Endemic Radiation of African Moonfish, Selene dorsalis (Gill 1863), in the Eastern Atlantic: Mitogenomic Characterization and Phylogenetic Implications of Carangids (Teleostei: Carangiformes)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection, Identification, and Preservation
2.2. Genomic DNA Extraction and Partial Gene Sequencing
2.3. Mitogenome Sequencing and Assembly
2.4. Characterization and Comparative Analyses
2.5. Dataset Preparation and Phylogenetic Analyses
3. Results and Discussion
3.1. Mitogenome Structure and Organization
3.2. Protein-Coding Genes
3.3. Codon Usage and Substitution Pattern
3.4. Ribosomal RNA and Transfer RNA
3.5. Control Regions
3.6. Phylogenetic Relationship of Carangidae
3.7. Lineage Diversification of Selene Species
3.8. Conservation Implication of Selene Species in the Eastern Atlantic Ocean
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boore, J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999, 27, 767–1780. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.; Bankier, A.T.; Barrell, B.G.; de Bruijn, M.H.; Coulson, A.R.; Drouin, J.; Eperon, I.C.; Nierlich, D.P.; Roe, B.A.; Sanger, F.; et al. Sequence and organization of the human mitochondrial genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Gissi, C.; Iannelli, F.; Pesole, G. Evolution of the mitochondrial genome of Metazoa as exemplified by comparison of congeneric species. Heredity 2008, 101, 301–320. [Google Scholar] [CrossRef] [PubMed]
- Dowling, D.K.; Wolff, J.N. Evolutionary genetics of the mitochondrial genome: Insights from Drosophila. Genetics 2023, 224, iyad036. [Google Scholar] [CrossRef]
- Bielawski, J.P.; Gold, J.R. Mutation Patterns of Mitochondrial H- and L-Strand DNA in Closely Related Cyprinid Fishes. Genetics 2002, 161, 1589–1597. [Google Scholar] [CrossRef]
- Rubinoff, D. Utility of mitochondrial DNA barcodes in species conservation. Conserv. Biol. 2006, 20, 1026–1033. [Google Scholar] [CrossRef]
- Tang, M.; Tan, M.; Meng, G.; Yang, S.; Su, X.; Liu, S.; Song, W.; Li, Y.; Wu, Q.; Zhang, A.; et al. Multiplex sequencing of pooled mitochondrial genomes—A crucial step toward biodiversity analysis using mito-metagenomics. Nucleic Acids Res. 2014, 42, e166. [Google Scholar] [CrossRef]
- Miya, M.; Takeshima, H.; Endo, H.; Ishiguro, N.B.; Inoue, J.G.; Mukai, T.; Satoh, T.P.; Yamaguchi, M.; Kawaguchi, A.; Mabuchi, K.; et al. Major patterns of higher teleostean phylogenies: A new perspective based on 100 complete mitochondrial DNA sequences. Mol. Phylogenet. Evol. 2003, 26, 121–138. [Google Scholar] [CrossRef]
- Amponsah, S.K.K.; Avornyo, S.Y.; Ofori-Danson, P.; Afranewaa, N.A.B. Assessing the population characteristics of Carangids in the coast of Ghana, West Africa. Res. Mar. Sci. 2021, 6, 965–976. [Google Scholar]
- Reuben, S.; Kasim, H.; Sivakami, S.; Radhakrishnan, P.N.; Kurup, K.N.; Sivadas, M.; Noble, A.; Nair, K.V.S.; Raje, S.G. Fishery, biology and stock assessment of carangid resources from the Indian seas. Indian J. Fish. 1992, 39, 195–234. [Google Scholar]
- Fricke, R.; Eschmeyer, W.N.; Van der Laan, R. (Eds.) Eschmeyer’s Catalog of Fishes: Genera, Species. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 11 July 2024).
- Vella, P.; Deidun, A. First record of Selene dorsalis (Osteichthyes: Carangidae) in the Mediterranean Sea, from coastal waters off the Maltese Islands. Mar. Biodivers. Rec. 2009, 2, e125. [Google Scholar] [CrossRef]
- Efe, O.; John, A.; Omoariagbon, B. Some aspects of biology of Selene dorsalis from Forcados River estuary. Res. Mar. Sci. 2019, 4, 15–16. [Google Scholar]
- Reed, D.L.; deGravelle, M.J.; Carpenter, K.E. Molecular systematics of Selene (Perciformes: Carangidae) based on cytochrome B sequences. Mol. Phylogenet. Evol. 2001, 21, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Castro-Hernández, J.J. Primera cita de Selene dorsalis (Gill; 1862) (Osteichthyes: Carangidae) en las islas Canarias (Atlántico centro-oriental). Bol. Inst. Esp. Oceanogr 2001, 17, 333–335. [Google Scholar]
- Juárez, A.; Silva, L.; Gill, J. First Record of Selene dorsalis (Osteichthyes: Carangidae) in the Spanish Waters of the Gulf of Cádiz (ICES Division IXa South). Mar. Biodivers. Rec. 2008, 1, e28. [Google Scholar] [CrossRef]
- Kovačić, M.; Lipej, L.; Dulčić, J.; Iglesias, S.P.; Goren, M. Evidence-based checklist of the Mediterranean Sea fishes. Zootaxa 2021, 4998, 1–115. [Google Scholar] [CrossRef]
- Tsagarakis, K.; Agius Darmanin, S.; Al Mabruk, S.A.; Auriemma, R.; Azzurro, E.; Badouvas, N.; Bakiu, R.; Bariche, M.; Battaglia, P.; Betti, F.; et al. New records of rare species in the Mediterranean Sea (October 2021). Mediterr. Mar. Sci. 2021, 22, 627–652. [Google Scholar] [CrossRef]
- Odekunle, T.O.; Eludoyin, A.O. Sea surface temperature patterns in the Gulf of Guinea: Their implications for the spatio-temporal variability of precipitation in West Africa. Int. J. Climatol. 2008, 28, 1507–1517. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2024-1. Available online: https://www.iucnredlist.org (accessed on 11 July 2024).
