Glycosphingolipids in Cardiovascular Disease: Insights from Molecular Mechanisms and Heart Failure Models
Abstract
1. Introduction
2. Biosynthesis and Metabolism of Glycosphingolipids
2.1. GSL Synthesis
2.2. Transport and Glycoslyation
2.3. GSL Degradation
3. Spatial Dynamics of Glycosphingolipid Signaling in Cardiac Cellular Compartments
3.1. GSLs in Membrane Microdomains and Mitochondria-Associated ER Membranes (MAMs)
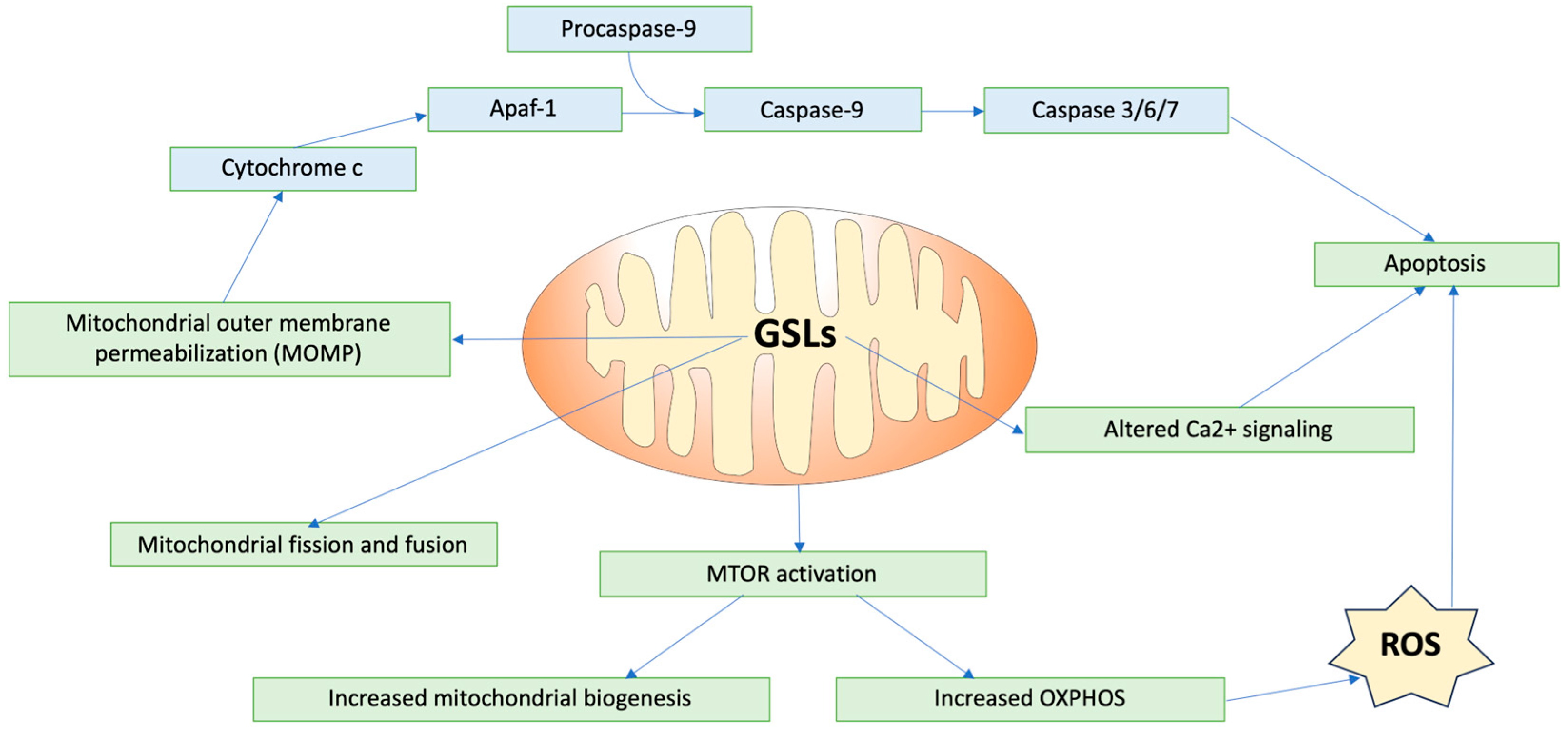
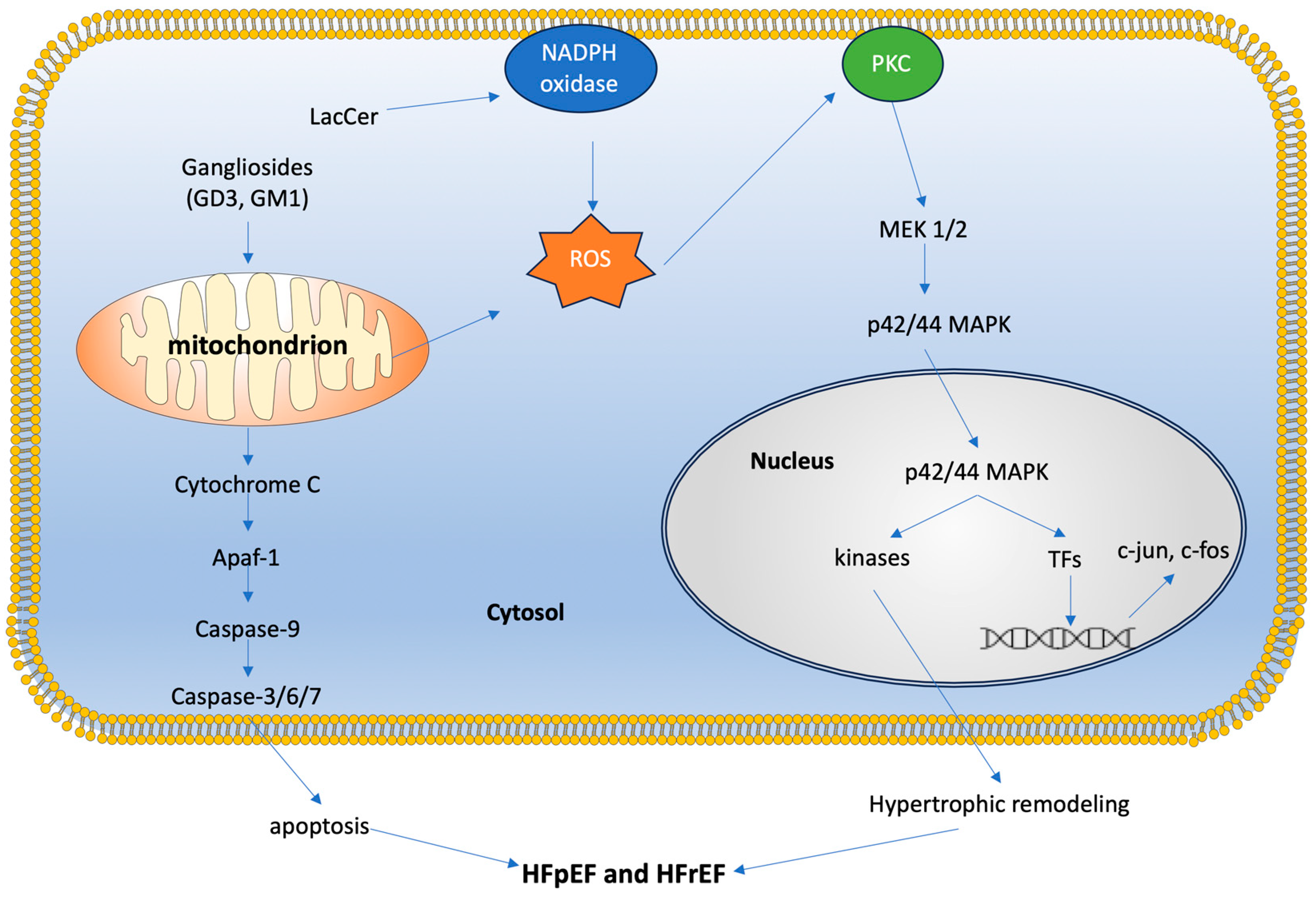
3.2. GSLs in Mitochondrial Function and Dynamics
3.3. GSLs in Cellular Signaling Pathways
4. Glycosphingolipids in Cardiovascular Disease Pathogenesis
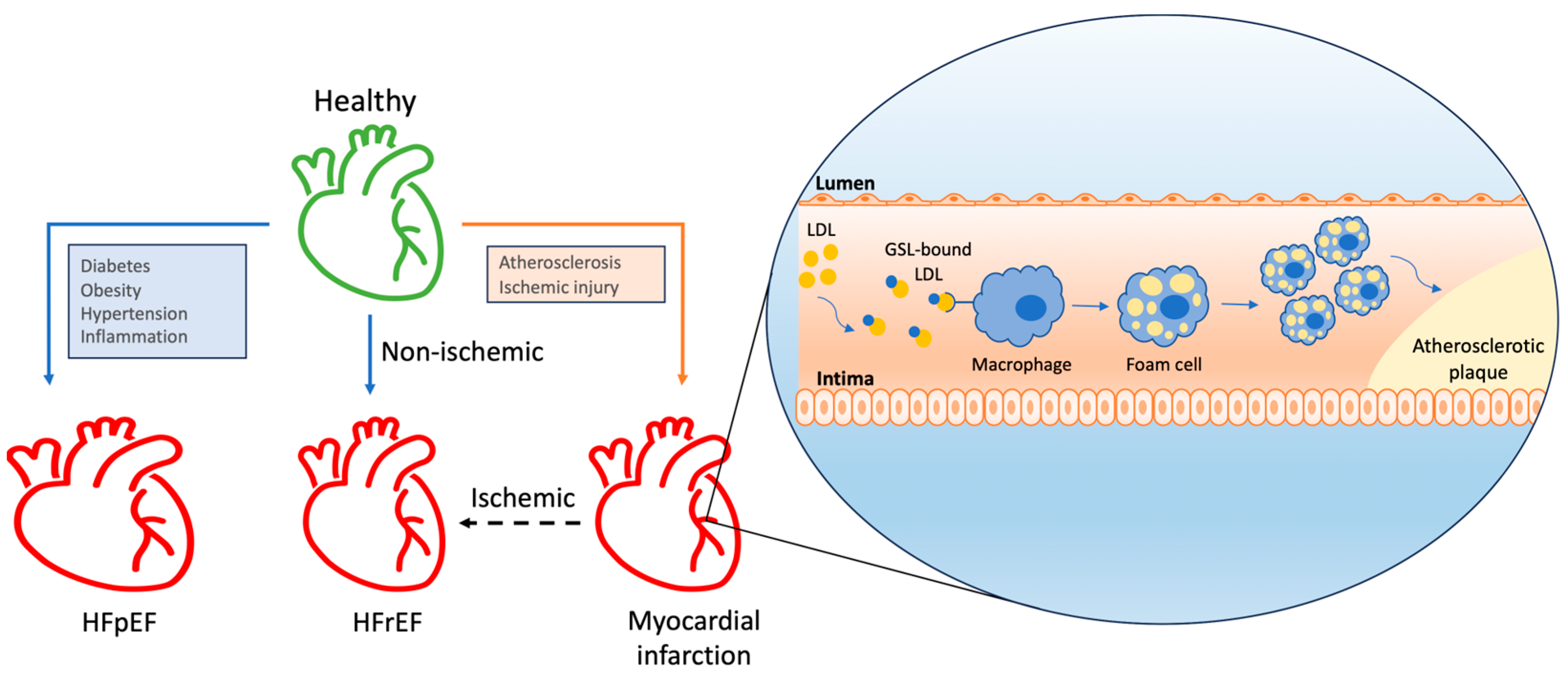
4.1. Atherosclerosis
4.2. GSLs in Angiogenesis and Inflammation
4.3. Hypertrophy and Heart Failure
4.3.1. GSL Insights from HFrEF
4.3.2. GSL Insights from Cardiometabolic Diseases and HFpEF
4.3.3. Diabetes
4.4. Fabry’s Disease
4.5. Gaucher’s Disease
4.6. Niemann-Pick Disease
5. Clinical Studies on Glycosphingolipids in Cardiovascular Disorders
6. Glycosphingolipids as Biomarkers in Cardiovascular Diseases
7. Therapeutic Potential of Targeting Glycosphingolipids
7.1. Enzyme Replacement Therapy (ERT)
7.2. Substrate Reduction Therapy (SRT)
7.3. Chaperone-Mediated Therapy (CMT) or Pharmacological Chaperone Therapy (PCT)
8. Methodologies for Determining GSLs
9. Conclusion and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, X.; Yang, G.Y. Pathophysiological roles and applications of glycosphingolipids in the diagnosis and treatment of cancer diseases. Prog. Lipid Res. 2023, 91, 101241. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, G.; Capasso, S.; Sticco, L.; Russo, D. Glycosphingolipids: Synthesis and functions. FEBS J. 2013, 280, 6338–6353. [Google Scholar] [CrossRef] [PubMed]
- Schnaar, R.L.; Sandhoff, R.; Tiemeyer, M.; Kinoshita, T. Glycosphingolipids. In Essentials of Glycobiology [Internet], 4th ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022; Chapter 11. Available online: https://www.ncbi.nlm.nih.gov/books/NBK579905/ (accessed on 3 October 2024).
