Role of Gonadal Steroid Hormones in the Eye: Therapeutic Implications
Abstract
:1. Gonadal Steroid Hormones, Synthesis
2. Neurosteroids
3. Steroid Hormones in the Eye
4. Role of Gonadal Steroid Hormones in Eye Diseases
4.1. Glaucoma
4.2. Age-Related Macular Degeneration
4.3. Diabetic Retinopathy
4.4. Retinitis Pigmentosa
4.5. Other Diseases
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.P.; Soldin, O.P.; Guo, T.; Soldin, S.J. Steroid hormones: Relevance and measurement in the clinical laboratory. Clin. Lab. Med. 2004, 24, 105–118. [Google Scholar] [CrossRef]
- Pillerová, M.; Borbélyová, V.; Hodosy, J.; Riljak, V.; Renczés, E.; Frick, K.M.; Tóthová, Ľ. On the role of sex steroids in biological functions by classical and non-classical pathways. An update. Front. Neuroendocrinol. 2021, 62, 100926. [Google Scholar] [CrossRef]
- Naamneh Elzenaty, R.; du Toit, T.; Flück, C.E. Basics of androgen synthesis and action. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101665. [Google Scholar] [CrossRef]
- Alemany, M. The Roles of Androgens in Humans: Biology, Metabolic Regulation and Health. Int. J. Mol. Sci. 2022, 23, 11952. [Google Scholar] [CrossRef]
- Nuzzi, R.; Caselgrandi, P. Sex Hormones and Their Effects on Ocular Disorders and Pathophysiology: Current Aspects and Our Experience. Int. J. Mol. Sci. 2022, 23, 3269. [Google Scholar] [CrossRef]
- Sumien, N.; Cunningham, J.T.; Davis, D.L.; Engelland, R.; Fadeyibi, O.; Farmer, G.E.; Mabry, S.; Mensah-Kane, P.; Trinh, O.T.P.; Vann, P.H.; et al. Neurodegenerative Disease: Roles for Sex, Hormones, and Oxidative Stress. Endocrinology 2021, 162, bqab185. [Google Scholar] [CrossRef]
- Sakamoto, H.; Takahashi, H.; Matsuda, K.; Nishi, M.; Takanami, K.; Ogoshi, M.; Sakamoto, T.; Kawata, M. Rapid signaling of steroid hormones in the vertebrate nervous system. Front. Biosci. (Landmark Ed.) 2012, 17, 996–1019. [Google Scholar] [CrossRef]
- Singh, M.; Su, C.; Ng, S. Non-genomic mechanisms of progesterone action in the brain. Front. Neurosci. 2013, 7, 159. [Google Scholar] [CrossRef]
- Conneely, O.M.; Lydon, J.P. Progesterone receptors in reproduction: Functional impact of the A and B isoforms. Steroids 2000, 65, 571–577. [Google Scholar] [CrossRef]
- Balasubramanian, B.; Portillo, W.; Reyna, A.; Chen, J.Z.; Moore, A.N.; Dash, P.K.; Mani, S.K. Nonclassical mechanisms of progesterone action in the brain: II. Role of calmodulin-dependent protein kinase II in progesterone-mediated signaling in the hypothalamus of female rats. Endocrinology 2008, 149, 5518–5526. [Google Scholar] [CrossRef] [PubMed]
- Boulware, M.I.; Kordasiewicz, H.; Mermelstein, P.G. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J. Neurosci. 2007, 27, 9941–9950. [Google Scholar] [CrossRef]
- Fester, L.; Rune, G.M. Sex neurosteroids: Hormones made by the brain for the brain. Neurosci. Lett. 2021, 753, 135849. [Google Scholar] [CrossRef] [PubMed]
