Repurposed Drugs and Plant-Derived Natural Products as Potential Host-Directed Therapeutic Candidates for Tuberculosis
Abstract
1. Introduction
2. Immune Responses Against Mtb
2.1. Innate and Adaptive Immunity
2.2. Death of Infected Phagocytes
2.3. Metabolism in Host Cells During Infection and Inflammation
3. Host-Directed Therapies in the Treatment of TB
3.1. Repurposed Medicines
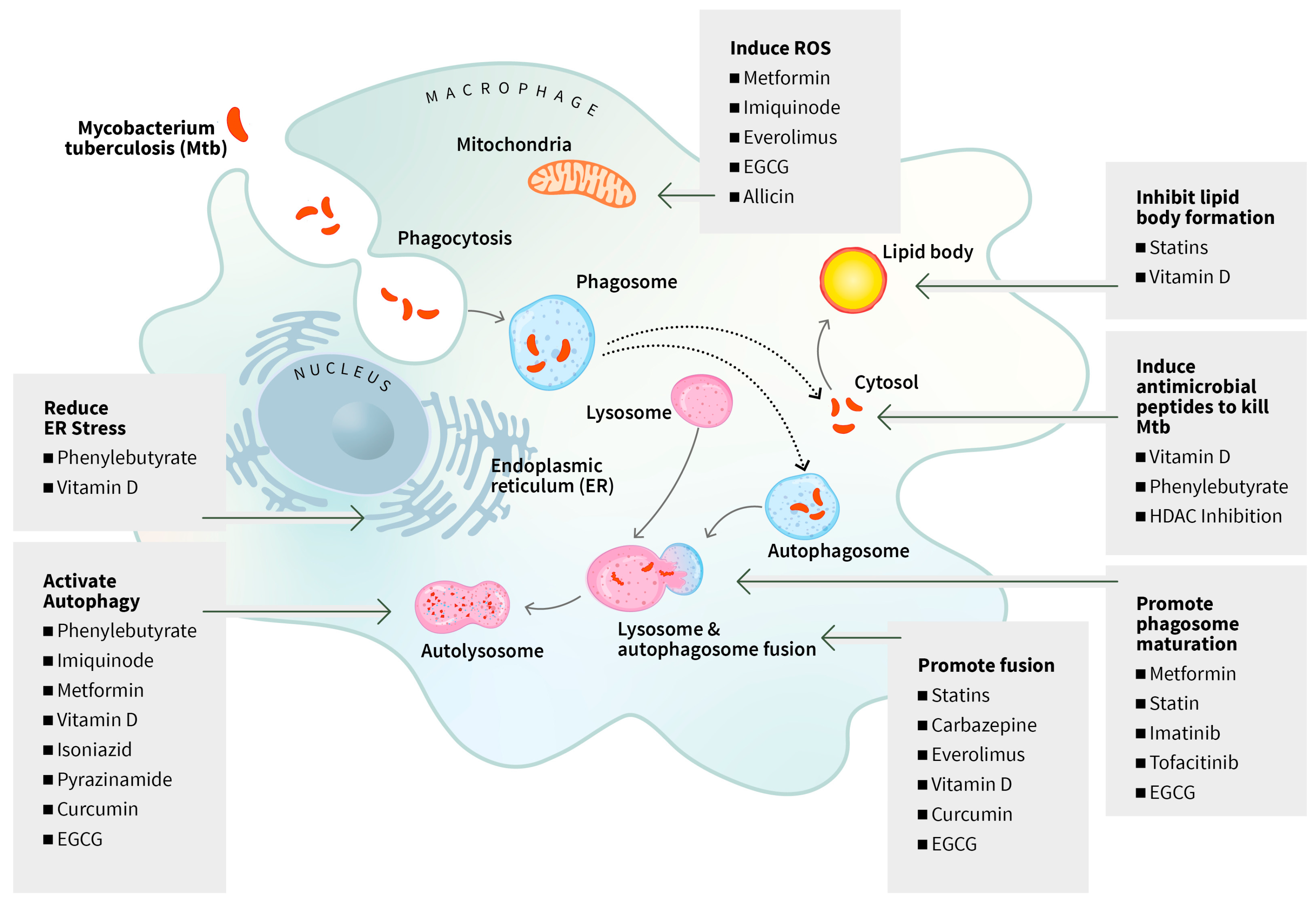
3.1.1. Targeting Death of Infected Phagocytes
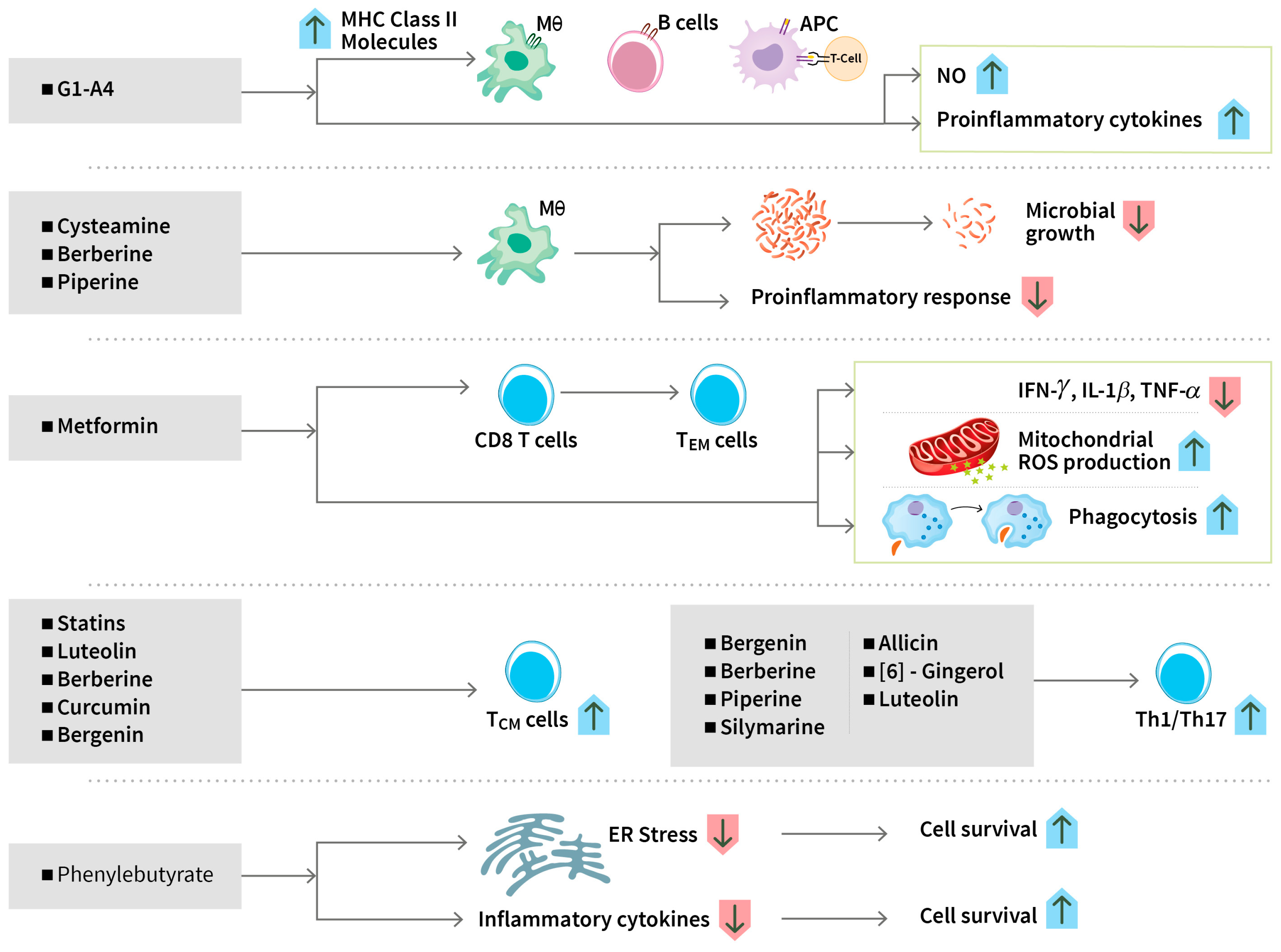
3.1.2. Modulating Immune Responses
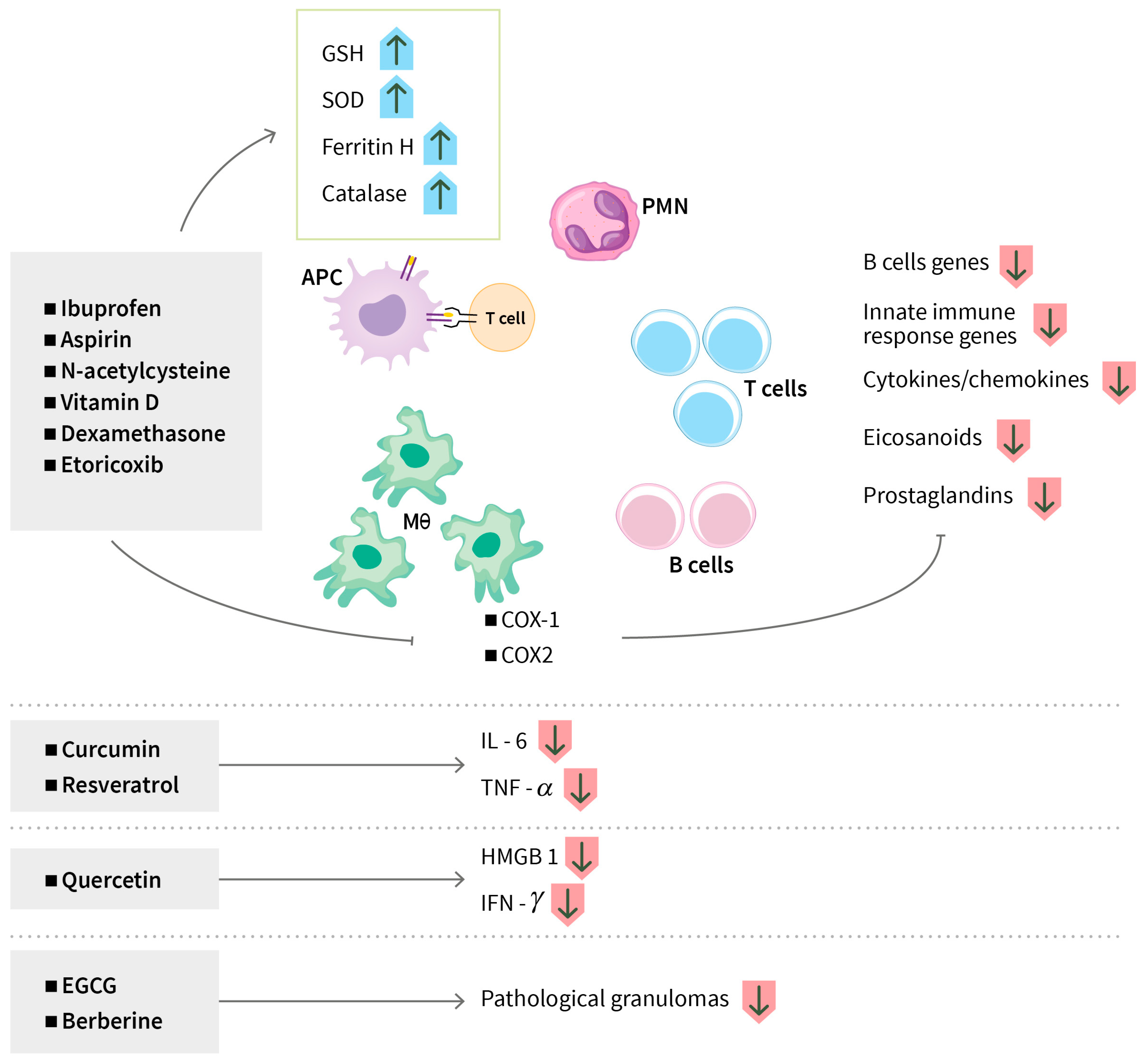
3.1.3. Modulating Anti-Inflammatory Responses
3.1.4. Regulating Metabolic Pathways
3.2. Plant-Derived Natural Products (Phytochemicals)
3.2.1. Host-Directed Therapeutic Effects of Phytochemicals for TB
Targeting Cell Death of Phagocytes and Autophagy
Modulating Immune Responses
Modulating Anti-Inflammatory Responses
Hepatoprotective Role of Natural Products
3.2.2. Potential Side Effects and Challenges Associated with Plant-Derived Natural Products
Adverse Effects
Challenges Regarding Chemical Stability and Bioavailability
4. Comparison of Therapeutic Potential of HDT Candidates
5. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO Guidelines Approved by the Guidelines Review Committee. In WHO Consolidated Guidelines on Tuberculosis: Module 6: Tuberculosis and Comorbidities; WHO: Geneva, Switzerland, 2024.