- Arra, S.; Sylla, S.; Kouame, A.C.; Zan-BI, T.T.; Ouattara, M. Reproductive biology of the African moonfish, Selene dorsalis (Gill, 1862) (Carangidae) in continental shelf of Côte d’Ivoire fishery (West Africa). Int. J. Fish. Aquat. Stud. 2018, 6, 358–363. [Google Scholar]
- Biro, P.A.; Post, J.R.; Booth, D.J. Mechanisms for climate-induced mortality of fish populations in whole-lake experiments. Proc. Natl. Acad. Sci. USA 2007, 104, 9715–9719. [Google Scholar] [CrossRef]
- Watanabe, Y.Y.; Goldman, K.J.; Caselle, J.E.; Chapman, D.D.; Papastamatiou, Y.P. Comparative analyses of animal-tracking data reveal ecological significance of endothermy in fishes. Proc. Natl. Acad. Sci. USA 2015, 112, 6104–6109. [Google Scholar] [CrossRef] [PubMed]
- Moritz, C. Applications of mitochondrial DNA analysis in conservation: A critical review. Mol. Ecol. 1994, 3, 401–411. [Google Scholar] [CrossRef]
- Kimura, S.; Takeuchi, S.; Yadome, T. Generic revision of the species formerly belonging to the genus Carangoides and its related genera (Carangiformes: Carangidae). Ichthyol. Res. 2022, 69, 433–487. [Google Scholar] [CrossRef]
- Li, B.; Dettaï, A.; Cruaud, C.; Couloux, A.; Desoutter-Meniger, M.; Lecointre, G. RNF213, a new nuclear marker for acanthomorph phylogeny. Mol. Phylogenet. Evol. 2009, 50, 345–363. [Google Scholar] [CrossRef] [PubMed]
- Damerau, M.; Freese, M.; Hanel, R. Multi-gene phylogeny of jacks and pompanos (Carangidae), including placement of monotypic vadigo Campogramma glaycos. J. Fish Biol. 2018, 92, 190–202. [Google Scholar] [CrossRef]
- Yancy, H.F.; Zemlak, T.S.; Mason, J.A.; Washington, J.D.; Tenge, B.J.; Nguyen, N.L.; Barnett, J.D.; Savary, W.E.; Hill, W.E.; Moore, M.M.; et al. Potential use of DNA barcodes in regulatory science: Applications of the Regulatory Fish Encyclopedia. J. Food Prot. 2008, 71, 210–217. [Google Scholar] [CrossRef]
- Steinke, D.; Zemlak, T.S.; Hebert, P.D.N. Barcoding nemo: DNA-based identifications for the ornamental fish trade. PLoS ONE 2009, 4, e6300. [Google Scholar] [CrossRef]
- Valdez-Moreno, M.; Vásquez-Yeomans, L.; Elías-Gutiérrez, M.; Ivanova, N.V.; Hebert, P.D.N. Using DNA barcodes to connect adults and early life stages of marine fishes from the Yucatan Peninsula, Mexico: Potential in fisheries management. Mar. Freshw. Res. 2010, 61, 665–671. [Google Scholar] [CrossRef]
- Ribeiro, A.O.; Caires, R.A.; Mariguela, T.C.; Pereira, L.H.; Hanner, R.; Oliveira, C. DNA barcodes identify marine fishes of São Paulo State, Brazil. Mol. Ecol. Resour. 2012, 12, 1012–1020. [Google Scholar] [CrossRef]
- Weigt, L.A.; Baldwin, C.C.; Driskell, A.; Smith, D.G.; Ormos, A.; Reyier, E.A. Using DNA barcoding to assess Caribbean reef fish biodiversity: Expanding taxonomic and geographic coverage. PLoS ONE 2012, 7, e41059. [Google Scholar] [CrossRef]
- McCusker, M.R.; Denti, D.; Van Guelpen, L.; Kenchington, E.; Bentzen, P. Barcoding Atlantic Canada’s commonly encountered marine fishes. Mol. Ecol. Resour. 2013, 13, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Betancur-R, R.; Broughton, R.E.; Wiley, E.O.; Carpenter, K.; López, J.A.; Li, C.; Holcroft, N.I.; Arcila, D.; Sanciangco, M.; Cureton, J.C., II; et al. The tree of life and a new classification of bony fishes. PLoS Curr. 2013, 5, ecurrents.tol.53ba26640df0ccaee75bb165c8c26288. [Google Scholar] [CrossRef] [PubMed]
