[18F]NaF PET/CT as a Marker for Fibrodysplasia Ossificans Progressiva: From Molecular Mechanisms to Clinical Applications in Bone Disorders
Abstract
:1. Introduction
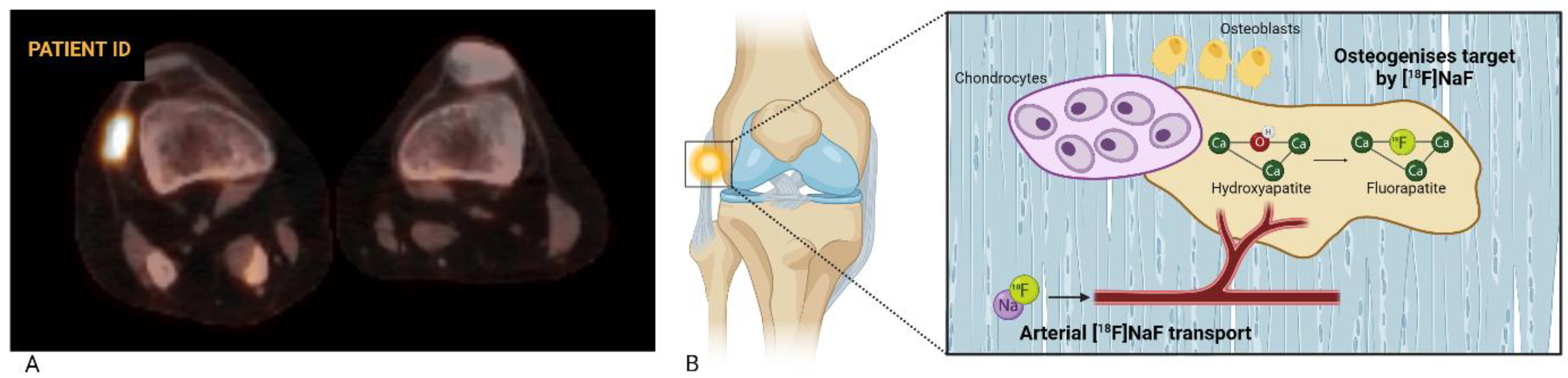
2. [18F]NaF as a Biological Marker of Bone Formation
2.1. Pharmacokinetics of [18F]NaF
2.2. [18F]NaF PET in Bone Disorders
3. [18F]NaF PET/CT in FOP
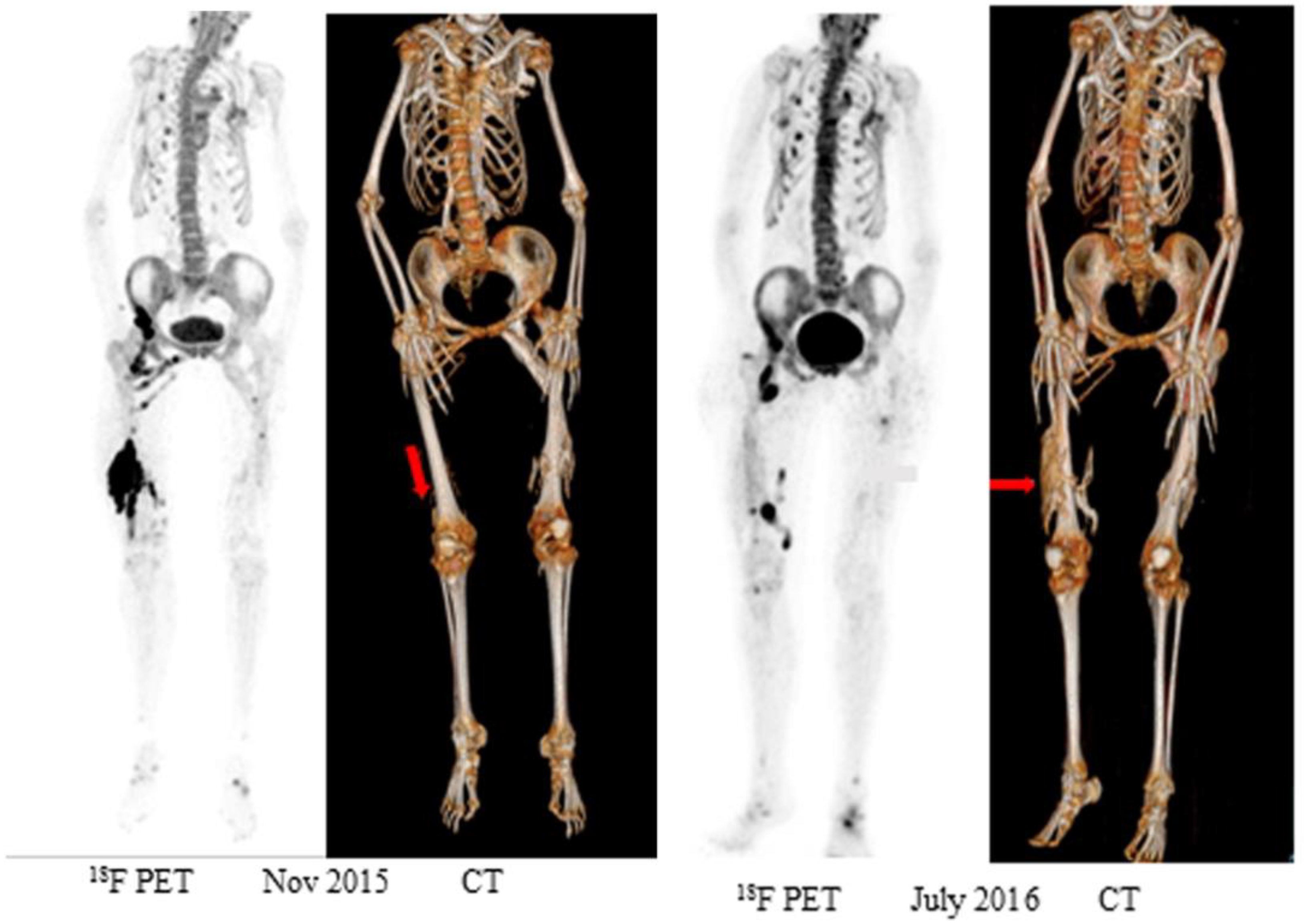
3.1. Early Detection of Ossification
3.2. Chronic Lesions and Clinical Trials
4. Quantitative [18F]NaF PET
5. Discussion
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shore, E.M.; Xu, M.; Feldman, G.J.; Fenstermacher, D.A.; Cho, T.J.; Choi, I.H.; Connor, J.M.; Delai, P.; Glaser, D.L.; LeMerrer, M.; et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat. Genet. 2006, 38, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Pignolo, R.J.; Hsiao, E.C.; Baujat, G.; Lapidus, D.; Sherman, A.; Kaplan, F.S. Prevalence of fibrodysplasia ossificans progressiva (FOP) in the United States: Estimate from three treatment centers and a patient organization. Orphanet J. Rare Dis. 2021, 16, 350. [Google Scholar] [CrossRef] [PubMed]
- Baujat, G.; Choquet, R.; Bouée, S.; Jeanbat, V.; Courouve, L.; Ruel, A.; Michot, C.; Le Quan Sang, K.H.; Lapidus, D.; Messiaen, C.; et al. Prevalence of fibrodysplasia ossificans progressiva (FOP) in France: An estimate based on a record linkage of two national databases. Orphanet J. Rare Dis. 2017, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Pignolo, R.J.; Bedford-Gay, C.; Liljesthröm, M.; Durbin-Johnson, B.P.; Shore, E.M.; Rocke, D.M.; Kaplan, F.S. The Natural History of Flare-Ups in Fibrodysplasia Ossificans Progressiva (FOP): A Comprehensive Global Assessment. J. Bone Miner. Res. 2016, 31, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.S.; Glaser, D.L. Thoracic insufficiency syndrome in patients with fibrodysplasia ossificans progressiva. Clin. Rev. Bone Miner. Metab. 2005, 3, 213–216. [Google Scholar] [CrossRef]
- Smilde, B.J.; Botman, E.; de Ruiter, R.D.; Smit, J.M.; Teunissen, B.P.; Lubbers, W.D.; Schwarte, L.A.; Schober, P.; Eekhoff, E.M.W. Monitoring and Management of Fibrodysplasia Ossificans Progressiva: Current Perspectives. Orthop. Res. Rev. 2022, 14, 113–120. [Google Scholar] [CrossRef]
- Pignolo, R.J.; McCarrick-Walmsley, R.; Wang, H.; Qiu, S.; Hunter, J.; Barr, S.; He, K.; Zhang, H.; Kaplan, F.S. Plasma-Soluble Biomarkers for Fibrodysplasia Ossificans Progressiva (FOP) Reflect Acute and Chronic States. J. Bone Miner. Res. 2022, 37, 475–483. [Google Scholar] [CrossRef]
