Role of Nicotinamide in the Pathogenesis of Actinic Keratosis: Implications for NAD+/SIRT1 Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Samples Collection and PBMCs Extraction
2.3. NAD/NADH and SIRT1 Immunoenzymatic Detection
2.4. SIRT1 Activity Assay
2.5. Statistical Analysis
3. Results
Study Population Demographics and Clinical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wood, D.L.A.; Lachner, N.; Tan, J.M.; Tang, S.; Angel, N.; Laino, A.; Linedale, R.; Le Cao, K.A.; Morrison, M.; Frazer, I.H.; et al. A Natural History of Actinic Keratosis and Cutaneous Squamous Cell Carcinoma Microbiomes. mBio 2018, 9, e01432-18. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Shin, S.; Jung, S.H.; Park, Y.M.; Park, G.S.; Lee, S.H.; Chung, Y.J. Genomic Progression of Precancerous Actinic Keratosis to Squamous Cell Carcinoma. J. Investig. Dermatol. 2022, 142, 528–538.e8. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, C.P.H.; Bakos, R.M. Actinic keratoses: Review of clinical, dermoscopic, and therapeutic aspects. An. Bras. Dermatol. 2019, 94, 637–657. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.N.; Stockfleth, E.; Connolly, S.M.; Correia, O.; Erdmann, R.; Foley, P.; Gupta, A.K.; Jacobs, A.; Kerl, H.; Lim, H.W.; et al. Evidence- and consensus-based (S3) Guidelines for the Treatment of Actinic Keratosis—International League of Dermatological Societies in cooperation with the European Dermatology Forum—Short version. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2069–2079. [Google Scholar] [CrossRef]
- Werner, R.N.; Sammain, A.; Erdmann, R.; Hartmann, V.; Stockfleth, E.; Nast, A. The natural history of actinic keratosis: A systematic review. Br. J. Dermatol. 2013, 169, 502–518. [Google Scholar] [CrossRef]
- Campione, E.; Rivieccio, A.; Gaeta Shumak, R.; Costanza, G.; Cosio, T.; Lambiase, S.; Garofalo, V.; Artosi, F.; Lozzi, F.; Freni, C.; et al. Preliminary Evidence of Efficacy, Safety, and Treatment Satisfaction with Tirbanibulin 1% Ointment: A Clinical Perspective on Actinic Keratoses. Pharmaceuticals 2023, 16, 1686. [Google Scholar] [CrossRef]
- Zalaudek, I.; Argenziano, G. Dermoscopy of actinic keratosis, intraepidermal carcinoma. In Actinic Keratosis; Current Problems in Dermatology; Karger Publishers: Basel, Switzerland, 2015; Volume 46, pp. 70–76. [Google Scholar] [CrossRef]
- Fernandez Figueras, M.T. From actinic keratosis to squamous cell carcinoma: Pathophysiology revisited. J. Eur. Acad. Dermatol. Venereol. 2017, 31 (Suppl. S2), 5–7. [Google Scholar] [CrossRef]
