Connecting the Dots: Telomere Shortening and Rheumatic Diseases
Abstract
:1. Introduction
Structure and Function of Telomere Complex
2. Factors Affecting Telomere Length
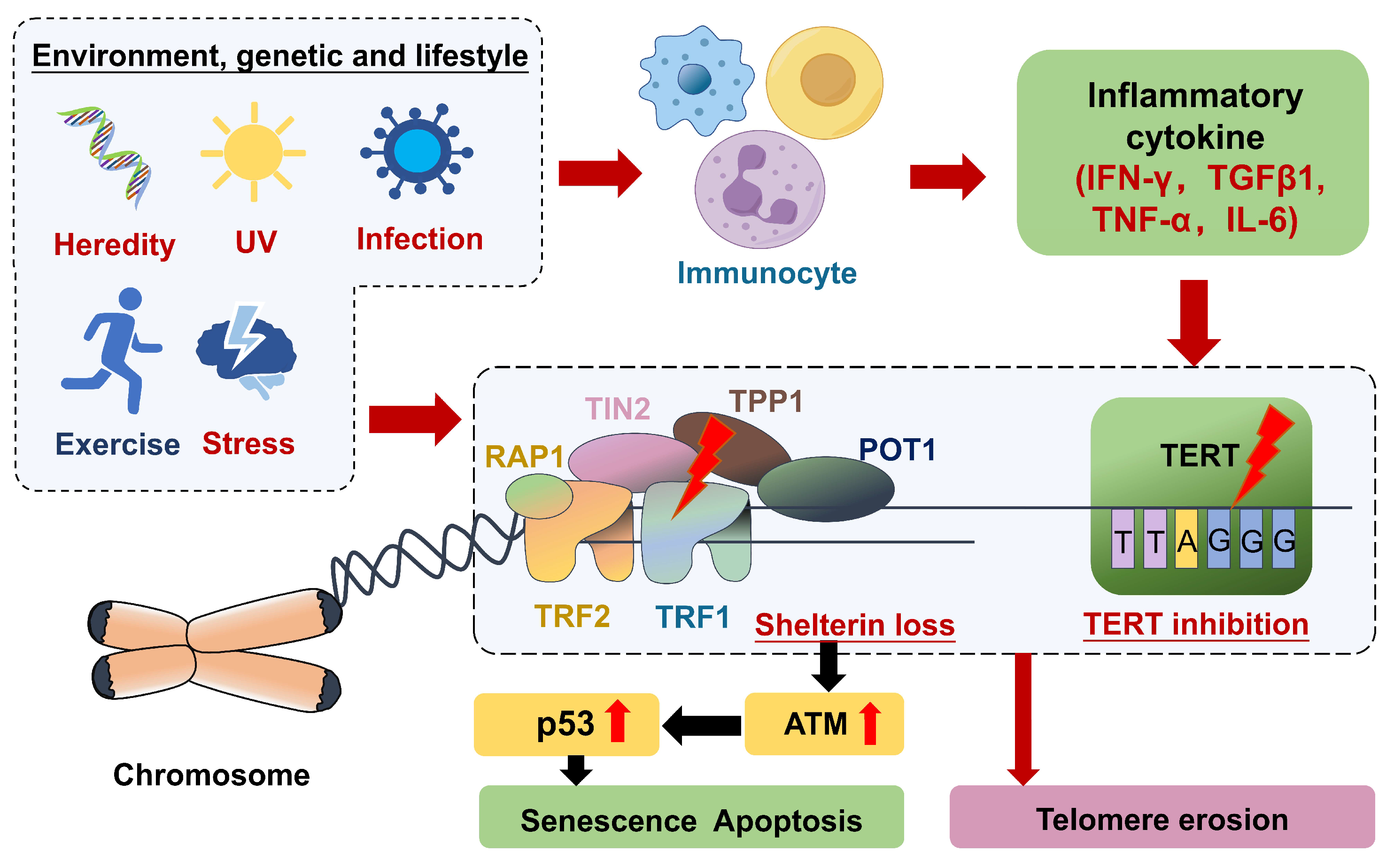
3. Possible Mechanisms of Telomere Shortening in Inflammation
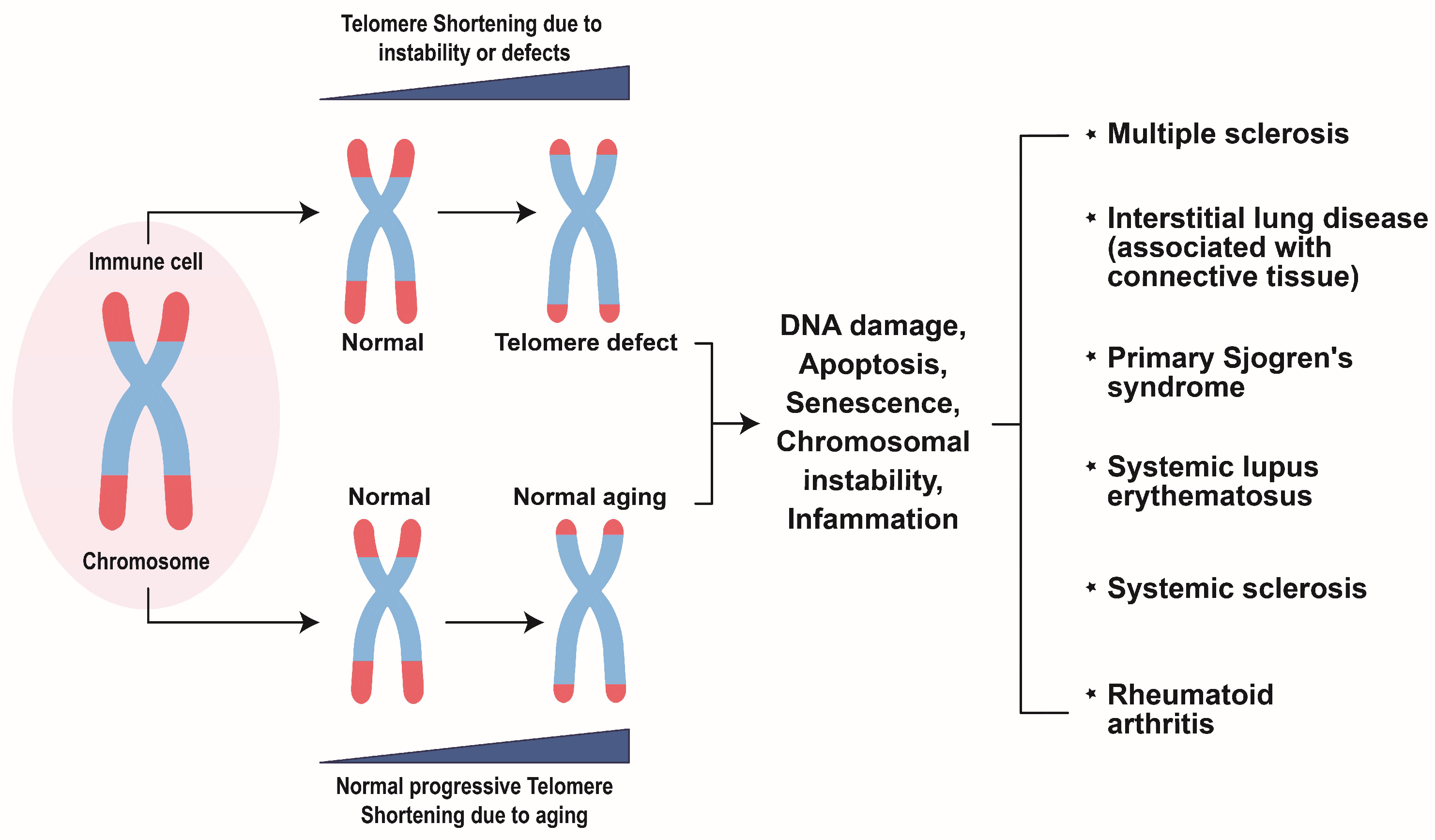
4. Telomere Erosion in Rheumatic Disease
4.1. Rheumatoid Arthritis
4.2. Systemic Lupus Erythematosus
4.3. Primary Sjögren’s Syndrome
4.4. Systemic Sclerosis
4.5. Other Rheumatic Diseases
4.6. Interstitial Lung Disease Associated with Connective Tissue Disease
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blackburn, E.H. Structure and function of telomeres. Nature 1991, 350, 569–573. [Google Scholar] [CrossRef]
- Moyzis, R.K.; Buckingham, J.M.; Cram, L.S.; Dani, M.; Deaven, L.L.; Jones, M.D.; Meyne, J.; Ratliff, R.L.; Wu, J.R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc. Natl. Acad. Sci. USA 1988, 85, 6622–6626. [Google Scholar] [CrossRef] [PubMed]
- Morin, G.B. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell 1989, 59, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Greider, C.W. Telomeres. Curr. Opin. Cell Biol. 1991, 3, 444–451. [Google Scholar] [CrossRef]
- Georgin-Lavialle, S.; Aouba, A.; Mouthon, L.; Londono-Vallejo, J.A.; Lepelletier, Y.; Gabet, A.-S.; Hermine, O. The telomere/telomerase system in autoimmune and systemic immune-mediated diseases. Autoimmun. Rev. 2010, 9, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Frydrychová, R.Č.; Konopová, B.; Peska, V.; Brejcha, M.; Sábová, M. Telomeres and telomerase: Active but complex players in life-history decisions. Biogerontology 2023, 25, 205–226. [Google Scholar] [CrossRef]
- Harley, C.B. Human ageing and telomeres. Ciba Found. Symp. 1997, 211, 129–139; Discussion 139–144. [Google Scholar] [CrossRef]
- Revy, P.; Kannengiesser, C.; Bertuch, A.A. Genetics of human telomere biology disorders. Nat. Rev. Genet. 2023, 24, 86–108. [Google Scholar] [CrossRef]
- d’Adda di Fagagna, F. Living on a break: Cellular senescence as a DNA-damage response. Nat. Rev. Cancer 2008, 8, 512–522. [Google Scholar] [CrossRef]
- de Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes. Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef] [PubMed]
- Greider, C.W.; Blackburn, E.H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 1985, 43 Pt 1, 405–413. [Google Scholar] [CrossRef]
- Wright, W.E.; Piatyszek, M.A.; Rainey, W.E.; Byrd, W.; Shay, J.W. Telomerase activity in human germline and embryonic tissues and cells. Dev. Genet. 1996, 18, 173–179. [Google Scholar] [CrossRef]
- Harley, C.B. Telomerases. Pathol. Biol. 1994, 42, 342–345. [Google Scholar] [PubMed]
- Grill, S.; Nandakumar, J. Molecular mechanisms of telomere biology disorders. J. Biol. Chem. 2021, 296, 100064. [Google Scholar] [CrossRef] [PubMed]
- Guterres, A.N.; Villanueva, J. Targeting telomerase for cancer therapy. Oncogene 2020, 39, 5811–5824. [Google Scholar] [CrossRef]
- Sagris, M.; Theofilis, P.; Antonopoulos, A.S.; Tsioufis, K.; Tousoulis, D. Telomere Length: A Cardiovascular Biomarker and a Novel Therapeutic Target. Int. J. Mol. Sci. 2022, 23, 16010. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Hagg, S. Telomere length and cardiovascular disease risk. Curr. Opin. Cardiol. 2019, 34, 270–274. [Google Scholar] [CrossRef]
- Schneider, C.V.; Schneider, K.M.; Teumer, A.; Rudolph, K.L.; Hartmann, D.; Rader, D.J.; Strnad, P. Association of Telomere Length with Risk of Disease and Mortality. JAMA Intern. Med. 2022, 182, 291–300. [Google Scholar] [CrossRef]
- Cawthon, R.M.; Smith, K.R.; O’Brien, E.; Sivatchenko, A.; Kerber, R.A. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 2003, 361, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Reichert, S.; Stier, A. Does oxidative stress shorten telomeres in vivo? A review. Biol. Lett. 2017, 13, 20170463. [Google Scholar] [CrossRef]
- von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002, 27, 339–344. [Google Scholar] [CrossRef]
- d’Adda di Fagagna, F.; Reaper, P.M.; Clay-Farrace, L.; Fiegler, H.; Carr, P.; Von Zglinicki, T.; Saretzki, G.; Carter, N.P.; Jackson, S.P. A DNA damage checkpoint response in telomere-initiated senescence. Nature 2003, 426, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.R.; Wood, E.; Collins, K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature 1999, 402, 551–555. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Calado, R.T.; Ly, H.; Kajigaya, S.; Baerlocher, G.M.; Chanock, S.J.; Lansdorp, P.M.; Young, N.S. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N. Engl. J. Med. 2005, 352, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Heba, A.C.; Toupance, S.; Arnone, D.; Peyrin-Biroulet, L.; Benetos, A.; Ndiaye, N.C. Telomeres: New players in immune-mediated inflammatory diseases? J. Autoimmun. 2021, 123, 102699. [Google Scholar] [CrossRef] [PubMed]
- Putman, R.K.; Axelsson, G.T.; Ash, S.Y.; Sanders, J.L.; Menon, A.A.; Araki, T.; Nishino, M.; Yanagawa, M.; Gudmundsson, E.F.; Qiao, D.; et al. Interstitial lung abnormalities are associated with decreased mean telomere length. Eur. Respir. J. 2022, 60, 2101814. [Google Scholar] [CrossRef]
- Stock, C.J.; Renzoni, E.A. Telomeres in interstitial lung disease. J. Clin. Med. 2021, 10, 1384. [Google Scholar] [CrossRef]
- Gamal, R.M.; Hammam, N.; Zakary, M.M.; Abdelaziz, M.M.; Razek, M.R.A.; Mohamed, M.S.E.; Emad, Y.; Elnaggar, M.G.; Furst, D.E. Telomere dysfunction-related serological markers and oxidative stress markers in rheumatoid arthritis patients: Correlation with diseases activity. Clin. Rheumatol. 2018, 37, 3239–3246. [Google Scholar] [CrossRef] [PubMed]