- Singh, R.; Dwivedi, S.P.; Gaharwar, U.S.; Meena, R.; Rajamani, P.; Prasad, T. Recent updates on drug resistance in Mycobacterium tuberculosis. J. Appl. Microbiol. 2020, 128, 1547–1567. [Google Scholar] [CrossRef] [PubMed]
- Bodke, H.; Wagh, V.; Kakar, G. Diabetes Mellitus and Prevalence of Other Comorbid Conditions: A Systematic Review. Cureus 2023, 15, e49374. [Google Scholar] [CrossRef] [PubMed]
- Nyasulu, P.S.; Doumbia, C.O.; Ngah, V.; Togo, A.C.G.; Diarra, B.; Chongwe, G. Multidrug-resistant tuberculosis: Latest opinions on epidemiology, rapid diagnosis and management. Curr. Opin. Pulm. Med. 2024, 30, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Diriba, G.; Alemu, A.; Yenew, B.; Tola, H.H.; Gamtesa, D.F.; Mollalign, H.; Eshetu, K.; Moga, S.; Abdella, S.; Tollera, G.; et al. Epidemiology of extensively drug-resistant tuberculosis among patients with multidrug-resistant tuberculosis: A systematic review and meta-analysis. Int. J. Infect. Dis. 2023, 132, 50–63. [Google Scholar] [CrossRef]
- WHO. Global Tuberculosis Report; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Ahmed, S.; Raqib, R.; Guethmundsson, G.H.; Bergman, P.; Agerberth, B.; Rekha, R.S. Host-Directed Therapy as a Novel Treatment Strategy to Overcome Tuberculosis: Targeting Immune Modulation. Antibiotics 2020, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.; Raqib, R.; Rekha, R.S.; Agerberth, B.; Gudmundsson, G.H. Host Directed Therapy Against Infection by Boosting Innate Immunity. Front. Immunol. 2020, 11, 1209. [Google Scholar] [CrossRef]
- Kaufmann, S.H.E.; Dorhoi, A.; Hotchkiss, R.S.; Bartenschlager, R. Host-directed therapies for bacterial and viral infections. Nat. Rev. Drug Discov. 2018, 17, 35–56. [Google Scholar] [CrossRef]
- Bussi, C.; Gutierrez, M.G. Mycobacterium tuberculosis infection of host cells in space and time. FEMS Microbiol. Rev. 2019, 43, 341–361. [Google Scholar] [CrossRef]
- Ravesloot-Chavez, M.M.; Van Dis, E.; Stanley, S.A. The Innate Immune Response to Mycobacterium tuberculosis Infection. Annu. Rev. Immunol. 2021, 39, 611–637. [Google Scholar] [CrossRef]
- Rodriguez-Carlos, A.; Jacobo-Delgado, Y.; Santos-Mena, A.O.; Garcia-Hernandez, M.H.; De Jesus-Gonzalez, L.A.; Lara-Ramirez, E.E.; Rivas-Santiago, B. Histone deacetylase (HDAC) inhibitors- based drugs are effective to control Mycobacterium tuberculosis infection and promote the sensibility for rifampicin in MDR strain. Mem. Inst. Oswaldo Cruz 2023, 118, e230143. [Google Scholar] [CrossRef]
- Mihret, A. The role of dendritic cells in Mycobacterium tuberculosis infection. Virulence 2012, 3, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, K.A.; Kirman, J.R. Dendritic cell subsets in mycobacterial infection: Control of bacterial growth and T cell responses. Tuberculosis 2013, 93, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Silva Miranda, M.; Breiman, A.; Allain, S.; Deknuydt, F.; Altare, F. The tuberculous granuloma: An unsuccessful host defence mechanism providing a safety shelter for the bacteria? Clin. Dev. Immunol. 2012, 2012, 139127. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.T.; Cambier, C.J.; Davis, J.M.; Hall, C.J.; Crosier, P.S.; Ramakrishnan, L. Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocytosed from infected macrophages. Cell Host Microbe 2012, 12, 301–312. [Google Scholar] [CrossRef]
- Jena, P.; Mohanty, S.; Mohanty, T.; Kallert, S.; Morgelin, M.; Lindstrom, T.; Borregaard, N.; Stenger, S.; Sonawane, A.; Sorensen, O.E. Azurophil granule proteins constitute the major mycobactericidal proteins in human neutrophils and enhance the killing of mycobacteria in macrophages. PLoS ONE 2012, 7, e50345. [Google Scholar] [CrossRef]
- Dallenga, T.; Repnik, U.; Corleis, B.; Eich, J.; Reimer, R.; Griffiths, G.W.; Schaible, U.E.M. tuberculosis-Induced Necrosis of Infected Neutrophils Promotes Bacterial Growth Following Phagocytosis by Macrophages. Cell Host Microbe 2017, 22, 519–530.e513. [Google Scholar] [CrossRef]
- Lu, C.C.; Wu, T.S.; Hsu, Y.J.; Chang, C.J.; Lin, C.S.; Chia, J.H.; Wu, T.L.; Huang, T.T.; Martel, J.; Ojcius, D.M.; et al. NK cells kill mycobacteria directly by releasing perforin and granulysin. J. Leukoc. Biol. 2014, 96, 1119–1129. [Google Scholar] [CrossRef]
- Larsen, S.E.; Williams, B.D.; Rais, M.; Coler, R.N.; Baldwin, S.L. It Takes a Village: The Multifaceted Immune Response to Mycobacterium tuberculosis Infection and Vaccine-Induced Immunity. Front. Immunol. 2022, 13, 840225. [Google Scholar] [CrossRef]
- Lewinsohn, D.M.; Lewinsohn, D.A. The Missing Link in Correlates of Protective Tuberculosis Immunity: Recognizing the Infected Cell. Front. Immunol. 2022, 13, 869057. [Google Scholar] [CrossRef]
- Lin, P.L.; Flynn, J.L. CD8 T cells and Mycobacterium tuberculosis infection. Semin. Immunopathol. 2015, 37, 239–249. [Google Scholar] [CrossRef]
- Aerts, L.; Selis, E.; Corbiere, V.; Smits, K.; Van Praet, A.; Dauby, N.; Petit, E.; Singh, M.; Locht, C.; Dirix, V.; et al. HBHA-Induced Polycytotoxic CD4(+) T Lymphocytes Are Associated with the Control of Mycobacterium tuberculosis Infection in Humans. J. Immunol. 2019, 202, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Chen, R.; Jiang, Y.; Zhu, H.; Chen, L.; Chen, Y.; Shen, M.; Lin, X. Multifunctional T cell response in active pulmonary tuberculosis patients. Int. Immunopharmacol. 2021, 99, 107898. [Google Scholar] [CrossRef] [PubMed]
- Maglione, P.J.; Xu, J.; Chan, J. B cells moderate inflammatory progression and enhance bacterial containment upon pulmonary challenge with Mycobacterium tuberculosis. J. Immunol. 2007, 178, 7222–7234. [Google Scholar] [CrossRef]
- Augenstreich, J.; Arbues, A.; Simeone, R.; Haanappel, E.; Wegener, A.; Sayes, F.; Le Chevalier, F.; Chalut, C.; Malaga, W.; Guilhot, C.; et al. ESX-1 and phthiocerol dimycocerosates of Mycobacterium tuberculosis act in concert to cause phagosomal rupture and host cell apoptosis. Cell Microbiol. 2017, 19, e12726. [Google Scholar] [CrossRef] [PubMed]
- Manzanillo, P.S.; Ayres, J.S.; Watson, R.O.; Collins, A.C.; Souza, G.; Rae, C.S.; Schneider, D.S.; Nakamura, K.; Shiloh, M.U.; Cox, J.S. The ubiquitin ligase parkin mediates resistance to intracellular pathogens. Nature 2013, 501, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.O.; Manzanillo, P.S.; Cox, J.S.; Extracellular, M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell 2012, 150, 803–815. [Google Scholar] [CrossRef]
- Braian, C.; Hogea, V.; Stendahl, O. Mycobacterium tuberculosis- induced neutrophil extracellular traps activate human macrophages. J. Innate Immun. 2013, 5, 591–602. [Google Scholar] [CrossRef]
- Jeong, E.K.; Lee, H.J.; Jung, Y.J. Host-Directed Therapies for Tuberculosis. Pathogens 2022, 11, 1291. [Google Scholar] [CrossRef]
- Brandenburg, J.; Marwitz, S.; Tazoll, S.C.; Waldow, F.; Kalsdorf, B.; Vierbuchen, T.; Scholzen, T.; Gross, A.; Goldenbaum, S.; Holscher, A.; et al. WNT6/ACC2-induced storage of triacylglycerols in macrophages is exploited by Mycobacterium tuberculosis. J. Clin. Investig. 2021, 131, e141833. [Google Scholar] [CrossRef]
- Wik, J.A.; Skalhegg, B.S. T Cell Metabolism in Infection. Front. Immunol. 2022, 13, 840610. [Google Scholar] [CrossRef]
- Tiberi, S.; du Plessis, N.; Walzl, G.; Vjecha, M.J.; Rao, M.; Ntoumi, F.; Mfinanga, S.; Kapata, N.; Mwaba, P.; McHugh, T.D.; et al. Tuberculosis: Progress and advances in development of new drugs, treatment regimens, and host-directed therapies. Lancet Infect. Dis. 2018, 18, e183–e198. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lowrie, D.B.; Fan, X.Y.; Hu, Z. Natural products in anti-tuberculosis host-directed therapy. Biomed. Pharmacother. 2024, 171, 116087. [Google Scholar] [CrossRef] [PubMed]
- Wallis, R.S.; O’Garra, A.; Sher, A.; Wack, A. Host-directed immunotherapy of viral and bacterial infections: Past, present and future. Nat. Rev. Immunol. 2023, 23, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Hawn, T.R.; Shah, J.A.; Kalman, D. New tricks for old dogs: Countering antibiotic resistance in tuberculosis with host-directed therapeutics. Immunol. Rev. 2015, 264, 344–362. [Google Scholar] [CrossRef]
- Guler, R.; Brombacher, F. Host-directed drug therapy for tuberculosis. Nat. Chem. Biol. 2015, 11, 748–751. [Google Scholar] [CrossRef]
- Cubillos-Angulo, J.M.; Nogueira, B.M.F.; Arriaga, M.B.; Barreto-Duarte, B.; Araujo-Pereira, M.; Fernandes, C.D.; Vinhaes, C.L.; Villalva-Serra, K.; Nunes, V.M.; Miguez-Pinto, J.P.; et al. Host-directed therapies in pulmonary tuberculosis: Updates on anti-inflammatory drugs. Front. Med. 2022, 9, 970408. [Google Scholar] [CrossRef]
- Mily, A.; Rekha, R.S.; Kamal, S.M.; Arifuzzaman, A.S.; Rahim, Z.; Khan, L.; Haq, M.A.; Zaman, K.; Bergman, P.; Brighenti, S.; et al. Significant Effects of Oral Phenylbutyrate and Vitamin D3 Adjunctive Therapy in Pulmonary Tuberculosis: A Randomized Controlled Trial. PLoS ONE 2015, 10, e0138340. [Google Scholar] [CrossRef]
- Rekha, R.S.; Mily, A.; Sultana, T.; Haq, A.; Ahmed, S.; Mostafa Kamal, S.M.; van Schadewijk, A.; Hiemstra, P.S.; Gudmundsson, G.H.; Agerberth, B.; et al. Immune responses in the treatment of drug-sensitive pulmonary tuberculosis with phenylbutyrate and vitamin D(3) as host directed therapy. BMC Infect. Dis. 2018, 18, 303. [Google Scholar] [CrossRef]