- Liang, Y.J. Glycosphingolipids in human embryonic stem cells and breast cancer stem cells, and potential cancer therapy strategies based on their structures and functions. Glycoconj. J. 2022, 39, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Chatterjee, S. Lactosylceramide promotes hypertrophy through ROS generation and activation of ERK1/2 in cardiomyocytes. Glycobiology 2014, 24, 518–531. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kojima, N.; Hakomori, S. Cell adhesion, spreading, and motility of GM3-expressing cells based on glycolipid-glycolipid interaction. J. Biol. Chem. 1991, 266, 17552–17558. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
- Handa, K.; Hakomori, S.I. Carbohydrate to carbohydrate interaction in development process and cancer progression. Glycoconj. J. 2012, 29, 627–637. [Google Scholar] [CrossRef]
- Ando, H.; Komura, N. Recent progress in the synthesis of glycosphingolipids. Curr. Opin. Chem. Biol. 2024, 78, 102423. [Google Scholar] [CrossRef]
- Prokazova, N.V.; Bergelson, L.D. Gangliosides and atherosclerosis. Lipids 1994, 29, 1–5. [Google Scholar] [CrossRef]
- Wang, S.H.; Wu, T.J.; Lee, C.W.; Yu, J. Dissecting the conformation of glycans and their interactions with proteins. J. Biomed. Sci. 2020, 27, 93. [Google Scholar] [CrossRef]
- Wang, X.Q.; Sun, P.; Paller, A.S. Ganglioside GM3 blocks the activation of epidermal growth factor receptor induced by integrin at specific tyrosine sites. J. Biol. Chem. 2003, 278, 48770–48778. [Google Scholar] [CrossRef] [PubMed]
- Regina Todeschini, A.; Hakomori, S.I. Functional role of glycosphingolipids and gangliosides in control of cell adhesion, motility, and growth, through glycosynaptic microdomains. Biochim. Biophys. Acta 2008, 1780, 421–433. [Google Scholar] [CrossRef]
- Cumin, C.; Huang, Y.L.; Everest-Dass, A.; Jacob, F. Deciphering the Importance of Glycosphingolipids on Cellular and Molecular Mechanisms Associated with Epithelial-to-Mesenchymal Transition in Cancer. Biomolecules 2021, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Dutta, D.; Lu, S.; Bellen, H.J. Sphingolipids in neurodegenerative diseases. Front. Neurosci. 2023, 17, 1137893. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Bedja, D.; Amuzie, C.; Avolio, A.; Chatterjee, S. Prevention of cardiac hypertrophy by the use of a glycosphingolipid synthesis inhibitor in ApoE-/- mice. Biochem. Biophys. Res. Commun. 2015, 465, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Bedja, D.; Amuzie, C.; Foss, C.A.; Pomper, M.G.; Bhattacharya, R.; Yarema, K.J.; Chatterjee, S. Improved intervention of atherosclerosis and cardiac hypertrophy through biodegradable polymer-encapsulated delivery of glycosphingolipid inhibitor. Biomaterials 2015, 64, 125–135. [Google Scholar] [CrossRef]
- Levy, M.; Futerman, A.H. Mammalian ceramide synthases. IUBMB Life 2010, 62, 347–356. [Google Scholar] [CrossRef]
- Hernández-Corbacho, M.J.; Salama, M.F.; Canals, D.; Senkal, C.E.; Obeid, L.M. Sphingolipids in mitochondria. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 56–68. [Google Scholar] [CrossRef]
- Michel, C.; van Echten-Deckert, G.; Rother, J.; Sandhoff, K.; Wang, E.; Merrill, A.H. Characterization of ceramide synthesis. A dihydroceramide desaturase introduces the 4,5-trans-double bond of sphingosine at the level of dihydroceramide. J. Biol. Chem. 1997, 272, 22432–22437. [Google Scholar] [CrossRef]
- Marchesini, N.; Hannun, Y.A. Acid and neutral sphingomyelinases: Roles and mechanisms of regulation. Biochem. Cell Biol. 2004, 82, 27–44. [Google Scholar] [CrossRef]
- Hanada, K.; Kumagai, K.; Yasuda, S.; Miura, Y.; Kawano, M.; Fukasawa, M.; Nishijima, M. Molecular machinery for non-vesicular trafficking of ceramide. Nature 2003, 426, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.H.; Cook, G.M.; Spratley, S.J.; Fawke, S.; Graham, S.C.; Deane, J.E. The mechanism of glycosphingolipid degradation revealed by a GALC-SapA complex structure. Nat. Commun. 2018, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Ryckman, A.E.; Brockhausen, I.; Walia, J.S. Metabolism of Glycosphingolipids and Their Role in the Pathophysiology of Lysosomal Storage Disorders. Int. J. Mol. Sci. 2020, 21, 6881. [Google Scholar] [CrossRef] [PubMed]
- Kleynerman, A.; Rybova, J.; Faber, M.L.; McKillop, W.M.; Levade, T.; Medin, J.A. Acid Ceramidase Deficiency: Bridging Gaps between Clinical Presentation, Mouse Models, and Future Therapeutic Interventions. Biomolecules 2023, 13, 274. [Google Scholar] [CrossRef] [PubMed]
- Coant, N.; Hannun, Y.A. Neutral ceramidase: Advances in mechanisms, cell regulation, and roles in cancer. Adv. Biol. Regul. 2019, 71, 141–146. [Google Scholar] [CrossRef]
- Coant, N.; Sakamoto, W.; Mao, C.; Hannun, Y.A. Ceramidases, roles in sphingolipid metabolism and in health and disease. Adv. Biol. Regul. 2017, 63, 122–131. [Google Scholar] [CrossRef]
