Transcutaneous Non-Invasive Vagus Nerve Stimulation: Changing the Paradigm for Stroke and Atrial Fibrillation Therapies?
Abstract
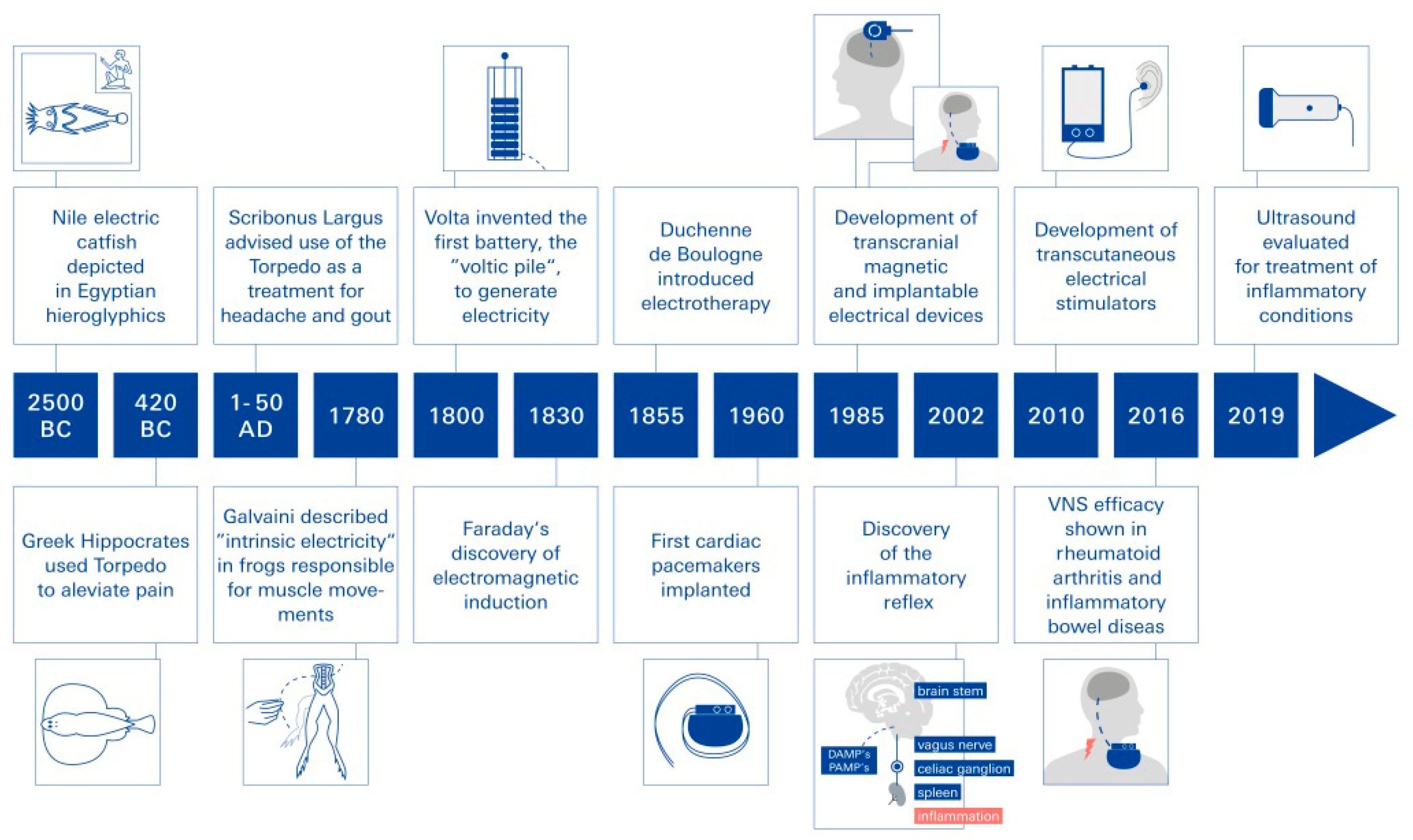
:1. Electrical Neuromodulation as Biophysical Medicine—Introduction and Historical Review
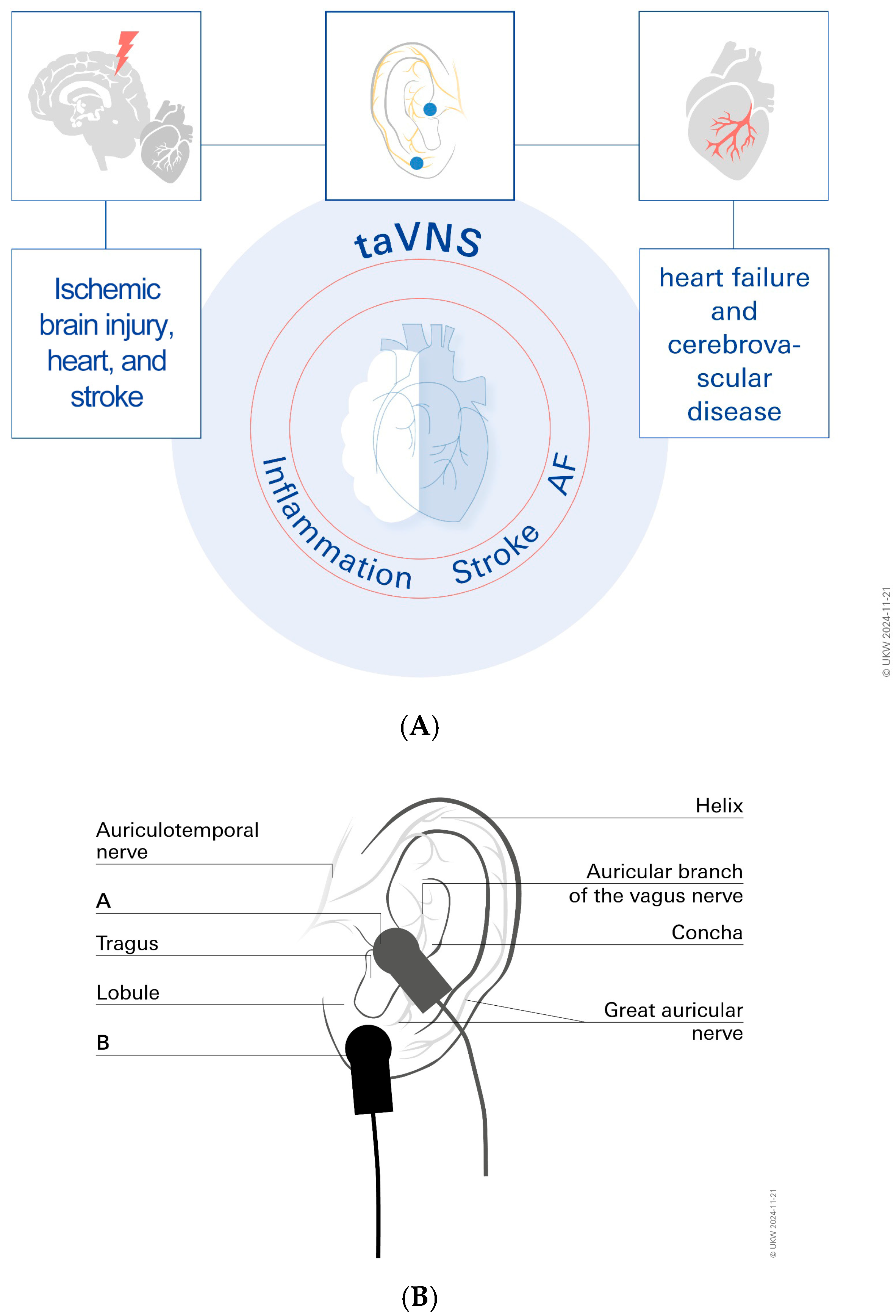
2. Vagus Nerve Stimulation (VNS)
3. taVNS for Stroke Treatment
4. taVNS Treatment Helps to Prevent Post-Stroke Impairments by Promoting Neurological Plasticity
5. taVNS for Treatment of Cardiac Arrhythmias
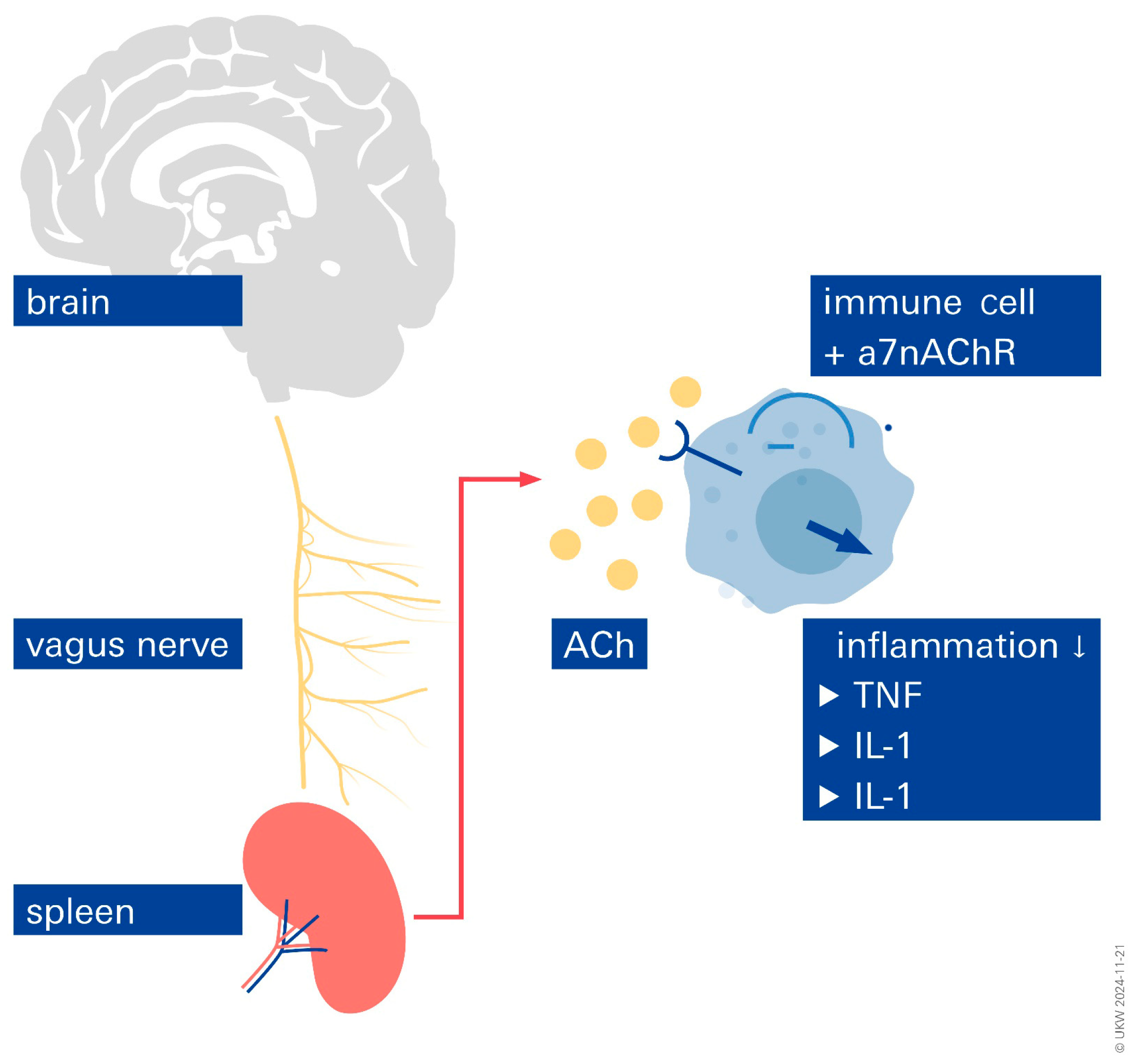
6. Inflammation
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- González-González, M.A.; Conde, S.V.; Latorre, R.; Thébault, S.C.; Pratelli, M.; Spitzer, N.C.; Verkhratsky, A.; Tremblay, M.; Akcora, C.G.; Hernández-Reynoso, A.G.; et al. Bioelectronic Medicine: A multidisciplinary roadmap from biophysics to precision therapies. Front. Integr. Neurosci. 2024, 18, 1321872. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, A.J.R. A Brief Review of the History of Electrotherapy and Its Union with Acupuncture. Acupunct. Med. 1993, 11, 66–75. [Google Scholar] [CrossRef]
- Salvador, E.; Kessler, A.F.; Domröse, D.; Hörmann, J.; Schaeffer, C.; Giniunaite, A.; Burek, M.; Tempel-Brami, C.; Voloshin, T.; Volodin, A.; et al. Tumor Treating Fields (TTFields) Reversibly Permeabilize the Blood–Brain Barrier In Vitro and In Vivo. Biomolecules 2022, 12, 1348. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Yan, L.; Zhang, J.; Sun, Y.; Qian, Y.; Wang, M.; Yu, T. Transcutaneous vagus nerve stimulation: A bibliometric study on current research hotspots and status. Front. Neurosci. 2024, 18, 1406135. [Google Scholar] [CrossRef]
- Foley, J.O.; DuBois, F.S. Quantitative studies of the vagus nerve in the cat. I. The ratio of sensory to motor fibers. J. Comp. Neurol. 1937, 67, 49–67. [Google Scholar] [CrossRef]
