Morphological Characterization, Polyphenolic Profile, and Bioactive Properties of Limoncella, an Ancient Mediterranean Variety of Sweet Citrus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PCR Amplification of ITS Region
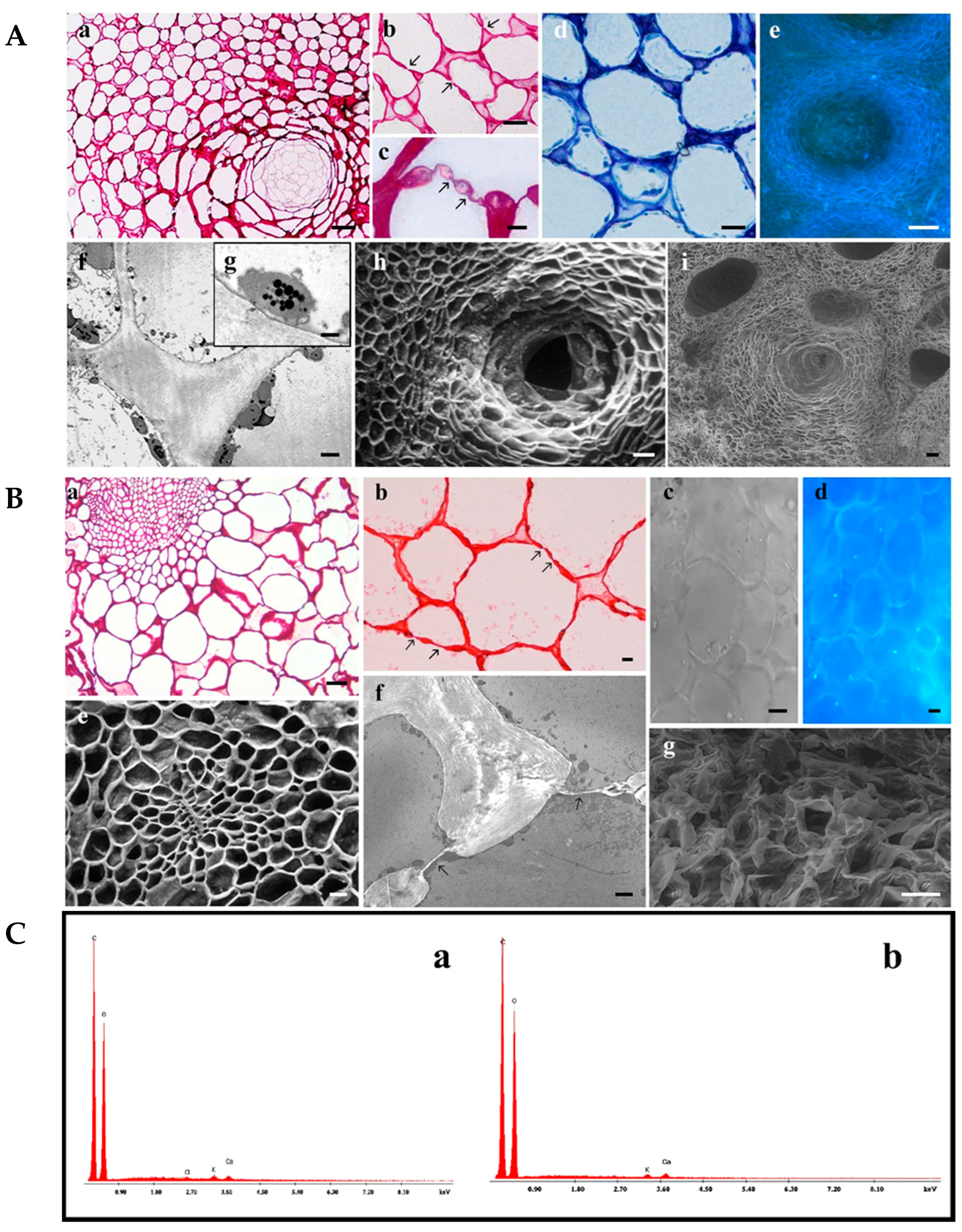
2.3. Light and Transmission Electron Microscopy
2.4. Scanning Electron Microscopy (SEM) and Environmental Scanning Electron Microscopy (ESEM)
2.5. Preparation of Albedo and Flavedo Extracts
2.6. Quantification of Total Phenolics by Folin–Ciocalteu Method
2.7. HPLC-ESI-MS/MS Analysis
2.8. Antioxidant Activity
2.8.1. DPPH (2,2-Diphenyl-1-picrylhydrazyl) Assay
2.8.2. ABTS, 2,2,-azino-bis(3-Ethylbenzothiazoline-6-sulphonic acid) Assay
2.8.3. Lipoxygenase Inhibition Assay
2.8.4. Chelating Capacity on Fe2+
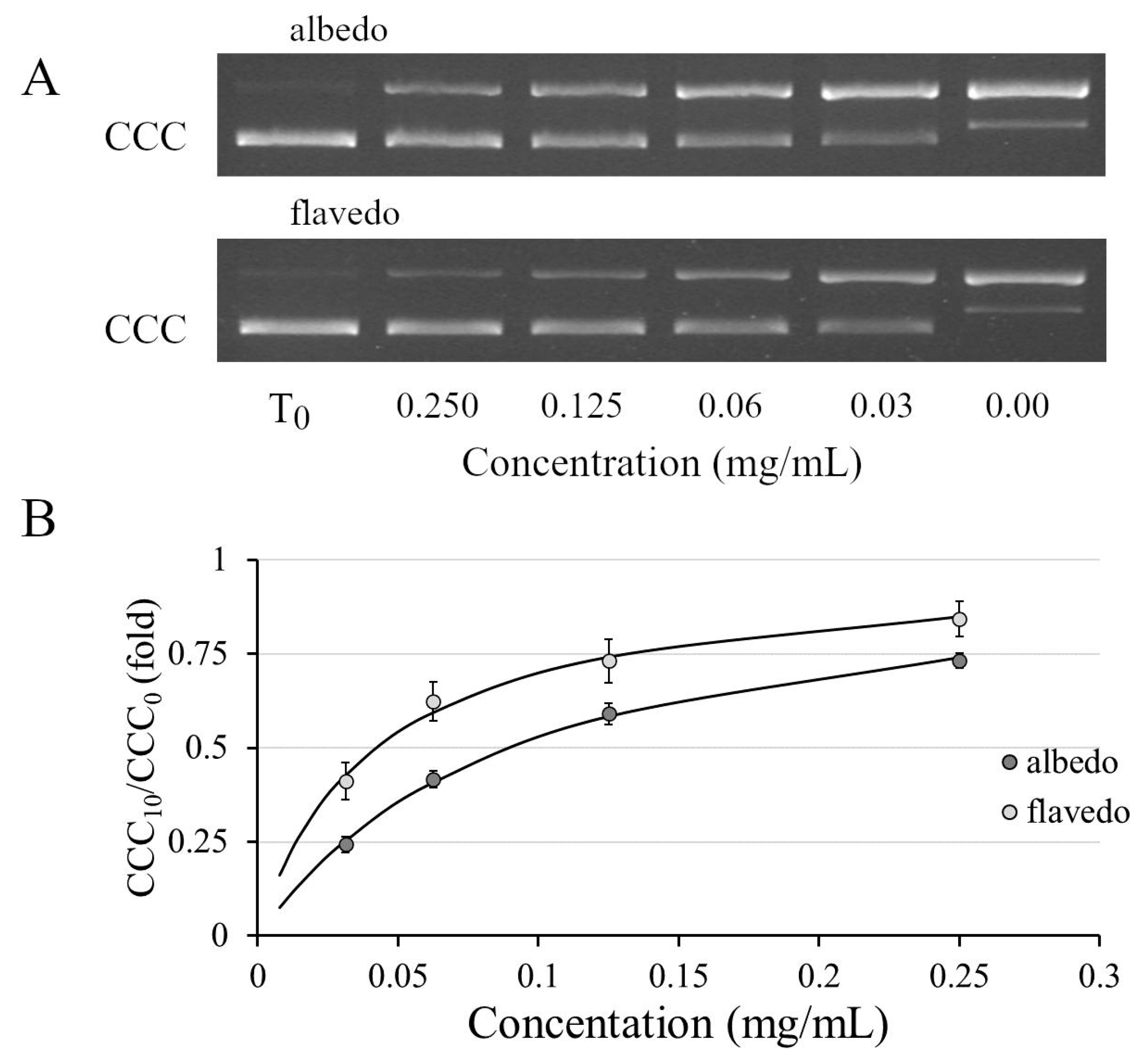
2.9. DNA Nicking Assay
2.10. Cell Cultures
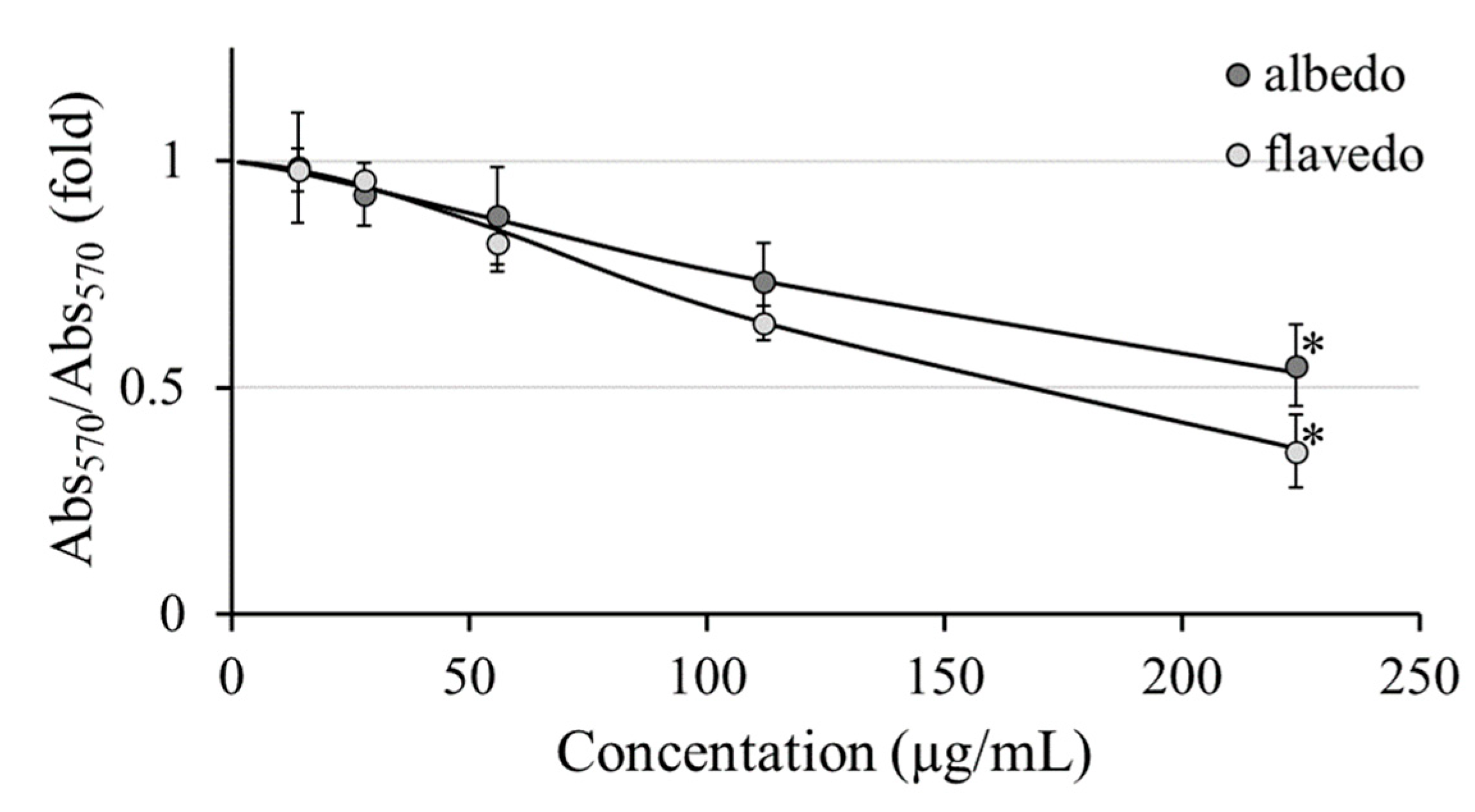
2.11. Cytotoxicity Assays
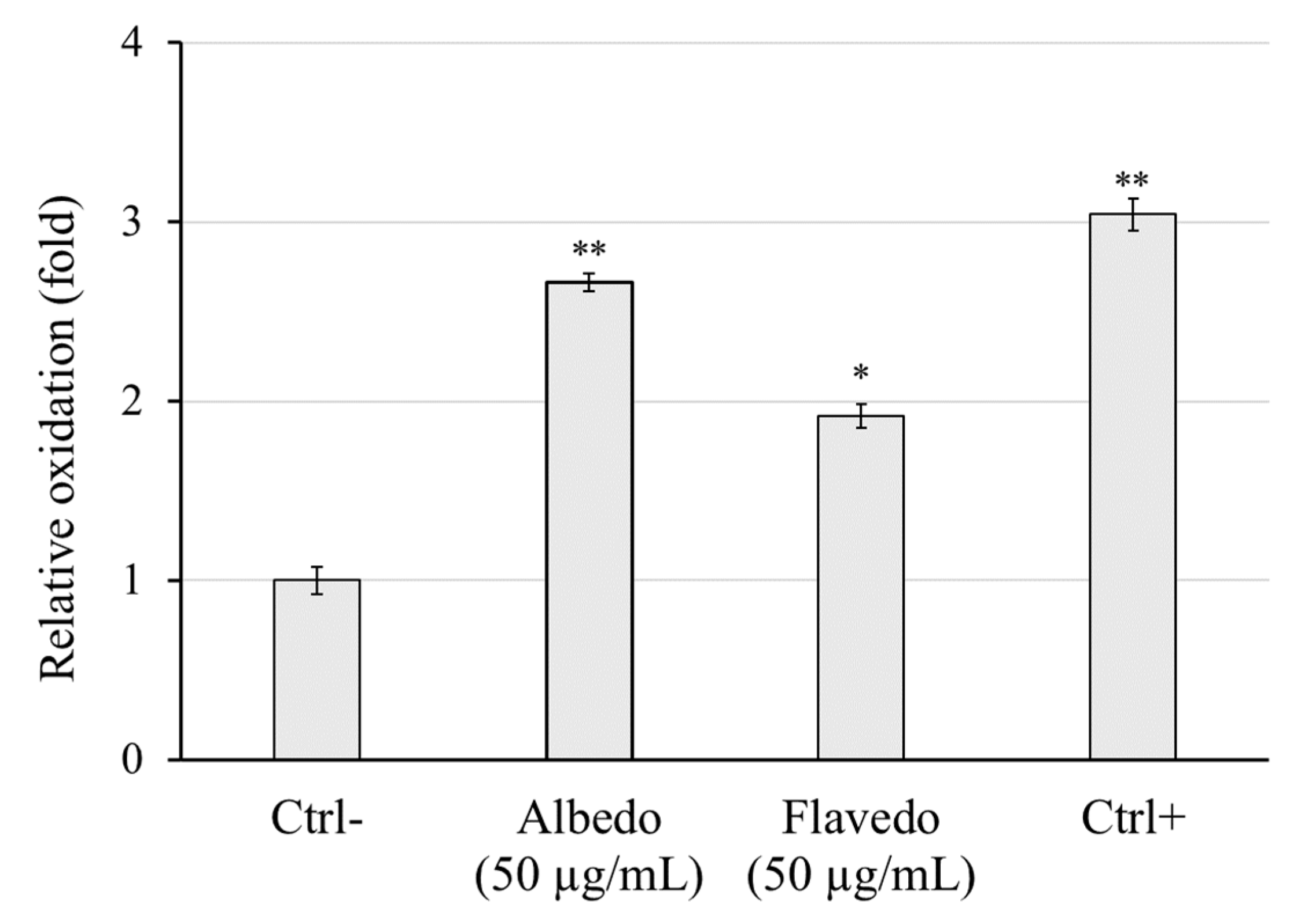
2.12. Evaluation of Antioxidant Properties by DCFH-DA Assay
2.13. Determination of Nitric Oxide Production
2.14. Statistical Analysis
3. Results
3.1. PCR Amplification, DNA Sequencing, and Sequence Analysis of the ITS Region
3.2. Morphological and Semi-Quantitative Data
3.3. Characterization and Quantification of Phenolic Compounds
3.4. Antioxidant Activities
3.5. Genoprotective Activity
3.6. Evaluation of the Cytotoxic Effects
3.7. Radical Scavenging Properties of Limoncella Extracts by Cell-Based Assay
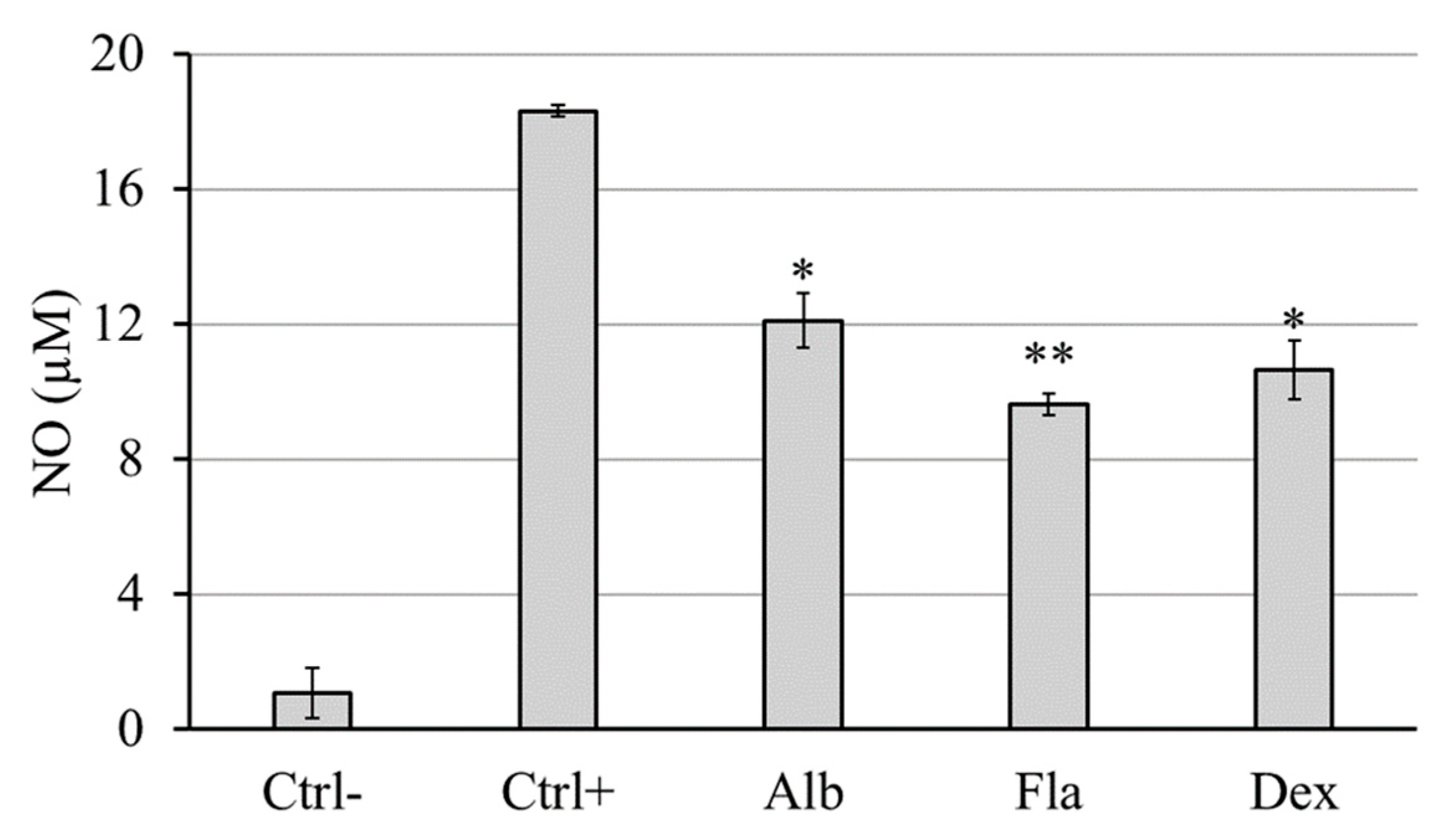
3.8. Anti-Inflammatory Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicolosi, E.; Deng, Z.N.; Gentile, A.; La Malfa, S.; Continella, G.; Tribulato, E. Citrus Phylogeny and Genetic Origin of Important Species as Investigated by Molecular Markers. Theor. Appl. Genet. 2000, 100, 1155–1166. [Google Scholar] [CrossRef]
- Zech-Matterne, V.; Fiorentino, G. AGRUMED: Archaeology and History of Citrus Fruit in the Mediterranean; Collection du Centre Jean Bérard, Publications du Centre Jean Bérard: Naples, Italy, 2017. [Google Scholar] [CrossRef]
- De Freitas, R.M.; Campêlo, L.M.L.; De Almeida, A.A.C.; De Freitas, R.L.M.; Cerqueira, G.S.; De Sousa, G.F.; Saldanha, G.B.; Feitosa, C.M. Antioxidant and Antinociceptive Effects of Citrus limon Essential Oil in Mice. J. Biomed Biotechnol. 2011, 2011, 678673. [Google Scholar] [CrossRef]
