Antioxidant Therapies in the Treatment of Multiple Sclerosis
Abstract
:1. Introduction
2. Antioxidant Therapies Tested in Experimental Models of or in Patients Diagnosed with Multiple Sclerosis
2.1. Alpha-Lipoic (Thioctic) Acid
2.1.1. Studies in Experimental Animal Models
2.1.2. Studies in Human Cell Cultures
2.1.3. Studies in Patients with Multiple Sclerosis
2.2. Melatonin
2.2.1. Studies in Experimental Animal Models
- Decreasing peripheral and central T helper1/T helper 17 lymphocytes (Th1/Th17) responses and increasing the T regulatory (Treg) frequency and the synthesis of IL-10 in the Central Nervous System (CNS), therefore reducing the pro-inflammatory response [38];
- Decreasing the levels of oxidative stress markers (decreased thiobarbituric acid reactive substances (TBARS) and ROS concentrations and increased the level of SOD and CAT in the brain) by activation of the transcription factor NF-E2 related factor (Nrf2) and antioxidant response elements (ARE) pathway, increasing the expression of the enzymes heme oxygenase-1 (HO-1) and nicotine adenine dinucleotide(phosphate) (NAD(P)H dehydrogenase [quinone] 1 (NQO1)) [39];
- Reversing the decrease in glutathione (GSH) partially, the increase in oxidized glutathione (GSSG), the decrease in GSH/GSSG ratio, the decrease in GPx, and the increase in lipoperoxides, nitric oxide (NO) metabolites, carbonylated proteins, and TNF-α, caused by the induction of EAE [40];
- Reducing the mRNA expression of several kynurenin regulatory enzymes (mainly indoleamine 2,3-dioxygenase 1 or IDO-1) and aryl hydrocarbon receptor (AhR) and inhibiting the enzyme Nicotinamide N-Methyltransferase (Nnmt) overexpression (which leads to an increase in NAD+ levels) [41].
2.2.2. Studies in Human Cell Cultures
2.2.3. Studies in Patients with Multiple Sclerosis
2.3. Epigallocatechin-3-Gallate (EGCG, Green Tea)
2.3.1. Studies in Experimental Animal Models
2.3.2. Studies in Patients with MS
2.4. Curcumin
2.4.1. Studies in Experimental Animal Models
2.4.2. Studies in Patients with MS
2.5. Resveratrol
2.6. Pentoxifylline
2.6.1. Studies in Experimental Animal Models
2.6.2. Studies in Patients with Multiple Sclerosis
2.7. Vegetable and Animal Oils
2.7.1. Studies in Experimental Animal Models
2.7.2. Studies in Patients with MS
2.8. Coenzyme Q10
2.8.1. Studies in Experimental Animal Models
2.8.2. Studies in Patients with MS
2.9. Antioxidant Vitamins
2.9.1. Studies in Experimental Animal Models
2.9.2. Studies in Patients with MS
2.10. Uric Acid and Bilirubin
2.10.1. Studies in Experimental Animal Models
2.10.2. Studies in Patients with MS
2.11. Nitric Oxide Synthase (NOS) Inhibitors and NO Scavengers and Precursors
2.12. N-Acetyl-Cysteine
2.12.1. Studies in Experimental Animal Models
2.12.2. Studies in Patients with MS
2.13. Flavonoids
2.14. Peroxisome Proliferation Activator Receptor (PPAR)-Gamma Agonists
2.15. Carnitine and Carnosine
2.15.1. Studies in Experimental Animal Models
2.15.2. Studies in Patients with Multiple Sclerosis
2.16. Edaravone
2.17. Phycocyanine/Phycocyanobiline
2.18. Antidiabetic Drugs
2.19. Methallothioneine
2.20. Caffeic Acid
2.21. Histone Deacetylase (HDAC) Inhibitors
2.22. Other Antioxidants
3. Discussion, Conclusions, and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Patsopoulos, N.A.; De Jager, P.-L. Genetic and gene expression signatures in multiple sclerosis. Mult. Scler. J. 2020, 26, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Patsopoulos, N.A. Genetics and functional genomics of multiple sclerosis. Semin. Immunopathol. 2022, 44, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Mechelli, R.; Umeton, R.; Manfrè, G.; Romano, S.; Buscarinu, M.C.; Rinaldi, V.; Bellucci, G.; Bigi, R.; Ferraldeschi, M.; Salvetti, M.; et al. Reworking GWAS Data to Understand the Role of Nongenetic Factors in MS Etiopathogenesis. Genes 2020, 11, 97. [Google Scholar] [CrossRef]
- Zarghami, A.; Li, Y.; Claflin, S.B.; van der Mei, I.; Taylor, B.V. Role of environmental factors in multiple sclerosis. Expert Rev. Neurother. 2021, 21, 1389–1408. [Google Scholar] [CrossRef]
- Waubant, E.; Lucas, R.; Mowry, E.; Graves, J.; Olsson, T.; Alfredsson, L.; Langer-Gould, A. Environmental and genetic risk factors for MS: An integrated review. Ann. Clin. Transl. Neurol. 2019, 6, 1905–1922. [Google Scholar] [CrossRef]
- McGarry, T.; Biniecka, M.; Veale, D.-J.; Fearon, U. Hypoxia, oxidative stress and inflammation. Free Radic. Biol. Med. 2018, 125, 15–24. [Google Scholar] [CrossRef]
- Gambini, J.; Stromsnes, K. Oxidative Stress and Inflammation, From Mechanisms to Therapeutic Approaches. Biomedicines. 2022, 10, 753. [Google Scholar] [CrossRef]
- Ramos-González, E.J.; Bitzer-Quintero, O.K.; Ortiz, G.; Hernández-Cruz, J.J.; Ramírez-Jirano, L.J. Relationship between inflammation and oxidative stress and its effect on multiple sclerosis. Neurologia (Engl. Ed.) 2024, 39, 292–301. [Google Scholar] [PubMed]
- Ibitoye, R.; Kemp, K.; Rice, C.; Hares, K.; Scolding, N.; Wilkins, A. Oxidative stress-related biomarkers in multiple sclerosis, a review. Biomark. Med. 2016, 10, 889–902. [Google Scholar] [CrossRef]
- Hollen, C.; Neilson, L.E.; Barajas, R.F., Jr.; Greenhouse, I.; Spain, R.I. Oxidative stress in multiple sclerosis-Emerging imaging techniques. Front. Neurol. 2023, 13, 1025659. [Google Scholar] [CrossRef]
- Sanabria-Castro, A.; Alape-Girón, A.; Flores-Díaz, M.; Echeverri-McCandless, A.; Parajeles-Vindas, A. Oxidative stress involvement in the molecular pathogenesis and progression of multiple sclerosis, a literature review. Rev. Neurosci. 2024, 35, 355–371. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, F.J.; Alonso-Navarro, H.; Salgado-Cámara, P.; García-Martín, E.; Agúndez, J.A.G. Oxidative Stress Markers in Multiple Sclerosis. Int. J. Mol. Sci. 2024, 25, 6289. [Google Scholar] [CrossRef]
- Nguyen, H.; Pellegrini, M.V.; Gupta, V. Alpha-Lipoic Acid. 2024 Jan 26. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Yadav, V.; Marracci, G.H.; Munar, M.Y.; Cherala, G.; Stuber, L.E.; Alvarez, L.; Shinto, L.; Koop, D.R.; Bourdette, D.N. Pharmacokinetic study of lipoic acid in multiple sclerosis, comparing mice and human pharmacokinetic parameters. Mult. Scler. J. 2010, 16, 387–397. [Google Scholar] [CrossRef]
- Morini, M.; Roccatagliata, L.; Dell’Eva, R.; Pedemonte, E.; Furlan, R.; Minghelli, S.; Giunti, D.; Pfeffer, U.; Marchese, M.; Noonan, D.; et al. Alpha-lipoic acid is effective in prevention and treatment of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2004, 148, 146–153. [Google Scholar] [CrossRef]
- Marracci, G.H.; Jones, R.E.; McKeon, G.P.; Bourdette, D.N. Alpha lipoic acid inhibits T cell migration into the spinal cord and suppresses and treats experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2002, 131, 104–114. [Google Scholar] [CrossRef]
- Chaudhary, P.; Marracci, G.H.; Bourdette, D.N. Lipoic acid inhibits expression of ICAM-1 and VCAM-1 by CNS endothelial cells and T cell migration into the spinal cord in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2006, 175, 87–96. [Google Scholar] [CrossRef]
- Schreibelt, G.; Musters, R.J.; Reijerkerk, A.; de Groot, L.R.; van der Pol, S.M.; Hendrikx, E.M.; Döpp, E.D.; Dijkstra, C.D.; Drukarch, B.; de Vries, H.E. Lipoic acid affects cellular migration into the central nervous system and stabilizes blood-brain barrier integrity. J. Immunol. 2006, 177, 2630–2637. [Google Scholar] [CrossRef]
- Wang, K.C.; Tsai, C.P.; Lee, C.L.; Chen, S.Y.; Lin, G.J.; Yen, M.H.; Sytwu, H.K.; Chen, S.J. α-Lipoic acid enhances endogenous peroxisome-proliferator-activated receptor-γ to ameliorate experimental autoimmune encephalomyelitis in mice. Clin. Sci. 2013, 125, 329–340. [Google Scholar] [CrossRef]
- Chaudhary, P.; Marracci, G.; Galipeau, D.; Pocius, E.; Morris, B.; Bourdette, D. Lipoic acid reduces inflammation in a mouse focal cortical experimental autoimmune encephalomyelitis model. J. Neuroimmunol. 2015, 289, 68–74. [Google Scholar] [CrossRef]
- Li, B.; Tan, G.J.; Lin, H.Q.; Zhang, J.N.; Guo, L.; Chen, L.P. Neuroprotective effects of α-lipoic acid on long-term experimental autoimmune encephalomyelitis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6517–6528. [Google Scholar]
- Peres, D.S.; Theisen, M.C.; Fialho, M.F.P.; Dalenogare, D.P.; Rodrigues, P.; Kudsi, S.Q.; Bernardes, L.B.; Ruviaro da Silva, N.A.; Lückemeyer, D.D.; Sampaio, T.B.; et al. TRPA1 involvement in depression- and anxiety-like behaviors in a progressive multiple sclerosis model in mice. Brain Res. Bull. 2021, 175, 1–15. [Google Scholar] [CrossRef]
- Kong, D.; Saqer, A.A.; Carpinelli de Jesus, M.; Khan, N.; Jones, A.; Blanchfield, J.T.; Smith, M.T.; Williams, C.M. Design, synthesis and evaluation of alpha lipoic acid derivatives to treat multiple sclerosis-associated central neuropathic pain. Bioorg. Med. Chem. 2022, 69, 116889. [Google Scholar] [CrossRef]
- Dietrich, M.; Helling, N.; Hilla, A.; Heskamp, A.; Issberner, A.; Hildebrandt, T.; Kohne, Z.; Küry, P.; Berndt, C.; Aktas, O.; et al. Early alpha-lipoic acid therapy protects from degeneration of the inner retinal layers and vision loss in an experimental autoimmune encephalomyelitis-optic neuritis model. J. Neuroinflammation. 2018, 15, 71. [Google Scholar] [CrossRef]
- Sanadgol, N.; Golab, F.; Askari, H.; Moradi, F.; Ajdary, M.; Mehdizadeh, M. Alpha-lipoic acid mitigates toxic-induced demyelination in the corpus callosum by lessening of oxidative stress and stimulation of polydendrocytes proliferation. Metab. Brain Dis. 2018, 33, 27–37. [Google Scholar] [CrossRef]
- Ibrahim Fouad, G.; Ahmed, K.A. Remyelinating activities of Carvedilol or alpha lipoic acid in the Cuprizone-Induced rat model of demyelination. Int. Immunopharmacol. 2023, 118, 110125. [Google Scholar] [CrossRef]
- Marracci, G.H.; McKeon, G.P.; Marquardt, W.E.; Winter, R.W.; Riscoe, M.K.; Bourdette, D.N. Alpha lipoic acid inhibits human T-cell migration, implications for multiple sclerosis. J. Neurosci. Res. 2004, 78, 362–370. [Google Scholar] [CrossRef]
- Marracci, G.H.; Marquardt, W.E.; Strehlow, A.; McKeon, G.P.; Gross, J.; Buck, D.C.; Kozell, L.B.; Bourdette, D.N. Lipoic acid downmodulates CD4 from human T lymphocytes by dissociation of p56(Lck). Biochem. Biophys. Res. Commun. 2006, 344, 963–971. [Google Scholar] [CrossRef]
- Salinthone, S.; Schillace, R.V.; Tsang, C.; Regan, J.W.; Bourdette, D.N.; Carr, D.W. Lipoic acid stimulates cAMP production via G protein-coupled receptor-dependent and -independent mechanisms. J. Nutr. Biochem. 2011, 22, 681–690. [Google Scholar] [CrossRef]
- George, J.D.; Kim, E.; Spain, R.; Bourdette, D.; Salinthone, S. Effects of lipoic acid on migration of human B cells and monocyte-enriched peripheral blood mononuclear cells in relapsing remitting multiple sclerosis. J. Neuroimmunol. 2018, 315, 24–27. [Google Scholar] [CrossRef]
- Fiedler, S.E.; Spain, R.I.; Kim, E.; Salinthone, S. Lipoic acid modulates inflammatory responses of monocytes and monocyte-derived macrophages from healthy and relapsing-remitting multiple sclerosis patients. Immunol. Cell Biol. 2021, 99, 107–115. [Google Scholar] [CrossRef]
- Mattmann, E. Die Behandlung Der Multiplen Sklerose Mit Thioctsaeure [Treatment of Multiple Sclerosis with Thioctic Acid]. Schweiz Med. Wochenschr. 1963, 93, 1334–1336. [Google Scholar]
- Yadav, V.; Marracci, G.; Lovera, J.; Woodward, W.; Bogardus, K.; Marquardt, W.; Shinto, L.; Morris, C.; Bourdette, D. Lipoic acid in multiple sclerosis, a pilot study. Mult. Scler. 2005, 11, 159–165. [Google Scholar] [CrossRef]
- Khalili, M.; Eghtesadi, S.; Mirshafiey, A.; Eskandari, G.; Sanoobar, M.; Sahraian, M.A.; Motevalian, A.; Norouzi, A.; Moftakhar, S.; Azimi, A. Effect of lipoic acid consumption on oxidative stress among multiple sclerosis patients: A randomized controlled clinical trial. Nutr. Neurosci. 2014, 17, 16–20. [Google Scholar] [CrossRef]
- Khalili, M.; Azimi, A.; Izadi, V.; Eghtesadi, S.; Mirshafiey, A.; Sahraian, M.A.; Motevalian, A.; Norouzi, A.; Sanoobar, M.; Eskandari, G.; et al. Does lipoic acid consumption affect the cytokine profile in multiple sclerosis patients: A double-blind, placebo-controlled, randomized clinical trial. Neuroimmunomodulation 2014, 21, 291–296. [Google Scholar] [CrossRef]
- Khalili, M.; Soltani, M.; Moghadam, S.A.; Dehghan, P.; Azimi, A.; Abbaszadeh, O. Effect of alpha-lipoic acid on asymmetric dimethylarginine and disability in multiple sclerosis patients, A randomized clinical trial. Electron. Physician 2017, 9, 4899–4905. [Google Scholar] [CrossRef]
- Fiedler, S.E.; Yadav, V.; Kerns, A.R.; Tsang, C.; Markwardt, S.; Kim, E.; Spain, R.; Bourdette, D.; Salinthone, S. Lipoic Acid Stimulates cAMP Production in Healthy Control and Secondary Progressive MS Subjects. Mol. Neurobiol. 2018, 55, 6037–6049. [Google Scholar] [CrossRef]
- Álvarez-Sánchez, N.; Cruz-Chamorro, I.; López-González, A.; Utrilla, J.C.; Fernández-Santos, J.M.; Martínez-López, A.; Lardone, P.J.; Guerrero, J.M.; Carrillo-Vico, A. Melatonin controls experimental autoimmune encephalomyelitis by altering the T effector/regulatory balance. Brain Behav. Immun. 2015, 50, 101–114. [Google Scholar] [CrossRef]
- Long, T.; Yang, Y.; Peng, L.; Li, Z. Neuroprotective Effects of Melatonin on Experimental Allergic Encephalomyelitis Mice Via Anti-Oxidative Stress Activity. J. Mol. Neurosci. 2018, 64, 233–241. [Google Scholar] [CrossRef]
- Escribano, B.M.; Muñoz-Jurado, A.; Caballero-Villarraso, J.; Valdelvira, M.E.; Giraldo, A.I.; Paz-Rojas, E.; Gascón, F.; Santamaría, A.; Agüera, E.; Túnez, I. Protective effects of melatonin on changes occurring in the experimental autoimmune encephalomyelitis model of multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 58, 103520. [Google Scholar] [CrossRef]
- Jand, Y.; Ghahremani, M.H.; Ghanbari, A.; Ejtemaei-Mehr, S.; Guillemin, G.J.; Ghazi-Khansari, M. Melatonin ameliorates disease severity in a mouse model of multiple sclerosis by modulating the kynurenine pathway. Sci. Rep. 2022, 12, 15963. [Google Scholar] [CrossRef]
- Ortiz, G.G.; Briones-Torres, A.L.; Benitez-King, G.; González-Ortíz, L.J.; Palacios-Magaña, C.V.; Pacheco-Moisés, F.P. Beneficial Effect of Melatonin Alone or in Combination with Glatiramer Acetate and Interferon β-1b on Experimental Autoimmune Encephalomyelitis. Molecules 2022, 27, 4217. [Google Scholar] [CrossRef]
- Chen, S.J.; Huang, S.H.; Chen, J.W.; Wang, K.C.; Yang, Y.R.; Liu, P.F.; Lin, G.J.; Sytwu, H.K. Melatonin enhances interleukin-10 expression and suppresses chemotaxis to inhibit inflammation in situ and reduce the severity of experimental autoimmune encephalomyelitis. Int. Immunopharmacol. 2016, 31, 169–177. [Google Scholar] [CrossRef]
- Ghareghani, M.; Dokoohaki, S.; Ghanbari, A.; Farhadi, N.; Zibara, K.; Khodadoust, S.; Parishani, M.; Ghavamizadeh, M.; Sadeghi, H. Melatonin exacerbates acute experimental autoimmune encephalomyelitis by enhancing the serum levels of lactate, A potential biomarker of multiple sclerosis progression. Clin. Exp. Pharmacol. Physiol. 2017, 44, 52–61. [Google Scholar] [CrossRef]
- Ghareghani, M.; Farhadi, Z.; Rivest, S.; Zibara, K. PDK4 Inhibition Ameliorates Melatonin Therapy by Modulating Cerebral Metabolism and Remyelination in an EAE Demyelinating Mouse Model of Multiple Sclerosis. Front. Immunol. 2022, 13, 862316. [Google Scholar] [CrossRef]
- Abo Taleb, H.A.; Alghamdi, B.S. Neuroprotective Effects of Melatonin during Demyelination and Remyelination Stages in a Mouse Model of Multiple Sclerosis. J. Mol. Neurosci. 2020, 70, 386–402. [Google Scholar] [CrossRef]
- Emamgholipour, S.; Hossein-Nezhad, A.; Sahraian, M.A.; Askarisadr, F.; Ansari, M. Evidence for possible role of melatonin in reducing oxidative stress in multiple sclerosis through its effect on SIRT1 and antioxidant enzymes. Life Sci. 2016, 145, 34–41. [Google Scholar] [CrossRef]
