Determining the Effect of Non-Thermal Plasma on the Transmembrane Kinetics of Melittin through Molecular Explorations
Abstract
1. Introduction
2. Methods and Materials
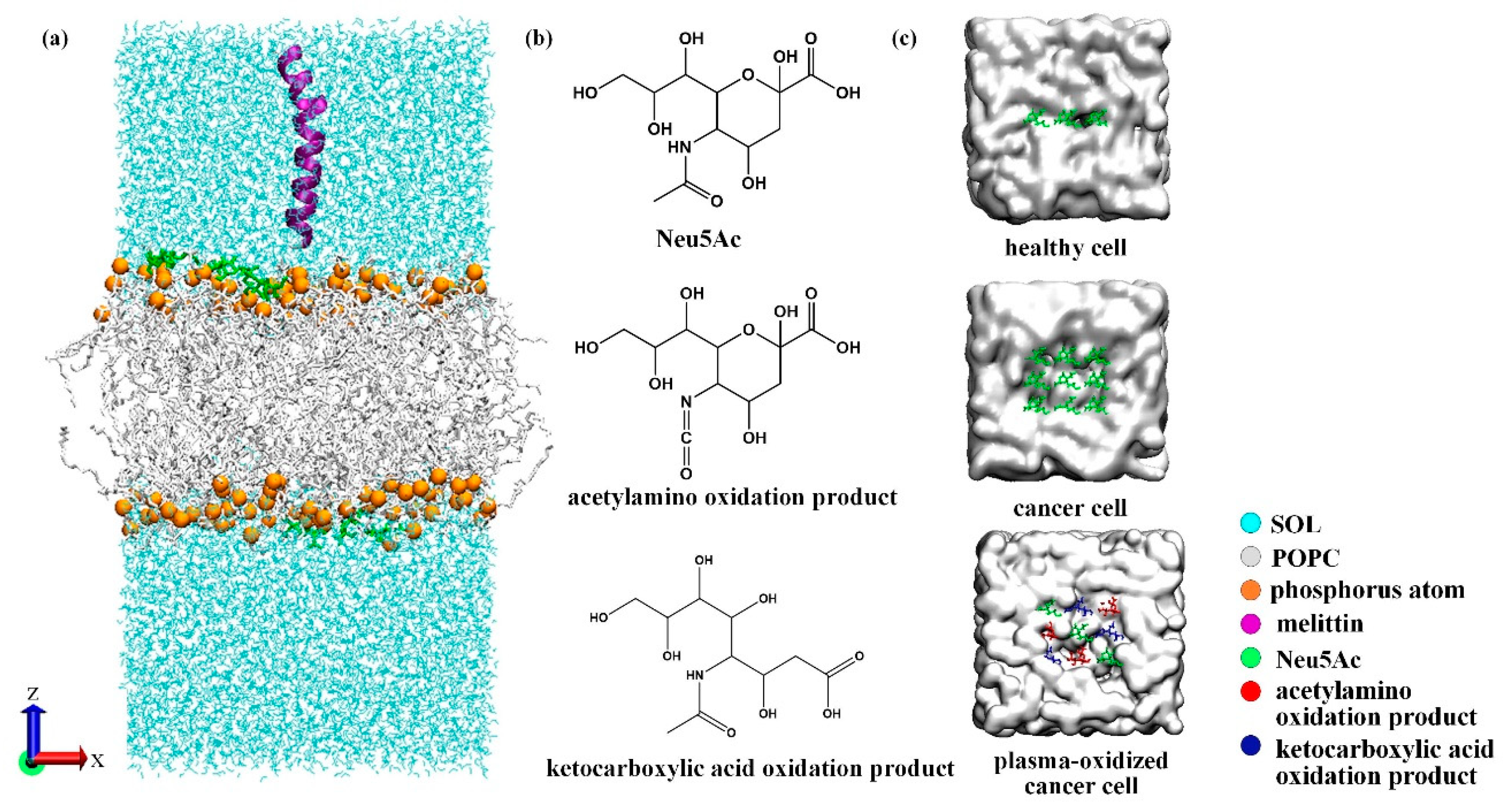
2.1. Simulation Setup and Model Introduction
2.2. MD Trajectory Analysis
2.3. Umbrella Sampling
3. Results
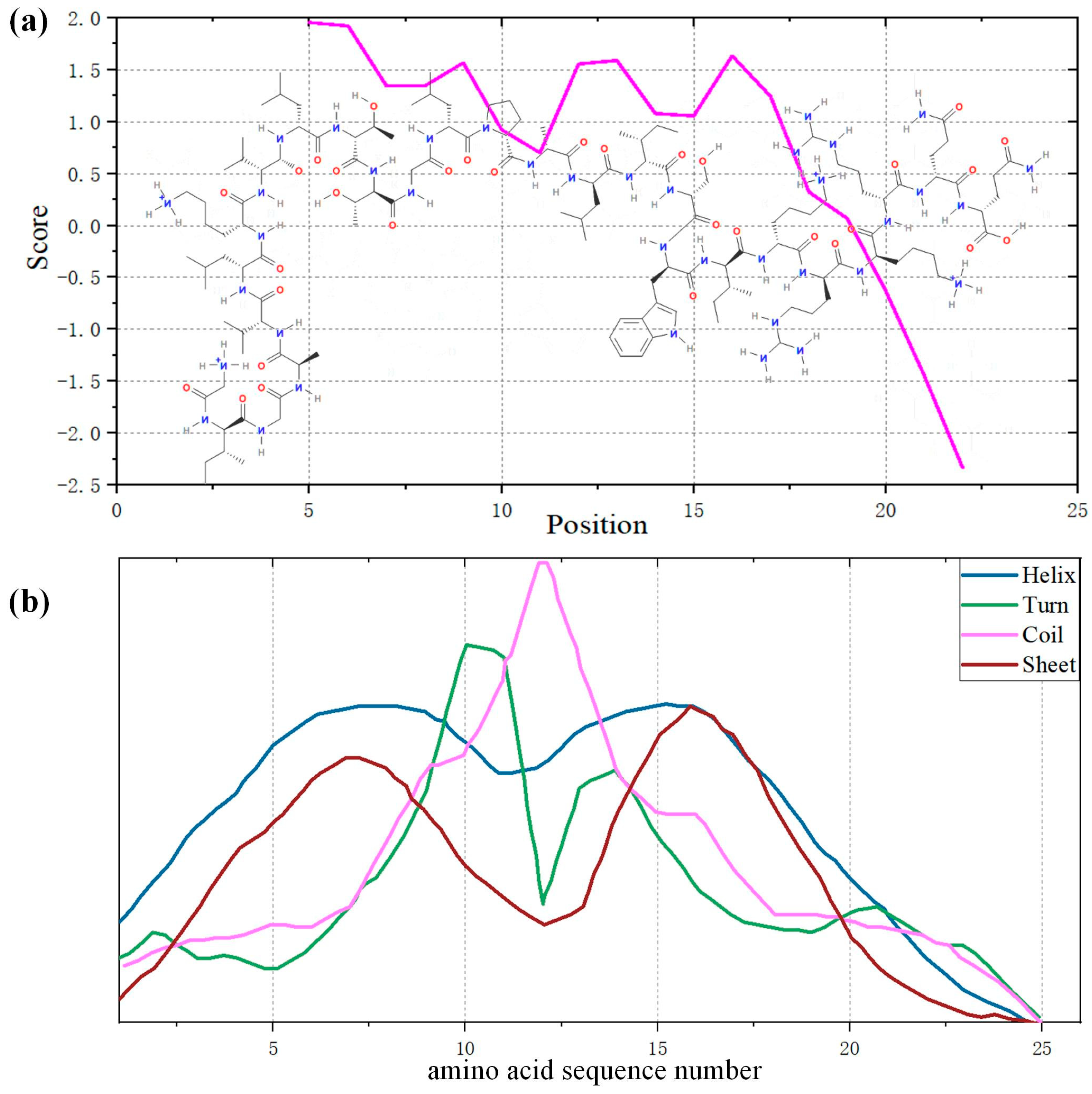
3.1. Analysis of Physicochemical Properties of Melittin
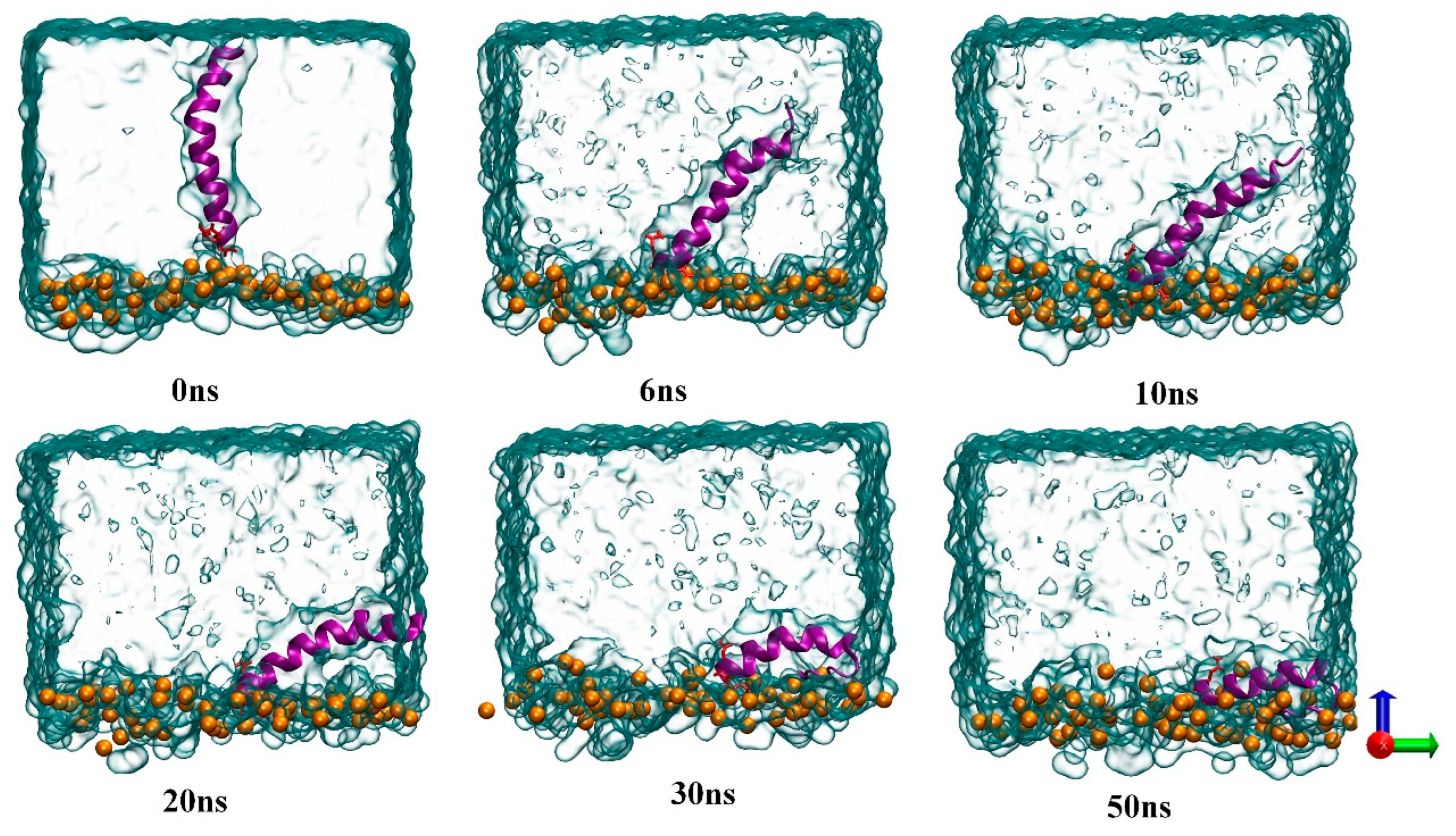
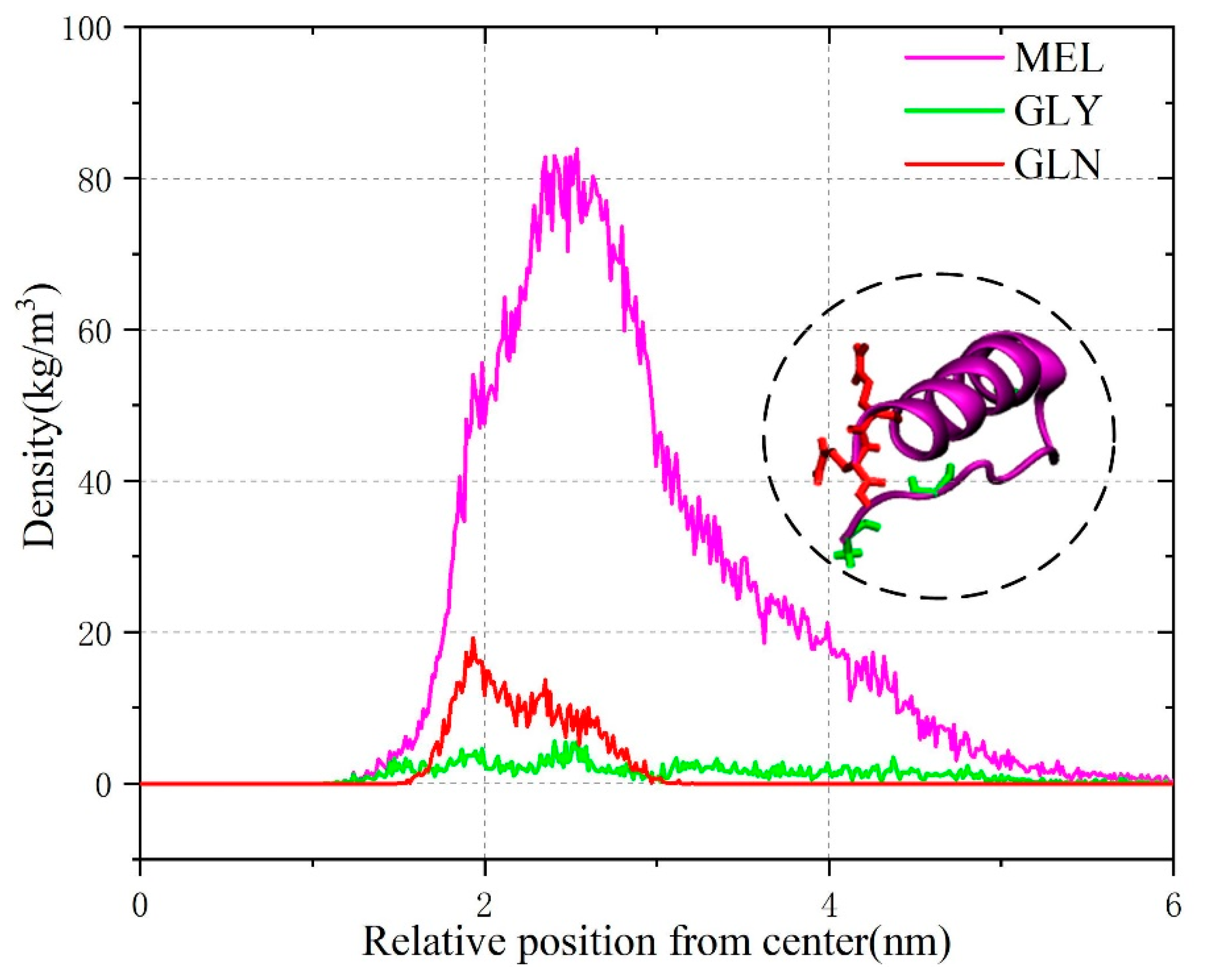
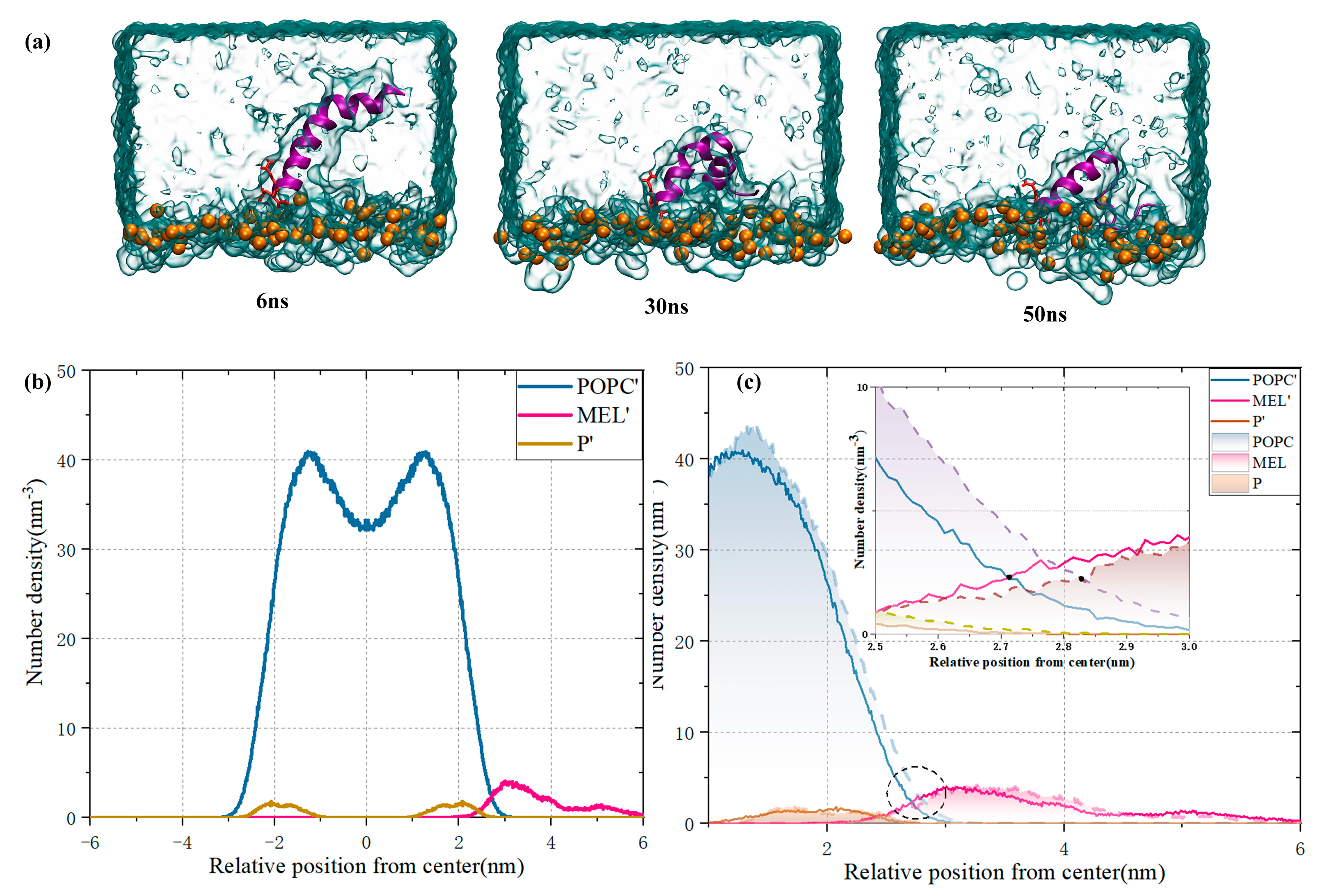
3.2. Membrane Lipid–Melittin Interaction
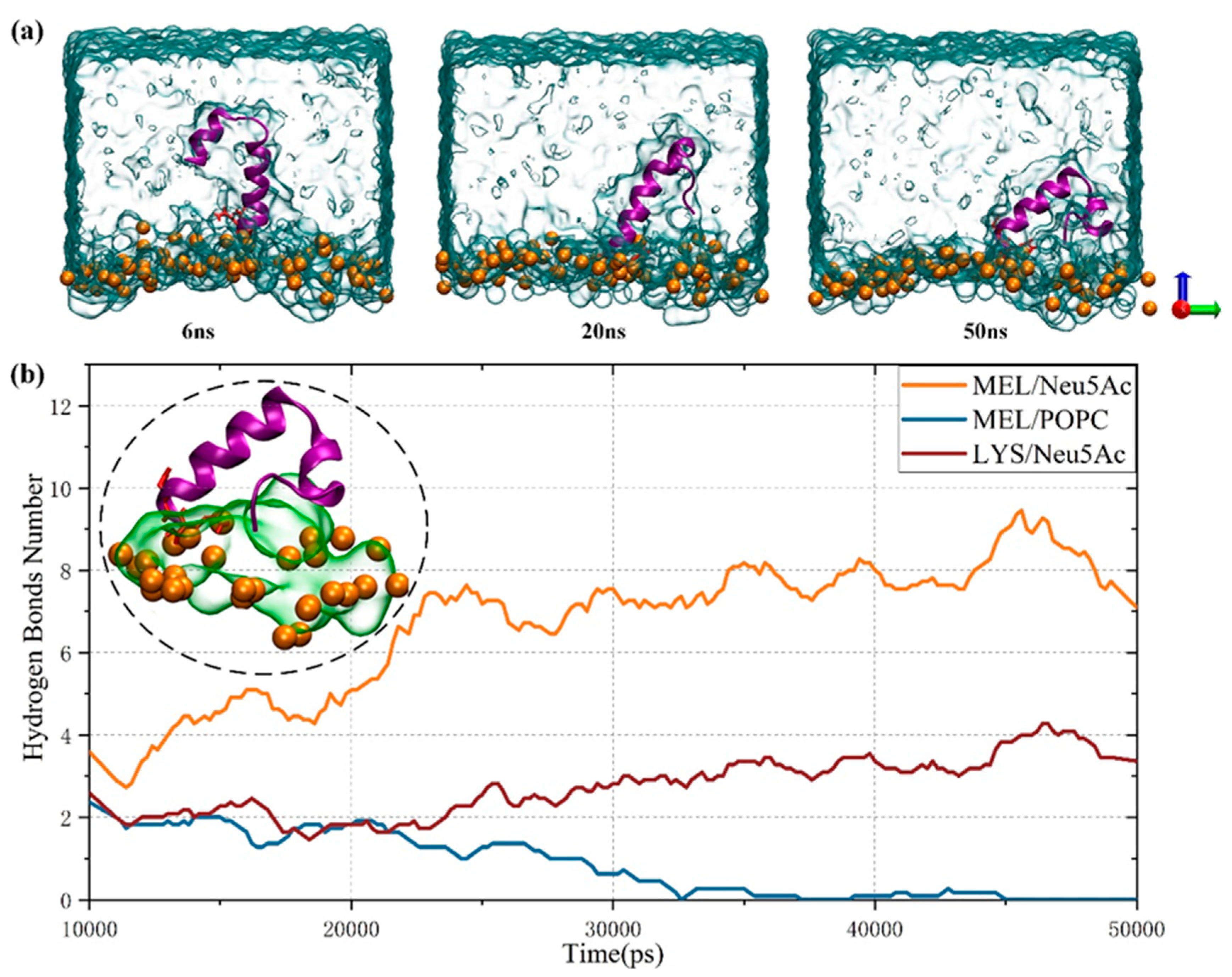
3.3. Effect of Sialic Acid on the Transmembrane Behavior of MEL
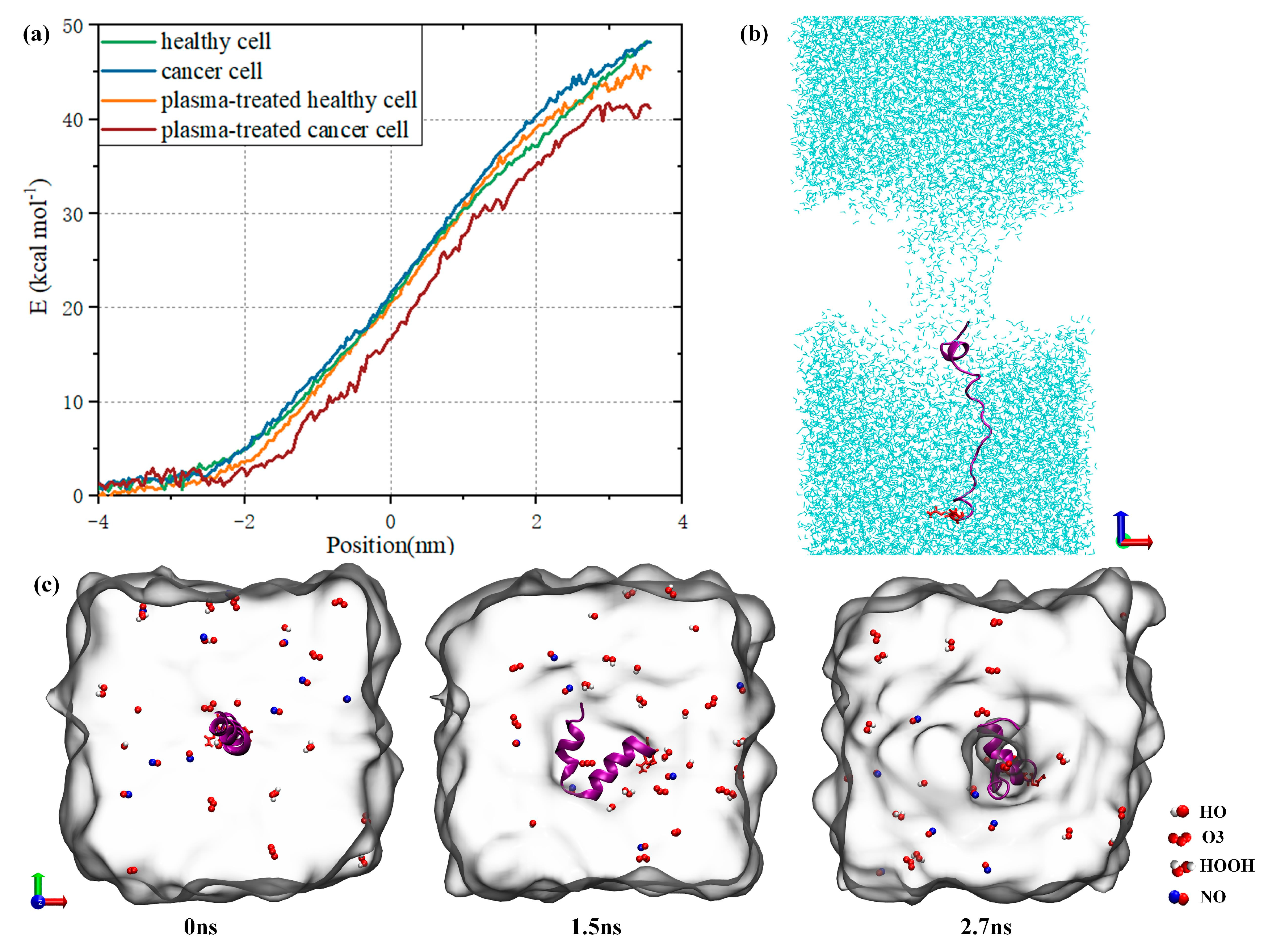
3.4. Synergistic Effect of Plasma and Melittin across Membranes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chauvin, J.; Judée, F.; Yousfi, M.; Vicendo, P.; Merbahi, N. Analysis of reactive oxygen and nitrogen species generated in three liquid media by low temperature helium plasma jet. Sci. Rep. 2017, 7, 4562. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys. Rep. 2016, 630, 1–84. [Google Scholar] [CrossRef]