- Yuan, H.; Silberstein, S.D. Vagus Nerve and Vagus Nerve Stimulation, a Comprehensive Review: Part I. Headache J. Head Face Pain 2016, 56, 71–78. [Google Scholar] [CrossRef]
- Howland, R.H. Vagus nerve stimulation. Curr. Behav. Neurosci. Rep. 2014, 1, 64–73. [Google Scholar] [CrossRef]
- Berthoud, H.-R.; Neuhuber, W.L. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 2000, 85, 1–17. [Google Scholar] [CrossRef]
- Polak, T.; Markulin, F.; Ehlis, A.-C.; Langer, J.B.M.; Ringel, T.M.; Fallgatter, A.J. Far field potentials from brain stem after transcutaneous Vagus nerve stimulation: Optimization of stimulation and recording parameters. J. Neural Transm. 2009, 116, 1237–1242. [Google Scholar] [CrossRef]
- Nemeroff, C.B.; Mayberg, H.S.; Krahl, S.E.; McNamara, J.; Frazer, A.; Henry, T.R.; George, M.S.; Charney, D.S.; Brannan, S.K. VNS Therapy in Treatment-Resistant Depression: Clinical Evidence and Putative Neurobiological Mechanisms. Neuropsychopharmacology 2006, 31, 1345–1355. [Google Scholar] [CrossRef]
- Johnson, R.L.; Wilson, C.G. A review of vagus nerve stimulation as a therapeutic intervention. J. Inflamm. Res. 2018, 11, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Hein, E.; Nowak, M.; Kiess, O.; Biermann, T.; Bayerlein, K.; Kornhuber, J.; Kraus, T. Auricular transcutaneous electrical nerve stimulation in depressed patients: A randomized controlled pilot study. J. Neural Transm. 2013, 120, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, S.; Martin, J.; Rennaker, R.L., II; Kilgard, M.P. Kilgard, Pairing sound with vagus nerve stimulation modulates cortical synchrony and phase coherence in tinnitus: An exploratory retrospective study. Sci. Rep. 2017, 7, 17345. [Google Scholar] [CrossRef]
- Krahl, S.; Clark, K. Vagus nerve stimulation for epilepsy: A review of central mechanisms. Surg. Neurol. Int. 2012, 3 (Suppl. S4), S255–S259. [Google Scholar] [CrossRef]