- Ladaniya, M.S. Nutritive and Medicinal Value of Citrus Fruits. In Citrus Fruit; Biology, Technology and Evaluation; Elsevier: Amsterdam, The Netherlands, 2008; pp. 501–514. [Google Scholar] [CrossRef]
- Ballistreri, G.; Fabroni, S.; Romeo, F.V.; Timpanaro, N.; Amenta, M.; Rapisarda, P. Anthocyanins and Other Polyphenols in Citrus Genus: Biosynthesis, Chemical Profile, and Biological Activity. In Polyphenols in Plants, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 191–215. [Google Scholar] [CrossRef]
- Zou, Z.; Xi, W.; Hu, Y.; Nie, C.; Zhou, Z. Antioxidant Activity of Citrus Fruits. Food Chem. 2016, 196, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Castro-Vazquez, L.; Alañón, M.E.; Rodríguez-Robledo, V.; Pérez-Coello, M.S.; Hermosín-Gutierrez, I.; Díaz-Maroto, M.C.; Jordán, J.; Galindo, M.F.; Arroyo-Jiménez, M.D.M. Bioactive Flavonoids, Antioxidant Behaviour, and Cytoprotective Effects of Dried Grapefruit Peels (Citrus paradisi Macf.). Oxidative Med. Cell. Longev. 2016, 2016, 8915729. [Google Scholar] [CrossRef] [PubMed]
- Esparza-Martínez, F.J.; Miranda-López, R.; Mata-Sánchez, S.M.; Guzmán-Maldonado, S.H. Extractable and Non-Extractable Phenolics and Antioxidant Capacity of Mandarin Waste Dried at Different Temperatures. Plant Foods Hum. Nutr. 2016, 71, 294–300. [Google Scholar] [CrossRef]
- Pepe, G.; Pagano, F.; Adesso, S.; Sommella, E.; Ostacolo, C.; Manfra, M.; Chieppa, M.; Sala, M.; Russo, M.; Marzocco, S.; et al. Bioavailable Citrus Sinensis Extract: Polyphenolic Composition and Biological Activity. Molecules 2017, 22, 623. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting Citrus Wastes into Value-Added Products: Economic and Environmently Friendly Approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef]
- Diab, K.A.E. In Vitro Studies on Phytochemical Content, Antioxidant, Anticancer, Immunomodulatory, and Antigenotoxic Activities of Lemon, Grapefruit, and Mandarin Citrus Peels. Asian Pac. J. Cancer Prev. 2016, 17, 3559–3567. [Google Scholar] [PubMed]