- Varela, A.R.; Gonçalves da Silva, A.M.; Fedorov, A.; Futerman, A.H.; Prieto, M.; Silva, L.C. Effect of glucosylceramide on the biophysical properties of fluid membranes. Biochim. Biophys. Acta 2013, 1828, 1122–1130. [Google Scholar] [CrossRef][Green Version]
- Sonnino, S.; Mauri, L.; Chigorno, V.; Prinetti, A. Gangliosides as components of lipid membrane domains. Glycobiology 2007, 17, 1R–13R. [Google Scholar] [CrossRef]
- Horbay, R.; Hamraghani, A.; Ermini, L.; Holcik, S.; Beug, S.T.; Yeganeh, B. Role of Ceramides and Lysosomes in Extracellular Vesicle Biogenesis, Cargo Sorting and Release. Int. J. Mol. Sci. 2022, 23, 15317. [Google Scholar] [CrossRef]
- Das, M.; Das, D.K. Lipid raft in cardiac health and disease. Curr. Cardiol. Rev. 2009, 5, 105–111. [Google Scholar] [CrossRef]
- Annunziata, I.; Sano, R.; d’Azzo, A. Mitochondria-associated ER membranes (MAMs) and lysosomal storage diseases. Cell Death Dis. 2018, 9, 328. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, T.; Matarrese, P.; Manganelli, V.; Marconi, M.; Tinari, A.; Gambardella, L.; Faggioni, A.; Misasi, R.; Sorice, M.; Malorni, W. Evidence for the involvement of lipid rafts localized at the ER-mitochondria associated membranes in autophagosome formation. Autophagy 2016, 12, 917–935. [Google Scholar] [CrossRef] [PubMed]
- Sano, R.; Annunziata, I.; Patterson, A.; Moshiach, S.; Gomero, E.; Opferman, J.; Forte, M.; d’Azzo, A. GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca(2+)-dependent mitochondrial apoptosis. Mol. Cell 2009, 36, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Sessa, W.C. eNOS at a glance. J. Cell Sci. 2004, 117, 2427–2429. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Silva, C.; Rotellar, F.; Gil, M.J.; Cienfuegos, J.A.; Salvador, J.; Frühbeck, G. Expression of caveolin-1 in human adipose tissue is upregulated in obesity and obesity-associated type 2 diabetes mellitus and related to inflammation. Clin. Endocrinol. 2008, 68, 213–219. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Q.; Wang, J.; Yu, H.; Zhen, X.; Li, L.; Qu, Y.; He, Y.; Zhang, J.; Li, C.; et al. Novel Indel Variation of NPC1 Gene Associates With Risk of Sudden Cardiac Death. Front. Genet. 2022, 13, 869859. [Google Scholar] [CrossRef]
- Higuchi, Y.; Miura, T.; Kajimoto, T.; Ohta, Y. Effects of disialoganglioside GD3 on the mitochondrial membrane potential. FEBS Lett. 2005, 579, 3009–3013. [Google Scholar] [CrossRef]
- Park, L.K.; Garr Barry, V.; Hong, J.; Heebink, J.; Sah, R.; Peterson, L.R. Links between ceramides and cardiac function. Curr. Opin. Lipidol. 2022, 33, 47–56. [Google Scholar] [CrossRef]
- Scheffer, D.D.L.; Garcia, A.A.; Lee, L.; Mochly-Rosen, D.; Ferreira, J.C.B. Mitochondrial Fusion, Fission, and Mitophagy in Cardiac Diseases: Challenges and Therapeutic Opportunities. Antioxid. Redox Signal. 2022, 36, 844–863. [Google Scholar] [CrossRef]
- Ciarlo, L.; Manganelli, V.; Garofalo, T.; Matarrese, P.; Tinari, A.; Misasi, R.; Malorni, W.; Sorice, M. Association of fission proteins with mitochondrial raft-like domains. Cell Death Differ. 2010, 17, 1047–1058. [Google Scholar] [CrossRef]
- Schömel, N.; Geisslinger, G.; Wegner, M.S. Influence of glycosphingolipids on cancer cell energy metabolism. Prog. Lipid Res. 2020, 79, 101050. [Google Scholar] [CrossRef] [PubMed]
- Dany, M.; Ogretmen, B. Ceramide induced mitophagy and tumor suppression. Biochim. Biophys. Acta 2015, 1853, 2834–2845. [Google Scholar] [CrossRef] [PubMed]
- Novgorodov, S.A.; Riley, C.L.; Yu, J.; Keffler, J.A.; Clarke, C.J.; Van Laer, A.O.; Baicu, C.F.; Zile, M.R.; Gudz, T.I. Lactosylceramide contributes to mitochondrial dysfunction in diabetes. J. Lipid Res. 2016, 57, 546–562. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Schuchman, E.H. Ceramide and Ischemia/Reperfusion Injury. J. Lipids 2018, 2018, 3646725. [Google Scholar] [CrossRef]
- Wang, N.; Li, J.Y.; Zeng, B.; Chen, G.L. Sphingosine-1-Phosphate Signaling in Cardiovascular Diseases. Biomolecules 2023, 13, 818. [Google Scholar] [CrossRef]
- Egom, E.E.; Bae, J.S.; Capel, R.; Richards, M.; Ke, Y.; Pharithi, R.B.; Maher, V.; Kruzliak, P.; Lei, M. Effect of sphingosine-1-phosphate on L-type calcium current and Ca(2+) transient in rat ventricular myocytes. Mol. Cell Biochem. 2016, 419, 83–92. [Google Scholar] [CrossRef]
- Brizuela, L.; Rábano, M.; Peña, A.; Gangoiti, P.; Macarulla, J.M.; Trueba, M.; Gómez-Muñoz, A. Sphingosine 1-phosphate: A novel stimulator of aldosterone secretion. J. Lipid Res. 2006, 47, 1238–1249. [Google Scholar] [CrossRef]
- Mutoh, T.; Tokuda, A.; Miyadai, T.; Hamaguchi, M.; Fujiki, N. Ganglioside GM1 binds to the Trk protein and regulates receptor function. Proc. Natl. Acad. Sci. USA 1995, 92, 5087–5091. [Google Scholar] [CrossRef]
- Mishra, S.; Kass, D.A. Cellular and molecular pathobiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2021, 18, 400–423. [Google Scholar] [CrossRef]
- Heart Disease Facts. Heart Disease 2023. Available online: https://www.cdc.gov/heart-disease/data-research/facts-stats/index.html (accessed on 23 January 2024).