- Stefan, H.; Kreiselmeyer, G.; Kerling, F.; Kurzbuch, K.; Rauch, C.; Heers, M.; Kasper, B.S.; Hammen, T.; Rzonsa, M.; Pauli, E.; et al. Transcutaneous vagus nerve stimulation (t-VNS) in pharmacoresistant epilepsies: A proof of concept trial. Epilepsia 2012, 53, e115–e118. [Google Scholar] [CrossRef]
- Ventureyra, E.C. Transcutaneous vagus nerve stimulation for partial onset seizure therapy. A new concept. Child’s Nerv. Syst. 2000, 16, 101–102. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.-Y.; Wang, D.; Wu, M.-Z.; He, J.-K.; Zhang, J.-L.; Zhao, B.; Hou, L.-W.; Wang, J.-Y.; Wang, L.; et al. Transcutaneous Auricular Vagus Nerve Stimulation: From Concept to Application. Neurosci. Bull. 2021, 37, 853–862. [Google Scholar] [CrossRef]
- Deuchars, S.A.; Lall, V.K.; Clancy, J.; Mahadi, M.; Murray, A.; Peers, L.; Deuchars, J. Mechanisms underpinning sympathetic nervous activity and its modulation using transcutaneous vagus nerve stimulation. Exp. Physiol. 2018, 103, 326–331. [Google Scholar] [CrossRef]
- Sorski, L.; Gidron, Y. The Vagal Nerve, Inflammation, and Diabetes-A Holy Triangle. Cells 2023, 12, 1632. [Google Scholar] [CrossRef]
- Du, L.; He, X.; Xiong, X.; Zhang, X.; Jian, Z.; Yang, Z. Vagus nerve stimulation in cerebral stroke: Biological mechanisms, therapeutic modalities, clinical applications, and future directions. Neural Regen. Res. 2024, 19, 1707–1717. [Google Scholar] [CrossRef]
- Xia, X.M.; Duan, Y.; Wang, Y.P.; Han, R.X.; Dong, Y.F.; Jiang, S.Y.; Zheng, Y.; Quiao, C.; Cao, L.; Lu, X.; et al. Vagus nerve stimulation as a promising neuroprotection for ischemic stroke via alpha7nAchR-dependent inactivation of microglial NLRP3 inflammasome. Acta Pharmacol. Sin. 2024, 45, 1349–1365. [Google Scholar] [CrossRef] [PubMed]