- Hildebrand, L.; Gaber, T.; Kühnen, P.; Morhart, R.; Unterbörsch, H.; Schomburg, L.; Seemann, P. Trace element and cytokine concentrations in patients with Fibrodysplasia Ossificans Progressiva (FOP): A case control study. J. Trace Elem. Med. Biol. 2017, 39, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Pignolo, R.J.; Kimel, M.; Whalen, J.; Kawata, A.K.; Artyomenko, A.; Kaplan, F.S. The Fibrodysplasia Ossificans Progressiva Physical Function Questionnaire (FOP-PFQ): A patient-reported, disease-specific measure. Bone 2023, 168, 116642. [Google Scholar] [CrossRef]
- Kaplan, F.S.; Al Mukaddam, M.; Pignolo, R.J. A cumulative analogue joint involvement scale (CAJIS) for fibrodysplasia ossificans progressiva (FOP). Bone 2017, 101, 123–128. [Google Scholar] [CrossRef]
- Al Mukaddam, M.; Rajapakse, C.S.; Pignolo, R.J.; Kaplan, F.S.; Smith, S.E. Imaging assessment of fibrodysplasia ossificans progressiva: Qualitative, quantitative and questionable. Bone 2018, 109, 147–152. [Google Scholar] [CrossRef]
- Botman, E.; Teunissen, B.P.; Raijmakers, P.; de Graaf, P.; Yaqub, M.; Treurniet, S.; Schoenmaker, T.; Bravenboer, N.; Micha, D.; Pals, G.; et al. Diagnostic Value of Magnetic Resonance Imaging in Fibrodysplasia Ossificans Progressiva. JBMR Plus 2020, 4, e10363. [Google Scholar] [CrossRef] [PubMed]
- Eekhoff, E.M.W.; Netelenbos, J.C.; de Graaf, P.; Hoebink, M.; Bravenboer, N.; Micha, D.; Pals, G.; de Vries, T.J.; Lammertsma, A.A.; Raijmakers, P.G.; et al. Flare-Up After Maxillofacial Surgery in a Patient With Fibrodysplasia Ossificans Progressiva: An [(18)F]-NaF PET/CT Study and a Systematic Review. JBMR Plus 2018, 2, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Eekhoff, E.M.W.; Botman, E.; Coen Netelenbos, J.; de Graaf, P.; Bravenboer, N.; Micha, D.; Pals, G.; de Vries, T.J.; Schoenmaker, T.; Hoebink, M.; et al. [18F]NaF PET/CT scan as an early marker of heterotopic ossification in fibrodysplasia ossificans progressiva. Bone 2018, 109, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.S.; Tabas, J.A.; Gannon, F.H.; Finkel, G.; Hahn, G.V.; Zasloff, M.A. The histopathology of fibrodysplasia ossificans progressiva. An endochondral process. J. Bone Jt. Surg. Am. 1993, 75, 220–230. [Google Scholar] [CrossRef]
- Kaplan, F.S.; Strear, C.M.; Zasloff, M.A. Radiographic and scintigraphic features of modeling and remodeling in the heterotopic skeleton of patients who have fibrodysplasia ossificans progressiva. Clin. Orthop. Relat. Res. 1994, 304, 238–247. [Google Scholar]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast Differentiation and Bone Matrix Formation In Vivo and In Vitro. Tissue Eng. Part. B Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, H.M. Developmental regulation of the growth plate. Nature 2003, 423, 332–336. [Google Scholar] [CrossRef]
- Posner, A.S. The Mineral of Bone. Clin. Orthop. Relat. Res.® 1985, 200, 87–99. [Google Scholar] [CrossRef]
- Grynpas, M.D.; Cheng, P.T. Fluoride reduces the rate of dissolution of bone. Bone Miner. 1988, 5, 1–9. [Google Scholar] [CrossRef]
