Interaction Between Nitric Oxide and Silicon on Leghaemoglobin and S-Nitrosothiol Levels in Soybean Nodules
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growth Conditions, and Experimental Design
2.2. Chlorophyll Content Measurement
2.3. Estimation of Phenylalanine Ammonia-Lyase Activity
2.4. Determination of Leghaemoglobin Content
2.5. Measurement of S-Nitrosothiol
2.6. Relative Gene Expression Analysis
2.7. Statistical Analysis
3. Results
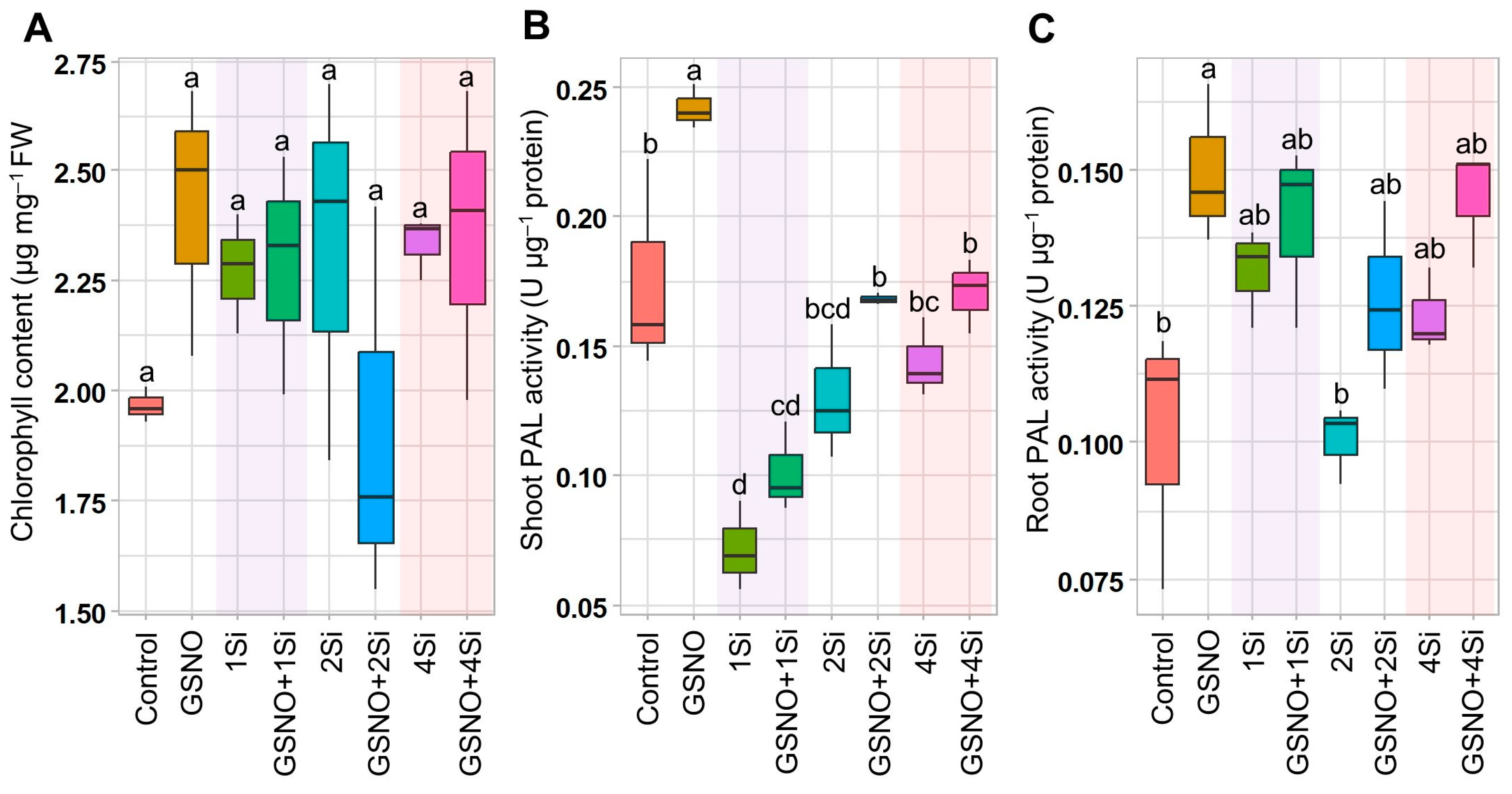
3.1. Effects of S-Nitrosoglutathione and Silicon Supplementation on Chlorophyll Content and Phenylalanine Ammonia-Lyase Activity
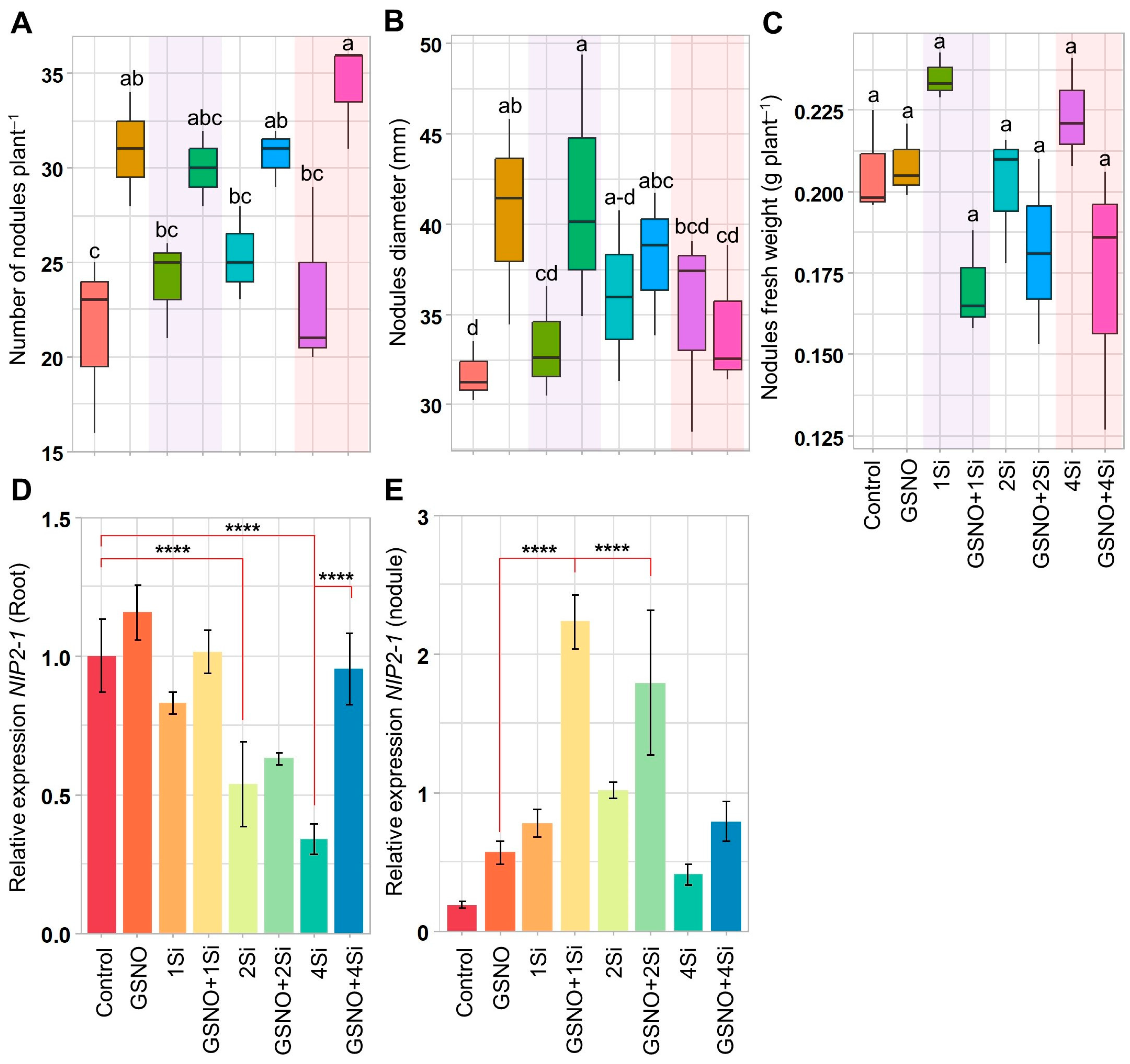
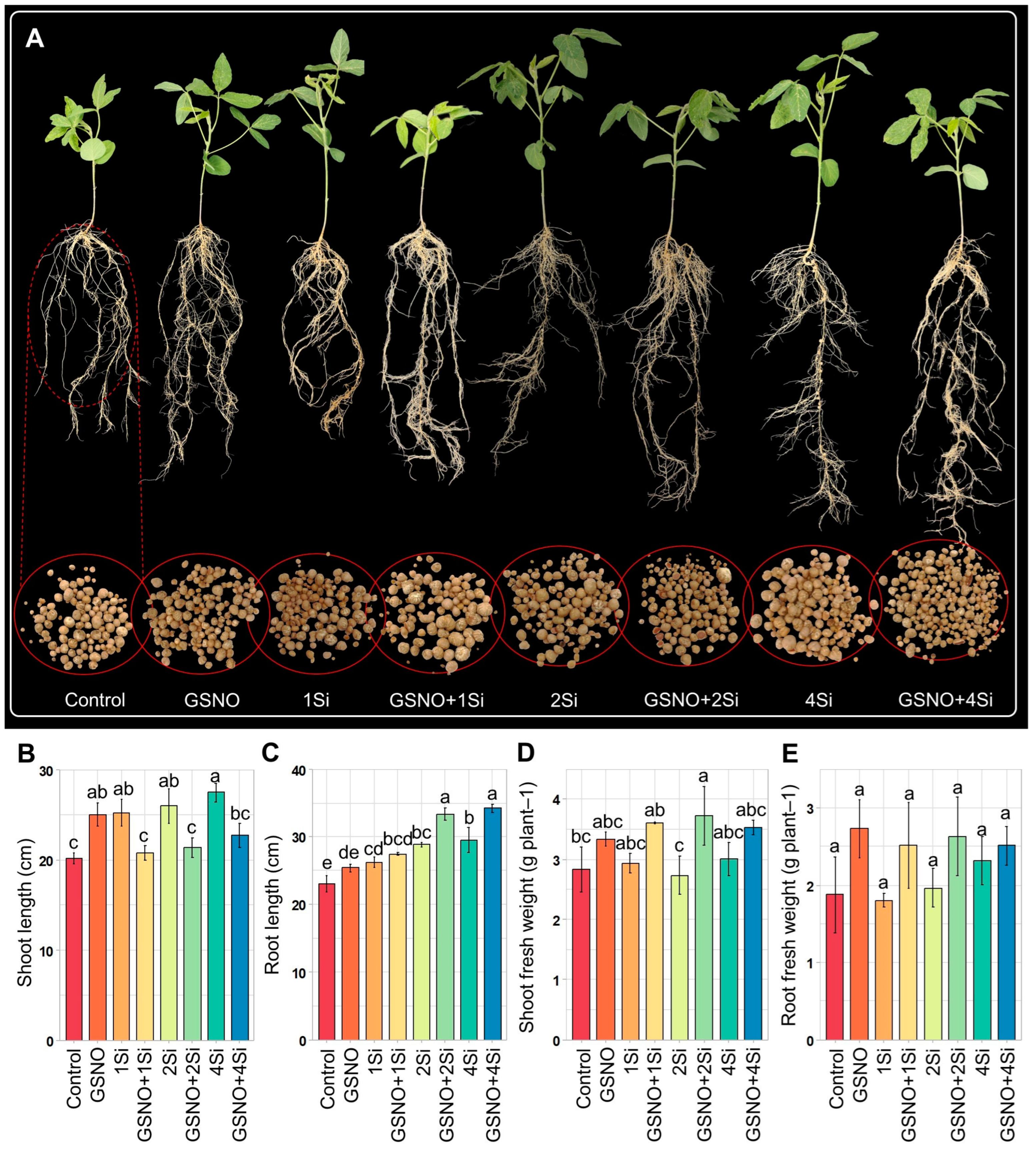
3.2. Application of S-Nitrosoglutathione and Silicon Regulates Soybean Nodulation
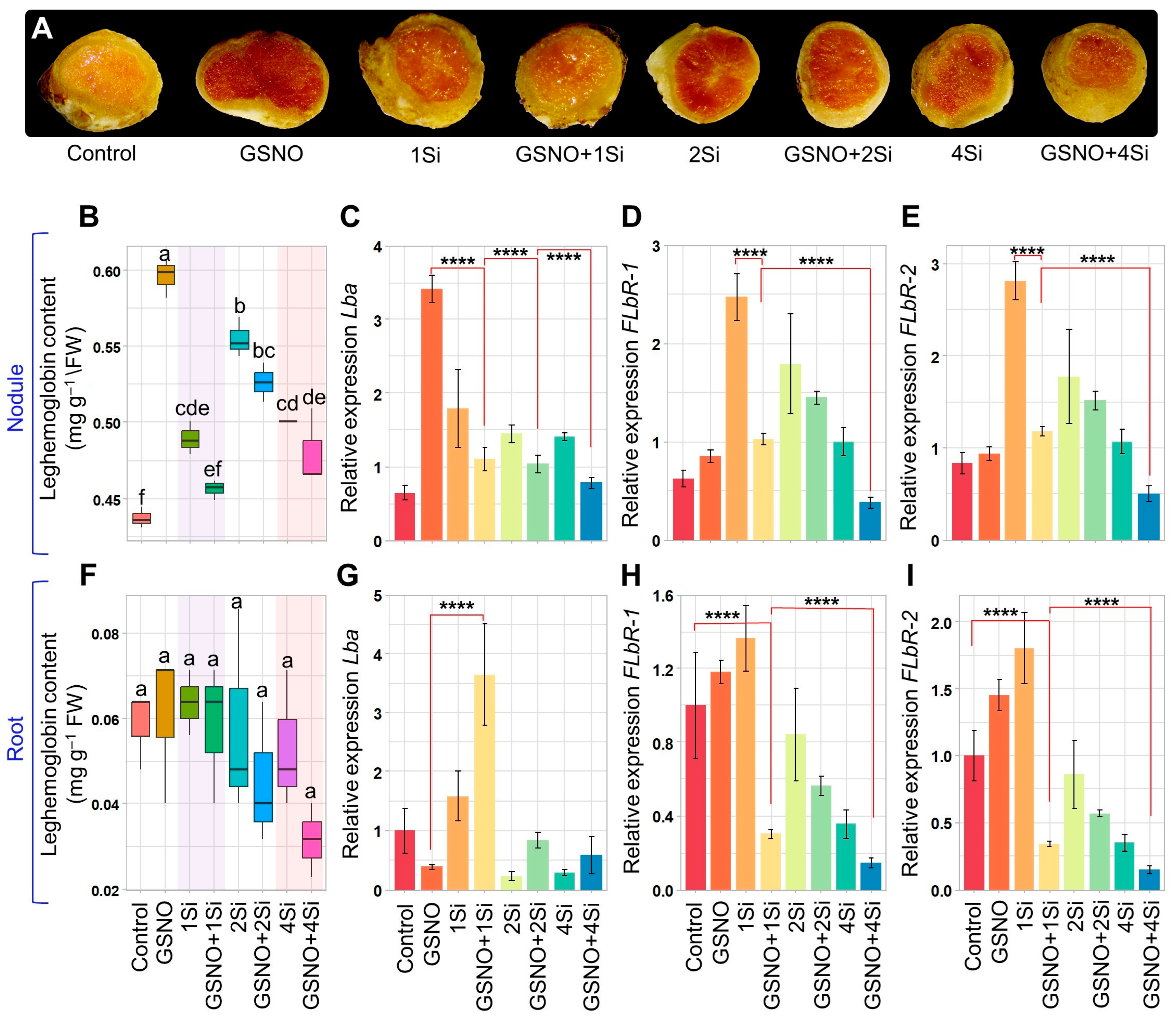
3.3. Response of S-Nitrosoglutathione and Silicon to Leghaemoglobin Levels in Soybean Roots and Nodules
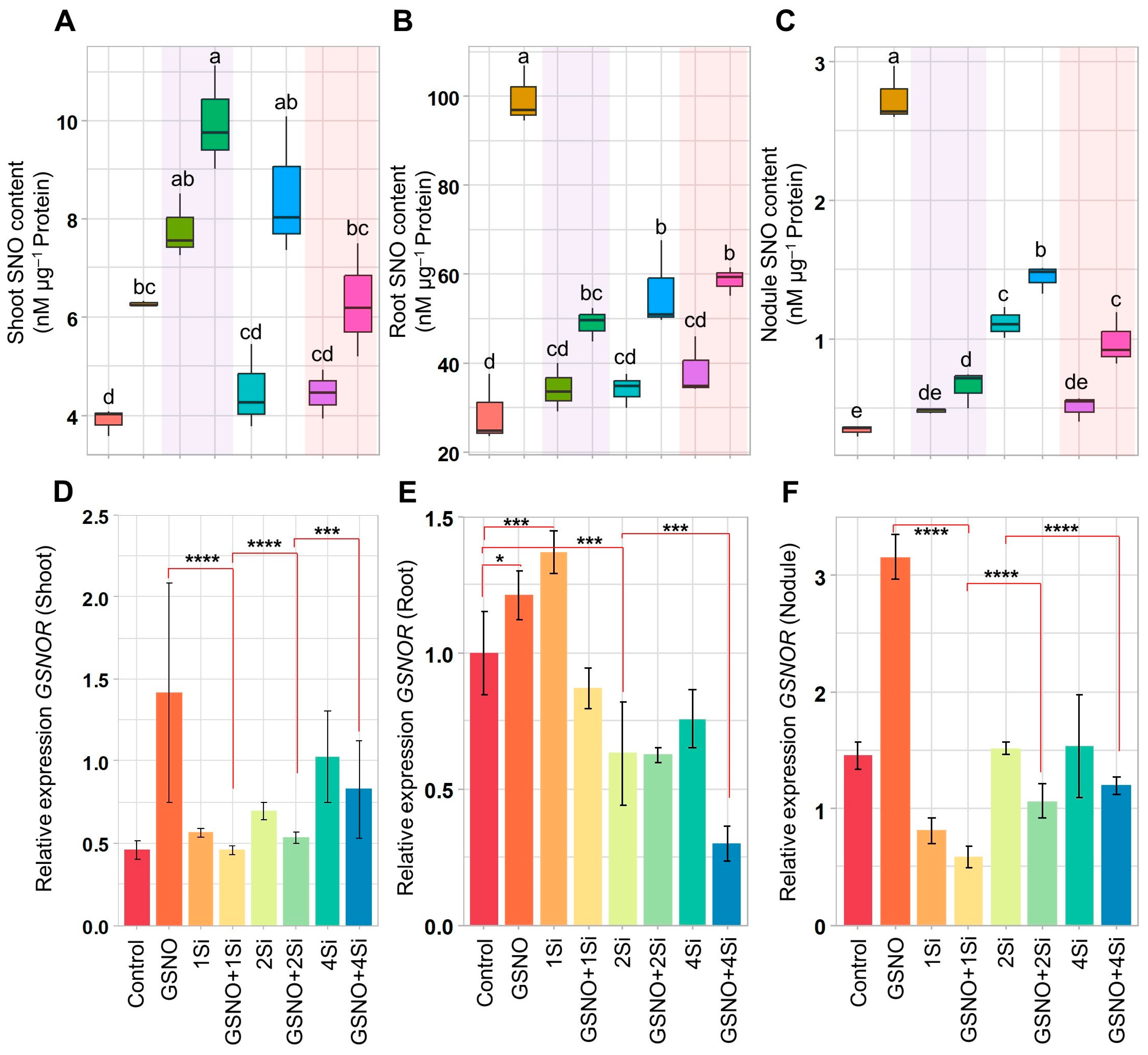
3.4. Effect of S-Nitrosoglutathione and Silicon on S-Nitrosothiol Levels
3.5. Effect of S-Nitrosoglutathione and Silicon on Soybean Shoot and Root Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, A.K.; Rahman, M.A.; Mitra, P.; Sukhwani, V.; Shaw, R.; Mitra, B.K.; Sharma, D.; Deshkar, S.; Morey, B. Up-scaling organic agriculture to enhance food and water security in South Asia. Org. Agric. 2022, 12, 475–494. [Google Scholar] [CrossRef]
- Brichi, L.; Fernandes, J.V.; Silva, B.M.; Vizú, J.d.F.; Junior, J.N.; Cherubin, M.R. Organic residues and their impact on soil health, crop production and sustainable agriculture: A review including bibliographic analysis. Soil Use Manag. 2023, 39, 686–706. [Google Scholar] [CrossRef]
- Maitra, S.; Praharaj, S.; Brestic, M.; Sahoo, R.K.; Sagar, L.; Shankar, T.; Palai, J.B.; Sahoo, U.; Sairam, M.; Pramanick, B. Rhizobium as biotechnological tools for green solutions: An environment-friendly approach for sustainable crop production in the modern era of climate change. Curr. Microbiol. 2023, 80, 219. [Google Scholar] [CrossRef] [PubMed]