- Near, T.J.; Dornburg, A.; Eytan, R.I.; Keck, B.P.; Smith, W.L.; Kuhn, K.L.; Moore, J.A.; Price, S.A.; Burbrink, F.T.; Friedman, M.; et al. Phylogeny and tempo of diversification in the superradiation of spiny-rayed fishes. Proc. Natl. Acad. Sci. USA 2013, 110, 12738–12743. [Google Scholar] [CrossRef] [PubMed]
- Sanciangco, M.D.; Carpenter, K.E.; Betancur-R, R. Phylogenetic placement of enigmatic percomorph families (Teleostei: Percomorphaceae). Mol. Phylogenet. Evol. 2016, 94, 565–576. [Google Scholar] [CrossRef]
- Deeds, J.R.; Handy, S.M.; Fry, F., Jr.; Granade, H.; Williams, J.T.; Powers, M.; Shipp, R.; Weigt, L.A. Protocol for building a reference standard sequence library for DNA-based seafood identification. J. AOAC Int. 2014, 97, 1626–1633. [Google Scholar] [CrossRef]
- Miya, M.; Sato, Y.; Fukunaga, T.; Sado, T.; Poulsen, J.Y.; Sato, K.; Minamoto, T.; Yamamoto, S.; Yamanaka, H.; Araki, H.; et al. MiFish, a set of universal PCR primers for metabarcoding environmental DNA from fishes: Detection of more than 230 subtropical marine species. R. Soc. Open Sci. 2015, 2, 150088. [Google Scholar] [CrossRef]
- Aguilar, R.; Ogburn, M.B.; Driskell, A.C.; Weigt, L.A.; Groves, M.C.; Hines, A.H. Gutsy genetics: Identification of digested piscine prey items in the stomach contents of sympatric native and introduced warm water catfishes via DNA barcoding. Environ. Biol. Fishes 2017, 100, 325–336. [Google Scholar] [CrossRef]
- Stoeckle, M.Y.; Das Mishu, M.; Charlop-Powers, Z. GoFish: A versatile nested PCR strategy for environmental DNA assays for marine vertebrates. PLoS ONE 2018, 13, e0198717. [Google Scholar] [CrossRef]
- Sarmiento-Camacho, S.; Valdez-Moreno, M. DNA barcode identification of commercial fish sold in Mexican markets. Genome 2018, 61, 457–466. [Google Scholar] [CrossRef]
- Gold, Z.; Curd, E.E.; Goodwin, K.D.; Choi, E.S.; Frable, B.W.; Thompson, A.R.; Walker, H.J., Jr.; Burton, R.S.; Kacev, D.; Martz, L.D.; et al. Improving metabarcoding taxonomic assignment: A case study of fishes in a large marine ecosystem. Mol. Ecol. Resour. 2021, 21, 2546–2564. [Google Scholar] [CrossRef]
- Díaz-Ferguson, E.; Chial, M.; Gonzalez, M.; Muñoz, E.; Chen, O.; Durán, O.; Vega, A.J.; Delgado, C.R. Building a teleost fish traceability program based on genetic data from Pacific Panama fish markets. Animals 2023, 13, 2272. [Google Scholar] [CrossRef] [PubMed]
- Hoban, M.L.; Whitney, J.; Collins, A.G.; Meyer, C.; Murphy, K.R.; Reft, A.J.; Bemis, K.E. Skimming for barcodes: Rapid production of mitochondrial genome and nuclear ribosomal repeat reference markers through shallow shotgun sequencing. PeerJ 2022, 10, e13790. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, M.; D’Elia, A.K.P.; Rocha, G.; Arantes, C.A.; Henning, F.; de Vasconcelos, A.T.R.; Solé-Cava, A.M. Mitochondrial genome structure and composition in 70 fishes: A key resource for fisheries management in the South Atlantic. BMC Genom. 2024, 25, 215. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.P.; Miya, M.; Mabuchi, K.; Nishida, M. Structure and variation of the mitochondrial genome of fishes. BMC Genom. 2016, 17, 719. [Google Scholar] [CrossRef] [PubMed]
