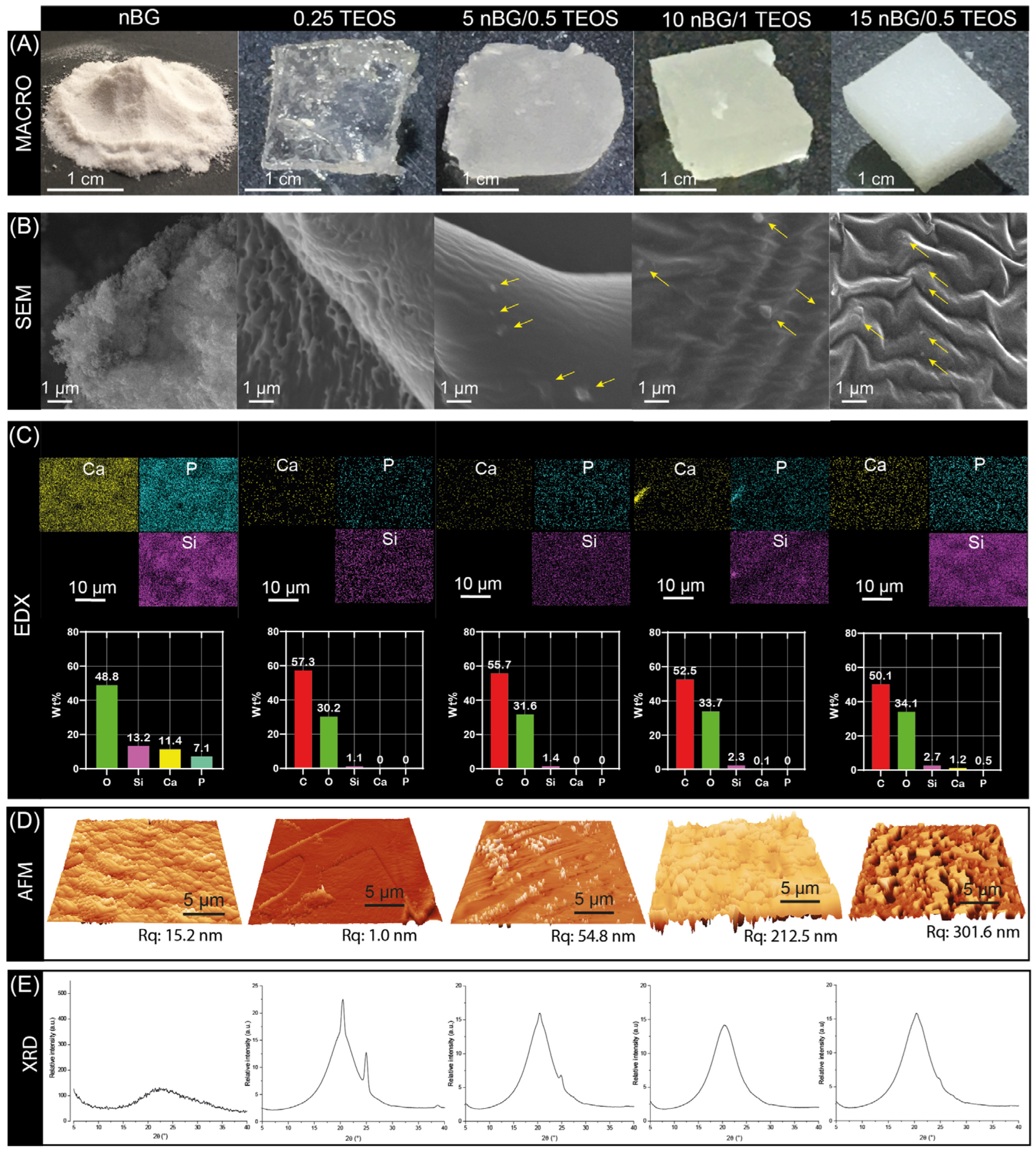
3.2. Mechanical Properties of Synthesized Hybrid Materials
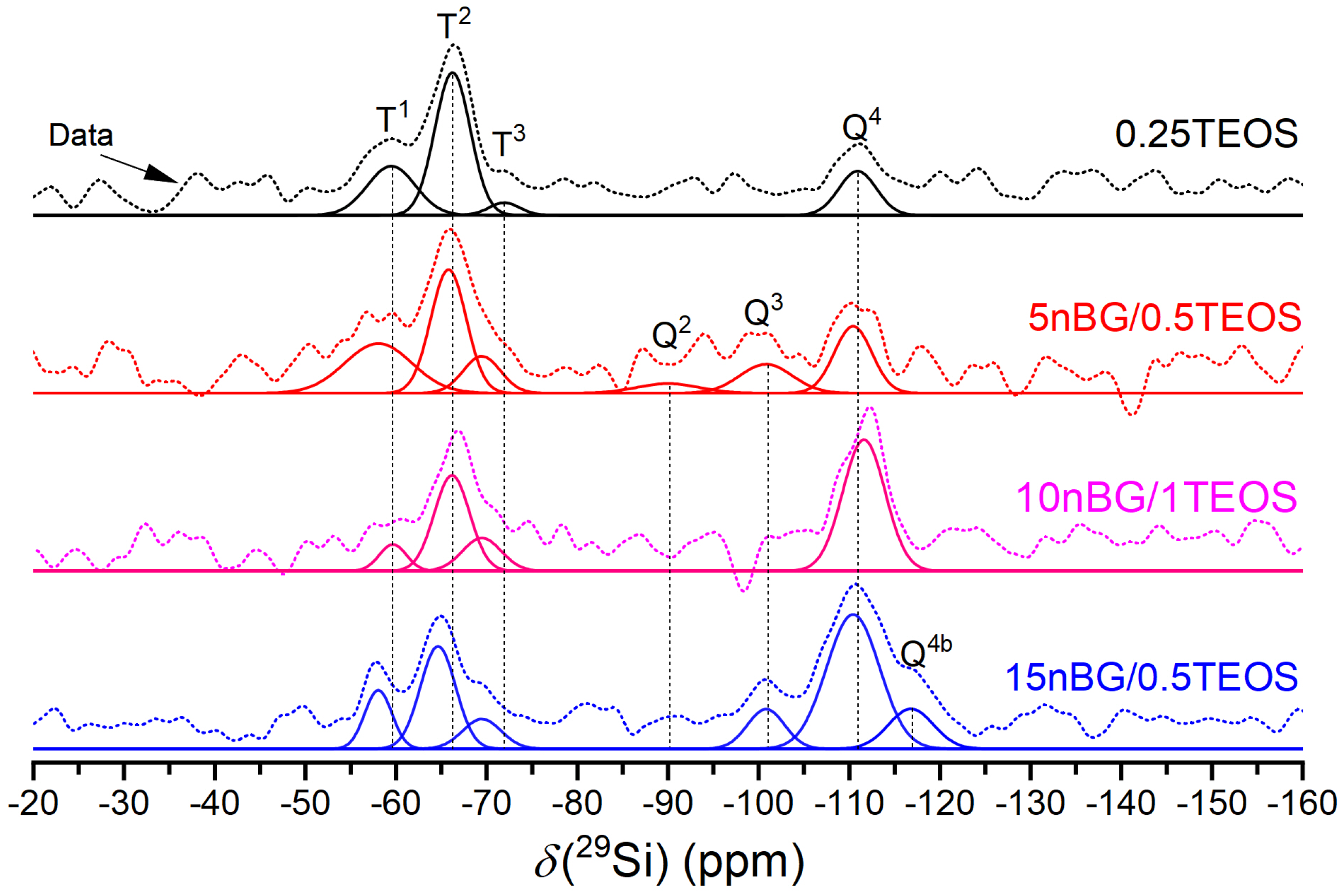
3.3. Structural Characterization of Selected Hybrids
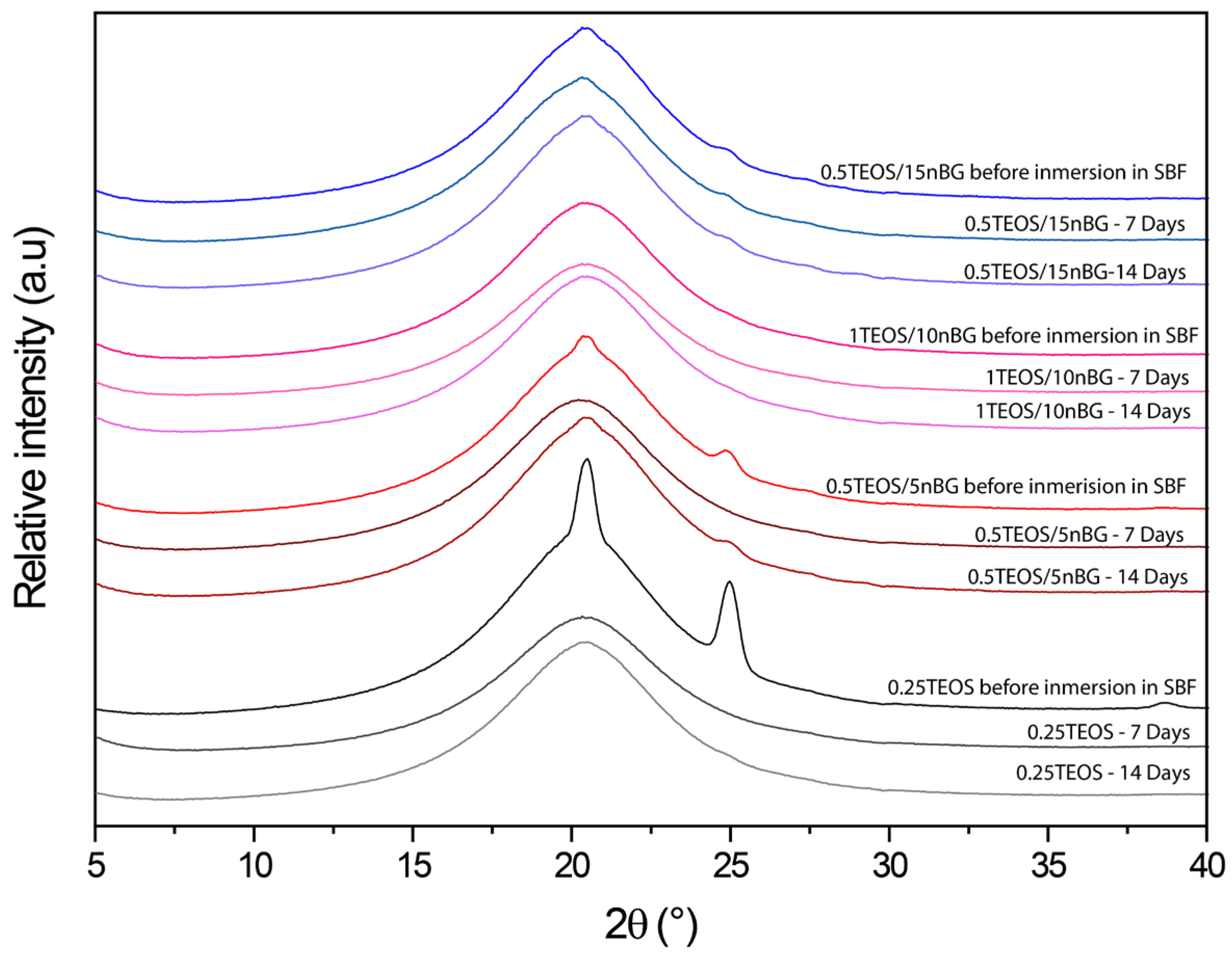
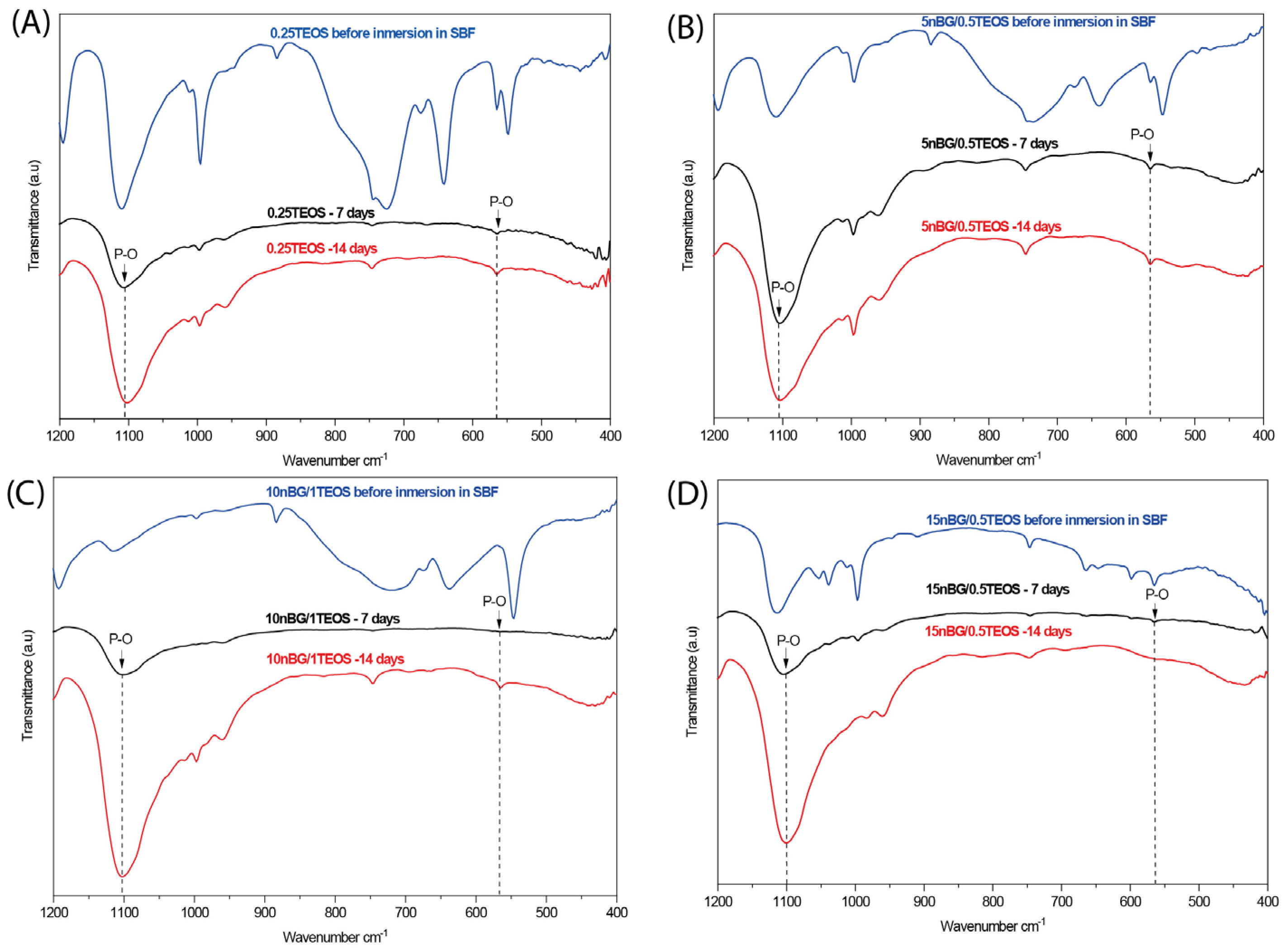
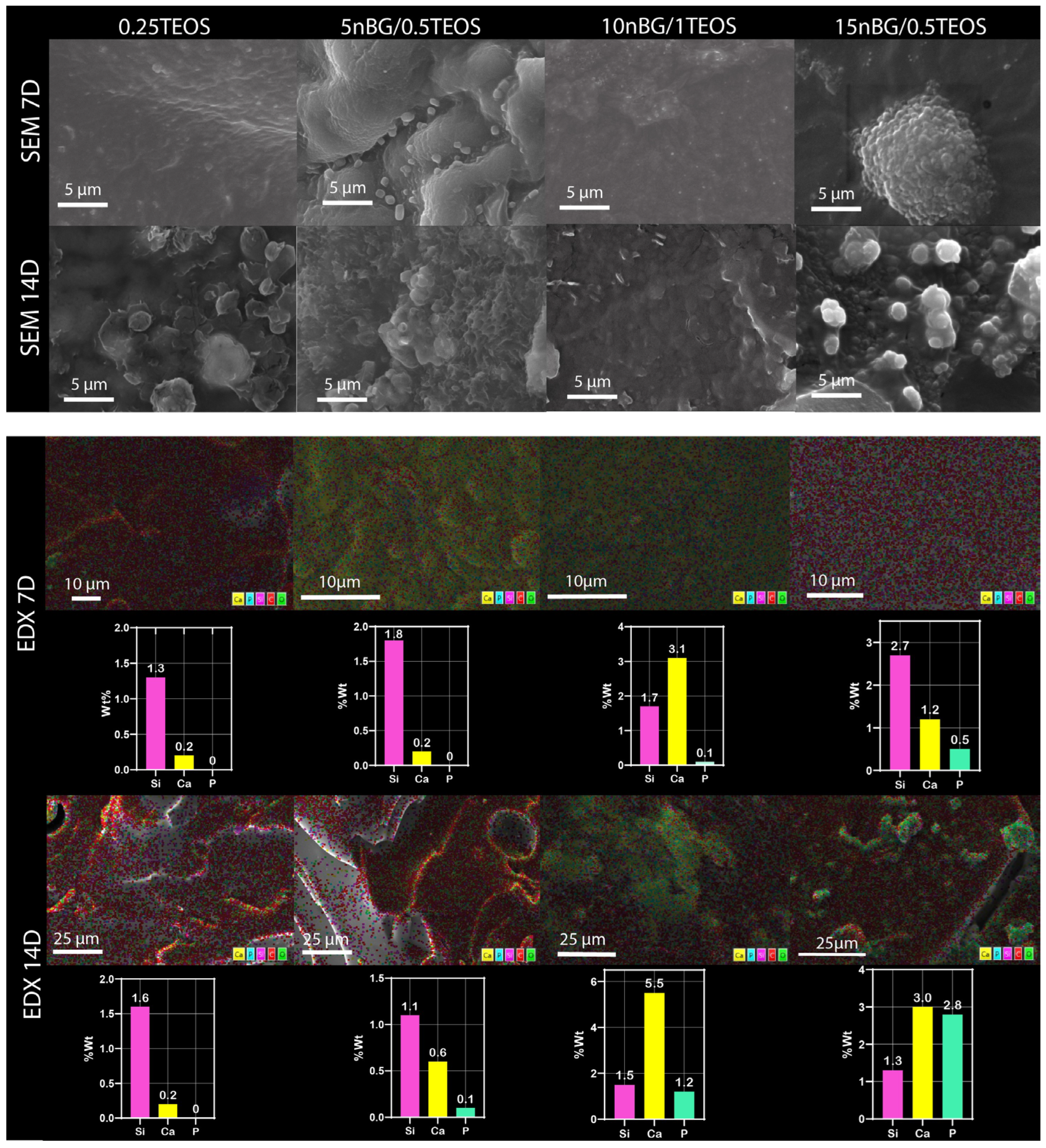
3.4. In Vitro Bioactivity in SBF
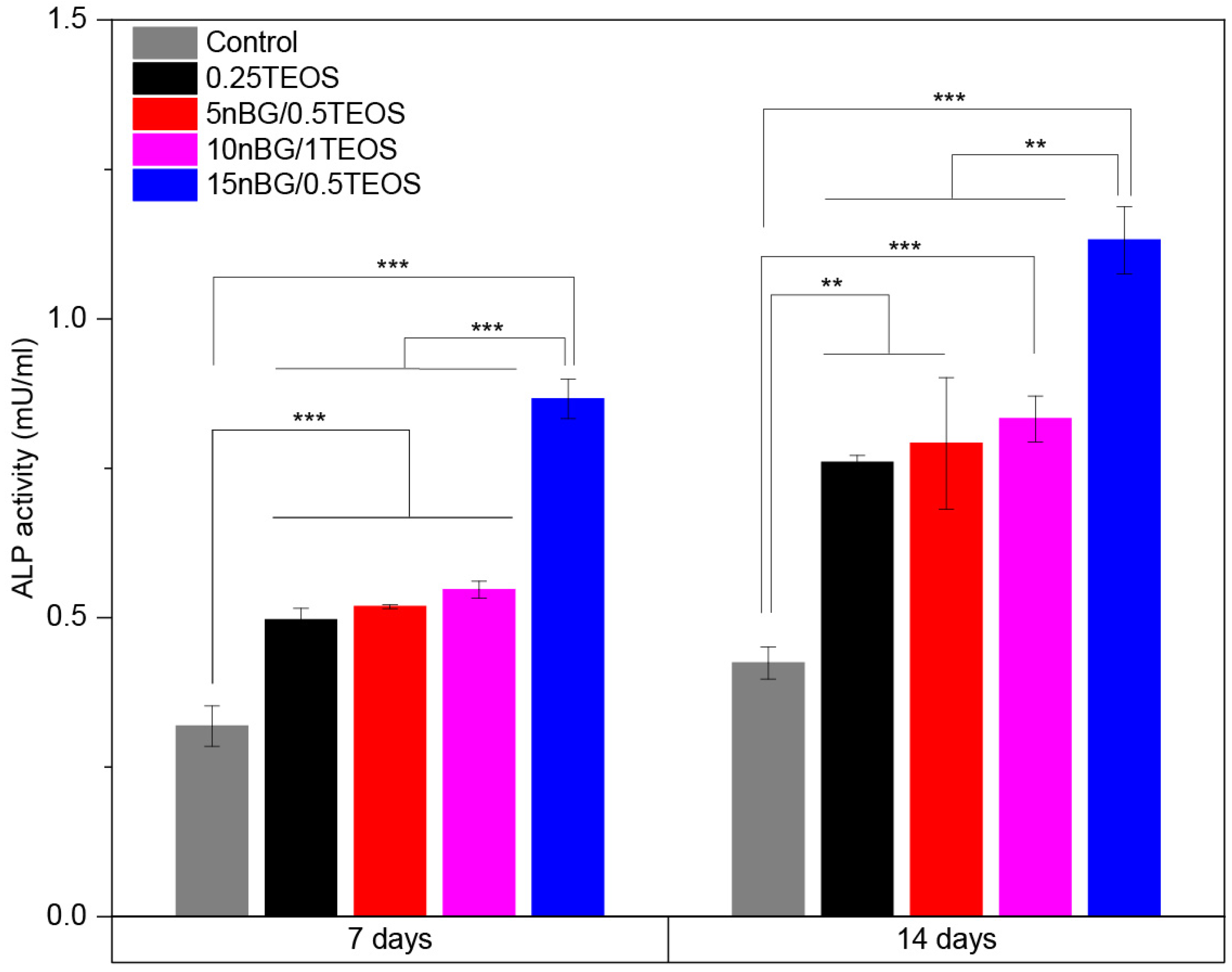
3.5. Cytocompatibility and Osteogenic Differentiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive glasses: Where are we and where are we going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Houreh, A.B.; Labbaf, S.; Ting, H.-K.; Ejeian, F.; Jones, J.R.; Esfahani, M.-H.N. Influence of calcium and phosphorus release from bioactive glasses on viability and differentiation of dental pulp stem cells. J. Mater. Sci. 2017, 52, 8928–8941. [Google Scholar] [CrossRef]
- Thompson, I.D.; Hench, L.L. Mechanical properties of bioactive glasses, glass-ceramics and composites. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1998, 212, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R. Reprint of: Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2015, 23, S53–S82. [Google Scholar] [CrossRef] [PubMed]
- Innocenzi, P.; Brusatin, G.; Guglielmi, M.; Bertani, R. New Synthetic Route to (3-Glycidoxypropyl)trimethoxysilane-Based Hybrid Organic−Inorganic Materials. Chem. Mater. 1999, 11, 1672–1679. [Google Scholar] [CrossRef]
- Ren, L.; Tsuru, K.; Hayakawa, S.; Osaka, A. Synthesis and Characterization of Gelatin-Siloxane Hybrids Derived through Sol-Gel Procedure. J. Sol-Gel Sci. Technol. 2001, 21, 115–121. [Google Scholar] [CrossRef]
- Novak, B.M. Hybrid Nanocomposite Materials—Between inorganic glasses and organic polymers. Adv. Mater. 1993, 5, 422–433. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Mahony, O.; Yue, S.; Turdean-Ionescu, C.; Hanna, J.V.; Smith, M.E.; Lee, P.D.; Jones, J.R. Silica–gelatin hybrids for tissue regeneration: Inter-relationships between the process variables. J. Sol-Gel Sci. Technol. 2013, 69, 288–298. [Google Scholar] [CrossRef]
- Valliant, E.M.; Jones, J.R. Softening bioactive glass for bone regeneration: Sol-gel hybrid materials. Soft Matter 2011, 7, 5083–5095. [Google Scholar] [CrossRef]
- Nuyken, O.; Pask, S.D. Ring-Opening Polymerization—An Introductory Review. Polymers 2013, 5, 361–403. [Google Scholar] [CrossRef]
- Valliant, E.M.; Romer, F.; Wang, D.; McPhail, D.S.; Smith, M.E.; Hanna, J.V.; Jones, J.R. Bioactivity in silica/poly(γ-glutamic acid) sol-gel hybrids through calcium chelation. Acta Biomater. 2013, 9, 7662–7671. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Romer, F.; Connell, L.; Walter, C.; Saiz, E.; Yue, S.; Lee, P.D.; McPhail, D.S.; Hanna, J.V.; Jones, J.R. Highly flexible silica/chitosan hybrid scaffolds with oriented pores for tissue regeneration. J. Mater. Chem. B 2015, 3, 7560–7576. [Google Scholar] [CrossRef] [PubMed]
- Poologasundarampillai, G.; Yu, B.; Tsigkou, O.; Valliant, E.; Yue, S.; Lee, P.D.; Hamilton, R.W.; Stevens, M.M.; Kasuga, T.; Jones, J.R. Bioactive silica–poly(γ-glutamic acid) hybrids for bone regeneration: Effect of covalent coupling on dissolution and mechanical properties and fabrication of porous scaffolds. Soft Matter 2012, 8, 4822–4832. [Google Scholar] [CrossRef]