- Svyryd, Y.; Pascual-Ramos, V.; Contreras-Yañez, I.; Muñoz-Tellez, L.A.; Luna-Muñoz, L.; López-Hernández, M.A.; Aguayo-Gómez, A.; Mutchinick, O.M. Telomeres Length Variations in a Rheumatoid Arthritis Patients Cohort at Early Disease Onset and after Follow-Up. Rev. Investig. Clin. 2022, 74, 202–211. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Shen, C.-Y.; Liao, H.-T.; Li, K.-J.; Lee, H.-T.; Lu, C.-S.; Wu, C.-H.; Kuo, Y.-M.; Hsieh, S.-C.; Yu, C.-L. Molecular and Cellular Bases of Immunosenescence, Inflammation, and Cardiovascular Complications Mimicking “Inflammaging” in Patients with Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2019, 20, 3878. [Google Scholar] [CrossRef]
- Gao, Y.; Cai, W.; Zhou, Y.; Li, Y.; Cheng, J.; Wei, F. Immunosenescence of T cells: A key player in rheumatoid arthritis. Inflamm. Res. 2022, 71, 1449–1462. [Google Scholar] [CrossRef] [PubMed]
- Onuora, S. Gout: Short telomeres in gout linked with flares and CVD. Nat. Rev. Rheumatol. 2017, 13, 324. [Google Scholar] [CrossRef]
- Griffith, J.D.; Comeau, L.; Rosenfield, S.; Stansel, R.M.; Bianchi, A.; Moss, H.; de Lange, T. Mammalian telomeres end in a large duplex loop. Cell 1999, 97, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H.; Greider, C.W.; Szostak, J.W. Telomeres and telomerase: The path from maize, Tetrahymena and yeast to human cancer and aging. Nat. Med. 2006, 12, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Lulkiewicz, M.; Bajsert, J.; Kopczynski, P.; Barczak, W.; Rubis, B. Telomere length: How the length makes a difference. Mol. Biol. Rep. 2020, 47, 7181–7188. [Google Scholar] [CrossRef]
- Srinivas, N.; Rachakonda, S.; Kumar, R. Telomeres and telomere length: A general overview. Cancers 2020, 12, 558. [Google Scholar] [CrossRef]
- Wu, X.; Amos, C.I.; Zhu, Y.; Zhao, H.; Grossman, B.H.; Shay, J.W.; Luo, S.; Hong, W.K.; Spitz, M.R. Telomere dysfunction: A potential cancer predisposition factor. J. Natl. Cancer Inst. 2003, 95, 1211–1218. [Google Scholar] [CrossRef]
- Roka, K.; Solomou, E.E.; Kattamis, A. Telomere biology: From disorders to hematological diseases. Front. Oncol. 2023, 13, 1167848. [Google Scholar] [CrossRef]
- Tsoukalas, D.; Fragkiadaki, P.; Docea, A.O.; Alegakis, A.K.; Sarandi, E.; Thanasoula, M.; Spandidos, D.A.; Tsatsakis, A.; Razgonova, M.P.; Calina, D. Discovery of potent telomerase activators: Unfolding new therapeutic and anti-aging perspectives. Mol. Med. Rep. 2019, 20, 3701–3708. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.R.; Garcia, A.; Dalberto, D.; Picinini, J.; Touguinha, L.; Salvador, M.; da Silva, J. Multiple factors influence telomere length and DNA damage in individuals residing in proximity to a coal-burning power plant. Mutat Res Genet Toxicol Environ Mutagen.. 2024, 898, 503793. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Gasevic, D.; Wen, B.; Yu, P.; Xu, R.; Zhou, G.; Zhang, Y.; Song, J.; Liu, H.; Li, S. Association between air pollution and telomere length:: A study of 471,808 UK Biobank participants. Innov. Med. 2023, 1, 100017. [Google Scholar] [CrossRef]
- Ikeda, H.; Aida, J.; Hatamochi, A.; Hamasaki, Y.; Izumiyama-Shimomura, N.; Nakamura, K.-I.; Ishikawa, N.; Poon, S.S.; Fujiwara, M.; Tomita, K.-I.; et al. Quantitative fluorescence in situ hybridization measurement of telomere length in skin with/without sun exposure or actinic keratosis. Hum. Pathol. 2014, 45, 473–480. [Google Scholar] [CrossRef]
- Valdes, A.M.; Andrew, T.; Gardner, J.P.; Kimura, M.; Oelsner, E.; Cherkas, L.F.; Aviv, A.; Spector, T.D. Obesity, cigarette smoking, and telomere length in women. Lancet 2005, 366, 662–664. [Google Scholar] [CrossRef]
- McGrath, M.; Wong, J.Y.; Michaud, D.; Hunter, D.J.; De Vivo, I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol. Biomark. Prev. 2007, 16, 815–819. [Google Scholar] [CrossRef]
- Morlá, M.; Busquets, X.; Pons, J.; Sauleda, J.; MacNee, W.; Agustí, A.G. Telomere shortening in smokers with and without COPD. Eur. Respir. J. 2006, 27, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Aubert, G.; Lansdorp, P.M. Telomeres and aging. Physiol. Rev. 2008, 88, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Abrahamyan, S.; Eberspächer, B.; Hoshi, M.M.; Aly, L.; Luessi, F.; Groppa, S.; Klotz, L.; Meuth, S.G.; Schroeder, C.; Grüter, T.; et al. Complete Epstein-Barr virus seropositivity in a large cohort of patients with early multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2020, 91, 681–686. [Google Scholar] [CrossRef]
- Kamranvar, S.A.; Masucci, M.G. Regulation of Telomere Homeostasis during Epstein-Barr virus Infection and Immortalization. Viruses 2017, 9, 217. [Google Scholar] [CrossRef]
- Shammas, M.A. Telomeres, lifestyle, cancer, and aging. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.A.; Tian, J.; Gandelman, M.; Cheng, H.; Tsapekos, M.; Crego, S.R.; Maddela, R.; Sinnott, R. A Multivitamin Mixture Protects against Oxidative Stress-Mediated Telomere Shortening. J. Diet. Suppl. 2023, 21, 53–70. [Google Scholar] [CrossRef]
- Galiè, S.; Canudas, S.; Muralidharan, J.; García-Gavilán, J.; Bulló, M.; Salas-Salvadó, J. Impact of Nutrition on Telomere Health: Systematic Review of Observational Cohort Studies and Randomized Clinical Trials. Adv. Nutr. 2020, 11, 576–601. [Google Scholar] [CrossRef] [PubMed]
- Barcın-Güzeldere, H.K.; Aksoy, M.; Demircan, T.; Yavuz, M.; Beler, M. Association between the anthropometric measurements and dietary habits on telomere shortening in healthy older adults: A-cross-sectional study. Geriatr. Gerontol. Int. 2023, 23, 565–572. [Google Scholar] [CrossRef]