- Baig, S.S.; Kamarova, M.; Bell, S.M.; Ali, A.N.; Su, L.; Dimairo, M.; Dawson, J.; Redgrave, J.N.; Majid, A. tVNS in Stroke: A Narrative Review on the Current State and the Future. Stroke 2023, 54, 2676–2687. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, D.; Pan, H.; Huang, L.; Sun, X.; He, C.; Wei, Q. Non-invasive Vagus Nerve Stimulation in Cerebral Stroke: Current Status and Future Perspectives. Front. Neurosci. 2022, 16, 820665. [Google Scholar] [CrossRef] [PubMed]
- Baig, S.S.; Kamarova, M.; Ali, A.; Su, L.; Dawson, J.; Redgrave, J.N.; Majid, A. Transcutaneous vagus nerve stimulation (tVNS) in stroke: The evidence, challenges and future directions. Auton. Neurosci. 2022, 237, 102909. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, L.Y.; Orban, L.; Cuylear, D.; Thompson, J.; Simon, B.; Yang, Y. Non-invasive vagus nerve stimulation reduces blood-brain barrier disruption in a rat model of ischemic stroke. Brain Stimul. 2018, 11, 689–698. [Google Scholar] [CrossRef]
- Baig, S.S.; Falidas, K.; Laud, P.J.; Snowdon, N.; Farooq, M.U.; Ali, A.; Majid, A.; Redgrave, J.N. Transcutaneous Auricular Vagus Nerve Stimulation with Upper Limb Repetitive Task Practice May Improve Sensory Recovery in Chronic Stroke. J. Stroke Cerebrovasc. Dis. 2019, 28, 104348. [Google Scholar] [CrossRef]
- Zhang, R.L.; Zhang, Z.G.; Chopp, M. Neurogenesis in the adult ischemic brain: Generation, migration, survival, and restorative therapy. Neuroscientist 2005, 11, 408–416. [Google Scholar] [CrossRef]
- Fang, Y.-T.; Lin, Y.-T.; Tseng, W.-L.; Tseng, P.; Hua, G.-L.; Chao, Y.-J.; Wu, Y.-J. Neuroimmunomodulation of vagus nerve stimulation and the therapeutic implications. Front. Aging Neurosci. 2023, 15, 1173987. [Google Scholar] [CrossRef]
- Li, Z.-D.; Qiu, H.-J.; Wang, X.-Q.; Zhang, C.-C.; Zhang, Y.-J. Transcutaneous auricular vagus nerve stimulation in poststroke cognitive impairment: Protocol for a randomised controlled trial. BMJ Open 2022, 12, e063803. [Google Scholar] [CrossRef]