- Balram, A.; Thapa, S.; Chatterjee, S. Glycosphingolipids in Diabetes, Oxidative Stress, and Cardiovascular Disease: Prevention in Experimental Animal Models. Int. J. Mol. Sci. 2022, 23, 15442. [Google Scholar] [CrossRef]
- Choi, R.H.; Tatum, S.M.; Symons, J.D.; Summers, S.A.; Holland, W.L. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat. Rev. Cardiol. 2021, 18, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Mukhin, D.N.; Chao, F.F.; Kruth, H.S. Glycosphingolipid accumulation in the aortic wall is another feature of human atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Glaros, E.N.; Kim, W.S.; Rye, K.A.; Shayman, J.A.; Garner, B. Reduction of plasma glycosphingolipid levels has no impact on atherosclerosis in apolipoprotein E-null mice. J. Lipid Res. 2008, 49, 1677–1681. [Google Scholar] [CrossRef] [PubMed]
- Borodzicz-Jażdżyk, S.; Jażdżyk, P.; Łysik, W.; Cudnoch-Jȩdrzejewska, A.; Czarzasta, K. Sphingolipid metabolism and signaling in cardiovascular diseases. Front. Cardiovasc. Med. 2022, 9, 915961. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, M.; Cirillo, F.; Ghiroldi, A.; Rota, P.; Coviello, S.; Tarantino, A.; La Rocca, P.; Lavota, I.; Creo, P.; Signorelli, P.; et al. Sphingolipids and Atherosclerosis: The Dual Role of Ceramide and Sphingosine-1-Phosphate. Antioxidants 2023, 12, 143. [Google Scholar] [CrossRef]
- Iwabuchi, K. Involvement of glycosphingolipid-enriched lipid rafts in inflammatory responses. Front. Biosci. (Landmark. Ed.) 2015, 20, 325–334. [Google Scholar] [CrossRef]
- Song, B.; Zheng, Y.; Chi, H.; Zhu, Y.; Cui, Z.; Chen, L.; Chen, G.; Gao, B.; Du, Y.; Yu, Z. Revealing the roles of glycosphingolipid metabolism pathway in the development of keloid: A conjoint analysis of single-cell and machine learning. Front. Immunol. 2023, 14, 1139775. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, S.H.; Shin, M.J.; Hwang, G.S. Alteration in metabolic signature and lipid metabolism in patients with angina pectoris and myocardial infarction. PLoS ONE 2015, 10, e0135228. [Google Scholar] [CrossRef]
- Lozanski, G.; Berthier, F.; Kushner, I. The sphingomyelin-ceramide pathway participates in cytokine regulation of C-reactive protein and serum amyloid A, but not alpha-fibrinogen. Biochem. J. 1997, 328 Pt 1, 271–275. [Google Scholar] [CrossRef]
- Yokoyama, N.; Hanafusa, K.; Hotta, T.; Oshima, E.; Iwabuchi, K.; Nakayama, H. Multiplicity of Glycosphingolipid-Enriched Microdomain-Driven Immune Signaling. Int. J. Mol. Sci. 2021, 22, 9565. [Google Scholar] [CrossRef]
- Coskun, Ü.; Grzybek, M.; Drechsel, D.; Simons, K. Regulation of human EGF receptor by lipids. Proc. Natl. Acad. Sci. USA 2011, 108, 9044–9048. [Google Scholar] [CrossRef] [PubMed]
- Mobarak, E.; Håversen, L.; Manna, M.; Rutberg, M.; Levin, M.; Perkins, R.; Rog, T.; Vattulainen, I.; Borén, J. Glucosylceramide modifies the LPS-induced inflammatory response in macrophages and the orientation of the LPS/TLR4 complex in silico. Sci. Rep. 2018, 8, 13600. [Google Scholar] [CrossRef] [PubMed]
- Chiricozzi, E.; Di Biase, E.; Lunghi, G.; Fazzari, M.; Loberto, N.; Aureli, M.; Mauri, L.; Sonnino, S. Turning the spotlight on the oligosaccharide chain of GM1 ganglioside. Glycoconj. J. 2021, 38, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.B.; Tidhar, R.; Futerman, A.H.; Cowart, L.A. Myristate-derived d16:0 sphingolipids constitute a cardiac sphingolipid pool with distinct synthetic routes and functional properties. J. Biol. Chem. 2013, 288, 13397–13409. [Google Scholar] [CrossRef]
- Sasset, L.; Manzo, O.L.; Zhang, Y.; Marino, A.; Rubinelli, L.; Riemma, M.A.; Chalasani, M.L.S.; Dasoveanu, D.C.; Roviezzo, F.; Jankauskas, S.S.; et al. Nogo-A reduces ceramide de novo biosynthesis to protect from heart failure. Cardiovasc. Res. 2023, 119, 506–519. [Google Scholar] [CrossRef]
- Sansbury, B.E.; DeMartino, A.M.; Xie, Z.; Brooks, A.C.; Brainard, R.E.; Watson, L.J.; DeFilippis, A.P.; Cummins, T.D.; Harbeson, M.A.; Brittian, K.R.; et al. Metabolomic analysis of pressure-overloaded and infarcted mouse hearts. Circ. Heart Fail. 2014, 7, 634–642. [Google Scholar] [CrossRef]
- Pellieux, C.; Montessuit, C.; Papageorgiou, I.; Pedrazzini, T.; Lerch, R. Differential effects of high-fat diet on myocardial lipid metabolism in failing and nonfailing hearts with angiotensin II-mediated cardiac remodeling in mice. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1795–H1805. [Google Scholar] [CrossRef]
- Cui, S.; Zhang, X.; Li, Y.; Hu, S.; Wu, B.; Fang, Z.; Gao, J.; Li, M.; Wu, H.; Tao, B.; et al. UGCG modulates heart hypertrophy through B4GalT5-mediated mitochondrial oxidative stress and the ERK signaling pathway. Cell Mol. Biol. Lett. 2023, 28, 71. [Google Scholar] [CrossRef]
- Andersson, L.; Cinato, M.; Mardani, I.; Miljanovic, A.; Arif, M.; Koh, A.; Lindbom, M.; Laudette, M.; Bollano, E.; Omerovic, E.; et al. Glucosylceramide synthase deficiency in the heart compromises β1-adrenergic receptor trafficking. Eur. Heart J. 2021, 42, 4481–4492. [Google Scholar] [CrossRef]
- Brady, E.M.; Cao, T.H.; Moss, A.J.; Athithan, L.; Ayton, S.L.; Redman, E.; Argyridou, S.; Graham-Brown, M.P.M.; Maxwell, C.B.; Jones, D.J.L.; et al. Circulating sphingolipids and relationship to cardiac remodelling before and following a low-energy diet in asymptomatic Type 2 Diabetes. BMC Cardiovasc. Disord. 2024, 24, 25. [Google Scholar] [CrossRef]