- Baulieu, E.E.; Schumacher, M.; Koenig, H.; Jung-Testas, I.; Akwa, Y. Progesterone as a neurosteroid: Actions within the nervous system. Cell Mol. Neurobiol. 1996, 16, 143–154. [Google Scholar] [CrossRef]
- Baulieu, E.E.; Robel, P.; Schumacher, M. Neurosteroids: Beginning of the story. Int. Rev. Neurobiol. 2001, 46, 1–32. [Google Scholar]
- Mendell, A.L.; MacLusky, N.J. Neurosteroid Metabolites of Gonadal Steroid Hormones in Neuroprotection: Implications for Sex Differences in Neurodegenerative Disease. Front. Mol. Neurosci. 2018, 11, 359. [Google Scholar] [CrossRef]
- Mogi, K.; Takanashi, H.; Nagasawa, M.; Kikusui, T. Sex differences in spatiotemporal expression of AR, ERα, and ERβ mRNA in the perinatal mouse brain. Neurosci. Lett. 2015, 584, 88–92. [Google Scholar] [CrossRef]
- Gupta, P.D.; Johar, K.S.; Nagpal, K.; Vasavada, A.R. Sex hormone receptors in the human eye. Surv. Ophthalmol. 2005, 50, 274–284. [Google Scholar] [CrossRef]
- Cascio, C.; Deidda, I.; Russo, D.; Guarneri, P. The estrogenic retina: The potential contribution to healthy aging and age-related neurodegenerative diseases of the retina. Steroids 2015, 103, 31–41. [Google Scholar] [CrossRef]
- Ramachandra Rao, S.; Fliesler, S.J. Cholesterol homeostasis in the vertebrate retina: Biology and pathobiology. J. Lipid Res. 2021, 62, 100057. [Google Scholar] [CrossRef]
- Fliesler, S.J.; Bretillon, L. The ins and outs of cholesterol in the vertebrate retina. J. Lipid Res. 2010, 51, 3399–3413. [Google Scholar] [CrossRef] [PubMed]
- Mast, N.; El-Darzi, N.; Li, Y.; Pikuleva, I.A. Quantitative characterizations of the cholesterol-related pathways in the retina and brain of hamsters. J. Lipid Res. 2023, 64, 100401. [Google Scholar] [CrossRef] [PubMed]
- Guarneri, P.; Guarneri, R.; Cascio, C.; Pavasant, P.; Piccoli, F.; Papadopoulos, V. Neurosteroidogenesis in rat retinas. J. Neurochem. 1994, 63, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Cascio, C.; Russo, D.; Drago, G.; Galizzi, G.; Passantino, R.; Guarneri, R.; Guarneri, P. 17beta-estradiol synthesis in the adult male rat retina. Exp. Eye Res. 2007, 85, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Mellon, S.H.; Griffin, L.D.; Compagnone, N.A. Biosynthesis and action of neurosteroids. Brain Res. Brain Res. Rev. 2001, 37, 3–12. [Google Scholar] [CrossRef]
- Wickham, L.A.; Gao, J.; Toda, I.; Rocha, E.M.; Ono, M.; Sullivan, D.A. Identification of androgen, estrogen and progesterone receptor mRNAs in the eye. Acta Ophthalmol. Scand. 2000, 78, 146–153. [Google Scholar] [CrossRef]
- Ishikawa, M.; Yoshitomi, T.; Zorumski, C.F.; Izumi, Y. Neurosteroids are endogenous neuroprotectants in an ex vivo glaucoma model. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8531–8541. [Google Scholar] [CrossRef]
- Siesky, B.A.; Harris, A.; Patel, C.; Klaas, C.L.; Harris, M.; McCranor, L.J.; Lauer, J.; Kaplan, B. Comparison of visual function and ocular hemodynamics between pre- and post-menopausal women. Eur. J. Ophthalmol. 2008, 18, 320–323. [Google Scholar] [CrossRef]