- Molina-Quirós, J.L.; Hernández-Muñoz, S.; Baeza, J.A. The complete mitochondrial genome of the roosterfish Nematistius pectoralis Gill 1862: Purifying selection in protein-coding genes, organization of the control region, and insights into family-level phylogenomic relationships in the recently erected order Carangiformes. Gene 2022, 845, 146847. [Google Scholar] [PubMed]
- Kundu, S.; Kang, H.-E.; Kim, A.R.; Lee, S.R.; Kim, E.-B.; Amin, M.H.F.; Andriyono, S.; Kim, H.-W.; Kang, K. Mitogenomic characterization and phylogenetic placement of African Hind, Cephalopholis taeniops: Shedding light on the evolution of groupers (Serranidae: Epinephelinae). Int. J. Mol. Sci. 2024, 25, 1822. [Google Scholar] [CrossRef]
- Wirtz, P.; Brito, A.; Falcón, J.M.; Freitas, R.; Fricke, R.; Monteiro, V.; Reiner, F.; Tariche, O. The coastal fishes of the Cape Verde Islands–new records and an annotated checklist. Spixiana 2013, 36, 113–142. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef]
- Iwasaki, W.; Fukunaga, T.; Isagozawa, R.; Yamada, K.; Maeda, Y.; Satoh, T.P.; Sado, T.; Mabuchi, K.; Takeshima, H.; Miya, M.; et al. MitoFish and MitoAnnotator: A mitochondrial genome database of fish with an accurate and automatic annotation pipeline. Mol. Biol. Evol. 2013, 30, 2531–2540. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Perna, N.T.; Kocher, T.D. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J. Mol. Evol. 1995, 41, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Xia, X. DAMBE6: New tools for microbial genomics, phylogenetics, and molecular evolution. J. Hered. 2017, 108, 431–437. [Google Scholar] [CrossRef]
- Laslett, D.; Canbäck, B. ARWEN: A program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics 2008, 24, 172–175. [Google Scholar] [CrossRef]
- Chan, P.P.; Lowe, T.M. GtRNAdb 2.0: An expanded database of transfer RNA genes identified in complete and draft genomes. Nucleic Acids Res. 2016, 44, D184–D189. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Kundu, S.; Kim, H.W.; Lee, J.; Chung, S.; Lee, S.R.; Gietbong, F.Z.; Kang, K. Mitogenomic architecture and phylogenetic relationship of European barracuda, Sphyraena sphyraena (Teleostei: Sphyraenidae) from the Atlantic Ocean. Fishes 2023, 8, 573. [Google Scholar] [CrossRef]
- Santini, F.; Carnevale, G. First multilocus and densely sampled timetree of trevallies, pompanos and allies (Carangoidei, Percomorpha) suggests a Cretaceous origin and Eocene radiation of a major clade of piscivores. Mol. Phylogenet. Evol. 2015, 83, 33–39. [Google Scholar] [CrossRef]
- Glass, J.R.; Harrington, R.C.; Cowman, P.F.; Faircloth, B.C.; Near, T.J. Widespread sympatry in a species-rich clade of marine fishes (Carangoidei). Proc. Biol. Sci. 2023, 290, 20230657. [Google Scholar] [CrossRef] [PubMed]
- Vences, M.; Miralles, A.; Brouillet, S.; Ducasse, J.; Fedosov, A.; Kharchev, V.; Renner, S.S. iTaxoTools 0.1: Kickstarting a specimen-based software toolkit for taxonomists. Megataxa 2021, 6, 77–92. [Google Scholar] [CrossRef]
