Ex Vivo 13C-Metabolic Flux Analysis of Porcine Circulating Immune Cells Reveals Cell Type-Specific Metabolic Patterns and Sex Differences in the Pentose Phosphate Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Granulocytes and PBMCs from Whole Blood
2.2. Measurement of Immune Cell Superoxide Anion Production and Mitochondrial Respiration
2.3. Ex Vivo 13C-Tracer Experiments of PBMCs and Granulocytes
2.4. Extraction of Intracellular Metabolites
2.5. Derivatization and GC-MS Analysis of Intracellular Metabolites
2.6. GC-MS Analysis of 13CO2 Enrichment
2.7. Derivatization and GC-MS Analysis of Secreted Lactate
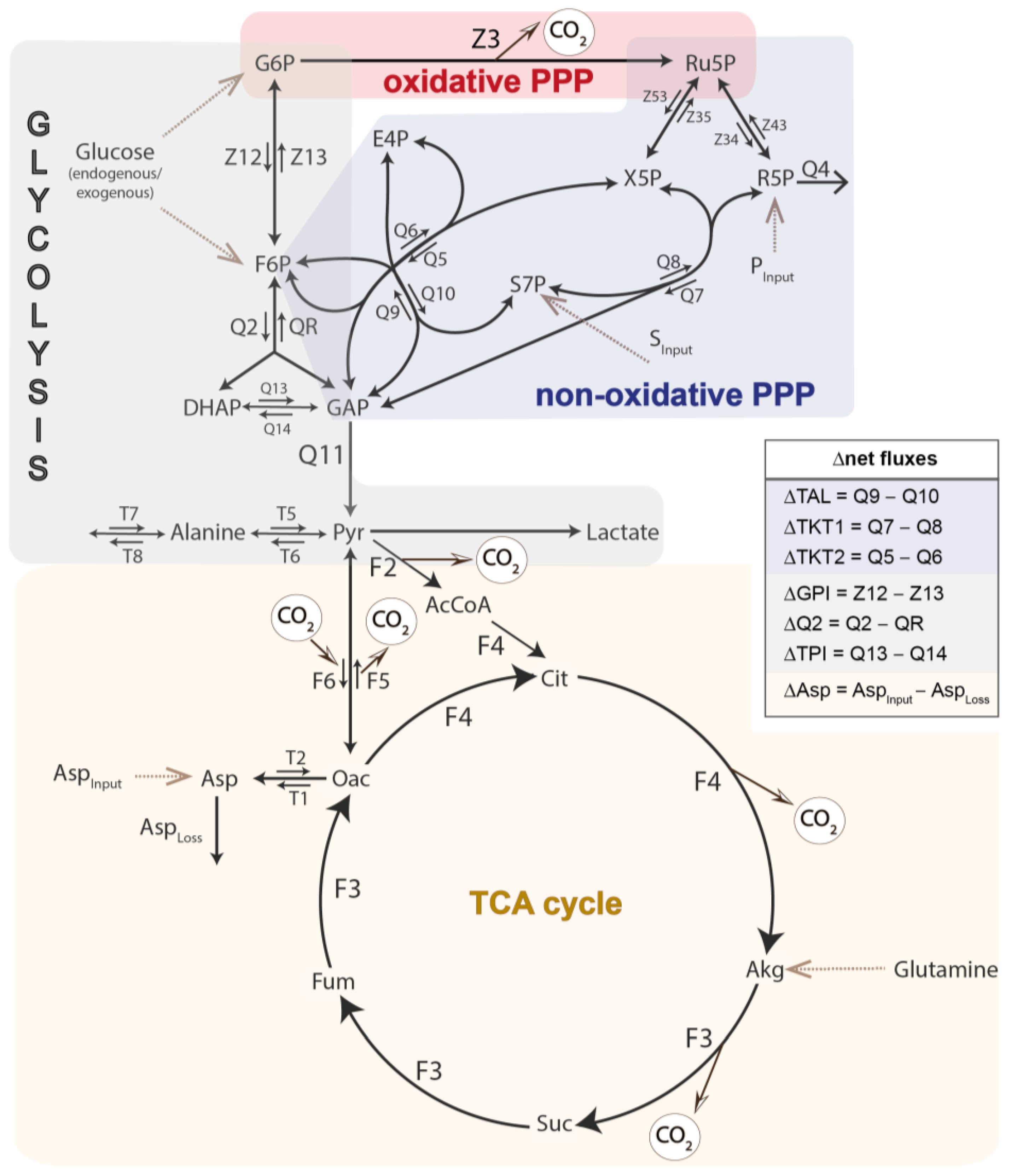
2.8. Metabolic Flux Analysis
2.9. Measurement of Circulating Steroid Levels and Cytokine Plasma Levels
2.10. Flow Cytometry
2.11. Statistical Analysis
3. Results
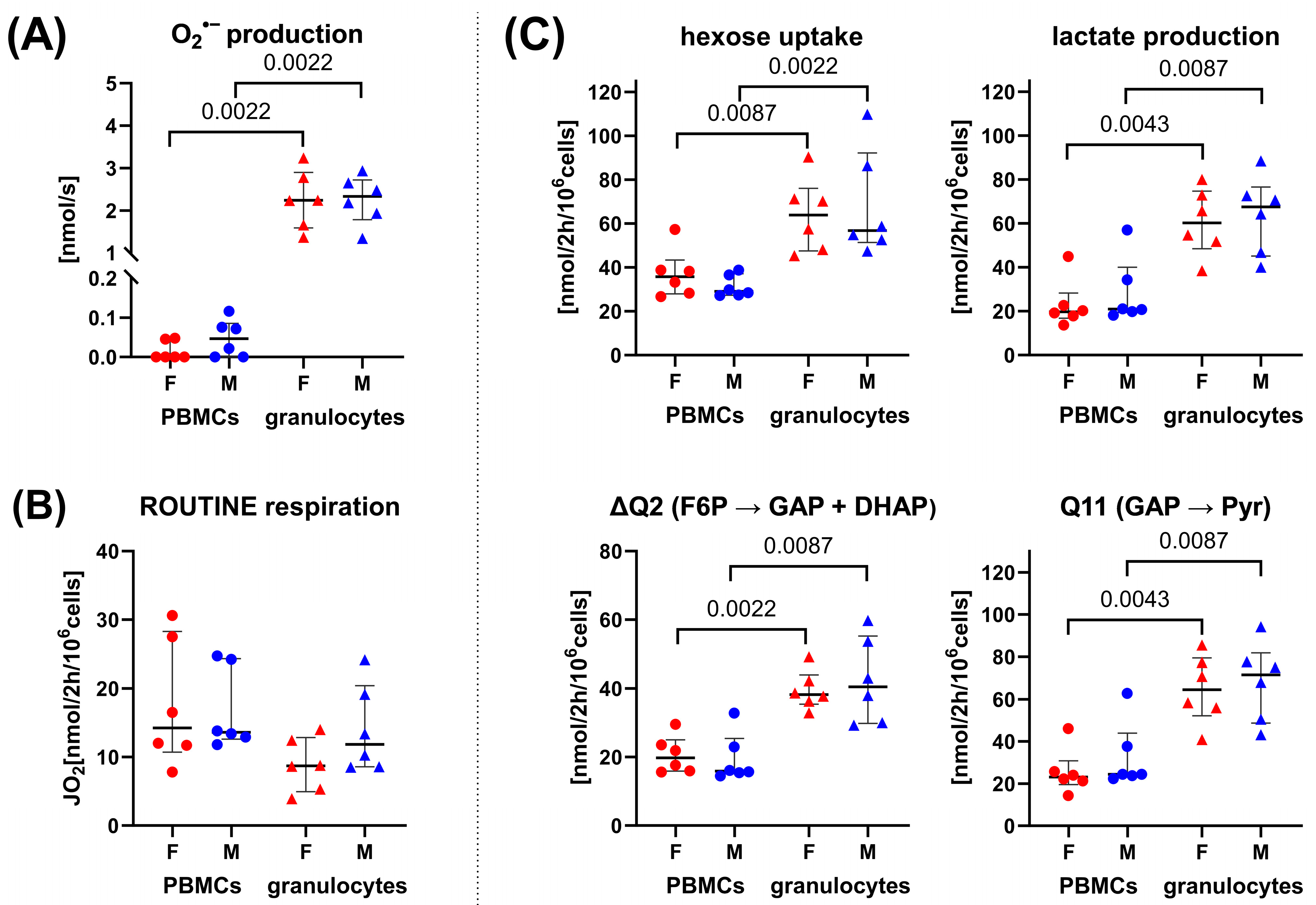
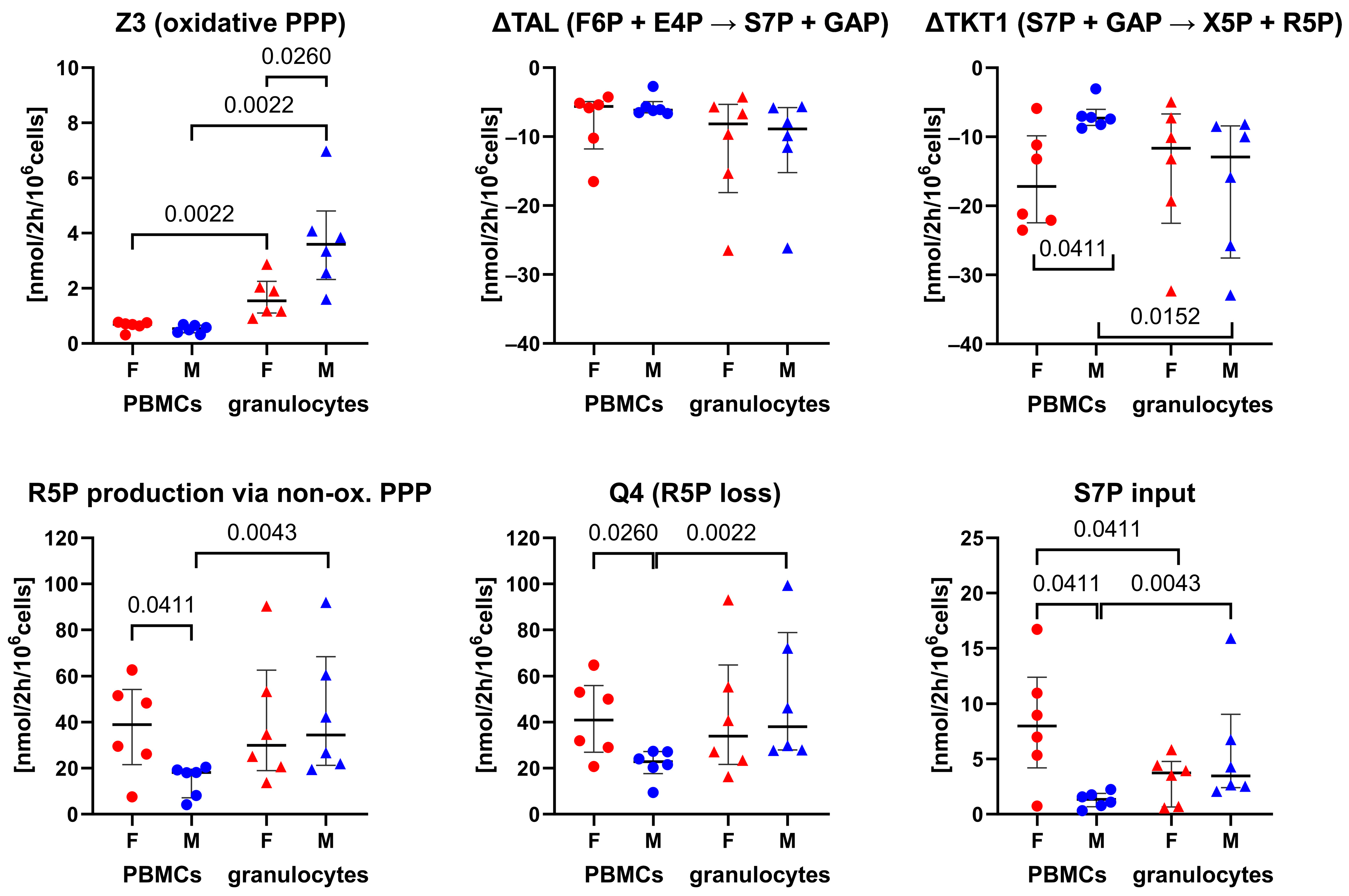
3.1. 13C-MFA of Granulocytes and PBMCs: Sex-Specific Differences in the Pentose Phosphate Pathway
3.2. Theoretical Calculation of ATP Generation Rates Based on 13C-MFA Results
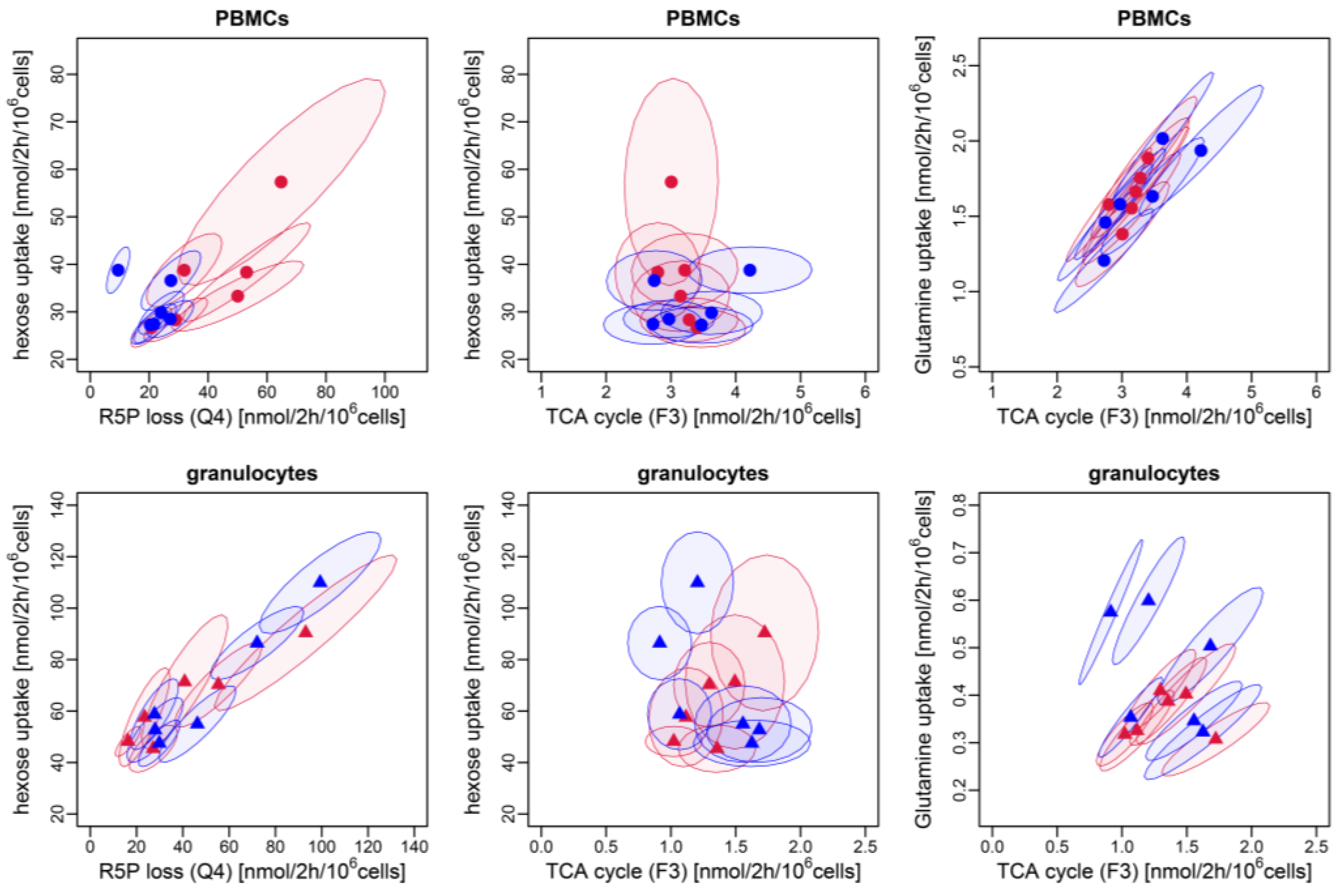
3.3. Identification of Metabolic Patterns with PCA: Granulocytes and PBMCs Differ in Their Metabolic Profile
3.4. Average Percentage of T Helper Cells (CD3+CD4+) in PBMCs Was Higher in Females Than Males
3.5. Sex-Specific Differences of Circulating Steroid Hormone Levels and Their Association with Metabolic Fluxes of Immune Cells
4. Discussion
Limitations and Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borrás, C.; Sastre, J.; García-Sala, D.; Lloret, A.; Pallardó, F.V.; Viña, J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative. Free Radic. Biol. Med. 2003, 34, 546–552. [Google Scholar] [CrossRef]
- Klein, S.L. The effects of hormones on sex differences in infection: From genes to behavior. Neurosci. Biobehav. Rev. 2000, 24, 627–638. [Google Scholar] [CrossRef]
- Bouman, A.; Heineman, M.J.; Faas, M.M. Sex hormones and the immune response in humans. Hum. Reprod. Update 2005, 11, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Asai, H.; Hiki, N.; Mimura, Y.; Ogawa, T.; Unou, K.; Kaminishi, M. Gender differences in cytokine secretion by human peripheral blood mononuclear cells: Role of estrogen in modulating LPS-induced cytokine secretion in an ex vivo septic model. Shock 2001, 16, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Békési, G.; Kakucs, R.; Várbíró, S.; Rácz, K.; Sprintz, D.; Fehér, J.; Székács, B. In vitro effects of different steroid hormones on superoxide anion production of human neutrophil granulocytes granulocytes. Steroids 2000, 65, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Boje, A.; Moesby, L.; Timm, M.; Hansen, E.W. Immunomodulatory effects of testosterone evaluated in all-trans retinoic acid differentiated HL-60 cells, granulocytes, and monocytes. Int. Immunopharmacol. 