- Ozawa, G.Y.; Bearse, M.A.; Harrison, W.W.; Bronson-Castain, K.W.; Schneck, M.E.; Barez, S.; Adams, A.J. Differences in neuroretinal function between adult males and females. Optom. Vis. Sci. 2014, 91, 602–607. [Google Scholar] [CrossRef]
- Olakowska, E.; Rodak, P.; Pacwa, A.; Machowicz, J.; Machna, B.; Lewin-Kowalik, J.; Smedowski, A. Surgical Menopause Impairs Retinal Conductivity and Worsens Prognosis in an Acute Model of Rat Optic Neuropathy. Cells 2022, 11, 3062. [Google Scholar] [CrossRef]
- Pang, I.H.; Clark, A.F. Rodent models for glaucoma retinopathy and optic neuropathy. J. Glaucoma 2007, 16, 483–505. [Google Scholar] [CrossRef] [PubMed]
- Hulsman, C.A.; Westendorp, I.C.; Ramrattan, R.S.; Wolfs, R.C.; Witteman, J.C.; Vingerling, J.R.; Hofman, A.; de Jong, P.T. Is open-angle glaucoma associated with early menopause? The Rotterdam Study. Am. J. Epidemiol. 2001, 154, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Vajaranant, T.S.; Grossardt, B.R.; Maki, P.M.; Pasquale, L.R.; Sit, A.J.; Shuster, L.T.; Rocca, W.A. Risk of glaucoma after early bilateral oophorectomy. Menopause 2014, 21, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Panchami: Pai, S.R.; Shenoy, J.P.; J, S.; Kole, S.B. Postmenopausal intraocular pressure changes in South Indian females. J. Clin. Diagn. Res. 2013, 7, 1322–1324. [Google Scholar]
- Lee, A.J.; Mitchell, P.; Rochtchina, E.; Healey, P.R.; Blue Mountains Eye Study. Female reproductive factors and open angle glaucoma: The Blue Mountains Eye Study. Br. J. Ophthalmol. 2003, 87, 1324–1328. [Google Scholar] [CrossRef]
- Newman-Casey, P.A.; Talwar, N.; Nan, B.; Much, D.C.; Pasquale, L.R.; Stein, J.D. The potential association between postmenopausal hormone use and primary open-angle glaucoma. JAMA Ophthalmol. 2014, 132, 298–303. [Google Scholar] [CrossRef]
- Prokai-Tatrai, K.; Xin, H.; Nguyen, V.; Szarka, S.; Blazics, B.; Prokai, L.; Koulen, P. 17β-estradiol eye drops protect the retinal ganglion cell layer and preserve visual function in an in vivo model of glaucoma. Mol. Pharm. 2013, 10, 3253–3261. [Google Scholar] [CrossRef]
- Armstrong, R.A.; Mousavi, M. Overview of Risk Factors for Age-Related Macular Degeneration (AMD). J. Stem Cells 2015, 10, 171–191. [Google Scholar]
- Klein, R.; Chou, C.F.; Klein, B.E.; Zhang, X.; Meuer, S.M.; Saaddine, J.B. Prevalence of age-related macular degeneration in the US population. Arch. Ophthalmol. 2011, 129, 75–80. [Google Scholar] [CrossRef]
- Ferris, F.L.; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R.; Beckman Initiative for Macular Research Classification Committee. Clinical classification of age-related macular degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar]