- Tallia, F.; Russo, L.; Li, S.; Orrin, A.L.H.; Shi, X.; Chen, S.; Steele, J.A.M.; Meille, S.; Chevalier, J.; Lee, P.D.; et al. Bouncing and 3D printable hybrids with self-healing properties. Mater. Horiz. 2018, 5, 849–860. [Google Scholar] [CrossRef]
- Lei, B.; Shin, K.-H.; Koh, Y.-H.; Kim, H.-E. Porous gelatin-siloxane hybrid scaffolds with biomimetic structure and properties for bone tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.R.; Brauer, D.S.; Hupa, L. Bioactive Glasses; Royal Society of Chemistry: Cambridge, UK, 2016. [Google Scholar]
- Chung, J.J.; Li, S.; Stevens, M.M.; Georgiou, T.K.; Jones, J.R. Tailoring Mechanical Properties of Sol-gel Hybrids for Bone Regeneration through Polymer Structure. Chem. Mater. 2016, 28, 6127–6135. [Google Scholar] [CrossRef]
- Rhee, S.-H.; Hwang, M.-H.; Si, H.-J.; Choi, J.-Y. Biological activities of osteoblasts on poly(methyl methacrylate)/silica hybrid containing calcium salt. Biomaterials 2002, 24, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Tsuru, K.; Hayakawa, S.; Osaka, A. Novel approach to fabricate porous gelatin–siloxane hybrids for bone tissue engineering. Biomaterials 2002, 23, 4765–4773. [Google Scholar] [CrossRef]
- Mondal, D.; Lin, S.; Rizkalla, A.S.; Mequanint, K.; Dixon, S.J. Porous and biodegradable polycaprolactone-borophosphosilicate hybrid scaffolds for osteoblast infiltration and stem cell differentiation. J. Mech. Behav. Biomed. Mater. 2019, 92, 162–171. [Google Scholar] [CrossRef]
- Gomide, V.S.; Zonari, A.; Ocarino, N.M.; Goes, A.M.; Serakides, R.; Pereira, M.M. In vitro and in vivo osteogenic potential of bioactive glass–PVA hybrid scaffolds colonized by mesenchymal stem cells. Biomed. Mater. 2012, 7, 015004. [Google Scholar] [CrossRef] [PubMed]
- Allo, B.A.; Lin, S.; Mequanint, K.; Rizkalla, A.S. Role of Bioactive 3D Hybrid Fibrous Scaffolds on Mechanical Behavior and Spatiotemporal Osteoblast Gene Expression. ACS Appl. Mater. Interfaces 2013, 5, 7574–7583. [Google Scholar] [CrossRef] [PubMed]
- Ravarian, R.; Murphy, C.M.; Schindeler, A.; Rawal, A.; Hook, J.M.; Dehghani, F. Bioactive poly(methyl methacrylate) for bone fixation. RSC Adv. 2015, 5, 60681–60690. [Google Scholar] [CrossRef]
- Aslankoohi, N.; Mondal, D.; Rizkalla, A.S.; Mequanint, K. Bone Repair and Regenerative Biomaterials: Towards Recapitulating the Microenvironment. Polymers 2019, 11, 1437. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Tsuru, K.; Hayakawa, S.; Osaka, A. In vitro Evaluation of Osteoblast Response to Sol-Gel Derived Gelatin-Siloxane Hybrids. J. Sol-Gel Sci. Technol. 2003, 26, 1137–1140. [Google Scholar] [CrossRef]
- Houaoui, A.; Szczodra, A.; Lallukka, M.; El-Guermah, L.; Agniel, R.; Pauthe, E.; Massera, J.; Boissiere, M. New generation of hybrid materials based on gelatin and bioactive glass particles for bone tissue regeneration. Biomolecules 2021, 11, 444. [Google Scholar] [CrossRef] [PubMed]
- Caridade, S.G.; Merino, E.G.; Alves, N.M.; Bermudez, V.d.Z.; Boccaccini, A.R.; Mano, J.F. Chitosan membranes containing micro or nano-size bioactive glass particles: Evolution of biomineralization followed by in situ dynamic mechanical analysis. J. Mech. Behav. Biomed. Mater. 2013, 20, 173–183. [Google Scholar] [CrossRef]
