Advancing Roles and Therapeutic Potentials of Pyroptosis in Host Immune Defenses against Tuberculosis
Abstract
:1. Introduction
2. The Role of Pyroptosis in Tuberculosis
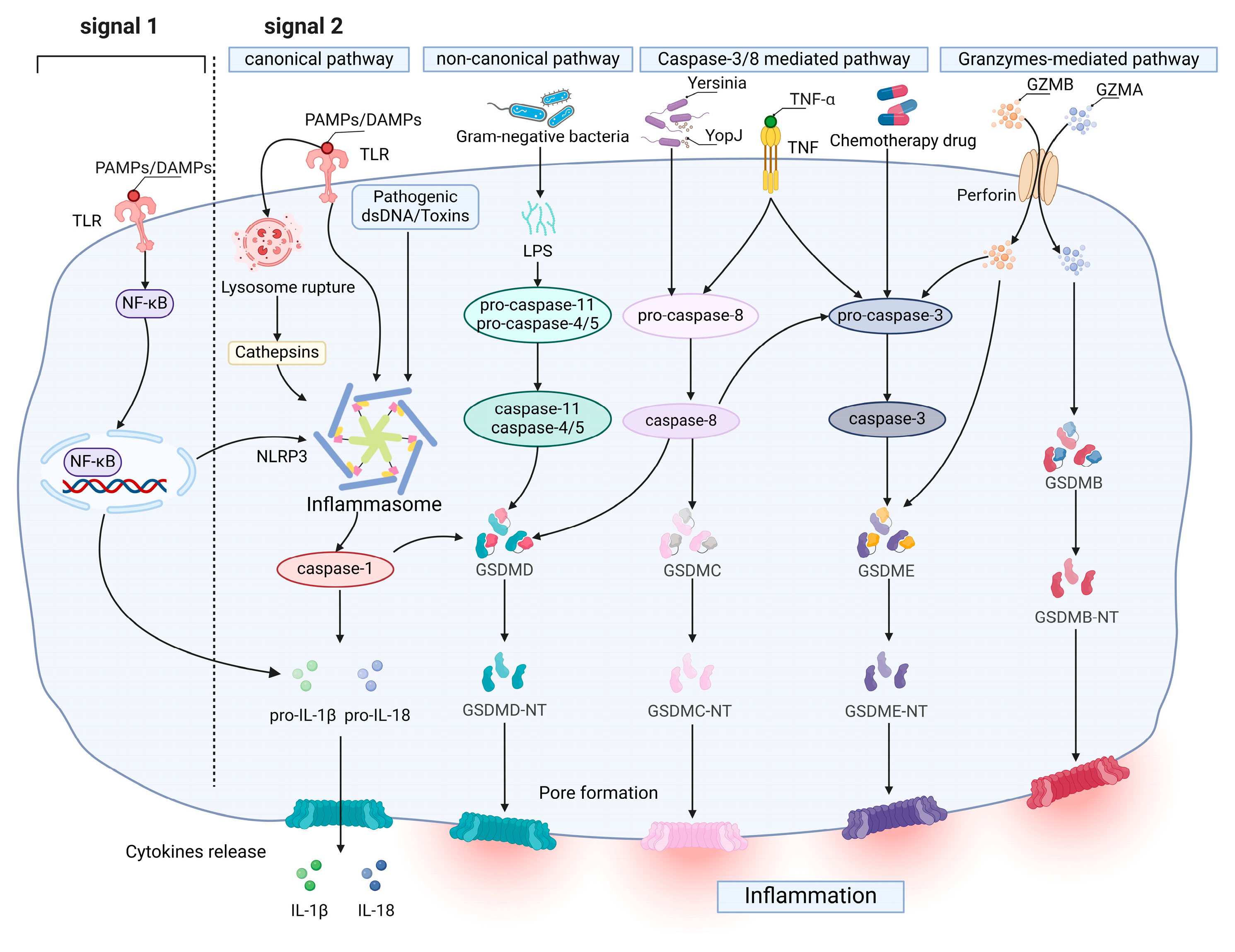
2.1. Molecular Mechanism of Pyroptosis
2.2. The Impact of Pyroptosis on Tuberculosis
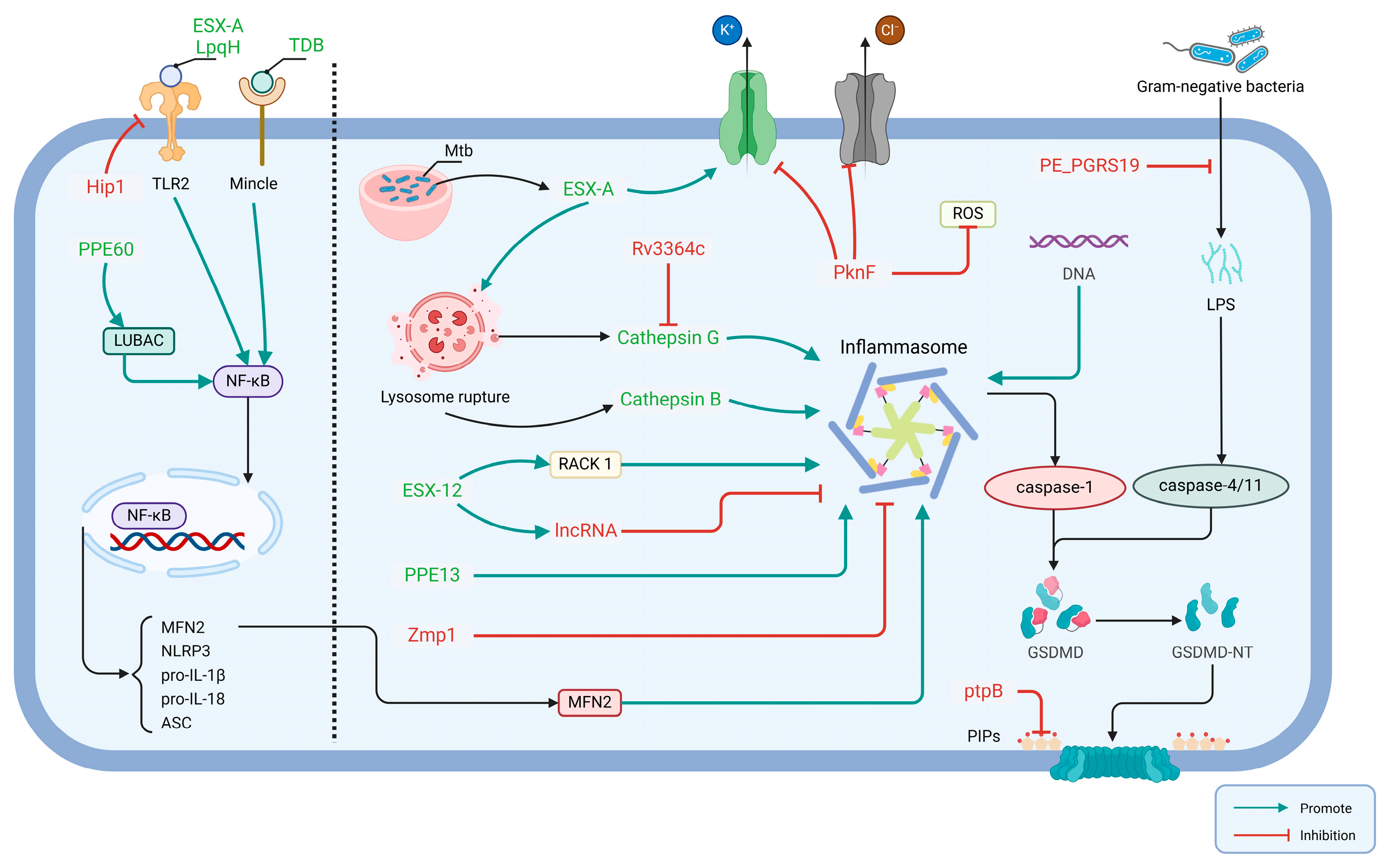
2.2.1. Mtb Effector Involved in Activation of Host Pyroptosis
2.2.2. Mtb Effector Involved in Inhibition of Host Pyroptosis
3. Potential of Pyroptosis as Anti-TB Target
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Furin, J.; Cox, H.; Pai, M. Tuberculosis. Lancet 2019, 393, 1642–1656. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2023; World Health Organization: Geneva, Switzerland, 2023; ISBN 978-92-4-008385-1. [Google Scholar]
- Singh, V.; Chibale, K. Strategies to Combat Multi-Drug Resistance in Tuberculosis. Acc. Chem. Res. 2021, 54, 2361–2376. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.; Behr, M.A.; Dowdy, D.; Dheda, K.; Divangahi, M.; Boehme, C.C.; Ginsberg, A.; Swaminathan, S.; Spigelman, M.; Getahun, H.; et al. Tuberculosis. Nat. Rev. Dis. Primer 2016, 2, 16076. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.B.; Gern, B.H.; Delahaye, J.L.; Adams, K.N.; Plumlee, C.R.; Winkler, J.K.; Sherman, D.R.; Gerner, M.Y.; Urdahl, K.B. Alveolar Macrophages Provide an Early Mycobacterium tuberculosis Niche and Initiate Dissemination. Cell Host Microbe 2018, 24, 439–446.e4. [Google Scholar] [CrossRef]
- Carabalí-Isajar, M.L.; Rodríguez-Bejarano, O.H.; Amado, T.; Patarroyo, M.A.; Izquierdo, M.A.; Lutz, J.R.; Ocampo, M. Clinical Manifestations and Immune Response to Tuberculosis. World J. Microbiol. Biotechnol. 2023, 39, 206. [Google Scholar] [CrossRef]
- Kroon, E.E.; Coussens, A.K.; Kinnear, C.; Orlova, M.; Möller, M.; Seeger, A.; Wilkinson, R.J.; Hoal, E.G.; Schurr, E. Neutrophils: Innate Effectors of TB Resistance? Front. Immunol. 2018, 9, 2637. [Google Scholar] [CrossRef]
- Blanc, L.; Gilleron, M.; Prandi, J.; Song, O.; Jang, M.-S.; Gicquel, B.; Drocourt, D.; Neyrolles, O.; Brodin, P.; Tiraby, G.; et al. Mycobacterium tuberculosis Inhibits Human Innate Immune Responses via the Production of TLR2 Antagonist Glycolipids. Proc. Natl. Acad. Sci. USA 2017, 114, 11205–11210. [Google Scholar] [CrossRef]
- Patankar, Y.R.; Sutiwisesak, R.; Boyce, S.; Lai, R.; Arlehamn, C.S.L.; Sette, A.; Behar, S.M. Limited Recognition of Mycobacterium tuberculosis-Infected Macrophages by Polyclonal CD4 and CD8 T Cells from the Lungs of Infected Mice. Mucosal Immunol. 2020, 13, 140–148. [Google Scholar] [CrossRef]
- Mahon, R.N.; Sande, O.J.; Rojas, R.E.; Levine, A.D.; Harding, C.V.; Henry Boom, W. Mycobacterium tuberculosis ManLAM Inhibits T-Cell-Receptor Signaling by Interference with ZAP-70, Lck and LAT Phosphorylation. Cell. Immunol. 2012, 275, 98–105. [Google Scholar] [CrossRef]
- Goldberg, M.F.; Saini, N.K.; Porcelli, S.A. Evasion of Innate and Adaptive Immunity by Mycobacterium tuberculosis. Microbiol. Spectr. 2014, 2, 1–172. [Google Scholar] [CrossRef]
- De Leon, J.; Jiang, G.; Ma, Y.; Rubin, E.; Fortune, S.; Sun, J. Mycobacterium tuberculosis ESAT-6 Exhibits a Unique Membrane-Interacting Activity That Is Not Found in Its Ortholog from Non-Pathogenic Mycobacterium smegmatis. J. Biol. Chem. 2012, 287, 44184–44191. [Google Scholar] [CrossRef] [PubMed]
