Evolution of Repetitive Elements, Their Roles in Homeostasis and Human Disease, and Potential Therapeutic Applications
Abstract
:1. Introduction
2. Classification and Evolution of REs
2.1. Tandem Repeat Classification and Evolution
2.2. Interspersed Repeat Classification
Interspersed Repeat Evolution
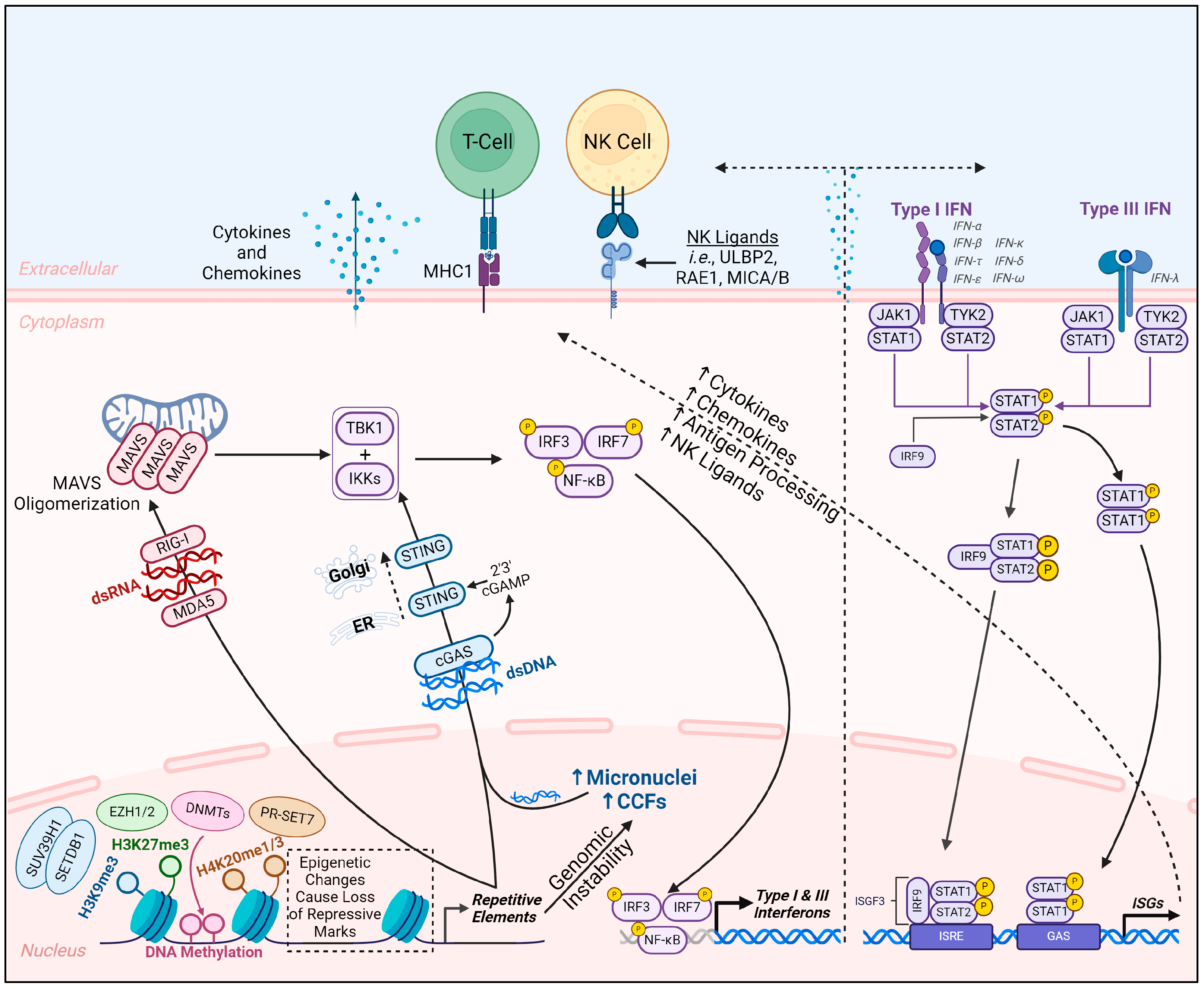
3. RE Regulation and the Roles of REs in Both Beneficial and Pathological Processes
3.1. General Methods of RE Regulation

3.2. Beneficial Activities of REs
3.3. Harmful RE Activities
4. Derepression of REs in Cancer, Aging, and Neurological Disorders
4.1. Implication of REs in Carcinogenesis
4.1.1. TEs Can Inactivate Tumor Suppressor Genes
4.1.2. TEs Can Activate Oncogenes
4.1.3. REs and Their Role in DNA Damage and Genomic Instability
4.2. REs in Aging, Neurological Disorders, and Neurodevelopmental Diseases
4.2.1. Role of REs in Aging
4.2.2. Role of TEs in Neurological Diseases
Role of TEs in Neurodegenerative Diseases
Role of TEs in Neurodevelopmental Diseases
5. Therapeutic Applications of RE Modulation
5.1. Modulating RE Activity in Anti-Cancer Therapies
5.1.1. Activation of REs in Combination with Checkpoint Blockade Inhibitors
5.1.2. Antigens Derived from REs as Vaccination Targets
5.1.3. REs and the Development of Cellular Immunotherapies
5.1.4. REs as Targets for Monoclonal Antibody-Based Therapies
5.1.5. Challenges to Be Addressed for RE-Based Immunotherapeutic Applications
5.1.6. RE Activation and DNA Damage Response Inhibitors
5.2. Targeting TEs in Aging and Neurological Disorders
6. Concluding Remarks and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Avery, O.T.; MacLeod, C.M.; McCarty, M. Studies on the Chemical Nature of the Substance Inducing Transformation of Pneumococcal Types. J. Exp. Med. 1944, 79, 137–158. [Google Scholar] [CrossRef] [PubMed]
- McClintock, B. The Origin and Behavior of Mutable Loci in Maize. Proc. Natl. Acad. Sci. USA 1950, 36, 344–355. [Google Scholar] [CrossRef]
- Britten, R.J.; Kohne, D.E. Repeated Sequences in DNA: Hundreds of Thousands of Copies of DNA Sequences Have Been Incorporated into the Genomes of Higher Organisms. Science 1968, 161, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Kit, S. Equilibrium Sedimentation in Density Gradients of DNA Preparations from Animal Tissues. J. Mol. Biol. 1961, 3, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R. Common Repeat Elements in the Mitochondrial and Plastid Genomes of Green Algae. Front. Genet. 2020, 11, 465. [Google Scholar] [CrossRef]
- Čechová, J.; Lýsek, J.; Bartas, M.; Brázda, V. Complex Analyses of Inverted Repeats in Mitochondrial Genomes Revealed Their Importance and Variability. Bioinformatics 2018, 34, 1081–1085. [Google Scholar] [CrossRef]
- Brázda, V.; Lýsek, J.; Bartas, M.; Fojta, M. Complex Analyses of Short Inverted Repeats in All Sequenced Chloroplast DNAs. BioMed Res. Int. 2018, 2018, 1097018. [Google Scholar] [CrossRef]
- Delihas, N. Impact of Small Repeat Sequences on Bacterial Genome Evolution. Genome Biol. Evol. 2011, 3, 959–973. [Google Scholar] [CrossRef]
- Wickstead, B.; Ersfeld, K.; Gull, K. Repetitive Elements in Genomes of Parasitic Protozoa. Microbiol. Mol. Biol. Rev. 2003, 67, 360–375. [Google Scholar] [CrossRef]
- Möller, M.; Stukenbrock, E.H. Evolution and Genome Architecture in Fungal Plant Pathogens. Nat. Rev. Microbiol. 2017, 15, 756–771. [Google Scholar] [CrossRef]
- Chinwalla, A.T.; Cook, L.L.; Delehaunty, K.D.; Fewell, G.A.; Fulton, L.A.; Fulton, R.S.; Graves, T.A.; Hillier, L.W.; Mardis, E.R.; McPherson, J.D.; et al. Initial Sequencing and Comparative Analysis of the Mouse Genome. Nature 2002, 420, 520–562. [Google Scholar] [CrossRef]
- Waterson, R.H.; Lander, E.S.; Wilson, R.K. The Chimpanzee Sequencing and Analysis Consortium Initial Sequence of the Chimpanzee Genome and Comparison with the Human Genome. Nature 2005, 437, 69–87. [Google Scholar] [CrossRef]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial Sequencing and Analysis of the Human Genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR Provides Acquired Resistance Against Viruses in Prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef]
- Marraffini, L.A.; Sontheimer, E.J. CRISPR Interference: RNA-Directed Adaptive Immunity in Bacteria and Archaea. Nat. Rev. Genet. 2010, 11, 181–190. [Google Scholar] [CrossRef]
- Padeken, J.; Zeller, P.; Gasser, S.M. Repeat DNA in Genome Organization and Stability. Curr. Opin. Genet. Dev. 2015, 31, 12–19. [Google Scholar] [CrossRef]
- de Koning, A.P.J.; Gu, W.; Castoe, T.A.; Batzer, M.A.; Pollock, D.D. Repetitive Elements May Comprise Over Two-Thirds of the Human Genome. PLoS Genet. 2011, 7, e1002384. [Google Scholar] [CrossRef] [PubMed]
- Nurk, S.; Koren, S.; Rhie, A.; Rautiainen, M.; Bzikadze, A.V.; Mikheenko, A.; Vollger, M.R.; Altemose, N.; Uralsky, L.; Gershman, A.; et al. The Complete Sequence of a Human Genome. Science 2022, 376, 44–53. [Google Scholar] [CrossRef]
- Lee, H.; Zhang, Z.; Krause, H.M. Long Noncoding RNAs and Repetitive Elements: Junk or Intimate Evolutionary Partners? Trends Genet. 2019, 35, 892–902. [Google Scholar] [CrossRef]
- Hall, A.N.; Morton, E.; Queitsch, C. First Discovered, Long out of Sight, Finally Visible: Ribosomal DNA. Trends Genet. 2022, 38, 587–597. [Google Scholar] [CrossRef]
- Altemose, N. A Classical Revival: Human Satellite DNAs Enter the Genomics Era. Semin. Cell Dev. Biol. 2022, 128, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Thakur, J.; Packiaraj, J.; Henikoff, S. Sequence, Chromatin and Evolution of Satellite DNA. Int. J. Mol. Sci. 2021, 22, 4309. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.N.; Feschotte, C. A Field Guide to Eukaryotic Transposable Elements. Annu. Rev. Genet. 2020, 54, 539–561. [Google Scholar] [CrossRef] [PubMed]
- Slotkin, R.K.; Martienssen, R. Transposable Elements and the Epigenetic Regulation of the Genome. Nat. Rev. Genet. 2007, 8, 272–285. Available online: https://www.nature.com/articles/nrg2072 (accessed on 16 July 2024). [CrossRef]
- Kojima, K.K. Human Transposable Elements in Repbase: Genomic Footprints from Fish to Humans. Mob. DNA 2018, 9, 2. [Google Scholar] [CrossRef]
- Chuong, E.B.; Elde, N.C.; Feschotte, C. Regulatory Activities of Transposable Elements: From Conflicts to Benefits. Nat. Rev. Genet. 2017, 18, 71–86. [Google Scholar] [CrossRef]
- Fueyo, R.; Judd, J.; Feschotte, C.; Wysocka, J. Roles of Transposable Elements in the Regulation of Mammalian Transcription. Nat. Rev. Mol. Cell Biol. 2022, 23, 481–497. [Google Scholar] [CrossRef]
- Kazazian, H.H.; Moran, J.V. Mobile DNA in Health and Disease. N. Engl. J. Med. 2017, 377, 361–370. [Google Scholar] [CrossRef]
- Burns, K.H. Transposable Elements in Cancer. Nat. Rev. Cancer 2017, 17, 415–424. [Google Scholar] [CrossRef]
- Liehr, T. Repetitive Elements in Humans. Int. J. Mol. Sci. 2021, 22, 2072. [Google Scholar] [CrossRef]