- Weltmann, K.; Von Woedtke, T. Plasma medicine—Current state of research and medical application. Plasma Phys. Control. Fusion 2016, 59, 014031. [Google Scholar] [CrossRef]
- Attri, P.; Koga, K.; Okumura, T.; Chawarambwa, F.L.; Putri, T.E.; Tsukada, Y.; Kamataki, K.; Itagaki, N.; Shiratani, M. Treatment of organic wastewater by a combination of non-thermal plasma and catalyst: A review. Rev. Mod. Plasma Phys. 2022, 6, 17. [Google Scholar] [CrossRef]
- Dobslaw, D.; Ortlinghaus, O. Biological Waste Air and Waste Gas Treatment: Overview, Challenges, Operational Efficiency, and Current Trends. Sustainability 2020, 12, 8577. [Google Scholar] [CrossRef]
- Kyere-Yeboah, K.; Bique, I.K.; Qiao, X.C. Advances of non-thermal plasma discharge technology in degrading recalcitrant wastewater pollutants. A comprehensive review. Chemosphere 2023, 320, 138061. [Google Scholar] [CrossRef]
- Kumar, N.; Attri, P.; Dewilde, S.; Bogaerts, A. Inactivation of human pancreatic ductal adenocarcinoma with atmospheric plasma treated media and water: A comparative study. J. Phys. D Appl. Phys. 2018, 51, 255401. [Google Scholar] [CrossRef]
- Ratovitski, E.A.; Cheng, X.; Yan, D.; Sherman, J.H.; Canady, J.; Trink, B.; Keidar, M. Anti-cancer therapies of 21st century: Novel approach to treat human cancers using cold atmospheric plasma. Plasma Process. Polym. 2014, 11, 1128–1137. [Google Scholar] [CrossRef]
- Ghasemitarei, M.; Ghorbi, T.; Yusupov, M.; Zhang, Y.; Zhao, T.; Shali, P.; Bogaerts, A. Effects of Nitro-Oxidative Stress on Biomolecules: Part 1—Non-Reactive Molecular Dynamics Simulations. Biomolecules 2023, 13, 1371. [Google Scholar] [CrossRef]
- Iseki, S.; Nakamura, K.; Hayashi, M.; Tanaka, H.; Kondo, H.; Kajiyama, H.; Kano, H.; Kikkawa, F.; Hori, M. Selective killing of ovarian cancer cells through induction of apoptosis by nonequilibrium atmospheric pressure plasma. App. Phys. Lett. 2012, 100. [Google Scholar] [CrossRef]