- Li, J.; Kemp, B.A.; Howell, N.L.; Massey, J.; Mińczuk, K.; Huang, Q.; Chordia, M.D.; Roy, R.J.; Patrie, J.T.; Davogustto, G.E.; et al. Metabolic Changes in Spontaneously Hypertensive Rat Hearts Precede Cardiac Dysfunction and Left Ventricular Hypertrophy. J. Am. Heart Assoc. 2019, 8, e010926. [Google Scholar] [CrossRef] [PubMed]
- Mikhalkova, D.; Holman, S.R.; Jiang, H.; Saghir, M.; Novak, E.; Coggan, A.R.; O’Connor, R.; Bashir, A.; Jamal, A.; Ory, D.S.; et al. Bariatric Surgery-Induced Cardiac and Lipidomic Changes in Obesity-Related Heart Failure with Preserved Ejection Fraction. Obesity 2018, 26, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Stratford, S.; Hoehn, K.L.; Liu, F.; Summers, S.A. Regulation of insulin action by ceramide: Dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J. Biol. Chem. 2004, 279, 36608–36615. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Lin, C.F.; Chang, W.T.; Huang, W.C.; Teng, C.F.; Lin, Y.S. Ceramide induces p38 MAPK and JNK activation through a mechanism involving a thioredoxin-interacting protein-mediated pathway. Blood 2008, 111, 4365–4374. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.J.; Cases, S.; Jensen, D.R.; Chen, H.C.; Sande, E.; Tow, B.; Sanan, D.A.; Raber, J.; Eckel, R.H.; Farese, R.V. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat. Genet. 2000, 25, 87–90. [Google Scholar] [CrossRef]
- Liu, L.; Trent, C.M.; Fang, X.; Son, N.H.; Jiang, H.; Blaner, W.S.; Hu, Y.; Yin, Y.X.; Farese, R.V.; Homma, S.; et al. Cardiomyocyte-specific loss of diacylglycerol acyltransferase 1 (DGAT1) reproduces the abnormalities in lipids found in severe heart failure. J. Biol. Chem. 2014, 289, 29881–29891. [Google Scholar] [CrossRef]
- Chavez, J.A.; Siddique, M.M.; Wang, S.T.; Ching, J.; Shayman, J.A.; Summers, S.A. Ceramides and glucosylceramides are independent antagonists of insulin signaling. J. Biol. Chem. 2014, 289, 723–734. [Google Scholar] [CrossRef]
- Lenders, M.; Brand, E. Fabry Disease: The Current Treatment Landscape. Drugs 2021, 81, 635–645. [Google Scholar] [CrossRef]
- Bernardes, T.P.; Foresto, R.D.; Kirsztajn, G.M. Fabry disease: Genetics, pathology, and treatment. Rev. Assoc. Med. Bras. (1992) 2020, 66 (Suppl. S1), s10–s16. [Google Scholar] [CrossRef]
- Germain, D.P. Fabry disease. Orphanet J. Rare Dis. 2010, 5, 30. [Google Scholar] [CrossRef]
- Kovilakath, A.; Cowart, L.A. Sphingolipid Mediators of Myocardial Pathology. J. Lipid Atheroscler. 2020, 9, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Machann, W.; Breunig, F.; Weidemann, F.; Sandstede, J.; Hahn, D.; Köstler, H.; Neubauer, S.; Wanner, C.; Beer, M. Cardiac energy metabolism is disturbed in Fabry disease and improves with enzyme replacement therapy using recombinant human galactosidase A. Eur. J. Heart Fail. 2011, 13, 278–283. [Google Scholar] [CrossRef]
- Liebau, M.C.; Braun, F.; Höpker, K.; Weitbrecht, C.; Bartels, V.; Müller, R.U.; Brodesser, S.; Saleem, M.A.; Benzing, T.; Schermer, B.; et al. Dysregulated autophagy contributes to podocyte damage in Fabry’s disease. PLoS ONE 2013, 8, e63506. [Google Scholar] [CrossRef] [PubMed]
- Birket, M.J.; Raibaud, S.; Lettieri, M.; Adamson, A.D.; Letang, V.; Cervello, P.; Redon, N.; Ret, G.; Viale, S.; Wang, B.; et al. A Human Stem Cell Model of Fabry Disease Implicates LIMP-2 Accumulation in Cardiomyocyte Pathology. Stem Cell Rep. 2019, 13, 380–393. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, P.N.; Mucci, J.M.; Ceci, R.; Fossati, C.A.; Rozenfeld, P.A. Higher apoptotic state in Fabry disease peripheral blood mononuclear cells.: Effect of globotriaosylceramide. Mol. Genet. Metab. 2011, 104, 319–324. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, P.N.; Mucci, J.M.; Ceci, R.; Fossati, C.A.; Rozenfeld, P.A. Fabry disease peripheral blood immune cells release inflammatory cytokines: Role of globotriaosylceramide. Mol. Genet. Metab. 2013, 109, 93–99. [Google Scholar] [CrossRef]
- Yogasundaram, H.; Nikhanj, A.; Putko, B.N.; Boutin, M.; Jain-Ghai, S.; Khan, A.; Auray-Blais, C.; West, M.L.; Oudit, G.Y. Elevated Inflammatory Plasma Biomarkers in Patients with Fabry Disease: A Critical Link to Heart Failure with Preserved Ejection Fraction. J. Am. Heart Assoc. 2018, 7, e009098. [Google Scholar] [CrossRef]
- Morales, L.E. Gaucher’s disease: A review. Ann. Pharmacother. 1996, 30, 381–388. [Google Scholar] [CrossRef]
- Ivanova, M. Altered Sphingolipids Metabolism Damaged Mitochondrial Functions: Lessons Learned From Gaucher and Fabry Diseases. J. Clin. Med. 2020, 9, 1116. [Google Scholar] [CrossRef]
- Gözdaşoğlu, S. Gaucher Disease and Gaucher Cells. Turk. J. Haematol. 2015, 32, 187–188. [Google Scholar] [CrossRef]
- Kurolap, A.; Del Toro, M.; Spiegel, R.; Gutstein, A.; Shafir, G.; Cohen, I.J.; Barrabés, J.A.; Feldman, H.B. Gaucher disease type 3c: New patients with unique presentations and review of the literature. Mol. Genet. Metab. 2019, 127, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Solanich, X.; Claver, E.; Carreras, F.; Giraldo, P.; Vidaller, A.; Aguilar, R.; Cequier, A. Myocardial infiltration in Gaucher’s disease detected by cardiac MRI. Int. J. Cardiol. 2012, 155, e5–e6. [Google Scholar] [CrossRef] [PubMed]
- Vanier, M.T. Niemann-Pick diseases. Handb. Clin. Neurol. 2013, 113, 1717–1721. [Google Scholar] [CrossRef] [PubMed]