- Farley, J.R.; Wergedal, J.E.; Baylink, D.J. Fluoride directly stimulates proliferation and alkaline phosphatase activity of bone-forming cells. Science 1983, 222, 330–332. [Google Scholar] [CrossRef] [PubMed]
- Blau, M.; Ganatra, R.; Bender, M.A. 18 F-fluoride for bone imaging. Semin. Nucl. Med. 1972, 2, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Grant, F.D.; Fahey, F.H.; Packard, A.B.; Davis, R.T.; Alavi, A.; Treves, S.T. Skeletal PET with 18F-fluoride: Applying new technology to an old tracer. J. Nucl. Med. 2008, 49, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, M.; Mottaghy, F.M.; Paycha, F.; Behrendt, F.F.F.; Van den Wyngaert, T.; Fogelman, I.; Strobel, K.; Celli, M.; Fanti, S.; Giammarile, F.; et al. (18)F-NaF PET/CT: EANM procedure guidelines for bone imaging. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1767–1777. [Google Scholar] [CrossRef]
- Segall, G.; Delbeke, D.; Stabin, M.G.; Even-Sapir, E.; Fair, J.; Sajdak, R.; Smith, G.T. SNM Practice Guideline for Sodium <sup>18</sup>F-Fluoride PET/CT Bone Scans 1.0. J. Nucl. Med. 2010, 51, 1813–1820. [Google Scholar] [CrossRef]
- Hsu, W.K.; Feeley, B.T.; Krenek, L.; Stout, D.B.; Chatziioannou, A.F.; Lieberman, J.R. The use of 18F-fluoride and 18F-FDG PET scans to assess fracture healing in a rat femur model. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1291–1301. [Google Scholar] [CrossRef]
- Park, P.S.U.; Raynor, W.Y.; Sun, Y.; Werner, T.J.; Rajapakse, C.S.; Alavi, A. (18)F-Sodium Fluoride PET as a Diagnostic Modality for Metabolic, Autoimmune, and Osteogenic Bone Disorders: Cellular Mechanisms and Clinical Applications. Int. J. Mol. Sci. 2021, 22, 6504. [Google Scholar] [CrossRef]
- van der Bruggen, W.; Hagelstein-Rotman, M.; de Geus-Oei, L.-F.; Smit, F.; Dijkstra, P.D.S.; Appelman-Dijkstra, N.M.; Vriens, D. Quantifying skeletal burden in fibrous dysplasia using sodium fluoride PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1527–1537. [Google Scholar] [CrossRef]
- Papadakis, G.Z.; Manikis, G.C.; Karantanas, A.H.; Florenzano, P.; Bagci, U.; Marias, K.; Collins, M.T.; Boyce, A.M. 18F-NaF PET/CT imaging in fibrous dysplasia of bone. J. Bone Miner. Res. 2019, 34, 1619–1631. [Google Scholar] [CrossRef]
- van der Bruggen, W.; Vriens, D.; Meier, M.E.; Smit, F.; Winter, E.M.; de Geus-Oei, L.F.; Appelman-Dijkstra, N.M. Denosumab Reduces Lesional Fluoride Skeletal Burden on Na[18F]F PET-CT in Patients With Fibrous Dysplasia/McCune-Albright Syndrome. J. Clin. Endocrinol. Metab. 2021, 106, e2980–e2994. [Google Scholar] [CrossRef]
- Messa, C.; Goodman, W.G.; Hoh, C.K.; Choi, Y.; Nissenson, A.R.; Salusky, I.B.; Phelps, M.E.; Hawkins, R.A. Bone metabolic activity measured with positron emission tomography and [18F]fluoride ion in renal osteodystrophy: Correlation with bone histomorphometry. J. Clin. Endocrinol. Metab. 1993, 77, 949–955. [Google Scholar] [CrossRef]