- Kulkarni, K.; Singh, J.P.; Parks, K.A.; Katritsis, D.G.; Stavrakis, S.; Armoundas, A.A. Low-Level Tragus Stimulation Modulates Atrial Alternans and Fibrillation Burden in Patients with Paroxysmal Atrial Fibrillation. J. Am. Heart Assoc. 2021, 10, e020865. [Google Scholar] [CrossRef]
- Ahammed, M.R.; Ananya, F.N. Impact of Weight Loss on Atrial Fibrillation. Cureus 2023, 15, e46232. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, S.; Das, S.; Aronow, W.S. Risk factors modification in atrial fibrillation: A brief review. Expert Rev. Cardiovasc. Ther. 2023, 22, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Marsetti, P.S.; Milagro, F.I.; Zulet, M.; Martínez, J.A.; Lorente-Cebrián, S. Changes in miRNA expression with two weight-loss dietary strategies in a population with metabolic syndrome. Nutrition 2021, 83, 111085. [Google Scholar] [CrossRef]
- Garnier, L.F.; Rouesnel, P.; Espitalier, F. Atrial fibrillation and anticoagulation. Arch. Mal. Coeur Vaiss. 2004, 97, 1001–1005. [Google Scholar]
- Ahn, H.-J.; Lee, S.-R.; Choi, E.-K.; Han, K.-D.; Rhee, T.-M.; Kwon, S.; Kim, S.; Oh, S.; Lip, G.Y.H. Associations between obesity parameters and the risk of incident atrial fibrillation and ischaemic stroke in the different age groups. Front. Cardiovasc. Med. 2022, 9, 906844. [Google Scholar] [CrossRef]
- Stavrakis, S.; Humphrey, M.B.; Scherlag, B.J.; Hu, Y.; Jackman, W.M.; Nakagawa, H.; Lockwood, D.; Lazzara, R.; Po, S.S. Low-Level Transcutaneous Electrical Vagus Nerve Stimulation Suppresses Atrial Fibrillation. J. Am. Coll. Cardiol. 2015, 65, 867–875. [Google Scholar] [CrossRef]
- Stavrakis, S.; Stoner, J.A.; Humphrey, M.B.; Morris, L.; Filiberti, A.; Reynolds, J.C.; Elkholey, K.; Javed, I.; Twidale, N.; Riha, P.; et al. TREAT AF (Transcutaneous Electrical Vagus Nerve Stimulation to Suppress Atrial Fibrillation): A Randomized Clinical Trial. Clin. Electrophysiol. 2020, 6, 282–291. [Google Scholar] [CrossRef]