2012, 12, 573–579. [Google Scholar] [CrossRef]
- Kovats, S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell. Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef]
- Dai, R.; Cowan, C.; Heid, B.; Khan, D.; Liang, Z.; Pham, C.T.N.; Ahmed, S.A. Neutrophils and neutrophil serine proteases are increased in the spleens of estrogen-treated C57BL/6 mice and several strains of spontaneous lupus-prone mice. PLoS ONE 2017, 12, e0172105. [Google Scholar] [CrossRef]
- Silaidos, C.; Pilatus, U.; Grewal, R.; Matura, S.; Lienerth, B.; Pantel, J.; Eckert, G.P. Sex-associated differences in mitochondrial function in human peripheral blood mononuclear cells (PBMCs) and brain. Biol. Sex Differ. 2018, 9, 34. [Google Scholar] [CrossRef]
- Schmidt, C.A.; Fisher-Wellman, K.H.; Neufer, P.D. From OCR and ECAR to energy: Perspectives on the design and interpretation of bioenergetics studies. J. Biol. Chem. 2021, 297, 101140. [Google Scholar] [CrossRef]
- Alexovič, M.; Uličná, C.; Sabo, J.; Davalieva, K. Human peripheral blood mononuclear cells as a valuable source of disease-related biomarkers: Evidence from comparative proteomics studies. Proteom.-Clin. Appl. 2023; e2300072, Early View. [Google Scholar] [CrossRef]
- Costa, A.; Reynés, B.; Konieczna, J.; Martín, M.; Fiol, M.; Palou, A.; Romaguera, D.; Oliver, P. Use of human PBMC to analyse the impact of obesity on lipid metabolism and metabolic status: A proof-of-concept pilot study. Sci. Rep. 2021, 11, 18329. [Google Scholar] [CrossRef]
- Kramer, P.A.; Ravi, S.; Chacko, B.; Johnson, M.S.; Darley-Usmar, V.M. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: Implications for their use as bioenergetic biomarkers. Redox Biol. 2014, 2, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, B.; Sun, F.; Li, Y.; Li, Q.; Lang, H.; Zhao, Z.; Gao, P.; Zhao, Y.; Shang, Q.; et al. Mitochondrial respiratory dysfunctions of blood mononuclear cells link with cardiac disturbance in patients with early-stage heart failure. Sci. Rep. 2015, 5, 10229. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.L.; Selak, M.A.; Tuluc, F.; Perales Villarroel, J.; Nadkarni, V.M.; Deutschman, C.S.; Becker, L.B. Mitochondrial dysfunction in peripheral blood mononuclear cells in pediatric septic shock. Pediatr. Crit. Care Med. 2015, 16, e4–e12. [Google Scholar] [CrossRef]