- Covarrubias, C.; Agüero, A.; Maureira, M.; Morelli, E.; Escobar, G.; Cuadra, F.; Peñafiel, C.; Von Marttens, A. In situ preparation and osteogenic properties of bionanocomposite scaffolds based on aliphatic polyurethane and bioactive glass nanoparticles. Mater. Sci. Eng. C 2018, 96, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Lee, S.S.; Sivashanmugam, A.; Kwon, J.; Kim, S.H.L.; Noh, M.Y.; Kwon, S.K.; Jayakumar, R.; Hwang, N.S. Bioglass-incorporated methacrylated gelatin cryogel for regeneration of bone defects. Polymers 2018, 10, 914. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, C.; Arroyo, F.; Balanda, C.; Neira, M.; Von Marttens, A.; Caviedes, P.; Rodríguez, J.P.; Urra, C. The Effect of the Nanoscale Structure of Nanobioceramics on Their In Vitro Bioactivity and Cell Differentiation Properties. J. Nanomater. 2015, 2015, 1–14. [Google Scholar] [CrossRef]
- Covarrubias, C.; Cádiz, M.; Maureira, M.; Celhay, I.; Cuadra, F.; von Marttens, A. Bionanocomposite scaffolds based on chitosan–gelatin and nanodimensional bioactive glass particles: In vitro properties and in vivo bone regeneration. J. Biomater. Appl. 2017, 32, 1155–1163. [Google Scholar] [CrossRef]
- Ajita, J.; Saravanan, S.; Selvamurugan, N. Effect of size of bioactive glass nanoparticles on mesenchymal stem cell proliferation for dental and orthopedic applications. Mater. Sci. Eng. C 2015, 53, 142–149. [Google Scholar] [CrossRef]
- Ardeshirylajimi, A.; Farhadian, S.; Adegani, F.J.; Mirzaei, S.; Zomorrod, M.S.; Langroudi, L.; Doostmohammadi, A.; Seyedjafari, E.; Soleimani, M. Enhanced osteoconductivity of polyethersulphone nanofibres loaded with bioactive glass nanoparticles in in vitro and in vivo models. Cell Prolif. 2015, 48, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, H.; Chen, X. The preparation of hollow mesoporous bioglass nanoparticles with excellent drug delivery capacity for bone tissue regeneration. Front. Chem. 2019, 7, 283. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, A.; Murthy, A.A.; Thipperudrappa, S.; Bharath, K.N. Nanoparticles Filled Polymer Nanocomposites: A Technological Review. Cogent Eng. 2021, 8, 1991229. [Google Scholar] [CrossRef]
- Jumahat, A.; Soutis, C.; Jones, F.R.; Hodzic, A. Effect of silica nanoparticles on compressive properties of an epoxy polymer. J. Mater. Sci. 2010, 45, 5973–5983. [Google Scholar] [CrossRef]
- Poologasundarampillai, G.; Ionescu, C.; Tsigkou, O.; Murugesan, M.; Hill, R.G.; Stevens, M.M.; Hanna, J.V.; Smith, M.E.; Jones, J.R. Synthesis of bioactive class II poly(γ-glutamic acid)/silica hybrids for bone regeneration. J. Mater. Chem. 2010, 20, 8952–8961. [Google Scholar] [CrossRef]
- MacKenzie, M.E.S.; Kenneth, J.D. Multinuclear Solid-State NMR of Inorganic Materials. In Pergamon Materials Series; Elsevier: Amsterdam, The Netherlands, 2002; Volume 6, p. 748. [Google Scholar]
- Oyane, A.; Kim, H.; Furuya, T.; Kokubo, T.; Miyazaki, T.; Nakamura, T. Preparation and assessment of revised simulated body fluids. J. Biomed. Mater. Res. Part A 2002, 65, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Furuike, T.; Nagahama, H.; Chaochai, T.; Tamura, H. Preparation and Characterization of Chitosan-Coated Poly(l-Lactic Acid) Fibers and Their Braided Rope. Fibers 2015, 3, 380–393. [Google Scholar] [CrossRef]
- Padmanabhan, K. Mechanical properties of nanostructured materials. Mater. Sci. Eng. A 2001, 304–306, 200–205. [Google Scholar] [CrossRef]