- Dolasia, K.; Nazar, F.; Mukhopadhyay, S. Mycobacterium tuberculosis PPE18 Protein Inhibits MHC Class II Antigen Presentation and B Cell Response in Mice. Eur. J. Immunol. 2021, 51, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Noss, E.H.; Pai, R.K.; Sellati, T.J.; Radolf, J.D.; Belisle, J.; Golenbock, D.T.; Boom, W.H.; Harding, C.V. Toll-like Receptor 2-Dependent Inhibition of Macrophage Class II MHC Expression and Antigen Processing by 19-kDa Lipoprotein of Mycobacterium tuberculosis1. J. Immunol. 2001, 167, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.N. Pulmonary Tuberculosis: The Essentials. Radiology 1999, 210, 307–322. [Google Scholar] [CrossRef]
- Huppertz, C.; Jäger, B.; Wieczorek, G.; Engelhard, P.; Oliver, S.J.; Bauernfeind, F.-G.; Littlewood-Evans, A.; Welte, T.; Hornung, V.; Prasse, A. The NLRP3 Inflammasome Pathway Is Activated in Sarcoidosis and Involved in Granuloma Formation. Eur. Respir. J. 2020, 55, 1900119. [Google Scholar] [CrossRef]
- Chai, Q.; Yu, S.; Zhong, Y.; Lu, Z.; Qiu, C.; Yu, Y.; Zhang, X.; Zhang, Y.; Lei, Z.; Qiang, L.; et al. A Bacterial Phospholipid Phosphatase Inhibits Host Pyroptosis by Hijacking Ubiquitin. Science 2022, 378, eabq0132. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Man, S.M.; Karki, R.; Kanneganti, T.-D. Molecular Mechanisms and Functions of Pyroptosis, Inflammatory Caspases and Inflammasomes in Infectious Diseases. Immunol. Rev. 2017, 277, 61–75. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by Inflammatory Caspases Determines Pyroptotic Cell Death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Kesavardhana, S.; Malireddi, R.K.S.; Kanneganti, T.-D. Caspases in Cell Death, Inflammation, and Pyroptosis. Annu. Rev. Immunol. 2020, 38, 567–595. [Google Scholar] [CrossRef] [PubMed]
- Orning, P.; Lien, E.; Fitzgerald, K.A. Gasdermins and Their Role in Immunity and Inflammation. J. Exp. Med. 2019, 216, 2453–2465. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, W.; Hu, L.; Li, J.; Fang, Y.; Wang, X.; Xu, X.; Wang, Z.; Huang, K.; Han, J. Pyroptosis Is Driven by Non-Selective Gasdermin-D Pore and Its Morphology Is Different from MLKL Channel-Mediated Necroptosis. Cell Res. 2016, 26, 1007–1020. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, Q.; Li, X.; Zeng, Z.; Xiong, W.; Li, G.; Li, X.; Yang, J.; Xiang, B.; Yi, M. Pyroptosis: A New Paradigm of Cell Death for Fighting against Cancer. J. Exp. Clin. Cancer Res. CR 2021, 40, 153. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Hsu, J.-M.; Hung, M.-C. Molecular Mechanisms and Functions of Pyroptosis in Inflammation and Antitumor Immunity. Mol. Cell 2021, 81, 4579–4590. [Google Scholar] [CrossRef]
- Li, L.; Jiang, M.; Qi, L.; Wu, Y.; Song, D.; Gan, J.; Li, Y.; Bai, Y. Pyroptosis, a New Bridge to Tumor Immunity. Cancer Sci. 2021, 112, 3979–3994. [Google Scholar] [CrossRef]
- Hsu, S.-K.; Li, C.-Y.; Lin, I.-L.; Syue, W.-J.; Chen, Y.-F.; Cheng, K.-C.; Teng, Y.-N.; Lin, Y.-H.; Yen, C.-H.; Chiu, C.-C. Inflammation-Related Pyroptosis, a Novel Programmed Cell Death Pathway, and Its Crosstalk with Immune Therapy in Cancer Treatment. Theranostics 2021, 11, 8813–8835. [Google Scholar] [CrossRef]
- Wei, X.; Xie, F.; Zhou, X.; Wu, Y.; Yan, H.; Liu, T.; Huang, J.; Wang, F.; Zhou, F.; Zhang, L. Role of Pyroptosis in Inflammation and Cancer. Cell. Mol. Immunol. 2022, 19, 971–992. [Google Scholar] [CrossRef]
- Yarovinsky, T.O.; Su, M.; Chen, C.; Xiang, Y.; Tang, W.H.; Hwa, J. Pyroptosis in Cardiovascular Diseases: Pumping Gasdermin on the Fire. Semin. Immunol. 2023, 69, 101809. [Google Scholar] [CrossRef]
- He, X.; Fan, X.; Bai, B.; Lu, N.; Zhang, S.; Zhang, L. Pyroptosis Is a Critical Immune-Inflammatory Response Involved in Atherosclerosis. Pharmacol. Res. 2021, 165, 105447. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, B.; Li, S.; Yang, S. Pyroptosis, and Its Role in Central Nervous System Disease. J. Mol. Biol. 2022, 434, 167379. [Google Scholar] [CrossRef] [PubMed]