- Toller-Kawahisa, J.E.; O’Neill, L.A.J. How neutrophil metabolism affects bacterial killing. Open Biol. 2022, 12, 220248. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-H.; Hong, C.-W.; Kim, E.Y.; Lee, J.M. Current Understanding on the Metabolism of Neutrophils. Immune Netw. 2020, 20, e46. [Google Scholar] [CrossRef]
- Zhang, X.; Zink, F.; Hezel, F.; Vogt, J.; Wachter, U.; Wepler, M.; Loconte, M.; Kranz, C.; Hellmann, A.; Mizaikoff, B.; et al. Metabolic substrate utilization in stress-induced immune cells. Intensive Care Med. Exp. 2020, 8, 28. [Google Scholar] [CrossRef]
- Britt, E.C.; Lika, J.; Giese, M.A.; Schoen, T.J.; Seim, G.L.; Huang, Z.; Lee, P.Y.; Huttenlocher, A.; Fan, J. Switching to the cyclic pentose phosphate pathway powers the oxidative burst in activated neutrophils. Nat. Metab. 2022, 4, 389–403. [Google Scholar] [CrossRef]
- TeSlaa, T.; Ralser, M.; Fan, J.; Rabinowitz, J.D. The pentose phosphate pathway in health and disease. Nat. Metab. 2023, 5, 1275–1289. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, F.; Liu, X.; Lu, Y.; Yao, K.; Tian, N.; Tong, L.; Figge, D.A.; Wang, X.; Han, Y.; et al. Non-oxidative pentose phosphate pathway controls regulatory T cell function by integrating metabolism and epigenetics. Nat. Metab. 2022, 4, 559–574. [Google Scholar] [CrossRef]
- Bekesi, G.; Tulassay, Z.; Racz, K.; Feher, J.; Szekacs, B.; Kakucs, R.; Dinya, E.; Riss, E.; Magyar, Z.; Rigo, J. The effect of estrogens on superoxide anion generation by human neutrophil granulocytes: Possible consequences of the antioxidant defense. Gynecol. Endocrinol. 2007, 23, 451–454. [Google Scholar] [CrossRef]
- Marin, D.P.; Bolin, A.P.; dos Santos, R.d.C.M.; Curi, R.; Otton, R. Testosterone suppresses oxidative stress in human neutrophils. Cell Biochem. Funct. 2010, 28, 394–402. [Google Scholar] [CrossRef]
- Wiechert, W. 13C metabolic flux analysis. Metab. Eng. 2001, 3, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Moon, S.J.; Kelleher, J.K.; Stephanopoulos, G. Dissecting Mammalian Cell Metabolism through 13 C- and 2 H-Isotope Tracing: Interpretations at the Molecular and Systems Levels. Ind. Eng. Chem. Res. 2020, 59, 2593–2610. [Google Scholar] [CrossRef]
- Antoniewicz, M.R. A guide to 13C metabolic flux analysis for the cancer biologist. Exp. Mol. Med. 2018, 50, 1–13. [Google Scholar] [CrossRef]