- Kiran; Govindaraju, H.; Jayaraju, T.; Kumar, N. Review-Effect of Fillers on Mechanical Properties of Polymer Matrix Composites. Mater. Today Proc. 2018, 5, 22421–22424. [Google Scholar] [CrossRef]
- de Paiva, J.M.F.; Frollini, E. Unmodified and Modified Surface Sisal Fibers as Reinforcement of Phenolic and Lignophenolic Matrices Composites: Thermal Analyses of Fibers and Composites. Macromol. Mater. Eng. 2006, 291, 405–417. [Google Scholar] [CrossRef]
- Khouloud, J.; Chehimi, M.M.; Thomas, S. Clay Polymer Nanocomposites; Elsevier B.V: Amsterdam, The Netherlands, 2019. [Google Scholar]
- White, J.L. Interpretation of infrared spectra of soil minerals. Soil Sci. 1971, 112, 22–31. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; He, S.; Yang, D. Structural characterization of sol-gel composites using TEOS/MEMO as precursors. Surf. Coat. Technol. 2007, 201, 6051–6058. [Google Scholar] [CrossRef]
- Tian, D.; Dubois, P.; Jérôme, R. Biodegradable and biocompatible inorganic-organic hybrid materials. I. Synthesis and characterization. J. Polym. Sci. Part A Polym. Chem. 1997, 35, 2295–2309. [Google Scholar] [CrossRef]
- Lee, H.J.; Oh, K.S.; Choi, C.K. The mechanical properties of the SiOC(H) composite thin films with a low dielectric constant. Surf. Coat. Technol. 2003, 171, 296–301. [Google Scholar] [CrossRef]
- Medda, S.K.; Kundu, D.; De, G. Inorganic–organic hybrid coatings on polycarbonate. Spectroscopic studies on the simultaneous polymerizations of methacrylate and silica networks. J. Non-Cryst. Solids 2003, 318, 149–156. [Google Scholar] [CrossRef]
- Vinet, L.; Zhedanov, A. A ‘missing’ family of classical orthogonal polynomials. J. Phys. A Math. Theor. 2011, 44, 085201. [Google Scholar] [CrossRef]
- Fidalgo, A.; Ilharco, L.M. The defect structure of sol-gel-derived silica/polytetrahydrofuran hybrid films by FTIR. J. Non-Cryst. Solids 2001, 283, 144–154. [Google Scholar] [CrossRef]
- Guo, S.Z.; Zhang, C.; Wang, W.Z.; Liu, T.X. Preparation and characterization of organic-inorganic hybrid nanomaterials using polyurethane-b-poly[3-(trimethoxysilyl) propyl methacrylate] via RAFT polymerization. Express Polym. Lett. 2010, 4, 17–25. [Google Scholar] [CrossRef]
- Freund, F.; Knobel, R.M. Distribution of fluorine in hydroxyapatite studied by infrared spectroscopy. J. Chem. Soc. Dalton Trans. 1977, 11, 1136–1140. [Google Scholar] [CrossRef]
- Fowler, B.O. Infrared studies of apatites. I. Vibrational assignments for calcium, strontium, and barium hydroxyapatites utilizing isotopic substitution. Inorg. Chem. 1974, 13, 194–207. [Google Scholar] [CrossRef]
- Tallia, F.; Ting, H.-K.; Page, S.J.; Clark, J.P.; Li, S.; Sang, T.; Russo, L.; Stevens, M.M.; Hanna, J.V.; Jones, J.R. Bioactive, Degradable and Tough Hybrids Through Calcium and Phosphate Incorporation. Front. Mater. 2022, 9, 901196. [Google Scholar] [CrossRef]
- Fan, W.; Du, T.; Droce, A.; Jensen, L.R.; Youngman, R.E.; Ren, X.; Gurevich, L.; Bauchy, M.; Kristensen, P.; Xing, B.; et al. Resolving the Conflict between Strength and Toughness in Bioactive Silica–Polymer Hybrid Materials. ACS Nano 2022, 16, 9748–9761. [Google Scholar] [CrossRef] [PubMed]
- Almond, G.G. A Nuclear Magnetic Resonance Study of Hydrous Layer Nuclear Magnetic Resonance Study of Hydrous Layer Silicates; Durham University: Durham, UK, 1995. [Google Scholar]