- Zervas, M.; Somers, K.L.; Thrall, M.A.; Walkley, S.U. Critical role for glycosphingolipids in Niemann-Pick disease type C. Curr. Biol. 2001, 11, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- te Vruchte, D.; Lloyd-Evans, E.; Veldman, R.J.; Neville, D.C.; Dwek, R.A.; Platt, F.M.; van Blitterswijk, W.J.; Sillence, D.J. Accumulation of glycosphingolipids in Niemann-Pick C disease disrupts endosomal transport. J. Biol. Chem. 2004, 279, 26167–26175. [Google Scholar] [CrossRef]
- Welch, C.L.; Sun, Y.; Arey, B.J.; Lemaitre, V.; Sharma, N.; Ishibashi, M.; Sayers, S.; Li, R.; Gorelik, A.; Pleskac, N.; et al. Spontaneous atherothrombosis and medial degradation in Apoe−/−, Npc1−/− mice. Circulation 2007, 116, 2444–2452. [Google Scholar] [CrossRef]
- Feng, B.; Zhang, D.; Kuriakose, G.; Devlin, C.M.; Kockx, M.; Tabas, I. Niemann-Pick C heterozygosity confers resistance to lesional necrosis and macrophage apoptosis in murine atherosclerosis. Proc. Natl. Acad. Sci. USA 2003, 100, 10423–10428. [Google Scholar] [CrossRef]
- Afzali, M.; Nakhaee, A.; Tabatabaei, S.P.; Tirgar-Fakheri, K.; Hashemi, M. Aberrant promoter methylation profile of Niemann-pick type C1 gene in cardiovascular disease. Iran. Biomed. J. 2013, 17, 77–83. [Google Scholar] [CrossRef]
- Ma, W.; Xu, J.; Wang, Q.; Xin, Y.; Zhang, L.; Zheng, X.; Wang, H.; Sun, K.; Hui, R.; Huang, X. Interaction of functional NPC1 gene polymorphism with smoking on coronary heart disease. BMC Med. Genet. 2010, 11, 149. [Google Scholar] [CrossRef]
- Knapp, M.; Lisowska, A.; Knapp, P.; Baranowski, M. Dose-dependent effect of aspirin on the level of sphingolipids in human blood. Adv. Med. Sci. 2013, 58, 274–281. [Google Scholar] [CrossRef]
- Spijkers, L.J.; van den Akker, R.F.; Janssen, B.J.; Debets, J.J.; De Mey, J.G.; Stroes, E.S.; van den Born, B.J.; Wijesinghe, D.S.; Chalfant, C.E.; MacAleese, L.; et al. Hypertension is associated with marked alterations in sphingolipid biology: A potential role for ceramide. PLoS ONE 2011, 6, e21817. [Google Scholar] [CrossRef] [PubMed]
- Daidone, M.; Casuccio, A.; Puleo, M.G.; Del Cuore, A.; Pacinella, G.; Di Chiara, T.; Di Raimondo, D.; Immordino, P.; Tuttolomondo, A. Mediterranean diet effects on vascular health and serum levels of adipokines and ceramides. PLoS ONE 2024, 19, e0300844. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Dugo, C. Ceramides and risk of major adverse cardiovascular events: A meta-analysis of longitudinal studies. J. Clin. Lipidol. 2020, 14, 176–185. [Google Scholar] [CrossRef]
- Wang, D.D.; Toledo, E.; Hruby, A.; Rosner, B.A.; Willett, W.C.; Sun, Q.; Razquin, C.; Zheng, Y.; Ruiz-Canela, M.; Guasch-Ferré, M.; et al. Plasma Ceramides, Mediterranean Diet, and Incident Cardiovascular Disease in the PREDIMED Trial (Prevención con Dieta Mediterránea). Circulation 2017, 135, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Li, F.; Tan, X.; Wang, H.; Jiang, W.; Wang, X.; Li, S.; Zhang, Y.; Han, Q.; Wang, Y.; et al. Plasma Ceramides and Cardiovascular Events in Hypertensive Patients at High Cardiovascular Risk. Am. J. Hypertens. 2021, 34, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Hilvo, M.; Wallentin, L.; Ghukasyan Lakic, T.; Held, C.; Kauhanen, D.; Jylhä, A.; Lindbäck, J.; Siegbahn, A.; Granger, C.B.; Koenig, W.; et al. Prediction of Residual Risk by Ceramide-Phospholipid Score in Patients With Stable Coronary Heart Disease on Optimal Medical Therapy. J. Am. Heart Assoc. 2020, 9, e015258. [Google Scholar] [CrossRef]
- Saleem, M.; Bandaru, V.V.; Herrmann, N.; Swardfager, W.; Mielke, M.M.; Oh, P.I.; Shammi, P.; Kiss, A.; Haughey, N.J.; Rovinski, R.; et al. Ceramides predict verbal memory performance in coronary artery disease patients undertaking exercise: A prospective cohort pilot study. BMC Geriatr. 2013, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Yu, J.; Shi, R.; Yan, L.; Yang, T.; Li, Y.; Zhang, Z.; Yu, G.; Bai, Y.; Schuchman, E.H.; et al. Elevation of ceramide and activation of secretory acid sphingomyelinase in patients with acute coronary syndromes. Coron. Artery Dis. 2014, 25, 230–235. [Google Scholar] [CrossRef]
- Wittenbecher, C.; Eichelmann, F.; Toledo, E.; Guasch-Ferré, M.; Ruiz-Canela, M.; Li, J.; Arós, F.; Lee, C.H.; Liang, L.; Salas-Salvadó, J.; et al. Lipid Profiles and Heart Failure Risk: Results From Two Prospective Studies. Circ. Res. 2021, 128, 309–320. [Google Scholar] [CrossRef]
- Titievsky, L.; Schuster, T.; Wang, R.; Younus, M.; Palladino, A.; Quazi, K.; Wajnrajch, M.P.; Hernandez, B.; Becker, P.S.; Weinreb, N.J.; et al. Safety and effectiveness of taliglucerase alfa in patients with Gaucher disease: An interim analysis of real-world data from a multinational drug registry (TALIAS). Orphanet J. Rare Dis. 2022, 17, 145. [Google Scholar] [CrossRef]