- Smeriglio, A.; Cornara, L.; Denaro, M.; Barreca, D.; Burlando, B.; Xiao, J.; Trombetta, D. Antioxidant and Cytoprotective Activities of an Ancient Mediterranean Citrus (Citrus lumia Risso) Albedo Extract: Microscopic Observations and Polyphenol Characterization. Food Chem. 2019, 279, 347–355. [Google Scholar] [CrossRef]
- Liang, X.; Wang, H.; Xu, W.; Liu, X.; Zhao, C.; Chen, J.; Wang, D.; Xu, S.; Cao, J.; Sun, C.; et al. Metabolome and Transcriptome Analysis Revealed the Basis of the Difference in Antioxidant Capacity in Different Tissues of Citrus Reticulata ‘Ponkan’. Antioxidants 2024, 13, 243. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Methods in Enzymology—Oxidants and Antioxidants Part A. Methods Enzym. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Angeloni, S.; Abouelenein, D.; Acquaticci, L.; Xiao, J.; Sagratini, G.; Maggi, F.; Vittori, S.; Caprioli, G. A New HPLC-MS/MS Method for the Simultaneous Determination of 36 Polyphenols in Blueberry, Strawberry and Their Commercial Products and Determination of Antioxidant Activity. Food Chem. 2022, 367, 130743. [Google Scholar] [CrossRef]
- Santanatoglia, A.; Cespi, M.; Perinelli, D.R.; Fioretti, L.; Sagratini, G.; Vittori, S.; Caprioli, G. Impact of the Human Factor on the Reproducibility of Different Coffee Brewing Methods. J. Food Compos. Anal. 2023, 124, 105698. [Google Scholar] [CrossRef]
- Santanatoglia, A.; Caprioli, G.; Cespi, M.; Ciarlantini, D.; Cognigni, L.; Fioretti, L.; Maggi, F.; Mustafa, A.M.; Nzekoue, F.; Vittori, S. A Comprehensive Comparative Study among the Newly Developed Pure Brew Method and Classical Ones for Filter Coffee Production. LWT 2023, 175, 114471. [Google Scholar] [CrossRef]
- Saltarelli, R.; Palma, F.; Gioacchini, A.M.; Calcabrini, C.; Mancini, U.; De Bellis, R.; Stocchi, V.; Potenza, L. Phytochemical Composition, Antioxidant and Antiproliferative Activities and Effects on Nuclear DNA of Ethanolic Extract from an Italian Mycelial Isolate of Ganoderma Lucidum. J. Ethnopharmacol. 2019, 231, 464–473. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Sicari, V.; Tundis, R.; Leporini, M.; Falco, T.; Calabrò, V. The Influence of Ultrafiltration of Citrus limon L. Burm. cv Femminello Comune Juice on Its Chemical Composition and Antioxidant and Hypoglycemic Properties. Antioxidants 2019, 8, 23. [Google Scholar] [CrossRef]