- De Paul, S.M.; Ernst, M.; Shore, J.S.; Stebbins, J.F.; Pines, A. Cross-Polarization from Quadrupolar Nuclei to Silicon Using Low-Radio-Frequency Amplitudes during Magic-Angle Spinning. J. Phys. Chem. B 1997, 101, 3240–3249. [Google Scholar] [CrossRef]
- Lin, S.; Ionescu, C.; Pike, K.J.; Smith, M.E.; Jones, J.R. Nanostructure evolution and calcium distribution in sol-gel derived bioactive glass. J. Mater. Chem. 2009, 19, 1276–1282. [Google Scholar] [CrossRef]
- Fandzloch, M.; Bodylska, W.; Augustyniak, A.W.; Roszek, K.; Jaromin, A.; Lukowiak, A. Bioactive nanoglasses and xerogels (SiO2–CaO and SiO2–CaO–P2O5) as promising candidates for biomedical applications. Ceram. Int. 2023, 49, 7438–7451. [Google Scholar] [CrossRef]
- Anselme, K.; Bigerelle, M.; Noel, B.; Dufresne, E.; Judas, D.; Iost, A.; Hardouin, P. Qualitative and quantitative study of human osteoblast adhesion on materials with various surface roughnesses. J. Biomed. Mater. Res. 2000, 49, 155–166. [Google Scholar] [CrossRef]
- Anselme, K.; Bigerelle, M.; Noël, B.; Iost, A.; Hardouin, P. Effect of grooved titanium substratum on human osteoblastic cell growth. J. Biomed. Mater. Res. 2002, 60, 529–540. [Google Scholar] [CrossRef]
- Chen, S.; Guo, Y.; Liu, R.; Wu, S.; Fang, J.; Huang, B.; Li, Z.; Chen, Z.; Chen, Z. Tuning surface properties of bone biomaterials to manipulate osteoblastic cell adhesion and the signaling pathways for the enhancement of early osseointegration. Colloids Surf. B Biointerfaces 2018, 164, 58–69. [Google Scholar] [CrossRef]
- Takeshita, H.; Poovarodom, M.; Kiya, T.; Arai, F.; Takenaka, K.; Miya, M.; Shiomi, T. Crystallization behavior and chain folding manner of cyclic, star and linear poly(tetrahydrofuran)s. Polymer 2012, 53, 5375–5384. [Google Scholar] [CrossRef]
- Lempesis, N.; Veld, P.J.I.; Rutledge, G.C. Atomistic Simulation of the Structure and Mechanics of a Semicrystalline Polyether. Macromolecules 2016, 49, 5714–5726. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Irska, I.; Zubkiewicz, A.; Szymczyk, A.; Piesowicz, E.; Rozwadowski, Z.; Goracy, K. Biobased Thermoplastic Elastomers: Structure-Property Relationship of Poly(hexamethylene 2,5-furanodicarboxylate)-Block-Poly(tetrahydrofuran) Copolymers Prepared by Melt Polycondensation. Polymers 2021, 13, 397. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, C.; Quijada, R. Preparation of aluminophosphate/polyethylene nanocomposite membranes and their gas permeation properties. J. Membr. Sci. 2010, 358, 33–42. [Google Scholar] [CrossRef]
- Ciardelli, F.; Coiai, S.; Passaglia, E.; Pucci, A.; Ruggeri, G. Nanocomposites based on polyolefins and functional thermoplastic materials. Polym. Int. 2008, 57, 805–836. [Google Scholar] [CrossRef]
- Xie, Y.; Yu, D.; Kong, J.; Fan, X.; Qiao, W. Study on morphology, crystallization behaviors of highly filled maleated polyethylene-layered silicate nanocomposites. J. Appl. Polym. Sci. 2006, 100, 4004–4011. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Harris, R.H.; Zhang, X.; Briber, R.M.; Cipriano, B.H.; Raghavan, S.R.; Awad, W.H.; Shields, J.R. Flame retardant mechanism of polyamide 6–clay nanocomposites. Polymer 2004, 45, 881–891. [Google Scholar] [CrossRef]