- Cassidy, A.; De Vivo, I.; Liu, Y.; Han, J.; Prescott, J.; Hunter, D.J.; Rimm, E.B. Associations between diet, lifestyle factors, and telomere length in women. Am. J. Clin. Nutr. 2010, 91, 1273–1280. [Google Scholar] [CrossRef]
- DeBoy, E.A.; Tassia, M.G.; Schratz, K.E.; Yan, S.M.; Cosner, Z.L.; McNally, E.J.; Gable, D.L.; Xiang, Z.; Lombard, D.B.; Antonarakis, E.S. Familial Clonal Hematopoiesis in a Long Telomere Syndrome. N. Engl. J. Med. 2023, 388, 2422–2433. [Google Scholar] [CrossRef]
- Stewart, S.A.; Weinberg, R.A. Telomerase and human tumorigenesis. Semin. Cancer Biol. 2000, 10, 399–406. [Google Scholar] [CrossRef]
- De Lange, T. Shelterin-Mediated Telomere Protection. Annu. Rev. Genet. 2018, 52, 223–247. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, F.; Sun, B.; Du, J.; Sun, C.; Yuan, J.; Wang, Y.; Tao, L.; Kota, K.; Liu, X. Telomerase enzymatic component hTERT shortens long telomeres in human cells. Cell Cycle 2014, 13, 1765–1776. [Google Scholar] [CrossRef]
- Bryan, T.M. Nucleotide metabolism regulates human telomere length via telomerase activation. Nat. Genet. 2023, 55, 532–533. [Google Scholar] [CrossRef] [PubMed]
- Mannherz, W.; Agarwal, S. Thymidine nucleotide metabolism controls human telomere length. Nat. Genet. 2023, 55, 568–580. [Google Scholar] [CrossRef]
- Ysrraelit, M.C.; Correale, J. Impact of sex hormones on immune function and multiple sclerosis development. Immunology 2019, 156, 9–22. [Google Scholar] [CrossRef]
- Kyo, S.; Takakura, M.; Kanaya, T.; Zhuo, W.; Fujimoto, K.; Nishio, Y.; Orimo, A.; Inoue, M. Estrogen activates telomerase. Cancer Res. 1999, 59, 5917–5921. [Google Scholar]
- Barrett, E.L.; Richardson, D.S. Sex differences in telomeres and lifespan. Aging Cell 2011, 10, 913–921. [Google Scholar] [CrossRef]
- Blackburn, E.H. Telomere states and cell fates. Nature 2000, 408, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Vajen, B.; Thomay, K.; Schlegelberger, B. Induction of Chromosomal Instability via Telomere Dysfunction and Epigenetic Alterations in Myeloid Neoplasia. Cancers 2013, 5, 857–874. [Google Scholar] [CrossRef] [PubMed]
- Cleal, K.; Norris, K.; Baird, D. Telomere Length Dynamics and the Evolution of Cancer Genome Architecture. Int. J. Mol. Sci. 2018, 19, 482. [Google Scholar] [CrossRef]
- Maestroni, L.; Matmati, S.; Coulon, S. Solving the Telomere Replication Problem. Genes 2017, 8, 55. [Google Scholar] [CrossRef]
- Röth, A.; Baerlocher, G.M. Telomere Biology in T Cells: An Important Brake on the Road of Their Life Span? Clin. Leuk. 2009, 3, 41–46. [Google Scholar] [CrossRef]
- Fujii, H.; Shao, L.; Colmegna, I.; Goronzy, J.J.; Weyand, C.M. Telomerase insufficiency in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2009, 106, 4360–4365. [Google Scholar] [CrossRef]
- Wallace, D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Annu. Rev. Genet. 2005, 39, 359–407. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Free radicals in aging. Mol. Cell Biochem. 1988, 84, 155–161. [Google Scholar] [CrossRef]
- Amorim, J.A.; Coppotelli, G.; Rolo, A.P.; Palmeira, C.M.; Ross, J.M.; Sinclair, D.A. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258. [Google Scholar] [CrossRef]
- Metcalfe, J.A.; Parkhill, J.; Campbell, L.; Stacey, M.; Biggs, P.; Byrd, P.J.; Taylor, A.M. Accelerated telomere shortening in ataxia telangiectasia. Nat. Genet. 1996, 13, 350–353. [Google Scholar] [CrossRef]
- Donehower, L.A. Does p53 affect organismal aging? J. Cell Physiol. 2002, 192, 23–33. [Google Scholar] [CrossRef]
- Matheu, A.; Maraver, A.; Klatt, P.; Flores, I.; Garcia-Cao, I.; Borras, C.; Flores, J.M.; Viña, J.; Blasco, M.A.; Serrano, M. Delayed ageing through damage protection by the Arf/p53 pathway. Nature 2007, 448, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.S.; Wright, W.E.; Shay, J.W. Human telomerase and its regulation. Microbiol. Mol. Biol. Rev. 2002, 66, 407–425, table of contents. [Google Scholar] [CrossRef]
- Najarro, K.; Nguyen, H.; Chen, G.; Xu, M.; Alcorta, S.; Yao, X.; Zukley, L.; Metter, E.J.; Truong, T.; Lin, Y.; et al. Telomere Length as an Indicator of the Robustness of B- and T-Cell Response to Influenza in Older Adults. J. Infect. Dis. 2015, 212, 1261–1269. [Google Scholar] [CrossRef]
- Gavia-García, G.; Rosado-Pérez, J.; Arista-Ugalde, T.L.; Aguiñiga-Sánchez, I.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Telomere Length and Oxidative Stress and Its Relation with Metabolic Syndrome Components in the Aging. Biology 2021, 10, 253. [Google Scholar] [CrossRef]
- Rodriguez, I.J.; Lalinde Ruiz, N.; Llano León, M.; Martínez Enríquez, L.; Montilla Velásquez, M.D.P.; Ortiz Aguirre, J.P.; Rodríguez Bohórquez, O.M.; Velandia Vargas, E.A.; Hernández, E.D.; Parra López, C.A. Immunosenescence Study of T Cells: A Systematic Review. Front. Immunol. 2020, 11, 604591. [Google Scholar] [CrossRef]
- Agrawal, A.; Gupta, S. Impact of aging on dendritic cell functions in humans. Ageing Res. Rev. 2011, 10, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Sebastián, C.; Herrero, C.; Serra, M.; Lloberas, J.; Blasco, M.A.; Celada, A. Telomere shortening and oxidative stress in aged macrophages results in impaired STAT5a phosphorylation. J. Immunol. 2009, 183, 2356–2364. [Google Scholar] [CrossRef]
- Guimarães, G.R.; Almeida, P.P.; de Oliveira Santos, L.; Rodrigues, L.P.; de Carvalho, J.L.; Boroni, M. Hallmarks of Aging in Macrophages: Consequences to Skin Inflammaging. Cells 2021, 10, 1323. [Google Scholar] [CrossRef] [PubMed]