- Boeckmann, L.; Schäfer, M.; Bernhardt, T.; Semmler, M.L.; Jung, O.; Ojak, G.; Fischer, T.; Peters, K.; Nebe, B.; Müller-Hilke, B.; et al. Cold atmospheric pressure plasma in wound healing and cancer treatment. App.Sci. 2020, 10, 6898. [Google Scholar] [CrossRef]
- Oršolić, N. Bee venom in cancer therapy. Cancer Metastasis Rev. 2012, 31, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Hansel, W.; Enright, F.; Leuschner, C. Destruction of Breast Cancers and Their Metastases by Lytic Peptide Conjugates in vitro and in vivo. Mol. Cell. Endocrinol. 2007, 260, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.F.; Chen, Z. Melittin exerts an antitumor effect on non-small cell lung cancer cells. Mol. Med. Rep. 2017, 16, 3581–3586. [Google Scholar] [CrossRef]
- Zhou, C.; Ma, J.; Lu, Y.; Zhao, W.; Xu, B.; Lin, J.; Ma, Y.; Tian, Y.; Zhang, Q.; Wang, W.; et al. TERT promoter regulating melittin expression induces apoptosis and G 0/G 1 cell cycle arrest in esophageal carcinoma cells. Oncol. Lett. 2021, 21, 1. [Google Scholar] [CrossRef]