- Flemmig, J.; Arnhold, J. Ferrous Ion-Induced Strand Breaks in the DNA Plasmid PBR322 Are Not Mediated by Hydrogen Peroxide. Eur. Biophys. J. 2007, 36, 377–384. [Google Scholar] [CrossRef]
- Benedetti, S.; Catalani, S.; Palma, F.; Canestrari, F. The Antioxidant Protection of CELLFOOD® against Oxidative Damage in Vitro. Food Chem. Toxicol. 2011, 49, 2292–2298. [Google Scholar] [CrossRef]
- Adan, A.; Kiraz, Y.; Baran, Y. Cell Proliferation and Cytotoxicity Assays. Curr. Pharm. Biotechnol. 2016, 17, 1213–1221. [Google Scholar] [CrossRef]
- Catalani, S.; Palma, F.; Battistelli, S.; Benedetti, S. Oxidative Stress and Apoptosis Induction in Human Thyroid Carcinoma Cells Exposed to the Essential Oil from Pistacia lentiscus Aerial Parts. PLoS ONE 2017, 12, e0172138. [Google Scholar] [CrossRef]
- Benedetti, S.; Catalani, S.; Canonico, B.; Nasoni, M.G.; Luchetti, F.; Papa, S.; Potenza, L.; Palma, F. The Effects of Acyclovir Administration to NCI-H1975 Non-Small Cell Lung Cancer Cells. Toxicol. Vitr. 2022, 79, 105301. [Google Scholar] [CrossRef]
- Bryan, N.S.; Grisham, M.B. Methods to Detect Nitric Oxide and Its Metabolites in Biological Samples. Free. Radic. Biol. Med. 2007, 43, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Borghi, S.M.; Pavanelli, W.R. Antioxidant Compounds and Health Benefits of Citrus Fruits. Antioxidants 2023, 12, 1526. [Google Scholar] [CrossRef]
- Richa, R.; Kohli, D.; Vishwakarma, D.; Mishra, A.; Kabdal, B.; Kothakota, A.; Richa, S.; Sirohi, R.; Kumar, R.; Naik, B. Citrus Fruit: Classification, Value Addition, Nutritional and Medicinal Values, and Relation with Pandemic and Hidden Hunger. J. Agric. Food Res. 2023, 14, 100718. [Google Scholar] [CrossRef]
- González-Molina, E.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Natural Bioactive Compounds of Citrus limon for Food and Health. J. Pharm. Biomed. Anal. 2010, 51, 327–345. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Composition, Antioxidant Potential and Health Benefits of Citrus Peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef]
- De Moraes Barros, H.R.; De Castro Ferreira, T.A.P.; Genovese, M.I. Antioxidant Capacity and Mineral Content of Pulp and Peel from Commercial Cultivars of Citrus from Brazil. Food Chem. 2012, 134, 1892–1898. [Google Scholar] [CrossRef]