- Chopp, L.; Redmond, C.; O’Shea, J.J.; Schwartz, D.M. From thymus to tissues and tumors: A review of T-cell biology. J. Allergy Clin. Immunol. 2023, 151, 81–97. [Google Scholar] [CrossRef]
- Ebina-Shibuya, R.; Leonard, W.J. Role of thymic stromal lymphopoietin in allergy and beyond. Nat. Rev. Immunol. 2023, 23, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Nabi, M.; Noor, R.; Zahid, A.; Zulfiqar, T.; Khalid, A.; Ri, S. Grave’s disease: Pathophysiology of a model autoimmune disease. Arch. Microbiol. Immunol. 2022, 6, 149–164. [Google Scholar] [CrossRef]
- Young, A.R.; Narita, M. SASP reflects senescence. EMBO Rep. 2009, 10, 228–230. [Google Scholar] [CrossRef]
- Armanios, M.Y.; Chen, J.J.L.; Cogan, J.D.; Alder, J.K.; Ingersoll, R.G.; Markin, C.; Lawson, W.E.; Xie, M.; Vulto, I.; Phillips, J.A.; et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2007, 356, 1317–1326. [Google Scholar] [CrossRef]
- Demanelis, K.; Jasmine, F.; Chen, L.S.; Chernoff, M.; Tong, L.; Delgado, D.; Zhang, C.; Shinkle, J.; Sabarinathan, M.; Lin, H.; et al. Determinants of telomere length across human tissues. Science 2020, 369, eaaz6876. [Google Scholar] [CrossRef]
- Gilson, E.; Géli, V. How telomeres are replicated. Nat. Rev. Mol. Cell Biol. 2007, 8, 825–838. [Google Scholar] [CrossRef]
- Lansdorp, P.M. Telomeres, aging, and cancer: The big picture. Blood J. Am. Soc. Hematol. 2022, 139, 813–821. [Google Scholar] [CrossRef]
- Allsopp, R.C.; Chang, E.; Kashefi-Aazam, M.; Rogaev, E.I.; Piatyszek, M.A.; Shay, J.W.; Harley, C.B. Telomere shortening is associated with cell division in vitro and in vivo. Exp. Cell Res. 1995, 220, 194–200. [Google Scholar] [CrossRef]
- Dewhurst, S.M.; Yao, X.; Rosiene, J.; Tian, H.; Behr, J.; Bosco, N.; Takai, K.K.; de Lange, T.; Imieliński, M. Structural variant evolution after telomere crisis. Nat. Commun. 2021, 12, 2093. [Google Scholar] [CrossRef]
- Wang, Z.; Lieberman, P.M. The crosstalk of telomere dysfunction and inflammation through cell-free TERRA containing exosomes. RNA Biol. 2016, 13, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Nassour, J.; Aguiar, L.G.; Correia, A.; Schmidt, T.T.; Mainz, L.; Przetocka, S.; Haggblom, C.; Tadepalle, N.; Williams, A.; Shokhirev, M.N. Telomere-to-mitochondria signalling by ZBP1 mediates replicative crisis. Nature 2023, 614, 767–773. [Google Scholar] [CrossRef]
- Psarras, A.; Alase, A.; Antanaviciute, A.; Carr, I.M.; Md Yusof, M.Y.; Wittmann, M.; Emery, P.; Tsokos, G.C.; Vital, E.M. Functionally impaired plasmacytoid dendritic cells and non-haematopoietic sources of type I interferon characterize human autoimmunity. Nat. Commun. 2020, 11, 6149. [Google Scholar] [CrossRef]
- Contreras-Galindo, R.; Paul, S.; McCourt, P.M. Chromosome Segregation Defects in Scleroderma; IntechOpen: London, UK, 2023. [Google Scholar]
- Jiang, J.; Zhao, M.; Chang, C.; Wu, H.; Lu, Q. Type I interferons in the pathogenesis and treatment of autoimmune diseases. Clin. Rev. Allergy Immunol. 2020, 59, 248–272. [Google Scholar] [CrossRef] [PubMed]
- Gravallese, E.M.; Firestein, G.S. Rheumatoid Arthritis—Common Origins, Divergent Mechanisms. N. Engl. J. Med. 2023, 388, 529–542. [Google Scholar] [CrossRef]
- Weyand, C.M.; Goronzy, J.J. The immunology of rheumatoid arthritis. Nat. Immunol. 2021, 22, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Schönland, S.O.; Lopez, C.; Widmann, T.; Zimmer, J.; Bryl, E.; Goronzy, J.J.; Weyand, C.M. Premature telomeric loss in rheumatoid arthritis is genetically determined and involves both myeloid and lymphoid cell lineages. Proc. Natl. Acad. Sci. USA 2003, 100, 13471–13476. [Google Scholar] [CrossRef] [PubMed]
- Steer, S.E.; Williams, F.M.K.; Kato, B.; Gardner, J.P.; Norman, P.J.; Hall, M.A.; Kimura, M.; Vaughan, R.; Aviv, A.; Spector, T.D. Reduced telomere length in rheumatoid arthritis is independent of disease activity and duration. Ann. Rheum. Dis. 2007, 66, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Ormseth, M.J.; Solus, J.F.; Oeser, A.M.; Bian, A.; Gebretsadik, T.; Shintani, A.; Raggi, P.; Stein, C.M. Telomere Length and Coronary Atherosclerosis in Rheumatoid Arthritis. J. Rheumatol. 2016, 43, 1469–1474. [Google Scholar] [CrossRef]
- Salmon, M.; Akbar, A.N. Telomere erosion: A new link between HLA DR4 and rheumatoid arthritis? Trends Immunol. 2004, 25, 339–341. [Google Scholar] [CrossRef]
- Yudoh, K.; Nguyen, V.; Nakamura, H.; Hongo-Masuko, K.; Kato, T.; Nishioka, K. Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: Oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res. Ther. 2005, 7, R380–R391. [Google Scholar] [CrossRef]
- Natalini, J.G.; England, B.R.; Baker, J.F.; Chen, Q.; Singh, N.; Mahajan, T.D.; Roul, P.; Thiele, G.M.; Sauer, B.C.; Mikuls, T.R.; et al. Associations between shortened telomeres and rheumatoid arthritis-associated interstitial lung disease among U.S. Veterans. Respir. Med. 2022, 201, 106943. [Google Scholar] [CrossRef]
- Prescott, J.; Karlson, E.W.; Orr, E.H.; Zee, R.Y.L.; De Vivo, I.; Costenbader, K.H. A Prospective Study Investigating Prediagnostic Leukocyte Telomere Length and Risk of Developing Rheumatoid Arthritis in Women. J. Rheumatol. 2016, 43, 282–288. [Google Scholar] [CrossRef]
- Koetz, K.; Bryl, E.; Spickschen, K.; O’Fallon, W.M.; Goronzy, J.J.; Weyand, C.M. T cell homeostasis in patients with rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2000, 97, 9203–9208. [Google Scholar] [CrossRef]