- Lee, W.N.; Boros, L.G.; Puigjaner, J.; Bassilian, S.; Lim, S.; Cascante, M. Mass isotopomer study of the nonoxidative pathways of the pentose cycle with 1,2-13C2glucose. Am. J. Physiol. 1998, 274, E843–E851. [Google Scholar] [CrossRef]
- Wittmann, C. Fluxome analysis using GC-MS. Microb. Cell Fact. 2007, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- de Falco, B.; Giannino, F.; Carteni, F.; Mazzoleni, S.; Kim, D.-H. Metabolic flux analysis: A comprehensive review on sample preparation, analytical techniques, data analysis, computational modelling, and main application areas. RSC Adv. 2022, 12, 25528–25548. [Google Scholar] [CrossRef] [PubMed]
- Bruntz, R.C.; Lane, A.N.; Higashi, R.M.; Fan, T.W.-M. Exploring cancer metabolism using stable isotope-resolved metabolomics (SIRM). J. Biol. Chem. 2017, 292, 11601–11609. [Google Scholar] [CrossRef]
- Sadiku, P.; Willson, J.A.; Ryan, E.M.; Sammut, D.; Coelho, P.; Watts, E.R.; Grecian, R.; Young, J.M.; Bewley, M.; Arienti, S.; et al. Neutrophils Fuel Effective Immune Responses through Gluconeogenesis and Glycogenesis. Cell Metab. 2021, 33, 411–423.e4. [Google Scholar] [CrossRef] [PubMed]
- Kohlstedt, M.; Wittmann, C. GC-MS-based 13C metabolic flux analysis resolves the parallel and cyclic glucose metabolism of Pseudomonas putida KT2440 and Pseudomonas aeruginosa PAO1. Metab. Eng. 2019, 54, 35–53. [Google Scholar] [CrossRef]
- Hogg, M.; Wolfschmitt, E.-M.; Wachter, U.; Zink, F.; Radermacher, P.; Vogt, J.A. Bayesian 13C-Metabolic Flux Analysis of Parallel Tracer Experiments in Granulocytes: A Directional Shift within the Non-Oxidative Pentose Phosphate Pathway Supports Phagocytosis. Metabolites 2024, 14, 24. [Google Scholar] [CrossRef]
- Wolfschmitt, E.-M.; Hogg, M.; Vogt, J.A.; Zink, F.; Wachter, U.; Hezel, F.; Zhang, X.; Hoffmann, A.; Gröger, M.; Hartmann, C.; et al. The effect of sodium thiosulfate on immune cell metabolism during porcine hemorrhage and resuscitation. Front. Immunol. 2023, 14, 1125594. [Google Scholar] [CrossRef]
- Münz, F.; Wolfschmitt, E.-M.; Zink, F.; Abele, N.; Hogg, M.; Hoffmann, A.; Gröger, M.; Calzia, E.; Waller, C.; Radermacher, P.; et al. Porcine blood cell and brain tissue energy metabolism: Effects of “early life stress”. Front. Mol. Biosci. 2023, 10, 1113570. [Google Scholar] [CrossRef] [PubMed]
- van Winden, W.A.; Wittmann, C.; Heinzle, E.; Heijnen, J.J. Correcting mass isotopomer distributions for naturally occurring isotopes. Biotechnol. Bioeng. 2002, 80, 477–479. [Google Scholar] [CrossRef]