- Bekele, A.; Gebreselassie, N.; Ashenafi, S.; Kassa, E.; Aseffa, G.; Amogne, W.; Getachew, M.; Aseffa, A.; Worku, A.; Raqib, R.; et al. Daily adjunctive therapy with vitamin D(3) and phenylbutyrate supports clinical recovery from pulmonary tuberculosis: A randomized controlled trial in Ethiopia. J. Intern. Med. 2018, 284, 292–306. [Google Scholar] [CrossRef]
- Tamara, L.; Kartasasmita, C.B.; Alam, A.; Gurnida, D.A. Effects of Vitamin D supplementation on resolution of fever and cough in children with pulmonary tuberculosis: A randomized double-blind controlled trial in Indonesia. J. Glob. Health 2022, 12, 04015. [Google Scholar] [CrossRef]
- Giver, C.R.; Shaw, P.A.; Fletcher, H.; Kaushal, D.; Pamela, G.; Omoyege, D.; Bisson, G.; Gumbo, T.; Wallis, R.; Waller, E.K.; et al. IMPACT-TB*: A Phase II Trial Assessing the Capacity of Low Dose Imatinib to Induce Myelopoiesis and Enhance Host Anti-Microbial Immunity Against Tuberculosis. *Imatinib Mesylate per Oral As a Clinical Therapeutic for TB. Blood 2019, 134, 1050. [Google Scholar] [CrossRef]
- Cleverley, T.L.; Peddineni, S.; Guarner, J.; Cingolani, F.; Garcia, P.K.; Koehler, H.; Mocarski, E.S.; Kalman, D. The host-directed therapeutic imatinib mesylate accelerates immune responses to Mycobacterium marinum infection and limits pathology associated with granulomas. PLoS Pathog. 2023, 19, e1011387. [Google Scholar] [CrossRef] [PubMed]
- Schoeman, J.F.; Janse van Rensburg, A.; Laubscher, J.A.; Springer, P. The role of aspirin in childhood tuberculous meningitis. J. Child. Neurol. 2011, 26, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Maitre, T.; Bonnet, M.; Calmy, A.; Raberahona, M.; Rakotoarivelo, R.A.; Rakotosamimanana, N.; Ambrosioni, J.; Miro, J.M.; Debeaudrap, P.; Muzoora, C.; et al. Intensified tuberculosis treatment to reduce the mortality of HIV-infected and uninfected patients with tuberculosis meningitis (INTENSE-TBM): Study protocol for a phase III randomized controlled trial. Trials 2022, 23, 928. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, L.R.; Manesh, A.; Ponnuraja, C.; Bhaskar, A.; Srinivasalu, V.A.; Daniel, B.D. Comparative evaluation of intensified short course regimen and standard regimen for adults TB meningitis: A protocol for an open label, multi-center, parallel arms, randomized controlled superiority trial (INSHORT trial). Trials 2024, 25, 294. [Google Scholar] [CrossRef]
- Mai, N.T.H.; Dobbs, N.; Phu, N.H.; Colas, R.A.; Thao, L.T.P.; Thuong, N.T.T.; Nghia, H.D.T.; Hanh, N.H.H.; Hang, N.T.; Heemskerk, A.D.; et al. A randomised double blind placebo controlled phase 2 trial of adjunctive aspirin for tuberculous meningitis in HIV-uninfected adults. eLife 2018, 7, e33478. [Google Scholar] [CrossRef]
- Misra, U.K.; Kalita, J.; Sagar, B.; Bhoi, S.K. Does adjunctive corticosteroid and aspirin therapy improve the outcome of tuberculous meningitis? Neurol. India 2018, 66, 1672–1677. [Google Scholar] [CrossRef]
- Arias, L.; Otwombe, K.; Waja, Z.; Tukvadze, N.; Korinteli, T.; Moloantoa, T.; Fonseca, K.L.; Pillay, N.; Seiphetlo, T.; Ouchi-Vernet, D.; et al. SMA-TB: Study protocol for the phase 2b randomized double-blind, placebo-controlled trial to estimate the potential efficacy and safety of two repurposed drugs, acetylsalicylic acid and ibuprofen, for use as adjunct therapy added to, and compared with, the standard WHO recommended TB regimen. Trials 2023, 24, 435. [Google Scholar] [CrossRef]
- Donovan, J.; Bang, N.D.; Imran, D.; Nghia, H.D.T.; Burhan, E.; Huong, D.T.T.; Hiep, N.T.T.; Ngoc, L.H.B.; Thanh, D.V.; Thanh, N.T.; et al. Adjunctive Dexamethasone for Tuberculous Meningitis in HIV-Positive Adults. N. Engl. J. Med. 2023, 389, 1357–1367. [Google Scholar] [CrossRef]
- Fu, L.; Wang, W.; Xiong, J.; Zhang, P.; Li, H.; Zhang, X.; Liang, H.; Yang, Q.; Wang, Z.; Chen, X.; et al. Evaluation of Sulfasalazine as an Adjunctive Therapy in Treating Pulmonary Pre-XDR-TB: Efficacy, Safety, and Treatment Implication. Infect. Drug Resist. 2024, 17, 595–604. [Google Scholar] [CrossRef]
- Jenum, S.; Tonby, K.; Rueegg, C.S.; Ruhwald, M.; Kristiansen, M.P.; Bang, P.; Olsen, I.C.; Sellaeg, K.; Rostad, K.; Mustafa, T.; et al. A Phase I/II randomized trial of H56:IC31 vaccination and adjunctive cyclooxygenase-2-inhibitor treatment in tuberculosis patients. Nat. Commun. 2021, 12, 6774. [Google Scholar] [CrossRef] [PubMed]
- Miow, Q.H.; Vallejo, A.F.; Wang, Y.; Hong, J.M.; Bai, C.; Teo, F.S.; Wang, A.D.; Loh, H.R.; Tan, T.Z.; Ding, Y.; et al. Doxycycline host-directed therapy in human pulmonary tuberculosis. J. Clin. Investig. 2021, 131, e141895. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.; Wu, X.; Liang, J. The advances in adjuvant therapy for tuberculosis with immunoregulatory compounds. Front. Microbiol. 2024, 15, 1380848. [Google Scholar] [CrossRef] [PubMed]
- Safe, I.P.; Amaral, E.P.; Araujo-Pereira, M.; Lacerda, M.V.G.; Printes, V.S.; Souza, A.B.; Beraldi-Magalhaes, F.; Monteiro, W.M.; Sampaio, V.S.; Barreto-Duarte, B.; et al. Adjunct N-Acetylcysteine Treatment in Hospitalized Patients with HIV-Associated Tuberculosis Dampens the Oxidative Stress in Peripheral Blood: Results From the RIPENACTB Study Trial. Front. Immunol. 2020, 11, 602589. [Google Scholar] [CrossRef] [PubMed]
- Wallis, R.S.; Sabi, I.; Lalashowi, J.; Bakuli, A.; Mapamba, D.; Olomi, W.; Siyame, E.; Ngaraguza, B.; Chimbe, O.; Charalambous, S.; et al. Adjunctive N-Acetylcysteine and Lung Function in Pulmonary Tuberculosis. NEJM Evid. 2024, 3, EVIDoa2300332. [Google Scholar] [CrossRef]
- Cross, G.B.; Sari, I.P.; Kityo, C.; Lu, Q.; Pokharkar, Y.; Moorakonda, R.B.; Thi, H.N.; Do, Q.; Dalay, V.B.; Gutierrez, E.; et al. Rosuvastatin adjunctive therapy for rifampicin-susceptible pulmonary tuberculosis: A phase 2b, randomised, open-label, multicentre trial. Lancet Infect. Dis. 2023, 23, 847–855. [Google Scholar] [CrossRef]
- Parihar, S.P.; Guler, R.; Brombacher, F. Statins: A viable candidate for host-directed therapy against infectious diseases. Nat. Rev. Immunol. 2019, 19, 104–117. [Google Scholar] [CrossRef]
- Padmapriydarsini, C.; Mamulwar, M.; Mohan, A.; Shanmugam, P.; Gomathy, N.S.; Mane, A.; Singh, U.B.; Pavankumar, N.; Kadam, A.; Kumar, H.; et al. Randomized Trial of Metformin with Anti-Tuberculosis Drugs for Early Sputum Conversion in Adults with Pulmonary Tuberculosis. Clin. Infect. Dis. 2022, 75, 425–434. [Google Scholar] [CrossRef]
- Pavan Kumar, N.; Padmapriyadarsini, C.; Nancy, A.; Tamizhselvan, M.; Mohan, A.; Reddy, D.; Ganga Devi, N.P.; Rathinam, P.; Jeyadeepa, B.; Shandil, R.K.; et al. Effect of Metformin on systemic chemokine responses during anti-tuberculosis chemotherapy. Tuberculosis 2024, 148, 102523. [Google Scholar] [CrossRef]
- Krismawati, H.; Muchtar, S.V.; Rahardjani, M.; Utami, N.N.; Oktaviani, M.; Puspatriani, K.; Syamsiah; Imbiri, N.; Hasvitasari, D.E.; Fajrianti, D.R.; et al. Metformin as adjunctive therapy in combination with multidrug treatment for multibacillary leprosy: A protocol for a randomized double-blind, controlled Phase 2 trial in Indonesia (MetLep Trial). Wellcome Open Res. 2023, 8, 289. [Google Scholar] [CrossRef]
- Wallis, R.S.; Ginindza, S.; Beattie, T.; Arjun, N.; Likoti, M.; Edward, V.A.; Rassool, M.; Ahmed, K.; Fielding, K.; Ahidjo, B.A.; et al. Adjunctive host-directed therapies for pulmonary tuberculosis: A prospective, open-label, phase 2, randomised controlled trial. Lancet Respir. Med. 2021, 9, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Wallis, R.S.; Ginindza, S.; Beattie, T.; Arjun, N.; Likoti, M.; Sebe, M.; Edward, V.A.; Rassool, M.; Ahmed, K.; Fielding, K.; et al. Lung and blood early biomarkers for host-directed tuberculosis therapies: Secondary outcome measures from a randomized controlled trial. PLoS ONE 2022, 17, e0252097. [Google Scholar] [CrossRef] [PubMed]
- Steiger, J.; Stephan, A.; Inkeles, M.S.; Realegeno, S.; Bruns, H.; Kroll, P.; de Castro Kroner, J.; Sommer, A.; Batinica, M.; Pitzler, L.; et al. Imatinib Triggers Phagolysosome Acidification and Antimicrobial Activity against Mycobacterium bovis Bacille Calmette-Guerin in Glucocorticoid-Treated Human Macrophages. J. Immunol. 2016, 197, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Adikesavalu, H.; Gopalaswamy, R.; Kumar, A.; Ranganathan, U.D.; Shanmugam, S. Autophagy Induction as a Host-Directed Therapeutic Strategy against Mycobacterium tuberculosis Infection. Medicina 2021, 57, 522. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.E.; Lee, H.K. Pattern recognition receptors and autophagy. Front. Immunol. 2014, 5, 300. [Google Scholar] [CrossRef]
- Cho, J.H.; Lee, H.J.; Ko, H.J.; Yoon, B.I.; Choe, J.; Kim, K.C.; Hahn, T.W.; Han, J.A.; Choi, S.S.; Jung, Y.M.; et al. The TLR7 agonist imiquimod induces anti-cancer effects via autophagic cell death and enhances anti-tumoral and systemic immunity during radiotherapy for melanoma. Oncotarget 2017, 8, 24932–24948. [Google Scholar] [CrossRef]
- Lee, H.J.; Kang, S.J.; Woo, Y.; Hahn, T.W.; Ko, H.J.; Jung, Y.J. TLR7 Stimulation with Imiquimod Induces Selective Autophagy and Controls Mycobacterium tuberculosis Growth in Mouse Macrophages. Front. Microbiol. 2020, 11, 1684. [Google Scholar] [CrossRef]
- Periyasamy, K.M.; Ranganathan, U.D.; Tripathy, S.P.; Bethunaickan, R. Vitamin D—A host directed autophagy mediated therapy for tuberculosis. Mol. Immunol. 2020, 127, 238–244. [Google Scholar] [CrossRef]