- Colmegna, I.; Diaz-Borjon, A.; Fujii, H.; Schaefer, L.; Goronzy, J.J.; Weyand, C.M. Defective proliferative capacity and accelerated telomeric loss of hematopoietic progenitor cells in rheumatoid arthritis. Arthritis Rheum. 2008, 58, 990–1000. [Google Scholar] [CrossRef]
- Blinova, E.A.; Zinnatova, E.V.; Barkovskaya, M.S.; Borisov, V.I.; Sizikov, A.E.; Kozhevnikov, V.S.; Rubtsov, N.B.; Kozlov, V.A. Telomere Length of Individual Chromosomes in Patients with Rheumatoid Arthritis. Bull. Exp. Biol. Med. 2016, 160, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, M.; Mosquera, A.; Rego, J.I.; Fernández-Sueiro, J.L.; Blanco, F.J.; Fernández, J.L. Differing patterns of peripheral blood leukocyte telomere length in rheumatologic diseases. Mutat. Res. 2010, 683, 68–73. [Google Scholar] [CrossRef]
- Yudoh, K.; Matsuno, H.; Nezuka, T.; Kimura, T. Different mechanisms of synovial hyperplasia in rheumatoid arthritis and pigmented villonodular synovitis: The role of telomerase activity in synovial proliferation. Arthritis Rheum. 1999, 42, 669–677. [Google Scholar] [CrossRef]
- Bridges, J.; Chung, K.W.; Martz, C.D.; Smitherman, E.A.; Drenkard, C.; Wu, C.; Lin, J.; Lim, S.S.; Chae, D.H. Leukocyte Telomere Length and Childhood Onset of Systemic Lupus Erythematosus in the Black Women’s Experiences Living with Lupus Study. ACR Open Rheumatol. 2022, 4, 426–431. [Google Scholar] [CrossRef]
- Qi, Y.-Y.; Liu, X.-R.; He, Y.-X.; Zhou, M.; Ning, X.-H.; Zhai, Y.-L.; Zhang, X.-X.; Wang, X.-Y.; Zhao, Y.-F.; Cui, Y.; et al. Association of the Variant rs6984094, Which Lengthens Telomeres, with Systemic Lupus Erythematosus Susceptibility in Chinese Populations. J. Immunol. Res. 2021, 2021, 7079359. [Google Scholar] [CrossRef] [PubMed]
- Qi, A.; Zhou, H.; Zhou, Z.; Huang, X.; Ma, L.; Wang, H.; Yang, Y.; Zhang, D.; Li, H.; Ren, R.; et al. Telomerase activity increased and telomere length shortened in peripheral blood cells from patients with immune thrombocytopenia. J. Clin. Immunol. 2013, 33, 577–585. [Google Scholar] [CrossRef]
- Hoffecker, B.M.; Raffield, L.M.; Kamen, D.L.; Nowling, T.K. Systemic lupus erythematosus and vitamin D deficiency are associated with shorter telomere length among African Americans: A case-control study. PLoS ONE 2013, 8, e63725. [Google Scholar] [CrossRef]
- Skamra, C.; Romero-Diaz, J.; Sandhu, A.; Huang, Q.; Lee, J.; Pearce, W.; McPherson, D.D.; Sutton-Tyrrell, K.; Pope, R.; Ramsey-Goldman, R. Telomere length in patients with systemic lupus erythematosus and its associations with carotid plaque. Rheumatology 2013, 52, 1101–1108. [Google Scholar] [CrossRef]
- Haque, S.; Rakieh, C.; Marriage, F.; Ho, P.; Gorodkin, R.; Teh, L.S.; Snowden, N.; Day, P.J.; Bruce, I.N. Shortened telomere length in patients with systemic lupus erythematosus. Arthritis Rheum. 2013, 65, 1319–1323. [Google Scholar] [CrossRef]
- Beier, F.; Balabanov, S.; Amberger, C.C.; Hartmann, U.; Manger, K.; Dietz, K.; Kötter, I.; Brummendorf, T.H. Telomere length analysis in monocytes and lymphocytes from patients with systemic lupus erythematosus using multi-color flow-FISH. Lupus 2007, 16, 955–962. [Google Scholar] [CrossRef]
- Wu, C.H.; Hsieh, S.C.; Li, K.J.; Lu, M.C.; Yu, C.L. Premature telomere shortening in polymorphonuclear neutrophils from patients with systemic lupus erythematosus is related to the lupus disease activity. Lupus 2007, 16, 265–272. [Google Scholar] [CrossRef]
- Kurosaka, D.; Yasuda, J.; Yoshida, K.; Yoneda, A.; Yasuda, C.; Kingetsu, I.; Toyokawa, Y.; Yokoyama, T.; Saito, S.; Yamada, A. Abnormal telomerase activity and telomere length in T and B cells from patients with systemic lupus erythematosus. J. Rheumatol. 2006, 33, 1102–1107. [Google Scholar]
- Lin, J.; Xie, J.; Qian, W.B. Telomerase activity and telomere length in CD4+, CD8+ and CD19+ lymphocytes from patients with systemic lupus erythematosus. Zhejiang Da Xue Xue Bao Yi Xue Ban 2005, 34, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Klapper, W.; Moosig, F.; Sotnikova, A.; Qian, W.; Schröder, J.O.; Parwaresch, R. Telomerase activity in B and T lymphocytes of patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2004, 63, 1681–1683. [Google Scholar] [CrossRef]
- Kurosaka, D.; Yasuda, J.; Yoshida, K.; Yokoyama, T.; Ozawa, Y.; Obayashi, Y.; Kingetsu, I.; Saito, S.; Yamada, A. Telomerase activity and telomere length of peripheral blood mononuclear cells in SLE patients. Lupus 2003, 12, 591–599. [Google Scholar] [CrossRef]
- Honda, M.; Mengesha, E.; Albano, S.; Nichols, W.S.; Wallace, D.J.; Metzger, A.; Klinenberg, J.R.; Linker-Israeli, M. Telomere shortening and decreased replicative potential, contrasted by continued proliferation of telomerase-positive CD8+CD28(lo) T cells in patients with systemic lupus erythematosus. Clin. Immunol. 2001, 99, 211–221. [Google Scholar] [CrossRef]
- Fessler, J.; Fasching, P.; Raicht, A.; Hammerl, S.; Weber, J.; Lackner, A.; Hermann, J.; Dejaco, C.; Graninger, W.B.; Schwinger, W.; et al. Lymphopenia in primary Sjögren’s syndrome is associated with premature aging of naïve CD4+ T cells. Rheumatology 2021, 60, 588–597. [Google Scholar] [CrossRef]
- Noll, B.; Bahrani Mougeot, F.; Brennan, M.T.; Mougeot, J.-L.C. Telomere erosion in Sjögren’s syndrome: A multi-tissue comparative analysis. J. Oral. Pathol. Med. 2020, 49, 63–71. [Google Scholar] [CrossRef]
- Pringle, S.; Wang, X.; Verstappen, G.M.P.J.; Terpstra, J.H.; Zhang, C.K.; He, A.; Patel, V.; Jones, R.E.; Baird, D.M.; Spijkervet, F.K.L.; et al. Salivary Gland Stem Cells Age Prematurely in Primary Sjögren’s Syndrome. Arthritis Rheumatol. 2019, 71, 133–142. [Google Scholar] [CrossRef]