- Álvarez-Sánchez, N.; Cruz-Chamorro, I.; Díaz-Sánchez, M.; Sarmiento-Soto, H.; Medrano-Campillo, P.; Martínez-López, A.; Lardone, P.J.; Guerrero, J.M.; Carrillo-Vico, A. Melatonin reduces inflammatory response in peripheral T helper lymphocytes from relapsing-remitting multiple sclerosis patients. J. Pineal Res. 2017, 63, e12442. [Google Scholar] [CrossRef]
- Miller, E.; Walczak, A.; Majsterek, I.; Kędziora, J. Melatonin reduces oxidative stress in the erythrocytes of multiple sclerosis patients with secondary progressive clinical course. J. Neuroimmunol. 2013, 257, 97–101. [Google Scholar] [CrossRef]
- Adamczyk-Sowa, M.; Pierzchala, K.; Sowa, P.; Mucha, S.; Sadowska-Bartosz, I.; Adamczyk, J.; Hartel, M. Melatonin acts as antioxidant and improves sleep in MS patients. Neurochem. Res. 2014, 39, 1585–1593. [Google Scholar] [CrossRef]
- Adamczyk-Sowa, M.; Sowa, P.; Mucha, S.; Zostawa, J.; Mazur, B.; Owczarek, M.; Pierzchała, K. Changes in Serum Ceruloplasmin Levels Based on Immunomodulatory Treatments and Melatonin Supplementation in Multiple Sclerosis Patients. Med. Sci. Monit. 2016, 22, 2484–2491. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk-Sowa, M.; Sowa, P.; Adamczyk, J.; Niedziela, N.; Misiolek, H.; Owczarek, M.; Zwirska-Korczala, K. Effect of melatonin supplementation on plasma lipid hydroperoxides.; homocysteine concentration and chronic fatigue syndrome in multiple sclerosis patients treated with interferons-beta and mitoxantrone. J. Physiol. Pharmacol. 2016, 67, 235–242. [Google Scholar]
- Adamczyk-Sowa, M.; Pierzchala, K.; Sowa, P.; Polaniak, R.; Kukla, M.; Hartel, M. Influence of melatonin supplementation on serum antioxidative properties and impact of the quality of life in multiple sclerosis patients. J. Physiol. Pharmacol. 2014, 65, 543–550. [Google Scholar]
- Roostaei, T.; Sahraian, M.A.; Hajeaghaee, S.; Gholipour, T.; Togha, M.; Siroos, B.; Mansouri, S.; Mohammadshirazi, Z.; Aghazadeh Alasti, M.; Harirchian, M.H. Impact of Melatonin on Motor.; Cognitive and Neuroimaging Indices in Patients with Multiple Sclerosis. Iran. J. Allergy Asthma Immunol. 2015, 14, 589–595. [Google Scholar]
- Sánchez-López, A.L.; Ortiz, G.G.; Pacheco-Moises, F.P.; Mireles-Ramírez, M.A.; Bitzer-Quintero, O.K.; Delgado-Lara, D.L.C.; Ramírez-Jirano, L.J.; Velázquez-Brizuela, I.E. Efficacy of Melatonin on Serum Pro-inflammatory Cytokines and Oxidative Stress Markers in Relapsing Remitting Multiple Sclerosis. Arch. Med. Res. 2018, 49, 391–398. [Google Scholar] [CrossRef]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef]
- Wang, J.; Ren, Z.; Xu, Y.; Xiao, S.; Meydani, S.N.; Wu, D. Epigallocatechin-3-gallate ameliorates experimental autoimmune encephalomyelitis by altering balance among CD4+ T-cell subsets. Am. J. Pathol. 2012, 180, 221–234. [Google Scholar] [CrossRef]
- Cai, F.; Liu, S.; Lei, Y.; Jin, S.; Guo, Z.; Zhu, D.; Guo, X.; Zhao, H.; Niu, X.; Xi, Y.; et al. Epigallocatechin-3 gallate regulates macrophage subtypes and immunometabolism to ameliorate experimental autoimmune encephalomyelitis. Cell. Immunol. 2021, 368, 104421. [Google Scholar] [CrossRef]
- Aktas, O.; Prozorovski, T.; Smorodchenko, A.; Savaskan, N.E.; Lauster, R.; Kloetzel, P.M.; Infante-Duarte, C.; Brocke, S.; Zipp, F. Green tea epigallocatechin-3-gallate mediates T cellular NF-kappa B inhibition and exerts neuroprotection in autoimmune encephalomyelitis. J. Immunol. 2004, 173, 5794–5800. [Google Scholar] [CrossRef]
- Semnani, M.; Mashayekhi, F.; Azarnia, M.; Salehi, Z. Effects of green tea epigallocatechin-3-gallate on the proteolipid protein and oligodendrocyte transcription factor 1 messenger RNA gene expression in a mouse model of multiple sclerosis. Folia Neuropathol. 2017, 55, 199–205. [Google Scholar] [CrossRef]
- Lovera, J.; Ramos, A.; Devier, D.; Garrison, V.; Kovner, B.; Reza, T.; Koop, D.; Rooney, W.; Foundas, A.; Bourdette, D. Polyphenon E, non-futile at neuroprotection in multiple sclerosis but unpredictably hepatotoxic, Phase I single group and phase II randomized placebo-controlled studies. J. Neurol. Sci. 2015, 358, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Rust, R.; Chien, C.; Scheel, M.; Brandt, A.U.; Dörr, J.; Wuerfel, J.; Klumbies, K.; Zimmermann, H.; Lorenz, M.; Wernecke, K.D.; et al. Epigallocatechin Gallate in Progressive MS: A Randomized, Placebo-Controlled Trial. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e964. [Google Scholar] [CrossRef]
- Bellmann-Strobl, J.; Paul, F.; Wuerfel, J.; Dörr, J.; Infante-Duarte, C.; Heidrich, E.; Körtgen, B.; Brandt, A.; Pfüller, C.; Radbruch, H.; et al. Epigallocatechin Gallate in Relapsing-Remitting Multiple Sclerosis; A Randomized, Placebo-Controlled Trial. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e981. [Google Scholar] [CrossRef] [PubMed]
- Cuerda-Ballester, M.; Proaño, B.; Alarcón-Jimenez, J.; de Bernardo, N.; Villaron-Casales, C.; Lajara Romance, J.M.; de la Rubia Ortí, J.E. Improvements in gait and balance in patients with multiple sclerosis after treatment with coconut oil and epigallocatechin gallate. A pilot study. Food Funct. 2023, 14, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Mähler, A.; Steiniger, J.; Bock, M.; Klug, L.; Parreidt, N.; Lorenz, M.; Zimmermann, B.F.; Krannich, A.; Paul, F.; Boschmann, M. Metabolic response to epigallocatechin-3-gallate in relapsing-remitting multiple sclerosis, a randomized clinical trial. Am. J. Clin. Nutr. 2015, 101, 487–495. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Drużga, A.; Katarzyna, J.; Skonieczna-Żydecka, K. Antioxidant Potential of Curcumin-A Meta-Analysis of Randomized Clinical Trials. Antioxidants 2020, 9, 1092. [Google Scholar] [CrossRef]
- Esmaeilzadeh, E.; Soleimani, M.; Zare-Abdollahi, D.; Jameie, B.; Khorram Khorshid, H.R. Curcumin ameliorates experimental autoimmune encephalomyelitis in a C57BL/6 mouse model. Drug Dev. Res. 2019, 80, 629–636. [Google Scholar] [CrossRef]
- Kanakasabai, S.; Casalini, E.; Walline, C.C.; Mo, C.; Chearwae, W.; Bright, J.J. Differential regulation of CD4(+) T helper cell responses by curcumin in experimental autoimmune encephalomyelitis. J. Nutr. Biochem. 2012, 23, 1498–1507. [Google Scholar] [CrossRef]
- Mohajeri, M.; Sadeghizadeh, M.; Najafi, F.; Javan, M. Polymerized nano-curcumin attenuates neurological symptoms in EAE model of multiple sclerosis through down regulation of inflammatory and oxidative processes and enhancing neuroprotection and myelin repair. Neuropharmacology 2015, 99, 156–167. [Google Scholar] [CrossRef]
- Motavaf, M.; Sadeghizadeh, M.; Babashah, S.; Zare, L.; Javan, M. Protective Effects of a Nano-Formulation of Curcumin against Cuprizone-Induced Demyelination in the Mouse Corpus Callosum. Iran. J. Pharm. Res. 2020, 19, 310–320. [Google Scholar]
- Alam, M.Z.; Bagabir, H.A.; Zaher, M.A.F.; Alqurashi, T.M.A.; Alghamdi, B.S.; Kazi, M.; Ashraf, G.M.; Alshahrany, G.A.; Alzahrani, N.A.; Bakhalgi, R.M.; et al. Black Seed Oil-Based Curcumin Nanoformulations Ameliorated Cuprizone-Induced Demyelination in the Mouse Hippocampus. Mol. Neurobiol. 2024, 1–22. [Google Scholar] [CrossRef]
- Barzegarzadeh, B.; Hatami, H.; Dehghan, G.; Khajehnasiri, N.; Khoobi, M.; Sadeghian, R. Conjugated Linoleic Acid-Curcumin Attenuates Cognitive Deficits and Oxidative Stress Parameters in the Ethidium Bromide-Induced Model of Demyelination. Neurotox. Res. 2021, 39, 815–825. [Google Scholar] [CrossRef]
- Petracca, M.; Quarantelli, M.; Moccia, M.; Vacca, G.; Satelliti, B.; D’Ambrosio, G.; Carotenuto, A.; Ragucci, M.; Assogna, F.; Capacchione, A.; et al. ProspeCtive study to evaluate efficacy.; safety and tOlerability of dietary supplemeNT of Curcumin (BCM95) in subjects with Active relapsing MultIple Sclerosis treated with subcutaNeous Interferon beta 1a 44 mcg TIW (CONTAIN): A randomized, controlled trial. Mult. Scler. Relat. Disord. 2021, 56, 103274. [Google Scholar]
- Meng, X.; Zhou, J.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health Benefits and Molecular Mechanisms of Resveratrol, A Narrative Review. Foods 2020, 9, 340. [Google Scholar] [CrossRef]
- Singh, N.P.; Hegde, V.L.; Hofseth, L.J.; Nagarkatti, M.; Nagarkatti, P. Resveratrol (trans-3.;5.;4′-trihydroxystilbene) ameliorates experimental allergic encephalomyelitis, primarily via induction of apoptosis in T cells involving activation of aryl hydrocarbon receptor and estrogen receptor. Mol. Pharmacol. 2007, 72, 1508–1521. [Google Scholar] [CrossRef]
- Imler, T.J., Jr.; Petro, T.M. Decreased severity of experimental autoimmune encephalomyelitis during resveratrol administration is associated with increased IL-17+IL-10+ T cells, CD4(-) IFN-gamma+ cells, and decreased macrophage IL-6 expression. Int. Immunopharmacol. 2009, 9, 134–143. [Google Scholar] [CrossRef]
- Wang, D.; Li, S.P.; Fu, J.S.; Bai, L.; Guo, L. Resveratrol augments therapeutic efficiency of mouse bone marrow mesenchymal stem cell-based therapy in experimental autoimmune encephalomyelitis. Int. J. Dev. Neurosci. 2016, 49, 60–66. [Google Scholar] [CrossRef]
- Wang, D.; Li, S.P.; Fu, J.S.; Zhang, S.; Bai, L.; Guo, L. Resveratrol defends blood-brain barrier integrity in experimental autoimmune encephalomyelitis mice. J. Neurophysiol. 2016, 116, 2173–2179. [Google Scholar] [CrossRef]
- Gandy, K.A.O.; Zhang, J.; Nagarkatti, P.; Nagarkatti, M. Resveratrol (3, 5, 4′-Trihydroxy-trans-Stilbene) Attenuates a Mouse Model of Multiple Sclerosis by Altering the miR-124/Sphingosine Kinase 1 Axis in Encephalitogenic T Cells in the Brain. J. Neuroimmune Pharmacol. 2019, 14, 462–477. [Google Scholar] [CrossRef]
- Shindler, K.S.; Ventura, E.; Dutt, M.; Elliott, P.; Fitzgerald, D.C.; Rostami, A. Oral resveratrol reduces neuronal damage in a model of multiple sclerosis. J. Neuroophthalmol. 2010, 30, 328–339. [Google Scholar] [CrossRef]
- Zheng, X.; Sun, K.; Liu, Y.; Yin, X.; Zhu, H.; Yu, F.; Zhao, W. Resveratrol-loaded macrophage exosomes alleviate multiple sclerosis through targeting microglia. J. Control. Release 2023, 353, 675–684. [Google Scholar] [CrossRef]
- Shamsher, E.; Khan, R.S.; Davis, B.M.; Dine, K.; Luong, V.; Cordeiro, M.F.; Shindler, K.S. Intranasal Resveratrol Nanoparticles Enhance Neuroprotection in a Model of Multiple Sclerosis. Int. J. Mol. Sci. 2024, 25, 4047. [Google Scholar] [CrossRef]
- Ghaiad, H.R.; Nooh, M.M.; El-Sawalhi, M.M.; Shaheen, A.A. Resveratrol Promotes Remyelination in Cuprizone Model of Multiple Sclerosis, Biochemical and Histological Study. Mol. Neurobiol. 2017, 54, 3219–3229. [Google Scholar] [CrossRef]
- Sato, F.; Martinez, N.E.; Shahid, M.; Rose, J.W.; Carlson, N.G.; Tsunoda, I. Resveratrol exacerbates both autoimmune and viral models of multiple sclerosis. Am. J. Pathol. 2013, 183, 1390–1396. [Google Scholar] [CrossRef]
- Wangm, Y.; Kang, Y.; Qi, C.; Zhang, T.; Zhao, H.; Ji, X.; Yan, W.; Huang, Y.; Cui, R.; Zhang, G.; et al. Pentoxifylline enhances antioxidative capability and promotes mitochondrial biogenesis for improving age-related behavioral deficits. Aging 2020, 12, 25487–25504. [Google Scholar] [CrossRef]
- Okuda, Y.; Sakoda, S.; Fujimura, H.; Yanagihara, T. Pentoxifylline delays the onset of experimental allergic encephalomyelitis in mice by modulating cytokine production in peripheral blood mononuclear cells. Immunopharmacology 1996, 35, 141–148. [Google Scholar] [CrossRef]
- Grassin, M.; Brochet, B.; Coussemacq, M.; Brochet, H. Controlled therapeutic trials of pentoxifylline in relapsing-experimental auto-immune encephalomyelitis. Acta Neurol. Scand. 1998, 97, 404–408. [Google Scholar] [CrossRef]
- Corrêa, J.O.; Aarestrup, B.J.; Aarestrup, F.M. Effect of thalidomide and pentoxifylline on experimental autoimmune encephalomyelitis (EAE). Exp. Neurol. 2010, 226, 15–23. [Google Scholar] [CrossRef]
- Du, C.; Cooper, J.C.; Klaus, S.J.; Sriram, S. Amelioration of CR-EAE with lisofylline, effects on mRNA levels of IL-12 and IFN-gamma in the CNS. J. Neuroimmunol. 2000, 110, 13–19. [Google Scholar] [CrossRef]
- van Oosten, B.W.; Rep, M.H.; van Lier, R.A.; Scholten, P.E.; von Blomberg, B.M.; Pflughaupt, K.W.; Hartung, H.P.; Adèr, H.J.; Polman, C.H. A pilot study investigating the effects of orally administered pentoxifylline on selected immune variables in patients with multiple sclerosis. J. Neuroimmunol. 1996, 66, 49–55. [Google Scholar] [CrossRef]
- Friedman, J.E.; Zabriskie, J.; Bourganskaia, E. A pilot study of pentoxifylline in multiple sclerosis. Arch. Neurol. 1996, 53, 956–957. [Google Scholar] [CrossRef]
- Prieto, J.M.; Dapena, D.; Lema, M.; Ares, B.; Cacabelos, P.; Noya, M. Pentoxifilina, es útil en la esclerosis múltiple ? [Pentoxifylline, is it useful in multiple sclerosis?]. Rev. Neurol. 2001, 32, 529–531. [Google Scholar] [PubMed]
- Myers, L.W.; Ellison, G.W.; Merrill, J.E.; El Hajjar, A.; St Pierre, B.; Hijazin, M.; Leake, B.D.; Bentson, J.R.; Nuwer, M.R.; Tourtellotte, W.W.; et al. Pentoxifylline is not a promising treatment for multiple sclerosis in progression phase. Neurology 1998, 51, 1483–1486. [Google Scholar] [CrossRef]
- Rieckmann, P.; Weber, F.; Günther, A.; Poser, S. The phosphodiesterase inhibitor pentoxifylline reduces early side effects of interferon-beta 1b treatment in patients with multiple sclerosis. Neurology 1996, 47, 604. [Google Scholar] [CrossRef]
- Weber, F.; Polak, T.; Günther, A.; Kubuschok, B.; Janovskaja, J.; Bitsch, A.; Poser, S.; Rieckmann, P. Synergistic immunomodulatory effects of interferon-beta1b and the phosphodiesterase inhibitor pentoxifylline in patients with relapsing-remitting multiple sclerosis. Ann. Neurol. 1998, 44, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ozugurlu, F.; Sahin, S.; Idiz, N.; Akyol, O.; Ilhan, A.; Yigitoglu, R.; Isik, B. The effect of Nigella sativa oil against experimental allergic encephalomyelitis via nitric oxide and other oxidative stress parameters. Cell. Mol. Biol. 2005, 51, 337–342. [Google Scholar] [PubMed]
- Binyamin, O.; Larush, L.; Frid, K.; Keller, G.; Friedman-Levi, Y.; Ovadia, H.; Abramsky, O.; Magdassi, S.; Gabizon, R. Treatment of a multiple sclerosis animal model by a novel nanodrop formulation of a natural antioxidant. Int. J. Nanomedicine. 2015, 10, 7165–7174. [Google Scholar] [CrossRef]
- Ganji, A.; Farahani, I.; Palizvan, M.R.; Ghazavi, A.; Ejtehadifar, M.; Ebrahimimonfared, M.; Shojapour, M.; Mosayebi, G. Therapeutic effects of walnut oil on the animal model of multiple sclerosis. Nutr. Neurosci. 2019, 22, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.S.; Fontes, L.B.; Crotti, A.E.; Aarestrup, B.J.; Aarestrup, F.M.; da Silva Filho, A.A.; Corrêa, J.O. Copaiba oil suppresses inflammatory cytokines in splenocytes of C57Bl/6 mice induced with experimental autoimmune encephalomyelitis (EAE). Molecules 2014, 19, 12814–12826. [Google Scholar] [CrossRef]
- Conde, C.; Escribano, B.M.; Luque, E.; Aguilar-Luque, M.; Feijóo, M.; Ochoa, J.J.; LaTorre, M.; Giraldo, A.I.; Lillo, R.; Agüera, E.; et al. The protective effect of extra-virgin olive oil in the experimental model of multiple sclerosis in the rat. Nutr. Neurosci. 2020, 23, 37–48. [Google Scholar] [CrossRef]
- Giacometti, J.; Grubić-Kezele, T. Olive Leaf Polyphenols Attenuate the Clinical Course of Experimental Autoimmune Encephalomyelitis and Provide Neuroprotection by Reducing Oxidative Stress, Regulating Microglia and SIRT1, and Preserving Myelin Integrity. Oxid. Med. Cell. Longev. 2020, 2020, 6125638. [Google Scholar] [CrossRef]
- Gutiérrez-Miranda, B.; Gallardo, I.; Melliou, E.; Cabero, I.; Álvarez, Y.; Hernández, M.; Magiatis, P.; Hernández, M.; Nieto, M.L. Treatment with the Olive Secoiridoid Oleacein Protects against the Intestinal Alterations Associated with EAE. Int. J. Mol. Sci. 2023, 24, 4977. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Miranda, B.; Gallardo, I.; Melliou, E.; Cabero, I.; Álvarez, Y.; Magiatis, P.; Hernández, M.; Nieto, M.L. Oleacein Attenuates the Pathogenesis of Experimental Autoimmune Encephalomyelitis through Both Antioxidant and Anti-Inflammatory Effects. Antioxidants 2020, 21, 1161. [Google Scholar] [CrossRef] [PubMed]