- Zgadzaj, R.; Garrido-Oter, R.; Jensen, D.B.; Koprivova, A.; Schulze-Lefert, P.; Radutoiu, S. Root nodule symbiosis in Lotus japonicus drives the establishment of distinctive rhizosphere, root, and nodule bacterial communities. Proc. Natl. Acad. Sci. USA 2016, 113, E7996–E8005. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Wang, L.; Kong, F. miR172: A messenger between nodulation and flowering. Trends Plant Sci. 2023, 28, 623–625. [Google Scholar] [CrossRef]
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Signorelli, S.; Sainz, M.; Tabares-da Rosa, S.; Monza, J. The role of nitric oxide in nitrogen fixation by legumes. Front. Plant Sci. 2020, 11, 521. [Google Scholar] [CrossRef]
- Yun, J.; Wang, C.; Zhang, F.; Chen, L.; Sun, Z.; Cai, Y.; Luo, Y.; Liao, J.; Wang, Y.; Cha, Y. A nitrogen fixing symbiosis-specific pathway required for legume flowering. Sci. Adv. 2023, 9, eade1150. [Google Scholar] [CrossRef]
- Hirsch, A.M. Developmental biology of legume nodulation. New Phytol. 1992, 122, 211–237. [Google Scholar] [CrossRef]
- Larrainzar, E.; Villar, I.; Rubio, M.C.; Pérez-Rontomé, C.; Huertas, R.; Sato, S.; Mun, J.H.; Becana, M. Hemoglobins in the legume–Rhizobium symbiosis. New Phytol. 2020, 228, 472–484. [Google Scholar] [CrossRef]
- Jiang, S.; Jardinaud, M.-F.; Gao, J.; Pecrix, Y.; Wen, J.; Mysore, K.; Xu, P.; Sanchez-Canizares, C.; Ruan, Y.; Li, Q. NIN-like protein transcription factors regulate leghemoglobin genes in legume nodules. Science 2021, 374, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Fuchsman, W.H.; Appleby, C.A. Separation and determination of the relative concentrations of the homogeneous components of soybean leghemoglobin by isoelectric focusing. Biochim. Biophys. Acta (BBA)-Protein Struct. 1979, 579, 314–324. [Google Scholar] [CrossRef]
- Yun, B.W.; Skelly, M.J.; Yin, M.; Yu, M.; Mun, B.G.; Lee, S.U.; Hussain, A.; Spoel, S.H.; Loake, G.J. Nitric oxide and S-nitrosoglutathione function additively during plant immunity. New Phytol. 2016, 211, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-K.; Klucas, R.V. Reduction of ferric leghemoglobin in soybean root nodules. Plant Physiol. 1984, 74, 984–988. [Google Scholar] [CrossRef]
- Ji, L.; Becana, M.; Sarath, G.; Shearman, L.; Klucas, R.V. Overproduction in Escherichia coli and characterization of a soybean ferric leghemoglobin reductase. Plant Physiol. 1994, 106, 203–209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luan, P.; Aréchaga-Ocampo, E.; Sarath, G.; Arredondo-Peter, R.; Klucas, R.V. Analysis of a ferric leghemoglobin reductase from cowpea (Vigna unguiculata) root nodules. Plant Sci. 2000, 154, 161–170. [Google Scholar] [CrossRef]