- Multari, S.; Licciardello, C.; Caruso, M.; Anesi, A.; Martens, S. Flavedo and Albedo of Five Citrus Fruits from Southern Italy: Physicochemical Characteristics and Enzyme-Assisted Extraction of Phenolic Compounds. J. Food Meas. Charact. 2021, 15, 1754–1762. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, J.; Cao, J.; Wang, D.; Liu, C.; Yang, R.; Li, X.; Sun, C. Antioxidant Capacity, Anticancer Ability and Flavonoids Composition of 35 Citrus (Citrus reticulata Blanco) Varieties. Molecules 2017, 22, 1114. [Google Scholar] [CrossRef] [PubMed]
- Mateus, A.R.S.; Teixeira, J.D.; Barros, S.C.; Almeida, C.; Silva, S.; Sanches-Silva, A. Unlocking the Potential of Citrus medica L.: Antioxidant Capacity and Phenolic Profile across Peel, Pulp, and Seeds. Molecules 2024, 29, 3533. [Google Scholar] [CrossRef]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid Composition of Fruit Tissues of Citrus Species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef]
- Chhikara, N.; Kour, R.; Jaglan, S.; Gupta, P.; Gat, Y.; Panghal, A. Citrus Medica: Nutritional, Phytochemical Composition and Health Benefits—A Review. Food Funct. 2018, 9, 1978–1992. [Google Scholar] [CrossRef]
- Batool, S.; Javaid, S.; Javed, H.; Asim, L.; Shahid, I.; Khan, M.; Muhammad, A. Addressing Artifacts of Colorimetric Anticancer Assays for Plant-Based Drug Development. Med. Oncol. 2022, 39, 198. [Google Scholar] [CrossRef]
- Geran, R.I.; Greenberg, N.H.; Macdonald, M.M.; Schumacher, A. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother. Rep. 1972, 3, 59–61. [Google Scholar]
- Gözcü, S.; Polat, H.K.; Gültekin, Y.; Ünal, S.; Karakuyu, N.F.; Şafak, E.K.; Doğan, O.; Pezik, E.; Haydar, M.K.; Aytekin, E.; et al. Formulation of Hesperidin-Loaded in Situ Gel for Ocular Drug Delivery: A Comprehensive Study. J. Sci. Food Agric. 2024, 104, 5846–5859. [Google Scholar] [CrossRef]
- Lončarić, M.; Strelec, I.; Moslavac, T.; Šubarić, D.; Pavić, V.; Molnar, M. Lipoxygenase Inhibition by Plant Extracts. Biomolecules 2021, 11, 152. [Google Scholar] [CrossRef]
- Taciak, B.; Białasek, M.; Braniewska, A.; Sas, Z.; Sawicka, P.; Kiraga, Ł.; Rygiel, T.; Król, M. Evaluation of Phenotypic and Functional Stability of RAW 264.7 Cell Line through Serial Passages. PLoS ONE 2018, 13, e0198943. [Google Scholar] [CrossRef]