- Forina, M.; Armanino, C.; Lanteri, S.; Leardi, R. Methods of varimax rotation in factor analysis with applications in clinical and food chemistry. J. Chemom. 1989, 3, 115–125. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 7 December 2023).
- Abdi, H.; Williams, L.J. Principal component analysis. WIREs Comp. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Ahmad, M.; Wolberg, A.; Kahwaji, C.I. Biochemistry, Electron Transport Chain; StatPearls Publishing: Treasure Island, FL, USA, 2022; Available online: https://europepmc.org/article/NBK/nbk526105 (accessed on 7 December 2023).
- Soede, N.M.; Langendijk, P.; Kemp, B. Reproductive cycles in pigs. Anim. Reprod. Sci. 2011, 124, 251–258. [Google Scholar] [CrossRef]
- Gurol, T.; Zhou, W.; Deng, Q. MicroRNAs in neutrophils: Potential next generation therapeutics for inflammatory ailments. Immunol. Rev. 2016, 273, 29–47. [Google Scholar] [CrossRef]
- Walczak, C.; Gaignier, F.; Gilet, A.; Zou, F.; Thornton, S.N.; Ropars, A. Aldosterone increases VEGF-A production in human neutrophils through PI3K, ERK1/2 and p38 pathways. Biochim. Biophys. Acta 2011, 1813, 2125–2132. [Google Scholar] [CrossRef]
- Gilet, A.; Zou, F.; Boumenir, M.; Frippiat, J.-P.; Thornton, S.N.; Lacolley, P.; Ropars, A. Aldosterone up-regulates MMP-9 and MMP-9/NGAL expression in human neutrophils through p38, ERK1/2 and PI3K pathways. Exp. Cell Res. 2015, 331, 152–163. [Google Scholar] [CrossRef]
- Gupta, S.; Nakabo, S.; Blanco, L.P.; O’Neil, L.J.; Wigerblad, G.; Goel, R.R.; Mistry, P.; Jiang, K.; Carmona-Rivera, C.; Chan, D.W.; et al. Sex differences in neutrophil biology modulate response to type I interferons and immunometabolism. Proc. Natl. Acad. Sci. USA 2020, 117, 16481–16491. [Google Scholar] [CrossRef] [PubMed]
- Clapper, J.A.; Clark, T.M.; Rempel, L.A. Serum concentrations of IGF-I, estradiol-17beta, testosterone, and relative amounts of IGF binding proteins (IGFBP) in growing boars, barrows, and gilts. J. Anim. Sci. 2000, 78, 2581–2588. [Google Scholar] [CrossRef] [PubMed]
- Paclet, M.-H.; Laurans, S.; Dupré-Crochet, S. Regulation of Neutrophil NADPH Oxidase, NOX2: A Crucial Effector in Neutrophil Phenotype and Function. Front. Cell Dev. Biol. 2022, 10, 945749. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Levine, J.D.; Green, P.G. Sexual dimorphism in the effect of sound stress on neutrophil function. J. Neuroimmunol. 2008, 205, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Chai, P.-S.; Chong, M.-Y.; Tohit, E.R.M.; Ramasamy, R.; Pei, C.P.; Vidyadaran, S. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell. Immunol. 2012, 272, 214–219. [Google Scholar] [CrossRef]