- Becana, M.; Dalton, D.A.; Moran, J.F.; Iturbe-Ormaetxe, I.; Matamoros, M.A.; Rubio, M.C. Reactive oxygen species and antioxidants in legume nodules. Physiol. Plant. 2000, 109, 372–381. [Google Scholar] [CrossRef]
- Trinchant, J.-C.; Rigaud, J. Nitrite and nitric oxide as inhibitors of nitrogenase from soybean bacteroids. Appl. Environ. Microbiol. 1982, 44, 1385–1388. [Google Scholar] [CrossRef]
- Sasakura, F.; Uchiumi, T.; Shimoda, Y.; Suzuki, A.; Takenouchi, K.; Higashi, S.; Abe, M. A class 1 hemoglobin gene from Alnus firma functions in symbiotic and nonsymbiotic tissues to detoxify nitric oxide. Mol. Plant-Microbe Interact. 2006, 19, 441–450. [Google Scholar] [CrossRef]
- Kato, K.; Kanahama, K.; Kanayama, Y. Involvement of nitric oxide in the inhibition of nitrogenase activity by nitrate in Lotus root nodules. J. Plant Physiol. 2010, 167, 238–241. [Google Scholar] [CrossRef]
- Shimoda, Y.; Nagata, M.; Suzuki, A.; Abe, M.; Sato, S.; Kato, T.; Tabata, S.; Higashi, S.; Uchiumi, T. Symbiotic rhizobium and nitric oxide induce gene expression of non-symbiotic hemoglobin in Lotus japonicus. Plant Cell Physiol. 2005, 46, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Cam, Y.; Pierre, O.; Boncompagni, E.; Hérouart, D.; Meilhoc, E.; Bruand, C. Nitric oxide (NO): A key player in the senescence of Medicago truncatula root nodules. New Phytol. 2012, 196, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Feechan, A.; Kwon, E.; Yun, B.-W.; Wang, Y.; Pallas, J.A.; Loake, G.J. A central role for S-nitrosothiols in plant disease resistance. Proc. Natl. Acad. Sci. 2005, 102, 8054–8059. [Google Scholar] [CrossRef] [PubMed]
- Maiti, D.; Sarkar, T.S.; Ghosh, S. Detection of S-nitrosothiol and nitrosylated proteins in Arachis hypogaea functional nodule: Response of the nitrogen fixing symbiont. PLoS ONE 2012, 7, e45526. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Puppo, A.; Pauly, N.; Boscari, A.; Mandon, K.; Brouquisse, R. Hydrogen peroxide and nitric oxide: Key regulators of the legume—Rhizobium and mycorrhizal symbioses. Antioxid. Redox Signal. 2013, 18, 2202–2219. [Google Scholar] [CrossRef]