- Miller, M.A.; Schwartz, T.; Pickett, B.E.; He, S.; Klem, E.B.; Scheuermann, R.H.; Passarotti, M.; Kaufman, S.; O’Leary, M.A. A RESTful API for access to phylogenetic tools via the CIPRES Science Gateway. Evol. Bioinform. 2015, 11, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL): An online tool for phylogenetic tree display and annotation. Bioinformatics 2007, 23, 127–128. [Google Scholar] [CrossRef]
- Brown, K.H. Fish mitochondrial genomics: Sequence, inheritance and functional variation. J. Fish Biol. 2008, 72, 355–374. [Google Scholar] [CrossRef]
- Ojala, D.; Montoya, J.; Attardi, G. tRNA punctuation model of RNA processing in human mitochondria. Nature 1981, 290, 470–474. [Google Scholar] [CrossRef]
- Consuegra, S.; John, E.; Verspoor, E.; de Leaniz, C.G. Patterns of natural selection acting on the mitochondrial genome of a locally adapted fish species. Genet. Sel. Evol. 2015, 47, 58. [Google Scholar] [CrossRef]
- Hill, J.; Enbody, E.D.; Pettersson, M.E.; Sprehn, C.G.; Bekkevold, D.; Folkvord, A.; Andersson, L. Recurrent convergent evolution at amino acid residue 261 in fish rhodopsin. Proc. Natl. Acad. Sci. USA 2019, 116, 18473–18478. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, M.; Xu, S.; Liu, L.; Chen, Z.; Zou, K. Complete mitogenomes of three Carangidae (Perciformes) fishes: Genome description and phylogenetic considerations. Int. J. Mol. Sci. 2020, 21, 4685. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Nielsen, R.; Goldman, N.; Pedersen, A.M.K. Codon-substitution models for heterogeneous selection pressure at amino acid sites. Genetics 2000, 155, 431–449. [Google Scholar] [CrossRef]
- Elmer, K.R.; Fan, S.; Gunter, H.M.; Jones, J.C.; Boekhoff, S.; Kuraku, S.; Meyer, A. Rapid evolution and selection inferred from the transcriptomes of sympatric crater lake cichlid fishes. Mol. Ecol. 2010, 19, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Rbbani, G.; Nedoluzhko, A.; Galindo-Villegas, J.; Fernandes, J.M. Function of circular RNAs in fish and their potential application as biomarkers. Int. J. Mol. Sci. 2021, 22, 7119. [Google Scholar] [CrossRef] [PubMed]
- Quraishia, S.F.; Panneerchelvam, S.; Zainuddin, Z.; Abd Rashid, N.H. Molecular characterization of Malaysian marine fish species using partial sequence of mitochondrial DNA 12S and 16S rRNA markers. Sains Malays. 2015, 44, 1119–1123. [Google Scholar] [CrossRef]
- Thornlow, B.P.; Hough, J.; Roger, J.M.; Gong, H.; Lowe, T.M.; Corbett-Detig, R.B. Transfer RNA genes experience exceptionally elevated mutation rates. Proc. Natl. Acad. Sci. USA 2018, 115, 8996–9001. [Google Scholar] [CrossRef]
- Cantatore, P.; Gadaleta, M.N.; Roberti, M.; Saccone, C.; Wilson, A.C. Duplication and remoulding of tRNA genes during the evolutionary rearrangement of mitochondrial genomes. Nature 1987, 329, 853–855. [Google Scholar] [CrossRef]
- Kundu, S.; Binarao, J.D.; De Alwis, P.S.; Kim, A.R.; Lee, S.R.; Andriyono, S.; Kim, H.W. First mitogenome of endangered Enteromius thysi (Actinopterygii: Cypriniformes: Cyprinidae) from Africa: Characterization and phylogeny. Fishes 2022, 8, 25. [Google Scholar] [CrossRef]