- Mily, A.; Rekha, R.S.; Kamal, S.M.; Akhtar, E.; Sarker, P.; Rahim, Z.; Gudmundsson, G.H.; Agerberth, B.; Raqib, R. Oral intake of phenylbutyrate with or without vitamin D3 upregulates the cathelicidin LL-37 in human macrophages: A dose finding study for treatment of tuberculosis. BMC Pulm. Med. 2013, 13, 23. [Google Scholar] [CrossRef]
- Rekha, R.S.; Rao Muvva, S.S.; Wan, M.; Raqib, R.; Bergman, P.; Brighenti, S.; Gudmundsson, G.H.; Agerberth, B. Phenylbutyrate induces LL-37-dependent autophagy and intracellular killing of Mycobacterium tuberculosis in human macrophages. Autophagy 2015, 11, 1688–1699. [Google Scholar] [CrossRef]
- Rao Muvva, J.; Ahmed, S.; Rekha, R.S.; Kalsum, S.; Groenheit, R.; Schon, T.; Agerberth, B.; Bergman, P.; Brighenti, S. Immunomodulatory Agents Combat Multidrug-Resistant Tuberculosis by Improving Antimicrobial Immunity. J. Infect. Dis. 2021, 224, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Lee, H.M.; Shin, D.M.; Kim, W.; Yuk, J.M.; Jin, H.S.; Lee, S.H.; Cha, G.H.; Kim, J.M.; Lee, Z.W.; et al. Host cell autophagy activated by antibiotics is required for their effective antimycobacterial drug action. Cell Host Microbe 2012, 11, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Krug, S.; Gupta, M.; Kumar, P.; Feller, L.; Ihms, E.A.; Kang, B.G.; Srikrishna, G.; Dawson, T.M.; Dawson, V.L.; Bishai, W.R. Inhibition of host PARP1 contributes to the anti-inflammatory and antitubercular activity of pyrazinamide. Nat. Commun. 2023, 14, 8161. [Google Scholar] [CrossRef] [PubMed]
- Schiebler, M.; Brown, K.; Hegyi, K.; Newton, S.M.; Renna, M.; Hepburn, L.; Klapholz, C.; Coulter, S.; Obregon-Henao, A.; Henao Tamayo, M.; et al. Functional drug screening reveals anticonvulsants as enhancers of mTOR-independent autophagic killing of Mycobacterium tuberculosis through inositol depletion. EMBO Mol. Med. 2015, 7, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Choi, J.A.; Choi, H.H.; Cho, S.N.; Kim, H.J.; Jo, E.K.; Park, J.K.; Song, C.H. Endoplasmic reticulum stress pathway-mediated apoptosis in macrophages contributes to the survival of Mycobacterium tuberculosis. PLoS ONE 2011, 6, e28531. [Google Scholar] [CrossRef]
- Xu, P.; Tang, J.; He, Z.G. Induction of Endoplasmic Reticulum Stress by CdhM Mediates Apoptosis of Macrophage During Mycobacterium tuberculosis Infection. Front. Cell Infect. Microbiol. 2022, 12, 877265. [Google Scholar] [CrossRef]
- Pennini, M.E.; Liu, Y.; Yang, J.; Croniger, C.M.; Boom, W.H.; Harding, C.V. CCAAT/enhancer-binding protein beta and delta binding to CIITA promoters is associated with the inhibition of CIITA expression in response to Mycobacterium tuberculosis 19-kDa lipoprotein. J. Immunol. 2007, 179, 6910–6918. [Google Scholar] [CrossRef]
- Gupta, P.K.; Chakraborty, P.; Kumar, S.; Singh, P.K.; Rajan, M.G.; Sainis, K.B.; Kulkarni, S. G1-4A, a Polysaccharide from Tinospora cordifolia Inhibits the Survival of Mycobacterium tuberculosis by Modulating Host Immune Responses in TLR4 Dependent Manner. PLoS ONE 2016, 11, e0154725. [Google Scholar] [CrossRef]
- Alonzi, T.; Aiello, A.; Sali, M.; Delogu, G.; Villella, V.R.; Raia, V.; Nicastri, E.; Piacentini, M.; Goletti, D. Multiple antimicrobial and immune-modulating activities of cysteamine in infectious diseases. Biomed. Pharmacother. 2024, 178, 117153. [Google Scholar] [CrossRef]
- Boland, R.; Heemskerk, M.T.; Forn-Cuni, G.; Korbee, C.J.; Walburg, K.V.; Esselink, J.J.; Carvalho Dos Santos, C.; de Waal, A.M.; van der Hoeven, D.C.M.; van der Sar, E.; et al. Repurposing Tamoxifen as Potential Host-Directed Therapeutic for Tuberculosis. mBio 2023, 14, e0302422. [Google Scholar] [CrossRef]
- Singhal, A.; Jie, L.; Kumar, P.; Hong, G.S.; Leow, M.K.; Paleja, B.; Tsenova, L.; Kurepina, N.; Chen, J.; Zolezzi, F.; et al. Metformin as adjunct antituberculosis therapy. Sci. Transl. Med. 2014, 6, 263ra159. [Google Scholar] [CrossRef] [PubMed]
- Lachmandas, E.; Eckold, C.; Bohme, J.; Koeken, V.; Marzuki, M.B.; Blok, B.; Arts, R.J.W.; Chen, J.; Teng, K.W.W.; Ratter, J.; et al. Metformin Alters Human Host Responses to Mycobacterium tuberculosis in Healthy Subjects. J. Infect. Dis. 2019, 220, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Bohme, J.; Martinez, N.; Li, S.; Lee, A.; Marzuki, M.; Tizazu, A.M.; Ackart, D.; Frenkel, J.H.; Todd, A.; Lachmandas, E.; et al. Metformin enhances anti-mycobacterial responses by educating CD8+ T-cell immunometabolic circuits. Nat. Commun. 2020, 11, 5225. [Google Scholar] [CrossRef] [PubMed]
- Safe, I.P.; Lacerda, M.V.G.; Printes, V.S.; Praia Marins, A.F.; Rebelo Rabelo, A.L.; Costa, A.A.; Tavares, M.A.; Jesus, J.S.; Souza, A.B.; Beraldi-Magalhaes, F.; et al. Safety and efficacy of N-acetylcysteine in hospitalized patients with HIV-associated tuberculosis: An open-label, randomized, phase II trial (RIPENACTB Study). PLoS ONE 2020, 15, e0235381. [Google Scholar] [CrossRef]
- Mannick, J.B.; Del Giudice, G.; Lattanzi, M.; Valiante, N.M.; Praestgaard, J.; Huang, B.; Lonetto, M.A.; Maecker, H.T.; Kovarik, J.; Carson, S.; et al. mTOR inhibition improves immune function in the elderly. Sci. Transl. Med. 2014, 6, 268ra179. [Google Scholar] [CrossRef]
- Raien, A.; Davis, S.; Zhang, M.; Zitser, D.; Lin, M.; Pitcher, G.; Bhalodia, K.; Subbian, S.; Venketaraman, V. Effects of Everolimus in Modulating the Host Immune Responses against Mycobacterium tuberculosis Infection. Cells 2023, 12, 2653. [Google Scholar] [CrossRef]
- de Almeida, P.E.; Pereira de Sousa, N.M.; Rampinelli, P.G.; Silva, R.V.S.; Correa, J.R.; D’Avila, H. Lipid droplets as multifunctional organelles related to the mechanism of evasion during mycobacterial infection. Front. Cell Infect. Microbiol. 2023, 13, 1102643. [Google Scholar] [CrossRef]
- Gabor, K.A.; Fessler, M.B. Roles of the Mevalonate Pathway and Cholesterol Trafficking in Pulmonary Host Defense. Curr. Mol. Pharmacol. 2017, 10, 27–45. [Google Scholar] [CrossRef]
- Adewole, O.O.; Omotoso, B.A.; Ogunsina, M.; Aminu, A.; Ayoola, O.; Adedeji, T.; Awopeju, O.F.; Sogaolu, O.M.; Adewole, T.O.; Odeyemi, A.O.; et al. Atorvastatin improves sputum conversion and chest X-ray severity score. Int. J. Tuberc. Lung Dis. 2023, 27, 912–917. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, L.; Qiao, W.; Luo, Y. Mycobacterium tuberculosis: Pathogenesis and therapeutic targets. MedComm 2023, 4, e353. [Google Scholar] [CrossRef]
- Babunovic, G.H.; DeJesus, M.A.; Bosch, B.; Chase, M.R.; Barbier, T.; Dickey, A.K.; Bryson, B.D.; Rock, J.M.; Fortune, S.M. CRISPR Interference Reveals That All-Trans-Retinoic Acid Promotes Macrophage Control of Mycobacterium tuberculosis by Limiting Bacterial Access to Cholesterol and Propionyl Coenzyme A. mBio 2022, 13, e0368321. [Google Scholar] [CrossRef] [PubMed]
- Stan, D.; Enciu, A.M.; Mateescu, A.L.; Ion, A.C.; Brezeanu, A.C.; Stan, D.; Tanase, C. Natural Compounds with Antimicrobial and Antiviral Effect and Nanocarriers Used for Their Transportation. Front. Pharmacol. 2021, 12, 723233. [Google Scholar] [CrossRef] [PubMed]
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-derived natural products for drug discovery: Current approaches and prospects. Nucleus 2022, 65, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Prasad, R.; Jain, A. Effect of green tea extract (catechins) in reducing oxidative stress seen in patients of pulmonary tuberculosis on DOTS Cat I regimen. Phytomedicine 2010, 17, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Butov, D.; Zaitseva, S.; Butova, T.; Stepanenko, G.; Pogorelova, O.; Zhelezniakova, N. Efficacy and safety of quercetin and polyvinylpyrrolidone in treatment of patients with newly diagnosed destructive pulmonary tuberculosis in comparison with standard antimycobacterial therapy. Int. J. Mycobacteriol. 2016, 5, 446–453. [Google Scholar] [CrossRef]
- Talebi, A.; Soltani, R.; Khorvash, F.; Jouabadi, S.M. The Effectiveness of Silymarin in the Prevention of Anti-tuberculosis Drug-induced Hepatotoxicity: A Randomized Controlled Clinical Trial. Int. J. Prev. Med. 2023, 14, 48. [Google Scholar] [CrossRef]
- Luangchosiri, C.; Thakkinstian, A.; Chitphuk, S.; Stitchantrakul, W.; Petraksa, S.; Sobhonslidsuk, A. A double-blinded randomized controlled trial of silymarin for the prevention of antituberculosis drug-induced liver injury. BMC Complement. Altern. Med. 2015, 15, 334. [Google Scholar] [CrossRef]
- Lara-Espinosa, J.V.; Arce-Aceves, M.F.; Lopez-Torres, M.O.; Lozano-Ordaz, V.; Mata-Espinosa, D.; Barrios-Payan, J.; Silva-Islas, C.A.; Maldonado, P.D.; Marquina-Castillo, B.; Hernandez-Pando, R. Effect of Curcumin in Experimental Pulmonary Tuberculosis: Antimycobacterial Activity in the Lungs and Anti-Inflammatory Effect in the Brain. Int. J. Mol. Sci. 2022, 23, 1964. [Google Scholar] [CrossRef]
- Gupta, P.K.; Jahagirdar, P.; Tripathi, D.; Devarajan, P.V.; Kulkarni, S. Macrophage targeted polymeric curcumin nanoparticles limit intracellular survival of Mycobacterium tuberculosis through induction of autophagy and augment anti-TB activity of isoniazid in RAW 264.7 macrophages. Front. Immunol. 2023, 14, 1233630. [Google Scholar] [CrossRef]
- Tousif, S.; Singh, D.K.; Mukherjee, S.; Ahmad, S.; Arya, R.; Nanda, R.; Ranganathan, A.; Bhattacharyya, M.; Van Kaer, L.; Kar, S.K.; et al. Nanoparticle-Formulated Curcumin Prevents Posttherapeutic Disease Reactivation and Reinfection with Mycobacterium tuberculosis following Isoniazid Therapy. Front. Immunol. 2017, 8, 739. [Google Scholar] [CrossRef]