- Baudouin, E.; Pieuchot, L.; Engler, G.; Pauly, N.; Puppo, A. Nitric oxide is formed in Medicago truncatula-Sinorhizobium meliloti functional nodules. Mol. Plant-Microbe Interact. 2006, 19, 970–975. [Google Scholar] [CrossRef]
- Calvo-Begueria, L.; Rubio, M.C.; Martínez, J.I.; Pérez-Rontomé, C.; Delgado, M.J.; Bedmar, E.J.; Becana, M. Redefining nitric oxide production in legume nodules through complementary insights from electron paramagnetic resonance spectroscopy and specific fluorescent probes. J. Exp. Bot. 2018, 69, 3703–3714. [Google Scholar] [CrossRef]
- Fukudome, M.; Watanabe, E.; Osuki, K.-I.; Imaizumi, R.; Aoki, T.; Becana, M.; Uchiumi, T. Stably transformed Lotus japonicus plants overexpressing phytoglobin LjGlb1-1 show decreased nitric oxide levels in roots and nodules as well as delayed nodule senescence. Plant Cell Physiol. 2019, 60, 816–825. [Google Scholar] [CrossRef]
- Epstein, E. Silicon. Annu. Rev. Plant Biol. 1999, 50, 641–664. [Google Scholar] [CrossRef]
- Tubana, B.S.; Babu, T.; Datnoff, L.E. A review of silicon in soils and plants and its role in US agriculture: History and future perspectives. Soil Sci. 2016, 181, 393–411. [Google Scholar] [CrossRef]
- Nelwamondo, A.; Dakora, F. Silicon promotes nodule formation and nodule function in symbiotic cowpea (Vigna unguiculata). New Phytol. 1999, 142, 463–467. [Google Scholar] [CrossRef]
- Mali, M.; Aery, A.N.C. Silicon effects on nodule growth, dry-matter production, and mineral nutrition of cowpea (Vigna unguiculata). J. Plant Nutr. Soil Sci. 2008, 171, 835–840. [Google Scholar] [CrossRef]
- Steiner, F.; Zuffo, A.M.; Bush, A.; Santos, D.M.d.S. Silicate fertilization potentiates the nodule formation and symbiotic nitrogen fixation in soybean1. Pesqui. Agropecu. Trop. 2018, 48, 212–221. [Google Scholar] [CrossRef]
- Putra, R.; Waterman, J.M.; Mathesius, U.; Wojtalewicz, D.; Powell, J.R.; Hartley, S.E.; Johnson, S.N. Benefits of silicon-enhanced root nodulation in a model legume are contingent upon rhizobial efficacy. Plant Soil 2022, 477, 201–217. [Google Scholar] [CrossRef]