- Cook, G.J.R.; Blake, G.M.; Marsden, P.K.; Cronin, B.; Fogelman, I. Quantification of Skeletal Kinetic Indices in Paget’s Disease Using Dynamic18F-Fluoride Positron Emission Tomography. J. Bone Miner. Res. 2002, 17, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Aaltonen, L.; Koivuviita, N.; Seppänen, M.; Tong, X.; Kröger, H.; Löyttyniemi, E.; Metsärinne, K. Correlation between 18F-Sodium Fluoride positron emission tomography and bone histomorphometry in dialysis patients. Bone 2020, 134, 115267. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, G.Z.; Jha, S.; Bhattacharyya, T.; Millo, C.; Tu, T.W.; Bagci, U.; Marias, K.; Karantanas, A.H.; Patronas, N.J. 18F-NaF PET/CT in Extensive Melorheostosis of the Axial and Appendicular Skeleton With Soft-Tissue Involvement. Clin. Nucl. Med. 2017, 42, 537–539. [Google Scholar] [CrossRef]
- Kang, H.; Jha, S.; Deng, Z.; Fratzl-Zelman, N.; Cabral, W.A.; Ivovic, A.; Meylan, F.; Hanson, E.P.; Lange, E.; Katz, J.; et al. Somatic activating mutations in MAP2K1 cause melorheostosis. Nat. Commun. 2018, 9, 1390. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Fratzl-Zelman, N.; Roschger, P.; Papadakis, G.Z.; Cowen, E.W.; Kang, H.; Lehky, T.J.; Alter, K.; Deng, Z.; Ivovic, A.; et al. Distinct Clinical and Pathological Features of Melorheostosis Associated With Somatic MAP2K1 Mutations. J. Bone Miner. Res. 2019, 34, 145–156. [Google Scholar] [CrossRef]
- Strobel, K.; Fischer, D.R.; Tamborrini, G.; Kyburz, D.; Stumpe, K.D.M.; Hesselmann, R.G.X.; Johayem, A.; von Schulthess, G.K.; Michel, B.A.; Ciurea, A. 18F-Fluoride PET/CT for detection of sacroiliitis in ankylosing spondylitis. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1760–1765. [Google Scholar] [CrossRef]
- Bruijnen, S.T.; van der Weijden, M.A.; Klein, J.P.; Hoekstra, O.S.; Boellaard, R.; van Denderen, J.C.; Dijkmans, B.A.; Voskuyl, A.E.; van der Horst-Bruinsma, I.E.; van der Laken, C.J. Bone formation rather than inflammation reflects ankylosing spondylitis activity on PET-CT: A pilot study. Arthritis Res. Ther. 2012, 14, R71. [Google Scholar] [CrossRef]
- Bruijnen, S.T.G.; Verweij, N.J.F.; van Duivenvoorde, L.M.; Bravenboer, N.; Baeten, D.L.P.; van Denderen, C.J.; van der Horst-Bruinsma, I.E.; Voskuyl, A.E.; Custers, M.; van de Ven, P.M.; et al. Bone formation in ankylosing spondylitis during anti-tumour necrosis factor therapy imaged by 18F-fluoride positron emission tomography. Rheumatology 2018, 57, 631–638. [Google Scholar] [CrossRef]
- Watanabe, T.; Takase-Minegishi, K.; Ihata, A.; Kunishita, Y.; Kishimoto, D.; Kamiyama, R.; Hama, M.; Yoshimi, R.; Kirino, Y.; Asami, Y.; et al. (18)F-FDG and (18)F-NaF PET/CT demonstrate coupling of inflammation and accelerated bone turnover in rheumatoid arthritis. Mod. Rheumatol. 2016, 26, 180–187. [Google Scholar] [CrossRef]