- Brokatzky, D.; Mostowy, S. Pyroptosis in Host Defence against Bacterial Infection. Dis. Model. Mech. 2022, 15, dmm049414. [Google Scholar] [CrossRef]
- Li, L.; Dickinson, M.S.; Coers, J.; Miao, E.A. Pyroptosis in Defense against Intracellular Bacteria. Semin. Immunol. 2023, 69, 101805. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Zhu, Y.; Yang, P.; Chen, Z.; Xia, Y.; Qiao, C.; Liu, W.; Deng, H.; Li, J.; Ning, P.; et al. Pyroptosis in Inflammatory Diseases and Cancer. Theranostics 2022, 12, 4310–4329. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xia, S.; Zhang, Z.; Wu, H.; Lieberman, J. Channelling Inflammation: Gasdermins in Physiology and Disease. Nat. Rev. Drug Discov. 2021, 20, 384–405. [Google Scholar] [CrossRef]
- Toldo, S.; Mezzaroma, E.; Buckley, L.F.; Potere, N.; Di Nisio, M.; Biondi-Zoccai, G.; Van Tassell, B.W.; Abbate, A. Targeting the NLRP3 Inflammasome in Cardiovascular Diseases. Pharmacol. Ther. 2022, 236, 108053. [Google Scholar] [CrossRef]
- Toldo, S.; Mauro, A.G.; Cutter, Z.; Abbate, A. Inflammasome, Pyroptosis, and Cytokines in Myocardial Ischemia-Reperfusion Injury. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1553–H1568. [Google Scholar] [CrossRef]
- Li, G.Q.; Gao, S.X.; Wang, F.H.; Kang, L.; Tang, Z.Y.; Ma, X.D. Anticancer Mechanisms on Pyroptosis Induced by Oridonin: New Potential Targeted Therapeutic Strategies. Biomed. Pharmacother. 2023, 165, 115019. [Google Scholar] [CrossRef]
- Miao, E.A.; Leaf, I.A.; Treuting, P.M.; Mao, D.P.; Dors, M.; Sarkar, A.; Warren, S.E.; Wewers, M.D.; Aderem, A. Caspase-1-Induced Pyroptosis Is an Innate Immune Effector Mechanism against Intracellular Bacteria. Nat. Immunol. 2010, 11, 1136–1142. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, Y.; Gao, W.; Ding, J.; Li, P.; Hu, L.; Shao, F. Inflammatory Caspases Are Innate Immune Receptors for Intracellular LPS. Nature 2014, 514, 187–192. [Google Scholar] [CrossRef]
- Kayagaki, N.; Stowe, I.B.; Lee, B.L.; O’Rourke, K.; Anderson, K.; Warming, S.; Cuellar, T.; Haley, B.; Roose-Girma, M.; Phung, Q.T.; et al. Caspase-11 Cleaves Gasdermin D for Non-Canonical Inflammasome Signalling. Nature 2015, 526, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Wong, M.T.; Stowe, I.B.; Ramani, S.R.; Gonzalez, L.C.; Akashi-Takamura, S.; Miyake, K.; Zhang, J.; Lee, W.P.; Muszyński, A.; et al. Noncanonical Inflammasome Activation by Intracellular LPS Independent of TLR4. Science 2013, 341, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Burgk, J.L.; Gaidt, M.M.; Schmidt, T.; Ebert, T.S.; Bartok, E.; Hornung, V. Caspase-4 Mediates Non-Canonical Activation of the NLRP3 Inflammasome in Human Myeloid Cells. Eur. J. Immunol. 2015, 45, 2911–2917. [Google Scholar] [CrossRef] [PubMed]
- Rühl, S.; Broz, P. Caspase-11 Activates a Canonical NLRP3 Inflammasome by Promoting K(+) Efflux. Eur. J. Immunol. 2015, 45, 2927–2936. [Google Scholar] [CrossRef]
- Baker, P.J.; Boucher, D.; Bierschenk, D.; Tebartz, C.; Whitney, P.G.; D’Silva, D.B.; Tanzer, M.C.; Monteleone, M.; Robertson, A.A.B.; Cooper, M.A.; et al. NLRP3 Inflammasome Activation Downstream of Cytoplasmic LPS Recognition by Both Caspase-4 and Caspase-5. Eur. J. Immunol. 2015, 45, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Chen, M.; Chen, X.; Zhao, C.; Fang, Z.; Wang, H.; Dai, H. Chemotherapy-Induced Pyroptosis Is Mediated by BAK/BAX-Caspase-3-GSDME Pathway and Inhibited by 2-Bromopalmitate. Cell Death Dis. 2020, 11, 281. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.; Shi, X.; Ding, J.; Liu, W.; He, H.; Wang, K.; Shao, F. Chemotherapy Drugs Induce Pyroptosis through Caspase-3 Cleavage of a Gasdermin. Nature 2017, 547, 99–103. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Xia, S.; Kong, Q.; Li, S.; Liu, X.; Junqueira, C.; Meza-Sosa, K.F.; Mok, T.M.Y.; Ansara, J.; et al. Gasdermin E Suppresses Tumour Growth by Activating Anti-Tumour Immunity. Nature 2020, 579, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, S.; Chen, W.; Lian, G.; Wu, W.; Chen, A.; Sagor, M.I.H.; Luo, L.; Wang, H.; Xie, L. TNF-α Contributes to Sarcopenia through Caspase-8/Caspase-3/GSDME-Mediated Pyroptosis. Cell Death Discov. 2023, 9, 76. [Google Scholar] [CrossRef]
- Rogers, C.; Erkes, D.A.; Nardone, A.; Aplin, A.E.; Fernandes-Alnemri, T.; Alnemri, E.S. Gasdermin Pores Permeabilize Mitochondria to Augment Caspase-3 Activation during Apoptosis and Inflammasome Activation. Nat. Commun. 2019, 10, 1689. [Google Scholar] [CrossRef]
- Chao, K.L.; Kulakova, L.; Herzberg, O. Gene Polymorphism Linked to Increased Asthma and IBD Risk Alters Gasdermin-B Structure, a Sulfatide and Phosphoinositide Binding Protein. Proc. Nat. Acad. Sci. USA 2017, 114, E1128–E1137. [Google Scholar] [CrossRef]
- Hou, J.; Zhao, R.; Xia, W.; Chang, C.-W.; You, Y.; Hsu, J.-M.; Nie, L.; Chen, Y.; Wang, Y.-C.; Liu, C.; et al. PD-L1-Mediated Gasdermin C Expression Switches Apoptosis to Pyroptosis in Cancer Cells and Facilitates Tumour Necrosis. Nat. Cell Biol. 2020, 22, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Orning, P.; Weng, D.; Starheim, K.; Ratner, D.; Best, Z.; Lee, B.; Brooks, A.; Xia, S.; Wu, H.; Kelliher, M.A.; et al. Pathogen Blockade of TAK1 Triggers Caspase-8-Dependent Cleavage of Gasdermin D and Cell Death. Science 2018, 362, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; He, H.; Wang, K.; Shi, X.; Wang, Y.; Su, Y.; Wang, Y.; Li, D.; Liu, W.; Zhang, Y.; et al. Granzyme A from Cytotoxic Lymphocytes Cleaves GSDMB to Trigger Pyroptosis in Target Cells. Science 2020, 368, eaaz7548. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, Y.; Chen, X.; Wang, Z.; Liang, X.; Zhang, T.; Liu, M.; Zhou, N.; Lv, J.; Tang, K.; et al. Gasdermin E-Mediated Target Cell Pyroptosis by CAR T Cells Triggers Cytokine Release Syndrome. Sci. Immunol. 2020, 5, eaax7969. [Google Scholar] [CrossRef]
- Abdallah, A.M.; Gey van Pittius, N.C.; DiGiuseppe Champion, P.A.; Cox, J.; Luirink, J.; Vandenbroucke-Grauls, C.M.J.E.; Appelmelk, B.J.; Bitter, W. Type VII Secretion—Mycobacteria Show the Way. Nat. Rev. Microbiol. 2007, 5, 883–891. [Google Scholar] [CrossRef]
- Majlessi, L.; Prados-Rosales, R.; Casadevall, A.; Brosch, R. Release of Mycobacterial Antigens. Immunol. Rev. 2015, 264, 25–45. [Google Scholar] [CrossRef]
- Beckwith, K.S.; Beckwith, M.S.; Ullmann, S.; Sætra, R.S.; Kim, H.; Marstad, A.; Åsberg, S.E.; Strand, T.A.; Haug, M.; Niederweis, M.; et al. Plasma Membrane Damage Causes NLRP3 Activation and Pyroptosis during Mycobacterium tuberculosis Infection. Nat. Commun. 2020, 11, 2270. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.-W.; Jacobs, W.R. Critical Role for NLRP3 in Necrotic Death Triggered by Mycobacterium tuberculosis. Cell. Microbiol. 2011, 13, 1371–1384. [Google Scholar] [CrossRef]
- Saiga, H.; Kitada, S.; Shimada, Y.; Kamiyama, N.; Okuyama, M.; Makino, M.; Yamamoto, M.; Takeda, K. Critical Role of AIM2 in Mycobacterium tuberculosis Infection. Int. Immunol. 2012, 24, 637–644. [Google Scholar] [CrossRef]