- Mansoor, S.; Tripathi, P.; Ghimire, A.; Hamid, S.; Abd El-moniem, D.; Chung, Y.S.; Kim, Y. Comparative transcriptomic analysis of the nodulation-competent zone and inference of transcription regulatory network in silicon applied Glycine max [L.]-Merr. Roots. Plant Cell Rep. 2024, 43, 169. [Google Scholar] [CrossRef] [PubMed]
- Coquerel, R.; Arkoun, M.; Trouverie, J.; Bernay, B.; Laîné, P.; Etienne, P. Ionomic and proteomic changes highlight the effect of silicon supply on the functioning of Trifolium incarnatum L. nodules subjected to nitrogen starvation. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Garg, N.; Singh, S. Arbuscular mycorrhiza Rhizophagus irregularis and silicon modulate growth, proline biosynthesis and yield in Cajanus cajan L. Millsp.(pigeonpea) genotypes under cadmium and zinc stress. J. Plant Growth Regul. 2018, 37, 46–63. [Google Scholar] [CrossRef]
- Ahmad, P.; Alyemeni, M.N.; Wijaya, L.; Ahanger, M.A.; Ashraf, M.; Alam, P.; Paray, B.A.; Rinklebe, J. Nitric oxide donor, sodium nitroprusside, mitigates mercury toxicity in different cultivars of soybean. J. Hazard. Mater. 2021, 408, 124852. [Google Scholar] [CrossRef]
- Methela, N.J.; Islam, M.S.; Lee, D.-S.; Yun, B.-W.; Mun, B.-G. S-nitrosoglutathione (GSNO)-mediated lead detoxification in soybean through the regulation of ROS and metal-related transcripts. Int. J. Mol. Sci. 2023, 24, 9901. [Google Scholar] [CrossRef]
- Methela, N.J.; Pande, A.; Islam, M.S.; Rahim, W.; Hussain, A.; Lee, D.-S.; Mun, B.-G.; Maria Joseph Raj, N.P.; Kim, S.-J.; Kim, Y. Chitosan-GSNO nanoparticles: A positive modulator of drought stress tolerance in soybean. BMC Plant Biol. 2023, 23, 639. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Rahman, M.M.; Ansary, M.M.U.; Keya, S.S.; Abdelrahman, M.; Miah, M.G.; Phan Tran, L.-S. Silicon in mitigation of abiotic stress-induced oxidative damage in plants. Crit. Rev. Biotechnol. 2021, 41, 918–934. [Google Scholar] [CrossRef] [PubMed]
- Hiscox, J.; Israelstam, G. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Fan, C.; Hu, H.; Wang, L.; Zhou, Q.; Huang, X. Enzymological mechanism for the regulation of lanthanum chloride on flavonoid synthesis of soybean seedlings under enhanced ultraviolet-B radiation. Environ. Sci. Pollut. Res. 2014, 21, 8792–8800. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Reisenauer, H. Determination of leghemoglobin in legume nodules. Anal. Biochem. 1963, 6, 27–30. [Google Scholar] [CrossRef]