- Sterner, T.; Pink, R.; Freudenberg, L.; Jentzen, T.; Quitmann, H.; Bockisch, A.; Löer, F. The role of [18F]fluoride positron emission tomography in the early detection of aseptic loosening of total knee arthroplasty. Int. J. Surg. 2007, 5, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Koob, S.; Gaertner, F.C.; Jansen, T.R.; Schmolders, J.; Gravius, S.; Strunk, H.; Wirtz, D.C.; Essler, M. Diagnosis of peri-prosthetic loosening of total hip and knee arthroplasty using (18)F-Fluoride PET/CT. Oncotarget 2019, 10, 2203–2211. [Google Scholar] [CrossRef] [PubMed]
- Temmerman, O.P.; Raijmakers, P.G.; Heyligers, I.C.; Comans, E.F.; Lubberink, M.; Teule, G.J.; Lammertsma, A.A. Bone metabolism after total hip revision surgery with impacted grafting: Evaluation using H2 15O and [18F]fluoride PET; a pilot study. Mol. Imaging Biol. 2008, 10, 288–293. [Google Scholar] [CrossRef]
- Seraj, S.M.; Al-Zaghal, A.; Østergaard, B.; Høilund-Carlsen, P.F.; Alavi, A. Identification of Heterotopic Ossification Using 18F-NaF PET/CT. Clin. Nucl. Med. 2019, 44, 319–320. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, K.; Song, L.; Pang, J.; Ma, H.; Shore, E.M.; Kaplan, F.S.; Wang, P. The phenotype and genotype of fibrodysplasia ossificans progressiva in China: A report of 72 cases. Bone 2013, 57, 386–391. [Google Scholar] [CrossRef]
- Warner, S.E.; Kaplan, F.S.; Pignolo, R.J.; Smith, S.E.; Hsiao, E.C.; De Cunto, C.; Di Rocco, M.; Harnett, K.; Grogan, D.; Genant, H.K. Whole-body Computed Tomography Versus Dual Energy X-ray Absorptiometry for Assessing Heterotopic Ossification in Fibrodysplasia Ossificans Progressiva. Calcif. Tissue Int. 2021, 109, 615–625. [Google Scholar] [CrossRef]
- Upadhyay, J.; Xie, L.; Huang, L.; Das, N.; Stewart, R.C.; Lyon, M.C.; Palmer, K.; Rajamani, S.; Graul, C.; Lobo, M.; et al. The Expansion of Heterotopic Bone in Fibrodysplasia Ossificans Progressiva Is Activin A-Dependent. J. Bone Miner. Res. 2017, 32, 2489–2499. [Google Scholar] [CrossRef]
- Botman, E.; Raijmakers, P.; Yaqub, M.; Teunissen, B.; Netelenbos, C.; Lubbers, W.; Schwarte, L.A.; Micha, D.; Bravenboer, N.; Schoenmaker, T.; et al. Evolution of heterotopic bone in fibrodysplasia ossificans progressiva: An [(18)F]NaF PET/CT study. Bone 2019, 124, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Botman, E.; Netelenbos, J.C.; Rustemeyer, T.; Schoonmade, L.J.; Nieuwenhuijzen, J.A.; Teunissen, B.P.; Visser, M.; Raijmakers, P.; Lammertsma, A.A.; Dahele, M.; et al. Radiotherapy in Fibrodysplasia Ossificans Progressiva: A Case Report and Systematic Review of the Literature. Front. Endocrinol. 2020, 11, 6. [Google Scholar] [CrossRef]