- Li, Y.; Luo, W.W.; Cheng, X.; Xiang, H.R.; He, B.; Zhang, Q.Z.; Peng, W.X. Curcumin attenuates isoniazid-induced hepatotoxicity by upregulating the SIRT1/PGC-1alpha/NRF1 pathway. J. Appl. Toxicol. 2022, 42, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hu, J.; Chen, Y.J.; Ge, B. Role of Sirt1 in innate immune mechanisms against Mycobacterium tuberculosis via the inhibition of TAK1 activation. Arch. Biochem. Biophys. 2019, 667, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Tousif, S.; Bhaskar, A.; Devi, A.; Negi, K.; Moitra, B.; Ranganathan, A.; Dwivedi, V.P.; Das, G. Luteolin as a potential host-directed immunotherapy adjunct to isoniazid treatment of tuberculosis. PLoS Pathog. 2021, 17, e1009805. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, V.P.; Bhattacharya, D.; Yadav, V.; Singh, D.K.; Kumar, S.; Singh, M.; Ojha, D.; Ranganathan, A.; Van Kaer, L.; Chattopadhyay, D.; et al. The Phytochemical Bergenin Enhances T Helper 1 Responses and Anti-Mycobacterial Immunity by Activating the MAP Kinase Pathway in Macrophages. Front. Cell Infect. Microbiol. 2017, 7, 149. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, C.; Kaushik, S.R.; Kulshreshtha, A.; Chaturvedi, S.; Nanda, R.K.; Bhaskar, A.; Chattopadhyay, D.; Das, G.; Dwivedi, V.P. The phytochemical bergenin as an adjunct immunotherapy for tuberculosis in mice. J. Biol. Chem. 2019, 294, 8555–8563. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, A.; Kumari, A.; Singh, M.; Kumar, S.; Kumar, S.; Dabla, A.; Chaturvedi, S.; Yadav, V.; Chattopadhyay, D.; Prakash Dwivedi, V. [6]-Gingerol exhibits potent anti-mycobacterial and immunomodulatory activity against tuberculosis. Int. Immunopharmacol. 2020, 87, 106809. [Google Scholar] [CrossRef]
- Patel, N.; Jagannath, K.; Vora, A.; Patel, M.; Patel, A. A Randomized, Controlled, Phase III Clinical Trial to Evaluate the Efficacy and Tolerability of Risorine with Conventional Rifampicin in the Treatment of Newly Diagnosed Pulmonary Tuberculosis Patients. J. Assoc. Physicians India 2017, 65, 48–54. [Google Scholar]
- Ayman, M.; Mahmoud, M.C.G.a.A.S.S. Berberine Attenuates Isoniazid-lnduced Hepatotoxicity by Modulating Peroxisome Proliferator-Activated Receptor y, Oxidative Stress and Inflammation. Int. J. Pharmacol. 2014, 10, 10. [Google Scholar] [CrossRef]
- Pahuja, I.; Negi, K.; Kumari, A.; Agarwal, M.; Mukhopadhyay, S.; Mathew, B.; Chaturvedi, S.; Maras, J.S.; Bhaskar, A.; Dwivedi, V.P. Berberine governs NOTCH3/AKT signaling to enrich lung-resident memory T cells during tuberculosis. PLoS Pathog. 2023, 19, e1011165. [Google Scholar] [CrossRef]
- Ozturk, M.; Chia, J.E.; Hazra, R.; Saqib, M.; Maine, R.A.; Guler, R.; Suzuki, H.; Mishra, B.B.; Brombacher, F.; Parihar, S.P. Evaluation of Berberine as an Adjunct to TB Treatment. Front. Immunol. 2021, 12, 656419. [Google Scholar] [CrossRef]
- Dwivedi, V.P.; Bhattacharya, D.; Singh, M.; Bhaskar, A.; Kumar, S.; Fatima, S.; Sobia, P.; Kaer, L.V.; Das, G. Allicin enhances antimicrobial activity of macrophages during Mycobacterium tuberculosis infection. J. Ethnopharmacol. 2019, 243, 111634. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.K.; Kaul, D.; Sharma, M. Green tea polyphenol inhibits Mycobacterium tuberculosis survival within human macrophages. Int. J. Biochem. Cell Biol. 2006, 38, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Vaghasiya, K.; Ray, E.; Gupta, P.; Gupta, U.D.; Singh, A.K.; Verma, R.K. Targeted Pulmonary Delivery of the Green Tea Polyphenol Epigallocatechin Gallate Controls the Growth of Mycobacterium tuberculosis by Enhancing the Autophagy and Suppressing Bacterial Burden. ACS Biomater. Sci. Eng. 2020, 6, 4126–4140. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Deng, J.; Li, W.; Lin, D.; Su, C.; Wang, M.; Li, X.; Abuaku, B.K.; Tan, H.; Wen, S.W. Impact of tea drinking upon tuberculosis: A neglected issue. BMC Public Health 2015, 15, 515. [Google Scholar] [CrossRef] [PubMed]
- Soh, A.Z.; Pan, A.; Chee, C.B.E.; Wang, Y.T.; Yuan, J.M.; Koh, W.P. Tea Drinking and Its Association with Active Tuberculosis Incidence among Middle-Aged and Elderly Adults: The Singapore Chinese Health Study. Nutrients 2017, 9, 544. [Google Scholar] [CrossRef]
- Bai, X.; Oberley-Deegan, R.E.; Bai, A.; Ovrutsky, A.R.; Kinney, W.H.; Weaver, M.; Zhang, G.; Honda, J.R.; Chan, E.D. Curcumin enhances human macrophage control of Mycobacterium tuberculosis infection. Respirology 2016, 21, 951–957. [Google Scholar] [CrossRef]
- Jahagirdar, P.S.; Gupta, P.K.; Kulkarni, S.P.; Devarajan, P.V. Intramacrophage Delivery of Dual Drug Loaded Nanoparticles for Effective Clearance of Mycobacterium tuberculosis. J. Pharm. Sci. 2020, 109, 2262–2270. [Google Scholar] [CrossRef]
- Yang, H.; Chen, J.; Chen, Y.; Jiang, Y.; Ge, B.; Hong, L. Sirtuin inhibits M. tuberculosis -induced apoptosis in macrophage through glycogen synthase kinase-3beta. Arch. Biochem. Biophys. 2020, 694, 108612. [Google Scholar] [CrossRef]
- Yang, H.; Chen, J.; Chen, Y.; Jiang, Y.; Ge, B.; Hong, L. Sirt1 activation negatively regulates overt apoptosis in Mtb-infected macrophage through Bax. Int. Immunopharmacol. 2021, 91, 107283. [Google Scholar] [CrossRef]
- Ahmad, S.; Bhattacharya, D.; Kar, S.; Ranganathan, A.; Van Kaer, L.; Das, G. Curcumin Nanoparticles Enhance Mycobacterium bovis BCG Vaccine Efficacy by Modulating Host Immune Responses. Infect. Immun. 2019, 87, 10–1128. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Pahuja, I.; Okieh, A.A.; Pandey, D.; Yadav, V.; Bhaskar, A.; Dwivedi, V.P. Bergenin potentiates BCG efficacy by enriching mycobacteria-specific adaptive memory responses via the Akt-Foxo-Stat4 axis. Tuberculosis 2024, 147, 102517. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kalia, N.P.; Suden, P.; Chauhan, P.S.; Kumar, M.; Ram, A.B.; Khajuria, A.; Bani, S.; Khan, I.A. Protective efficacy of piperine against Mycobacterium tuberculosis. Tuberculosis 2014, 94, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Vora, A.; Patel, S.; Patel, K. Role of Risorine in the Treatment of Drug—Susceptible Pulmonary Tuberculosis: A Pilot Study. J. Assoc. Physicians India 2016, 64, 20–24. [Google Scholar] [PubMed]
- Rodriguez-Flores, E.M.; Mata-Espinosa, D.; Barrios-Payan, J.; Marquina-Castillo, B.; Castanon-Arreola, M.; Hernandez-Pando, R. A significant therapeutic effect of silymarin administered alone, or in combination with chemotherapy, in experimental pulmonary tuberculosis caused by drug-sensitive or drug-resistant strains: In vitro and in vivo studies. PLoS ONE 2019, 14, e0217457. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, Z.; Niu, W.; Wan, Y.; Zhang, L.; Shi, G.; Xi, X. The protective effect of curcumin against the 19-kDa Mycobacterium tuberculosis protein-induced inflammation and apoptosis in human macrophages. Mol. Med. Rep. 2014, 10, 3261–3267. [Google Scholar] [CrossRef]
- Hasan, N.; Yusuf, N.; Toossi, Z.; Islam, N. Suppression of Mycobacterium tuberculosis induced reactive oxygen species (ROS) and TNF-alpha mRNA expression in human monocytes by allicin. FEBS Lett. 2006, 580, 2517–2522. [Google Scholar] [CrossRef]
- Butova, T.; Zaitseva, S.; Butov, D.; Stepanenko, G. Morphological changes in experimental tuberculosis resulting from treatment with quercetin and polyvinylpyrrolidone. Int. J. Mycobacteriol. 2016, 5 (Suppl. S1), S103–S104. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, X.; Gao, H.; Zhai, J.; Tao, L.; Sun, J.; Song, Y.; Zhang, J. Quercetin attenuates NLRP3 inflammasome activation and apoptosis to protect INH-induced liver injury via regulating SIRT1 pathway. Int. Immunopharmacol. 2020, 85, 106634. [Google Scholar] [CrossRef]
- Sanjay, S.; Girish, C.; Toi, P.C.; Bobby, Z. Quercetin modulates NRF2 and NF-kappaB/TLR-4 pathways to protect against isoniazid- and rifampicin-induced hepatotoxicity in vivo. Can. J. Physiol. Pharmacol. 2021, 99, 952–963. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Tee, V. Hepatoprotective effects of silymarin in management of liver injury caused by tuberculosis treatment. Drugs Context 2023, 12. eCollection 2023. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, A.; Ma, A.; Jiang, F.; Xiao, Y.; Chen, Y.; Huang, R.; Yang, T.; Zhou, J. Effectiveness of Prophylactic Use of Hepatoprotectants for Tuberculosis Drug-Induced Liver Injury: A Population-Based Cohort Analysis Involving 6,743 Chinese Patients. Front. Pharmacol. 2022, 13, 813682. [Google Scholar] [CrossRef] [PubMed]
- Marjani, M.; Fahim, F.; Sadr, M.; Kazempour Dizaji, M.; Moniri, A.; Khabiri, S.; Tabarsi, P.; Velayati, A.A. Evaluation of Silymarin for management of anti-tuberculosis drug induced liver injury: A randomized clinical trial. Gastroenterol. Hepatol. Bed Bench 2019, 12, 138–142. [Google Scholar] [PubMed]
- Heo, E.; Kim, D.K.; Oh, S.H.; Lee, J.K.; Park, J.H.; Chung, H.S. Effect of Prophylactic Use of Silymarin on Anti-tuberculosis Drugs Induced Hepatotoxicity. Tuberc. Respir. Dis. 2017, 80, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Marjani, M.; Baghaei, P.; Kazempour Dizaji, M.; Gorji Bayani, P.; Fahimi, F.; Tabarsi, P.; Velayati, A.A. Evaluation of Hepatoprotective Effect of Silymarin Among Under Treatment Tuberculosis Patients: A Randomized Clinical Trial. Iran. J. Pharm. Res. 2016, 15, 247–252. [Google Scholar]