- Saberwal, G.; Nagaraj, R. Cell-lytic and antibacterial peptides that act by perturbing the barrier function of membranes: Facets of their conformational features, structure-function correlations and membrane-perturbing abilities. Biochim. Biophys. Acta (BBA)-Rev. Biomembr. 1994, 1197, 109–131. [Google Scholar] [CrossRef]
- Mihajlovic, M.; Lazaridis, T. Antimicrobial peptides in toroidal and cylindrical pores. Biophys. J. 2010, 98, 281a. [Google Scholar] [CrossRef][Green Version]
- Rady, I.; Siddiqui, I.A.; Rady, M.; Mukhtar, H. Melittin, a major peptide component of bee venom, and its conjugates in cancer therapy. Cancer Lett. 2017, 402, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Kumar, N.; Hammerschmid, D.; Privat-Maldonado, A.; Dewilde, S.; Bogaerts, A. Synergistic effects of melittin and plasma treatment: A promising approach for cancer therapy. Cancers 2019, 11, 1109. [Google Scholar] [CrossRef]
- Varki, A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature 2007, 446, 1023–1029. [Google Scholar] [CrossRef]
- Gruszewska, E.; Chrostek, L.; Cylwik, B.; Tobolczyk, J.; Szmitkowski, M.; Kuklinski, A.; Kedra, B. Serum sialic acid as a marker of pancreatic cancers. Clin. Lab. 2013, 59, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Visser, E.A.; Moons, S.J.; Timmermans, S.B.; De Jong, H.; Boltje, T.J.; Büll, C. Sialic acid O-acetylation: From biosynthesis to roles in health and disease. J. Biol. Chem. 2021, 297, 100906. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ozhegov, E.; Wang, L.; Zhou, A.; Nie, H.; Li, Y.; Xue, L. Sialylation and desialylation dynamics of monocytes upon differentiation and polarization to macrophages. Glycoconj. J. 2016, 33. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, X.; Wang, L.; Wang, S.; Sun, X.L. Quantification of free sialic acid in human plasma through a robust quinoxalinone derivatization and LC–MS/MS using isotope-labeled standard calibration. J. Chromatogr. 2014, 944, 75–81. [Google Scholar] [CrossRef][Green Version]
- Yusupov, M.; Privat-Maldonado, A.; Cordeiro, R.M.; Verswyvel, H.; Shaw, P.; Razzokov, J.; Smits, E.; Bogaerts, A. Oxidative damage to hyaluronan–CD44 interactions as an underlying mechanism of action of oxidative stress-inducing cancer therapy. Redox Biol. 2021, 43, 101968. [Google Scholar] [CrossRef]
- Cordeiro, R.M. Reactive oxygen species at phospholipid bilayers: Distribution, mobility and permeation. Biochim. Biophys. Acta (BBA)-Biomembr. 2014, 1838, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. Packmol: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Macyk, W.; Franke, A.; Stochel, G. Metal compounds and small molecules activation–case studies. Coord. Chem. Rev. 2005, 249, 2437–2457. [Google Scholar] [CrossRef]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. App. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. Software 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Isralewitz, B. Timeline: A VMD plugin for trajectory analysis. Mars 2011. Available online: https://www.ks.uiuc.edu/Training/TutorialsOverview/science/timeline/tutorial_timeline.pdf (accessed on 27 August 2024).