- Flynn, J.M.; Yamashita, Y.M. The Implications of Satellite DNA Instability on Cellular Function and Evolution. Semin. Cell Dev. Biol. 2024, 156, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Alaguponniah, S.; Velayudhan Krishna, D.; Paul, S.; Christyraj, J.R.S.S.; Nallaperumal, K.; Sivasubramaniam, S. Finding of Novel Telomeric Repeats and Their Distribution in the Human Genome. Genomics 2020, 112, 3565–3570. [Google Scholar] [CrossRef]
- Liao, X.; Zhu, W.; Zhou, J.; Li, H.; Xu, X.; Zhang, B.; Gao, X. Repetitive DNA Sequence Detection and Its Role in the Human Genome. Commun. Biol. 2023, 6, 1–21. [Google Scholar] [CrossRef]
- Garrido-Ramos, M.A. Satellite DNA: An Evolving Topic. Genes 2017, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Craig-Holmes, A.P.; Shaw, M.W. Polymorphism of Human Constitutive Heterochromatin. Science 1971, 174, 702–704. [Google Scholar] [CrossRef]
- Kim, J.C.; Mirkin, S.M. The Balancing Act of DNA Repeat Expansions. Curr. Opin. Genet. Dev. 2013, 23, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Gadgil, R.; Barthelemy, J.; Lewis, T.; Leffak, M. Replication Stalling and DNA Microsatellite Instability. Biophys. Chem. 2017, 225, 38–48. [Google Scholar] [CrossRef]
- Šatović-Vukšić, E.; Plohl, M. Satellite DNAs—From Localized to Highly Dispersed Genome Components. Genes. 2023, 14, 742. [Google Scholar] [CrossRef]
- Louzada, S.; Lopes, M.; Ferreira, D.; Adega, F.; Escudeiro, A.; Gama-Carvalho, M.; Chaves, R. Decoding the Role of Satellite DNA in Genome Architecture and Plasticity—An Evolutionary and Clinical Affair. Genes 2020, 11, 72. [Google Scholar] [CrossRef]
- Iwata-Otsubo, A.; Dawicki-McKenna, J.M.; Akera, T.; Falk, S.J.; Chmátal, L.; Yang, K.; Sullivan, B.A.; Schultz, R.M.; Lampson, M.A.; Black, B.E. Expanded Satellite Repeats Amplify a Discrete CENP-A Nucleosome Assembly Site on Chromosomes That Drive in Female Meiosis. Curr. Biol. 2017, 27, 2365–2373.e8. [Google Scholar] [CrossRef]
- Shin, W.; Mun, S.; Han, K. Human Endogenous Retrovirus-K (HML-2)-Related Genetic Variation: Human Genome Diversity and Disease. Genes 2023, 14, 2150. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.A. Human Endogenous Retroviruses: Friend or Foe? Apmis 2016, 124, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Belshaw, R.; Watson, J.; Katzourakis, A.; Howe, A.; Woolven-Allen, J.; Burt, A.; Tristem, M. Rate of Recombinational Deletion among Human Endogenous Retroviruses. J. Virol. 2007, 81, 9437–9442. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.J.; Macfarlan, T.S.; Lorincz, M.C. Long Terminal Repeats: From Parasitic Elements to Building Blocks of the Transcriptional Regulatory Repertoire. Mol. Cell 2016, 62, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Friedli, M.; Trono, D. The Developmental Control of Transposable Elements and the Evolution of Higher Species. Annu. Rev. Cell Dev. Biol. 2015, 31, 429–451. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Montojo, M.; Doucet-O’Hare, T.; Henderson, L.; Nath, A. Human Endogenous Retrovirus-K (HML-2): A Comprehensive Review. Crit. Rev. Microbiol. 2018, 44, 715–738. [Google Scholar] [CrossRef]
- Richardson, S.R.; Doucet, A.J.; Kopera, H.C.; Moldovan, J.B.; Garcia-Perez, J.L.; Moran, J.V. The Influence of LINE-1 and SINE Retrotransposons on Mammalian Genomes. Microbiol. Spectr. 2015, 3, MDNA3-0061-2014. [Google Scholar] [CrossRef]
- Denli, A.M.; Narvaiza, I.; Kerman, B.E.; Pena, M.; Benner, C.; Marchetto, M.C.N.; Diedrich, J.K.; Aslanian, A.; Ma, J.; Moresco, J.J.; et al. Primate-Specific ORF0 Contributes to Retrotransposon-Mediated Diversity. Cell 2015, 163, 583–593. [Google Scholar] [CrossRef]
- Brouha, B.; Schustak, J.; Badge, R.M.; Lutz-Prigge, S.; Farley, A.H.; Moran, J.V.; Kazazian, H.H. Hot L1s Account for the Bulk of Retrotransposition in the Human Population. Proc. Natl. Acad. Sci. USA 2003, 100, 5280–5285. [Google Scholar] [CrossRef]
- Goodwin, T.J.D.; Poulter, R.T.M. The DIRS1 Group of Retrotransposons. Mol. Biol. Evol. 2001, 18, 2067–2082. [Google Scholar] [CrossRef]
- Abascal, F.; Tress, M.L.; Valencia, A. Alternative Splicing and Co-Option of Transposable Elements: The Case of TMPO/LAP2α and ZNF451 in Mammals. Bioinformatics 2015, 31, 2257–2261. [Google Scholar] [CrossRef] [PubMed]
- Curcio, M.J.; Derbyshire, K.M. The Outs and Ins of Transposition: From Mu to Kangaroo. Nat. Rev. Mol. Cell Biol. 2003, 4, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Chénais, B. Transposable Elements and Human Diseases: Mechanisms and Implication in the Response to Environmental Pollutants. Int. J. Mol. Sci. 2022, 23, 2551. [Google Scholar] [CrossRef] [PubMed]
- Pace, J.K.; Feschotte, C. The Evolutionary History of Human DNA Transposons: Evidence for Intense Activity in the Primate Lineage. Genome Res. 2007, 17, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Spradling, A.C.; Bellen, H.J.; Hoskins, R.A.; Drosophila, P. Elements Preferentially Transpose to Replication Origins. Proc. Natl. Acad. Sci. USA 2011, 108, 15948–15953. [Google Scholar] [CrossRef]
- Fricker, A.D.; Peters, J.E. Vulnerabilities on the Lagging-Strand Template: Opportunities for Mobile Elements. Annu. Rev. Genet. 2014, 48, 167–186. [Google Scholar] [CrossRef]
- Engels, W.R.; Johnson-Schlitz, D.M.; Eggleston, W.B.; Sved, J. High-Frequency P Element Loss in Drosophila Is Homolog Dependent. Cell 1990, 62, 515–525. [Google Scholar] [CrossRef]
- Hsia, A.-P.; Schnable, P.S. DNA Sequence Analyses Support the Role of Interrupted Gap Repair in the Origin of Internal Deletions of the Maize Transposon, MuDR. Genetics 1996, 142, 603–618. [Google Scholar] [CrossRef]
- Feschotte, C.; Zhang, X.; Wessler, S.R. Miniature Inverted-Repeat Transposable Elements and Their Relationship to Established DNA Transposons. In Mobile DNA II; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 1145–1158. ISBN 978-1-68367-415-3. [Google Scholar]
- Hayward, A.; Gilbert, C. Transposable Elements. Curr. Biol. 2022, 32, R904–R909. [Google Scholar] [CrossRef]
- Gilbert, C.; Feschotte, C. Horizontal Acquisition of Transposable Elements and Viral Sequences: Patterns and Consequences. Curr. Opin. Genet. Dev. 2018, 49, 15–24. [Google Scholar] [CrossRef]
- Krupovic, M.; Dolja, V.V.; Koonin, E.V. Origin of Viruses: Primordial Replicators Recruiting Capsids from Hosts. Nat. Rev. Microbiol. 2019, 17, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Emera, D.; Casola, C.; Lynch, V.J.; Wildman, D.E.; Agnew, D.; Wagner, G.P. Convergent Evolution of Endometrial Prolactin Expression in Primates, Mice, and Elephants through the Independent Recruitment of Transposable Elements. Mol. Biol. Evol. 2012, 29, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Bourc’his, D.; Bestor, T.H. Meiotic Catastrophe and Retrotransposon Reactivation in Male Germ Cells Lacking Dnmt3L. Nature 2004, 431, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacol. 2013, 38, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xu, Y.; Ren, S.; Yang, N.; Sun, Y.; Yang, Q.; Zhang, Y.; Cai, H.; Deng, W.; Chen, J.; et al. Trophoblast PR-SET7 Dysfunction Induces Viral Mimicry Response and Necroptosis Associated with Recurrent Miscarriage. Proc. Natl. Acad. Sci. USA 2023, 120, e2216206120. [Google Scholar] [CrossRef]