- Rezapour-Firouzi, S.; Mohammadian, M.; Sadeghzadeh, M.; Mazloomi, E. Effects of co-administration of rapamycin and evening primrose/hemp seed oil supplement on immunologic factors and cell membrane fatty acids in experimental autoimmune encephalomyelitis. Gene 2020, 759, 144987. [Google Scholar] [CrossRef]
- Pan, R.Y.; Kong, X.X.; Cheng, Y.; Du, L.; Wang, Z.C.; Yuan, C.; Cheng, J.B.; Yuan, Z.Q.; Zhang, H.Y.; Liao, Y.J. 1, 2, 4-Trimethoxybenzene selectively inhibits NLRP3 inflammasome activation and attenuates experimental autoimmune encephalomyelitis. Acta Pharmacol. Sin. 2021, 42, 1769–1779. [Google Scholar] [CrossRef]
- Sell, L.B.; Ramelow, C.C.; Kohl, H.M.; Hoffman, K.; Bains, J.K.; Doyle, W.J.; Strawn, K.D.; Hevrin, T.; Kirby, T.O.; Gibson, K.M.; et al. Farnesol induces protection against murine CNS inflammatory demyelination and modifies gut microbiome. Clin. Immunol. 2022, 235, 108766. [Google Scholar] [CrossRef] [PubMed]
- Moradi, V.; Ghanadian, S.M.; Rashidi, B.; Ghasemi, N.; Dashti, G.; Esfandiari, E. The preventive effect of Zingiber officinale essential oil on demyelination of corpus callosum in a cuprizone rat model of multiple sclerosis. Avicenna J. Phytomed. 2023, 13, 675–687. [Google Scholar]
- Lee, J.; Hong, S.; Ahn, M.; Kim, J.; Moon, C.; Matsuda, H.; Tanaka, A.; Nomura, Y.; Jung, K.; Shin, T. Eugenol alleviates the symptoms of experimental autoimmune encephalomyelitis in mice by suppressing inflammatory responses. Int. Immunopharmacol. 2024, 128, 111479. [Google Scholar] [CrossRef]
- Ramirez-Ramirez, V.; Macias-Islas, M.A.; Ortiz, G.G.; Pacheco-Moises, F.; Torres-Sanchez, E.D.; Sorto-Gomez, T.E.; Cruz-Ramos, J.A.; Orozco-Aviña, G.; Celis de la Rosa, A.J. Efficacy of fish oil on serum of TNF α, IL-1 β, and IL-6 oxidative stress markers in multiple sclerosis treated with interferon beta-1b. Oxid. Med. Cell. Longev. 2013, 2013, 709493. [Google Scholar] [CrossRef]
- Zandi-Esfahan, S.; Fazeli, M.; Shaygannejad, V.; Hasheminia, J.; Badihian, S.; Aghayerashti, M.; Maghzi, H. Evaluating the effect of adding Fish oil to Fingolimod on TNF-α, IL1β, IL6, and IFN-γ in patients with relapsing-remitting multiple sclerosis: A double-blind randomized placebo-controlled trial. Clin. Neurol. Neurosurg. 2017, 163, 173–178. [Google Scholar] [CrossRef]
- Torres-Sánchez, E.D.; Pacheco-Moisés, F.P.; Macias-Islas, M.A.; Morales-Sánchez, E.W.; Ramírez-Ramírez, V.; Celis de la Rosa, A.J.; Cid-Hernández, M.; Sorto-Gómez, T.E.; Ortiz, G.G. Effect of fish and olive oil on mitochondrial ATPase activity and membrane fluidity in patients with relapsing-remitting multiple sclerosis treated with interferon beta 1-b. Nutr Hosp. 2018, 35, 162–168. [Google Scholar]
- Petrou, P.; Ginzberg, A.; Binyamin, O.; Karussis, D. Beneficial effects of a nano formulation of pomegranate seed oil, GranaGard, on the cognitive function of multiple sclerosis patients. Mult. Scler. Relat. Disord. 2021, 54, 103103. [Google Scholar] [CrossRef] [PubMed]
- Fiebiger, S.M.; Bros, H.; Grobosch, T.; Janssen, A.; Chanvillard, C.; Paul, F.; Dörr, J.; Millward, J.M.; Infante-Duarte, C. The antioxidant idebenone fails to prevent or attenuate chronic experimental autoimmune encephalomyelitis in the mouse. J. Neuroimmunol. 2013, 262, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Jameie, S.B.; Barati, M.; Mehdizadeh, M.; Kerdari, M. Effects of coenzyme Q10 on the ratio of TH1/TH2 in experimental autoimmune encephalomyelitis model of multiple sclerosis in C57BL/6. Iran. Biomed. J. 2014, 18, 203–211. [Google Scholar]
- Khalilian, B.; Madadi, S.; Fattahi, N.; Abouhamzeh, B. Coenzyme Q10 enhances remyelination and regulate inflammation effects of cuprizone in corpus callosum of chronic model of multiple sclerosis. J. Mol. Histol. 2021, 52, 125–134. [Google Scholar] [CrossRef]
- Sanoobar, M.; Eghtesadi, S.; Azimi, A.; Khalili, M.; Jazayeri, S.; Reza Gohari, M. Coenzyme Q10 supplementation reduces oxidative stress and increases antioxidant enzyme activity in patients with relapsing-remitting multiple sclerosis. Int. J. Neurosci. 2013, 123, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Sanoobar, M.; Eghtesadi, S.; Azimi, A.; Khalili, M.; Khodadadi, B.; Jazayeri, S.; Gohari, M.R.; Aryaeian, N. Coenzyme Q10 supplementation ameliorates inflammatory markers in patients with multiple sclerosis, a double blind, placebo, controlled randomized clinical trial. Nutr. Neurosci. 2015, 18, 169–176. [Google Scholar] [CrossRef]
- Sanoobar, M.; Dehghan, P.; Khalili, M.; Azimi, A.; Seifar, F. Coenzyme Q10 as a treatment for fatigue and depression in multiple sclerosis patients, A double blind randomized clinical trial. Nutr. Neurosci. 2016, 19, 138–143. [Google Scholar] [CrossRef]
- Hossein Haghighi, A.; Ahmadi, A.; Carotenuto, A.; Askari, R.; Nikkhah, K.; Bagherzadeh-Rahmani, B.; Sharabadi, H.; Souza, D.; Gentil, P. Effects of concurrent training and CoQ10 on neurotrophic factors and physical function in people with Multiple Sclerosis, a pilot study. Eur. J Transl. Myol. 2023, 33, 11253. [Google Scholar] [CrossRef]
- Kosa, P.; Wu, T.; Phillips, J.; Leinonen, M.; Masvekar, R.; Komori, M.; Wichman, A.; Sandford, M.; Bielekova, B. Idebenone does not inhibit disability progression in primary progressive MS. Mult. Scler. Relat. Disord. 2020, 45, 102434. [Google Scholar] [CrossRef]
- Xue, H.; Ren, H.; Zhang, L.; Sun, X.; Wang, W.; Zhang, S.; Zhao, J.; Ming, L. Alpha-tocopherol ameliorates experimental autoimmune encephalomyelitis through the regulation of Th1 cells. Iran. J. Basic Med. Sci. 2016, 19, 561–566. [Google Scholar]
- Blanchard, B.; Heurtaux, T.; Garcia, C.; Moll, N.M.; Caillava, C.; Grandbarbe, L.; Klosptein, A.; Kerninon, C.; Frah, M.; Coowar, D.; et al. Tocopherol derivative TFA-12 promotes myelin repair in experimental models of multiple sclerosis. J. Neurosci. 2013, 33, 11633–11642. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.D.; Christopher, M.A.; Thati, S.; Salash, J.R.; Pressnall, M.M.; Weerasekara, D.B.; Lunte, S.M.; Berkland, C.J. Tocopherol Emulsions as Functional Autoantigen Delivery Vehicles Evoke Therapeutic Efficacy in Experimental Autoimmune Encephalomyelitis. Mol. Pharm. 2019, 16, 607–617. [Google Scholar] [CrossRef]
- Navidhamidi, M.; Nazari, A.; Dehghan, S.; Ebrahimpour, A.; Nasrnezhad, R.; Pourabdolhossein, F. Therapeutic Potential of Combined Therapy of Vitamin A and Vitamin C in the Experimental Autoimmune Encephalomyelitis (EAE) in Lewis Rats. Mol. Neurobiol. 2022, 59, 2328–2347. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, D.M.; Li, J.; Deng, S.H.; Liu, T.; Zhang, T.; He, M.; Zhao, Y.Y.; Xu, Y. Bixin Attenuates Experimental Autoimmune Encephalomyelitis by Suppressing TXNIP/NLRP3 Inflammasome Activity and Activating NRF2 Signaling. Front. Immunol. 2020, 11, 593368. [Google Scholar] [CrossRef] [PubMed]
- Deslauriers, A.M.; Afkhami-Goli, A.; Paul, A.M.; Bhat, R.K.; Acharjee, S.; Ellestad, K.K.; Noorbakhsh, F.; Michalak, M.; Power, C. Neuroinflammation and endoplasmic reticulum stress are coregulated by crocin to prevent demyelination and neurodegeneration. J. Immunol. 2011, 187, 4788–4799. [Google Scholar] [CrossRef] [PubMed]
- Tashakori, A.; Hassanpour, S.; Vazir, B. Protective effect of crocin on cuprizone-induced model of multiple sclerosis in mice. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 1713–1725. [Google Scholar] [CrossRef]
- Banaeeyeh, S.; Afkhami-Goli, A.; Moosavi, Z.; Razavi, B.M.; Hosseinzadeh, H. Anti-inflammatory.; antioxidant and anti-mitophagy effects of trans sodium crocetinate on experimental autoimmune encephalomyelitis in BALB/C57 mice. Metab. Brain Dis. 2024, 39, 783–801. [Google Scholar] [CrossRef]
- Aristotelous, P.; Stefanakis, M.; Pantzaris, M.; Pattichis, C.S.; Calder, P.C.; Patrikios, I.S.; Sakkas, G.K.; Giannaki, C.D. The Effects of Specific Omega-3 and Omega-6 Polyunsaturated Fatty Acids and Antioxidant Vitamins on Gait and Functional Capacity Parameters in Patients with Relapsing-Remitting Multiple Sclerosis. Nutrients 2021, 13, 3661. [Google Scholar] [CrossRef]
- Mai, J.; Sørensen, P.S.; Hansen, J.C. High dose antioxidant supplementation to MS patients. Effects on glutathione peroxidase, clinical safety, and absorption of selenium. Biol. Trace Elem. Res. 1990, 24, 109–117. [Google Scholar] [CrossRef]
- Guan, J.Z.; Guan, W.P.; Maeda, T. Vitamin E administration erases an enhanced oxidation in multiple sclerosis. Can. J. Physiol. Pharmacol. 2018, 96, 1181–1183. [Google Scholar] [CrossRef]
- Bitarafan, S.; Saboor-Yaraghi, A.; Sahraian, M.A.; Nafissi, S.; Togha, M.; Beladi Moghadam, N.; Roostaei, T.; Siassi, F.; Eshraghian, M.R.; Ghanaati, H.; et al. Impact of Vitamin A Supplementation on Disease Progression in Patients with Multiple Sclerosis. Arch. Iran. Med. 2015, 18, 435–440. [Google Scholar] [PubMed]
- Bitarafan, S.; Saboor-Yaraghi, A.; Sahraian, M.A.; Soltani, D.; Nafissi, S.; Togha, M.; Beladi Moghadam, N.; Roostaei, T.; Mohammadzadeh Honarvar, N.; Harirchian, M.H. Effect of Vitamin A Supplementation on fatigue and depression in Multiple Sclerosis patients, A Double-Blind Placebo-Controlled Clinical Trial. Iran. J. Allergy Asthma Immunol. 2016, 15, 13–19. [Google Scholar]
- Mohammadzadeh Honarvar, N.; Harirchian, M.H.; Abdolahi, M.; Abedi, E.; Bitarafan, S.; Koohdani, F.; Siassi, F.; Sahraian, M.A.; Chahardoli, R.; Zareei, M.; et al. Retinyl Palmitate Supplementation Modulates T-bet and Interferon Gamma Gene Expression in Multiple Sclerosis Patients. J. Mol. Neurosci. 2016, 59, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Ghiasian, M.; Khamisabadi, F.; Kheiripour, N.; Karami, M.; Haddadi, R.; Ghaleiha, A.; Taghvaei, B.; Oliaie, S.S.; Salehi, M.; Samadi, P.; et al. Effects of crocin in reducing DNA damage, inflammation, and oxidative stress in multiple sclerosis patients, A double-blind, randomized, and placebo-controlled trial. J. Biochem. Mol. Toxicol. 2019, 33, e22410. [Google Scholar] [CrossRef] [PubMed]
- Kouchaki, E.; Rafiei, H.; Ghaderi, A.; Azadchehr, M.J.; Safa, F.; Omidian, K.; Khodabakhshi, A.; Vahid, F.; Rezapoor-Kafteroodi, B.; Banafshe, H.R.; et al. Effects of crocin on inflammatory biomarkers and mental health status in patients with multiple sclerosis: A randomized, double-blinded clinical trial. Mult. Scler. Relat. Disord. 2024, 83, 105454. [Google Scholar] [CrossRef]
- Hooper, D.C.; Spitsin, S.; Kean, R.B.; Champion, J.M.; Dickson, G.M.; Chaudhry, I.; Koprowski, H. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc. Natl. Acad. Sci. USA 1998, 95, 675–680. [Google Scholar] [CrossRef]
- Hooper, D.C.; Scott, G.S.; Zborek, A.; Mikheeva, T.; Kean, R.B.; Koprowski, H.; Spitsin, S.V. Uric acid, a peroxynitrite scavenger, inhibits CNS inflammation, blood-CNS barrier permeability changes, and tissue damage in a mouse model of multiple sclerosis. FASEB J. 2000, 14, 691–698. [Google Scholar] [CrossRef]
- Spitsin, S.V.; Scott, G.S.; Kean, R.B.; Mikheeva, T.; Hooper, D.C. Protection of myelin basic protein immunized mice from free-radical mediated inflammatory cell invasion of the central nervous system by the natural peroxynitrite scavenger uric acid. Neurosci. Lett. 2000, 292, 137–141. [Google Scholar] [CrossRef]
- Kean, R.B.; Spitsin, S.V.; Mikheeva, T.; Scott, G.S.; Hooper, D.C. The peroxynitrite scavenger uric acid prevents inflammatory cell invasion into the central nervous system in experimental allergic encephalomyelitis through maintenance of blood-central nervous system barrier integrity. J. Immunol. 2000, 165, 6511–6518. [Google Scholar] [CrossRef]
- Scott, G.S.; Spitsin, S.V.; Kean, R.B.; Mikheeva, T.; Koprowski, H.; Hooper, D.C. Therapeutic intervention in experimental allergic encephalomyelitis by administration of uric acid precursors. Proc. Natl. Acad. Sci. USA 2002, 99, 16303–16308. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, B.; Wang, X.; Luo, L.; Li, P.; Paty, D.W.; Cynader, M.S. Bilirubin as a potent antioxidant suppresses experimental autoimmune encephalomyelitis, implications for the role of oxidative stress in the development of multiple sclerosis. J. Neuroimmunol. 2003, 139, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Tetzlaff, W.; Paty, D.W.; Cynader, M.S. Biliverdin reductase, a major physiologic cytoprotectant, suppresses experimental autoimmune encephalomyelitis. Free Radic. Biol. Med. 2006, 40, 960–967. [Google Scholar] [CrossRef]
- Liu, Y.; Li, P.; Lu, J.; Xiong, W.; Oger, J.; Tetzlaff, W.; Cynader, M. Bilirubin possesses powerful immunomodulatory activity and suppresses experimental autoimmune encephalomyelitis. J. Immunol. 2008, 181, 1887–1897. [Google Scholar] [CrossRef]
- Koprowski, H.; Spitsin, S.V.; Hooper, D.C. Prospects for the treatment of multiple sclerosis by raising serum levels of uric acid.; a scavenger of peroxynitrite. Ann. Neurol. 2001, 49, 139. [Google Scholar] [CrossRef]
- Spitsin, S.; Hooper, D.C.; Leist, T.; Streletz, L.J.; Mikheeva, T.; Koprowskil, H. Inactivation of peroxynitrite in multiple sclerosis patients after oral administration of inosine may suggest possible approaches to therapy of the disease. Mult. Scler. J. 2001, 7, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, C.E.; Spitsin, S.; Zimmerman, V.; Jacobs, D.; Udupa, J.K.; Hooper, D.C.; Koprowski, H. The treatment of multiple sclerosis with inosine. J. Altern. Complement. Med. 2009, 15, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Gonsette, R.E.; Sindic, C.; D’hooghe, M.B.; De Deyn, P.P.; Medaer, R.; Michotte, A.; Seeldrayers, P.; Guillaume, D.; ASIIMS Study Group. Boosting endogenous neuroprotection in multiple sclerosis, the ASsociation of Inosine and Interferon beta in relapsing- remitting Multiple Sclerosis (ASIIMS) trial. Mult. Scler. J. 2010, 16, 455–462. [Google Scholar] [CrossRef]
- Muñoz García, D.; Midaglia, L.; Martinez Vilela, J.; Marín Sánchez, M.; López González, F.J.; Arias Gómez, M.; Dapena Bolaño, D.; Iglesias Castañón, A.; Alonso Alonso, M.; Romero López, J. Associated Inosine to interferon: Results of a clinical trial in multiple sclerosis. Acta Neurol. Scand. 2015, 131, 405–410. [Google Scholar] [CrossRef]
- Cross, A.H.; Misko, T.P.; Lin, R.F.; Hickey, W.F.; Trotter, J.L.; Tilton, R.G. Aminoguanidine, an inhibitor of inducible nitric oxide synthase, ameliorates experimental autoimmune encephalomyelitis in SJL mice. J. Clin. Investig. 1994, 93, 2684–2690. [Google Scholar] [CrossRef]
- Ding, M.; Zhang, M.; Wong, J.L.; Rogers, N.E.; Ignarro, L.J.; Voskuhl, R.R. Antisense knockdown of inducible nitric oxide synthase inhibits induction of experimental autoimmune encephalomyelitis in SJL/J mice. J. Immunol. 1998, 160, 2560–2564. [Google Scholar] [CrossRef]
- Jolivalt, C.G.; Howard, R.B.; Chen, L.S.; Mizisin, A.P.; Lai, C.S. A novel nitric oxide scavenger in combination with cyclosporine A ameliorates experimental autoimmune encephalomyelitis progression in mice. J. Neuroimmunol. 2003, 138, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Pozza, M.; Bettelli, C.; Aloe, L.; Giardino, L.; Calzà, L. Further evidence for a role of nitric oxide in experimental allergic encephalomyelitis, aminoguanidine treatment modifies its clinical evolution. Brain Res. 2000, 855, 39–46. [Google Scholar] [CrossRef]
- Ljubisavljevic, S.; Stojanovic, I.; Pavlovic, D.; Sokolovic, D.; Stevanovic, I. Aminoguanidine and N-acetyl-cysteine supress oxidative and nitrosative stress in EAE rat brains. Redox Rep. 2011, 16, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.S.; Kean, R.B.; Southan, G.J.; Szabó, C.; Hooper, D.C. Effect of mercaptoethylguanidine scavengers of peroxynitrite on the development of experimental allergic encephalomyelitis in PLSJL mice. Neurosci. Lett. 2001, 311, 125–128. [Google Scholar] [CrossRef]