- Pragasam, S.J.; Venkatesan, V.; Rasool, M. Immunomodulatory and Anti-Inflammatory Effect of p-Coumaric Acid, a Common Dietary Polyphenol on Experimental Inflammation in Rats. Inflammation 2013, 36, 169–176. [Google Scholar] [CrossRef]
- Iriti, M.; Varoni, E.M.; Vitalini, S. Bioactive Compounds in Health and Disease—Focus on Rutin. Bioact. Compd. Health Dis. 2023, 6, 235–242. [Google Scholar] [CrossRef]
- Mari, G.; Catalani, S.; Antonini, E.; De Crescentini, L.; Mantellini, F.; Santeusanio, S.; Lombardi, P.; Amicucci, A.; Battistelli, S.; Benedetti, S.; et al. Synthesis and Biological Evaluation of Novel Heteroring-Annulated Pyrrolino-Tetrahydroberberine Analogues as Antioxidant Agents. Bioorg. Med. Chem. 2018, 26, 5037–5044. [Google Scholar] [CrossRef]
- Zhao, Z. Hydroxyl Radical Generations Form the Physiologically Relevant Fenton-like Reactions. Free Radic. Biol. Med. 2023, 208, 510–515. [Google Scholar] [CrossRef]
- Benedetti, S.; Nasoni, M.G.; Luchetti, F.; Palma, F. New Insights into the Cytotoxic Effects of Thymus vulgaris Essential Oil on the Human Triple-Negative Breast Cancer Cell Line MDA-MB-231. Toxicol. Vitr. 2023, 93, 105705. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, Oxidants, and Aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef]
- Entezari, M.; Majd, A.; Falahian, F.; Mehrabian, S.; Hashemi, M.; Lajimi, A.A. Antimutagenicity and Anticancer Effects of Citrus Medica Fruit Juice. Acta Med. Iran. 2009, 47, 373–377. [Google Scholar]
- Koolaji, N.; Shammugasamy, B.; Schindeler, A.; Dong, Q.; Dehghani, F.; Valtchev, P. Citrus Peel Flavonoids as Potential Cancer Prevention Agents. Curr. Dev. Nutr. 2020, 4, nzaa025. [Google Scholar] [CrossRef]
- Ahmad, A.; Afzaal, M.; Saeed, F.; Ali, S.W.; Imran, A.; Zaidi, S.Y.R.; Saleem, M.A.; Hussain, M.; Al Jbawi, E. A Comprehensive Review of the Therapeutic Potential of Citrus Bioflavonoid Hesperidin against Lifestyle-Related Disorders. Cogent Food Agric. 2023, 9, 2226427. [Google Scholar] [CrossRef]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and Anti-Inflammatory Properties of the Citrus Flavonoids Hesperidin and Hesperetin: An Updated Review of Their Molecular Mechanisms and Experimental Models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef]