- Wassermann, R.; Gulen, M.F.; Sala, C.; Perin, S.G.; Lou, Y.; Rybniker, J.; Schmid-Burgk, J.L.; Schmidt, T.; Hornung, V.; Cole, S.T.; et al. Mycobacterium tuberculosis Differentially Activates cGAS- and Inflammasome-Dependent Intracellular Immune Responses through ESX-1. Cell Host Microbe 2015, 17, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.B.; Moura-Alves, P.; Sonawane, A.; Hacohen, N.; Griffiths, G.; Moita, L.F.; Anes, E. Mycobacterium tuberculosis Protein ESAT-6 Is a Potent Activator of the NLRP3/ASC Inflammasome. Cell. Microbiol. 2010, 12, 1046–1063. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Briken, V. Interaction of Mycobacteria With Host Cell Inflammasomes. Front. Immunol. 2022, 13, 791136. [Google Scholar] [CrossRef]
- Qu, Z.; Zhou, J.; Zhou, Y.; Xie, Y.; Jiang, Y.; Wu, J.; Luo, Z.; Liu, G.; Yin, L.; Zhang, X.-L. Mycobacterial EST12 Activates a RACK1-NLRP3-Gasdermin D Pyroptosis-IL-1β Immune Pathway. Sci. Adv. 2020, 6, eaba4733. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Luo, F.-L.; Xie, Y.; Xiong, H.; Gao, Y.; Liu, G.; Zhang, X.-L. EST12 Regulates Myc Expression and Enhances Anti-Mycobacterial Inflammatory Response via RACK1-JNK-AP1-Myc Immune Pathway. Front. Immunol. 2022, 13, 943174. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, P.; He, P.; Shi, F.; Tang, Y.; Guan, C.; Zeng, H.; Zhou, Y.; Song, Q.; Zhou, B.; et al. Mycobacterial PPE13 Activates Inflammasome by Interacting with the NATCH and LRR Domains of NLRP3. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 12820–12833. [Google Scholar] [CrossRef]
- Gong, Z.; Kuang, Z.; Li, H.; Li, C.; Ali, M.K.; Huang, F.; Li, P.; Li, Q.; Huang, X.; Ren, S.; et al. Regulation of Host Cell Pyroptosis and Cytokines Production by Mycobacterium tuberculosis Effector PPE60 Requires LUBAC Mediated NF-κB Signaling. Cell. Immunol. 2019, 335, 41–50. [Google Scholar] [CrossRef]
- Qian, J.; Hu, Y.; Zhang, X.; Chi, M.; Xu, S.; Wang, H.; Zhang, X. Mycobacterium tuberculosis PE_PGRS19 Induces Pyroptosis through a Non-Classical Caspase-11/GSDMD Pathway in Macrophages. Microorganisms 2022, 10, 2473. [Google Scholar] [CrossRef]
- Chevriaux, A.; Pilot, T.; Derangère, V.; Simonin, H.; Martine, P.; Chalmin, F.; Ghiringhelli, F.; Rébé, C. Cathepsin B Is Required for NLRP3 Inflammasome Activation in Macrophages, Through NLRP3 Interaction. Front. Cell Dev. Biol. 2020, 8, 167. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhang, X.; Xu, Y.; Chen, L.; Zhang, W.; Liu, E.; Xiao, C.; Kou, Q. Cathepsin B Aggravates Acute Pancreatitis by Activating the NLRP3 Inflammasome and Promoting the Caspase-1-Induced Pyroptosis. Int. Immunopharmacol. 2021, 94, 107496. [Google Scholar] [CrossRef]
- Liu, L.; Zhai, K.; Chen, Y.; Chen, X.; Wang, G.; Wu, L. Effect and Mechanism of Mycobacterium tuberculosis Lipoprotein LpqH in NLRP3 Inflammasome Activation in Mouse Ana-1 Macrophage. BioMed Res. Int. 2021, 2021, 8239135. [Google Scholar] [CrossRef] [PubMed]
- Schweneker, K.; Gorka, O.; Schweneker, M.; Poeck, H.; Tschopp, J.; Peschel, C.; Ruland, J.; Gross, O. The Mycobacterial Cord Factor Adjuvant Analogue Trehalose-6,6′-Dibehenate (TDB) Activates the Nlrp3 Inflammasome. Immunobiology 2013, 218, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Bohsali, A.; Ahlbrand, S.E.; Srinivasan, L.; Rathinam, V.A.K.; Vogel, S.N.; Fitzgerald, K.A.; Sutterwala, F.S.; Briken, V. Mycobacterium tuberculosis but Not Non-Virulent Mycobacteria Inhibit IFN-β and AIM2-Inflammasome Dependent IL-1β Production via Their ESX-1 Secretion System. J. Immunol. Baltim. Md. 1950 2013, 191, 3514–3518. [Google Scholar] [CrossRef]
- Prisic, S.; Husson, R.N. Mycobacterium tuberculosis Serine/Threonine Protein Kinases. Microbiol. Spectr. 2014, 2, 681–708. [Google Scholar] [CrossRef]