- Yun, B.-W.; Feechan, A.; Yin, M.; Saidi, N.B.; Le Bihan, T.; Yu, M.; Moore, J.W.; Kang, J.-G.; Kwon, E.; Spoel, S.H. S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 2011, 478, 264–268. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, Z.; Gao, X.; Jin, C.; Wen, L.; Yao, X.; Ren, J. GPS-SNO: Computational prediction of protein S-nitrosylation sites with a modified GPS algorithm. PLoS ONE 2010, 5, e11290. [Google Scholar] [CrossRef]
- Wickham, H. Data Analysis. In ggplot2: Elegant Graphics for Data Analysis; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- USDA. Department of Agriculture Foreign Agricultural Services. 2024. Available online: https://fas.usda.gov/data/production/commodity/2222000 (accessed on 1 September 2024).
- Herridge, D.F.; Giller, K.E.; Jensen, E.S.; Peoples, M.B. Quantifying country-to-global scale nitrogen fixation for grain legumes II. Coefficients, templates and estimates for soybean, groundnut and pulses. Plant Soil 2022, 474, 1–15. [Google Scholar] [CrossRef]
- Du, M.; Gao, Z.; Li, X.; Liao, H. Excess nitrate induces nodule greening and reduces transcript and protein expression levels of soybean leghaemoglobins. Ann. Bot. 2020, 126, 61–72. [Google Scholar] [CrossRef]
- Pueppke, S.G.; Bolanos-Vásquez, M.C.; Werner, D.; Bec-Ferté, M.-P.; Promé, J.-C.; Krishnan, H.B. Release of flavonoids by the soybean cultivars McCall and Peking and their perception as signals by the nitrogen-fixing symbiont Sinorhizobium fredii. Plant Physiol. 1998, 117, 599–606. [Google Scholar] [CrossRef]
- Cullimore, J.V.; Ranjeva, R.; Bono, J.-J. Perception of lipo-chitooligosaccharidic Nod factors in legumes. Trends Plant Sci. 2001, 6, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Radutoiu, S.; Madsen, L.H.; Madsen, E.B.; Felle, H.H.; Umehara, Y.; Grønlund, M.; Sato, S.; Nakamura, Y.; Tabata, S.; Sandal, N. Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature 2003, 425, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Broghammer, A.; Krusell, L.; Blaise, M.; Sauer, J.; Sullivan, J.T.; Maolanon, N.; Vinther, M.; Lorentzen, A.; Madsen, E.B.; Jensen, K.J. Legume receptors perceive the rhizobial lipochitin oligosaccharide signal molecules by direct binding. Proc. Natl. Acad. Sci. USA 2012, 109, 13859–13864. [Google Scholar] [CrossRef] [PubMed]