- Taanman, J.W. The mitochondrial genome: Structure, transcription, translation and replication. Biochim. Biophys. Acta 1999, 1410, 103–123. [Google Scholar] [CrossRef]
- Jose, A.; Sukumaran, S.; Mukundan, L.P.; Raj, N.; Mary, S.; Nisha, K.; Gopalakrishnan, A. Comparative mitogenomics and phylogenetics of the family Carangidae with special emphasis on the mitogenome of the Indian Scad Decapterus russelli. Sci. Rep. 2022, 12, 5642. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, H.; Yang, L.; Liu, S.; Zhuang, Z. Complete mitochondrial genome of Pseudocaranx dentex (Carangidae, Perciformes) provides insight into phylogenetic and evolutionary relationship among Carangidae family. Genes 2021, 12, 1234. [Google Scholar] [CrossRef] [PubMed]
- Swart, B.L.; von der Heyden, S.; Bester-van der Merwe, A.; Roodt-Wilding, R. Molecular systematics and biogeography of the circumglobally distributed genus Seriola (Pisces: Carangidae). Mol. Phylogenet. Evol. 2015, 93, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Leprieur, F.; Descombes, P.; Gaboriau, T.; Cowman, P.F.; Parravicini, V.; Kulbicki, M.; Melián, C.J.; de Santana, C.N.; Heine, C.; Mouillot, D.; et al. Plate tectonics drive tropical reef biodiversity dynamics. Nat. Commun. 2016, 7, 11461. [Google Scholar] [CrossRef]
- Lepprieur, F.; Pellissier, L.; Mouillot, D.; Gaboriau, T. Influence of historical changes in tropical reef habitat on the diversification of coral reef fishes. Sci. Rep. 2021, 11, 20731. [Google Scholar] [CrossRef] [PubMed]
- Caley, M.J.; Carr, M.H.; Hixon, M.A.; Hughes, T.P.; Jones, G.P.; Menge, B.A. Recruitment and the local dynamics of open marine populations. Annu. Rev. Ecol. Syst. 1996, 27, 477–500. [Google Scholar] [CrossRef]
- Harrison, T.D.; Whitfield, A.K. Temperature and salinity as primary determinants influencing the biogeography of fishes in South African estuaries. Estuar. Coast. Shelf Sci. 2006, 66, 335–345. [Google Scholar] [CrossRef]
- Dahms, C.; Killen, S. Temperature change effects on marine fish range shifts: A meta-analysis of ecological and methodological predictors. Glob. Chang. Biol. 2023, 29, 4459–4479. [Google Scholar] [CrossRef]
- Brierley, A.S.; Kingsford, M.J. Impacts of climate change on marine organisms and ecosystems. Curr. Biol. 2009, 19, R602–R614. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Bruno, J.F. The impact of climate change on the world’s marine ecosystems. Science 2010, 328, 1523–1528. [Google Scholar] [CrossRef]
- Mat Jaafar, T.N.; Taylor, M.I.; Mohd Nor, S.A.; Bruyn, M.D.; Carvalho, G.R. Comparative genetic stock structure in three species of commercially exploited Indo-Malay Carangidae (Teleosteii, Perciformes). J. Fish Biol. 2020, 96, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Andaloro, F.; Falautano, M.; Sinopoli, M.; Passarelli, F.M.; Pipitone, C.; Addis, P.; Castriota, L. The Lesser Amberjack Seriola fasciata (Perciformes: Carangidae) in the Mediterranean: A recent colonist. Cybium 2005, 29, 141–145. [Google Scholar]
- Di Blasi, D.; Bava, S.; Desiderà, E.; Merotto, L.; Poli, F.; Guidetti, P. The northernmost records of Caranx crysos (Osteichthyes: Carangidae) in the NW Mediterranean Sea. Thalassas 2024, 40, 7–11. [Google Scholar] [CrossRef]