- Yu, L.; Scherlag, B.J.; Li, S.; Sheng, X.; Lu, Z.; Nakagawa, H.; Zhang, Y.; Jackman, W.M.; Lazzara, R.; Jiang, H.; et al. Low-Level Vagosympathetic Nerve Stimulation Inhibits Atrial Fibrillation Inducibility: Direct Evidence by Neural Recordings from Intrinsic Cardiac Ganglia. J. Cardiovasc. Electrophysiol. 2011, 22, 455–463. [Google Scholar] [CrossRef]
- Choudhary, R.C.; Ahmed, U.; Shoaib, M.; Alper, E.; Rehman, A.; Kim, J.; Shinozaki, K.; Volpe, B.T.; Chavan, S.; Zanos, S.; et al. Threshold adjusted vagus nerve stimulation after asphyxial cardiac arrest results in neuroprotection and improved survival. Bioelectron. Med. 2022, 8, 10. [Google Scholar] [CrossRef]
- Tracey, K.J. Reflex control of immunity. Nat. Rev. Immunol. 2009, 9, 418–428. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Tracey, K.J. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Investig. 2007, 117, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Kaniusas, E.; Kampusch, S.; Tittgemeyer, M.; Panetsos, F.; Gines, R.F.; Papa, M.; Kiss, A.; Podesser, B.; Cassara, A.M.; Tanghe, E.; et al. Current Directions in the Auricular Vagus Nerve Stimulation I—A Physiological Perspective. Front. Neurosci. 2019, 13, 854. [Google Scholar] [CrossRef] [PubMed]
- Okdahl, T.; Bertoli, D.; Brock, B.; Krogh, K.; Knop, F.K.; Brock, C.; Drewes, A.M. Study protocol for a multicentre, randomised, parallel group, sham-controlled clinical trial investigating the effect of transcutaneous vagal nerve stimulation on gastrointestinal symptoms in people with diabetes complicated with diabetic autonomic neuropathy: The DAN-VNS Study. BMJ Open 2021, 11, e038677. [Google Scholar] [CrossRef]
- Frank, N.; Nagai, M.; Förster, C.Y. Exploration of transcutaneous vagus nerve stimulation as a treatment option for adjuvant cancer and heart failure therapy. Explor. Neuroprotective Ther. 2023, 3, 363–397. [Google Scholar] [CrossRef]