- Zhang, S.; Pan, H.; Peng, X.; Lu, H.; Fan, H.; Zheng, X.; Xu, G.; Wang, M.; Wang, J. Preventive use of a hepatoprotectant against anti-tuberculosis drug-induced liver injury: A randomized controlled trial. J. Gastroenterol. Hepatol. 2016, 31, 409–416. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Scientific opinion on the safety of green tea catechins. EFSA J. 2018, 16, e05239. [Google Scholar] [CrossRef]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The safety of green tea and green tea extract consumption in adults—Results of a systematic review. Regul. Toxicol. Pharmacol. 2018, 95, 412–433. [Google Scholar] [CrossRef]
- Ouyang, J.; Zhu, K.; Liu, Z.; Huang, J. Prooxidant Effects of Epigallocatechin-3-Gallate in Health Benefits and Potential Adverse Effect. Oxid. Med. Cell Longev. 2020, 2020, 9723686. [Google Scholar] [CrossRef]
- Bellmann-Strobl, J.; Paul, F.; Wuerfel, J.; Dorr, J.; Infante-Duarte, C.; Heidrich, E.; Kortgen, B.; Brandt, A.; Pfuller, C.; Radbruch, H.; et al. Epigallocatechin Gallate in Relapsing-Remitting Multiple Sclerosis: A Randomized, Placebo-Controlled Trial. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e981. [Google Scholar] [CrossRef]
- Chatree, S.; Sitticharoon, C.; Maikaew, P.; Pongwattanapakin, K.; Keadkraichaiwat, I.; Churintaraphan, M.; Sripong, C.; Sririwichitchai, R.; Tapechum, S. Epigallocatechin gallate decreases plasma triglyceride, blood pressure, and serum kisspeptin in obese human subjects. Exp. Biol. Med. 2021, 246, 163–176. [Google Scholar] [CrossRef]
- Rust, R.; Chien, C.; Scheel, M.; Brandt, A.U.; Dorr, J.; Wuerfel, J.; Klumbies, K.; Zimmermann, H.; Lorenz, M.; Wernecke, K.D.; et al. Epigallocatechin Gallate in Progressive MS: A Randomized, Placebo-Controlled Trial. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e964. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhu, W.; Tang, X.; Yang, G.; Zhao, X.; Zhao, K.; Jiang, L.; Li, X.; Zhao, H.; Wang, X.; et al. Phase I/II clinical trial of efficacy and safety of EGCG oxygen nebulization inhalation in the treatment of COVID-19 pneumonia patients with cancer. BMC Cancer 2024, 24, 486. [Google Scholar] [CrossRef] [PubMed]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schafer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Iqtadar, S.; Khan, A.; Ullah Mumtaz, S.; Masud Chaudhry, M.; Bertuccioli, A.; Derosa, G.; Maffioli, P.; Togni, S.; Riva, A.; et al. Potential Clinical Benefits of Quercetin in the Early Stage of COVID-19: Results of a Second, Pilot, Randomized, Controlled and Open-Label Clinical Trial. Int. J. Gen. Med. 2021, 14, 2807–2816. [Google Scholar] [CrossRef]
- Han, M.K.; Barreto, T.A.; Martinez, F.J.; Comstock, A.T.; Sajjan, U.S. Randomised clinical trial to determine the safety of quercetin supplementation in patients with chronic obstructive pulmonary disease. BMJ Open Respir. Res. 2020, 7, e000392. [Google Scholar] [CrossRef]
- Kozhukhov, S.; Parkhomenko, A.; Lutay, Y.; Dovganych, N.; Study, I. Impact of quercetin in patients with myocardial infarction. A multicenter, randomized, and open-label pilot study. Hellenic J. Cardiol. 2024, 76, 68–74. [Google Scholar] [CrossRef]
- Yang, K.; Chen, J.; Zhang, T.; Yuan, X.; Ge, A.; Wang, S.; Xu, H.; Zeng, L.; Ge, J. Efficacy and safety of dietary polyphenol supplementation in the treatment of non-alcoholic fatty liver disease: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 949746. [Google Scholar] [CrossRef]
- Flaig, T.W.; Gustafson, D.L.; Su, L.J.; Zirrolli, J.A.; Crighton, F.; Harrison, G.S.; Pierson, A.S.; Agarwal, R.; Glode, L.M. A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Investig. New Drugs 2007, 25, 139–146. [Google Scholar] [CrossRef]
- Aryan, H.; Farahani, R.H.; Chamanara, M.; Elyasi, S.; Jaafari, M.R.; Haddad, M.; Sani, A.T.; Ardalan, M.A.; Mosaed, R. Evaluation of the efficacy of oral nano-silymarin formulation in hospitalized patients with COVID-19: A double-blind placebo-controlled clinical trial. Phytother. Res. 2022, 36, 3924–3931. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocinska, K.; Zielinska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer. Res. 2001, 21, 2895–2900. [Google Scholar] [PubMed]
- Doostkam, A.; Iravani, K.; Malekmakan, L.; Gholamabbas, G.; Roozbeh, J.; Soltaniesmaeili, A. The effectiveness of curcumin as a safe agent on hearing threshold improvement in patients with chronic kidney disease: A double-blind, placebo-controlled trial. Sci. Rep. 2024, 14, 17576. [Google Scholar] [CrossRef] [PubMed]
- Noppakun, K.; Jitraknatee, J.; Suteeka, Y.; Ruengorn, C.; Nochaiwong, S.; Gunaparn, S.; Phrommintikul, A.; Wongcharoen, W. Effect of Curcuminoids on Contrast-Induced Acute Kidney Injury after Elective Coronary Angiography or Intervention: A Pilot Randomized, Double-Blind, Placebo-Controlled Study. Cardiorenal Med. 2024, 14, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M.; Hosseinzadeh-Attar, M.J.; Rezaei, M.; Alipoor, E.; Vasheghani-Farahani, A.; Yaseri, M.; Rezayat, S.M. Effect of nano-curcumin supplementation on cardiometabolic risk factors, physical and psychological quality of life, and depression in patients with coronary slow flow phenomenon: A randomized double-blind clinical trial. Trials 2024, 25, 515. [Google Scholar] [CrossRef] [PubMed]
- Yaikwawong, M.; Jansarikit, L.; Jirawatnotai, S.; Chuengsamarn, S. Curcumin extract improves beta cell functions in obese patients with type 2 diabetes: A randomized controlled trial. Nutr. J. 2024, 23, 119. [Google Scholar] [CrossRef]
- Yaikwawong, M.; Jansarikit, L.; Jirawatnotai, S.; Chuengsamarn, S. The Effect of Curcumin on Reducing Atherogenic Risks in Obese Patients with Type 2 Diabetes: A Randomized Controlled Trial. Nutrients 2024, 16, 2441. [Google Scholar] [CrossRef]
- Garcia-Martinez, B.I.; Ruiz-Ramos, M.; Pedraza-Chaverri, J.; Santiago-Osorio, E.; Mendoza-Nunez, V.M. Effect of Resveratrol on Markers of Oxidative Stress and Sirtuin 1 in Elderly Adults with Type 2 Diabetes. Int. J. Mol. Sci. 2023, 24, 7422. [Google Scholar] [CrossRef]
- Mahjabeen, W.; Khan, D.A.; Mirza, S.A. Role of resveratrol supplementation in regulation of glucose hemostasis, inflammation and oxidative stress in patients with diabetes mellitus type 2: A randomized, placebo-controlled trial. Complement. Ther. Med. 2022, 66, 102819. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, Y.; Pan, Z.; Jin, Y.; Li, Q.; Pang, J.; Wang, X.; Chen, Y.; Yang, Y.; Ling, W. A Randomized Trial on Resveratrol Supplement Affecting Lipid Profile and Other Metabolic Markers in Subjects with Dyslipidemia. Nutrients 2023, 15, 492. [Google Scholar] [CrossRef]
- Popat, R.; Plesner, T.; Davies, F.; Cook, G.; Cook, M.; Elliott, P.; Jacobson, E.; Gumbleton, T.; Oakervee, H.; Cavenagh, J. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br. J. Haematol. 2013, 160, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Mankowski, R.T.; You, L.; Buford, T.W.; Leeuwenburgh, C.; Manini, T.M.; Schneider, S.; Qiu, P.; Anton, S.D. Higher dose of resveratrol elevated cardiovascular disease risk biomarker levels in overweight older adults—A pilot study. Exp. Gerontol. 2020, 131, 110821. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, C.; Xu, Y.; Yang, L. Efficacy and safety of berberine plus 5-ASA for ulcerative colitis: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0309144. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.; Yu, X.; Xu, X.; Wang, L.; Ding, C.; Wang, Z.; Xie, X.; Li, S.; Yang, W.; Luo, S.; et al. Effectiveness and safety of Bifidobacterium and berberine in human hyperglycemia and their regulatory effect on the gut microbiota: A multi-center, double-blind, randomized, parallel-controlled study. Genome Med. 2021, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, A.; Mohanty, S. Efficacy and safety of HIMABERB(R) Berberine on glycemic control in patients with prediabetes: Double-blind, placebo-controlled, and randomized pilot trial. BMC Endocr. Disord. 2023, 23, 190. [Google Scholar] [CrossRef]
- Rondanelli, M.; Gasparri, C.; Petrangolini, G.; Allegrini, P.; Avenoso, D.; Fazia, T.; Bernardinelli, L.; Peroni, G.; Patelli, Z.; Mansueto, F.; et al. Berberine phospholipid exerts a positive effect on the glycemic profile of overweight subjects with impaired fasting blood glucose (IFG): A randomized double-blind placebo-controlled clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 6718–6727. [Google Scholar] [CrossRef]
- Zhao, J.V.; Yeung, W.F.; Chan, Y.H.; Vackova, D.; Leung, J.Y.Y.; Ip, D.K.M.; Zhao, J.; Ho, W.K.; Tse, H.F.; Schooling, C.M. Effect of Berberine on Cardiovascular Disease Risk Factors: A Mechanistic Randomized Controlled Trial. Nutrients 2021, 13, 2550. [Google Scholar] [CrossRef]
- Bandala, C.; Carro-Rodriguez, J.; Cardenas-Rodriguez, N.; Pena-Montero, I.; Gomez-Lopez, M.; Hernandez-Roldan, A.P.; Huerta-Cruz, J.C.; Munoz-Gonzalez, F.; Ignacio-Mejia, I.; Dominguez, B.; et al. Comparative Effects of Gymnema sylvestre and Berberine on Adipokines, Body Composition, and Metabolic Parameters in Obese Patients: A Randomized Study. Nutrients 2024, 16, 2284. [Google Scholar] [CrossRef]