- Botman, E.; Treurniet, S.; Lubbers, W.D.; Schwarte, L.A.; Schober, P.R.; Sabelis, L.; Peters, E.J.G.; van Schie, A.; de Vries, R.; Grunwald, Z.; et al. When Limb Surgery Has Become the Only Life-Saving Therapy in FOP: A Case Report and Systematic Review of the Literature. Front. Endocrinol. 2020, 11, 570. [Google Scholar] [CrossRef]
- Di Rocco, M.; Forleo-Neto, E.; Pignolo, R.J.; Keen, R.; Orcel, P.; Funck-Brentano, T.; Roux, C.; Kolta, S.; Madeo, A.; Bubbear, J.S.; et al. Garetosmab in fibrodysplasia ossificans progressiva: A randomized, double-blind, placebo-controlled phase 2 trial. Nat. Med. 2023, 29, 2615–2624. [Google Scholar] [CrossRef] [PubMed]
- de Ruiter, R.D.; Botman, E.; Teunissen, B.; Lammertsma, A.A.; Boellaard, R.; Raijmakers, P.; Schwarte, L.A.; Nieuwenhuijzen, J.A.; Gonzalez Trotter, D.; Eekhoff, E.M.; et al. Performance of simplified methods for quantification of [18F]NaF uptake in fibrodysplasia ossificans progressiva. Front. Nucl. Med. 2024, 4, 1406947. [Google Scholar] [CrossRef] [PubMed]
- Smilde, B.J.; Stockklausner, C.; Keen, R.; Whittaker, A.; Bullock, A.N.; von Delft, A.; van Schoor, N.M.; Yu, P.B.; Eekhoff, E.M.W. Protocol paper: A multi-center, double-blinded, randomized, 6-month, placebo-controlled study followed by 12-month open label extension to evaluate the safety and efficacy of Saracatinib in Fibrodysplasia Ossificans Progressiva (STOPFOP). BMC Musculoskelet. Disord. 2022, 23, 519. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.A.; Choi, Y.; Huang, S.C.; Hoh, C.K.; Dahlbom, M.; Schiepers, C.; Satyamurthy, N.; Barrio, J.R.; Phelps, M.E. Evaluation of the skeletal kinetics of fluorine-18-fluoride ion with PET. J. Nucl. Med. 1992, 33, 633–642. [Google Scholar] [PubMed]
- Regelink, J.C.; Raijmakers, P.G.; Bravenboer, N.; Milek, R.; Hoetjes, N.J.; de Kreuk, A.M.; van Duin, M.; Wondergem, M.J.; Lips, P.; Sonneveld, P.; et al. (18)F-fluoride-PET for dynamic in vivo monitoring of bone formation in multiple myeloma. EJNMMI Res. 2016, 6, 46. [Google Scholar] [CrossRef]
- Kaplan, F.S.; Al Mukaddam, M.; Baujat, G.; Bravo, A.H.; Brown, M.; Cali, A.; Cho, T.-J.; Crowe, C.; De Cunto, C.L.; Delai, P.; et al. The Medical Management of Fibrodysplasia Ossificans Progressiva: Current Treatment Considerations. Proc. Intl. Clin. Counc. FOP 2024, 3, 1–159. Available online: https://www.iccfop.org/dvlp/wp-content/uploads/2024/07/FOP-GUIDELINES-FINAL-2024.pdf (accessed on 10 August 2024).
- Boellaard, R.; Krak, N.C.; Hoekstra, O.S.; Lammertsma, A.A. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: A simulation study. J. Nucl. Med. 2004, 45, 1519–1527. [Google Scholar]
- Raijmakers, P.; Temmerman, O.P.; Saridin, C.P.; Heyligers, I.C.; Becking, A.G.; van Lingen, A.; Lammertsma, A.A. Quantification of 18F-Fluoride Kinetics: Evaluation of Simplified Methods. J. Nucl. Med. 2014, 55, 1122–1127. [Google Scholar] [CrossRef]