- Li, X.; Huang, W.; Tan, R.; Xu, C.; Chen, X.; Li, S.; Liu, Y.; Qiu, H.; Cao, H.; Cheng, Q. The Benefits of Hesperidin in Central Nervous System Disorders, Based on the Neuroprotective Effect. Biomed. Pharmacother. 2023, 159, 114222. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health Effects of Quercetin: From Antioxidant to Nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Morgulis, A.; Coulouris, G.; Raytselis, Y.; Madden, T.L.; Agarwala, R.; Schäffer, A.A. Database Indexing for Production MegaBLAST Searches. Bioinformatics 2008, 24, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A Greedy Algorithm for Aligning DNA Sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef] [PubMed]

| No | Compounds | Albedo Concentration mg kg−1 | Flavedo Concentration mg kg−1 |
|---|---|---|---|
| 1 | Gallic acid | 5.57 | 41.20 |
| 2 | Neochlorogenic acid | n.d. a | n.d. |
| 3 | Delphinidin-3-galactoside | n.d. | 7.35 |
| 4 | (+)-Catechin | n.d. | n.d. |
| 5 | Procyanidin B2 | n.d. | n.d. |
| 6 | Chlorogenic acid | 0.80 | 1.54 |
| 7 | p-Hydroxybenzoic acid | 411.34 | 895.60 |
| 8 | (-)-Epicatechin | n.d. | 0.69 |
| 9 | Cyanidin-3-glucoside | n.d. | n.d. |
| 10 | Petunidin-3-glucoside | 0.45 | 1.82 |
| 11 | 3-Hydroxybenzoic acid | n.d. | n.d. |
| 12 | Caffeic acid | 6.92 | n.d. |
| 13 | Vanillic acid | 134.39 | n.d. |
| 14 | Resveratrol | n.d. | n.d. |
| 15 | Pelargonidin-3-glucoside | n.d. | 0.50 |
| 16 | Pelagonidin-3-rutinoside | n.d. | n.d. |
| 17 | Malvidin-3-galactoside | n.d. | n.d. |
| 18 | Syringic acid | n.d. | 36.43 |
| 19 | Procyanidin A2 | n.d. | n.d. |
| 20 | p-Coumaric acid | 143.89 | 1566.65 |
| 21 | Ferulic acid | 147.86 | 609.96 |
| 22 | 3,5-Dicaffeoylquinic acid | n.d. | n.d. |
| 23 | Rutin | 137.04 | 5996.78 |
| 24 | Hyperoside | n.d. | 245.49 |
| 25 | Isoquercitrin | 9.73 | 216.05 |
| 26 | Delphindin-3,5-diglucoside | 68.98 | 212.24 |
| 27 | Phloridzin | 0.82 | n.d. |
| 28 | Quercitrin | 1961.17 | 267.76 |
| 29 | Myricetin | 0.41 | n.d. |
| 30 | Naringin | 3.86 | 1.65 |
| 31 | Kaempferol-3-glucoside | 47.45 | 4.73 |
| 32 | Hesperidin | 11,625.45 | 778.88 |
| 33 | Ellagic acid | n.d. | 24.49 |