- Hyun, K.; Jeon, J.; Park, K.; Kim, J. Writing, Erasing and Reading Histone Lysine Methylations. Exp. Mol. Med. 2017, 49, e324. [Google Scholar] [CrossRef]
- Karimi, M.M.; Goyal, P.; Maksakova, I.A.; Bilenky, M.; Leung, D.; Tang, J.X.; Shinkai, Y.; Mager, D.L.; Jones, S.; Hirst, M.; et al. DNA Methylation and SETDB1/H3K9me3 Regulate Predominantly Distinct Sets of Genes, Retroelements, and Chimeric Transcripts in mESCs. Cell Stem Cell 2011, 8, 676–687. [Google Scholar] [CrossRef]
- Chen, R.; Ishak, C.A.; De Carvalho, D.D. Endogenous Retroelements and the Viral Mimicry Response in Cancer Therapy and Cellular Homeostasis. Cancer Discov. 2021, 11, 2707–2725. [Google Scholar] [CrossRef]
- Yu, L.; Liu, P. Cytosolic DNA Sensing by cGAS: Regulation, Function, and Human Diseases. Sig. Transduct. Target. Ther. 2021, 6, 170. [Google Scholar] [CrossRef]
- Thoresen, D.; Wang, W.; Galls, D.; Guo, R.; Xu, L.; Pyle, A.M. The Molecular Mechanism of RIG-I Activation and Signaling. Immunol. Rev. 2021, 304, 154–168. [Google Scholar] [CrossRef]
- Taguchi, T.; Mukai, K.; Takaya, E.; Shindo, R. STING Operation at the ER/Golgi Interface. Front. Immunol. 2021, 12, 646304. [Google Scholar] [CrossRef] [PubMed]
- Rehwinkel, J.; Gack, M.U. RIG-I-like Receptors: Their Regulation and Roles in RNA Sensing. Nat. Rev. Immunol. 2020, 20, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Decout, A.; Katz, J.D.; Venkatraman, S.; Ablasser, A. The cGAS–STING Pathway as a Therapeutic Target in Inflammatory Diseases. Nat. Rev. Immunol. 2021, 21, 548–569. [Google Scholar] [CrossRef]
- Yang, N.; Kazazian, H.H. L1 Retrotransposition Is Suppressed by Endogenously Encoded Small Interfering RNAs in Human Cultured Cells. Nat. Struct. Mol. Biol. 2006, 13, 763–771. [Google Scholar] [CrossRef]
- Velazquez Camacho, O.; Galan, C.; Swist-Rosowska, K.; Ching, R.; Gamalinda, M.; Karabiber, F.; De La Rosa-Velazquez, I.; Engist, B.; Koschorz, B.; Shukeir, N.; et al. Major Satellite Repeat RNA Stabilize Heterochromatin Retention of Suv39h Enzymes by RNA-Nucleosome Association and RNA:DNA Hybrid Formation. eLife 2017, 6, e25293. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.R.; Narvaiza, I.; Planegger, R.A.; Weitzman, M.D.; Moran, J.V. APOBEC3A Deaminates Transiently Exposed Single-Strand DNA during LINE-1 Retrotransposition. eLife 2014, 3, e02008. [Google Scholar] [CrossRef]
- Miller, W.J.; McDonald, J.F.; Nouaud, D.; Anxolabéhère, D. Molecular Domestication—More than a Sporadic Episode in Evolution. Genetica 1999, 107, 197–207. [Google Scholar] [CrossRef]
- Rebollo, R.; Romanish, M.T.; Mager, D.L. Transposable Elements: An Abundant and Natural Source of Regulatory Sequences for Host Genes. Annu. Rev. Genet. 2012, 46, 21–42. [Google Scholar] [CrossRef]
- Marasca, F.; Sinha, S.; Vadalà, R.; Polimeni, B.; Ranzani, V.; Paraboschi, E.M.; Burattin, F.V.; Ghilotti, M.; Crosti, M.; Negri, M.L.; et al. LINE1 Are Spliced in Non-Canonical Transcript Variants to Regulate T Cell Quiescence and Exhaustion. Nat. Genet. 2022, 54, 180–193. [Google Scholar] [CrossRef]
- Chuong, E.B.; Elde, N.C.; Feschotte, C. Regulatory Evolution of Innate Immunity through Co-Option of Endogenous Retroviruses. Science 2016, 351, 1083–1087. [Google Scholar] [CrossRef]
- Sundaram, V.; Cheng, Y.; Ma, Z.; Li, D.; Xing, X.; Edge, P.; Snyder, M.P.; Wang, T. Widespread Contribution of Transposable Elements to the Innovation of Gene Regulatory Networks. Genome Res. 2014, 24, 1963. [Google Scholar] [CrossRef] [PubMed]
- Bourque, G.; Leong, B.; Vega, V.B.; Chen, X.; Lee, Y.L.; Srinivasan, K.G.; Chew, J.-L.; Ruan, Y.; Wei, C.-L.; Ng, H.H.; et al. Evolution of the Mammalian Transcription Factor Binding Repertoire via Transposable Elements. Genome Res. 2008, 18, 1752–1762. [Google Scholar] [CrossRef] [PubMed]
- Kapitonov, V.V.; Koonin, E.V. Evolution of the RAG1-RAG2 Locus: Both Proteins Came from the Same Transposon. Biol. Direct 2015, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Kapitonov, V.V.; Jurka, J. RAG1 Core and V(D)J Recombination Signal Sequences Were Derived from Transib Transposons. PLoS Biol. 2005, 3, e181. [Google Scholar] [CrossRef]
- Cam, H.P.; Noma, K.; Ebina, H.; Levin, H.L.; Grewal, S.I.S. Host Genome Surveillance for Retrotransposons by Transposon-Derived Proteins. Nature 2008, 451, 431–436. [Google Scholar] [CrossRef]
- Nakamura, T.M.; Cech, T.R. Reversing Time: Origin of Telomerase. Cell 1998, 92, 587–590. [Google Scholar] [CrossRef]
- Feschotte, C. Transposable Elements and the Evolution of Regulatory Networks. Nat. Rev. Genet. 2008, 9, 397–405. [Google Scholar] [CrossRef]
- Johnson, R.; Guigó, R. The RIDL Hypothesis: Transposable Elements as Functional Domains of Long Noncoding RNAs. RNA 2014, 20, 959–976. [Google Scholar] [CrossRef]
- Durruthy-Durruthy, J.; Sebastiano, V.; Wossidlo, M.; Cepeda, D.; Cui, J.; Grow, E.J.; Davila, J.; Mall, M.; Wong, W.H.; Wysocka, J.; et al. The Primate-Specific Noncoding RNA HPAT5 Regulates Pluripotency during Human Preimplantation Development and Nuclear Reprogramming. Nat. Genet. 2016, 48, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.N.; Friedman, R.Z.; Wang, J.T.; Jang, H.S.; Zhuo, X.; Wang, T. Co-Opted Transposons Help Perpetuate Conserved Higher-Order Chromosomal Structures. Genome Biol. 2020, 21, 16. [Google Scholar] [CrossRef]
- Choudhary, M.N.K.; Quaid, K.; Xing, X.; Schmidt, H.; Wang, T. Widespread Contribution of Transposable Elements to the Rewiring of Mammalian 3D Genomes. Nat. Commun. 2023, 14, 634. [Google Scholar] [CrossRef] [PubMed]
- Bzymek, M.; Lovett, S.T. Instability of Repetitive DNA Sequences: The Role of Replication in Multiple Mechanisms. Proc. Natl. Acad. Sci. USA 2001, 98, 8319–8325. [Google Scholar] [CrossRef] [PubMed]
- Fournier, A.; McLeer-Florin, A.; Lefebvre, C.; Duley, S.; Barki, L.; Ribeyron, J.; Alboukadel, K.; Hamaidia, S.; Granjon, A.; Gressin, R.; et al. 1q12 Chromosome Translocations Form Aberrant Heterochromatic Foci Associated with Changes in Nuclear Architecture and Gene Expression in B Cell Lymphoma. EMBO Mol. Med. 2010, 2, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Paulson, H. Chapter 9—Repeat Expansion Diseases. In Handbook of Clinical Neurology; Geschwind, D.H., Paulson, H.L., Klein, C., Eds.; Neurogenetics, Part I; Elsevier: Amsterdam, The Netherlands, 2018; Volume 147, pp. 105–123. [Google Scholar]