- Madsen, E.B.; Madsen, L.H.; Radutoiu, S.; Olbryt, M.; Rakwalska, M.; Szczyglowski, K.; Sato, S.; Kaneko, T.; Tabata, S.; Sandal, N. A receptor kinase gene of the LysM type is involved in legumeperception of rhizobial signals. Nature 2003, 425, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.S.; Thomas, M.; Clarke, D.J. The gene stlA encodes a phenylalanine ammonia-lyase that is involved in the production of a stilbene antibiotic in Photorhabdus luminescens TT01. Microbiology 2005, 151, 2543–2550. [Google Scholar] [CrossRef]
- Liu, C.-W.; Murray, J.D. The role of flavonoids in nodulation host-range specificity: An update. Plants 2016, 5, 33. [Google Scholar] [CrossRef]
- Pii, Y.; Crimi, M.; Cremonese, G.; Spena, A.; Pandolfini, T. Auxin and nitric oxide control indeterminate nodule formation. BMC Plant Biol. 2007, 7, 1–11. [Google Scholar] [CrossRef]
- Hichri, I.; Boscari, A.; Castella, C.; Rovere, M.; Puppo, A.; Brouquisse, R. Nitric oxide: A multifaceted regulator of the nitrogen-fixing symbiosis. J. Exp. Bot. 2015, 66, 2877–2887. [Google Scholar] [CrossRef]
- Kubo, H. Uber hamoprotein aus den wurzelknollchen von leguminosen. Acta Phytochim. 1939, 11, 195–200. [Google Scholar]
- Cutting, J.; Schulman, H. Leghemoglobin-concerning the sites of biosynthesis of its components. Fed. Proc. 1968, 27, 768. [Google Scholar]
- Vieweg, M.F.; Frühling, M.; Quandt, H.-J.; Heim, U.; Bäumlein, H.; Pühler, A.; Küster, H.; Perlick, A.M. The promoter of the Vicia faba L. leghemoglobin gene VfLb29 is specifically activated in the infected cells of root nodules and in the arbuscule-containing cells of mycorrhizal roots from different legume and nonlegume plants. Mol. Plant-Microbe Interact. 2004, 17, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Becana, M.; Sprent, J.I. Effect of nitrate on components of nodule leghaemoglobins. J. Exp. Bot. 1989, 40, 725–731. [Google Scholar] [CrossRef]
- Kanayama, Y.; Yamamoto, Y. Inhibition of nitrogen fixation in soybean plants supplied with nitrate III. Kinetics of the formation of nitrosylleghemoglobin and of the inhibition of formation of oxyleghemoglobin. Plant Cell Physiol. 1990, 31, 603–608. [Google Scholar] [CrossRef]