- Ferreira, N.S.; Tostes, R.C.; Paradis, P.; Schiffrin, E.L. Aldosterone, Inflammation, Immune System, and Hypertension. Am. J. Hypertens. 2021, 34, 15–27. [Google Scholar] [CrossRef]
- Miura, R.; Nakamura, K.; Miura, D.; Miura, A.; Hisamatsu, K.; Kajiya, M.; Hashimoto, K.; Nagase, S.; Morita, H.; Fukushima Kusano, K.; et al. Aldosterone synthesis and cytokine production in human peripheral blood mononuclear cells. J. Pharmacol. Sci. 2006, 102, 288–295. [Google Scholar] [CrossRef]
- Kerstens, M.N.; Kobold, A.C.M.; Volmer, M.; Koerts, J.; Sluiter, W.J.; Dullaart, R.P.F. Reference values for aldosterone-renin ratios in normotensive individuals and effect of changes in dietary sodium consumption. Clin. Chem. 2011, 57, 1607–1611. [Google Scholar] [CrossRef]
- Solanki, P.; Gwini, S.M.; Doery, J.C.G.; Choy, K.W.; Shen, J.; Young, M.J.; Fuller, P.J.; Yang, J. Age- and sex-specific reference ranges are needed for the aldosterone/renin ratio. Clin. Endocrinol. 2020, 93, 221–228. [Google Scholar] [CrossRef]
- Kau, M.M.; Lo, M.J.; Wang, S.W.; Tsai, S.C.; Chen, J.J.; Chiao, Y.C.; Yeh, J.Y.; Lin, H.; Shum, A.Y.; Fang, V.S.; et al. Inhibition of Aldosterone Production by Testosterone in Male Rats. Metabolism 1999, 48, 1108–1114. [Google Scholar] [CrossRef]
- Vina, J.; Gambini, J.; Lopez-Grueso, R.; Abdelaziz, K.M.; Jove, M.; Borras, C. Females Live Longer than Males: Role of Oxidative Stress. Curr. Pharm. Des. 2011, 17, 3959–3965. [Google Scholar] [CrossRef]
- Jayachandran, M.; Okano, H.; Chatrath, R.; Owen, W.G.; McConnell, J.P.; Miller, V.M. Sex-specific changes in platelet aggregation and secretion with sexual maturity in pigs. J. Appl. Physiol. 2004, 97, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D. A review of the role of estrogen in dermal aging and facial attractiveness in women. J. Cosmet. Dermatol. 2018, 17, 282–288. [Google Scholar] [CrossRef]
- Dimitrakakis, C.; Bondy, C. Androgens and the breast. Breast Cancer Res. 2009, 11, 212. [Google Scholar] [CrossRef]
- Kowaltowski, A.J.; de Souza-Pinto, N.C.; Castilho, R.F.; Vercesi, A.E. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009, 47, 333–343. [Google Scholar] [CrossRef]