- Kawashima, M.; Kawakita, T.; Maida, Y.; Kamoi, M.; Ogawa, Y.; Shimmura, S.; Masutomi, K.; Tsubota, K. Comparison of telomere length and association with progenitor cell markers in lacrimal gland between Sjögren syndrome and non-Sjögren syndrome dry eye patients. Mol. Vis. 2011, 17, 1397–1404. [Google Scholar] [PubMed]
- Usategui, A.; Municio, C.; Arias-Salgado, E.G.; Martín, M.; Fernández-Varas, B.; Del Rey, M.J.; Carreira, P.; González, A.; Criado, G.; Perona, R.; et al. Evidence of telomere attrition and a potential role for DNA damage in systemic sclerosis. Immun. Ageing 2022, 19, 7. [Google Scholar] [CrossRef]
- Liu, S.; Chung, M.P.; Ley, B.; French, S.; Elicker, B.M.; Fiorentino, D.F.; Chung, L.S.; Boin, F.; Wolters, P.J. Peripheral blood leucocyte telomere length is associated with progression of interstitial lung disease in systemic sclerosis. Thorax 2021, 76, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Adler, B.L.; Boin, F.; Wolters, P.J.; Bingham, C.O.; Shah, A.A.; Greider, C.; Casciola-Rosen, L.; Rosen, A. Autoantibodies targeting telomere-associated proteins in systemic sclerosis. Ann. Rheum. Dis. 2021, 80, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Lakota, K.; Hanumanthu, V.S.; Agrawal, R.; Carns, M.; Armanios, M.; Varga, J. Short lymphocyte, but not granulocyte, telomere length in a subset of patients with systemic sclerosis. Ann. Rheum. Dis. 2019, 78, 1142–1144. [Google Scholar] [CrossRef]
- Tarhan, F.; Vural, F.; Kosova, B.; Aksu, K.; Cogulu, O.; Keser, G.; Gündüz, C.; Tombuloglu, M.; Oder, G.; Karaca, E.; et al. Telomerase activity in connective tissue diseases: Elevated in rheumatoid arthritis, but markedly decreased in systemic sclerosis. Rheumatol. Int. 2008, 28, 579–583. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, A.; Brouilette, S.W.; Lamb, K.; Radhakrishnan, K.; McGlynn, L.; Chee, M.M.; Parkinson, E.K.; Freeman, D.; Madhok, R.; Shiels, P.G. Association of increased telomere lengths in limited scleroderma, with a lack of age-related telomere erosion. Ann. Rheum. Dis. 2008, 67, 1780–1782. [Google Scholar] [CrossRef]
- Artlett, C.M.; Black, C.M.; Briggs, D.C.; Stevens, C.O.; Welsh, K.I. Telomere reduction in scleroderma patients: A possible cause for chromosomal instability. Br. J. Rheumatol. 1996, 35, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Guillén, R.; Otero, F.; Mosquera, A.; Vázquez-Mosquera, M.; Rego-Pérez, I.; Blanco, F.J.; Fernández, J.L. Association of accelerated dynamics of telomere sequence loss in peripheral blood leukocytes with incident knee osteoarthritis in Osteoarthritis Initiative cohort. Sci. Rep. 2021, 11, 15914. [Google Scholar] [CrossRef]
- Fajardo, R.G.; Fariña, F.O.; Rey, A.M.; Rego-Pérez, I.; Blanco, F.J.; García, J.L.F. Relationship Between the Dynamics of Telomere Loss in Peripheral Blood Leukocytes from Knee Osteoarthritis Patients and Mitochondrial DNA Haplogroups. J. Rheumatol. 2021, 48, 1603–1607. [Google Scholar] [CrossRef]
- Mosquera, A.; Rego-Pérez, I.; Blanco, F.J.; Fernández, J.L. Leukocyte Telomere Length in Patients with Radiographic Knee Osteoarthritis. Environ. Mol. Mutagen. 2019, 60, 298–301. [Google Scholar] [CrossRef]
- Poonpet, T.; Saetan, N.; Tanavalee, A.; Wilairatana, V.; Yuktanandana, P.; Honsawek, S. Association between leukocyte telomere length and angiogenic cytokines in knee osteoarthritis. Int. J. Rheum. Dis. 2018, 21, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Wiwanitkit, V. Telomere length and angiogenic cytokines in knee osteoarthritis. Int. J. Rheum. Dis. 2017, 20, 2141. [Google Scholar] [CrossRef]
- Zhai, G.; Aviv, A.; Hunter, D.J.; Hart, D.J.; Gardner, J.P.; Kimura, M.; Lu, X.; Valdes, A.M.; Spector, T.D. Reduction of leucocyte telomere length in radiographic hand osteoarthritis: A population-based study. Ann. Rheum. Dis. 2006, 65, 1444–1448. [Google Scholar] [CrossRef]
- Fessler, J.; Raicht, A.; Husic, R.; Ficjan, A.; Duftner, C.; Schwinger, W.; Dejaco, C.; Schirmer, M. Premature senescence of T-cell subsets in axial spondyloarthritis. Ann. Rheum. Dis. 2016, 75, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, M.; Pértega, S.; Mosquera, A.; Rodríguez, M.; Blanco, F.J.; Fernández-Sueiro, J.L.; Gosálvez, J.; Fernández, J.L. Individual telomere length decay in patients with spondyloarthritis. Mutat. Res. 2014, 765, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Vazirpanah, N.; Kienhorst, L.B.E.; Van Lochem, E.; Wichers, C.; Rossato, M.; Shiels, P.G.; Dalbeth, N.; Stamp, L.K.; Merriman, T.R.; Janssen, M.; et al. Patients with gout have short telomeres compared with healthy participants: Association of telomere length with flare frequency and cardiovascular disease in gout. Ann. Rheum. Dis. 2017, 76, 1313–1319. [Google Scholar] [CrossRef]
- Vogt, S.; Iking-Konert, C.; Hug, F.; Andrassy, K.; Hänsch, G.M. Shortening of telomeres: Evidence for replicative senescence of T cells derived from patients with Wegener’s granulomatosis. Kidney Int. 2003, 63, 2144–2151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, Y.; Ye, M.; Mao, Y.; Zhan, Y. Telomere length and its association with systemic lupus erythematosus in an Asian population: A Mendelian randomization study. Lupus 2023, 32, 1222–1226. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Jung, J.H.; Seo, Y.H.; Kim, J.H.; Choi, S.J.; Ji, J.D.; Song, G.G. Association between shortened telomere length and systemic lupus erythematosus: A meta-analysis. Lupus 2017, 26, 282–288. [Google Scholar] [CrossRef]