- Ambati, J.; Fowler, B.J. Mechanisms of age-related macular degeneration. Neuron 2012, 75, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E.; Linton, K.L.; DeMets, D.L. The Beaver Dam Eye Study: The relation of age-related maculopathy to smoking. Am. J. Epidemiol. 1993, 137, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.S.; O’Colmain, B.J.; Muñoz, B.; Tomany, S.C.; McCarty, C.; de Jong, P.T.; Nemesure, B.; Mitchell, P.; Kempen, J.; Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004, 122, 564–572, Erratum in Arch Ophthalmol. 2011, 129, 1188. [Google Scholar]
- Seddon, J.M.; Cote, J.; Page, W.F.; Aggen, S.H.; Neale, M.C. The US twin study of age-related macular degeneration: Relative roles of genetic and environmental influences. Arch. Ophthalmol. 2005, 123, 321–327. [Google Scholar] [CrossRef]
- Yuk, J.S.; Hwang, J.H. Menopause and the Risk of Developing Age-Related Macular Degeneration in Korean Women. J. Clin. Med. 2022, 11, 1899. [Google Scholar] [CrossRef]
- Erke, M.G.; Bertelsen, G.; Peto, T.; Sjølie, A.K.; Lindekleiv, H.; Njølstad, I. Lactation, female hormones and age-related macular degeneration: The Tromsø Study. Br. J. Ophthalmol. 2013, 97, 1036–1039. [Google Scholar] [CrossRef]
- Abramov, Y.; Borik, S.; Yahalom, C.; Fatum, M.; Avgil, G.; Brzezinski, A.; Banin, E. The effect of hormone therapy on the risk for age-related maculopathy in postmenopausal women. Menopause 2004, 11, 62–68. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Machalińska, A.; Veréb, Z.; Salminen, A.; Petrovski, G.; Kauppinen, A. Estrogen signalling in the pathogenesis of age-related macular degeneration. Curr. Eye Res. 2015, 40, 226–233. [Google Scholar] [CrossRef]
- Feskanich, D.; Cho, E.; Schaumberg, D.A.; Colditz, G.A.; Hankinson, S.E. Menopausal and reproductive factors and risk of age-related macular degeneration. Arch. Ophthalmol. 2008, 126, 519–524. [Google Scholar] [CrossRef]
- Snow, K.K.; Cote, J.; Yang, W.; Davis, N.J.; Seddon, J.M. Association between reproductive and hormonal factors and age-related maculopathy in postmenopausal women. Am. J. Ophthalmol. 2002, 134, 842–848. [Google Scholar] [CrossRef]
- Haan, M.N.; Klein, R.; Klein, B.E.; Deng, Y.; Blythe, L.K.; Seddon, J.M.; Musch, D.C.; Kuller, L.H.; Hyman, L.G.; Wallace, R.B. Hormone therapy and age-related macular degeneration: The Women’s Health Initiative Sight Exam Study. Arch. Ophthalmol. 2006, 124, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.E.; Muñoz, B.; Bressler, S.B.; West, S.K. Hormone replacement therapy, reproductive factors, and age-related macular degeneration: The Salisbury Eye Evaluation Project. Ophthalmic Epidemiol. 2005, 12, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Kang, S.W.; Han, J.; Han, K.; Kim, D.; Lee, K.N.; Jeon, K.H.; Yoo, J.E.; Lee, D.Y.; Shin, D.W.; et al. Femle reproductive factors and the risk of exudative age-related macular degeneration: A Nationwide Cohort Study. Retina 2021, 41, 2088–2097. [Google Scholar] [CrossRef] [PubMed]
- Pardue, M.T.; Allen, R.S. Neuroprotective strategies for retinal disease. Prog. Retin. Eye Res. 2018, 65, 50–76. [Google Scholar] [CrossRef]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Siddiqui, K.; George, T.P.; Alosaimi, J.; Bukhari, K.O.; Rubeaan, K.A. Level of hormones in menopause in relation to diabetic retinopathy among type 2 diabetic women. Health Care Women Int. 2021, 42, 58–66. [Google Scholar] [CrossRef]