- Hall, L.L.; Byron, M.; Carone, D.M.; Whitfield, T.W.; Pouliot, G.P.; Fischer, A.; Jones, P.; Lawrence, J.B. Demethylated HSATII DNA and HSATII RNA Foci Sequester PRC1 and MeCP2 into Cancer-Specific Nuclear Bodies. Cell Rep. 2017, 18, 2943–2956. [Google Scholar] [CrossRef]
- Della Valle, F.; Reddy, P.; Yamamoto, M.; Liu, P.; Saera-Vila, A.; Bensaddek, D.; Zhang, H.; Prieto Martinez, J.; Abassi, L.; Celii, M.; et al. LINE-1 RNA Causes Heterochromatin Erosion and Is a Target for Amelioration of Senescent Phenotypes in Progeroid Syndromes. Sci. Transl. Med. 2022, 14, eabl6057. [Google Scholar] [CrossRef] [PubMed]
- Payer, L.M.; Burns, K.H. Transposable Elements in Human Genetic Disease. Nat. Rev. Genet. 2019, 20, 760–772. [Google Scholar] [CrossRef]
- Ayarpadikannan, S.; Kim, H.-S. The Impact of Transposable Elements in Genome Evolution and Genetic Instability and Their Implications in Various Diseases. Genom. Inform. 2014, 12, 98–104. [Google Scholar] [CrossRef]
- Blake, D.; Lynch, K.W. The Three As: Alternative Splicing, Alternative Polyadenylation and Their Impact on Apoptosis in Immune Function. Immunol. Rev. 2021, 304, 30–50. [Google Scholar] [CrossRef]
- Gallicchio, L.; Olivares, G.H.; Berry, C.W.; Fuller, M.T. Regulation and Function of Alternative Polyadenylation in Development and Differentiation. RNA Biol. 2023, 20, 908–925. [Google Scholar] [CrossRef]
- Konkel, M.K.; Batzer, M.A. A Mobile Threat to Genome Stability: The Impact of Non-LTR Retrotransposons upon the Human Genome. Semin. Cancer Biol. 2010, 20, 211–221. [Google Scholar] [CrossRef]
- Hancks, D.C.; Kazazian, H.H. Roles for Retrotransposon Insertions in Human Disease. Mob. DNA 2016, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Gorbunova, V.; Seluanov, A.; Mita, P.; McKerrow, W.; Fenyö, D.; Boeke, J.D.; Linker, S.B.; Gage, F.H.; Kreiling, J.A.; Petrashen, A.P.; et al. The Role of Retrotransposable Elements in Ageing and Age-Associated Diseases. Nature 2021, 596, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Dodig, S.; Čepelak, I.; Pavić, I. Hallmarks of Senescence and Aging. Biochem. Med. 2019, 29, 030501. [Google Scholar] [CrossRef]
- Dou, Z.; Ghosh, K.; Vizioli, M.G.; Zhu, J.; Sen, P.; Wangensteen, K.J.; Simithy, J.; Lan, Y.; Lin, Y.; Zhou, Z.; et al. Cytoplasmic Chromatin Triggers Inflammation in Senescence and Cancer. Nature 2017, 550, 402–406. [Google Scholar] [CrossRef]
- De Cecco, M.; Ito, T.; Petrashen, A.P.; Elias, A.E.; Skvir, N.J.; Criscione, S.W.; Caligiana, A.; Brocculi, G.; Adney, E.M.; Boeke, J.D.; et al. L1 Drives IFN in Senescent Cells and Promotes Age-Associated Inflammation. Nature 2019, 566, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Z.; Wu, Z.; Ren, J.; Fan, Y.; Sun, L.; Cao, G.; Niu, Y.; Zhang, B.; Ji, Q.; et al. Resurrection of Endogenous Retroviruses during Aging Reinforces Senescence. Cell 2023, 186, 287–304.e26. [Google Scholar] [CrossRef]
- Admasu, T.D.; Rae, M.J.; Stolzing, A. Dissecting Primary and Secondary Senescence to Enable New Senotherapeutic Strategies. Ageing Res. Rev. 2021, 70, 101412. [Google Scholar] [CrossRef]
- Lee, E.; Iskow, R.; Yang, L.; Gokcumen, O.; Haseley, P.; Luquette, L.J.; Lohr, J.G.; Harris, C.C.; Ding, L.; Wilson, R.K.; et al. Landscape of Somatic Retrotransposition in Human Cancers. Science 2012, 337, 967–971. [Google Scholar] [CrossRef]
- Criscione, S.W.; Zhang, Y.; Thompson, W.; Sedivy, J.M.; Neretti, N. Transcriptional Landscape of Repetitive Elements in Normal and Cancer Human Cells. BMC Genom. 2014, 15, 583. [Google Scholar] [CrossRef]
- Ardeljan, D.; Taylor, M.S.; Ting, D.T.; Burns, K.H. The Human Long Interspersed Element-1 Retrotransposon: An Emerging Biomarker of Neoplasia. Clin. Chem. 2017, 63, 816–822. [Google Scholar] [CrossRef]
- Wang-Johanning, F.; Frost, A.R.; Jian, B.; Epp, L.; Lu, D.W.; Johanning, G.L. Quantitation of HERV-K Env Gene Expression and Splicing in Human Breast Cancer. Oncogene 2003, 22, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Wang-Johanning, F.; Frost, A.R.; Jian, B.; Azerou, R.; Lu, D.W.; Chen, D.-T.; Johanning, G.L. Detecting the Expression of Human Endogenous Retrovirus E Envelope Transcripts in Human Prostate Adenocarcinoma. Cancer 2003, 98, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Büscher, K.; Trefzer, U.; Hofmann, M.; Sterry, W.; Kurth, R.; Denner, J. Expression of Human Endogenous Retrovirus K in Melanomas and Melanoma Cell Lines. Cancer Res. 2005, 65, 4172–4180. [Google Scholar] [CrossRef] [PubMed]
- Engel, K.; Wieland, L.; Krüger, A.; Volkmer, I.; Cynis, H.; Emmer, A.; Staege, M.S. Identification of Differentially Expressed Human Endogenous Retrovirus Families in Human Leukemia and Lymphoma Cell Lines and Stem Cells. Front. Oncol. 2021, 11, 637981. [Google Scholar] [CrossRef]
- Yuan, Z.; Yang, Y.; Zhang, N.; Soto, C.; Jiang, X.; An, Z.; Zheng, W.J. Human Endogenous Retroviruses in Glioblastoma Multiforme. Microorganisms 2021, 9, 764. [Google Scholar] [CrossRef]
- Alves, G.; Tatro, A.; Fanning, T. Differential Methylation of Human LINE-1 Retrotransposons in Malignant Cells. Gene 1996, 176, 39–44. [Google Scholar] [CrossRef]
- Iskow, R.C.; McCabe, M.T.; Mills, R.E.; Torene, S.; Pittard, W.S.; Neuwald, A.F.; Meir, E.G.V.; Vertino, P.M.; Devine, S.E. Natural Mutagenesis of Human Genomes by Endogenous Retrotransposons. Cell 2010, 141, 1253–1261. [Google Scholar] [CrossRef]
- Swets, M.; Zaalberg, A.; Boot, A.; van Wezel, T.; Frouws, M.A.; Bastiaannet, E.; Gelderblom, H.; van de Velde, C.J.H.; Kuppen, P.J.K. Tumor LINE-1 Methylation Level in Association with Survival of Patients with Stage II Colon Cancer. Int. J. Mol. Sci. 2016, 18, 36. [Google Scholar] [CrossRef]
- Rodić, N.; Sharma, R.; Sharma, R.; Zampella, J.; Dai, L.; Taylor, M.S.; Hruban, R.H.; Iacobuzio-Donahue, C.A.; Maitra, A.; Torbenson, M.S.; et al. Long Interspersed Element-1 Protein Expression Is a Hallmark of Many Human Cancers. Am. J. Pathol. 2014, 184, 1280–1286. [Google Scholar] [CrossRef]
- Miki, Y.; Nishisho, I.; Horii, A.; Miyoshi, Y.; Utsunomiya, J.; Kinzler, K.W.; Vogelstein, B.; Nakamura, Y. Disruption of the APC Gene by a Retrotransposal Insertion of L1 Sequence in a Colon Cancer. Cancer Res. 1992, 52, 643–645. [Google Scholar]
- Zhang, R.; Zhang, F.; Sun, Z.; Liu, P.; Zhang, X.; Ye, Y.; Cai, B.; Walsh, M.J.; Ren, X.; Hao, X.; et al. LINE-1 Retrotransposition Promotes the Development and Progression of Lung Squamous Cell Carcinoma by Disrupting the Tumor-Suppressor Gene FGGY. Cancer Res. 2019, 79, 4453–4465. [Google Scholar] [CrossRef] [PubMed]
- Slebos, R.J.; Resnick, M.A.; Taylor, J.A. Inactivation of the P53 Tumor Suppressor Gene via a Novel Alu Rearrangement. Cancer Res. 1998, 58, 5333–5336. [Google Scholar] [PubMed]