- Wang, X.-F.; Xu, W.-J.; Wang, F.-F.; Leng, R.; Yang, X.-K.; Ling, H.-Z.; Fan, Y.-G.; Tao, J.-H.; Shuai, Z.-W.; Zhang, L.; et al. Telomere length and development of systemic lupus erythematosus: A Mendelian randomization study. Arthritis Rheumatol. 2022, 74, 1984–1990. [Google Scholar] [CrossRef]
- Vulsteke, J.-B.; Smith, V.; Bonroy, C.; Derua, R.; Blockmans, D.; De Haes, P.; Vanderschueren, S.; Lenaerts, J.L.; Claeys, K.G.; Wuyts, W.A. Identification of new telomere-and telomerase-associated autoantigens in systemic sclerosis. J. Autoimmun. 2023, 135, 102988. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Tsou, P.-S.; Ma, F.; Mariani, M.P.; Mattichak, M.N.; LeBrasseur, N.K.; Chini, E.N.; Lafyatis, R.; Khanna, D.; Whitfield, M.L. Senescent cells accumulate in systemic sclerosis skin. J. Investig. Dermatol. 2023, 143, 661. [Google Scholar] [CrossRef] [PubMed]
- Martyanov, V.; Whitfield, M.L.; Varga, J. Senescence Signature in Skin Biopsies From Systemic Sclerosis Patients Treated with Senolytic Therapy: Potential Predictor of Clinical Response? Arthritis Rheumatol. 2019, 71, 1766–1767. [Google Scholar] [CrossRef]
- Chini, C.C.S.; Peclat, T.R.; Warner, G.M.; Kashyap, S.; Espindola-Netto, J.M.; de Oliveira, G.C.; Gomez, L.S.; Hogan, K.A.; Tarragó, M.G.; Puranik, A.S.; et al. CD38 ecto-enzyme in immune cells is induced during aging and regulates NAD(+) and NMN levels. Nat. Metab. 2020, 2, 1284–1304. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Jiang, H.; Li, B.; Cao, L.; Shen, Q.; Yi, W.; Ju, Z.; Chen, L.; Han, F.; Appelgren, D.; et al. Telomere dysfunction promotes small vessel vasculitis via the LL37-NETs-dependent mechanism. Ann. Transl. Med. 2020, 8, 357. [Google Scholar] [CrossRef]
- Sambataro, G.; Ferro, F.; Orlandi, M.; Sambataro, D.; Torrisi, S.E.; Quartuccio, L.; Vancheri, C.; Baldini, C.; Matucci Cerinic, M. Clinical, morphological features and prognostic factors associated with interstitial lung disease in primary Sjögren’s syndrome: A systematic review from the Italian Society of Rheumatology. Autoimmun. Rev. 2020, 19, 102447. [Google Scholar] [CrossRef] [PubMed]
- Mathai, S.C.; Danoff, S.K. Management of interstitial lung disease associated with connective tissue disease. BMJ 2016, 352, h6819. [Google Scholar] [CrossRef] [PubMed]
- Luppi, F.; Sebastiani, M.; Sverzellati, N.; Cavazza, A.; Salvarani, C.; Manfredi, A. Lung complications of Sjogren syndrome. Eur. Respir. Rev. 2020, 29, 200021. [Google Scholar] [CrossRef]
- Tsakiri, K.D.; Cronkhite, J.T.; Kuan, P.J.; Xing, C.; Raghu, G.; Weissler, J.C.; Rosenblatt, R.L.; Shay, J.W.; Garcia, C.K. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc. Natl. Acad. Sci. USA 2007, 104, 7552–7557. [Google Scholar] [CrossRef] [PubMed]
- Akinnibosun, O.A.; Maier, M.C.; Eales, J.; Tomaszewski, M.; Charchar, F.J. Telomere therapy for chronic kidney disease. Epigenomics 2022, 14, 1039–1054. [Google Scholar] [CrossRef]
- Gao, J.; Pickett, H.A. Targeting telomeres: Advances in telomere maintenance mechanism-specific cancer therapies. Nat. Rev. Cancer 2022, 22, 515–532. [Google Scholar] [CrossRef]
- Rossiello, F.; Aguado, J.; Sepe, S.; Iannelli, F.; Nguyen, Q.; Pitchiaya, S.; Carninci, P.; d’Adda di Fagagna, F. DNA damage response inhibition at dysfunctional telomeres by modulation of telomeric DNA damage response RNAs. Nat. Commun. 2017, 8, 13980. [Google Scholar] [CrossRef] [PubMed]
- Coryell, P.R.; Diekman, B.O.; Loeser, R.F. Mechanisms and therapeutic implications of cellular senescence in osteoarthritis. Nat. Rev. Rheumatol. 2021, 17, 47–57. [Google Scholar] [CrossRef]
| Disease | Reference | Cells | Method | Authors’ Conclusion |
|---|---|---|---|---|
| RA | Svyryd et al. [29] | WBC | qPCR | rLTL was significantly shorter in RA patients than at admission. rLTL was shorter in early disease compared to controls. rLTL shortening effects were influenced by age, DET (disease exposure time), and natural rLTL. |
| Natalini et al. [104] | PBL | qPCR | Telomere shortening was strongly correlated with RA prevalence but did not lead to the progression of ILD in RA patients. | |
| Ormseth et al. [101] | PBL | qPCR | RA patients did not exhibit any significant difference in telomere length than healthy controls. | |
| Prescott et al. [105] | WB | qPCR | Longer prediagnostic LTL was associated with increased RA risk. | |
| Blinova et al. [108] | PBMC | Q-FISH | Patients with RA had significantly shorter chromosome 4p telomeres. | |
| Tamayo et al. [109] | PBL | qPCR | TL was significantly longer than controls in RA. | |
| Fujii et al. [68] | Naive CD4+ T cells | TRF | Sluggish cell cycle and growth factor nonresponsiveness. | |
| Colmegna et al. [107] | CD34+ hematopoietic precursor cells (HPCs) | Q-FISH | Marked telomere shortening, sluggish cell cycle progression, and growth factor nonresponsiveness were observed in HPCs from RA patients. This indicates proliferative and oxidative stress-stimulated cell senescence. | |
| Steer et al. [100] | WBC | TRF | Shortened TRF length in RA patients was not associated with disease markers and severity but was predisposed by the HLA-DRB1 genotype. | |
| Schonland et al. [99] | PBMC | TRF | Unwarranted loss of telomeres in CD4+ T cells was dependent on HLA-DRB1*04 alleles. | |
| Koetz et al. [106] | PBMC CD4+ (45RA/45RO); CD8+ | TRF | Usage of disease-modifying drugs and disease duration were not related to telomere loss. In contrast, telomere loss was increased in CD4+CD45ROnull (naive) T cells. | |
| Yudoh et al. [110] | PBL, synovial infiltrating lymphocytes, and synoviocytes | TRAP | Telomerase activity was elevated in synovial infiltrating lymphocytes and PBL from RA patients, but not in the synoviocytes. | |