- Richard, M.A.; Amici, J.M.; Basset-Seguin, N.; Claudel, J.P.; Cribier, B.; Dreno, B. Management of actinic keratosis at specific body sites in patients at high risk of carcinoma lesions: Expert consensus from the AKTeam of expert clinicians. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 339–346. [Google Scholar] [CrossRef]
- Conforti, C.; Giuffrida, R.; Dianzani, C.; Guarneri, F.; Marangi, G.F.; Neagu, N.; Persichetti, P.; Zalaudek, I.; di Meo, N. Effectiveness and tolerability of treatment for isolated actinic keratoses: A retrospective comparison between cryotherapy, CO(2) laser and 5-fluorouracil 0.5%/salicylic acid 10. Dermatol. Ther. 2021, 34, e14846. [Google Scholar] [CrossRef]
- Garofalo, V.; Geraci, F.; Di Prete, M.; Lanna, C.; Lozzi, F.; Cosio, T.; Lambiase, S.; Gaeta Shumak, R.; Di Raimondo, C.; Diluvio, L.; et al. Early clinical response to 5-fluorouracil 0.5% and salicylic acid 10% topical solution in the treatment of actinic keratoses of the head: An observational study. J. Dermatol. Treat. 2022, 33, 2664–2669. [Google Scholar] [CrossRef]
- Hanna, E.; Abadi, R.; Abbas, O. Imiquimod in dermatology: An overview. Int. J. Dermatol. 2016, 55, 831–844. [Google Scholar] [CrossRef]
- Babino, G.; Diluvio, L.; Bianchi, L.; Orlandi, A.; Di Prete, M.; Chimenti, S.; Milani, M.; Campione, E. Long-term use of a new topical formulation containing piroxicam 0.8% and sunscreen: Efficacy and tolerability on actinic keratosis. A proof of concept study. Curr. Med. Res. Opin. 2016, 32, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Paterno, E.J.; Candi, E.; Falconi, M.; Costanza, G.; Diluvio, L.; Terrinoni, A.; Bianchi, L.; Orlandi, A. The relevance of piroxicam for the prevention and treatment of nonmelanoma skin cancer and its precursors. Drug Des. Dev. Ther. 2015, 9, 5843–5850. [Google Scholar] [CrossRef] [PubMed]
- Segura, S.; Gadea, A.; Nonell, L.; Andrades, E.; Sanchez, S.; Pujol, R.; Hernandez-Munoz, I.; Toll, A. Identification of differentially expressed genes in actinic keratosis samples treated with ingenol mebutate gel. PLoS ONE 2020, 15, e0232146. [Google Scholar] [CrossRef]
- Chen, A.C.; Martin, A.J.; Choy, B.; Fernandez-Penas, P.; Dalziell, R.A.; McKenzie, C.A.; Scolyer, R.A.; Dhillon, H.M.; Vardy, J.L.; Kricker, A.; et al. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. N. Engl. J. Med. 2015, 373, 1618–1626. [Google Scholar] [CrossRef]
- Arcuri, D.; Ramchatesingh, B.; Lagace, F.; Iannattone, L.; Netchiporouk, E.; Lefrancois, P.; Litvinov, I.V. Pharmacological Agents Used in the Prevention and Treatment of Actinic Keratosis: A Review. Int. J. Mol. Sci. 2023, 24, 4989. [Google Scholar] [CrossRef]