- Polidoro, B.A.; Ralph, G.M.; Strongin, K.; Harvey, M.; Carpenter, K.E.; Arnold, R.; Williams, A. The status of marine biodiversity in the Eastern Central Atlantic (West and Central Africa). Aquat. Conserv. 2017, 27, 1021–1034. [Google Scholar] [CrossRef]
- Takyi, R.; Addo, C.; El Mahrad, B.; Adade, R.; ElHadary, M.; Nunoo, F.K.E.; Essandoh, J.; Chuku, E.O.; Iriarte-Ahon, F. Marine fisheries management in the Eastern Central Atlantic Ocean. Ocean Coast. Manag. 2023, 244, 106784. [Google Scholar] [CrossRef]
- Nunoo, F.K.; Asiedu, B. An investigation of fish catch data and its implications for management of small-scale fisheries of Ghana. Int. J. Fish. Aquat. Sci. 2013, 2, 46–57. [Google Scholar]
- Teletchea, F. Molecular identification methods of fish species: Reassessment and possible applications. Rev. Fish Biol. Fish. 2009, 19, 265–293. [Google Scholar] [CrossRef]

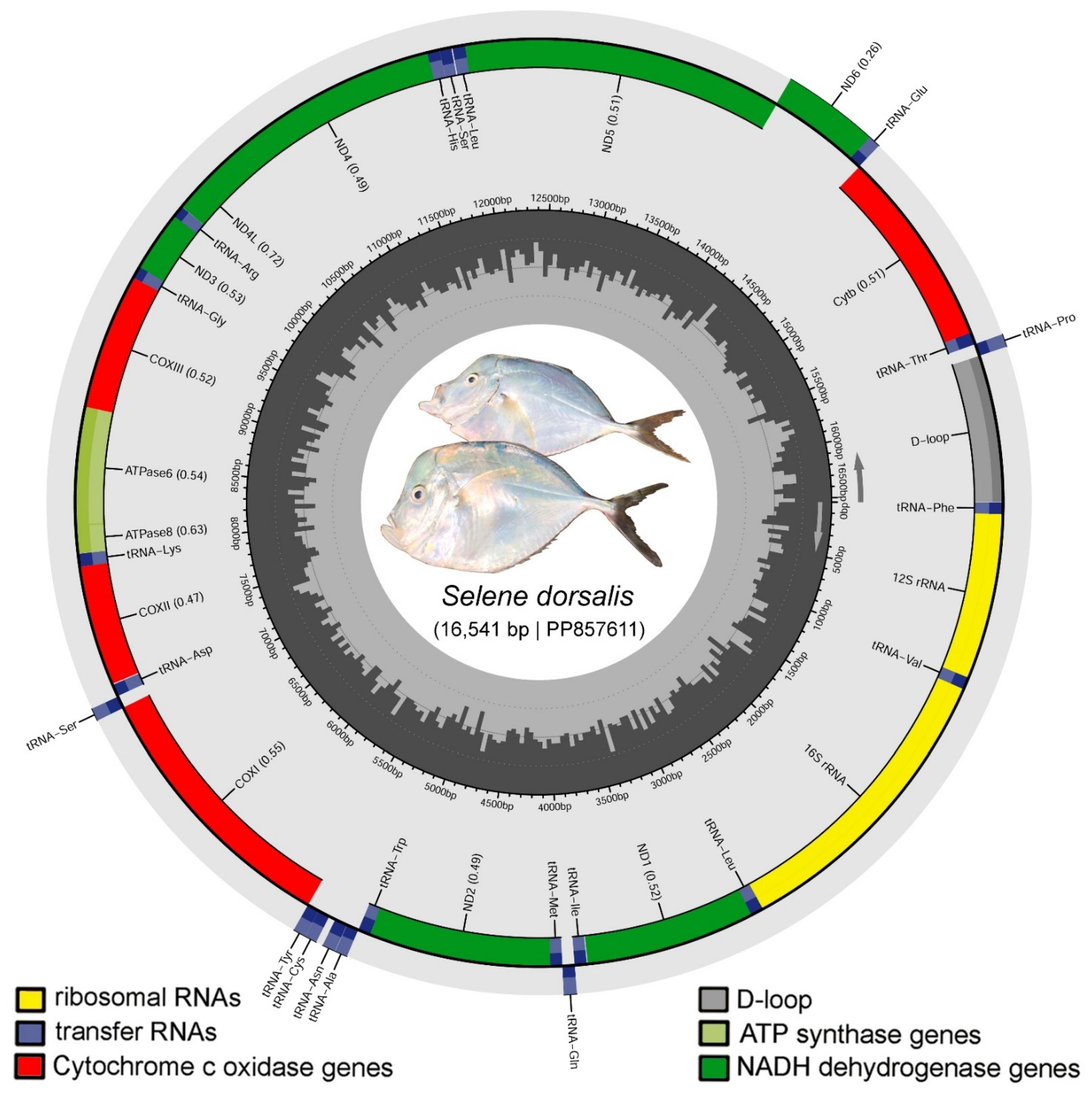
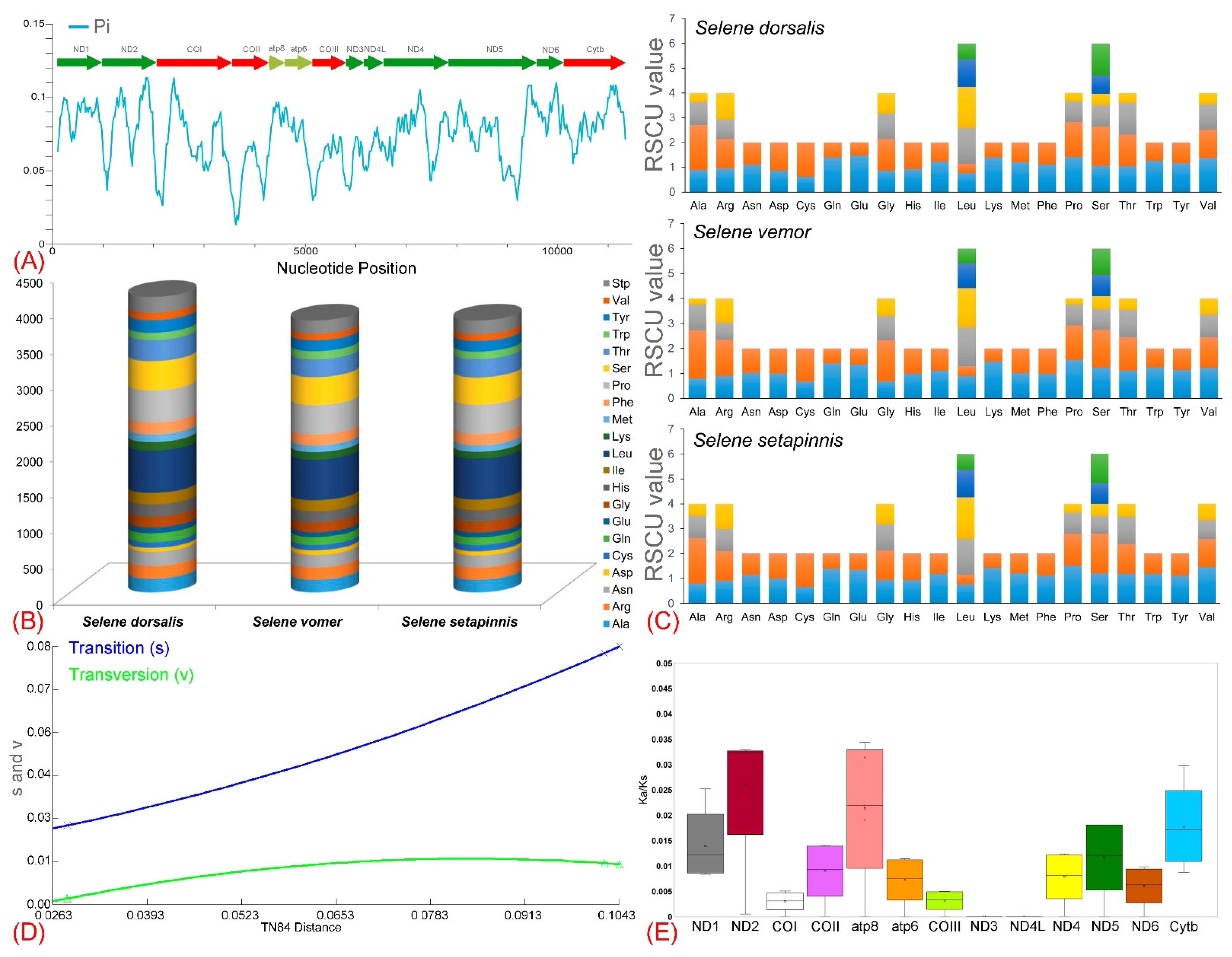

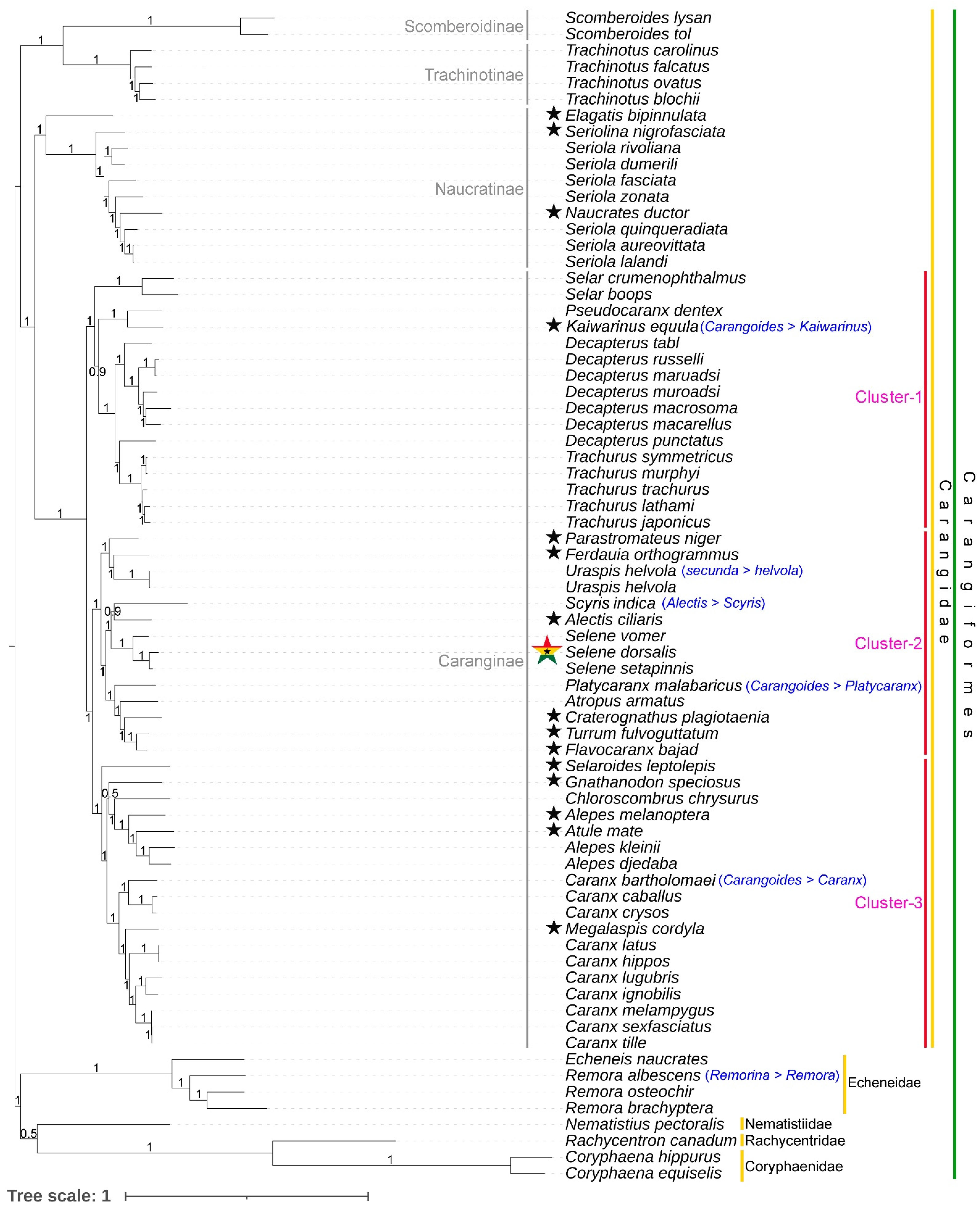

| Genes | Start | Stop | Size (bp) | Strand | IN | Start Codon | Stop Codon | Anticodon |
|---|---|---|---|---|---|---|---|---|
| tRNA-Phe (F) | 1 | 68 | 68 | + | −1 | . | . | GAA |
| 12S rRNA | 68 | 1022 | 955 | + | −1 | . | . | . |
| tRNA-Val (V) | 1022 | 1094 | 73 | + | 0 | . | . | TAC |
| 16S rRNA | 1095 | 2809 | 1715 | + | −1 | . | . | . |
| tRNA-Leu (L2) | 2809 | 2883 | 75 | + | 0 | . | . | TAA |
| ND1 | 2884 | 3858 | 975 | + | 4 | ATG | TAA | . |
| tRNA-Ile (I) | 3863 | 3933 | 71 | + | −2 | . | . | GAT |
| tRNA-Gln (Q) | 3932 | 4003 | 72 | − | −2 | . | . | TTG |
| tRNA-Met (M) | 4002 | 4072 | 71 | + | 0 | . | . | CAT |
| ND2 | 4073 | 5117 | 1045 | + | −1 | ATG | T-- | . |
| tRNA-Trp (W) | 5117 | 5188 | 72 | + | 0 | . | . | TCA |
| tRNA-Ala (A) | 5189 | 5258 | 70 | − | 0 | . | . | TGC |
| tRNA-Asn (N) | 5259 | 5332 | 74 | − | 37 | . | . | GTT |
| tRNA-Cys (C) | 5370 | 5437 | 68 | − | −1 | . | . | GCA |
| tRNA-Tyr (Y) | 5437 | 5507 | 71 | − | 1 | . | . | GTA |
| COI | 5509 | 7059 | 1551 | + | −1 | GTG | TAA | . |
| tRNA-Ser (S2) | 7059 | 7130 | 72 | − | 2 | . | . | TGA |
| tRNA-Asp (D) | 7133 | 7203 | 71 | + | 7 | . | . | GTC |
| COII | 7211 | 7901 | 691 | + | −1 | ATG | T-- | . |
| tRNA-Lys (K) | 7901 | 7976 | 76 | + | 1 | . | . | TTT |
| ATP8 | 7978 | 8145 | 168 | + | −10 | ATG | TAA | . |
| ATP6 | 8136 | 8818 | 683 | + | 0 | ATG | TA- | . |
| COIII | 8819 | 9603 | 785 | + | −1 | ATG | TAA | . |
| tRNA-Gly (G) | 9603 | 9673 | 71 | + | 0 | . | . | TCC |
| ND3 | 9674 | 10,022 | 349 | + | −1 | ATG | T-- | . |
| tRNA-Arg (R) | 10,022 | 10,091 | 70 | + | 1 | . | . | TCG |
| ND4L | 10,093 | 10,389 | 297 | + | −7 | ATG | TAA | . |
| ND4 | 10,383 | 11,763 | 1381 | + | −1 | ATG | T-- | . |
| tRNA-His (H) | 11,763 | 11,835 | 73 | + | −1 | . | . | GTG |
| tRNA-Ser (S1) | 11,835 | 11,903 | 69 | + | 5 | . | . | GCT |
| tRNA-Leu (L1) | 11,909 | 11,982 | 74 | + | 0 | . | . | TAG |
| ND5 | 11,983 | 13,821 | 1839 | + | −4 | ATG | TAA | . |
| ND6 | 13,818 | 14,339 | 522 | − | −1 | . | . | . |
| tRNA-Glu (E) | 14,339 | 14,408 | 70 | − | 3 | . | . | TTC |