- Yu, H.; Xiao, A.; Wu, J.; Li, H.; Duan, Y.; Chen, Q.; Zhu, H.; Cao, Y. GmNAC039 and GmNAC018 activate the expression of cysteine protease genes to promote soybean nodule senescence. Plant Cell 2023, 35, 2929–2951. [Google Scholar] [CrossRef] [PubMed]
- Appleby, C. Properties of leghaemoglobin in vivo, and its isolation as ferrous oxyleghaemoglobin. Biochim. Biophys. Acta (BBA)-Protein Struct. 1969, 188, 222–229. [Google Scholar] [CrossRef]
- Saari, L.L.; Klucas, R.V. Ferric leghemoglobin reductase from soybean root nodules. Arch. Biochem. Biophys. 1984, 231, 102–113. [Google Scholar] [CrossRef]
- Ji, L.; Wood, S.; Becana, M.; Klucas, R.V. Purification and characterization of soybean root nodule ferric leghemoglobin reductase. Plant Physiol. 1991, 96, 32–37. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Feechan, A.; Yun, B.-W.; Shafiei, R.; Hofmann, A.; Taylor, P.; Xue, P.; Yang, F.-Q.; Xie, Z.-S.; Pallas, J.A. S-nitrosylation of AtSABP3 antagonizes the expression of plant immunity. J. Biol. Chem. 2009, 284, 2131–2137. [Google Scholar] [CrossRef]
- Liu, L.; Hausladen, A.; Zeng, M.; Que, L.; Heitman, J.; Stamler, J.S. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 2001, 410, 490–494. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-S.; Das, A.K.; Methela, N.J.; Yun, B.-W. Interaction Between Nitric Oxide and Silicon on Leghaemoglobin and S-Nitrosothiol Levels in Soybean Nodules. Biomolecules 2024, 14, 1417. https://doi.org/10.3390/biom14111417
Lee D-S, Das AK, Methela NJ, Yun B-W. Interaction Between Nitric Oxide and Silicon on Leghaemoglobin and S-Nitrosothiol Levels in Soybean Nodules. Biomolecules. 2024; 14(11):1417. https://doi.org/10.3390/biom14111417
Chicago/Turabian StyleLee, Da-Sol, Ashim Kumar Das, Nusrat Jahan Methela, and Byung-Wook Yun. 2024. "Interaction Between Nitric Oxide and Silicon on Leghaemoglobin and S-Nitrosothiol Levels in Soybean Nodules" Biomolecules 14, no. 11: 1417. https://doi.org/10.3390/biom14111417
APA StyleLee, D.-S., Das, A. K., Methela, N. J., & Yun, B.-W. (2024). Interaction Between Nitric Oxide and Silicon on Leghaemoglobin and S-Nitrosothiol Levels in Soybean Nodules. Biomolecules, 14(11), 1417. https://doi.org/10.3390/biom14111417