- Franke, G.; Bausch, B.; Hoffmann, M.M.; Cybulla, M.; Wilhelm, C.; Kohlhase, J.; Scherer, G.; Neumann, H.P.H. Alu-Alu Recombination Underlies the Vast Majority of Large VHL Germline Deletions: Molecular Characterization and Genotype–Phenotype Correlations in VHL Patients. Human. Mutat. 2009, 30, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.L.; Wulaningsih, W.; Lehmann, U. Transposable Elements in Human Cancer: Causes and Consequences of Deregulation. Int. J. Mol. Sci. 2017, 18, 974. [Google Scholar] [CrossRef] [PubMed]
- Babaian, A.; Mager, D.L. Endogenous Retroviral Promoter Exaptation in Human Cancer. Mob. DNA 2016, 7, 24. [Google Scholar] [CrossRef]
- Morse, B.; Rotherg, P.G.; South, V.J.; Spandorfer, J.M.; Astrin, S.M. Insertional Mutagenesis of the Myc Locus by a LINE-1 Sequence in a Human Breast Carcinoma. Nature 1988, 333, 87–90. [Google Scholar] [CrossRef]
- Lu, X.J.; Xue, H.Y.; Qi, X.L.; Xu, J.; Ma, S.J. LINE-1 in Cancer: Multifaceted Functions and Potential Clinical Implications. Genet. Med. 2016, 18, 431–439. [Google Scholar] [CrossRef]
- Babaian, A.; Romanish, M.T.; Gagnier, L.; Kuo, L.Y.; Karimi, M.M.; Steidl, C.; Mager, D.L. Onco-Exaptation of an Endogenous Retroviral LTR Drives IRF5 Expression in Hodgkin Lymphoma. Oncogene 2016, 35, 2542–2546. [Google Scholar] [CrossRef]
- Lamprecht, B.; Walter, K.; Kreher, S.; Kumar, R.; Hummel, M.; Lenze, D.; Köchert, K.; Bouhlel, M.A.; Richter, J.; Soler, E.; et al. Derepression of an Endogenous Long Terminal Repeat Activates the CSF1R Proto-Oncogene in Human Lymphoma. Nat. Med. 2010, 16, 571–579. [Google Scholar] [CrossRef]
- Jiang, J.-C.; Upton, K.R. Human Transposons Are an Abundant Supply of Transcription Factor Binding Sites and Promoter Activities in Breast Cancer Cell Lines. Mob. DNA 2019, 10, 16. [Google Scholar] [CrossRef]
- Zeng, Y.; Cao, Y.; Halevy, R.S.; Nguyen, P.; Liu, D.; Zhang, X.; Ahituv, N.; Han, J.-D.J. Characterization of Functional Transposable Element Enhancers in Acute Myeloid Leukemia. Sci. China Life Sci. 2020, 63, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Deniz, Ö.; Ahmed, M.; Todd, C.D.; Rio-Machin, A.; Dawson, M.A.; Branco, M.R. Endogenous Retroviruses Are a Source of Enhancers with Oncogenic Potential in Acute Myeloid Leukaemia. Nat. Commun. 2020, 11, 3506. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.S.; Shah, N.M.; Du, A.Y.; Dailey, Z.Z.; Pehrsson, E.C.; Godoy, P.M.; Zhang, D.; Li, D.; Xing, X.; Kim, S.; et al. Transposable Elements Drive Widespread Expression of Oncogenes in Human Cancers. Nat. Genet. 2019, 51, 611–617. [Google Scholar] [CrossRef]
- Gasior, S.L.; Wakeman, T.P.; Xu, B.; Deininger, P.L. The Human LINE-1 Retrotransposon Creates DNA Double-Strand Breaks. J. Mol. Biol. 2006, 357, 1383–1393. [Google Scholar] [CrossRef]
- Hedges, D.J.; Deininger, P.L. Inviting Instability: Transposable Elements, Double-Strand Breaks, and the Maintenance of Genome Integrity. Mutat. Res. 2007, 616, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Belgnaoui, S.M.; Gosden, R.G.; Semmes, O.J.; Haoudi, A. Human LINE-1 Retrotransposon Induces DNA Damage and Apoptosis in Cancer Cells. Cancer Cell Int. 2006, 6, 13. [Google Scholar] [CrossRef]
- Voineagu, I.; Narayanan, V.; Lobachev, K.S.; Mirkin, S.M. Replication Stalling at Unstable Inverted Repeats: Interplay between DNA Hairpins and Fork Stabilizing Proteins. Proc. Natl. Acad. Sci. USA 2008, 105, 9936–9941. [Google Scholar] [CrossRef]
- Beck, C.R.; Collier, P.; Macfarlane, C.; Malig, M.; Kidd, J.M.; Eichler, E.E.; Badge, R.M.; Moran, J.V. LINE-1 Retrotransposition Activity in Human Genomes. Cell 2010, 141, 1159–1170. [Google Scholar] [CrossRef]
- Hsieh, S.-Y.; Chen, W.-Y.; Yeh, T.-S.; Sheen, I.-S.; Huang, S.-F. High-Frequency Alu-Mediated Genomic Recombination/Deletion within the Caspase-Activated DNase Gene in Human Hepatoma. Oncogene 2005, 24, 6584–6589. [Google Scholar] [CrossRef]
- Kanno, J.; Hutchin, T.; Kamada, F.; Narisawa, A.; Aoki, Y.; Matsubara, Y.; Kure, S. Genomic Deletion within GLDC Is a Major Cause of Non-ketotic Hyperglycinaemia. J. Med. Genet. 2007, 44, e69. [Google Scholar] [CrossRef]
- Chan, D.Y.L.; Moralli, D.; Khoja, S.; Monaco, Z.L. Noncoding Centromeric RNA Expression Impairs Chromosome Stability in Human and Murine Stem Cells. Dis. Markers 2017, 2017, 7506976. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.Z.; Qiu, Z.; Wu, Q.; Finlay, D.; Garcia, G.; Sun, D.; Rantala, J.; Barshop, W.; Hope, J.L.; Gimple, R.C.; et al. FBXO44 Promotes DNA Replication-Coupled Repetitive Element Silencing in Cancer Cells. Cell 2021, 184, 352–369.e23. [Google Scholar] [CrossRef] [PubMed]
- Teugels, E.; De Brakeleer, S.; Goelen, G.; Lissens, W.; Sermijn, E.; De Grève, J. De Novo Alu Element Insertions Targeted to a Sequence Common to the BRCA1 and BRCA2 Genes. Hum. Mutat. 2005, 26, 284. [Google Scholar] [CrossRef] [PubMed]
- De Brakeleer, S.; De Grève, J.; Lissens, W.; Teugels, E. Systematic Detection of Pathogenic Alu Element Insertions in NGS-Based Diagnostic Screens: The BRCA1/BRCA2 Example. Hum. Mutat. 2013, 34, 785–791. [Google Scholar] [CrossRef]
- Lobachev, K.S.; Stenger, J.E.; Kozyreva, O.G.; Jurka, J.; Gordenin, D.A.; Resnick, M.A. Inverted Alu Repeats Unstable in Yeast Are Excluded from the Human Genome. EMBO J. 2000, 19, 3822–3830. [Google Scholar] [CrossRef]
- Li, Y.; Salo-Mullen, E.; Varghese, A.; Trottier, M.; Stadler, Z.K.; Zhang, L. Insertion of an Alu-like Element in MLH1 Intron 7 as a Novel Cause of Lynch Syndrome. Mol. Genet. Genom. Med. 2020, 8, e1523. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Driver, C.J.; McKechnie, S.W. Transposable Elements as a Factor in the Aging of Drosophila Melanogaster. Ann. N. Y. Acad. Sci. 1992, 673, 83–91. [Google Scholar] [CrossRef]
- Wood, J.G.; Helfand, S.L. Chromatin Structure and Transposable Elements in Organismal Aging. Front. Genet. 2013, 4, 274. [Google Scholar] [CrossRef]
- Wood, J.G.; Hillenmeyer, S.; Lawrence, C.; Chang, C.; Hosier, S.; Lightfoot, W.; Mukherjee, E.; Jiang, N.; Schorl, C.; Brodsky, A.S.; et al. Chromatin Remodeling in the Aging Genome of Drosophila. Aging Cell 2010, 9, 971–978. [Google Scholar] [CrossRef]
- Larson, K.; Yan, S.-J.; Tsurumi, A.; Liu, J.; Zhou, J.; Gaur, K.; Guo, D.; Eickbush, T.H.; Li, W.X. Heterochromatin Formation Promotes Longevity and Represses Ribosomal RNA Synthesis. PLoS Genet. 2012, 8, e1002473. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Meter, M.V.; Ablaeva, J.; Ke, Z.; Gonzalez, R.S.; Taguchi, T.; Cecco, M.D.; Leonova, K.I.; Kogan, V.; Helfand, S.L.; et al. LINE1 Derepression in Aged Wild-Type and SIRT6-Deficient Mice Drives Inflammation. Cell Metab. 2019, 29, 871–885.e5. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.E.; Wahl, D.; Cavalier, A.N.; McWilliams, G.T.; Rossman, M.J.; Giordano, G.R.; Bryan, A.D.; Seals, D.R.; LaRocca, T.J. Repetitive Element Transcript Accumulation Is Associated with Inflammaging in Humans. GeroScience 2024. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Loba, A.; Flores, I.; Fernández-Marcos, P.J.; Cayuela, M.L.; Maraver, A.; Tejera, A.; Borrás, C.; Matheu, A.; Klatt, P.; Flores, J.M.; et al. Telomerase Reverse Transcriptase Delays Aging in Cancer-Resistant Mice. Cell 2008, 135, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Jaskelioff, M.; Muller, F.L.; Paik, J.-H.; Thomas, E.; Jiang, S.; Adams, A.C.; Sahin, E.; Kost-Alimova, M.; Protopopov, A.; Cadiñanos, J.; et al. Telomerase Reactivation Reverses Tissue Degeneration in Aged Telomerase-Deficient Mice. Nature 2011, 469, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, M.E.; Garza, R.; Johansson, P.A.; Jakobsson, J. Transposable Elements: A Common Feature of Neurodevelopmental and Neurodegenerative Disorders. Trends Genet. 2020, 36, 610–623. [Google Scholar] [CrossRef]