- Slart, R.; Tsoumpas, C.; Glaudemans, A.; Noordzij, W.; Willemsen, A.T.M.; Borra, R.J.H.; Dierckx, R.; Lammertsma, A.A. Long axial field of view PET scanners: A road map to implementation and new possibilities. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4236–4245. [Google Scholar] [CrossRef]
- van Sluis, J.; van Snick, J.H.; Brouwers, A.H.; Noordzij, W.; Dierckx, R.; Borra, R.J.H.; Lammertsma, A.A.; Glaudemans, A.; Slart, R.; Yaqub, M.; et al. Shortened duration whole body (18)F-FDG PET Patlak imaging on the Biograph Vision Quadra PET/CT using a population-averaged input function. EJNMMI Phys. 2022, 9, 74. [Google Scholar] [CrossRef]
| Ref. | Authors | Study type | Objective of [18F]NaF PET/CT use |
|---|---|---|---|
| [13] | Eekhoff et al. (2017) | Follow-up case study | To evaluate possible flare-ups after surgery |
| [47] | Upadhyay et al. (2017) | Follow-up animal study | To evaluate the mineralization of HO |
| [14] | Eekhoff et al. (2018) | Case study | To evaluate a flare-up over time |
| [48] | Botman et al. (2019) | Follow-up study | To monitor the natural progression of disease |
| [49] | Botman et al. (2020) | Case study | To evaluate a possible flare-up after radiotherapy |
| [12] | Botman et al. (2020) | Case study | To assess the diagnostic value of MRI compared to [18F]NaF PET |
| [50] | Botman et al. (2020) | Case study | To evaluate a possible flare-up after surgery |
| [51] | Di Rocco et al. (2023) | Randomized controlled trial | To measure treatment response (LUMINA-1) |
| [52] | De Ruiter et al. (2024) | Substudy LUMINA-1 | To assess the use of simplified uptake parameters compared to full kinetic analysis |
| Ref. | Authors | Study Type (n) | Quantitative [18F]NaF PET Parameters | Quantitative [18F]NaF PET and Follow-Up CT Results |
|---|---|---|---|---|
| [13] | Eekhoff et al. (2017) | Case study (1) | SUVmean | SUVmean measured in masseter muscle of 12.4, 23.3, 10.6 (left), and 19.0, 16.2, 9.6 (right), one month, six months and twelve months post-surgery, respectively. Both sites proceeded with HO formation. |
| [14] | Eekhoff et al. (2018) | Case study (1) | No quantitative assessment reported | - |
| [48] | Botman et al. (2019) | Retrospective follow-up study (5) | SUVpeak | Average SUVpeak of normotopic bone at the supra-acetabular region in five patients was measured at 5.5 (SD 1.4). A cutoff value of SUVpeak > 8.4 was established for PET active lesions indicating volumetric progression of HO. |
| [49] | Botman et al. (2020) | Case study (1) | No quantitative assessment reported | - |
| [12] | Botman et al. (2020) | Retrospective follow-up study (4) | SUVpeak | Flare-ups visualized by MRI without pathologically increased [18F]NaF uptake (SUVpeak > 8.4) did not result in HO progression. |