- Cui, Y.; Zhou, Q.; Jin, M.; Jiang, S.; Shang, P.; Dong, X.; Li, L. Research progress on pharmacological effects and bioavailability of berberine. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 8485–8514. [Google Scholar] [CrossRef]
- Harrison, S.A.; Gunn, N.; Neff, G.W.; Kohli, A.; Liu, L.; Flyer, A.; Goldkind, L.; Di Bisceglie, A.M. A phase 2, proof of concept, randomised controlled trial of berberine ursodeoxycholate in patients with presumed non-alcoholic steatohepatitis and type 2 diabetes. Nat. Commun. 2021, 12, 5503. [Google Scholar] [CrossRef]
- Qiao, M.; Lei, C.; Tan, C.; Lu, C.; Chen, Z.; Zhang, Q.; Wang, Z. Efficacy and safety of berberine for premature ventricular contractions: A meta-analysis and systematic review of randomized controlled trials. Pharm. Biol. 2023, 61, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Di Stadio, A.; D’Ascanio, L.; Vaira, L.A.; Cantone, E.; De Luca, P.; Cingolani, C.; Motta, G.; De Riu, G.; Vitelli, F.; Spriano, G.; et al. Ultramicronized Palmitoylethanolamide and Luteolin Supplement Combined with Olfactory Training to Treat Post-COVID-19 Olfactory Impairment: A Multi-Center Double-Blinded Randomized Placebo-Controlled Clinical Trial. Curr. Neuropharmacol. 2022, 20, 2001–2012. [Google Scholar] [CrossRef] [PubMed]
- Ntalouka, F.; Tsirivakou, A. Luteolin: A promising natural agent in management of pain in chronic conditions. Front. Pain Res. 2023, 4, 1114428. [Google Scholar] [CrossRef] [PubMed]
- Preisler, H.D.; Raza, A.; Larson, R.; Browman, G.; Goldberg, J.; Grunwald, H.; Vogler, R.; Bennett, J.; Gottlieb, A.; D’Arrigo, P. Limited efficacy of a four-day course of high-dose cytosine arabinoside in the treatment of poor-risk patients with acute nonlymphocytic leukemia. Cancer Chemother. Pharmacol. 1986, 18, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Terzo, S.; Amato, A.; Magan-Fernandez, A.; Castellino, G.; Calvi, P.; Chianetta, R.; Giglio, R.V.; Patti, A.M.; Nikolic, D.; Firenze, A.; et al. A Nutraceutical Containing Chlorogenic Acid and Luteolin Improves Cardiometabolic Parameters in Subjects with Pre-Obesity: A 6-Month Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2023, 15, 462. [Google Scholar] [CrossRef]
- Crichton, M.; Marshall, S.; Isenring, E.; Lohning, A.; McCarthy, A.L.; Molassiotis, A.; Bird, R.; Shannon, C.; Koh, A.; McPherson, I.; et al. Effect of a Standardized Ginger Root Powder Regimen on Chemotherapy-Induced Nausea and Vomiting: A Multicenter, Double-Blind, Placebo-Controlled Randomized Trial. J. Acad. Nutr. Diet. 2024, 124, 313–330.e316. [Google Scholar] [CrossRef]
- Crichton, M.; Marshall, S.; Marx, W.; Isenring, E.; Vazquez-Campos, X.; Dawson, S.L.; Lohning, A. Effect of Ginger Root Powder on Gastrointestinal Bacteria Composition, Gastrointestinal Symptoms, Mental Health, Fatigue, and Quality of Life: A Double-Blind Placebo-Controlled Trial. J. Nutr. 2023, 153, 3193–3206. [Google Scholar] [CrossRef]
- Ivashkin, V.T.; Kudryavtseva, A.V.; Krasnov, G.S.; Poluektov, Y.M.; Morozova, M.A.; Shifrin, O.S.; Beniashvili, A.G.; Mamieva, Z.A.; Kovaleva, A.L.; Ulyanin, A.I.; et al. Efficacy and safety of a food supplement with standardized menthol, limonene, and gingerol content in patients with irritable bowel syndrome: A double-blind, randomized, placebo-controlled trial. PLoS ONE 2022, 17, e0263880. [Google Scholar] [CrossRef]
- Dludla, P.V.; Cirilli, I.; Marcheggiani, F.; Silvestri, S.; Orlando, P.; Muvhulawa, N.; Moetlediwa, M.T.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Hlengwa, N.; et al. Bioactive Properties, Bioavailability Profiles, and Clinical Evidence of the Potential Benefits of Black Pepper (Piper nigrum) and Red Pepper (Capsicum annum) against Diverse Metabolic Complications. Molecules 2023, 28, 6569. [Google Scholar] [CrossRef]
- Ziegenhagen, R.; Heimberg, K.; Lampen, A.; Hirsch-Ernst, K.I. Safety Aspects of the Use of Isolated Piperine Ingested as a Bolus. Foods 2021, 10, 2121. [Google Scholar] [CrossRef]
- Salimo, Z.M.; Yakubu, M.N.; da Silva, E.L.; de Almeida, A.C.G.; Chaves, Y.O.; Costa, E.V.; da Silva, F.M.A.; Tavares, J.F.; Monteiro, W.M.; de Melo, G.C.; et al. Chemistry and Pharmacology of Bergenin or Its Derivatives: A Promising Molecule. Biomolecules 2023, 13, 403. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.V.; Pal, R.; Vaiphei, K.; Sharma, S.K.; Ola, R.P. Garlic in health and disease. Nutr. Res. Rev. 2011, 24, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Ho, C.T.; Lan, Y.; Xiao, J.; Lu, M. Bioavailability, Health Benefits, and Delivery Systems of Allicin: A Review. J. Agric. Food Chem. 2023, 71, 19207–19220. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, S.; Maqsood, M.; Mahtab, N.; Khan, M.I.; Sahar, A.; Zaib, S.; Gul, S. Epigallocatechin gallate: Phytochemistry, bioavailability, utilization challenges, and strategies. J. Food Biochem. 2022, 46, e14189. [Google Scholar] [CrossRef] [PubMed]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Carrillo-Martinez, E.J.; Flores-Hernandez, F.Y.; Salazar-Montes, A.M.; Nario-Chaidez, H.F.; Hernandez-Ortega, L.D. Quercetin, a Flavonoid with Great Pharmacological Capacity. Molecules 2024, 29, 1000. [Google Scholar] [CrossRef]
- Madaan, R.; Singla, R.K.; Kumar, S.; Dubey, A.K.; Kumar, D.; Sharma, P.; Bala, R.; Singla, S.; Shen, B. Bergenin—A Biologically Active Scaffold: Nanotechnological Perspectives. Curr. Top. Med. Chem. 2022, 22, 132–149. [Google Scholar] [CrossRef]
- Shen, L.; Liu, C.C.; An, C.Y.; Ji, H.F. How does curcumin work with poor bioavailability? Clues from experimental and theoretical studies. Sci. Rep. 2016, 6, 20872. [Google Scholar] [CrossRef]
- Yucel, C.; Karatoprak, G.S.; Acikara, O.B.; Akkol, E.K.; Barak, T.H.; Sobarzo-Sanchez, E.; Aschner, M.; Shirooie, S. Immunomodulatory and anti-inflammatory therapeutic potential of gingerols and their nanoformulations. Front. Pharmacol. 2022, 13, 902551. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, Q.; Song, M.; Xiao, J.; Cao, Y.; Huang, Q.; Ho, C.T.; Lu, M. A review on the bioavailability, bio-efficacies and novel delivery systems for piperine. Food Funct. 2021, 12, 8867–8881. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Wang, J.; Jin, Z.; Qiu, C. A review of nanostructured delivery systems for the encapsulation, protection, and delivery of silymarin: An emerging nutraceutical. Food Res. Int. 2022, 156, 111314. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, P.R.; Reeves, A.Z.; Powell, K.R.; Napier, R.J.; Swimm, A.I.; Sun, A.; Giesler, K.; Bommarius, B.; Shinnick, T.M.; Snyder, J.P.; et al. Monocarbonyl analogs of curcumin inhibit growth of antibiotic sensitive and resistant strains of Mycobacterium tuberculosis. Eur. J. Med. Chem. 2015, 92, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Pallathadka, H.; Gupta, J.; Ma, H.; Al-Shukri, H.H.K.; Kareem, A.K.; Zwamel, A.H.; Mustafa, Y.F. Berberine and berberine nanoformulations in cancer therapy: Focusing on lung cancer. Phytother. Res. 2024, 38, 4336–4350. [Google Scholar] [CrossRef] [PubMed]
- Saini, N.; Chopra, B.; Dhingra, A.K. Synergistic Effect of Piperine and its Derivatives: A Comprehensive Review. Curr. Drug Res. Rev. 2023, 15, 105–121. [Google Scholar] [CrossRef]

| FDA Approved Drugs | Clinical Indications | Probable Mechanisms and Outcomes/Endpoints | Study Site, Trial Phase, Sample Size | References |
|---|---|---|---|---|
| Targeting cell death | ||||
| Phenylbutyrate, Vitamin D3 | Urea cycle disorders, healthy bones, muscles, nerves, and to support the immune system | Induces LL-37 (antimicrobial peptide) expression, autophagy, reduction of proinflammatory cytokines/chemokines, inhibition of ER-stress related genes, intracellular killing of Mtb, and early sputum culture negative | Bangladesh Phase 2 n = 288 | A Mily, 2015 [39] NCT01580007 RS Rekha, 2018 [40] |
| Phenylbutyrate, Vitamin D3 | As above | Mitigate clinical TB symptoms and disease-specific complications | Ethiopia Phase 2 n = 348 | Bekele, 2018 [41] NCT01698476 |
| Vitamin D3 | Immunomodulatory, anti-inflammatory, modulation of cell growth, neuromuscular function, glucose metabolism | Faster resolution of fever, cough, and improvement in nutritional status | Indonesia Phase 2 n = 84 | L Tamara 2022 [42] NCT05073965 |
| Imatinib | Chronic myelogenous leukemia and other cancers | Human trial will assess safety, optimum dose identification, maximize bactericidal activity by immune cells; induce myelopoiesis; time to sputum culture conversion | Nepal and Vietnam Phase 2 n = 72 n = 180 | CR Giver, 2019 [43] Cleverley TL, 2023 [44] IMPACT-TB* NCT03891901 |
| Vitamin D3 | As above | Spondylitis Tuberculosis; clinical outcomes, serum levels of TLR-2, and TLR-4 | Indonesia Phase 2 Phase 3 n = 37 | Jainal Arifin, Firdaus Hamid, Andi Alfian Zainuddin NCT05376189 Not yet recruiting |
| Anti-inflammatory | ||||
| Acetylsalicylic acid (Aspirin) | Pain, inflammation or arthritis, risk of heart attack, stroke or blood clot | Favorable outcome in high dose group | South Africa Phase 2 n = 146 | JF Schoeman, 2011 [45] |
| Aspirin | As above | Primary outcome is all-cause death between inclusion and week 40 | Ivory Coast, Madagascar, Uganda, and South Africa Phase 3 n = 768 | Thomas Maitre, 2022 [46] NCT04145258 INTENSE-TBM |
| Aspirin | As above | To shorten the duration and improve the treatment outcomes; apply anti-inflammatory approaches to improve disability-free survival | India Phase 2 n = 372 | LR Inbaraj, 2024 [47] NCT05917340 |