- Neumann, M.; Sampathu, D.M.; Kwong, L.K.; Truax, A.C.; Micsenyi, M.C.; Chou, T.T.; Bruce, J.; Schuck, T.; Grossman, M.; Clark, C.M.; et al. Ubiquitinated TDP-43 in Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis. Science 2006, 314, 130–133. [Google Scholar] [CrossRef]
- Liu, E.Y.; Russ, J.; Cali, C.P.; Phan, J.M.; Amlie-Wolf, A.; Lee, E.B. Loss of Nuclear TDP-43 Is Associated with Decondensation of LINE Retrotransposons. Cell Rep. 2019, 27, 1409–1421.e6. [Google Scholar] [CrossRef]
- Tam, O.H.; Rozhkov, N.V.; Shaw, R.; Kim, D.; Hubbard, I.; Fennessey, S.; Propp, N.; Phatnani, H.; Kwan, J.; Sareen, D.; et al. Postmortem Cortex Samples Identify Distinct Molecular Subtypes of ALS: Retrotransposon Activation, Oxidative Stress, and Activated Glia. Cell Rep. 2019, 29, 1164–1177.e5. [Google Scholar] [CrossRef]
- Sun, W.; Samimi, H.; Gamez, M.; Zare, H.; Frost, B. Pathogenic Tau-Induced piRNA Depletion Promotes Neuronal Death through Transposable Element Dysregulation in Neurodegenerative Tauopathies. Nat. Neurosci. 2018, 21, 1038–1048. [Google Scholar] [CrossRef]
- Guo, C.; Jeong, H.-H.; Hsieh, Y.-C.; Klein, H.-U.; Bennett, D.A.; De Jager, P.L.; Liu, Z.; Shulman, J.M. Tau Activates Transposable Elements in Alzheimer’s Disease. Cell Rep. 2018, 23, 2874–2880. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, E.; Ramirez, P.; Gonzalez, E.; De Mange, J.; Ray, W.J.; Bieniek, K.F.; Frost, B. Pathogenic Tau–Induced Transposable Element–Derived dsRNA Drives Neuroinflammation. Sci. Adv. 2023, 9, eabq5423. [Google Scholar] [CrossRef] [PubMed]
- Mustafin, R.N.; Khusnutdinova, E.K. Involvement of Transposable Elements in Alzheimer’s Disease Pathogenesis. Vavilovskii Zhurnal Genet. Sel. 2024, 28, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Frost, B.; Hemberg, M.; Lewis, J.; Feany, M.B. Tau Promotes Neurodegeneration through Global Chromatin Relaxation. Nat. Neurosci. 2014, 17, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Grundman, J.; Spencer, B.; Sarsoza, F.; Rissman, R.A. Transcriptome Analyses Reveal Tau Isoform-Driven Changes in Transposable Element and Gene Expression. PLoS ONE 2021, 16, e0251611. [Google Scholar] [CrossRef]
- Hou, Y.; Li, Y.; Xiang, J.-F.; Tilahun, K.; Jiang, J.; Corces, V.G.; Yao, B. TDP-43 Chronic Deficiency Leads to Dysregulation of Transposable Elements and Gene Expression by Affecting R-Loop and 5hmC Crosstalk. Cell Rep. 2024, 43, 113662. [Google Scholar] [CrossRef]
- Dembny, P.; Newman, A.G.; Singh, M.; Hinz, M.; Szczepek, M.; Krüger, C.; Adalbert, R.; Dzaye, O.; Trimbuch, T.; Wallach, T.; et al. Human Endogenous Retrovirus HERV-K(HML-2) RNA Causes Neurodegeneration through Toll-like Receptors. JCI Insight 2020, 5, e131093. [Google Scholar] [CrossRef]
- Scopa, C.; Barnada, S.M.; Cicardi, M.E.; Singer, M.; Trotti, D.; Trizzino, M. JUN Upregulation Drives Aberrant Transposable Element Mobilization, Associated Innate Immune Response, and Impaired Neurogenesis in Alzheimer’s Disease. Nat. Commun. 2023, 14, 8021. [Google Scholar] [CrossRef]
- Evering, T.H.; Marston, J.L.; Gan, L.; Nixon, D.F. Transposable Elements and Alzheimer’s Disease Pathogenesis. Trends Neurosci. 2023, 46, 170–172. [Google Scholar] [CrossRef]
- Lang, A.E.; Lozano, A.M. Parkinson’s Disease. First of Two Parts. N. Engl. J. Med. 1998, 339, 1044–1053. [Google Scholar] [CrossRef]
- Beitz, J.M. Parkinson’s Disease: A Review. Front. Biosci. 2014, 6, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Baeken, M.W.; Moosmann, B.; Hajieva, P. Retrotransposon Activation by Distressed Mitochondria in Neurons. Biochem. Biophys. Res. Commun. 2020, 525, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Blaudin de Thé, F.; Rekaik, H.; Peze-Heidsieck, E.; Massiani-Beaudoin, O.; Joshi, R.L.; Fuchs, J.; Prochiantz, A. Engrailed Homeoprotein Blocks Degeneration in Adult Dopaminergic Neurons through LINE-1 Repression. EMBO J. 2018, 37, e97374. [Google Scholar] [CrossRef]
- Billingsley, K.J.; Lättekivi, F.; Planken, A.; Reimann, E.; Kurvits, L.; Kadastik-Eerme, L.; Kasterpalu, K.M.; Bubb, V.J.; Quinn, J.P.; Kõks, S.; et al. Analysis of Repetitive Element Expression in the Blood and Skin of Patients with Parkinson’s Disease Identifies Differential Expression of Satellite Elements. Sci. Rep. 2019, 9, 4369. [Google Scholar] [CrossRef] [PubMed]
- Loke, Y.J.; Hannan, A.J.; Craig, J.M. The Role of Epigenetic Change in Autism Spectrum Disorders. Front. Neurol. 2015, 6, 107. [Google Scholar] [CrossRef]
- LaSalle, J.M. Epigenomic Signatures Reveal Mechanistic Clues and Predictive Markers for Autism Spectrum Disorder. Mol. Psychiatry 2023, 28, 1890–1901. [Google Scholar] [CrossRef] [PubMed]
- Smigielski, L.; Jagannath, V.; Rössler, W.; Walitza, S.; Grünblatt, E. Epigenetic Mechanisms in Schizophrenia and Other Psychotic Disorders: A Systematic Review of Empirical Human Findings. Mol. Psychiatry 2020, 25, 1718–1748. [Google Scholar] [CrossRef] [PubMed]
- Muotri, A.R.; Marchetto, M.C.N.; Coufal, N.G.; Oefner, R.; Yeo, G.; Nakashima, K.; Gage, F.H. L1 Retrotransposition in Neurons Is Modulated by MeCP2. Nature 2010, 468, 443–446. [Google Scholar] [CrossRef]
- Neul, J.L.; Kaufmann, W.E.; Glaze, D.G.; Christodoulou, J.; Clarke, A.J.; Bahi-Buisson, N.; Leonard, H.; Bailey, M.E.S.; Schanen, N.C.; Zappella, M.; et al. Rett Syndrome: Revised Diagnostic Criteria and Nomenclature. Ann. Neurol. 2010, 68, 944–950. [Google Scholar] [CrossRef]
- Amir, R.E.; Van den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett Syndrome Is Caused by Mutations in X-Linked MECP2, Encoding Methyl-CpG-Binding Protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef]
- Bundo, M.; Toyoshima, M.; Okada, Y.; Akamatsu, W.; Ueda, J.; Nemoto-Miyauchi, T.; Sunaga, F.; Toritsuka, M.; Ikawa, D.; Kakita, A.; et al. Increased L1 Retrotransposition in the Neuronal Genome in Schizophrenia. Neuron 2014, 81, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Doyle, G.A.; Crist, R.C.; Karatas, E.T.; Hammond, M.J.; Ewing, A.D.; Ferraro, T.N.; Hahn, C.-G.; Berrettini, W.H. Analysis of LINE-1 Elements in DNA from Postmortem Brains of Individuals with Schizophrenia. Neuropsychopharmacology 2017, 42, 2602–2611. [Google Scholar] [CrossRef] [PubMed]