- Frank, N.; Herrmann, M.J.; Lauer, M.; Förster, C.Y. Exploratory Review of the Takotsubo Syndrome and the Possible Role of the Psychosocial Stress Response and Inflammaging. Biomolecules 2024, 14, 167. [Google Scholar] [CrossRef]
- Song, G.; Sclocco, R.; Sharma, A.; Guerrero-López, I.; Kuo, B. Electroceuticals and Magnetoceuticals in Gastroenterology. Biomolecules 2024, 14, 760. [Google Scholar] [CrossRef]
- Paplou, V.G.; Schubert, N.M.A.; van Tuinen, M.; Vijayakumar, S.; Pyott, S.J. Functional, Morphological and Molecular Changes Reveal the Mechanisms Associated with Age-Related Vestibular Loss. Biomolecules 2023, 13, 1429. [Google Scholar] [CrossRef]
- Lespérance, P.; Jodoin, V.D.; Drouin, D.; Racicot, F.; Miron, J.-P.; Longpré-Poirier, C.; Fournier-Gosselin, M.-P.; Thebault, P.; Lapointe, R.; Arbour, N.; et al. Vagus Nerve Stimulation Modulates Inflammation in Treatment-Resistant Depression Patients: A Pilot Study. Int. J. Mol. Sci. 2024, 25, 2679. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Förster, C.Y. Transcutaneous Non-Invasive Vagus Nerve Stimulation: Changing the Paradigm for Stroke and Atrial Fibrillation Therapies? Biomolecules 2024, 14, 1511. https://doi.org/10.3390/biom14121511
Förster CY. Transcutaneous Non-Invasive Vagus Nerve Stimulation: Changing the Paradigm for Stroke and Atrial Fibrillation Therapies? Biomolecules. 2024; 14(12):1511. https://doi.org/10.3390/biom14121511
Chicago/Turabian StyleFörster, Carola Y. 2024. "Transcutaneous Non-Invasive Vagus Nerve Stimulation: Changing the Paradigm for Stroke and Atrial Fibrillation Therapies?" Biomolecules 14, no. 12: 1511. https://doi.org/10.3390/biom14121511
APA StyleFörster, C. Y. (2024). Transcutaneous Non-Invasive Vagus Nerve Stimulation: Changing the Paradigm for Stroke and Atrial Fibrillation Therapies? Biomolecules, 14(12), 1511. https://doi.org/10.3390/biom14121511