| CYTB | 14,412 | 15,552 | 1141 | + | −1 | ATG | TAA | . |
| tRNA-Thr (T) | 15,552 | 15,624 | 73 | + | −2 | . | . | TGT |
| tRNA-Pro (P) | 15,623 | 15,694 | 72 | − | 0 | . | . | TGG |
| Control region | 15,695 | 16,541 | 847 | + | . | . | . | . |
| Species Name | Size (bp) | A% | T% | G% | C% | A + T% | AT-Skew | GC-Skew |
|---|---|---|---|---|---|---|---|---|
| Complete mitogenome | ||||||||
| S. dorsalis | 16,541 | 27.48 | 25.66 | 16.69 | 30.18 | 53.13 | 0.034 | −0.288 |
| S. vomer | 16,558 | 27.79 | 25.56 | 16.20 | 30.44 | 53.35 | 0.042 | −0.305 |
| PCGs | ||||||||
| S. dorsalis | 11,427 | 25.83 | 26.63 | 15.29 | 32.25 | 52.46 | −0.015 | −0.357 |
| S. vomer | 11,428 | 26.29 | 26.33 | 14.77 | 32.61 | 52.62 | −0.001 | −0.377 |
| S. setapinnis | 11,427 | 25.11 | 27.04 | 16.12 | 31.73 | 52.15 | −0.037 | −0.326 |
| tRNAs | ||||||||
| S. dorsalis | 1576 | 30.65 | 24.37 | 19.92 | 25.06 | 55.01 | 0.114 | −0.114 |
| S. vomer | 1556 | 30.59 | 24.23 | 20.18 | 25.00 | 54.82 | 0.116 | −0.107 |
| S. setapinnis | 1415 | 27.63 | 27.56 | 23.89 | 20.92 | 55.19 | 0.001 | 0.066 |
| rRNAs | ||||||||
| S. dorsalis | 2670 | 31.161 | 21.12 | 21.31 | 26.40 | 52.28 | 0.192 | −0.107 |
| S. vomer | 2669 | 31.285 | 21.06 | 21.06 | 26.60 | 52.34 | 0.195 | −0.116 |
| CRs | ||||||||
| S. dorsalis | 847 | 32.59 | 30.22 | 14.64 | 22.55 | 62.81 | 0.038 | −0.213 |
| S. vomer | 862 | 32.60 | 32.02 | 12.99 | 22.39 | 64.62 | 0.009 | −0.266 |
| S. setapinnis | 718 | 34.68 | 30.50 | 14.35 | 20.47 | 65.18 | 0.064 | −0.176 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ewusi, E.O.M.; Lee, S.R.; Kim, A.R.; Go, Y.; Htoo, H.; Chung, S.; Amin, M.H.F.; Andriyono, S.; Kim, H.-W.; Kundu, S. Endemic Radiation of African Moonfish, Selene dorsalis (Gill 1863), in the Eastern Atlantic: Mitogenomic Characterization and Phylogenetic Implications of Carangids (Teleostei: Carangiformes). Biomolecules 2024, 14, 1208. https://doi.org/10.3390/biom14101208
Ewusi EOM, Lee SR, Kim AR, Go Y, Htoo H, Chung S, Amin MHF, Andriyono S, Kim H-W, Kundu S. Endemic Radiation of African Moonfish, Selene dorsalis (Gill 1863), in the Eastern Atlantic: Mitogenomic Characterization and Phylogenetic Implications of Carangids (Teleostei: Carangiformes). Biomolecules. 2024; 14(10):1208. https://doi.org/10.3390/biom14101208
Chicago/Turabian StyleEwusi, Emmanuel Ofosu Mireku, Soo Rin Lee, Ah Ran Kim, Yunji Go, Hsu Htoo, Sangdeok Chung, Muhammad Hilman Fu’adil Amin, Sapto Andriyono, Hyun-Woo Kim, and Shantanu Kundu. 2024. "Endemic Radiation of African Moonfish, Selene dorsalis (Gill 1863), in the Eastern Atlantic: Mitogenomic Characterization and Phylogenetic Implications of Carangids (Teleostei: Carangiformes)" Biomolecules 14, no. 10: 1208. https://doi.org/10.3390/biom14101208
APA StyleEwusi, E. O. M., Lee, S. R., Kim, A. R., Go, Y., Htoo, H., Chung, S., Amin, M. H. F., Andriyono, S., Kim, H.-W., & Kundu, S. (2024). Endemic Radiation of African Moonfish, Selene dorsalis (Gill 1863), in the Eastern Atlantic: Mitogenomic Characterization and Phylogenetic Implications of Carangids (Teleostei: Carangiformes). Biomolecules, 14(10), 1208. https://doi.org/10.3390/biom14101208