- Rastogi, S.; Ellinwood, S.; Augenstreich, J.; Mayer-Barber, K.D.; Briken, V. Mycobacterium tuberculosis Inhibits the NLRP3 Inflammasome Activation via Its Phosphokinase PknF. PLoS Pathog. 2021, 17, e1009712. [Google Scholar] [CrossRef]
- Rastogi, S.; Ganesh, A.; Briken, V. Mycobacterium tuberculosis Utilizes Serine/Threonine Kinase PknF to Evade NLRP3 Inflammasome-Driven Caspase-1 and RIPK3/Caspase-8 Activation in Murine Dendritic Cells. J. Immunol. 2024, 213, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Madan-Lala, R.; Peixoto, K.V.; Re, F.; Rengarajan, J. Mycobacterium tuberculosis Hip1 Dampens Macrophage Proinflammatory Responses by Limiting Toll-like Receptor 2 Activation. Infect. Immun. 2011, 79, 4828–4838. [Google Scholar] [CrossRef]
- Master, S.S.; Rampini, S.K.; Davis, A.S.; Keller, C.; Ehlers, S.; Springer, B.; Timmins, G.S.; Sander, P.; Deretic, V. Mycobacterium tuberculosis Prevents Inflammasome Activation. Cell Host Microbe 2008, 3, 224–232. [Google Scholar] [CrossRef]
- Yao, Q.; Xie, Y.; Xu, D.; Qu, Z.; Wu, J.; Zhou, Y.; Wei, Y.; Xiong, H.; Zhang, X.-L. Lnc-EST12, Which Is Negatively Regulated by Mycobacterial EST12, Suppresses Antimycobacterial Innate Immunity through Its Interaction with FUBP3. Cell. Mol. Immunol. 2022, 19, 883–897. [Google Scholar] [CrossRef]
- Beresford, N.; Patel, S.; Armstrong, J.; Szöor, B.; Fordham-Skelton, A.P.; Tabernero, L. MptpB, a Virulence Factor from Mycobacterium tuberculosis, Exhibits Triple-Specificity Phosphatase Activity. Biochem. J. 2007, 406, 13–18. [Google Scholar] [CrossRef]
- Chai, Q.; Lei, Z.; Liu, C.H. Pyroptosis Modulation by Bacterial Effector Proteins. Semin. Immunol. 2023, 69, 101804. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Cao, S.; Li, Y.; Luo, Y.; Liu, J.; Chen, Y.; Bai, Q.; Chen, L. Pyroptosis in Microbial Infectious Diseases. Mol. Biol. Rep. 2023, 51, 42. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Wang, K.; Liu, W.; She, Y.; Sun, Q.; Shi, J.; Sun, H.; Wang, D.-C.; Shao, F. Pore-Forming Activity and Structural Autoinhibition of the Gasdermin Family. Nature 2016, 535, 111–116. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-Activated Gasdermin D Causes Pyroptosis by Forming Membrane Pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef]
- Wang, J.; Deobald, K.; Re, F. Gasdermin D Protects from Melioidosis through Pyroptosis and Direct Killing of Bacteria. J. Immunol. 2019, 202, 3468–3473. [Google Scholar] [CrossRef]
- Hansen, J.M.; de Jong, M.F.; Wu, Q.; Zhang, L.-S.; Heisler, D.B.; Alto, L.T.; Alto, N.M. Pathogenic Ubiquitination of GSDMB Inhibits NK Cell Bactericidal Functions. Cell 2021, 184, 3178–3191.e18. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.M.; Han, X.; Allaire, J.M.; Stahl, M.; Rauch, I.; Knodler, L.A.; Vallance, B.A. Intestinal Restriction of Salmonella Typhimurium Requires Caspase-1 and Caspase-11 Epithelial Intrinsic Inflammasomes. PLoS Pathog. 2020, 16, e1008498. [Google Scholar] [CrossRef]
- Forrellad, M.A.; Klepp, L.I.; Gioffré, A.; Sabio y García, J.; Morbidoni, H.R.; de la Paz Santangelo, M.; Cataldi, A.A.; Bigi, F. Virulence Factors of the Mycobacterium tuberculosis Complex. Virulence 2013, 4, 3–66. [Google Scholar] [CrossRef] [PubMed]
- Alshawwa, S.Z.; Kassem, A.A.; Farid, R.M.; Mostafa, S.K.; Labib, G.S. Nanocarrier Drug Delivery Systems: Characterization, Limitations, Future Perspectives and Implementation of Artificial Intelligence. Pharmaceutics 2022, 14, 883. [Google Scholar] [CrossRef]
- Zhou, K.; Li, Z.-Z.; Cai, Z.-M.; Zhong, N.-N.; Cao, L.-M.; Huo, F.-Y.; Liu, B.; Wu, Q.-J.; Bu, L.-L. Nanotheranostics in Cancer Lymph Node Metastasis: The Long Road Ahead. Pharmacol. Res. 2023, 198, 106989. [Google Scholar] [CrossRef]