- Hooper, D.C.; Bagasra, O.; Marini, J.C.; Zborek, A.; Ohnishi, S.T.; Kean, R.; Champion, J.M.; Sarker, A.B.; Bobroski, L.; Farber, J.L.; et al. Prevention of experimental allergic encephalomyelitis by targeting nitric oxide and peroxynitrite, implications for the treatment of multiple sclerosis. Proc. Natl. Acad. Sci. USA 1997, 94, 2528–2533. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, N.C.; Charlton, B.; Cowden, W.B.; Willenborg, D.O. Nitric oxide plays a critical role in the recovery of Lewis rats from experimental autoimmune encephalomyelitis and the maintenance of resistance to rei nduction. J. Immunol. 1999, 163, 6841–6847. [Google Scholar] [CrossRef]
- Kouhsar, S.S.; Karami, M.; Tafreshi, A.P.; Roghani, M.; Nadoushan, M.R. Microinjection of l-arginine into corpus callosum cause reduction in myelin concentration and neuroinflammation. Brain Res. 2011, 1392, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Molina-Holgado, F.; Hernanz, A.; De la Fuente, M.; Guaza, C. N-Acetyl-cysteine inhibition of encephalomyelitis Theiler’s virus-induced nitric oxide and tumour necrosis factor-alpha production by murine astrocyte cultures. Biofactors 1999, 10, 187–193. [Google Scholar] [CrossRef]
- Offen, D.; Gilgun-Sherki, Y.; Barhum, Y.; Benhar, M.; Grinberg, L.; Reich, R.; Melamed, E.; Atlas, D. A low molecular weight copper chelator crosses the blood-brain barrier and attenuates experimental autoimmune encephalomyelitis. J. Neurochem. 2004, 89, 1241–1251. [Google Scholar] [CrossRef]
- Escribano, B.M.; Muñoz-Jurado, A.; Luque, E.; Galván, A.; LaTorre, M.; Caballero-Villarraso, J.; Giraldo, A.I.; Agüera, E.; Túnez, I. Effect of the Combination of Different Therapies on Oxidative Stress in the Experimental Model of Multiple Sclerosis. Neuroscience 2023, 529, 116–128. [Google Scholar] [CrossRef]
- Monti, D.A.; Zabrecky, G.; Leist, T.P.; Wintering, N.; Bazzan, A.J.; Zhan, T.; Newberg, A.B. N-acetyl Cysteine Administration Is Associated With Increased Cerebral Glucose Metabolism in Patients With Multiple Sclerosis: An Exploratory Study. Front. Neurol. 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Khalatbari Mohseni, G.; Hosseini, S.A.; Majdinasab, N.; Cheraghian, B. Effects of N-acetyl-cysteine on oxidative stress biomarkers, depression. and anxiety symptoms in patients with multiple sclerosis. Neuropsychopharmacol. Rep. 2023, 43, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Krysko, K.M.; Bischof, A.; Nourbakhsh, B.; Henry, R.G.; Revirajan, N.; Manguinao, M.; Nguyen, K.; Akula, A.; Li, Y.; Waubant, E. A pilot study of oxidative pathways in MS fatigue: Randomized trial of N-acetyl cysteine. Ann. Clin. Transl. Neurol. 2021, 8, 811–824. [Google Scholar] [CrossRef]
- Schoeps, V.A.; Graves, J.S.; Stern, W.A.; Zhang, L.; Nourbakhsh, B.; Mowry, E.M.; Henry, R.G.; Waubant, E. N-Acetyl-Cysteine as a Neuroprotective Agent in Progressive Multiple Sclerosis (NACPMS) trial, Study protocol for a randomized, double-blind, placebo-controlled add-on phase 2 trial. Contemp. Clin. Trials 2022, 122, 106941. [Google Scholar] [CrossRef] [PubMed]
- Muthian, G.; Bright, J.J. Quercetin, a flavonoid phytoestrogen.; ameliorates experimental allergic encephalomyelitis by blocking IL-12 signaling through JAK-STAT pathway in T lymphocyte. J. Clin. Immunol. 2004, 24, 542–552. [Google Scholar] [CrossRef]
- Ginwala, R.; McTish, E.; Raman, C.; Singh, N.; Nagarkatti, M.; Nagarkatti, P.; Sagar, D.; Jain, P.; Khan, Z.K. Apigenin. a Natural Flavonoid. Attenuates EAE Severity Through the Modulation of Dendritic Cell and Other Immune Cell Functions. J. Neuroimmune Pharmacol. 2016, 11, 36–47. [Google Scholar] [CrossRef]
- Ma, X.; Wang, S.; Li, C.; Jia, X.; Wang, T.; Leng, Z.; Lu, R.; Kong, X.; Zhang, J.; Li, L. Baicalein inhibits the polarization of microglia/macrophages to the M1 phenotype by targeting STAT1 in EAE mice. Int. Immunopharmacol. 2022, 113 Pt A, 109373. [Google Scholar] [CrossRef]
- Ying, S.; Yang, H.; Gu, Q.; Wu, Z.; Zou, N.; Wang, C.Z.; Wan, C.; Yuan, C.S. The Small-Molecule compound baicalein alleviates experimental autoimmune encephalomyelitis by suppressing pathogenetic CXCR6+ CD4 cells. Int. Immunopharmacol. 2023, 114, 109562. [Google Scholar] [CrossRef] [PubMed]
- Fontes, L.B.; Dos Santos Dias, D.; de Carvalho, L.S.; Mesquita, H.L.; da Silva Reis, L.; Dias, A.T.; Da Silva Filho, A.A.; do Amaral Corrêa, J.O. Immunomodulatory effects of licochalcone A on experimental autoimmune encephalomyelitis. J. Pharm. Pharmacol. 2014, 66, 886–894. [Google Scholar] [CrossRef]
- Razeghi Jahromi, S.; Arrefhosseini, S.R.; Ghaemi, A.; Alizadeh, A.; Moradi Tabriz, H.; Togha, M. Alleviation of experimental allergic encephalomyelitis in C57BL/6 mice by soy daidzein. Iran. J. Allergy Asthma Immunol. 2014, 13, 256–264. [Google Scholar]
- Yarim, G.F.; Yarim, M.; Sozmen, M.; Gokceoglu, A.; Ertekin, A.; Kabak, Y.B.; Karaca, E. Nobiletin attenuates inflammation via modulating proinflammatory and antiinflammatory cytokine expressions in an autoimmune encephalomyelitis mouse model. Fitoterapia 2022, 156, 105099. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Guo, Y.X.; Gao, R.; Ji, X.Y.; Tang, Y.X.; Wang, L.B.; Zhang, Y.; Li, X. Quercetin regulates dendritic cell activation by targeting STAT4 in the treatment of experimental autoimmune encephalomyelitis. Toxicol. Appl. Pharmacol. 2024, 488, 116980. [Google Scholar] [CrossRef] [PubMed]
- Nicola, M.A.; Attaai, A.H.; Abdel-Raheem, M.H.; Mohammed, A.F.; Abu-Elhassan, Y.F. Neuroprotective effects of rutin against cuprizone-induced multiple sclerosis in mice. Inflammopharmacology 2024, 32, 1295–1315. [Google Scholar] [CrossRef]
- Omotoso, G.O.; Ukwubile, I.I.; Arietarhire, L.; Sulaimon, F.; Gbadamosi, I.T. Kolaviron protects the brain in cuprizone-induced model of experimental multiple sclerosis via enhancement of intrinsic antioxidant mechanisms, Possible therapeutic applications? Pathophysiology 2018, 25, 299–306. [Google Scholar] [CrossRef]
- Song, L.J.; Han, Q.X.; Ding, Z.B.; Liu, K.; Zhang, X.X.; Guo, M.F.; Ma, D.; Wang, Q.; Xiao, B.G.; Ma, C.G. Icariin ameliorates the cuprizone-induced demyelination associated with antioxidation and anti-inflammation. Inflammopharmacology 2024, 32, 809–823. [Google Scholar] [CrossRef]
- Karpov, S.M.; Shevchenko, P.P.; Nazarova, E.O.; Vyshlova, I.A.; Dolgova, I.N. Tsitoflavin v kompleksnoĭ terapii rasseiannogo skleroza [Cytoflavin in the complex therapy of multiple sclerosis]. Zh. Nevrol. Psikhiatr. Im. S S Korsakova 2018, 118, 37–39. (In Russian) [Google Scholar] [CrossRef]
- Diab, A.; Hussain, R.Z.; Lovett-Racke, A.E.; Chavis, J.A.; Drew, P.D.; Racke, M.K. Ligands for the peroxisome proliferator-activated receptor-gamma and the retinoid X receptor exert additive anti-inflammatory effects on experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2004, 148, 116–126. [Google Scholar] [CrossRef]
- Storer, P.D.; Xu, J.; Chavis, J.; Drew, P.D. Peroxisome proliferator-activated receptor-gamma agonists inhibit the activation of microglia and astrocytes, implications for multiple sclerosis. J. Neuroimmunol. 2005, 161, 113–122. [Google Scholar] [CrossRef]
- Xu, J.; Storer, P.D.; Chavis, J.A.; Racke, M.K.; Drew, P.D. Agonists for the peroxisome proliferator-activated receptor-alpha and the retinoid X receptor inhibit inflammatory responses of microglia. J. Neurosci. Res. 2005, 81, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chavis, J.A.; Racke, M.K.; Drew, P.D. Peroxisome proliferator-activated receptor-alpha and retinoid X receptor agonists inhibit inflammatory responses of astrocytes. J. Neuroimmunol. 2006, 176, 95–105. [Google Scholar] [CrossRef]
- Paintlia, A.S.; Paintlia, M.K.; Singh, I.; Singh, A.K. IL-4-induced peroxisome proliferator-activated receptor gamma activation inhibits NF-kappaB trans activation in central nervous system (CNS) glial cells and protects oligodendrocyte progenitors under neuroinflammatory disease conditions, implication for CNS-demyelinating diseases. J. Immunol. 2006, 176, 4385–4398. [Google Scholar] [PubMed]
- Zidan, A.; Hedya, S.E.; Elfeky, D.M.; Abdin, A.A. The possible anti-apoptotic and antioxidant effects of acetyl l-carnitine as an add-on therapy on a relapsing-remitting model of experimental autoimmune encephalomyelitis in rats. Biomed. Pharmacother. 2018, 103, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Mørkholt, A.S.; Trabjerg, M.S.; Oklinski, M.K.E.; Bolther, L.; Kroese, L.J.; Pritchard, C.E.J.; Huijbers, I.J.; Nieland, J.D.V. CPT1A plays a key role in the development and treatment of multiple sclerosis and experimental autoimmune encephalomyelitis. Sci. Rep. 2019, 9, 13299. [Google Scholar] [CrossRef] [PubMed]
- Safwat, S.M.; Aboonq, M.S.; El Tohamy, M.; Mojaddidi, M.; Al-Qahtani, S.A.M.; Zakari, M.O.; ElGendy, A.A.; Hussein, A.M. New Insight into the Possible Roles of L-Carnitine in a Rat Model of Multiple Sclerosis. Brain Sci. 2023, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Spaas, J.; Franssen, W.M.A.; Keytsman, C.; Blancquaert, L.; Vanmierlo, T.; Bogie, J.; Broux, B.; Hellings, N.; van Horssen, J.; Posa, D.K.; et al. Carnosine quenches the reactive carbonyl acrolein in the central nervous system and attenuates autoimmune neuroinflammation. J. Neuroinflamm 2021, 18, 255. [Google Scholar] [CrossRef]
- Zanini, D.; Jezdimirovic, T.; Stajer, V.; Ostojic, J.; Maksimovic, N.; Ostojic, S.M. Dietary supplementation with L-carnosine improves patient-reported outcomes.; autonomic nervous system performance.; and brain metabolism in 3 adult patients with multiple sclerosis. Nutr. Res. 2020, 84, 63–69. [Google Scholar] [CrossRef]
- Teixeira, C.F.; Azzolin, V.F.; Rodrigues Dos Passos, G.; Turra, B.O.; Alves, A.O.; Bressanim, A.C.M.; Canton, L.E.L.; Vieira Dos Santos, A.C.; Mastella, M.H.; Barbisan, F.; et al. A coffee enriched with guarana, selenium, and l-carnitine (GSC) has nutrigenomic effects on oxi-inflammatory markers of relapsing-remitting multiple sclerosis patients; A pilot study. Mult. Scler. Relat. Disord. 2023, 71, 104515. [Google Scholar] [CrossRef] [PubMed]
- Moriya, M.; Nakatsuji, Y.; Miyamoto, K.; Okuno, T.; Kinoshita, M.; Kumanogoh, A.; Kusunoki, S.; Sakoda, S. Edaravone, a free radical scavenger, ameliorates experimental autoimmune encephalomyelitis. Neurosci. Lett. 2008, 440, 323–326. [Google Scholar] [CrossRef]
- Bakhtiari, M.; Ghasemi, N.; Salehi, H.; Amirpour, N.; Kazemi, M.; Mardani, M. Evaluation of Edaravone effects on the differentiation of human adipose derived stem cells into oligodendrocyte cells in multiple sclerosis disease in rats. Life Sci. 2021, 282, 119812. [Google Scholar] [CrossRef]
- Villar-Delfino, P.H.; Gomes, N.A.O.; Christo, P.P.; Nogueira-Machado, J.A.; Volpe, C.M.O. Edaravone Inhibits the Production of Reactive Oxygen Species in Phagocytosis- and PKC-Stimulated Granulocytes from Multiple Sclerosis Patients Edaravone Modulate Oxidative Stress in Multiple Sclerosis. J. Cent. Nerv. Syst. Dis. 2022, 14, 11795735221092524. [Google Scholar] [CrossRef]
- Pentón-Rol, G.; Martínez-Sánchez, G.; Cervantes-Llanos, M.; Lagumersindez-Denis, N.; Acosta-Medina, E.F.; Falcón-Cama, V.; Alonso-Ramírez, R.; Valenzuela-Silva, C.; Rodríguez-Jiménez, E.; Llópiz-Arzuaga, A.; et al. C-Phycocyanin ameliorates experimental autoimmune encephalomyelitis and induces regulatory T cells. Int. Immunopharmacol. 2011, 11, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Pentón-Rol, G.; Lagumersindez-Denis, N.; Muzio, L.; Bergami, A.; Furlan, R.; Fernández-Massó, J.R.; Nazabal-Galvez, M.; Llópiz-Arzuaga, A.; Herrera-Rolo, T.; Veliz-Rodriguez, T.; et al. Comparative Neuroregenerative Effects of C-Phycocyanin and IFN-Beta in a Model of Multiple Sclerosis in Mice. J. Neuroimmune Pharmacol. 2016, 11, 153–167. [Google Scholar] [CrossRef]
- Gardón, D.P.; Cervantes-Llanos, M.; Matamoros, B.P.; Rodríguez, H.C.; Tan, C.Y.; Marín-Prida, J.; Falcón-Cama, V.; Pavón-Fuentes, N.; Lemus, J.G.; Ruiz, L.C.B.; et al. Positive effects of Phycocyanobilin on gene expression in glutamate-induced excitotoxicity in SH-SY5Y cells and animal models of multiple sclerosis and cerebral ischemia. Heliyon 2022, 8, e09769. [Google Scholar] [CrossRef]
- Marín-Prida, J.; Pavón-Fuentes, N.; Lagumersindez-Denis, N.; Camacho-Rodríguez, H.; García-Soca, A.M.; Sarduy-Chávez, R.C.; Vieira, É.L.M.; Carvalho-Tavares, J.; Falcón-Cama, V.; Fernández-Massó, J.R.; et al. Anti-inflammatory mechanisms and pharmacological actions of phycocyanobilin in a mouse model of experimental autoimmune encephalomyelitis: A therapeutic promise for multiple sclerosis. Front. Immunol. 2022, 13, 1036200. [Google Scholar] [CrossRef] [PubMed]
- DellaValle, B.; Brix, G.S.; Brock, B.; Gejl, M.; Landau, A.M.; Møller, A.; Rungby, J.; Larsen, A. Glucagon-Like Peptide-1 Analog, Liraglutide, Delays Onset of Experimental Autoimmune Encephalitis in Lewis Rats. Front. Pharmacol. 2016, 7, 433. [Google Scholar] [CrossRef]
- Sanadgol, N.; Barati, M.; Houshmand, F.; Hassani, S.; Clarner, T.; Shahlaei, M.; Golab, F. Metformin accelerates myelin recovery and ameliorates behavioral deficits in the animal model of multiple sclerosis via adjustment of AMPK/Nrf2/mTOR signaling and maintenance of endogenous oligodendrogenesis during brain self-repairing period. Pharmacol. Rep. 2020, 72, 641–658. [Google Scholar] [CrossRef]
- Penkowa, M.; Hidalgo, J. Metallothionein treatment reduces proinflammatory cytokines IL-6 and TNF-alpha and apoptotic cell death during experimental autoimmune encephalomyelitis (EAE). Exp. Neurol. 2001, 170, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, A.; Akyol, O.; Gurel, A.; Armutcu, F.; Iraz, M.; Oztas, E. Protective effects of caffeic acid phenethyl ester against experimental allergic encephalomyelitis-induced oxidative stress in rats. Free Radic. Biol. Med. 2004, 37, 386–394. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Chang, Y.; Li, R.; Sun, X.; Peng, L.; Zheng, W.; Qiu, W. Caffeic Acid Phenethyl Ester Protects against Experimental Autoimmune Encephalomyelitis by Regulating T Cell Activities. Oxid. Med. Cell. Longev. 2020, 2020, 7274342. [Google Scholar] [CrossRef]
- Camelo, S.; Iglesias, A.H.; Hwang, D.; Due, B.; Ryu, H.; Smith, K.; Gray, S.G.; Imitola, J.; Duran, G.; Assaf, B.; et al. Transcriptional therapy with the histone deacetylase inhibitor trichostatin A ameliorates experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2005, 164, 10–21. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, R.; Zhao, J.; Chen, M.; Chen, S.; Ji, B.; Chen, H.; Liu, D.; Li, L.; Du, G. The histone deacetylase inhibitor belinostat ameliorates experimental autoimmune encephalomyelitis in mice by inhibiting TLR2/MyD88 and HDAC3/NF-κB p65-mediated neuroinflammation. Pharmacol. Res. 2022, 176, 105969. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.M.; Cossins, J.A.; Wells, G.M.; Corkill, D.J.; Helfrich, K.; Wood, L.M.; Pigott, R.; Stabler, G.; Ward, G.A.; Gearing, A.J.; et al. Matrix metalloproteinase expression during experimental autoimmune encephalomyelitis and effects of a combined matrix metalloproteinase and tumour necrosis factor-alpha inhibitor. J. Neuroimmunol. 1997, 74, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, W.; Cannella, B.; Mazzaccaro, R.J.; Clements, J.M.; Miller, K.M.; Wucherpfennig, K.W.; Gearing, A.J.; Raine, C.S. Effective treatment of models of multiple sclerosis by matrix metalloproteinase inhibitors. Ann. Neurol. 1998, 44, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Malfroy, B.; Doctrow, S.R.; Orr, P.L.; Tocco, G.; Fedoseyeva, E.V.; Benichou, G. Prevention and suppression of autoimmune encephalomyelitis by EUK-8, a synthetic catalytic scavenger of oxygen-reactive metabolites. Cell. Immunol. 1997, 177, 62–68. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, B.; Luo, L.; Li, P.; Paty, D.W.; Cynader, M.S. Heme oxygenase-1 plays an important protective role in experimental autoimmune encephalomyelitis. Neuroreport 2001, 12, 1841–1845. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Avila, J.G.; Schültke, E.; Kamencic, H.; Skihar, V.; Obayan, A.; Juurlink, B.H. Amelioration of experimental allergic encephalitis (EAE) through phase 2 enzyme induction. Biomed. Sci. Instrum. 2002, 38, 9–13. [Google Scholar]