| Aspirin, Dexamethasone | Pain, inflammation or arthritis, risk of heart attack, stroke or blood clot | Significant inhibition of pro-thrombotic TXA2 and proinflammatory prostaglandins (PGD2, PGE2, and PGF2) and upregulation of pro-resolution protectins in TB meningitis | Vietnam Phase 2 n = 120 | NTH Mai, 2018 [48] |
| Aspirin Corticosteroid | Anti-inflammatory, immune-modulatory, rheumatoid arthritis, lupus or vasculitis | Anti-aggregation, anti-inflammatory, and antioxidant properties, and an antithrombotic effect | India Phase 2 n = 153 | UK Misra, 2018 [49] |
| Aspirin, Ibuprofen | Reducing pain, fever, inflammation, rheumatoid disorders, dysmenorrhea, and osteoarthritis | Inhibitors of cyclooxygenase-1 and cyclooxygenase-2, which are involved in synthesis of prostaglandins and leukotrienes | South Africa and Georgia Phase 2b n = 354 | L Arias, 2023 [50] NCT04575519 |
| Ibuprofen | - | Potential efficacy and safety when used as adjunctive therapy in XDR-TB patients; immune responses | Georgia, South Africa Phase 2 n = 24 | Cris Vilaplana NCT02781909 |
| Dexamethasone | Inflammation, certain forms of arthritis; severe allergies; asthma; certain types of cancer | Immune reconstitution inflammatory syndrome (IRIS); no favorable outcome | Vietnam Indonesia Phase 2 n = 520 | J Donova, 2023 [51] NCT03092817 |
| Dexamethasone | As above | In TB meningitis. Outcomes: Survival rate; Incidence of new neurological events; disability, frequency of severe and serious adverse events; need for rescue corticosteroids. | Vietnam Phase 3 n = 720 | Guy Thwaites NCT03100786 |
| Sulfasalazine | Lower inflammation in certain diseases and help prevent the need for steroids; used in ulcerative colitis, rheumatoid arthritis | Effective, safe, well-tolerated, and cost-effective; no treatment failure or death | China Phase 2 n = 44 | Liang Fu, 2024 [52] ChiCTR2000032298 |
| Immune-modulating | ||||
| Etoricoxib | Cyclooxygenase-2 inhibitor | Reduced circulating vaccine-responsive T-cells | Norway Phase 1/2 n = 222 | S Jenum, 2021 [53] NCT02503839 |
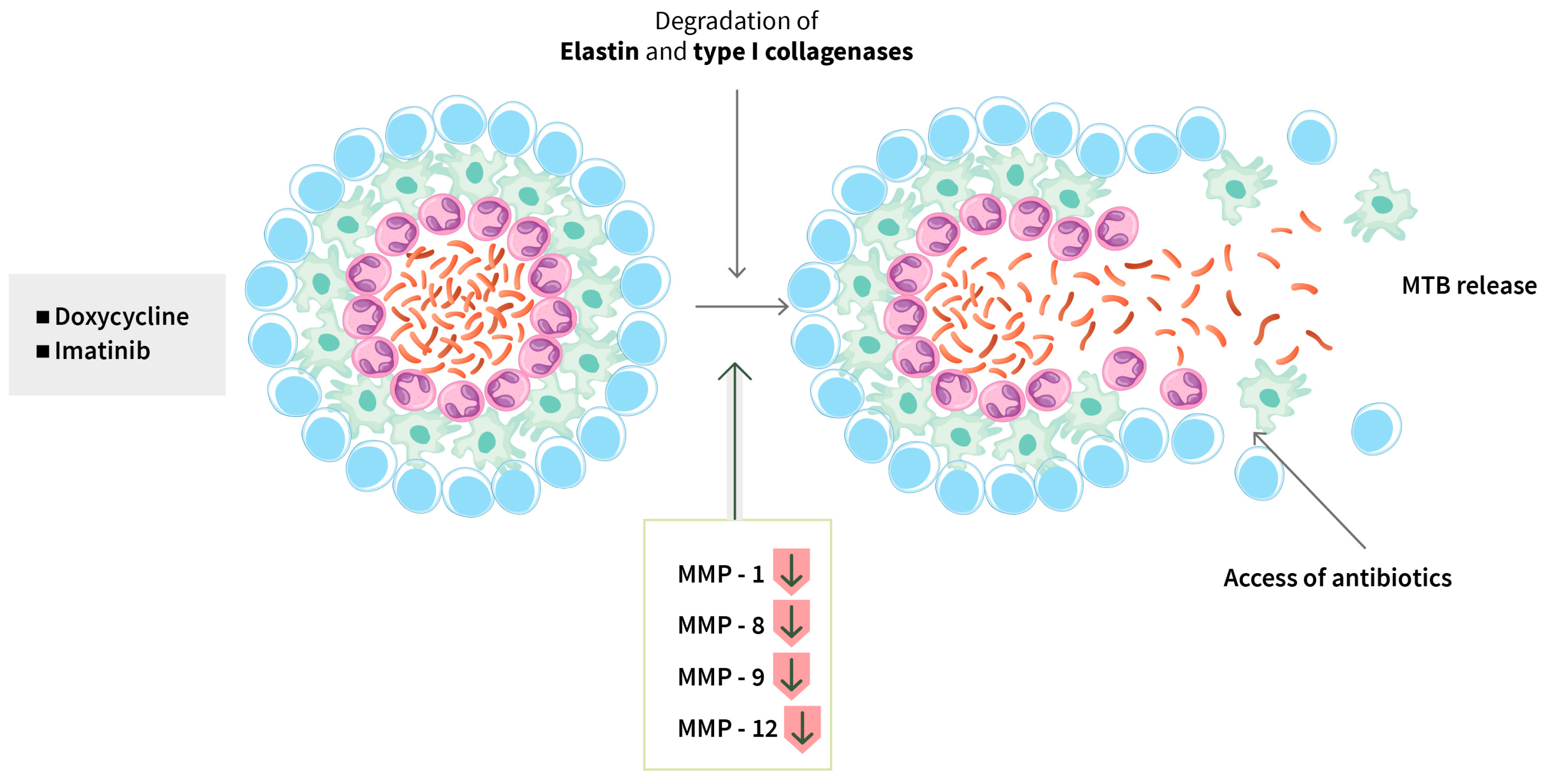
| Doxycycline | Preventing the growth and spread of bacteria, MMP inhibitor | MMP-1, -8, -9, -12 and -13, suppressed type I collagen, elastin destruction, reduced pulmonary cavity volume | Singapore Phase 2 n = 30 | QH Miow 2021 [54] NCT02774993 |
| Doxycycline | As above | Outcome: improvement of lung function and decrease in tissue destruction | Singapore, Malaysia Phase 3 n = 150 | J Mi, 2024 [55] NCT05473520 |
| N-acetylcysteine (NAC) | Treat acetaminophen overdose | Restore reduced form of glutathione, exert antioxidant effects, decreased peroxidation | Brazil Phase 2 n = 39 | IP Safe, 2021 [56] NCT03281226 RIPENACTB |
| N-acetylcysteine (NAC) | As above | Increased glutathione levels; improved recovery of lung function; no impact on sputum culture conversion | Tanzania Phase 2 n = 140 | RS Wallis, 2024 [57] NCT03702738 TB SEQUEL |
| Regulating metabolic pathways | ||||
| Statins Atorvastatin | Hypercholesterolemia | Induces phagosome and phagolysosome maturation, autophagy and apoptosis in Mtb-infected PBMCs | Cape Town Phase 2b n = 220 | K Wolmarans, 2022 NCT04147286 Poster |
| Rosuvastatin | Hypercholesterolemia, to prevent cardiovascular disease | Safe but did not demonstrate substantial benefits on culture conversion | Philippines, Vietnam, Uganda Phase 2b n = 137 | GB Cross, 2023 [58] NCT04504851 |
| Pravastatin | Hypercholesterolemia | Inhibits cellular cholesterol biosynthesis, phagolysosome maturation and induces autophagy | South Africa Phase 2 n = 16 | SP Parihar, 2019 [59] NCT03882177 StAT-TB |
| Pravastatin | Hypercholesterolemia | Safety and tolerance, severity of adverse outcomes | South Africa Phase 2b n = 40 | PC Karakousis; RE Chaisson; N Martinson, NCT03456102 StAT-TB |
| Atorvastatin | Hypercholesterolemia | Dose finding, outcome: Speedier sputum culture conversion, adverse events | Nigeria Phase 2 n = 440 | Olanisun O Adewole NCT06199921 StatinTB |
| Metformin | Diabetes | Did not speed up sputum culture conversion, reduced inflammatory markers and lung tissue damage. Reduced inflammatory chemokines. | India Phase 2 n = 322 | C Padmapriydarsini 2022 [60] CTRI/2018/01/011176 NP Kumar 2024 [61], H Krismawati 2024 [62] |
| Metformin | As above | Outcomes: % participants experiencing leprosy reactions; frequency of serious adverse events | Indonesia Phase 2 n = 166 | H Krismawati 2024 NCT05243654 MetLep Trial |
| Metformin | As above | Outcomes: Safety and tolerability; efficacy as measured by time to sputum conversion | South Africa Phase 2 n = 112 | Hardy Kornfeld NCT04930744 Recruiting |
| Everolimus, auranofin, CC-11050, vitamin D2 | Renal cell carcinoma | CC-11050 and everolimus arms were safe and relatively well tolerated; had increased recovery of FEV1 at day 180 | South Africa Phase 2 n = 200 | RS Wallis, 2021 [63] NCT02968927 |
| Everolimus | Renal cell carcinoma | Modulate autophagy via the inhibition of mTOR; higher recovery of lung function at day 180; peak glycolytic activity was reduced | South Africa Phase 2 Secondary analysis | RS Wallis, 2022 [64] |
| Phytochemicals | Categories (Source) | HDT Effects for TB | Developmental Stage as HDT for TB | References |
|---|---|---|---|---|
| Epigallocatechin gallate (EGCG) | Polyphenol (Green tea) | Induction of autophagy, phagosome maturation through autophagic flux, and reduction of inflammation and oxidative stress | Randomized clinical trial | Agarwal A, 2010 [96] |
| Quercetin | Polyphenolic flavonoid (Capers, red onions, kale) | Reduction of caseous necrosis of hepatocytes, inhibition of inflammatory mediators, suppression of oxidative stress, and boosting of endogenous antioxidants | Phase 2 clinical trial | Butov D, 2016 [97] |
| Silymarin | Polyphenolic flavonoid (Milk thistle seeds) | Reduction of anti-TB drug-induced hepatotoxicity and induction of Th-1 cytokines | Randomized double-blind clinical trial | Talebi A, 2023 [98], Luangchosiri C, 2015 [99] |
| Curcumin | Polyphenol (Turmeric) | Induction of apoptosis, autophagy, phagosome lysosome fusion, attenuation of isoniazide-induced hepatotoxicity, downregulation of oxidative stress and inflammation, reversion of isoniazide-induced dysfunction and apoptosis of T cells, restoration of isoniazide-induced suppression of antigen-specific cytokine responses, activation of Th and Tc cells, enhanced generation of central memory T cells, and reduction of the release of proinflammatory cytokines IL-6 and TNF-α | Preclinical trial (mouse model) | Lara-Espinosa JV, 2022 [100], Gupta PK, 2023 [101] Tousif S, 2017 [102], Li Y, 2022 [103] |
| Resveratrol | Stilbenoid polyphenol (Peanuts, grapes, cranberries) | Inhibition of Mtb-induced apoptosis and reversion of Mtb-induced secretion of IL-6 and TNF-α | Preclinical (mouse model) | Yang H, 2019 [104] |
| Luteolin | Polyphenolic flavonoid (Herbs, fruits and vegetables) | Alleviation of isoniazide-induced hepatotoxicity, promotion of central memory T cells, activation of NK and NKT cells, and induction of Th1 (IFN-γ and TNF-α) and Th17 (IL-17 and IL-22) cytokines | Preclinical (mouse model) | Singh DK, 2021 [105] |
| Bergenin | Polyphenol (Different parts of many plants) | Stimulation of Th1 and Th17 response and promotion of antigen-specific central memory T cell | Preclinical (mouse model) | Dwivedi VP, 2017 [106] Kumar S, 2019 [107] |
| 6-Gingerol | Polyphenol (Ginger) | Promotion of Th1/Th17 responses | Preclinical (mouse model) | Bhaskar A, 2020 [108] |
| Piperine | Alkaloid (Black pepper) | Promotion of the proliferation of T and B cells, Th-1 cytokines, and macrophage activation | Phase 3 clinical trial | Patel N, 2017 [109] |
| Berberine | Isoquinoline alkaloid (Medicinal plants) | Protection from isoniazide -induced liver injury, reduction of oxidative stress and inflammation, induction of macrophage activation, and Th1/Th17 polarization, T effector memory, T central memory, and tissue-resident memory T cell responses | Preclinical (mouse and rat models) | Mahmoud AM, 2014 [110], Pahuja I, 2023 [111], Ozturk M, 2021 [112] |
| Allicin | Organosulfur compound (Garlic) | Induction of protective Th1 response and reduction of Mtb-induced ROS and TNF-α | Preclinical (mouse model) | Dwivedi VP, 2019 [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raqib, R.; Sarker, P. Repurposed Drugs and Plant-Derived Natural Products as Potential Host-Directed Therapeutic Candidates for Tuberculosis. Biomolecules 2024, 14, 1497. https://doi.org/10.3390/biom14121497
Raqib R, Sarker P. Repurposed Drugs and Plant-Derived Natural Products as Potential Host-Directed Therapeutic Candidates for Tuberculosis. Biomolecules. 2024; 14(12):1497. https://doi.org/10.3390/biom14121497
Chicago/Turabian StyleRaqib, Rubhana, and Protim Sarker. 2024. "Repurposed Drugs and Plant-Derived Natural Products as Potential Host-Directed Therapeutic Candidates for Tuberculosis" Biomolecules 14, no. 12: 1497. https://doi.org/10.3390/biom14121497
APA StyleRaqib, R., & Sarker, P. (2024). Repurposed Drugs and Plant-Derived Natural Products as Potential Host-Directed Therapeutic Candidates for Tuberculosis. Biomolecules, 14(12), 1497. https://doi.org/10.3390/biom14121497