- Vakhitova, L. Fire retardant nanocoating for wood protection. In Nanotechnology in Eco-Efficient Construction; Pacheco-Torgal, F., Diamanti, M.V., Nazari, A., Granqvist, C.G., Pruna, A., Amirkhanian, S., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 361–391. [Google Scholar]
- Rhee, S.-H. Effect of molecular weight of poly(ε-caprolactone) on interpenetrating network structure, apatite-forming ability, and degradability of poly(ε-caprolactone)/silica nano-hybrid materials. Biomaterials 2003, 24, 1721–1727. [Google Scholar] [CrossRef]
- Tallia, F. 3-D Printed Flexible Hybrids for Tissue Regeneration; Imperial College London: London, UK, 2016. [Google Scholar]
- Zhang, X.; Sun, Y.; Zhang, C.; Zhang, X. Upcycling Polytetrahydrofuran to Polyester. CCS Chem. 2023, 5, 1233–1241. [Google Scholar] [CrossRef]
- Pol, B.J.M.; van der Does, L.; Bantjes, A.; van Wachem, P.B. In vivo testing of crosslinked polyethers. II. Weight loss, IR analysis, and swelling behavior after implantation. J. Biomed. Mater. Res. 1996, 32, 321–331. [Google Scholar] [CrossRef]
- Pajares-Chamorro, N.; Chatzistavrou, X. Bioactive Glass Nanoparticles for Tissue Regeneration. ACS Omega 2020, 5, 12716–12726. [Google Scholar] [CrossRef]
- Hoppe, A.; Sarker, B.; Detsch, R.; Hild, N.; Mohn, D.; Stark, W.; Boccaccini, A. In vitro reactivity of Sr-containing bioactive glass (type 1393) nanoparticles. J. Non-Cryst. Solids 2014, 387, 41–46. [Google Scholar] [CrossRef]
- Bejarano, J.; Boccaccini, A.R.; Covarrubias, C.; Palza, H. Effect of Cu- and Zn-doped bioactive glasses on the in vitro bioactivity, mechanical and degradation behavior of biodegradable PDLLA scaffolds. Materials 2020, 13, 2908. [Google Scholar] [CrossRef]
- Drouet, C. Apatite Formation: Why It May Not Work as Planned, and How to Conclusively Identify Apatite Compounds. BioMed Res. Int. 2013, 2013, 490946. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. An Introduction to Bioceramics. In Introduction to Biomaterials; Imperial College Press: London, UK, 2013; pp. 530–533. [Google Scholar]
- ISO23317: 2014(E); International Standard Implants for Surgery: In Vitro Evaluation for Apatite Forming Ability of Implant Material. International Organization for Standardization: Geneva, Switzerland, 2014.
- Meechoowas, E.; Tungsanguan, O.; Pamok, C.; Tapasa, K. Investigation of morphology, structure and bioactivity of bioactive glass. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Balamurugan, A.; Balossier, G.; Kannan, S.; Michel, J.; Rebelo, A.H.; Ferreira, J.M. Development and in vitro characterization of sol-gel derived CaO–P2O5–SiO2–ZnO bioglass. Acta Biomater. 2007, 3, 255–262. [Google Scholar] [CrossRef]
- Yu, Y.; Bacsik, Z.; Edén, M. Contrasting In Vitro Apatite Growth from Bioactive Glass Surfaces with that of Spontaneous Precipitation. Materials 2018, 11, 1690. [Google Scholar] [CrossRef]
- Covarrubias, C.; Mattmann, M.; Von Marttens, A.; Caviedes, P.; Arriagada, C.; Valenzuela, F.; Rodríguez, J.P.; Corral, C. Osseointegration properties of titanium dental implants modified with a nanostructured coating based on ordered porous silica and bioactive glass nanoparticles. Appl. Surf. Sci. 2016, 363, 286–295. [Google Scholar] [CrossRef]
- Corral Nunez, C.; Altamirano Gaete, D.; Maureira, M.; Martin, J.; Covarrubias, C. Nanoparticles of Bioactive Glass Enhance Biodentine Bioactivity on Dental Pulp Stem Cells. Materials 2021, 14, 2684. [Google Scholar] [CrossRef] [PubMed]