- Mohamed, A.; Shoker, A.; Bendjelloul, F.; Mare, A.; Alzrigh, M.; Benghuzzi, H.; Desin, T. Improvement of experimental allergic encephalomyelitis (EAE) by thymoquinone; an oxidative stress inhibitor. Biomed. Sci. Instrum. 2003, 39, 440–445. [Google Scholar] [PubMed]
- Min, K.; Yoon, W.K.; Kim, S.K.; Kim, B.H. Immunosuppressive effect of silibinin in experimental autoimmune encephalomyelitis. Arch. Pharm. Res. 2007, 30, 1265–1272. [Google Scholar] [CrossRef]
- Basso, A.S.; Frenkel, D.; Quintana, F.J.; Costa-Pinto, F.A.; Petrovic-Stojkovic, S.; Puckett, L.; Monsonego, A.; Bar-Shir, A.; Engel, Y.; Gozin, M.; et al. Reversal of axonal loss and disability in a mouse model of progressive multiple sclerosis. J. Clin. Investig. 2008, 118, 1532–1543. [Google Scholar] [CrossRef]
- Mangas, A.; Coveñas, R.; Bodet, D.; de León, M.; Duleu, S.; Geffard, M. Evaluation of the effects of a new drug candidate (GEMSP) in a chronic EAE model. Int. J. Biol. Sci. 2008, 4, 150–160. [Google Scholar] [CrossRef]
- De Paula, M.L.; Rodrigues, D.H.; Teixeira, H.C.; Barsante, M.M.; Souza, M.A.; Ferreira, A.P. Genistein down-modulates pro-inflammatory cytokines and reverses clinical signs of experimental autoimmune encephalomyelitis. Int. Immunopharmacol. 2008, 8, 1291–1297. [Google Scholar] [CrossRef]
- Kizelsztein, P.; Ovadia, H.; Garbuzenko, O.; Sigal, A.; Barenholz, Y. Pegylated nanoliposomes remote-loaded with the antioxidant tempamine ameliorate experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2009, 213, 20–25. [Google Scholar] [CrossRef]
- Ghazavi, A.; Mosayebi, G.; Salehi, H.; Abtahi, H. Effect of ethanol extract of saffron (Crocus sativus L.) on the inhibition of experimental autoimmune encephalomyelitis in C57bl/6 mice. Pak. J. Biol. Sci. 2009, 12, 690–695. [Google Scholar] [CrossRef]
- Mosayebi, G.; Haghmorad, D.; Namaki, S.; Ghazavi, A.; Ekhtiari, P.; Mirshafiey, A. Therapeutic effect of EDTA in experimental model of multiple sclerosis. Immunopharmacol. Immunotoxicol. 2010, 32, 321–326. [Google Scholar] [CrossRef]
- Mirshafiey, A.; Aghily, B.; Namaki, S.; Razavi, A.; Ghazavi, A.; Ekhtiari, P.; Mosayebi, G. Therapeutic approach by Aloe vera in experimental model of multiple sclerosis. Immunopharmacol. Immunotoxicol. 2010, 32, 410–415. [Google Scholar] [CrossRef]
- Chen, S.J.; Wang, Y.L.; Lo, W.T.; Wu, C.C.; Hsieh, C.W.; Huang, C.F.; Lan, Y.H.; Wang, C.C.; Chang, D.M.; Sytwu, H.K. Erythropoietin enhances endogenous haem oxygenase-1 and represses immune responses to ameliorate experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 2010, 162, 210–223. [Google Scholar] [CrossRef]
- Leung, G.; Sun, W.; Zheng, L.; Brookes, S.; Tully, M.; Shi, R. Anti-acrolein treatment improves behavioral outcome and alleviates myelin damage in experimental autoimmune encephalomyelitis mouse. Neuroscience 2011, 173, 150–155. [Google Scholar] [CrossRef]
- Guo, X.; Harada, C.; Namekata, K.; Kimura, A.; Mitamura, Y.; Yoshida, H.; Matsumoto, Y.; Harada, T. Spermidine alleviates severity of murine experimental autoimmune encephalomyelitis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2696–2703. [Google Scholar] [CrossRef] [PubMed]
- Schiffmann, S.; Ferreiros, N.; Birod, K.; Eberle, M.; Schreiber, Y.; Pfeilschifter, W.; Ziemann, U.; Pierre, S.; Scholich, K.; Grösch, S.; et al. Ceramide synthase 6 plays a critical role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2012, 188, 5723–5733. [Google Scholar] [CrossRef] [PubMed]
- Bowie, L.E.; Roscoe, W.A.; Lui, E.M.; Smith, R.; Karlik, S.J. Effects of an aqueous extract of North American ginseng on MOG(35-55)-induced EAE in mice. Can. J. Physiol. Pharmacol. 2012, 90, 933–939. [Google Scholar] [CrossRef]
- Morsali, D.; Bechtold, D.; Lee, W.; Chauhdry, S.; Palchaudhuri, U.; Hassoon, P.; Snell, D.M.; Malpass, K.; Piers, T.; Pocock, J.; et al. Safinamide and flecainide protect axons and reduce microglial activation in models of multiple sclerosis. Brain 2013, 136 Pt 4, 1067–1082. [Google Scholar] [CrossRef]
- Mao, P.; Manczak, M.; Shirendeb, U.P.; Reddy, P.H. MitoQ, a mitochondria-targeted antioxidant, delays disease progression and alleviates pathogenesis in an experimental autoimmune encephalomyelitis mouse model of multiple sclerosis. Biochim. Biophys. Acta 2013, 1832, 2322–2331. [Google Scholar] [CrossRef]
- Li, B.; Cui, W.; Liu, J.; Li, R.; Liu, Q.; Xie, X.H.; Ge, X.L.; Zhang, J.; Song, X.J.; Wang, Y.; et al. Sulforaphane ameliorates the development of experimental autoimmune encephalomyelitis by antagonizing oxidative stress and Th17-related inflammation in mice. Exp. Neurol. 2013, 250, 239–249. [Google Scholar] [CrossRef]
- He, Y.; Du, M.; Gao, Y.; Liu, H.; Wang, H.; Wu, X.; Wang, Z. Astragaloside IV attenuates experimental autoimmune encephalomyelitis of mice by counteracting oxidative stress at multiple levels. PLoS ONE 2013, 8, e76495. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, L.; Wang, J.; Xu, X.; Ni, S.; Liu, M.; Hu, K. Nose to brain delivery of Astragaloside IV by β-Asarone modified chitosan nanoparticles for multiple sclerosis therapy. Int. J. Pharm. 2023, 644, 123351. [Google Scholar] [CrossRef]
- Schmitz, K.; de Bruin, N.; Bishay, P.; Männich, J.; Häussler, A.; Altmann, C.; Ferreirós, N.; Lötsch, J.; Ultsch, A.; Parnham, M.J.; et al. R-flurbiprofen attenuates experimental autoimmune encephalomyelitis in mice. EMBO Mol. Med. 2014, 6, 1398–1422. [Google Scholar] [CrossRef]
- Choi, B.Y.; Kim, J.H.; Kho, A.R.; Kim, I.Y.; Lee, S.-H.; Lee, B.E.; Choi, E.; Sohn, M.; Stevenson, M.; Chung, T.N.; et al. Inhibition of NADPH oxidase activation reduces EAE-induced white matter damage in mice. J. Neuroinflammation. 2015, 12, 104. [Google Scholar] [CrossRef]
- Kong, W.; Hooper, K.M.; Ganea, D. The natural dual cyclooxygenase and 5-lipoxygenase inhibitor flavocoxid is protective in EAE through effects on Th1/Th17 differentiation and macrophage/microglia activation. Brain Behav. Immun. 2016, 53, 59–71. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, M.D.; Pu, Y.Y.; Wang, D.; Xie, Y.; Xue, G.C.; Jiang, Y.; Yang, Q.Q.; Sun, X.J.; Cao, L. Hydrogen-rich water improves neurological functional recovery in experimental autoimmune encephalomyelitis mice. J. Neuroimmunol. 2016, 294, 6–13. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Z.; Zhang, K.; Xue, Z.; Li, Y.; Zhang, Z.; Zhang, L.; Gu, C.; Zhang, Q.; Hao, J.; et al. Arctigenin Suppress Th17 Cells and Ameliorates Experimental Autoimmune Encephalomyelitis Through AMPK and PPAR-γ/ROR-γt Signaling. Mol. Neurobiol. 2016, 53, 5356–5366. [Google Scholar] [CrossRef]
- Kuo, P.C.; Brown, D.A.; Scofield, B.A.; Yu, I.C.; Chang, F.L.; Wang, P.Y.; Yen, J.H. 3H-1.;2-dithiole-3-thione as a novel therapeutic agent for the treatment of experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2016, 57, 173–186. [Google Scholar] [CrossRef]
- Lieberknecht, V.; Junqueira, S.C.; Cunha, M.P.; Barbosa, T.A.; de Souza, L.F.; Coelho, I.S.; Santos, A.R.; Rodrigues, A.L.; Dafré, A.L.; Dutra, R.C. Pramipexole, a Dopamine D2/D3 Receptor-Preferring Agonist, Prevents Experimental Autoimmune Encephalomyelitis Development in Mice. Mol. Neurobiol. 2017, 54, 1033–1045. [Google Scholar] [CrossRef]
- Buonvicino, D.; Ranieri, G.; Pratesi, S.; Gerace, E.; Muzzi, M.; Guasti, D.; Tofani, L.; Chiarugi, A. Neuroprotection induced by dexpramipexole delays disease progression in a mouse model of progressive multiple sclerosis. Br. J. Pharmacol. 2020, 177, 3342–3356. [Google Scholar] [CrossRef]
- Neil, S.; Huh, J.; Baronas, V.; Li, X.; McFarland, H.F.; Cherukuri, M.; Mitchell, J.B.; Quandt, J.A. Oral administration of the nitroxide radical TEMPOL exhibits immunomodulatory and therapeutic properties in multiple sclerosis models. Brain Behav. Immun. 2017, 62, 332–343. [Google Scholar] [CrossRef]
- Fontes, L.B.A.; Dias, D.D.S.; Aarestrup, B.J.V.; Aarestrup, F.M.; Da Silva Filho, A.A.; Corrêa, J.O.D.A. β-Caryophyllene ameliorates the development of experimental autoimmune encephalomyelitis in C57BL/6 mice. Biomed. Pharmacother. 2017, 91, 257–264. [Google Scholar] [CrossRef]
- You, Z.; Timilshina, M.; Jeong, B.S.; Chang, J.H. BJ-2266 ameliorates experimental autoimmune encephalomyelitis through down-regulation of the JAK/STAT signaling pathway. Eur. J. Immunol. 2017, 47, 1488–1500. [Google Scholar] [CrossRef]
- Afraei, S.; D’Aniello, A.; Sedaghat, R.; Ekhtiari, P.; Azizi, G.; Tabrizian, N.; Magliozzi, L.; Aghazadeh, Z.; Mirshafiey, A. Therapeutic effects of D-aspartate in a mouse model of multiple sclerosis. J. Food Drug Anal. 2017, 25, 699–708. [Google Scholar] [CrossRef]
- Kamisli, S.; Ciftci, O.; Taslidere, A.; Basak Turkmen, N.; Ozcan, C. The beneficial effects of 18β-glycyrrhetinic acid on the experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mouse model. Immunopharmacol. Immunotoxicol. 2018, 40, 344–352. [Google Scholar] [CrossRef]
- Yang, E.J.; Song, I.S.; Song, K.S. Ethanol extract of Glycyrrhizae Radix modulates the responses of antigen-specific splenocytes in experimental autoimmune encephalomyelitis. Phytomedicine 2019, 54, 56–65. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Yang, Z.; Sui, R.; Miao, Q.; Li, Y.; Yu, J.; Liu, C.; Zhang, G.; Xiao, B.; et al. Therapeutic effect of oligomeric proanthocyanidin in cuprizone-induced demyelination. Exp. Physiol. 2019, 104, 876–886. [Google Scholar] [CrossRef]
- Aghaie, T.; Jazayeri, M.H.; Avan, A.; Anissian, A.; Salari, A.A. Gold nanoparticles and polyethylene glycol alleviate clinical symptoms and alter cytokine secretion in a mouse model of experimental autoimmune encephalomyelitis. IUBMB Life 2019, 71, 1313–1321. [Google Scholar] [CrossRef]
- Selek, S.; Esrefoglu, M.; Meral, I.; Bulut, H.; Caglar, H.G.; Sonuc, G.; Yildiz, C.; Teloglu, E.S.; Dogan, N.; Yuce, B.; et al. Effects of Oenothera biennis L. and Hypericum perforatum L. extracts on some central nervous system myelin proteins, brain histopathology and oxidative stress in mice with experimental autoimmune encephalomyelitis. Biotech. Histochem. 2019, 94, 75–83. [Google Scholar] [CrossRef]
- Fetisova, E.K.; Muntyan, M.S.; Lyamzaev, K.G.; Chernyak, B.V. Therapeutic Effect of the Mitochondria-Targeted Antioxidant SkQ1 on the Culture Model of Multiple Sclerosis. Oxid. Med. Cell. Longev. 2019, 2019, 2082561. [Google Scholar] [CrossRef]
- Khodaei, F.; Rashedinia, M.; Heidari, R.; Rezaei, M.; Khoshnoud, M.J. Ellagic acid improves muscle dysfunction in cuprizone-induced demyelinated mice via mitochondrial Sirt3 regulation. Life Sci. 2019, 237, 116954. [Google Scholar] [CrossRef]
- Khodaei, F.; Khoshnoud, M.J.; Heidaryfar, S.; Heidari, R.; Karimpour Baseri, M.H.; Azarpira, N.; Rashedinia, M. The effect of ellagic acid on spinal cord and sciatica function in a mice model of multiple sclerosis. J. Biochem. Mol. Toxicol. 2020, 34, e22564. [Google Scholar] [CrossRef]
- Pejman, S.; Kamarehei, M.; Riazi, G.; Pooyan, S.; Balalaie, S. Ac-SDKP ameliorates the progression of experimental autoimmune encephalomyelitis via inhibition of ER stress and oxidative stress in the hippocampus of C57BL/6 mice. Brain Res. Bull. 2020, 154, 21–31. [Google Scholar] [CrossRef]
- Li, W.; Deng, R.; Jing, X.; Chen, J.; Yang, D.; Shen, J. Acteoside ameliorates experimental autoimmune encephalomyelitis through inhibiting peroxynitrite-mediated mitophagy activation. Free Radic. Biol. Med. 2020, 146, 79–91. [Google Scholar] [CrossRef]
- Lazarević, M.; Battaglia, G.; Jevtić, B.; Đedović, N.; Bruno, V.; Cavalli, E.; Miljković, Đ.; Nicoletti, F.; Momčilović, M.; Fagone, P. Upregulation of Tolerogenic Pathways by the Hydrogen Sulfide Donor GYY4137 and Impaired Expression of H2S-Producing Enzymes in Multiple Sclerosis. Antioxidants 2020, 9, 608. [Google Scholar] [CrossRef]
- Nasrollahzadeh Sabet, M.; Biglari, S.; Khorram Khorshid, H.R.; Esmaeilzadeh, E. Shikonin ameliorates experimental autoimmune encephalomyelitis (EAE) via immunomodulatory.; anti-apoptotic and antioxidative activity. J. Pharm. Pharmacol. 2020, 72, 1970–1976. [Google Scholar] [CrossRef]
- Yamamoto, S.; Sakemoto, C.; Iwasa, K.; Maruyama, K.; Shimizu, K.; Yoshikawa, K. Ursolic acid treatment suppresses cuprizone-induced demyelination and motor dysfunction via upregulation of IGF-1. J. Pharmacol. Sci. 2020, 144, 119–122. [Google Scholar] [CrossRef]
- Hassani, M.; Soleimani, M.; Esmaeilzadeh, E.; Zare-Abdollahi, D.; Khorram Khorshid, H.R. Healing Influence of Melilotus Officinalis Herbal Extract on Experimental Autoimmune Encephalomyelitis in C57BL/6 Mice. Iran. J. Pharm. Res. 2020, 19, 321–329. [Google Scholar]
- Nasrnezhad, R.; Halalkhor, S.; Sadeghi, F.; Pourabdolhossein, F. Piperine Improves Experimental Autoimmune Encephalomyelitis (EAE) in Lewis Rats Through its Neuroprotective, Anti-inflammatory, and Antioxidant Effects. Mol. Neurobiol. 2021, 58, 5473–5493. [Google Scholar] [CrossRef]
- Zeinali, H.; Baluchnejadmojarad, T.; Roghani, M. Diosgenin ameliorates cellular and molecular changes in multiple sclerosis in C57BL/6 mice. Mult. Scler. Relat. Disord. 2021, 55, 103211. [Google Scholar] [CrossRef]
- Khan, A.; Shal, B.; Khan, A.U.; Bibi, T.; Islam, S.U.; Baig, M.W.; Haq, I.U.; Ali, H.; Ahmad, S.; Khan, S. Withametelin, a novel phytosterol, alleviates neurological symptoms in EAE mouse model of multiple sclerosis via modulation of Nrf2/HO-1 and TLR4/NF-κB signaling. Neurochem. Int. 2021, 151, 105211. [Google Scholar] [CrossRef]
- Dąbrowska-Bouta, B.; Strużyńska, L.; Sidoryk-Węgrzynowicz, M.; Sulkowski, G. Memantine Modulates Oxidative Stress in the Rat Brain following Experimental Autoimmune Encephalomyelitis. Int. J. Mol. Sci. 2021, 22, 11330. [Google Scholar] [CrossRef]
- Mancino, D.N.J.; Lima, A.; Roig, P.; García Segura, L.M.; De Nicola, A.F.; Garay, L.I. Tibolone restrains neuroinflammation in mouse experimental autoimmune encephalomyelitis. J. Neuroendocrinol. 2022, 34, e13078. [Google Scholar] [CrossRef]
- Rasool, R.; Ullah, I.; Shahid, S.; Mubeen, B.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Alzarea, S.I.; Murtaza, B.N.; Nadeem, M.S.; et al. In Vivo Assessment of the Ameliorative Impact of Some Medicinal Plant Extracts on Lipopolysaccharide-Induced Multiple Sclerosis in Wistar Rats. Molecules 2022, 27, 1608. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Sarawi, W.; Attia, S.M.; Alanazi, W.A.; Ibrahim, K.E.; Alsanea, S.; Alqarni, S.A.; Alfardan, A.S.; et al. Acetyl-11-keto-β-boswellic acid improves clinical symptoms through modulation of Nrf2 and NF-κB pathways in SJL/J mouse model of experimental autoimmune encephalomyelitis. Int. Immunopharmacol. 2022, 107, 108703. [Google Scholar] [CrossRef]
- Upadhayay, S.; Mehan, S.; Prajapati, A.; Sethi, P.; Suri, M.; Zawawi, A.; Almashjary, M.N.; Tabrez, S. Nrf2/HO-1 Signaling Stimulation through Acetyl-11-Keto-Beta-Boswellic Acid (AKBA) Provides Neuroprotection in Ethidium Bromide-Induced Experimental Model of Multiple Sclerosis. Genes 2022, 13, 1324. [Google Scholar] [CrossRef]
- Namazi, F.; Bordbar, E.; Bakhshaei, F.; Nazifi, S. The effect of Urtica dioica extract on oxidative stress, heat shock proteins, and brain histopathology in multiple sclerosis model. Physiol. Rep. 2022, 10, e15404. [Google Scholar] [CrossRef]
- Naeem, A.G.; El-Naga, R.N.; Michel, H.E. Nebivolol elicits a neuroprotective effect in the cuprizone model of multiple sclerosis in mice, emphasis on M1/M2 polarization and inhibition of NLRP3 inflammasome activation. Inflammopharmacology 2022, 30, 2197–2209. [Google Scholar] [CrossRef]
- Esmaeilzadeh, E.; Soleimani, M.; Khorram Khorshid, H.R. Protective effects of Herbal Compound (IM253) on the inflammatory responses and oxidative stress in a mouse model of multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 67, 104076. [Google Scholar] [CrossRef]