- Gaytan, S.L.; Beaven, E.; Gadad, S.S.; Nurunnabi, M. Progress and Prospect of Nanotechnology for Cardiac Fibrosis Treatment. Interdiscip. Med. 2023, 1, e20230018. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liao, K.; Yang, E.; Yang, F.; Lin, W.; Wang, J.; Fan, S.; Huang, X.; Chen, L.; Shen, H.; et al. Macrophage Targeted Iron Oxide Nanodecoys Augment Innate Immunological and Drug Killings for More Effective Mycobacterium tuberculosis Clearance. J. Nanobiotechnol. 2023, 21, 369. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gou, R.; Liao, J.; Wang, Y.; Qu, R.; Tang, Q.; Gan, J.; Zou, L.Y.; Shi, S. Recent Advances in Nano-Targeting Drug Delivery Systems for Rheumatoid Arthritis Treatment. Acta Mater. Medica 2023, 2, 23–41. [Google Scholar] [CrossRef]
- Li, M.; Yin, B.; Gao, C.; Guo, J.; Zhao, C.; Jia, C.; Guo, X. Graphene: Preparation, Tailoring, and Modification. Exploration 2023, 3, 20210233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, L.; Li, Y.; Wan, S.; Yuan, Z.; Zu, G.; Peng, F.; Ding, X. Advanced Extracellular Vesicle Bioinformatic Nanomaterials: From Enrichment, Decoding to Clinical Diagnostics. J. Nanobiotechnol. 2023, 21, 366. [Google Scholar] [CrossRef]
- Dai, X.; Du, Y.; Li, Y.; Yan, F. Nanomaterials-Based Precision Sonodynamic Therapy Enhancing Immune Checkpoint Blockade: A Promising Strategy Targeting Solid Tumor. Mater. Today Bio 2023, 23, 100796. [Google Scholar] [CrossRef]
- Luo, W.; Li, Y.; Xiang, C.; Aizawa, T.; Niu, R.; Wang, Y.; Zhao, J.; Liu, Z.; Li, C.; Liu, W.; et al. Nanomaterials as Therapeutic Agents to Modulate Astrocyte-Mediated Inflammation in Spinal Cord Injury. Mater. Today Bio 2023, 23, 100888. [Google Scholar] [CrossRef]
- Yi, Q.; Xu, Z.; Thakur, A.; Zhang, K.; Liang, Q.; Liu, Y.; Yan, Y. Current Understanding of Plant-Derived Exosome-like Nanoparticles in Regulating the Inflammatory Response and Immune System Microenvironment. Pharmacol. Res. 2023, 190, 106733. [Google Scholar] [CrossRef]
- Hu, Y.; Xiong, Y.; Tao, R.; Xue, H.; Chen, L.; Lin, Z.; Panayi, A.; Mi, B.; Liu, G. Advances and Perspective on Animal Models and Hydrogel Biomaterials for Diabetic Wound Healing. Biomater. Transl. 2022, 3, 188–200. [Google Scholar] [CrossRef]
- Ding, B.; Sheng, J.; Zheng, P.; Li, C.; Li, D.; Cheng, Z.; Ma, P.; Lin, J. Biodegradable Upconversion Nanoparticles Induce Pyroptosis for Cancer Immunotherapy. Nano Lett. 2021, 21, 8281–8289. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, H.; Zhou, N.; Xu, P.; Wang, J.; Gao, Y.; Jin, X.; Liang, X.; Lv, J.; Zhang, Y.; et al. Methotrexate-Loaded Tumour-Cell-Derived Microvesicles Can Relieve Biliary Obstruction in Patients with Extrahepatic Cholangiocarcinoma. Nat. Biomed. Eng. 2020, 4, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, M.; Chen, M.; Chen, Z.; Peng, X.; Zhou, F.; Song, J.; Qu, J. Programming Cell Pyroptosis with Biomimetic Nanoparticles for Solid Tumor Immunotherapy. Biomaterials 2020, 254, 120142. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, X.; Chen, H.; Duan, Y.; Liu, J.; Pan, Y.; Liu, B. Activation of Pyroptosis by Membrane-Anchoring AIE Photosensitizer Design: New Prospect for Photodynamic Cancer Cell Ablation. Angew. Chem. Int. Ed. 2021, 60, 9093–9098. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Gong, L.; Li, Y.; Cao, S.; Zhao, W.; Hao, L.; Li, S.; Pang, B.; Zhang, C.; Li, S.; et al. BiW8O30 Exerts Antitumor Effect by Triggering Pyroptosis and Upregulating Reactive Oxygen Species. Angew. Chem. Int. Ed. 2021, 60, 21449–21456. [Google Scholar] [CrossRef]