- Liu, K.; Fan, H.; Hu, H.; Cheng, Y.; Liu, J.; You, Z. Genetic variation reveals the influence of steroid hormones on the risk of retinal neurodegenerative diseases. Front. Endocrinol. 2023, 13, 1088557. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Xu, N.; Feng, W.; Qiao, J.; Liu, M. Low serum dehydroepiandrosterone levels are associated with diabetic retinopathy in patients with type 2 diabetes mellitus. J. Diabetes Investig. 2023, 14, 675–685. [Google Scholar] [CrossRef]
- Sun, S.; Wang, Y.; Zhou, Y.; Ma, W.; Huang, Y.; Hu, J.; Wang, Y. Serum progesterone and retinopathy in male patients with type 2 diabetes: A cross-sectional study. J. Diabetes Investig. 2021, 12, 1228–1235. [Google Scholar] [CrossRef]
- Ozawa, G.Y.; Bearse, M.A.; Bronson-Castain, K.W.; Harrison, W.W.; Schneck, M.E.; Barez, S.; Adams, A.J. Neurodegenerative differences in the retinas of male and female patients with type 2 diabetes. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3040–3046. [Google Scholar] [CrossRef]
- Espinosa-Heidmann, D.G.; Marin-Castano, M.E.; Pereira-Simon, S.; Hernandez, E.P.; Elliot, S.; Cousins, S.W. Gender and estrogen supplementation increases severity of experimental choroidal neovascularization. Exp. Eye Res. 2005, 80, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Mangiamele, L.A.; Gomez, J.R.; Curtis, N.J.; Thompson, R.R. GPER/GPR30, a membrane estrogen receptor, is expressed in the brain and retina of a social fish (Carassius auratus) and colocalizes with isotocin. J. Comp. Neurol. 2017, 525, 252–270. [Google Scholar] [CrossRef] [PubMed]
- Farrar, G.J.; Kenna, P.F.; Humphries, P. On the genetics of retinitis pigmentosa and on mutation-independent approaches to therapeutic intervention. EMBO J. 2002, 21, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Berson, E.L. Retinitis pigmentosa. The Friedenwald Lecture. Investig. Ophthalmol. Vis. Sci. 1993, 34, 1659–1676. [Google Scholar]
- Bird, A.C. Retinal photoreceptor dystrophies LI. Edward Jackson Memorial Lecture. Am. J. Ophthalmol. 1995, 119, 543–562. [Google Scholar] [CrossRef]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Chizzolini, M.; Galan, A.; Milan, E.; Sebastiani, A.; Costagliola, C.; Parmeggiani, F. Good epidemiologic practice in retinitis pigmentosa: From phenotyping to biobanking. Curr. Genom. 2011, 12, 260–266. [Google Scholar]
- Phelan, J.K.; Bok, D. A brief review of retinitis pigmentosa and the identified retinitis pigmentosa genes. Mol. Vis. 2000, 6, 116–124. [Google Scholar]
- Kennan, A.; Aherne, A.; Humphries, P. Light in retinitis pigmentosa. Trends Genet. 2005, 21, 103–110. [Google Scholar] [CrossRef]
- Sahni, J.N.; Angi, M.; Irigoyen, C.; Semeraro, F.; Romano, M.R.; Parmeggiani, F. Therapeutic challenges to retinitis pigmentosa: From neuroprotection to gene therapy. Curr. Genom. 2011, 12, 276–284. [Google Scholar] [CrossRef]