- Moradi, V.; Esfandiary, E.; Ghanadian, M.; Ghasemi, N.; Rashidi, B. The effect of Zingiber Officinale Extract on Preventing Demyelination of Corpus Callosum in a Rat Model of Multiple Sclerosis. Iran. Biomed. J. 2022, 26, 330–339. [Google Scholar] [CrossRef]
- Kamankesh, F.; Ganji, A.; Ghazavi, A.; Mosayebi, G. The Anti-inflammatory Effect of Ginger Extract on the Animal Model of Multiple Sclerosis. Iran. J. Immunol. 2023, 20, 211–218. [Google Scholar]
- Mabrouk, M.; El Ayed, M.; Démosthènes, A.; Aissouni, Y.; Aouani, E.; Daulhac-Terrail, L.; Mokni, M.; Bégou, M. Antioxidant effect of grape seed extract corrects experimental autoimmune encephalomyelitis behavioral dysfunctions, demyelination, and glial activation. Front. Immunol. 2022, 13, 960355. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.Y.; Yang, Z.C.; Yuan, H.J.; Dong, Y.W.; Miao, Q.; Li, Y.Q.; Wang, J.; Yu, J.Z.; Xiao, B.G.; et al. Grape Seed Extract Attenuates Demyelination in Experimental Autoimmune Encephalomyelitis Mice by Inhibiting Inflammatory Response of Immune Cells. Chin. J. Integr. Med. 2023, 29, 394–404. [Google Scholar] [CrossRef]
- Soltanmohammadi, A.; Tavaf, M.J.; Zargarani, S.; Yazdanpanah, E.; Sadighi-Moghaddam, B.; Yousefi, B.; Sameni, H.R.; Haghmorad, D. Daphnetin alleviates experimental autoimmune encephalomyelitis by suppressing Th1 and Th17 cells and upregulating Th2 and regulatory T cells. Acta Neurobiol. Exp. 2022, 82, 273–283. [Google Scholar] [CrossRef]
- Stegnjaić, G.; Tsiailanis, A.D.; Lazarević, M.; Gkalpinos, V.K.; Djedovic, N.; Antoniou, T.; Stanisavljević, S.; Dimitrijević, M.; Momčilović, M.; Miljković, Đ.; et al. Phenethyl Ester of Gallic Acid Ameliorates Experimental Autoimmune Encephalomyelitis. Molecules 2022, 27, 8770. [Google Scholar] [CrossRef]
- Samani, S.A.; Moloudi, M.R.; Ramezanzadeh, R.; Abdi, M.; Nikkhoo, B.; Izadpanah, E.; Roshani, D.; Abdolahi, A.; Esmaili, P.; Hassanzadeh, K. Oral Administration of Probiotic Enterococcus durans to Ameliorate Experimental Autoimmune Encephalomyelitis in Mice. Basic Clin. Neurosci. 2022, 13, 35–46. [Google Scholar] [CrossRef]
- Xu, Z.; Lu, S.; Liu, X.; Tang, L.; Liu, Z.; Cui, J.; Wang, W.; Lu, W.; Huang, J. Drug repurposing of ilepcimide that ameliorates experimental autoimmune encephalomyelitis via restricting inflammatory response and oxidative stress. Toxicol. Appl. Pharmacol. 2023, 458, 116328. [Google Scholar] [CrossRef]
- Feinshtein, V.; Alfahel, L.; Israelson, A.; Bernstein, N.; Gorelick, J.; Ben-Shabat, S. Therapeutic Potential of Phytocannabinoid Cannabigerol for Multiple Sclerosis, Modulation of Microglial Activation In Vitro and In Vivo. Biomolecules 2023, 13, 376. [Google Scholar] [CrossRef]
- Khosravi-Nezhad, S.; Hassanpour, S.; Hesaraki, S. L-Theanine Improves Locomotor Function in a Model of Multiple Sclerosis Mice. Arch. Razi Inst. 2023, 78, 195–203. [Google Scholar]
- Haindl, M.T.; Üçal, M.; Wonisch, W.; Lang, M.; Nowakowska, M.; Adzemovic, M.Z.; Khalil, M.; Enzinger, C.; Hochmeister, S. Vitamin D-An Effective Antioxidant in an Animal Model of Progressive Multiple Sclerosis. Nutrients 2023, 15, 3309. [Google Scholar] [CrossRef]
- Lunin, S.M.; Novoselova, E.G.; Glushkova, O.V.; Parfenyuk, S.B.; Kuzekova, A.A.; Novoselova, T.V.; Sharapov, M.G.; Mubarakshina, E.K.; Goncharov, R.G.; Khrenov, M.O. Protective effect of exogenous and thymic peptide thymulin on BBB conditions in an experimental model of multiple sclerosis. Arch. Biochem. Biophys. 2023, 746, 109729. [Google Scholar] [CrossRef]
- Hasaniani, N.; Ghasemi-Kasman, M.; Halaji, M.; Rostami-Mansoor, S. Bifidobacterium breve Probiotic Compared to Lactobacillus casei Causes a Better Reduction in Demyelination and Oxidative Stress in Cuprizone-Induced Demyelination Model of Rat. Mol. Neurobiol. 2024, 61, 498–509. [Google Scholar] [CrossRef]
- Fan, H.; Yang, Y.; Bai, Q.; Wang, D.; Shi, X.; Zhang, L.; Yang, Y. Neuroprotective Effects of Sinomenine on Experimental Autoimmune Encephalomyelitis via Anti-Inflammatory and Nrf2-Dependent Anti-Oxidative Stress Activity. Neuromolecular Med. 2023, 25, 545–562. [Google Scholar] [CrossRef]
- Al-Kharashi, L.A.; Al-Harbi, N.O.; Ahmad, S.F.; Attia, S.M.; Algahtani, M.M.; Ibrahim, K.E.; Bakheet, S.A.; Alanazi, M.M.; Alqarni, S.A.; Alsanea, S.; et al. Auranofin Modulates Thioredoxin Reductase/Nrf2 Signaling in Peripheral Immune Cells and the CNS in a Mouse Model of Relapsing-Remitting EAE. Biomedicines 2023, 11, 2502. [Google Scholar] [CrossRef]
- Safwat, S.M.; El Tohamy, M.; Aboonq, M.S.; Alrehaili, A.; Assinnari, A.A.; Bahashwan, A.S.; ElGendy, A.A.; Hussein, A.M. Vanillic Acid Ameliorates Demyelination in a Cuprizone-Induced Multiple Sclerosis Rat Model: Possible Underlying Mechanisms. Brain Sci. 2023, 14, 12. [Google Scholar] [CrossRef]
- Muñoz-Jurado, A.; Escribano, B.M.; Galván, A.; Valdelvira, M.E.; Caballero-Villarraso, J.; Giraldo, A.I.; Santamaría, A.; Luque, E.; Agüera, E.; LaTorre, M.; et al. Neuroprotective and antioxidant effects of docosahexaenoic acid (DHA) in an experimental model of multiple sclerosis. J. Nutr. Biochem. 2024, 124, 109497. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Shaheen, A.M.; Zaki, H.F.; Rabie, M.A. Nicorandil and carvedilol mitigates motor deficits in experimental autoimmune encephalomyelitis-induced multiple sclerosis, Role of TLR4/TRAF6/MAPK/NF-κB signalling cascade. Int. Immunopharmacol. 2024, 127, 111387. [Google Scholar] [CrossRef]
- Prajapati, A.; Mehan, S.; Khan, Z.; Chhabra, S.; Das Gupta, G. Purmorphamine, a Smo-Shh/Gli Activator, Promotes Sonic Hedgehog-Mediated Neurogenesis and Restores Behavioural and Neurochemical Deficits in Experimental Model of Multiple Sclerosis. Neurochem. Res. 2024, 49, 1556–1576. [Google Scholar] [CrossRef]
- Ashrafpour, S.; Nasr-Taherabadi, M.J.; Sabouri-Rad, A.; Hosseinzadeh, S.; Pourabdolhossein, F. Arbutin intervention ameliorates memory impairment in a rat model of lysolecethin induced demyelination, Neuroprotective and anti-inflammatory effects. Behav. Brain Res. 2024, 469, 115041. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Phan, N.M.; Kim, J. Administration of ROS-Scavenging Cerium Oxide Nanoparticles Simply Mixed with Autoantigenic Peptides Induce Antigen-Specific Immune Tolerance against Autoimmune Encephalomyelitis. ACS Appl. Mater Interfaces 2024, 16, 33106–33120. [Google Scholar] [CrossRef]
- Ahmadi, A.; Habibi, G.; Farrokhnia, M. MS14, an Iranian herbal-marine compound for the treatment of multiple sclerosis. Chin. J. Integr. Med. 2010, 16, 270–271. [Google Scholar] [CrossRef]
- Mauriz, E.; Laliena, A.; Vallejo, D.; Tuñón, M.J.; Rodríguez-López, J.M.; Rodríguez-Pérez, R.; García-Fernández, M.C. Effects of a low-fat diet with antioxidant supplementation on biochemical markers of multiple sclerosis long-term care residents. Nutr. Hosp. 2013, 28, 2229–2235. [Google Scholar]
- Aghamohammadi, D.; Ayromlou, H.; Dolatkhah, N.; Jahanjoo, F.; Shakouri, S.K. The effects of probiotic Saccharomyces boulardii on the mental health, quality of life, fatigue, pain, and indices of inflammation and oxidative stress in patients with multiple sclerosis: Study protocol for a double-blind randomized controlled clinical trial. Trials 2019, 20, 379. [Google Scholar]
- Izadi, M.; Tahmasebi, S.; Pustokhina, I.; Yumashev, A.V.; Lakzaei, T.; Alvanegh, A.G.; Roshangar, L.; Dadashpour, M.; Yousefi, M.; Ahmadi, M. Changes in Th17 cells frequency and function after ozone therapy used to treat multiple sclerosis patients. Mult. Scler. Relat. Disord. 2020, 46, 102466. [Google Scholar] [CrossRef]
- Vezzoli, A.; Mrakic-Sposta, S.; Dellanoce, C.; Montorsi, M.; Vietti, D.; Ferrero, M.E. Chelation Therapy Associated with Antioxidant Supplementation Can Decrease Oxidative Stress and Inflammation in Multiple Sclerosis: Preliminary Results. Antioxidants 2023, 12, 1338. [Google Scholar] [CrossRef]
- Moravejolahkami, A.R.; Chitsaz, A.; Hassanzadeh, A.; Paknahad, Z. Anti-inflammatory-antioxidant modifications and synbiotics improved health-related conditions in patients with progressive forms of multiple sclerosis: A single-center, randomized clinical trial. Complement. Ther. Clin. Pract. 2023, 53, 101794. [Google Scholar] [CrossRef]
- Hajiluian, G.; Karegar, S.J.; Shidfar, F.; Aryaeian, N.; Salehi, M.; Lotfi, T.; Farhangnia, P.; Heshmati, J.; Delbandi, A.A. The effects of Ellagic acid supplementation on neurotrophic, inflammation, and oxidative stress factors, and indoleamine 2, 3-dioxygenase gene expression in multiple sclerosis patients with mild to moderate depressive symptoms: A randomized, triple-blind, placebo-controlled trial. Phytomedicine 2023, 121, 155094. [Google Scholar]
- Asghari, K.M.; Dolatkhah, N.; Ayromlou, H.; Mirnasiri, F.; Dadfar, T.; Hashemian, M. The effect of probiotic supplementation on the clinical and para-clinical findings of multiple sclerosis, a randomized clinical trial. Sci. Rep. 2023, 13, 18577. [Google Scholar] [CrossRef] [PubMed]
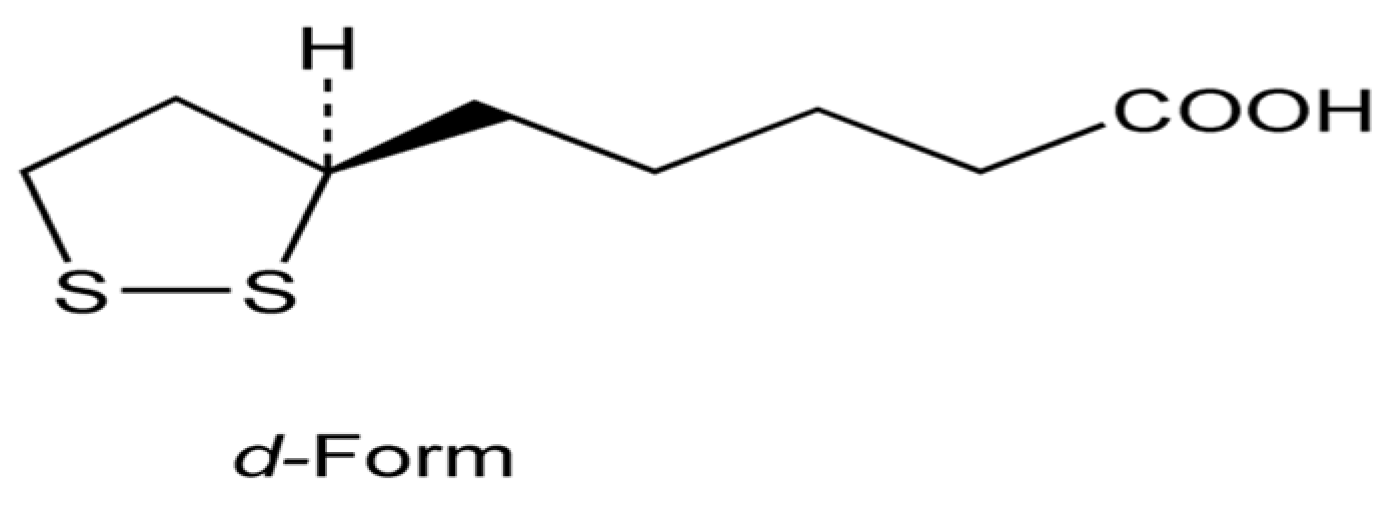




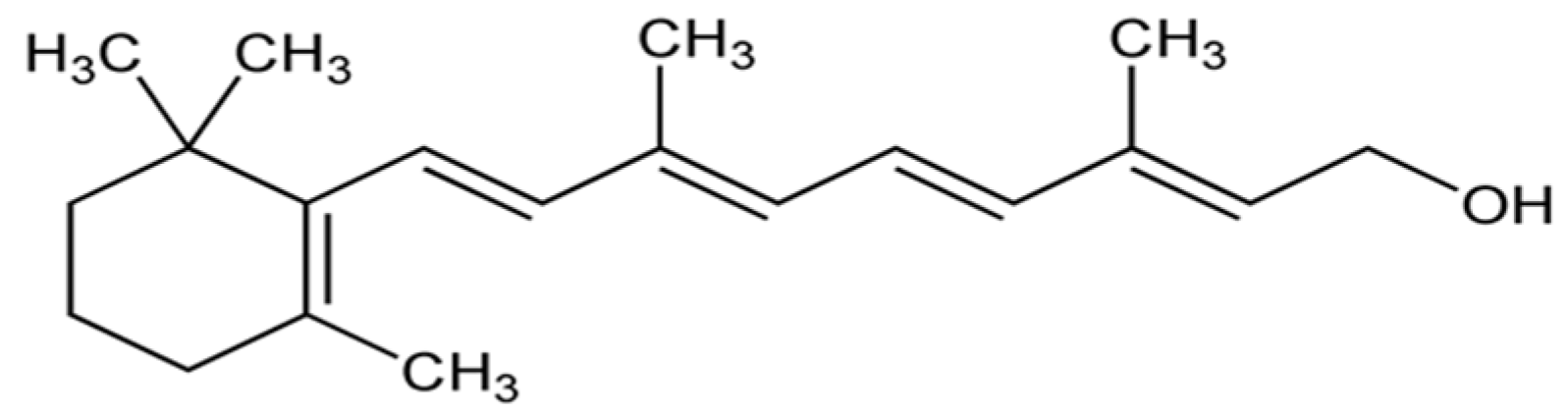

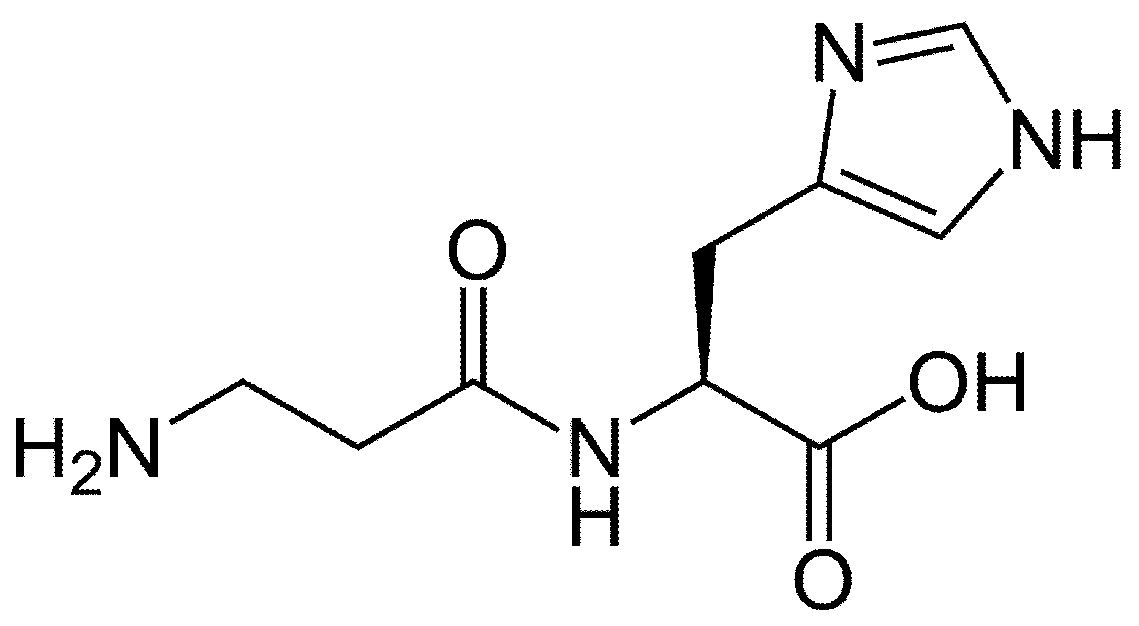

| Drug Class | Author, Year [Ref] | Animal Model | Main Findings |
|---|---|---|---|
| Combined matrix metalloproteinase (MMP) and TNF-alpha inhibitor | Clements et al., 1997 [203] | Male Lewis rats aged 5–8 weeks with EAE | Reduction of clinical signs and weight loss, a decrease in MMP activity in the CSF, a marked increase of the MMP matrilysin, and a modest increase in the MMP 92 kDa in the spinal cord. |
| Matrix metalloproteinase (MMP) inhibitor | Liedtke et al., 1998 [204] | SJL/J mice with EAE | Clinical improvement (blocking and reversal of acute disease, reduced number of relapses, improvement in clinical scores). Significant decrease in demyelination and glial scarring and in central nervous system gene expression for TNF alpha and fasL and an increase in IL-4 expression. |
| EUK-8 (synthetic catalytic scavenger of oxygen-reactive metabolites) | Malfroy et al., 1997 [205] | PL/J (H-2u) mice aged 3–8 months with EAE | Complete recovery after 40 days of EAE mice pretreated with EUK-8 and significant improvement after treatment with EUK-8 4 days after EAE induction. |
| Drugs acting on heme oxygenase 1 (HO-1) | Liu et al., 2001 [206] | Adult male Lewis rats with EAE | Hemin (HO-1 inducer) improved the clinical course of EAE and reduced inflammatory changes in the spinal cord, while tin mesoporphyrin (HO-1 inhibitor) worsened the clinical course of EAE and increased non-significantly inflammatory changes in the spinal cord. |
| t-butyl hydroxy anisole (phase 2 enzyme inducer) | Mohamed et al., 2002 [207] | Lewis rats aged 10–12 weeks with EAE | Clinical improvement of EAE, reduction in perivascular lymphocyte infiltration, and increase in red cell GSH concentrations. |
| Thymoquinone | Mohamed et al., 2002 [208] | Female Lewis rats with EAE | Prevention (if administered previously to EAE induction) or reversal (if administered after EAE induction) of clinical symptoms, perivascular inflammation, and decrease in spinal cord GSH levels. |
| Silibinin (Silybum marianum fruit extract) | Min et al., 2007 [209] | Female, 6-week-old C57BL/6 mice | Prevention or attenuation of clinical signs. Reduction of histological signs of demyelination and inflammation in the spinal cord. Down-regulation of the inflammatory cytokines IL-12 and IL-2 and slight upregulation of the anti-inflammatory cytokine IL-4 in a concentration-dependent manner in splenocytes. |
| ABS-75 (fullerene derivative) | Basso et al., 2008 [210] | Non-obese diabetic (NOD) mice with EAE | Clinical improvement associated with reduced axonal loss and demyelination, and halted oxidative injury, CD11b+ infiltration, and CCL2 expression in the spinal cord. |
| GEMSP (mixture of fatty acids, vitamins, and amino acids or their derivatives; and Poly-L-Lysine) | Mangas et al., 2008 [211] | 10–11 weeks Lewis 1A female rats with EAE | Complete clinical reversal of EAE, reduction in brain leukocyte infiltration, and preserved immunoreactivity to conjugated methionine antibodies in the motoneurons of the ventral horn. |
| Genistein (derivative of soy) | De Paula et al., 2008 [212] | 8–12 weeks old female C57BL/6 mice with EAE | Significant improvement in clinical symptoms, modulating pro- and anti-inflammatory cytokines in the CNS and splenocytes (upregulation of IL-10 and downregulation of IFN-gamma, TNF-alpha, and IL-12), and decreasing rolling and adhering of leukocytes to the CNS. |
| Tempamine (TMN) encapsulated in the intraliposomal aqueous phase of pegylated nanoliposomes | Kizelsztein et al., 2009 [213] | Female C57BL/6 mice with EAE | Significant improvement of clinical symptoms and reduction in the expression of mRNA for IFN-gamma and TNF-alpha in the brain. |
| Ethanol extract of saffron (Crocus sativus L.) | Ghazavi et al., 2009 [214] | 8-week-old male C57BL/6 mice with EAE | Significant clinical improvement, decrease of inflammatory changes in the brain, increase in serum TAC, and non-significant changes in serum NO levels. |
| Ethylene diamine tetra acetic acid (EDTA) | Mosayebi et al., 2010 [215] | 6–8-week-old male C57BL/6 mice with EAE | Significant delay in the time of onset and a significant reduction in severity of the EAE. Reduction in inflammatory changes and density of mononuclear infiltration in the CNS. Increase in total serum antioxidant power, decrease in serum NO levels and decrease in levels of IFN-gamma in cellular cultures of splenocytes. |
| Aloe vera | Mirshafiey et al., 2010 [216] | 6–8-week-old male C57BL/6 mice with EAE | Significant delay in the time of onset and a significant reduction in severity of the EAE. Reduction in inflammatory changes and density of mononuclear infiltration in the CNS. Increase in total serum antioxidant power, decrease in serum NO levels and decrease in levels of IFN-gamma in cellular cultures of splenocytes. |
| Erythropoietin | Chen et al.,, 2010 [217] | 6–8-week-old male C57BL/6 mice with EAE | Significant clinical improvement. Significant increase in expression of HO-1 mRNA in the spleen and the CNS. Significantly decrease in the expression of interferon-γ, interleukin (IL)-23, IL-6, and IL-17 mRNA, and increase in the expression of IL-4 and IL-10 mRNA in CNS. Significant decrease in the ratio of both T helper type 1 (Th1) and Th17 lymphocyte subsets isolated from CNS and an increase in the ratio of splenic regulatory CD4 T cells. |
| Hydralazine (acrolein scavenger) | Leung et al., 2011 [218] | 8-week-old male C57BL/6 mice with EAE | Significant delay and improvement of clinical symptoms. Significant decrease of demyelination in the spinal cord. |
| Spermidine | Guo et al., 2011 [219] | 6–8-week-old female C57BL/6 mice with EAE | Significant improvement of clinical symptoms, including optic neuritis. Significant improvement of demyelination in the spinal cord and optic nerve and prevention of cell loss and apoptosis in the retinal ganglion cell layer. |
| L-cycloserine (inhibitor of Ceramide synthase 6, CS6) | Schiffmann et al., 2012 [220] | 8–10 week-old female C57BL/6 or IFN-γ KO with EAE | Remission of the disease related to prevention of the increase in C(16:0)-Cer (reflecting a decrease in CS6 expression) and iNOS/TNF-α expression. |
| Aqueous extract of North American ginseng | Bowie et al., 2012 [221] | C57BL/6 6 week-olf female mice with EAE | Significant decrease in clinical signs of EAE, levels of circulating TNF-α, and CNS immunoreactive iNOS and demyelination scores. |
| Safinamide and flecainide (sodium channel-blocking agents) | Morsalli et al., 2013 [222] | Male and female Dark Agouti rats | Pretreatment and treatment with these drugs caused significant improvement of neurological deficits and protection against neurological deficit and axonal degeneration associated with reduction in the CNS of the activation of microglia/macrophages. |