- Fania, L.; Mazzanti, C.; Campione, E.; Candi, E.; Abeni, D.; Dellambra, E. Role of Nicotinamide in Genomic Stability and Skin Cancer Chemoprevention. Int. J. Mol. Sci. 2019, 20, 5946. [Google Scholar] [CrossRef] [PubMed]
- Cosio, T.; Di Prete, M.; Campione, E. Arsenic Trioxide, Itraconazole, All-Trans Retinoic Acid and Nicotinamide: A Proof of Concept for Combined Treatments with Hedgehog Inhibitors in Advanced Basal Cell Carcinoma. Biomedicines 2020, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Damian, D.L. Nicotinamide for skin cancer chemoprevention. Australas. J. Dermatol. 2017, 58, 174–180. [Google Scholar] [CrossRef]
- Drago, F.; Ciccarese, G.; Cogorno, L.; Calvi, C.; Marsano, L.A.; Parodi, A. Prevention of non-melanoma skin cancers with nicotinamide in transplant recipients: A case-control study. Eur. J. Dermatol. 2017, 27, 382–385. [Google Scholar] [CrossRef]
- Allen, N.C.; Martin, A.J.; Snaidr, V.A.; Eggins, R.; Chong, A.H.; Fernandez-Penas, P.; Gin, D.; Sidhu, S.; Paddon, V.L.; Banney, L.A.; et al. Nicotinamide for Skin-Cancer Chemoprevention in Transplant Recipients. N. Engl. J. Med. 2023, 388, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Lautrup, S.; Hou, Y.; Demarest, T.G.; Croteau, D.L.; Mattson, M.P.; Bohr, V.A. NAD(+) in Aging: Molecular Mechanisms and Translational Implications. Trends Mol. Med. 2017, 23, 899–916. [Google Scholar] [CrossRef] [PubMed]
- Surjana, D.; Halliday, G.M.; Damian, D.L. Role of nicotinamide in DNA damage, mutagenesis, and DNA repair. J. Nucleic Acids 2010, 2010, 157591. [Google Scholar] [CrossRef]
- Benavente, C.A.; Schnell, S.A.; Jacobson, E.L. Effects of niacin restriction on sirtuin and PARP responses to photodamage in human skin. PLoS ONE 2012, 7, e42276. [Google Scholar] [CrossRef]
- Aman, Y.; Qiu, Y.; Tao, J.; Fang, E.F. Therapeutic potential of boosting NAD+ in aging and age-related diseases. Transl. Med. Aging 2018, 2, 30–37. [Google Scholar] [CrossRef]
- Kobaisi, F.; Fayyad, N.; Rezvani, H.R.; Fayyad-Kazan, M.; Sulpice, E.; Badran, B.; Fayyad-Kazan, H.; Gidrol, X.; Rachidi, W. Signaling Pathways, Chemical and Biological Modulators of Nucleotide Excision Repair: The Faithful Shield against UV Genotoxicity. Oxidative Med. Cell. Longev. 2019, 2019, 4654206. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Schoonjans, K.; Auwerx, J. Sirtuin functions in health and disease. Mol. Endocrinol. 2007, 21, 1745–1755. [Google Scholar] [CrossRef]