- Originpro, V. OriginLab Corporation. Northampton, MA, USA, 2016. Available online: https://www.originlab.com/index.aspx?go=SUPPORT/Training&pid=2052 (accessed on 26 August 2024).
- Irudayam, S.J.; Berkowitz, M.L. Binding and reorientation of melittin in a POPC bilayer: Computer simulations. Biochim. Biophys. Acta (BBA)-Biomembr. 2012, 1818, 2975–2981. [Google Scholar] [CrossRef][Green Version]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, H.; Ioannidis, V.; Durinx, C. Expasy, the Swiss Bioinformatics Resource Portal, as designed by its users. Nucleic Acids Res. 2021, 49, W216–W227. [Google Scholar] [CrossRef] [PubMed]
- Manna, M.; Mukhopadhyay, C. Cause and effect of melittin-induced pore formation: A computational approach. Langmuir 2009, 25, 12235–12242. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Alexander-Katz, A. Cell membranes open “doors” for cationic nanoparticles/biomolecules: Insights into uptake kinetics. ACS Nano 2013, 7, 10799–10808. [Google Scholar] [CrossRef]
- Lu, X.; Xiong, Z.; Zhao, F.; Xian, Y.; Xiong, Q.; Gong, W.; Zou, C.; Jiang, Z.; Pan, Y. A simple atmospheric pressure room-temperature air plasma needle. Device Biomed. Appl. 2009, 95. [Google Scholar] [CrossRef]

| Name | Symbol | Properties |
|---|---|---|
| Glycine (Gly) | G | I/M |
| Isoleucine (Ile) | I | O/M |
| Alanine (Ala) | A | O/M |
| Valine (Val) | V | O/M |
| Leucine (Leu) | L | O/M |
| Lysine (Lys) | K | I/l+ |
| Proline (Pro) | P | O/M |
| Threonine (Thr) | T | I/M |
| Serine (Ser) | S | I/M |
| Tryptophan (Trp) | W | O/M |
| Arginine (Arg) | R | I/l+ |
| Glutamine (Gln) | Q | I/M |
| α-Helix | β-Turn | Random Coil | Extension Chain |
|---|---|---|---|
| 53.85% | 26.92% | 11.54% | 7.69% |
| Parameter | Healthy Cell | Cancer Cell |
|---|---|---|
| Surface tension/nN × m−1 | 5.453784 (1.679062) | 5.464252 (1.59166) |
| Membrane area/nm2 | 39.67764 (39.66392) | 40.83215 (39.86161) |
| Membrane thickness/nm | 3.91129 (3.99502) | 3.93175 (4.00525) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Zhao, T.; Niu, Y.; Wang, X.; Zhang, Y. Determining the Effect of Non-Thermal Plasma on the Transmembrane Kinetics of Melittin through Molecular Explorations. Biomolecules 2024, 14, 1207. https://doi.org/10.3390/biom14101207
Cui Y, Zhao T, Niu Y, Wang X, Zhang Y. Determining the Effect of Non-Thermal Plasma on the Transmembrane Kinetics of Melittin through Molecular Explorations. Biomolecules. 2024; 14(10):1207. https://doi.org/10.3390/biom14101207
Chicago/Turabian StyleCui, Yanxiu, Tong Zhao, Yanxiong Niu, Xiaolong Wang, and Yuantao Zhang. 2024. "Determining the Effect of Non-Thermal Plasma on the Transmembrane Kinetics of Melittin through Molecular Explorations" Biomolecules 14, no. 10: 1207. https://doi.org/10.3390/biom14101207
APA StyleCui, Y., Zhao, T., Niu, Y., Wang, X., & Zhang, Y. (2024). Determining the Effect of Non-Thermal Plasma on the Transmembrane Kinetics of Melittin through Molecular Explorations. Biomolecules, 14(10), 1207. https://doi.org/10.3390/biom14101207