- Hamel, C. Retinitis pigmentosa. Orphanet J. Rare Dis. 2006, 1, 40. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vallejo, V.; Benlloch-Navarro, S.; López-Pedrajas, R.; Romero, F.J.; Miranda, M. Neuroprotective actions of progesterone in an in vivo model of retinitis pigmentosa. Pharmacol. Res. 2015, 99, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Benlloch-Navarro, S.; Trachsel-Moncho, L.; Fernández-Carbonell, Á.; Olivar, T.; Soria, J.M.; Almansa, I.; Miranda, M. Progesterone anti-inflammatory properties in hereditary retinal degeneration. J. Steroid Biochem. Mol. Biol. 2019, 189, 291–301. [Google Scholar] [CrossRef]
- Guarneri, P.; Cascio, C.; Russo, D.; D’Agostino, S.; Drago, G.; Galizzi, G.; De Leo, G.; Piccoli, F.; Guarneri, M.; Guarneri, R. Neurosteroids in the retina: Neurodegenerative and neuroprotective agents in retinal degeneration. Ann. N. Y. Acad. Sci. 2003, 1007, 117–128. [Google Scholar] [CrossRef]
- Doonan, F.; O’Driscoll, C.; Kenna, P.; Cotter, T.G. Enhancing survival of photoreceptor cells in vivo using the synthetic progestin Norgestrel. J. Neurochem. 2011, 118, 915–927. [Google Scholar] [CrossRef]
- Gillson, G. Clarifying hormone terminology. Can. Fam. Physician 2007, 53, 29–30. [Google Scholar]
- Wei, Q.; Liang, X.; Peng, Y.; Yu, D.; Zhang, R.; Jin, H.; Fan, J.; Cai, W.; Ren, C.; Yu, J. 17β-estradiol ameliorates oxidative stress and blue light-emitting diode-induced retinal degeneration by decreasing apoptosis and enhancing autophagy. Drug Des. Devel Ther. 2018, 12, 2715–2730. [Google Scholar] [CrossRef]
- Xiong, Y.C.; Chen, T.; Yang, X.B.; Deng, C.L.; Ning, Q.L.; Quan, R.; Yu, X.R. 17β-Oestradiol attenuates the photoreceptor apoptosis in mce with retinitis pigmentosa by regulating N-myc downstream regulated gene 2 expression. Neuroscience 2021, 452, 280–294. [Google Scholar] [CrossRef]
- Marquioni-Ramella, M.D.; Cubilla, M.A.; Bermúdez, V.; Tate, P.S.; Marazita, M.C.; Suburo, A.M. Glucocorticoid and progesterone mechanisms in photoreceptor survival. Exp. Eye Res. 2020, 190, 107854. [Google Scholar] [CrossRef]
- Yu-Wai-Man, P.; Griffiths, P.G.; Chinnery, P.F. Mitochondrial optic neuropathies—Disease mechanisms and therapeutic strategies. Prog. Retin. Eye Res. 2011, 30, 81–114. [Google Scholar] [CrossRef]
- Harding, A.E.; Sweeney, M.G.; Govan, G.G.; Riordan-Eva, P. Pedigree analysis in Leber hereditary optic neuropathy families with a pathogenic mtDNA mutation. Am. J. Hum. Genet. 1995, 57, 77–86. [Google Scholar]
- Jankauskaitė, E.; Ambroziak, A.M.; Hajieva, P.; Ołdak, M.; Tońska, K.; Korwin, M.; Bartnik, E.; Kodroń, A. Testosterone increases apoptotic cell death and decreases mitophagy in Leber’s hereditary optic neuropathy cells. J. Appl. Genet. 2020, 61, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Nudleman, E.; Witmer, M.T.; Kiss, S.; Williams, G.A.; Wolfe, J.D. Central serous chorioretinopathy in patients receiving exogenous testosterone therapy. Retina 2014, 34, 2128–2132. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.; Wurm, A.; Linnertz, R.; Pannicke, T.; Iandiev, I.; Wiedemann, P.; Reichenbach, A.; Bringmann, A. Sex steroids inhibit osmotic swelling of retinal glial cells. Neurochem. Res. 2010, 35, 522–530. [Google Scholar] [CrossRef]