- Herrada, A.A.; Contreras, F.J.; Marini, N.P.; Amador, C.A.; González, P.A.; Cortés, C.M.; Riedel, C.A.; Carvajal, C.A.; Figueroa, F.; Michea, L.F.; et al. Aldosterone promotes autoimmune damage by enhancing Th17-mediated immunity. J. Immunol. 2010, 184, 191–202. [Google Scholar] [CrossRef]
- Muñoz-Durango, N.; Vecchiola, A.; Gonzalez-Gomez, L.M.; Simon, F.; Riedel, C.A.; Fardella, C.E.; Kalergis, A.M. Modulation of Immunity and Inflammation by the Mineralocorticoid Receptor and Aldosterone. BioMed Res. Int. 2015, 2015, 652738. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Papacosta, O.; Lawlor, D.A.; Whincup, P.H.; Lowe, G.D.; Ebrahim, S.; Sattar, N. Do women exhibit greater differences in established and novel risk factors between diabetes and non-diabetes than men? The British Regional Heart Study and British Women’s Heart Health Study. Diabetologia 2012, 55, 80–87. [Google Scholar] [CrossRef]
- Ramezankhani, A.; Azizi, F.; Hadaegh, F. Gender differences in changes in metabolic syndrome status and its components and risk of cardiovascular disease: A longitudinal cohort study. Cardiovasc. Diabetol. 2022, 21, 227. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Chen, Z.; Baudier, R.L.; Krousel-Wood, M.; Anderson, A.H.; Fonseca, V.A.; Mauvais-Jarvis, F. Sex Differences in the Progression of Metabolic Risk Factors in Diabetes Development. JAMA Netw. Open 2022, 5, e2222070. [Google Scholar] [CrossRef]
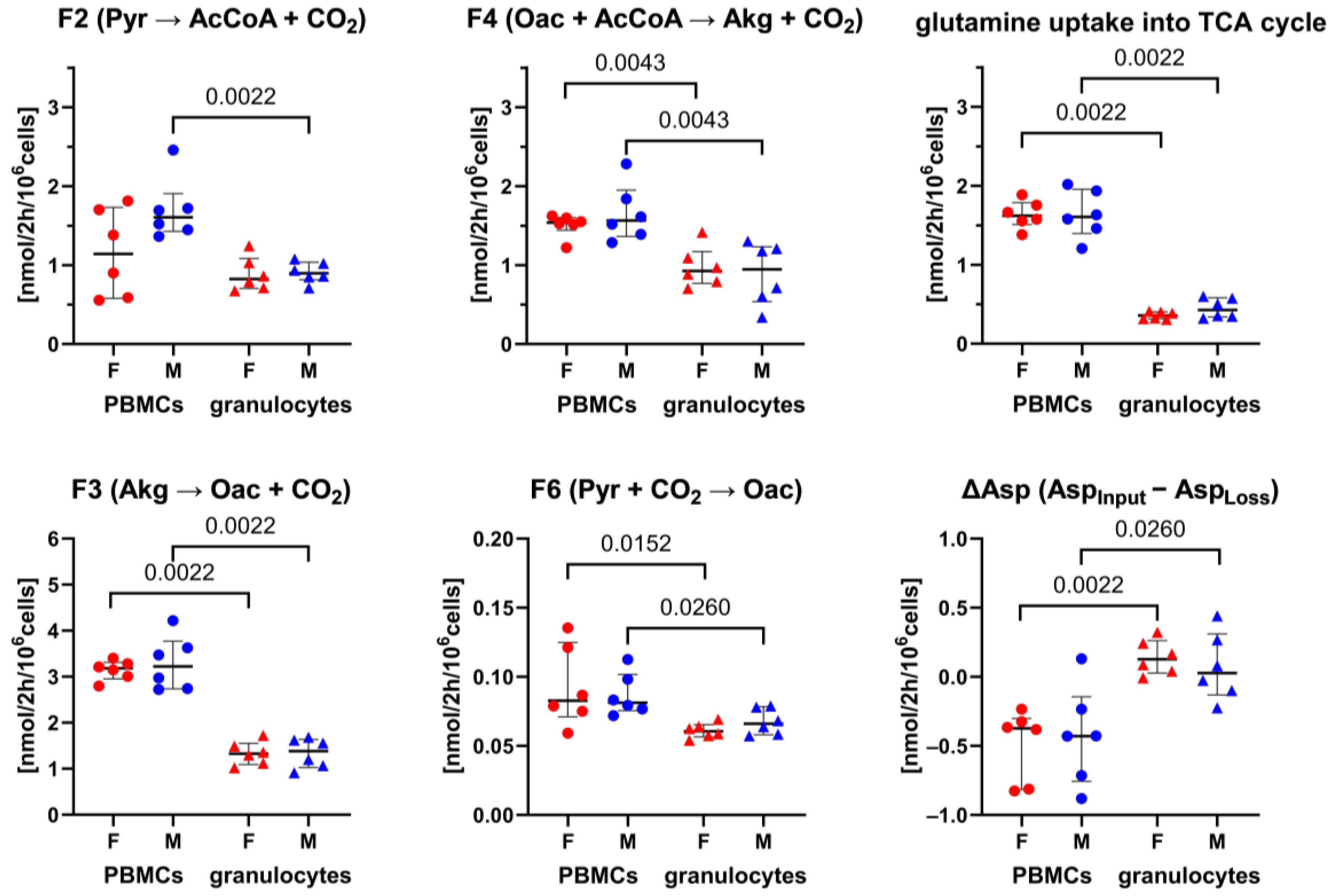

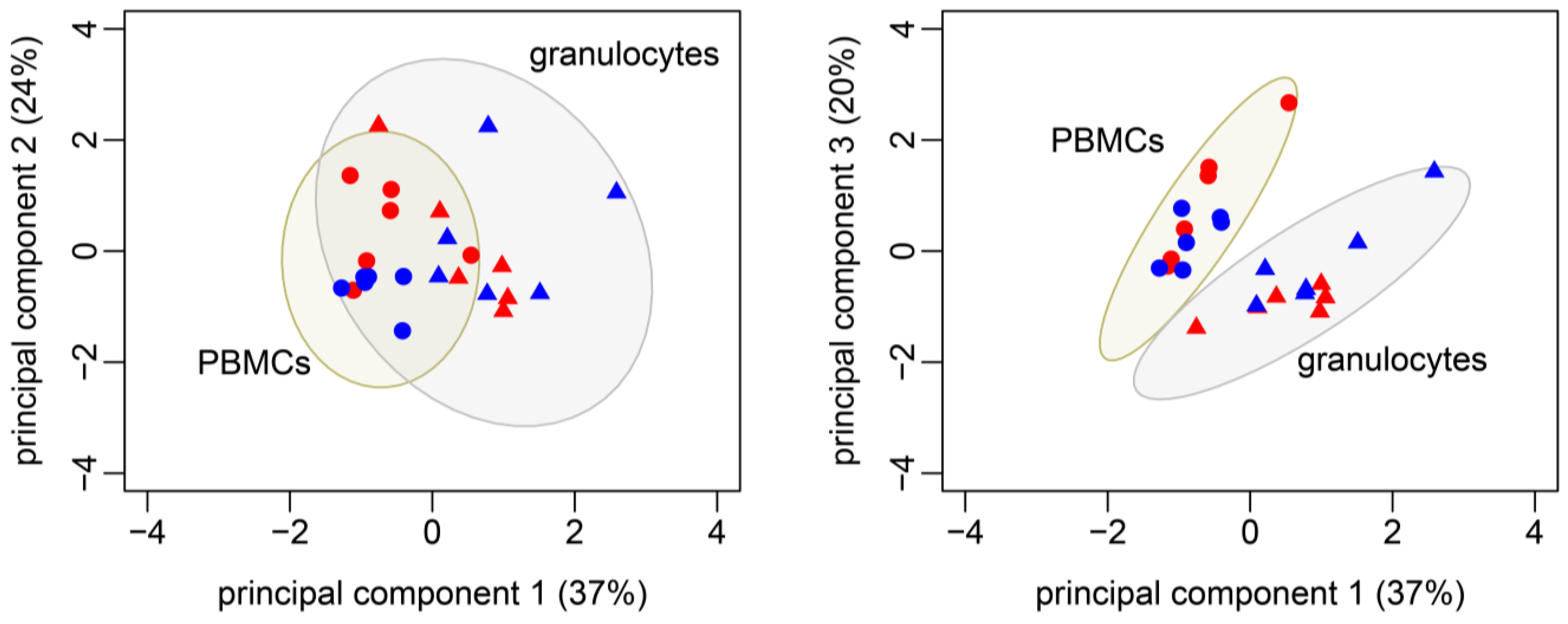

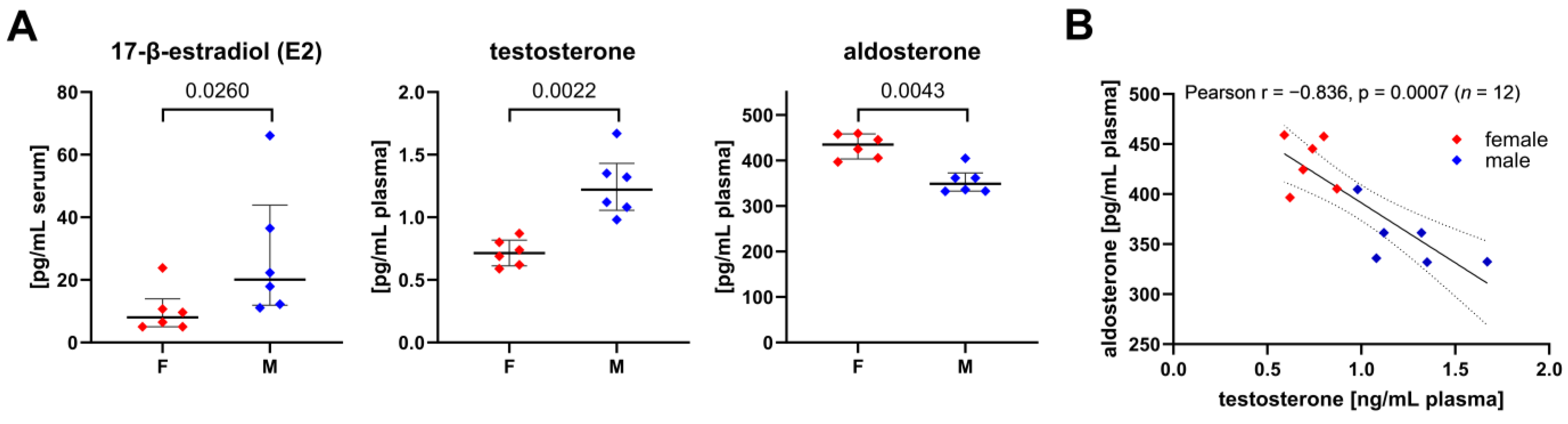

| Theoretical ATP Generation Rate (nmol/2 h/106 Cells) | |||
|---|---|---|---|
| PBMCs | Granulocytes | ||
| Glycolysis | both sexes (n = 12) | 13.8 (4.3; 23.1) | 49.3 (15.1; 64.8) |
| (hexose uptake, ∆Q2, Q11, lactate production) | female (n = 6) | 1.9 (−19.9; 18.8) | 54.3 (23.7; 65.6) |
| male (n = 6) | 14.4 (13.6; 21.6) | 43.6 (15.5; 55.8) | |
| TCA cycle | both sexes (n = 12) | 30.7 (28.5; 34.1) | 14.2 (12.2; 17.3) |
| (F2, F4, F3) | female (n = 6) | 30.7 (29.0; 31.9) | 14.2 (12.7; 16.5) |
| male (n = 6) | 47.9 (44.5; 51.1) | 14.9 (12.1; 17.8) | |
| Whole metabolism | both sexes (n = 12) | 45.4 (36.4; 53.0) | 62.5 (29.9; 78.6) |
| (hexose uptake, ∆Q2, Q11, lactate production, F2, F3, F4, F5, F6) | female (n = 6) | 34.0 (7.3; 52.0) | 68.8 (37.9; 79.1) |
| male (n = 6) | 47.9 (44.5; 51.1) | 58.5 (33.0; 71.3) | |
| Metabolic Flux/Effector Function | PC1 (37.4%) | PC2 (23.7%) | PC3 (20.0%) | |
|---|---|---|---|---|
| Effector function | O2•− production | 0.67 | −0.61 | |
| Mitochondrial respiration | JO2 of ROUTINE respiration | 0.63 | ||