| SLE | Bridges et al. [111] | Dried blood spots (DBSs) | qPCR | LTL shortening was accelerated in Black women suffering from childhood SLE. |
| Qi et al. [112,113] | PBMC | Increased expression of PINX1 (encoding PinX1 protein, which reduces telomerase enzyme activity and improves telomere length) mRNA in PBMCs. | ||
| Hoffecker et al. [114] | PBMC | qPCR | A higher titer of anti-telomere antibody and shorter telomeres. | |
| Skamra et al. [115] | WB | qPCR | Telomere shortening in SLE. | |
| Haque et al. [116] | WB | qPCR | Telomere shortening in SLE. | |
| Beier et al. [117] | PBMC: T cells (CD4+/CD8+); B cells (CD19+) and monocytes (CD14+) | Flow–FISH | SLE did not impact the TL. However, all three lymphocyte subsets exhibit shortened telomeres as compared to monocytes. | |
| Wu et al. [118] | polymorphonuclear neutrophils mononuclear cells | TRF | SLEDAI augmented telomere erosion. | |
| Kurosaka et al. [119] | PBMC, T and B lymphocytes | Flow–FISH | TL in T cells from the SLE group was reduced compared to controls but not in B cells. | |
| Lin et al. [120] | PBMC, T and B lymphocytes | TRAP | B cells from SLE individuals showed shortened TL but did not show differences in TL among T cells. | |
| Klapper et al. [121] | PBMC, T and B lymphocytes | TRAP | CD19+ B cells elevated telomerase activity in SLE patients. | |
| Kurosaka et al. [122] | PBMC | TRAP | Telomerase activity was significantly correlated with SLEDAI. Younger but not elderly SLE patients had substantially reduced TL. | |
| Honda et al. [123] | PBMC | TRF | In the early years, CD8+ CD4+ cells showed increased telomerase, ultimately shortening telomeres. | |
| pSS | Fessler et al. [124] | PBMC | qPCR | Aging signs were increased in naive CD4+ T cells. |
| Noll et al. [125] | PBMC; saliva; LSG | qPCR | pSS patients showed enhanced telomere erosion in saliva DNA. | |
| Pringle et al. [126] | SGSC | STELA | SGSCs from samples from patients with pSS were not only lower in number and less able to differentiate but were likely to be senescent. | |
| Kawashima et al. [127] | lacrimal gland tissue | Q-FISH | pSS patients showed shorter TL, linked with lower p63 and nucleostemin. | |
| SSc | Usategui et al. [128] | PBL | TRF | Shorter age-standardized TL in SSc patients. |
| Liu et al. [129] | PBL | qPCR | Dysfunction telomere was linked with SSc-ILD progression. | |
| Adler et al. [130] | PBL; PBMC | qPCR Flow–FISH | TERF1, an autoantibody against telomere-associated protein, was associated with short TL in lymphocytes and pulmonary fibrosis in patients with SSc. | |
| Lakota et al. [131] | PBMC | Flow–FISH | Acquired lineage-specific TL shortening in lymphocytes in SSc-associated ILD. | |
| Tarhan et al. [132] | PBMC | TRAP | Very low telomerase activity in the SSc group. | |
| MacIntyre et al. [133] | PBMC | TRF | TL was longer in lcSSc individuals, while age-related telomere erosion was not observed but diverged considerably from age-matched healthy individuals only after the age of 50 years. | |
| Artlett et al. [134] | Lymphocytes; fibroblasts | TRF | Telomeric erosion among SSc patients and family members. | |
| OA | Guillén et al. [135] | PBL | qPCR | Enhanced activity of telomere decay was observed in PBL from OA patients. |
| Fajardo et al. [136] | PBL | qPCR | Shortened telomere dynamics in PBL may be a persistent risk sign of knee OA occurrence. | |
| Mosquera et al. [137] | PBL | qPCR | Telomere size in PBL served as a risk factor for simultaneous knee OA. | |
| Poonpet et al. [138] | PBL | qPCR | Shortened TL in the knee of OA patients. | |
| Wiwanitkit et al. [139] | PBL | qPCR | PBL telomere length was associated with prevalent hand OA at baseline | |
| Zhai et al. [140] | PBL | TRF | Reduced TL in OA individuals. | |
| Tamayo et al. [109] | PBL | qPCR | No difference in TL was observed among OA patients and age-matched controls. | |
| SpA | Fessler et al. [141] | PBMC | qPCR | CD4+ and CD8+ T cells subsets showed reduced TL in young SpA patients. |
| AS | Tamayo et al. [142] | PBL | qPCR | AS patients had longer TL than controls. |
| AS, PsA | Tamayo et al. [109] | PBL | qPCR | PsA patients showed a higher telomere loss rate than AS patients. |
| GT | Vazirpanah et al. [143] | PBMC | qPCR | Patients with gout had shorter telomeres. |
| WG | Vogt et al. [144] | PBMC | TRF | Short telomeres were detected in patients with a disease course of >5 years. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, F.; Riaz, F.; Pu, J.; Gao, R.; Yang, L.; Wang, Y.; Song, J.; Liang, Y.; Wu, Z.; Li, C.; et al. Connecting the Dots: Telomere Shortening and Rheumatic Diseases. Biomolecules 2024, 14, 1261. https://doi.org/10.3390/biom14101261
Han F, Riaz F, Pu J, Gao R, Yang L, Wang Y, Song J, Liang Y, Wu Z, Li C, et al. Connecting the Dots: Telomere Shortening and Rheumatic Diseases. Biomolecules. 2024; 14(10):1261. https://doi.org/10.3390/biom14101261
Chicago/Turabian StyleHan, Fang, Farooq Riaz, Jincheng Pu, Ronglin Gao, Lufei Yang, Yanqing Wang, Jiamin Song, Yuanyuan Liang, Zhenzhen Wu, Chunrui Li, and et al. 2024. "Connecting the Dots: Telomere Shortening and Rheumatic Diseases" Biomolecules 14, no. 10: 1261. https://doi.org/10.3390/biom14101261
APA StyleHan, F., Riaz, F., Pu, J., Gao, R., Yang, L., Wang, Y., Song, J., Liang, Y., Wu, Z., Li, C., Tang, J., Xu, X., & Wang, X. (2024). Connecting the Dots: Telomere Shortening and Rheumatic Diseases. Biomolecules, 14(10), 1261. https://doi.org/10.3390/biom14101261