- Maureira, M.; Cuadra, F.; Cádiz, M.; Torres, M.; von Marttens, A.; Covarrubias, C. Preparation and osteogenic properties of nanocomposite hydrogel beads loaded with nanometric bioactive glass particles. Biomed. Mater. 2021, 16, 045043. [Google Scholar] [CrossRef]
- Zheng, K.; Boccaccini, A.R. Sol-gel processing of bioactive glass nanoparticles: A review. Adv. Colloid Interface Sci. 2017, 249, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.-H.; Choi, J.-Y.; Kim, H.-M. Preparation of a bioactive and degradable poly(ε-caprolactone)/silica hybrid through a sol-gel method. Biomaterials 2002, 23, 4915–4921. [Google Scholar] [CrossRef] [PubMed]
- Koh, M.-Y.; Kamitakahara, M.; Kim, I.Y.; Kikuta, K.; Ohtsuki, C. In vitro apatite formation on organic–inorganic hybrids in the CaO–SiO2–PO5/2–poly(tetramethylene oxide) system. J. Mater. Sci. Mater. Med. 2009, 21, 385–392. [Google Scholar] [CrossRef]
- Mondal, D.; Rizkalla, A.S.; Mequanint, K. Bioactive borophosphosilicate-polycaprolactone hybrid biomaterials via a non-aqueous sol gel process. RSC Adv. 2016, 6, 92824–92832. [Google Scholar] [CrossRef]
- Schreiber, R. Ca2+ Signaling, Intracellular pH and Cell Volume in Cell Proliferation. J. Membr. Biol. 2005, 205, 129–137. [Google Scholar] [CrossRef]
- Kennedy, S.B.; Washburn, N.R.; Simon, C.G., Jr.; Amis, E.J. Combinatorial screen of the effect of surface energy on fibronectin-mediated osteoblast adhesion, spreading and proliferation. Biomaterials 2006, 27, 3817–3824. [Google Scholar] [CrossRef]
- Trivedi, S.; Srivastava, K.; Gupta, A.; Saluja, T.S.; Kumar, S.; Mehrotra, D.; Singh, S.K. A quantitative method to determine osteogenic differentiation aptness of scaffold. J. Oral Biol. Craniofacial Res. 2020, 10, 158–160. [Google Scholar] [CrossRef]
- Shirosaki, Y.; Tsuru, K.; Hayakawa, S.; Osaka, A.; Lopes, M.A.; Santos, J.D.; Fernandes, M.H. In vitro cytocompatibility of MG63 cells on chitosan-organosiloxane hybrid membranes. Biomaterials 2005, 26, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Aghayan, M.; Alizadeh, P.; Keshavarz, M. Multifunctional polyethylene imine hybrids decorated by silica bioactive glass with enhanced mechanical properties, antibacterial, and osteogenesis for bone repair. Mater. Sci. Eng. C 2021, 131, 112534. [Google Scholar] [CrossRef]
- Shirosaki, Y.; Tsuru, K.; Moribayashi, H.; Hayakawa, S.; Nakamura, Y.; Gibson, I.R.; Osaka, A. Preparation of osteocompatible Si(IV)-enriched chitosan-silicate hybrids. J. Ceram. Soc. Jpn. 2010, 118, 989–992. [Google Scholar] [CrossRef]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.; Hench, L.L.; Polak, J.M. Ionic Products of Bioactive Glass Dissolution Increase Proliferation of Human Osteoblasts and Induce Insulin-like Growth Factor II mRNA Expression and Protein Synthesis. Biochem. Biophys. Res. Commun. 2000, 276, 461–465. [Google Scholar] [CrossRef]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.K.; Hench, L.L.; Polak, J.M. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass 45S5 dissolution. J. Biomed. Mater. Res. 2001, 55, 151–157. [Google Scholar] [CrossRef]