| Glycolysis | hexose (glucose/fructose) uptake | 0.59 | 0.66 | −0.36 |
| ∆Q2 (F6P → GAP+ DHAP) | 0.89 | 0.26 | ||
| Q11 (GAP → Pyr) | 0.91 | |||
| lactate production | 0.92 | |||
| Oxidative PPP | Z3 | 0.87 | ||
| Non-oxidative PPP | ∆TAL (F6P + E4P → S7P + GAP) | −0.88 | 0.39 | |
| ∆TKT1 (S7P + GAP → X5P + R5P) | −0.99 | |||
| input S7P | 0.64 | 0.55 | ||
| Q4 (R5P loss) | 0.97 | |||
| TCA cycle | F2 (Pyr → AcCoA + CO2) | −0.56 | ||
| F3 (Akg → Oac + CO2) | −0.78 | 0.49 | ||
| F4 (Oac + AcCoA → Akg + CO2) | −0.80 | |||
| F6 (Pyr + CO2 → Oac) | 0.75 | |||
| ΔAsp (AspInput − AspLoss) | −0.78 | |||
| glutamine input | −0.68 | 0.62 | ||
| Plasma Level [pg/mL] vs. Metabolic Flux [nmol/2 h/106 Granulocytes] | Sex | Spearman (n = 6) | Pearson (n = 6) | ||
|---|---|---|---|---|---|
| r | p | r | p | ||
| Aldosterone vs. hexose uptake | female | −0.886 | 0.033 * | −0.949 | 0.004 * |
| male | 0.886 | 0.033 * | 0.609 | 0.200 | |
| Aldosterone vs. ΔTAL | female | 1.000 | 0.003 * | 0.916 | 0.010 * |
| male | −0.657 | 0.175 | non-normal distribution | ||
| Aldosterone vs. ΔTKT1 | female | 0.904 | 0.013 * | 0.904 | 0.013 * |
| male | −0.726 | 0.102 | −0.726 | 0.102 | |
| Aldosterone vs. input S7P | female | −0.543 | 0.297 | −0.664 | 0.150 |
| male | 1.000 | 0.003 * | non-normal distribution | ||
| Aldosterone vs. R5P loss (Q4) | female | −0.943 | 0.017 * | −0.917 | 0.010 * |
| male | 0.714 | 0.136 | 0.672 | 0.144 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hogg, M.; Wolfschmitt, E.-M.; Wachter, U.; Zink, F.; Radermacher, P.; Vogt, J.A. Ex Vivo 13C-Metabolic Flux Analysis of Porcine Circulating Immune Cells Reveals Cell Type-Specific Metabolic Patterns and Sex Differences in the Pentose Phosphate Pathway. Biomolecules 2024, 14, 98. https://doi.org/10.3390/biom14010098
Hogg M, Wolfschmitt E-M, Wachter U, Zink F, Radermacher P, Vogt JA. Ex Vivo 13C-Metabolic Flux Analysis of Porcine Circulating Immune Cells Reveals Cell Type-Specific Metabolic Patterns and Sex Differences in the Pentose Phosphate Pathway. Biomolecules. 2024; 14(1):98. https://doi.org/10.3390/biom14010098
Chicago/Turabian StyleHogg, Melanie, Eva-Maria Wolfschmitt, Ulrich Wachter, Fabian Zink, Peter Radermacher, and Josef Albert Vogt. 2024. "Ex Vivo 13C-Metabolic Flux Analysis of Porcine Circulating Immune Cells Reveals Cell Type-Specific Metabolic Patterns and Sex Differences in the Pentose Phosphate Pathway" Biomolecules 14, no. 1: 98. https://doi.org/10.3390/biom14010098