- Viswanathan, V.; Pharande, R.; Bannalikar, A.; Gupta, P.; Gupta, U.; Mukne, A. Inhalable Liposomes of Glycyrrhiza Glabra Extract for Use in Tuberculosis: Formulation, In Vitro Characterization, In Vivo Lung Deposition, and In Vivo Pharmacodynamic Studies. Drug Dev. Ind. Pharm. 2019, 45, 11–20. [Google Scholar] [CrossRef]
- Cai, J.; Yi, M.; Tan, Y.; Li, X.; Li, G.; Zeng, Z.; Xiong, W.; Xiang, B. Correction to: Natural Product Triptolide Induces GSDME-Mediated Pyroptosis in Head and Neck Cancer through Suppressing Mitochondrial Hexokinase-ΙΙ. J. Exp. Clin. Cancer Res. CR 2021, 40, 298. [Google Scholar] [CrossRef]
- Estrada García, I.; Hernández Pando, R.; Ivanyi, J. Editorial: Advances in Immunotherapeutic Approaches to Tuberculosis. Front. Immunol. 2021, 12, 684200. [Google Scholar] [CrossRef]
- Van Deun, A.; Maug, A.K.J.; Salim, M.A.H.; Das, P.K.; Sarker, M.R.; Daru, P.; Rieder, H.L. Short, Highly Effective, and Inexpensive Standardized Treatment of Multidrug-Resistant Tuberculosis. Am. J. Respir. Crit. Care Med. 2010, 182, 684–692. [Google Scholar] [CrossRef]
- Fu, Y.; Shen, J.; Liu, F.; Zhang, H.; Zheng, Y.; Jiang, X. Andrographolide Suppresses Pyroptosis in Mycobacterium tuberculosis-Infected Macrophages via the microRNA-155/Nrf2 Axis. Oxid. Med. Cell. Longev. 2022, 2022, 1885066. [Google Scholar] [CrossRef]
- Ning, B.; Shen, J.; Liu, F.; Zhang, H.; Jiang, X. Baicalein Suppresses NLRP3 and AIM2 Inflammasome-Mediated Pyroptosis in Macrophages Infected by Mycobacterium tuberculosis via Induced Autophagy. Microbiol. Spectr. 2023, 11, e04711-22. [Google Scholar] [CrossRef]
- Cebani, L.; Mvubu, N.E. Can We Exploit Inflammasomes for Host-Directed Therapy in the Fight against Mycobacterium tuberculosis Infection? Int. J. Mol. Sci. 2024, 25, 8196. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, W.; Zhao, L.; Wu, Y.; Li, X.; Yan, D.; Gao, Q.; Yan, Y.; Zhang, J.; Feng, Y.; et al. Photothermal Therapy of Tuberculosis Using Targeting Pre-Activated Macrophage Membrane-Coated Nanoparticles. Nat. Nanotechnol. 2024, 19, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Lu, L.; Guo, K.; Lu, J.; Chen, H. Vitamin D Protects against High Glucose-Induced Pancreatic β-Cell Dysfunction via AMPK-NLRP3 Inflammasome Pathway. Mol. Cell. Endocrinol. 2022, 547, 111596. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Novick, D.; Kim, S.; Kaplanski, G. Interleukin-18 and IL-18 Binding Protein. Front. Immunol. 2013, 4, 289. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Ma, Y.; Yu, J.; Liu, Y.; Xia, J.; Kong, X.; Jin, X.; Li, J.; Lin, S.; Ruan, Y.; et al. Advancing Roles and Therapeutic Potentials of Pyroptosis in Host Immune Defenses against Tuberculosis. Biomolecules 2024, 14, 1255. https://doi.org/10.3390/biom14101255
Yang J, Ma Y, Yu J, Liu Y, Xia J, Kong X, Jin X, Li J, Lin S, Ruan Y, et al. Advancing Roles and Therapeutic Potentials of Pyroptosis in Host Immune Defenses against Tuberculosis. Biomolecules. 2024; 14(10):1255. https://doi.org/10.3390/biom14101255
Chicago/Turabian StyleYang, Jiayi, Yuhe Ma, Jiaqi Yu, Yilin Liu, Jiaojiao Xia, Xinen Kong, Xiaoying Jin, Jiaxiang Li, Siqi Lin, Yongdui Ruan, and et al. 2024. "Advancing Roles and Therapeutic Potentials of Pyroptosis in Host Immune Defenses against Tuberculosis" Biomolecules 14, no. 10: 1255. https://doi.org/10.3390/biom14101255
APA StyleYang, J., Ma, Y., Yu, J., Liu, Y., Xia, J., Kong, X., Jin, X., Li, J., Lin, S., Ruan, Y., Yang, F., & Pi, J. (2024). Advancing Roles and Therapeutic Potentials of Pyroptosis in Host Immune Defenses against Tuberculosis. Biomolecules, 14(10), 1255. https://doi.org/10.3390/biom14101255