- Aditi; Downing, S.M.; Schreiner, P.A.; Kwak, Y.D.; Li, Y.; Shaw, T.I.; Russell, H.R.; McKinnon, P.J. Genome Instability Independent of Type I Interferon Signaling Drives Neuropathology Caused by Impaired Ribonucleotide Excision Repair. Neuron 2021, 109, 3962–3979.e6. [Google Scholar] [CrossRef] [PubMed]
- Le Breton, A.; Bettencourt, M.P.; Gendrel, A.-V. Navigating the Brain and Aging: Exploring the Impact of Transposable Elements from Health to Disease. Front. Cell Dev. Biol. 2024, 12, 1357576. [Google Scholar] [CrossRef]
- Ravel-Godreuil, C.; Znaidi, R.; Bonnifet, T.; Joshi, R.L.; Fuchs, J. Transposable Elements as New Players in Neurodegenerative Diseases. FEBS Lett. 2021, 595, 2733–2755. [Google Scholar] [CrossRef]
- Laumont, C.M.; Vincent, K.; Hesnard, L.; Audemard, É.; Bonneil, É.; Laverdure, J.-P.; Gendron, P.; Courcelles, M.; Hardy, M.-P.; Côté, C.; et al. Noncoding Regions Are the Main Source of Targetable Tumor-Specific Antigens. Sci. Transl. Med. 2018, 10, eaau5516. [Google Scholar] [CrossRef]
- Jones, P.A.; Issa, J.-P.J.; Baylin, S. Targeting the Cancer Epigenome for Therapy. Nat. Rev. Genet. 2016, 17, 630–641. [Google Scholar] [CrossRef]
- Kong, Y.; Rose, C.M.; Cass, A.A.; Williams, A.G.; Darwish, M.; Lianoglou, S.; Haverty, P.M.; Tong, A.-J.; Blanchette, C.; Albert, M.L.; et al. Transposable Element Expression in Tumors Is Associated with Immune Infiltration and Increased Antigenicity. Nat. Commun. 2019, 10, 5228. [Google Scholar] [CrossRef]
- Shah, N.M.; Jang, H.J.; Liang, Y.; Maeng, J.H.; Tzeng, S.-C.; Wu, A.; Basri, N.L.; Qu, X.; Fan, C.; Li, A.; et al. Pan-Cancer Analysis Identifies Tumor-Specific Antigens Derived from Transposable Elements. Nat. Genet. 2023, 55, 631–639. [Google Scholar] [CrossRef]
- Liang, Y.; Qu, X.; Shah, N.M.; Wang, T. Towards Targeting Transposable Elements for Cancer Therapy. Nat. Rev. Cancer 2024, 24, 123–140. [Google Scholar] [CrossRef]
- Roulois, D.; Loo Yau, H.; Singhania, R.; Wang, Y.; Danesh, A.; Shen, S.Y.; Han, H.; Liang, G.; Jones, P.A.; Pugh, T.J.; et al. DNA-Demethylating Agents Target Colorectal Cancer Cells by Inducing Viral Mimicry by Endogenous Transcripts. Cell 2015, 162, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Chiappinelli, K.B.; Strissel, P.L.; Desrichard, A.; Li, H.; Henke, C.; Akman, B.; Hein, A.; Rote, N.S.; Cope, L.M.; Snyder, A.; et al. Inhibiting DNA Methylation Causes an Interferon Response in Cancer via dsRNA Including Endogenous Retroviruses. Cell 2015, 162, 974–986. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, L.; Wu, Z.; Yuan, K.; Hong, G.; Lian, Z.; Feng, J.; Li, N.; Li, D.; Wong, J.; et al. Loss of PHF8 Induces a Viral Mimicry Response by Activating Endogenous Retrotransposons. Nat. Commun. 2023, 14, 4225. [Google Scholar] [CrossRef]
- Wu, Q.; Nie, D.Y.; Ba-alawi, W.; Ji, Y.; Zhang, Z.; Cruickshank, J.; Haight, J.; Ciamponi, F.E.; Chen, J.; Duan, S.; et al. PRMT Inhibition Induces a Viral Mimicry Response in Triple-Negative Breast Cancer. Nat. Chem. Biol. 2022, 18, 821–830. [Google Scholar] [CrossRef]
- Appleton, E.; Hassan, J.; Chan Wah Hak, C.; Sivamanoharan, N.; Wilkins, A.; Samson, A.; Ono, M.; Harrington, K.J.; Melcher, A.; Wennerberg, E. Kickstarting Immunity in Cold Tumours: Localised Tumour Therapy Combinations with Immune Checkpoint Blockade. Front. Immunol. 2021, 12, 754436. [Google Scholar] [CrossRef] [PubMed]
- Morel, K.L.; Sheahan, A.V.; Burkhart, D.L.; Baca, S.C.; Boufaied, N.; Liu, Y.; Qiu, X.; Cañadas, I.; Roehle, K.; Heckler, M.; et al. EZH2 Inhibition Activates a dsRNA-STING-Interferon Stress Axis That Potentiates Response to PD-1 Checkpoint Blockade in Prostate Cancer. Nat. Cancer 2021, 2, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhu, Q.; Shi, Z.; Tu, Y.; Li, Q.; Zheng, W.; Yuan, Z.; Li, L.; Zu, X.; Hao, Y.; et al. Dual Inhibitors of DNMT and HDAC Induce Viral Mimicry to Induce Antitumour Immunity in Breast Cancer. Cell Death Discov. 2024, 10, 143. [Google Scholar] [CrossRef]
- Cortesi, A.; Gandolfi, F.; Arco, F.; Di Chiaro, P.; Valli, E.; Polletti, S.; Noberini, R.; Gualdrini, F.; Attanasio, S.; Citron, F.; et al. Activation of Endogenous Retroviruses and Induction of Viral Mimicry by MEK1/2 Inhibition in Pancreatic Cancer. Sci. Adv. 2024, 10, eadk5386. [Google Scholar] [CrossRef]
- Murayama, T.; Nakayama, J.; Jiang, X.; Miyata, K.; Morris, A.D.; Cai, K.Q.; Prasad, R.M.; Ma, X.; Efimov, A.; Belani, N.; et al. Targeting DHX9 Triggers Tumor-Intrinsic Interferon Response and Replication Stress in Small Cell Lung Cancer. Cancer Discov. 2024, 14, 468–491. [Google Scholar] [CrossRef]
- Goel, S.; DeCristo, M.J.; Watt, A.C.; BrinJones, H.; Sceneay, J.; Li, B.B.; Khan, N.; Ubellacker, J.M.; Xie, S.; Metzger-Filho, O.; et al. CDK4/6 Inhibition Triggers Anti-Tumour Immunity. Nature 2017, 548, 471–475. [Google Scholar] [CrossRef]
- McDonald, J.I.; Diab, N.; Arthofer, E.; Hadley, M.; Kanholm, T.; Rentia, U.; Gomez, S.; Yu, A.; Grundy, E.E.; Cox, O.; et al. Epigenetic Therapies in Ovarian Cancer Alter Repetitive Element Expression in a TP53-Dependent Manner. Cancer Res. 2021, 81, 5176–5189. [Google Scholar] [CrossRef] [PubMed]
- Wang-Johanning, F.; Radvanyi, L.; Rycaj, K.; Plummer, J.B.; Yan, P.; Sastry, K.J.; Piyathilake, C.J.; Hunt, K.K.; Johanning, G.L. Human Endogenous Retrovirus K Triggers an Antigen-Specific Immune Response in Breast Cancer Patients. Cancer Res. 2008, 68, 5869–5877. [Google Scholar] [CrossRef]
- Kraus, B.; Fischer, K.; Büchner, S.M.; Wels, W.S.; Löwer, R.; Sliva, K.; Schnierle, B.S. Vaccination Directed against the Human Endogenous Retrovirus-K Envelope Protein Inhibits Tumor Growth in a Murine Model System. PLoS ONE 2013, 8, e72756. [Google Scholar] [CrossRef]
- Rycaj, K.; Plummer, J.B.; Yin, B.; Li, M.; Garza, J.; Radvanyi, L.; Ramondetta, L.M.; Lin, K.; Johanning, G.L.; Tang, D.G.; et al. Cytotoxicity of Human Endogenous Retrovirus K-Specific T Cells toward Autologous Ovarian Cancer Cells. Clin. Cancer Res. 2015, 21, 471–483. [Google Scholar] [CrossRef]
- Cherkasova, E.; Scrivani, C.; Doh, S.; Weisman, Q.; Takahashi, Y.; Harashima, N.; Yokoyama, H.; Srinivasan, R.; Linehan, W.M.; Lerman, M.I.; et al. Detection of an Immunogenic HERV-E Envelope with Selective Expression in Clear Cell Kidney Cancer. Cancer Res. 2016, 76, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Sacha, J.B.; Kim, I.-J.; Chen, L.; Ullah, J.H.; Goodwin, D.A.; Simmons, H.A.; Schenkman, D.I.; von Pelchrzim, F.; Gifford, R.J.; Nimityongskul, F.A.; et al. Vaccination with Cancer- and HIV Infection-Associated Endogenous Retrotransposable Elements Is Safe and Immunogenic. J. Immunol. 2012, 189, 1467–1479. [Google Scholar] [CrossRef]