| Studies That Suggest a Higher Prevalence of AMD in Women | Studies That Suggest No Differences in the Risk of AMD in Premenopausal and Menopausal Women | Studies That Suggest That Estrogen Exposure Decrease Risk of AMD |
|---|---|---|
| Beaver Dam Eye Study: women more likely to develop AMD compared with men [42] | Incidence of AMD not different between menopausal and premenopausal groups [45] | Endogenous estrogen exposure reduces risk of AMD [48,49,50] |
| Eye Diseases Prevalence Research Group: higher prevalence among women [43] | No relationship between late AMD and natural exposure to estrogens and progesterone [46] | Estrogens alone or in combination with progestin lower the risk of neovascular AMD [51] |
| No relationship between AMD and hormone therapy [47] | hormone replacement therapy lower risk of advanced AMD [52] |
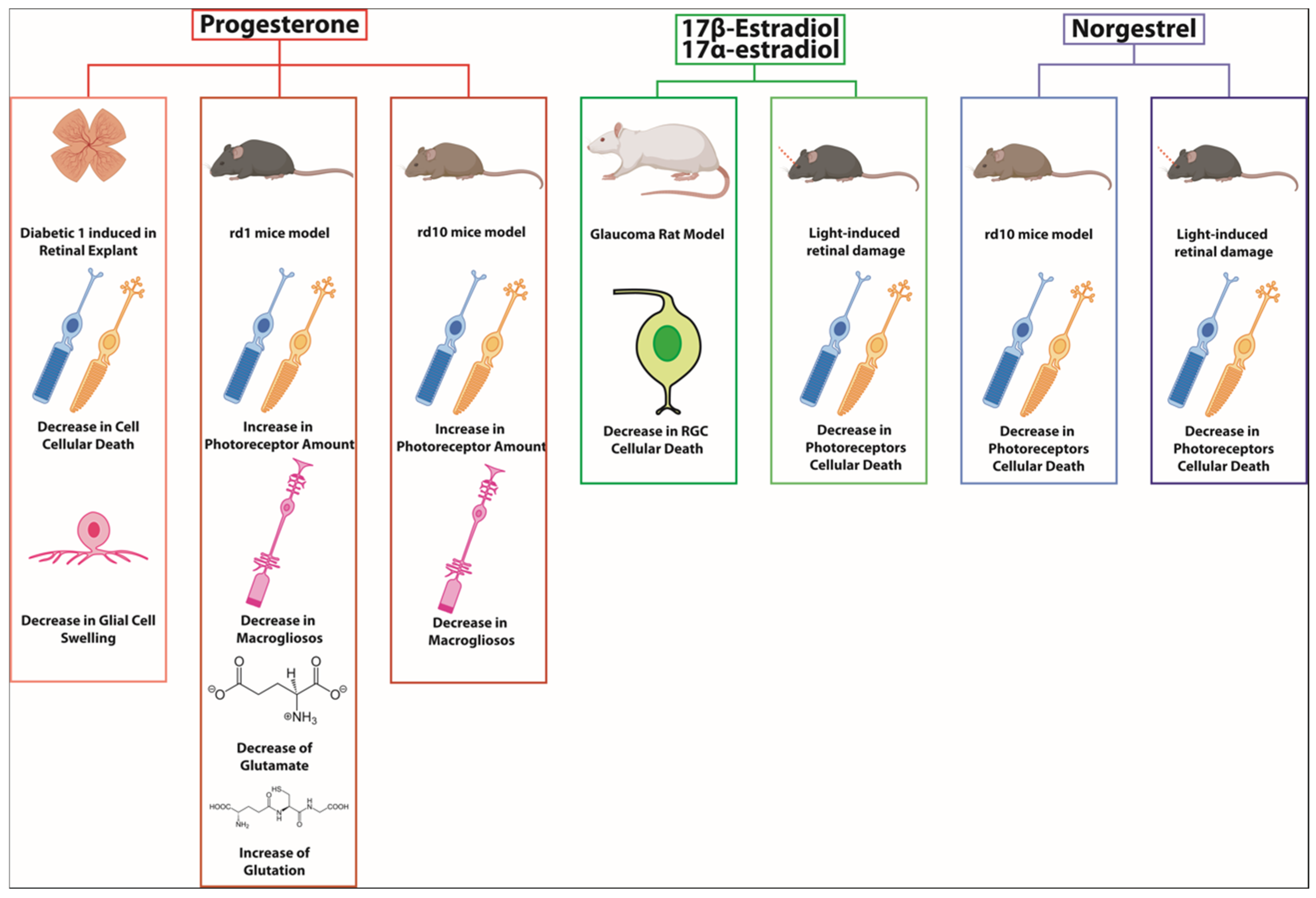
| Hormone | Retinal Disease and Disease Model | Observed Effect |
|---|---|---|
| Estradiol | Rat model of glaucoma | Prevented RGCs death |
| Progesterone | Retinal explants from induced type 1 diabetic rats | Reduced osmotic swelling of retinal glial cells which promote cell survival |
| Progesterone | Retinitis pigmentosa: rd1 mice | Preserves the number of photoreceptors; reduces gliosis; decreases glutamate, and increases GSH, |
| Progesterone | Retinitis pigmentosa: rd10 mice | Increases photoreceptor survival, decreases macrogliosis |
| Norgestrel | Light-induced degeneration and rd10 mice | Decreases in photoreceptor apoptosis and improves the electroretinogram |
| Estradiol | Light-induced retinal degeneration mice model | Reduces photoreceptors death |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valero-Ochando, J.; Cantó, A.; López-Pedrajas, R.; Almansa, I.; Miranda, M. Role of Gonadal Steroid Hormones in the Eye: Therapeutic Implications. Biomolecules 2024, 14, 1262. https://doi.org/10.3390/biom14101262
Valero-Ochando J, Cantó A, López-Pedrajas R, Almansa I, Miranda M. Role of Gonadal Steroid Hormones in the Eye: Therapeutic Implications. Biomolecules. 2024; 14(10):1262. https://doi.org/10.3390/biom14101262
Chicago/Turabian StyleValero-Ochando, Javier, Antolin Cantó, Rosa López-Pedrajas, Inmaculada Almansa, and María Miranda. 2024. "Role of Gonadal Steroid Hormones in the Eye: Therapeutic Implications" Biomolecules 14, no. 10: 1262. https://doi.org/10.3390/biom14101262
APA StyleValero-Ochando, J., Cantó, A., López-Pedrajas, R., Almansa, I., & Miranda, M. (2024). Role of Gonadal Steroid Hormones in the Eye: Therapeutic Implications. Biomolecules, 14(10), 1262. https://doi.org/10.3390/biom14101262