| [50] | Botman et al. (2020) | Case study (1) | SUVmax | SUVmax of 6.4 measured in distal femur fourteen days post through the knee amputation. Follow-up CT eight weeks post-surgery confirmed new HO formation. |
| [51] | Di Rocco et al. (2023) | Randomized controlled trial LUMINA-1 | SUVmax, SUVmean, SUVpeak, TLA | A patient-specific threshold value was used to identify PET active lesions. Normal uptake was measured in the supra-acetabular region using SUVmean. A lesion was considered active when SUVmax exceeded three times the normal uptake value. In addition to SUVmax, SUVmean, SUVpeak, and TLA were analyzed over time. |
| [52] | De Ruiter et al. (2024) | Substudy LUMINA-1 (7) | NLR derived Ki, SUVmean, TBRmean | Static SUVmean correlated with NLR-derived Ki at baseline and one year follow-up. Static TBRmean correlated with NLR-derived Ki at baseline and one year follow-up. Change in static SUVmean and TBRmean measured in HO did not correlate with change in NLR-derived Ki. Change in static SUVmean and TBRmean measured in PET active lesions (SUVpeak > 8.4) correlated with change in NLR-derived Ki. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zwama, J.; Rosenberg, N.M.; Verheij, V.A.; Raijmakers, P.G.H.M.; Yaqub, M.; Botman, E.; de Ruiter, R.D.; Garrelfs, M.R.; Bökenkamp, A.; Micha, D.; et al. [18F]NaF PET/CT as a Marker for Fibrodysplasia Ossificans Progressiva: From Molecular Mechanisms to Clinical Applications in Bone Disorders. Biomolecules 2024, 14, 1276. https://doi.org/10.3390/biom14101276
Zwama J, Rosenberg NM, Verheij VA, Raijmakers PGHM, Yaqub M, Botman E, de Ruiter RD, Garrelfs MR, Bökenkamp A, Micha D, et al. [18F]NaF PET/CT as a Marker for Fibrodysplasia Ossificans Progressiva: From Molecular Mechanisms to Clinical Applications in Bone Disorders. Biomolecules. 2024; 14(10):1276. https://doi.org/10.3390/biom14101276
Chicago/Turabian StyleZwama, Jolien, Neeltje M. Rosenberg, Vincent A. Verheij, Pieter G. H. M. Raijmakers, Maqsood Yaqub, Esmée Botman, Ruben D. de Ruiter, Mark R. Garrelfs, Arend Bökenkamp, Dimitra Micha, and et al. 2024. "[18F]NaF PET/CT as a Marker for Fibrodysplasia Ossificans Progressiva: From Molecular Mechanisms to Clinical Applications in Bone Disorders" Biomolecules 14, no. 10: 1276. https://doi.org/10.3390/biom14101276
APA StyleZwama, J., Rosenberg, N. M., Verheij, V. A., Raijmakers, P. G. H. M., Yaqub, M., Botman, E., de Ruiter, R. D., Garrelfs, M. R., Bökenkamp, A., Micha, D., Schwarte, L. A., Teunissen, B. P., Lammertsma, A. A., Boellaard, R., & Eekhoff, E. M. W. (2024). [18F]NaF PET/CT as a Marker for Fibrodysplasia Ossificans Progressiva: From Molecular Mechanisms to Clinical Applications in Bone Disorders. Biomolecules, 14(10), 1276. https://doi.org/10.3390/biom14101276