- Bosetti, M.; Cannas, M. The effect of bioactive glasses on bone marrow stromal cells differentiation. Biomaterials 2005, 26, 3873–3879. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.Y.; Dong, Y.M.; Chen, X.F.; Karabucak, B. Nano-sized 58S bioactive glass enhances proliferation and osteogenic genes expression of osteoblast-like cells. Chin. J. Dent. Res. 2012, 15, 145–152. [Google Scholar]
- Marelli, B.; Ghezzi, C.E.; Mohn, D.; Stark, W.J.; Barralet, J.E.; Boccaccini, A.R.; Nazhat, S.N. Accelerated mineralization of dense collagen-nano bioactive glass hybrid gels increases scaffold stiffness and regulates osteoblastic function. Biomaterials 2011, 32, 8915–8926. [Google Scholar] [CrossRef]
- Taygun, M.E.; Boccaccini, A.R. Nanoscaled bioactive glass particles and nanofibers. In Bioactive Glasses, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 235–283. [Google Scholar]






| Sample | nBGs (wt.%) | TEOS (mmol) |
|---|---|---|
| 0.25TEOS | - | 0.25 |
| 5nBG/0.25TEOS | 5 | 0.25 |
| 10nBG/0.25TEOS | 10 | 0.25 |
| 15nBG/0.25TEOS | 15 | 0.25 |
| 0.5TEOS | - | 0.50 |
| 5nBG/0.5TEOS | 5 | 0.50 |
| 10nBG/0.5TEOS | 10 | 0.50 |
| 15nBG/0.5TEOS | 15 | 0.50 |
| 1TEOS | - | 1.00 |
| 5nBG/1TEOS | 5 | 1.00 |
| 10nBG/1TEOS | 10 | 1.00 |
| 15nBG/1TEOS | 15 | 1.00 |
| Peak Position (cm−1) | Assignment | References |
|---|---|---|
| 3200 | Asymmetrical stretch –OH | [15,46] |
| 2800–2900 | Stretch CH2 | [15,46] |
| 1375–1365 | Stretch C-H | [15,46] |
| 1110–1080 | Stretch Si-O-C | [15,46,47,48,49,50] |
| 1100–1000 | Asymmetrical stretch Si–O | [15,46,47,51] |
| 990–950 | Stretch Si–OH | [15,47,50] |
| 880–800 | Symmetrical stretch Si–O | [15,52] |
| 720–700 | Cis Movement C-H | [46,51] |
| ~634 | Release mode –OH | [53,54,55] |
| ~540 | Si–O–Si asymmetrical bending | [15,47] |
| ~460 | Si–O–Si bending | [15,47] |
| Samples | Q2 | Q3. | Q4 | Q4b | T1 | T2 | T3 | %Dc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δiso (ppm) | f % | δiso (ppm) | f % | δiso (ppm) | f % | δiso (ppm) | f % | δiso (ppm) | f % | δiso (ppm) | f % | δiso (ppm) | f % | ||
| 0.25TEOS | - | - | - | - | −110.9 | 18 | - | - | −59.6 | 25 | −66.2 | 53 | −71.9 | 4 | 65.67 |
| 5nBG/0.5TEOS | −90.0 | 5 | −100.8 | 11 | −110.4 | 18 | - | - | −58.0 | 24 | −65.8 | 32 | −69.5 | 10 | 68.08 |
| 10nBG/1TEOS | - | - | - | - | −111.6 | 51 | - | - | −59.6 | 6 | −66.2 | 31 | −69.5 | 12 | 85.67 |
| 15nBG/0.5TEOS | - | - | −100.8 | 9 | −110.4 | 42 | −116.8 | 11 | −58.0 | 9 | −64.6 | 22 | −69.5 | 7 | 84.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohn, N.; Bradtmüller, H.; Zanotto, E.; von Marttens, A.; Covarrubias, C. Novel Organic–Inorganic Nanocomposite Hybrids Based on Bioactive Glass Nanoparticles and Their Enhanced Osteoinductive Properties. Biomolecules 2024, 14, 482. https://doi.org/10.3390/biom14040482
Cohn N, Bradtmüller H, Zanotto E, von Marttens A, Covarrubias C. Novel Organic–Inorganic Nanocomposite Hybrids Based on Bioactive Glass Nanoparticles and Their Enhanced Osteoinductive Properties. Biomolecules. 2024; 14(4):482. https://doi.org/10.3390/biom14040482
Chicago/Turabian StyleCohn, Nicolás, Henrik Bradtmüller, Edgar Zanotto, Alfredo von Marttens, and Cristian Covarrubias. 2024. "Novel Organic–Inorganic Nanocomposite Hybrids Based on Bioactive Glass Nanoparticles and Their Enhanced Osteoinductive Properties" Biomolecules 14, no. 4: 482. https://doi.org/10.3390/biom14040482