| 34 | trans-cinnamic acid | 13.23 | 4.09 |
| 35 | Quercetin | 12.92 | 73.08 |
| 36 | Phloretin | n.d. | n.d. |
| 37 | Kaempferol | 128.20 | 246.73 |
| 38 | Isorhamnetin | 3.22 | 158.56 |
| Total content (mg kg−1) | 14,863.69 | 11,392.24 | |
| Total content (%) | 1.48 | 1.14 | |
| Total content (mg kg−1) Folin–Ciocalteu method | 18,700 | 22,040 | |
| Part of Fruit | DPPH EC50 (mg/mL) | ABTS EC50 (mg/mL) | Lipoxygenase EC50 (mg/mL) | Total Antioxidant Capacity (µg TE/mg) |
|---|---|---|---|---|
| Albedo | 0.996 ± 0.17 | 0.063 ± 0.029 | 0.54 ± 0.10 | 35.42 ± 3.31 |
| Flavedo | 0.971 ± 0.34 | 0.063 ± 0.012 | 0.14 ± 0.06 * | 40.41 ± 10.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potenza, L.; Saltarelli, R.; Palma, F.; Di Patria, L.; Annibalini, G.; Burattini, S.; Gobbi, P.; Valentini, L.; Caprioli, G.; Santanatoglia, A.; et al. Morphological Characterization, Polyphenolic Profile, and Bioactive Properties of Limoncella, an Ancient Mediterranean Variety of Sweet Citrus. Biomolecules 2024, 14, 1275. https://doi.org/10.3390/biom14101275
Potenza L, Saltarelli R, Palma F, Di Patria L, Annibalini G, Burattini S, Gobbi P, Valentini L, Caprioli G, Santanatoglia A, et al. Morphological Characterization, Polyphenolic Profile, and Bioactive Properties of Limoncella, an Ancient Mediterranean Variety of Sweet Citrus. Biomolecules. 2024; 14(10):1275. https://doi.org/10.3390/biom14101275
Chicago/Turabian StylePotenza, Lucia, Roberta Saltarelli, Francesco Palma, Laura Di Patria, Giosuè Annibalini, Sabrina Burattini, Pietro Gobbi, Laura Valentini, Giovanni Caprioli, Agnese Santanatoglia, and et al. 2024. "Morphological Characterization, Polyphenolic Profile, and Bioactive Properties of Limoncella, an Ancient Mediterranean Variety of Sweet Citrus" Biomolecules 14, no. 10: 1275. https://doi.org/10.3390/biom14101275
APA StylePotenza, L., Saltarelli, R., Palma, F., Di Patria, L., Annibalini, G., Burattini, S., Gobbi, P., Valentini, L., Caprioli, G., Santanatoglia, A., Vittori, S., & Barbieri, E. (2024). Morphological Characterization, Polyphenolic Profile, and Bioactive Properties of Limoncella, an Ancient Mediterranean Variety of Sweet Citrus. Biomolecules, 14(10), 1275. https://doi.org/10.3390/biom14101275