- Tang, Y.; Ju, W.; Liu, Y.; Deng, Q. The role of SIRT1 in autophagy and drug resistance: Unveiling new targets and potential biomarkers in cancer therapy. Front. Pharmacol. 2024, 15, 1469830. [Google Scholar] [CrossRef]
- Yousafzai, N.A.; Jin, H.; Ullah, M.; Wang, X. Recent advances of SIRT1 and implications in chemotherapeutics resistance in cancer. Am. J. Cancer Res. 2021, 11, 5233–5248. [Google Scholar]
- Yu, L.; Li, Y.; Song, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Z.; Wang, Y. The dual role of sirtuins in cancer: Biological functions and implications. Front. Oncol. 2024, 14, 1384928. [Google Scholar] [CrossRef]
- Hosseninia, S.; Ameli, A.; Aslani, M.R.; Pourfarzi, F.; Ghobadi, H. Serum Levels of Sirtuin-1 in Patients with Lung Cancer and its Association with Karnofsky Performance Status. Acta Biomed. 2021, 92, e2021012. [Google Scholar] [CrossRef]
- Luna, A.; Aladjem, M.I.; Kohn, K.W. SIRT1/PARP1 crosstalk: Connecting DNA damage and metabolism. Genome Integr. 2013, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Dotto, G.P. Multifocal epithelial tumors and field cancerization: Stroma as a primary determinant. J. Clin. Investig. 2014, 124, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Loaiza, N.; Demaria, M. Cellular senescence and tumor promotion: Is aging the key? Biochim. Biophys. Acta 2016, 1865, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, R.; Morishita, H.; Iida, K.; Hayano, K.; Murakami, K.; Endo, S.; Toyozumi, T.; Matsumoto, Y.; Kurata, Y.; Kinoshita, K.; et al. Serum Versus Tissue SIRT1 as Potentially Valuable Biomarkers in Gastric Cancer. Anticancer Res. 2023, 43, 1485–1491. [Google Scholar] [CrossRef]
- Coppola, A.; Capuani, B.; Pacifici, F.; Pastore, D.; Arriga, R.; Bellia, A.; Andreadi, A.; Di Daniele, N.; Lauro, R.; Della-Morte, D.; et al. Activation of Peripheral Blood Mononuclear Cells and Leptin Secretion: New Potential Role of Interleukin-2 and High Mobility Group Box (HMGB)1. Int. J. Mol. Sci. 2021, 22, 7988. [Google Scholar] [CrossRef]
- Benavente, C.A.; Jacobson, M.K.; Jacobson, E.L. NAD in skin: Therapeutic approaches for niacin. Curr. Pharm. Des. 2009, 15, 29–38. [Google Scholar] [CrossRef]
- Houtkooper, R.H.; Pirinen, E.; Auwerx, J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 2012, 13, 225–238. [Google Scholar] [CrossRef]
- Borriello, F.; Granata, F.; Marone, G. Basophils and skin disorders. J. Investig. Dermatol. 2014, 134, 1202–1210. [Google Scholar] [CrossRef]
- Legrand, A.J.; Choul-Li, S.; Villeret, V.; Aumercier, M. Poly(ADP-ribose) Polyremase-1 (PARP-1) Inhibition: A Promising Therapeutic Strategy for ETS-Expressing Tumours. Int. J. Mol. Sci. 2023, 24, 13454. [Google Scholar] [CrossRef]