| MitoQ (mitochondria-targeted antioxidant) | Mao et al., 2013 [223] | 10-week-old C57BL/6 mice with EAE | Pretreatment and treatment with this drug caused significant improvement of neurological deficits, reversed the increase of mRNA expression of CD4, CD8, CD11b, IL1, IL6, IL10, IL17, TNFα, NOS2, and NFkB in the spinal cord, and induced upregulation of the anti-apoptotic gene STAT3 in the spinal cord. |
| Sulforaphane | Li et al., 2013 [224] | 8–10 week-old female C57BL/6 mice with EAE | Inhibition of development and severity of EAE. Decrease of inflammation and demyelination in the spinal cord. Prevention or reversion of the increased MDA levels expression of MMP-9 and decreased expression of Nrf2, HO-1, and NQO1 in the brain caused by EAE. Reversion of increased IL-17A-secreting Th17 cells in splenocytes. |
| Astragaloside IV (a natural saponin molecule) | He et al., 2013 [225] | 6-week-old female C57BL/6 mice with EAE | Improvement of clinical signs of EAE. Inhibition or reversion of the increase of ROS and pro-inflammatory cytokine levels, downregulation of SOD and GSH-Px activities, increase of iNOS, p53, and phosphorylated tau, and decrease of ratio of Bcl-2/Bax in CNS caused by EAE |
| Β-asarone modified astragaloside IV (a natural saponin molecule) | Zhao et al., 2023 [226] | 6–8 week-old female C57BL/6 mice | Improvement of clinical signs of EAE. Suppression of inflammatory infiltration and astrocyte/microglial activation and reduction of demyelination in the brain. |
| R-flurbiprofen | Schmitz et al., 2014 [227] | 10–12 week old female C57BL6/J mice with primary progressive EAE and 10–12 week female SLJ mice with relapsing-remitting EAE in SJL mice | Prevention of attenuation of clinical signs in both models of EAE. Decrease of immune cell infiltration and microglia activation and inflammation in the spinal cord, brain, and optic nerve and attenuation of myelin destruction and EAE-evoked hyperalgesia. Increase of CD4(+)CD25(+)FoxP3(+) regulatory T cells, CTLA4(+) inhibitory T cells, and IL-10, Important decrease of upregulation of pro-inflammatory genes in the spinal cord. Association of these effects to the increase of plasma and cortical endocannabinoids and decreased spinal prostaglandins. |
| Apocynin (NADPH oxidase assembly inhibitor) | Choi et al., 2015 [228] | Female C57BL/6 mice | Significantly marked clinical improvement of EAE associated with suppression of demyelination, reduced infiltration by CD4, CD8, CD20, and F4/80-positive cells, reduced induction of pro-inflammatory cytokines in cultured microglia, and inhibition of free radicals production and blood–brain barrier disruption. |
| Flavocoxid (dual cyclooxygenase and 5-lipoxygenase inhibitor) | Kong et al. 2016 [229] | Female C57BL/6 mice 6–10 weeks old | Significant clinical improvement of EAE. Decrease in COX2, 5-LO, IL12, IL23, IL17, IFNγ and increase in IL10 in the spinal cord. Decrease of MHCII, CD40, CD80, CD86, COX2, 5-LO, IL12 and IL6, and increase of IL-10 in primary macrophages. Increase of Arg1, Ym1, CD206, TGFβ1, IL10 and decrease of iNOS and IL-12 in macrophages and microglia. Inhibition of Th1/Th17 differentiation. |
| Hydrogen-rich water | Zhao et al., 2016 [230] | C57BL/6 mice 6–8 weeks old | Significant clinical improvement of EAE (delay and reduction of clinical severity). Inhibition of inflammatory infiltration and demyelination of CNS. Prevention of infiltration by CD4+ population and inhibition of Th17 cell differentiation in CNS and peripheral immune organs. |
| Arctigenin (lignan present in the extract from Arctium lappa) | Li et al., 2016 [231] | Female C57BL/6 mice 6–8 weeks old | Significant clinical improvement of EAE. Decreased inflammation and demyelination, inhibition of Th1 and Th17 cells in peripheral immune organs. Suppression of the Th1 cytokine IFN-γ, the transcription factor T-bet, the Th17 cytokines IL-17A, IL-17F, and the transcription factor ROR-γt. Inhibition of Th17 cells (but not Th1 cells) in the CNS. Activation of s AMPK, inhibition of phosphorylated p38, and upregulation of PPAR-γ. |
| 3H-1,2-dithiole-3-thione (D3T, a potent inducer of antioxidant genes and glutathione biosynthesis) | Kuo et al., 2016 [232] | C57BL/6 mice and Nrf2 deficient mice (Nrf2−/−) with its corresponding controls (Nrf2+/−) of 7–10 weeks of age | Significant clinical improvement of EAE. Suppression of the activation of dendritic cells. Suppression of IL-23 and co-stimulatory molecules in dendritic cells through induction of Nrf2. Inhibition of Th1 and Th17 differentiation and activation of microglia. |
| Pramipexole (D2/D3 receptor agonist with antioxidant actions) | Lieberknecht et al., 2017 [233] | Female C57BL/6 mice with EAE | Prevention of development of clinical signs of EAE. Blocking of neuroinflammatory response, demyelination, and astroglial activation in spinal cord. Inhibition of the production of inflammatory cytokines (IL-17, IL-1β, and TNF-α) in peripheral lymphoid tissue. Reversion of effects of EAE in reactive oxygen species, glutathione peroxidase, parkin, and α-synuclein in the spinal cord and striatum. |
| Despramipexole | Buonvicino et al., 2020 [234] | Female non-obese diabetic NOD/ShiLtj and C57BL/6 mice with EAE | Delay in progression of clinical symptoms. Increase in survival, counteracted reduction of spinal cord mitochondrial DNA content, and reduced spinal cord axonal loss of mice. |
| TEMPOL (4-hydroxy-2,2,6,6-tetramethyl-piperidine-1-oxyl, membrane-permeable radical scavenger) | Neil et al., 2017 [235] | Female C57BL/6J (Jackson Labs) or SJL/J of 8–10 weeks of age | Prevention of EAE by delayed onset, reduced incidence, and reduction of clinical severity of the disease. Decrease in microglial activation and fewer inflammatory infiltrates and axonal damage. Decrease in production of INF-γ and TNF-α by T-cells, decrease in levels of MHC class II expression and CD40 in myeloid and myeloid-dendritic cells. Increase in CD8+ T cell populations and CD4+FoxP3+ regulatory populations. |
| β-Caryophyllene (sesquiterpene derivative) | Fontes et al., 2017 [236] | Female C57BL/6 mice 8–12 weeks old | Significant improvement of clinical score and severity of EAE. Significant decrease in the number of inflammatory infiltrates and attenuation of neurological damage. Inhibition of H2O2, NO, TNF-α, IFN-γ, and IL-17 production. |
| BJ-2266 (6-thioureido-derivative) | You et al., 2017 [237] | C57BL/6 mice and OT-II mice | Significant suppression of EAE disease progression. Reduction of generation of Th1 and Th17 cells through inhibition of JAK/STAT phosphorylation. |
| D-aspartate | Afraei et al., 2017 [238] | Female C57BL/6 mice 8 weeks old | Significant attenuation in the severity and delay in the onset of the disease. Reduction of demyelination, inflammation, and degeneration in the CNS. Significant decrease in serum IL-6 levels. Non-significant increase in SOD and glutathione-reductase activities and TAC in serum. |
| 18β-glycyrrhetinic acid | Kamisli et al., 2018 [239] | Female C57BL/6 mice | Significant clinical improvement. Reversion of histological and immunological alterations caused by EAE. Increased brain levels of TBARS levels and decreased GPx, SOD, CAT, and GSH levels in brain tissue. Decreased caspase-3 and IL-17 activity in brain tissue. |
| Ethanol extract of Glycyrrhizae Radix | Yang et al., 2019 [240] | Female C57BL/6 mice aged 5 weeks | Clinical improvement of EAE signs associated with a decrease of antigen-specific TH1 and TC1 populations and an increase of CD8+IL-17A+ (TC17) population. Reduction in IFN-γ+ T cell populations, the expressions of cell adhesion molecules (CAMs), and secretions of cytokines containing IFN-γ and a chemokine IFN-γ-induced protein 10 (IP-10) in splenocytes in culture. Decrease in NO production and secretion of TNF-α and IP-10 in IFN-γ-stimulated BV2 cells in culture. |
| Oligomeric proanthocyanidin (OPC), the most effective component of grape seed extract | Wang et al., 2019 [241] | Male C57BL/6J mice with cuprizone-induced demyelination | Attenuation of abnormal behavior reduced demyelination and increased expression of myelin basic protein and expression of O4+ oligodendrocytes in the corpus callosum. Reduction in numbers of B and T cells, activation of microglia in the corpus callosum, and inhibition of secretion of inflammatory factors. |
| Gold nanoparticles and polyethylene glycol | Aghaie et al., 2019 [242] | Female C57BL/6 mice aged 8–10 weeks with EAE | Significant decrease in the severity of EAE symptoms. Significant decrease in the number and severity of cells’ infiltration and demyelinated lesions in the spinal cord. Significant increase in IL-27 and decrease in IL-23 levels (only for gold nanoparticles). |
| Oenothera biennis L. and Hypericum perforatum L. extracts | Selek et al., 2019 [243] | 8–10-week-old C57BL/6J mice | Significant decrease in the severity of EAE symptoms. Significant decrease in brain tissue MOG and MBP. Significant decrease in the TOS and OSI values and increase in TAS in brain tissue. Significant decrease in myelin loss and amyloid deposition on vascular walls, in the cytoplasm of the neurons, and in the intercellular space. |
| Mitochondria-Targeted Antioxidant SkQ1 | Fetisova et al., 2019 [244] | In vitro MS model of the primary oligodendrocyte culture of the cerebellum, challenged with lipopolysaccharide (LPS). | SkQ1 accumulated in the mitochondria of oligodendrocytes and microglial cells and prevented LPS-induced inhibition of myelin production in oligodendrocytes. |
| Ellagic acid (a polyphenol found in numerous fruits and vegetables with antioxidant actions) | Khodaei et al., 2019 [245,246] | 6–9 week-old male C57BL/6 mice with cuprizone-induced demyelination | Significant improvement of EAE symptoms. Decrease in MDA levels, increase in TAC, and GSH/GSSG ratio in muscle tissue. Increase in mitochondrial dehydrogenase. SOD and catalase activities, mitochondrial ATP levels and mitochondrial GSH/GSSG ratio, and decrease in mitochondrial depolarization in muscle tissue. Increase in mitochondrial complex I, II, and IV activities in Sirt3 level and Sirt3 expression in muscle tissue. |
| Tetrapeptide N-acetyl-ser-asp-lys-pro (Ac-SDKP) | Pegman et al., 2020 [247] | 10-week-old female C57BL/6 mice with EAE | Significant improvement of EAE symptoms. Decrease in inflammatory infiltration and demyelination in hippocampus oligodendrocytes. Reduction of reactive oxygen species, MDA levels, and NOS production. Increase in GSH levels, GPx and HO-1, and total antioxidant activities. Decrease in caspase-12 and CHOP expression in the hippocampus-resident oligodendrocytes. Decrease in the activation of the proinflammatory cytokines IL-6 and IL-1β and increase in levels of the anti-apoptotic proteinBcl2. |
| Acteoside (AC), an active compound from the medicinal herb Radix rehmanniae | Li et al., 2020 [248] | 10–14-week-olf female C57BL/6 N mice with EAE | Significant improvement and delay of EAE symptoms. Inhibition of inflammation/demyelination, and peripheral activation and CNS infiltration of encephalitogenic CD4+ T cells and CD11b+ activated microglia/macrophages in the spinal cord Decrease in peroxynitrite production down-regulation of the expression of iNOS and NADPH oxidases, and inhibition of neuronal apoptotic cell death and mitochondrial damage in the spinal cords. |
| GYY4137 (Hydrogen Sulfide Donor) | Lazarević et al., 2020 [249] | Dark Agouti rats or C57BL/6 mice with EAE | Increase in TGF-β expression and production in dendritic cells and significant reduction of IFN-γ and IL-17 production in the lymph node and spinal cord T cells obtained from mice immunized with CNS antigens. Decrease in the proportion of FoxP3+ regulatory CD4+ T cells in the lymph node cells and the percentage of IL-17+ CD4+ T cells in the spinal cord cells after culturing with GYY4137. |
| Shikonin (extract from plants of the Boraginaceae family) | Nasrollahzadeh Sabet et al. 2020 [250] | Female C57BL/6 mice aged 10–12 weeks with EAE | Significant improvement and delay of EAE symptoms. Significant decrease in the extent of demyelination. Significant reduction in the expression levels of TNF-α, IFN-γ, and Bax, and increase of levels of TGF-β and Bcl2 and the activity of Gpx1. |
| Ursolic acid (pentacyclic triterpenoid compound) | Yamamoto et al., 2020 [251] | Male C57BL/6J mice with cuprizone-induced demyelination | Significant improvement in motor dysfunction and demyelination. Increase of IGF-1 levels in the demyelinating lesions. |
| Herbal extract of Melilotus officinalis | Hassani et al., 2020 [252] | Female C57BL/6 mice with EAE | Prevention or attenuation of the clinical signs of the disease. Decreased demyelination in the corpus callosum. Significant decrease in the gene expression of pro-inflammatory cytokines (IL-6, TNF-α, and IFN-γ). Increase in the gene expression of GPx and CAT. |
| Piperine, a main bioactive alkaloid of black pepper | Nasrnezhad et al., 2021 [253] | Female Lewis rats with EAE | Significant improvement in neurological deficits and progression. Significant decrease in the extent of demyelination, inflammation, immune cell infiltration, microglia, and astrocyte activation in the spinal cord. Significant decrease in the gene of pro-inflammatory cytokines (TNF-α, IL-1β) and iNOS and enhanced IL-10, Nrf2, HO-1, and MBP expressions. Increase in TAC and reduction of MDA levels in the CNS. Reduction of caspase-3 (apoptosis marker) and enhancement of brain-derived neurotrophic factor (BDNF) and NeuN-expressing cells in the CNS. |
| Diosgenin (herbal from Dioscora species) | Zeinali et al., 2021 [254] | Female C57BL/6 mice aged 10–12 weeks with EAE | Significant clinical improvement. Decrease of inflammation, demyelination, and axonal degeneration severity in lumbar spinal cord in EAE. Lack of effect on modification of neuroprotective factors decreased by EAE. Decrease in serum and tissue levels of TNFα and MMP-9 with no effect on increased level of IL-17 in EAE. |
| Withametelin (phytosterol derivative) | Khan et al., 2021 [255] | Female Swiss mice of 8–12 weeks of age with EAE | Significant clinical improvement. Reversion of histopathological alteration of the brain, spinal cord, eye, and optic nerve. Reduction of H2O2-induced cytotoxicity and oxidative stress in a dose-dependent manner. Increase in the expression level of nuclear factor-erythroid-related factor-2 (Nrf2), heme-oxygenase-1 (HO-1), and reduction of the expression level of the Kelch-like-ECH-associated-protein-1 (keap-1), inducible-nitric-oxide-synthase (iNOS) in the CNS. Decrease in the expression level of toll-like-receptor 4 (TLR4), nuclear-factor-kappa-B (NF-κB), and increase in the expression level of IkB-α in the CNS. |
| Memantine (uncompetitive NMDA receptor antagonist) | Dąbrowska-Bouta et al. 2021 [256] | Female Lewis rats with EAE | Significant clinical improvement. Significant decrease in MDA levels and SOD1 and SOD2 expression, and increase in –SH groups levels in brain tissue. |
| Tibolone (synthetic steroid whose metabolites have potent antioxidant action) | Mancino et al., 2022 [257] | Female C57BL/6 mice with EAE | Significant clinical improvement. Reversion of the astrocytic and microglial reaction and reduction of the hyperexpression of TLR4, HMGB1, and NLR family pyrin domain containing 3-caspase 1-interleukin-1β inflammasome activation in the spinal cord. Attenuation of the Akt/nuclear factor kappa B pathway and decrease in the white matter demyelination area in the spinal cord. |