- Weinreb, N.J.; Camelo, J.S.; Charrow, J.; McClain, M.R.; Mistry, P.; Belmatoug, N.; for the International Collaborative Gaucher Group (ICGG) Gaucher Registry (NCT00358943) investigators. Gaucher disease type 1 patients from the ICGG Gaucher Registry sustain initial clinical improvements during twenty years of imiglucerase treatment. Mol. Genet. Metab. 2021, 132, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Zimran, A.; Durán, G.; Giraldo, P.; Rosenbaum, H.; Giona, F.; Petakov, M.; Terreros Muñoz, E.; Solorio-Meza, S.E.; Cooper, P.A.; Varughese, S.; et al. Long-term efficacy and safety results of taliglucerase alfa through 5years in adult treatment-naïve patients with Gaucher disease. Blood Cells Mol. Dis. 2019, 78, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, K.; Olivares-Medina, C.N.; Villagrana-Escareño, M.V.; Juárez-Moreno, K.; Cadena-Nava, R.D.; Rodríguez-Hernández, A.G.; Vazquez-Duhalt, R. Targeted Enzymatic VLP-Nanoreactors with β-Glucocerebrosidase Activity as Potential Enzyme Replacement Therapy for Gaucher’s Disease. ChemMedChem 2022, 17, e202200384. [Google Scholar] [CrossRef] [PubMed]
- Martín-Banderas, L.; Holgado, M.A.; Durán-Lobato, M.; Infante, J.J.; Álvarez-Fuentes, J.; Fernández-Arévalo, M. Role of Nanotechnology for Enzyme Replacement Therapy in Lysosomal Diseases. A Focus on Gaucher’s Disease. Curr. Med. Chem. 2016, 23, 929–952. [Google Scholar] [CrossRef]
- Banikazemi, M.; Bultas, J.; Waldek, S.; Wilcox, W.R.; Whitley, C.B.; McDonald, M.; Finkel, R.; Packman, S.; Bichet, D.G.; Warnock, D.G.; et al. Agalsidase-beta therapy for advanced Fabry disease: A randomized trial. Ann. Intern. Med. 2007, 146, 77–86. [Google Scholar] [CrossRef]
- West, M.; Nicholls, K.; Mehta, A.; Clarke, J.T.; Steiner, R.; Beck, M.; Barshop, B.A.; Rhead, W.; Mensah, R.; Ries, M.; et al. Agalsidase alfa and kidney dysfunction in Fabry disease. J. Am. Soc. Nephrol. 2009, 20, 1132–1139. [Google Scholar] [CrossRef]
- Linhart, A.; Dostálová, G.; Nicholls, K.; West, M.L.; Tøndel, C.; Jovanovic, A.; Giraldo, P.; Vujkovac, B.; Geberhiwot, T.; Brill-Almon, E.; et al. Safety and efficacy of pegunigalsidase alfa in patients with Fabry disease who were previously treated with agalsidase alfa: Results from BRIDGE, a phase 3 open-label study. Orphanet J. Rare Dis. 2023, 18, 332. [Google Scholar] [CrossRef]
- Kuter, D.J.; Mehta, A.; Hollak, C.E.; Giraldo, P.; Hughes, D.; Belmatoug, N.; Brand, M.; Muller, A.; Schaaf, B.; Giorgino, R.; et al. Miglustat therapy in type 1 Gaucher disease: Clinical and safety outcomes in a multicenter retrospective cohort study. Blood Cells Mol. Dis. 2013, 51, 116–124. [Google Scholar] [CrossRef]
- Bennett, L.L.; Turcotte, K. Eliglustat tartrate for the treatment of adults with type 1 Gaucher disease. Drug Des. Devel Ther. 2015, 9, 4639–4647. [Google Scholar] [CrossRef]
- van der Veen, S.J.; Hollak, C.E.M.; van Kuilenburg, A.B.P.; Langeveld, M. Developments in the treatment of Fabry disease. J. Inherit. Metab. Dis. 2020, 43, 908–921. [Google Scholar] [CrossRef]
- Baccam, G.C.; Xie, J.; Jin, X.; Park, H.; Wang, B.; Husson, H.; Ibraghimov-Beskrovnaya, O.; Huang, C.L. Glucosylceramide synthase inhibition protects against cardiac hypertrophy in chronic kidney disease. Sci. Rep. 2022, 12, 9340. [Google Scholar] [CrossRef] [PubMed]
- Jennemann, R.; Volz, M.; Bestvater, F.; Schmidt, C.; Richter, K.; Kaden, S.; Müthing, J.; Gröne, H.J.; Sandhoff, R. Blockade of Glycosphingolipid Synthesis Inhibits Cell Cycle and Spheroid Growth of Colon Cancer Cells In Vitro and Experimental Colon Cancer Incidence In Vivo. Int. J. Mol. Sci. 2021, 22, 10539. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, M.M.; Dao, J.; Kasaci, N.; Adewale, B.; Nazari, S.; Noll, L.; Fikry, J.; Sanati, A.H.; Goker-Alpan, O. Cellular and biochemical response to chaperone versus substrate reduction therapies in neuropathic Gaucher disease. PLoS ONE 2021, 16, e0247211. [Google Scholar] [CrossRef] [PubMed]
- Yam, G.H.; Bosshard, N.; Zuber, C.; Steinmann, B.; Roth, J. Pharmacological chaperone corrects lysosomal storage in Fabry disease caused by trafficking-incompetent variants. Am. J. Physiol. Cell Physiol. 2006, 290, C1076–C1082. [Google Scholar] [CrossRef]
- He, Z.; Chen, Q.; Chen, F.; Zhang, J.; Li, H.; Lin, J.M. DNA-mediated cell surface engineering for multiplexed glycan profiling using MALDI-TOF mass spectrometry. Chem. Sci. 2016, 7, 5448–5452. [Google Scholar] [CrossRef]
- Cummings, R.D.; Pierce, J.M. The challenge and promise of glycomics. Chem. Biol. 2014, 21, 1–15. [Google Scholar] [CrossRef]

| Series | Abbreviation | Core Structure |
|---|---|---|
| Ganglio- | Gg | Galb3GalNAcb4Galb4Glc- |
| Globo- | Gb | GalNAcb3Gala4Galb4Glc- |
| Isoglobo- | iGB | GalNAcb3Gala3Galb4Glc- |
| Lacto- | Lc | Galb3GlcNAcb3Galb4Glc- |
| Neolacto- | nLc | Galb4GlcNAcb3Galb4Glc- |
| Mollu- | Mu | GlcNAcb2Mana3Manb4Glc- |
| Arthro- | At | GalNAcb4GlcNAcb3Manb4Glc- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Abutaleb, K.; Mishra, S. Glycosphingolipids in Cardiovascular Disease: Insights from Molecular Mechanisms and Heart Failure Models. Biomolecules 2024, 14, 1265. https://doi.org/10.3390/biom14101265
Huang S, Abutaleb K, Mishra S. Glycosphingolipids in Cardiovascular Disease: Insights from Molecular Mechanisms and Heart Failure Models. Biomolecules. 2024; 14(10):1265. https://doi.org/10.3390/biom14101265
Chicago/Turabian StyleHuang, Sarah, Karima Abutaleb, and Sumita Mishra. 2024. "Glycosphingolipids in Cardiovascular Disease: Insights from Molecular Mechanisms and Heart Failure Models" Biomolecules 14, no. 10: 1265. https://doi.org/10.3390/biom14101265
APA StyleHuang, S., Abutaleb, K., & Mishra, S. (2024). Glycosphingolipids in Cardiovascular Disease: Insights from Molecular Mechanisms and Heart Failure Models. Biomolecules, 14(10), 1265. https://doi.org/10.3390/biom14101265