- Griffin, G.K.; Wu, J.; Iracheta-Vellve, A.; Patti, J.C.; Hsu, J.; Davis, T.; Dele-Oni, D.; Du, P.P.; Halawi, A.G.; Ishizuka, J.J.; et al. Epigenetic Silencing by SETDB1 Suppresses Tumour Intrinsic Immunogenicity. Nature 2021, 595, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, J.; Rabinovich, B.A.; Mi, T.; Switzer, K.C.; Olivares, S.; Maiti, S.N.; Plummer, J.B.; Singh, H.; Kumaresan, P.R.; Huls, H.M.; et al. Genetic Engineering of T Cells to Target HERV-K, an Ancient Retrovirus on Melanoma. Clin. Cancer Res. 2015, 21, 3241–3251. [Google Scholar] [CrossRef]
- Zhou, F.; Krishnamurthy, J.; Wei, Y.; Li, M.; Hunt, K.; Johanning, G.L.; Cooper, L.J.; Wang-Johanning, F. Chimeric Antigen Receptor T Cells Targeting HERV-K Inhibit Breast Cancer and Its Metastasis through Downregulation of Ras. Oncoimmunology 2015, 4, e1047582. [Google Scholar] [CrossRef]
- Li, M.; Radvanyi, L.; Yin, B.; Rycaj, K.; Li, J.; Chivukula, R.; Lin, K.; Lu, Y.; Shen, J.; Chang, D.Z.; et al. Downregulation of Human Endogenous Retrovirus Type K (HERV-K) Viral Env RNA in Pancreatic Cancer Cells Decreases Cell Proliferation and Tumor Growth. Clin. Cancer Res. 2017, 23, 5892–5911. [Google Scholar] [CrossRef]
- Hay, K.A. Cytokine Release Syndrome and Neurotoxicity after CD19 Chimeric Antigen Receptor-Modified (CAR-) T Cell Therapy. Br. J. Haematol. 2018, 183, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, K.; Rouce, R.; Liu, E.; Shpall, E. Engineering Natural Killer Cells for Cancer Immunotherapy. Mol. Ther. 2017, 25, 1769–1781. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Sharma, A.; Liu, T.; Hou, J.; Schmidt-Wolf, I.G. Synergistic Integration of Histone Deacetylase Inhibitors Apparently Enhances the Cytokine-induced Killer Cell Efficiency in Multiple Myeloma via the NKG2D Pathway. Clin. Transl. Immunol. 2024, 13, e1500. [Google Scholar] [CrossRef]
- Berger, G.; Knelson, E.H.; Jimenez-Macias, J.L.; Nowicki, M.O.; Han, S.; Panagioti, E.; Lizotte, P.H.; Adu-Berchie, K.; Stafford, A.; Dimitrakakis, N.; et al. STING Activation Promotes Robust Immune Response and NK Cell–Mediated Tumor Regression in Glioblastoma Models. Proc. Natl. Acad. Sci. USA 2022, 119, e2111003119. [Google Scholar] [CrossRef]
- Wang-Johanning, F.; Rycaj, K.; Plummer, J.B.; Li, M.; Yin, B.; Frerich, K.; Garza, J.G.; Shen, J.; Lin, K.; Yan, P.; et al. Immunotherapeutic Potential of Anti-Human Endogenous Retrovirus-K Envelope Protein Antibodies in Targeting Breast Tumors. J. Natl. Cancer Inst. 2012, 104, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.W.; Boumelha, J.; Enfield, K.S.S.; Almagro, J.; Cha, H.; Pich, O.; Karasaki, T.; Moore, D.A.; Salgado, R.; Sivakumar, M.; et al. Antibodies against Endogenous Retroviruses Promote Lung Cancer Immunotherapy. Nature 2023, 616, 563–573. [Google Scholar] [CrossRef]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody Drug Conjugate: The “Biological Missile” for Targeted Cancer Therapy. Sig. Transduct. Target. Ther. 2022, 7, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Zaretsky, J.M.; Garcia-Diaz, A.; Shin, D.S.; Escuin-Ordinas, H.; Hugo, W.; Hu-Lieskovan, S.; Torrejon, D.Y.; Abril-Rodriguez, G.; Sandoval, S.; Barthly, L.; et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N. Engl. J. Med. 2016, 375, 819–829. [Google Scholar] [CrossRef]
- Gao, J.; Shi, L.Z.; Zhao, H.; Chen, J.; Xiong, L.; He, Q.; Chen, T.; Roszik, J.; Bernatchez, C.; Woodman, S.E.; et al. Loss of IFN-γ Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell 2016, 167, 397–404.e9. [Google Scholar] [CrossRef]
- Kearney, C.J.; Vervoort, S.J.; Hogg, S.J.; Ramsbottom, K.M.; Freeman, A.J.; Lalaoui, N.; Pijpers, L.; Michie, J.; Brown, K.K.; Knight, D.A.; et al. Tumor Immune Evasion Arises through Loss of TNF Sensitivity. Sci. Immunol. 2018, 3, eaar3451. [Google Scholar] [CrossRef]
- Symer, D.E.; Connelly, C.; Szak, S.T.; Caputo, E.M.; Cost, G.J.; Parmigiani, G.; Boeke, J.D. Human L1 Retrotransposition Is Associated with Genetic Instability in Vivo. Cell 2002, 110, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, A.; García-Muse, T. R Loops: From Transcription Byproducts to Threats to Genome Stability. Mol. Cell 2012, 46, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Ardeljan, D.; Steranka, J.P.; Liu, C.; Li, Z.; Taylor, M.S.; Payer, L.M.; Gorbounov, M.; Sarnecki, J.S.; Deshpande, V.; Hruban, R.H.; et al. Cell Fitness Screens Reveal a Conflict between LINE-1 Retrotransposition and DNA Replication. Nat. Struct. Mol. Biol. 2020, 27, 168–178. [Google Scholar] [CrossRef]
- Feser, J.; Truong, D.; Das, C.; Carson, J.J.; Kieft, J.; Harkness, T.; Tyler, J.K. Elevated Histone Expression Promotes Life Span Extension. Mol. Cell 2010, 39, 724–735. [Google Scholar] [CrossRef]
- Pal, S.; Tyler, J.K. Epigenetics and Aging. Sci. Adv. 2016, 2, e1600584. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, H.; Hu, Q.; Wang, L.; Liu, J.; Zheng, Z.; Zhang, W.; Ren, J.; Zhu, F.; Liu, G.-H. Epigenetic Regulation of Aging: Implications for Interventions of Aging and Diseases. Sig. Transduct. Target. Ther. 2022, 7, 374. [Google Scholar] [CrossRef]
- Jung, M.; Pfeifer, G.P. Aging and DNA Methylation. BMC Biol. 2015, 13, 7. [Google Scholar] [CrossRef]
- Wilson, V.L.; Smith, R.A.; Ma, S.; Cutler, R.G. Genomic 5-Methyldeoxycytidine Decreases with Age. J. Biol. Chem. 1987, 262, 9948–9951. [Google Scholar] [CrossRef]
- Rinaldi, C.; Wood, M.J.A. Antisense Oligonucleotides: The next Frontier for Treatment of Neurological Disorders. Nat. Rev. Neurol. 2018, 14, 9–21. [Google Scholar] [CrossRef]
- Lee, C.; Longo, V. Dietary Restriction with and without Caloric Restriction for Healthy Aging. F1000Research 2016, 5. [Google Scholar] [CrossRef]
- Peze-Heidsieck, E.; Bonnifet, T.; Znaidi, R.; Ravel-Godreuil, C.; Massiani-Beaudoin, O.; Joshi, R.L.; Fuchs, J. Retrotransposons as a Source of DNA Damage in Neurodegeneration. Front. Aging Neurosci. 2022, 13, 786897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Tan, S.; Tang, N.; Li, Y.; Zhang, C.; Sun, J.; Guo, Y.; Gao, H.; Cai, Y.; Sun, W.; et al. Heterologous Survey of 130 DNA Transposons in Human Cells Highlights Their Functional Divergence and Expands the Genome Engineering Toolbox. Cell 2024, 187, 3741–3760.e30. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snowbarger, J.; Koganti, P.; Spruck, C. Evolution of Repetitive Elements, Their Roles in Homeostasis and Human Disease, and Potential Therapeutic Applications. Biomolecules 2024, 14, 1250. https://doi.org/10.3390/biom14101250
Snowbarger J, Koganti P, Spruck C. Evolution of Repetitive Elements, Their Roles in Homeostasis and Human Disease, and Potential Therapeutic Applications. Biomolecules. 2024; 14(10):1250. https://doi.org/10.3390/biom14101250
Chicago/Turabian StyleSnowbarger, Jeffrey, Praveen Koganti, and Charles Spruck. 2024. "Evolution of Repetitive Elements, Their Roles in Homeostasis and Human Disease, and Potential Therapeutic Applications" Biomolecules 14, no. 10: 1250. https://doi.org/10.3390/biom14101250