| Herbal plants Nepeta hindustana L., Vitex negundo L., and Argemone albiflora L. | Rasool et al., 2022 [258] | Wistar rats with lipopolysaccharide-induced MS | Neuroprotective effect regarding remyelination of axonal terminals and oligodendrocytes migration reduced lymphocytic infiltrations and reduced necrosis of Purkinje cells in histopathological examination. Reverse of changes in serum levels of inflammatory markers (MMP-6, TNF-α, AOPPs, AGEs, NO, IL-17 and IL-2), MDA, antioxidant (SOD, GSH, CAT, GPx), DNA damage markers (Isop-2α, 8OHdG), RAGE, caspase-8, and p38, induced by lipopolysaccharides. |
| Acetyl-11-keto-β-boswellic acid (a major component of Boswellia serrata) | Nadeem et al., 2022 [259] | Female SJL/J mice 8–9 weeks old with EAE | Significant clinical improvement. Downregulation of inflammatory markers in CD11c + DCs (p-NF-κB, iNOS, and nitrotyrosine) and CNS (p-NF-κB, iNOS, nitrotyrosine, lipid peroxides, and total antioxidant capacity). |
| Acetyl-11-keto-β-boswellic acid (a major component of Boswellia serrata) | Upadhayay et al., 2022 [260] | Wistar rats with EAE | Significant improvement in clinical parameters improvement and reversion of the EAE-induced histopathological changes in the brain. Reversal of the decreased Nrf2 and HO-1 levels in the CSF and brain homogenate caused by EAE-induction. Decrease in caspase-3 and Bax, and increased Bcl-2 Levels in brain homogenates. Reversion of decrease in acetylcholine, dopamine, and serotonin, and increase in glutamate in brain homogenates induced by EAE. Reversion of the increase in AchE, MDA, and nitrite levels and reduction of GSH, SOD, and catalase levels induced by EAE. Decrease in TNF-αand IL-1β levels in brain homogenates. |
| Urtica dioica extract | Namazi et al., 2022 [261] | Male C57BL/6J mice 8 weeks old with cuprizone-induced demyelination | Significant decrease of demyelination with high doses. Significant decrease of MDA and HSP-70 brain levels with no effect on HSP-60. |
| Nebivolol | Naeem et al., 2022 [262] | Male C57BL/6J mice with cuprizone-induced demyelination | Significant clinical improvement and reversion of the EAE-induced histopathological changes. Modulation of microglial activation status by suppressing M1 markers (Iba-1, CD86, iNOS, NO, and TNF-α) and increasing M2 markers (Arg-1 and IL-10). Inhibition of NLRP3/caspase-1/IL-18 inflammatory cascade. Decrease of MDA levels and increase of CAT and SOD activities. |
| IM253 (herbal compound) | Esmaeilzadeh et al., 2022 [263] | Female C57Bl/6 mice 10–12 weeks old with EAE | Significant clinical improvement and delay of symptoms of EAE. Significant decrease in inflammation and demyelination. Significant reduction in the expression of pro-inflammatory cytokine coding genes (IL-6, TNF-α, IFN-γ, and IL-17) and increase in the expression of anti-inflammatory and anti-oxidant enzyme coding genes (TGF-β, GPX-1, and CAT) and TAC. |
| Zingiber officinale (ginger) extract | Moradi et al., 2022 [264] | Male Wistar rats 8-week old with cuprizone-induced demyelination | Significant clinical improvement and decrease of demyelination with high doses. Significant expression and increased levels of MBP and OLIG2. |
| Ginger extract | Kamankesh et al. 2023 [265] | Female C57Bl/6 mice 8 weeks old with EAE | Significant clinical improvement without a decrease in lymphocyte infiltration. Reduction of gene expression levels of the inflammatory cytokines IL-17 and IFN-γ. Significant increase of Treg cells. Significant reduction of serum NO levels. |
| Grape seed extract | Mabrouk et al., 2022 [266] | Female C57Bl/6 mice 6 weeks old with EAE | Significant clinical improvement and decrease of demyelination induced by EAE. Significant decrease in MDA and carbonyl protein levels, increase in SOD1, SOD2, GPx, and CAT activities, decrease in GFAP expression, and increase in SIRT1 expression in the brain and spinal cord (reversing the effects of EAE). |
| Grape Seed Extract | Wang et al., 2023 [267] | C57BL/6 mice with EAE | Significant improvement of clinical symptoms of EAE. Inhibition of spinal cord demyelination and inflammatory cell infiltration. Inhibition of the secretion of TNF-α, IL-1 β, IL-6, IL-12, IL-17, and IFN-γ cells and reduction of the differentiation of Th1 and Th17 mediated by CD3 and CD28 factors in the spleen. Inhibition of the infiltration of CD45+CD11b+ and CD45+CD4+ cells, differentiation of Th1 and Th17 (p < 0.05), reduction of secretion of inflammatory factors, and prevented the activation of microglia in the CNS. |
| Daphnetin (7,8-dihydroxy-coumarin) | Soltanmoham-madi et al., 2022 [268] | Female C57Bl/6 mice 8 weeks old with EAE | Significant clinical improvement and decrease of demyelination and lymphocyte infiltration in the CNS. Increase in the expression of anti-inflammatory cytokines and transcription factors (IL-4, IL-10, IL-33, GATA3, TGF-β, FoxP3), and reduction in the production of pro-inflammatory cytokines and transcription factors (IFN-γ, STAT4, T-bet, IL-17, STAT3, ROR-γt, TNF-α) in the CNS. Significant increase of the ratio of spleen Treg cells and the levels of IL-4, IL-10, TGF-β, and IL-33, and reduction of the levels of IFN-γ, TNF-α, and IL-17 in splenocytes. |
| Gallic (3,4,5-tri hydroxybenzoic) acid | Stegnjaić et al., 2022 [269] | Dark Agouti (DA) rats 5–7 months old with EAE | Significant clinical improvement and decrease of demyelination and lymphocyte infiltration in the CNS. Decrease in the release of IL-17, IFN-γ, and NO, decrease in the proportion of OX40+ or CD25+ CD4+ and Th17 cells in the spinal cord. Inhibition of NO, IL-6, and TNF release from macrophages and microglia. |
| Enterococcus durans (probiotic) | Samani et al., 2022 [270] | Female C57Bl/6 mice 8–10 weeks old with EAE | Significant decrease of demyelination and lymphocyte infiltration in the CNS. Significant decrease of the inflammatory cytokines IL-17, IFN-γ, and MMP-9, and increase in the MBP levels at the spinal cord |
| Ilepcimide (antiepilepsirine) | Xu et al., 2023 [271] | Female C57Bl/6 mice 8–10 weeks old with EAE | Ameliorates demyelination, blood–brain barrier leakage, and infiltration of CD4+ and CD8+ T cells. |
| Cannabigerol (phytocannabinoid) | Feinshtein et al., 2023 [272] | Female C57Bl/6 mice 8 weeks old with EAE | Significant clinical improvement. Reversion of enhanced neuronal loss and areas stained for GFAP in the spinal cord. |
| L-Theanine (antioxidant present in green tea) | Khosravi-Nezhad et al., 2023 [273] | Female C57Bl/6 mice 4–6 weeks old with cuprizone-induced demyelination | Significant clinical improvement. Reversion of the increase of MDA and the decrease in SOD, GPx, and TAC serum levels induced by cuprizone. |
| Vitamin D | Haindl et al., 2023 [274] | Male Dark Agouti rats aged 10–12 weeks with EAE | Significant reduction in myelin loss, microglial activation, and number of apoptotic cells. Significant reduction of seri, levels of oxidized lipid markers and NfL, and increase in serum TAC and protective polyphenols. |
| Peroxiredoxin 6 | Lunin et al., 2023 [275] | Female SJL/J mice, aged 3 months | Significant clinical improvement. Decrease of EAE-induced NOX1 and NOX4 gene expression in brain tissue |
| Bifidobacterium breve and Lactobacillus casei (probiotics) | Hasaniani et al., 2023 [276] | Male Wistar rats aged 5–6 weeks with cuprizone-induced demyelination | Significant clinical improvement. Increase in HO-1 and Nrf-2 gene expression, reduction of MDA levels, and increase in TAC in demyelinated corpus callosum. |
| Sinomenine (isoquinoline alkaloid isolated from Sinomenii acutum) | Fan et al., 2023 [277] | C57Bl/6 mice 8 weeks old with EAE | Significant amelioration of EAE severity. Reduction in the demyelination, axonal damage, and inhibition of inflammatory cell infiltration in the spinal cord. Decrease in the inflammatory cytokines TNF-α and IL-1β, MCP-1, and GM-CSF expression in the spinal cord and serum, increase in anti-inflammatory cytokine IL-10 expression in the spinal cord, and suppression of microglia and astrocytes activation in EAE mice. Inhibition of oxidative stress (reduction in MDA and advanced oxidation protein products (AOPP) levels in the spinal cord) via the activation of the Nrf2 signaling pathway (increase in expression of HO-1, NQO-1, and Nrf2 expression in the spinal cord. Suppression of lipopolysaccharide (LPS)-induced microglial activation and the production of pro-inflammatory factors in vitro. |
| Auranofin (thiol-reactive gold-containing compound) | Al-Kharashi et al., 2023 [278] | Female SJL/J mice aged 9–10 weeks with EAE | Significant amelioration of the clinical features. Increase in thioredoxin reductase (TrxR) activity, decrease in Nrf2 signaling, downregulation of the p-NFkB, decrease of iNOS mRNA expression, IL-6 mRNA levels, lipid peroxides levels, and myeloperoxidase activity in the brain and Peripheral Myeloid Immune Cells. |
| Vanillic acid | Safwat et al., 2023 [279] | Male Sprague–Dawley 12–16 weeks with cuprizone-induced demyelination | Significant improvement in alterations of nerve conduction velocity and demyelination deterioration of the sciatic nerve fibers induced by cuprizone. Significant improvement of the increase in IL1-7 level, expression of INF-γ and caspase-3, and the reduction in the expression of MBP, Nrf2, and HO-1 induced by cuprizone in the sciatic nerve. |
| Docosahexaenoic acid | Muñoz-Jurado et al., 2024 [280] | Male Dark Agouti rats with EAE | Significant clinical improvement. Decrease in lipid peroxides, carbonylated proteins, nitric oxide, and GSSG, and reduction of GSH levels in brain, spinal cord, blood, and various tissues. |
| Nicorandil (ATP-sensitive potassium channels opener) + carvedilol (α and β adrenoceptor antagonist) | Mustafa et al., 2024 [281] | Male Sprague–Dawley rats with EAE | Significant clinical improvement. Significant improvement of demyelination. Downregulation of TLR4/MYD88/TRAF6 signaling cascade with inhibition of (pT183/Y185)-JNK/p38 (pT180/Y182)-MAPK axis with reduction of IL-1β, IL-6NF-κB, and TNF-α in the brain. Increase of SOD activity and Nrf2 content and reduction of MDA content of the brain. Inhibition of gene expression for CD4, IL-17, IL-23, and TGF-β. Anti-apoptotic effect by decreasing Bax protein expression and caspase-3 content and increasing Bcl-2. |
| Purmorphamine (Smo-Shh/Gli activator) | Prajapati et al., 2024 [282] | Wistar rats of both sexes with EAE | Significant improvement of clinical parameters and histological changes Significant decrease in the levels of Smo-Shh in brain homogenate, blood plasma, and cerebrospinal fluid. Decrease of brain and plasma TNF-α and IL-1β levels. Decrease of MDA, nitrite, and AchE and increase of reduced glutathione, SOD, and CAT brain levels. Reversion of the increase in the apoptosis-related markers caspase-3 and Bax and the decrease of Bcl-2 anti-apoptotic protein induced by EAE in the brain and plasma. Reversion of the increased neurofilament protein levels in the brain. Increased brain levels of acetylcholine, dopamine, and serotonin and decrease of glutamate. |
| Arbutin (glycosylated derivative of hydroquinone present in the leaves and bark of bearberry and other plants) | Ashrafpour et al., 2024 [283] | Adult male Wistar rats with demyelination induced by injection of lysolecithin into the hippocampus | Significant improvement of memory impairment. Reduction of demyelination and astrocyte activation, and increase of MBP. Suppression of pro-inflammatory markers (IL-1β, TNF-α) and increase of anti-inflammatory cytokine IL-10. Decreased iNOS and increase of anti-oxidative factors (Nrf2, HO-1) and BDNF expression. |
| Cerium oxide nanoparticles | Nguyen et al., 2024 [284] | Female BALB/c mice aged 6–8 weeks and female C57BL/6 mice aged 9–10 weeks with EAE | Significant clinical improvement. Reversion of the increase in expression of T cells, dendritic cells, and resident microglia in the CNS and in IFN-γ expression among CD4+ T cells sampled from spleen tissue induced by EAE. Reduction in demyelination in the spinal cord. |
| Drug | Author, Year [Ref] | Study Design | Main Findings |
|---|---|---|---|
| MS14 (natural herbal-marine drug) | Ahmadi et al., 2010 [285] | Open-label study involving 14 MS patients | Improvement of quality of life of patients. |
| Vegetable extract containing Lipia citriadora | Mauriz et al., 2013 [286] | Randomized prospective placebo-controlled study involving 9 MS patients (5 under low-fat diet and antioxidants, 4 under low-fat diet) | Decrease of serum levels of C reactive protein isoprostane 8-iso-PGF2α and interleukine IL-6 and increase or catalase activity in blood. |
| Saccharomyces boulardii (probiotic) | Aghamohammadi et al., 2019 [287] | 4-month, double-blind, randomized controlled two-group parallel trial involving 50 MS | This trial is ongoing. The study assesses changes in mental health evaluated by the 28-item General Health Questionnaire. Secondary endpoints include changes in life quality, fatigue, pain, serum markers of inflammatory stress, and oxidative stress. |
| Ozone therapy | Izadi et al., 2020 [288] | Open-label study involving 20 MS patients | Significant decrease in the frequency of Th17 cells, the expression levels of RORγt and IL-17, miR-141, and miR-155 in post-treatment compared to pre-treatment condition. Significant reduction in the secretion level of IL-17 in treated patients. |
| CaNa2EDTA and multivitamin complex with redox glutathione | Vezzoli et al., 2023 [289] | Open-label study involving 18 MS patients | Decrease in ROS production, and increase in TAC, SOD, and CAT activity in plasma. Decrease in GSSG and increase in reduced GSH in erythrocytes. Decrease of 8-iso PGF2α, 8-OH-dG, and NO metabolites in urine. Decrease in IL-6, TNF-α, and sICAM plasma levels. |
| Synbiotics supplement and anti-inflammatory antioxidant-rich diet compared to placebo and their usual diet | Moravejolahkami et al., 2023 [290] | 4-month single-center, single-blind, randomized, controlled clinical trial involving 70 MS patients with PPMS, SPMS, or progressive-relapsing MS | Clinical improvement in fatigue, pain, sexual function, and bowel/bladder status. |
| Ellagic acid (a polyphenol found in numerous fruits and vegetables with antioxidant actions) | Hajiluian et al., 2023 [291] | 12-week randomized, triple-blind, placebo-controlled trial involving 50 MS patients with mild to moderate depressive symptoms | Significant decrease in depression score, serum IFN-γ, NO, and cortisol levels, and indoleamine 2.3-dihydrooxygenase 1 (IDO) gene expression. Increase in serum levels of BDNF and serotonin. Lack of changes in serum Nrf2 levels. |
| Saccharomyces boulardii (probiotic) | Asghari et al., 2023 [292] | 4-month prospective randomized double-blinded clinical trial involving 40 RRMS patients | Significant improvement in scales measuring pain, fatigue, quality of life, and somatic and social dysfunction on a General Health Questionnaire. Significant decrease in plasma levels, high-sensitivity C-reactive protein (hs-CRP), and increase of TAC. Non-significant decrease in plasma MDA levels. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Jiménez, F.J.; Alonso-Navarro, H.; Salgado-Cámara, P.; García-Martín, E.; Agúndez, J.A.G. Antioxidant Therapies in the Treatment of Multiple Sclerosis. Biomolecules 2024, 14, 1266. https://doi.org/10.3390/biom14101266
Jiménez-Jiménez FJ, Alonso-Navarro H, Salgado-Cámara P, García-Martín E, Agúndez JAG. Antioxidant Therapies in the Treatment of Multiple Sclerosis. Biomolecules. 2024; 14(10):1266. https://doi.org/10.3390/biom14101266
Chicago/Turabian StyleJiménez-Jiménez, Félix Javier, Hortensia Alonso-Navarro, Paula Salgado-Cámara, Elena García-Martín, and José A. G. Agúndez. 2024. "Antioxidant Therapies in the Treatment of Multiple Sclerosis" Biomolecules 14, no. 10: 1266. https://doi.org/10.3390/biom14101266
APA StyleJiménez-Jiménez, F. J., Alonso-Navarro, H., Salgado-Cámara, P., García-Martín, E., & Agúndez, J. A. G. (2024). Antioxidant Therapies in the Treatment of Multiple Sclerosis. Biomolecules, 14(10), 1266. https://doi.org/10.3390/biom14101266