- Yaku, K.; Okabe, K.; Gulshan, M.; Takatsu, K.; Okamoto, H.; Nakagawa, T. Metabolism and biochemical properties of nicotinamide adenine dinucleotide (NAD) analogs, nicotinamide guanine dinucleotide (NGD) and nicotinamide hypoxanthine dinucleotide (NHD). Sci. Rep. 2019, 9, 13102. [Google Scholar] [CrossRef] [PubMed]
- Hida, Y.; Kubo, Y.; Murao, K.; Arase, S. Strong expression of a longevity-related protein, SIRT1, in Bowen’s disease. Arch. Dermatol. Res. 2007, 299, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.H.; Sengupta, K.; Li, C.; Kim, H.S.; Cao, L.; Xiao, C.; Kim, S.; Xu, X.; Zheng, Y.; Chilton, B.; et al. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell 2008, 14, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Byles, V.; Chmilewski, L.K.; Wang, J.; Zhu, L.; Forman, L.W.; Faller, D.V.; Dai, Y. Aberrant cytoplasm localization and protein stability of SIRT1 is regulated by PI3K/IGF-1R signaling in human cancer cells. Int. J. Biol. Sci. 2010, 6, 599–612. [Google Scholar] [CrossRef]
- Holmes, T.R.; Dindu, S.; Hansen, L.A. Aberrant localization of signaling proteins in skin cancer: Implications for treatment. Mol. Carcinog. 2019, 58, 1631–1639. [Google Scholar] [CrossRef]
- Jacobson, E.L.; Shieh, W.M.; Huang, A.C. Mapping the role of NAD metabolism in prevention and treatment of carcinogenesis. In ADP-Ribosylation Reactions: From Bacterial Pathogenesis to Cancer; Molecular and Cellular Biochemistry; Springer: Berlin/Heidelberg, Germany, 1999; Volume 193, pp. 69–74. [Google Scholar]
- Campagna, R.; Vignini, A. NAD(+) Homeostasis and NAD(+)-Consuming Enzymes: Implications for Vascular Health. Antioxidants 2023, 12, 376. [Google Scholar] [CrossRef]
- Campagna, R.; Pozzi, V.; Sartini, D.; Salvolini, E.; Brisigotti, V.; Molinelli, E.; Campanati, A.; Offidani, A.; Emanuelli, M. Beyond Nicotinamide Metabolism: Potential Role of Nicotinamide N-Methyltransferase as a Biomarker in Skin Cancers. Cancers 2021, 13, 4943. [Google Scholar] [CrossRef]
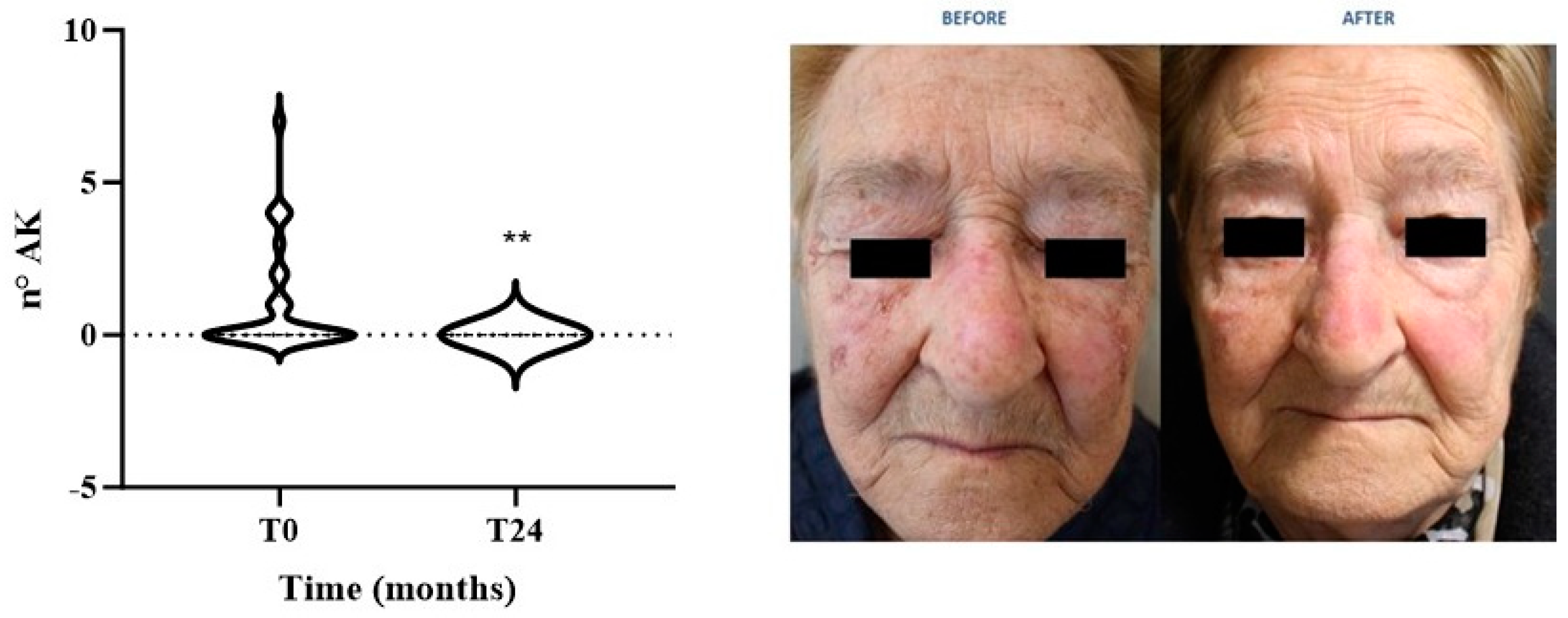
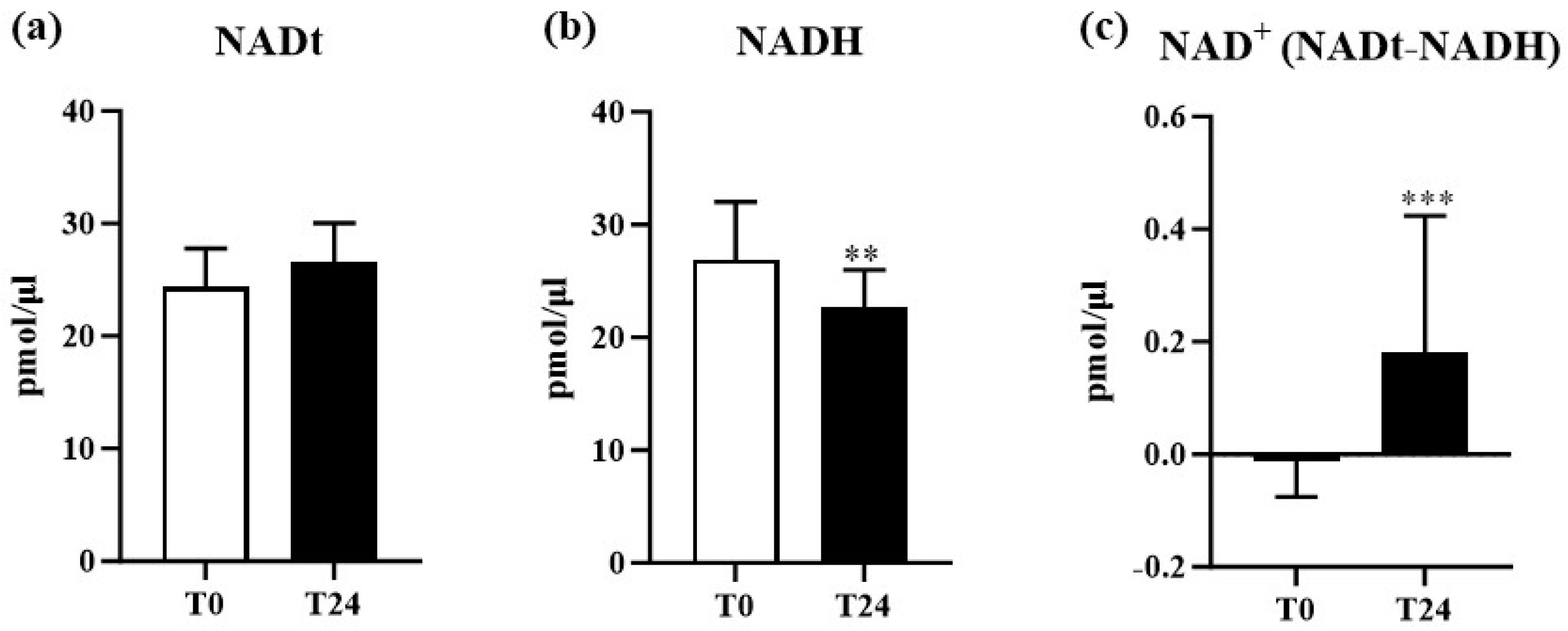

| Variables | Total (n = 30) |
|---|---|
| Age (years) | 66.4 ± 12.0 |
| Gender: | |
| Male % | 76.5 |
| Female % | 23.5 |
| Comorbidities | |
| Cardiovascular Diseases | |
| Hypertension % | 52.9 |
| Atrial Fibrillation % | 17.6 |
| Dyslipidemia | |
| Hypercholesterolemia % | 26.5 |
| Hypertriglyceridemia % | 8.8 |
| Others | |
| Benign Prostatic Hypertrophy % | 17.6 |
| Diabetes % | 11.8 |
| Vitamin B Deficiency % | 8.8 |
| Hyperuricemia % | 5.9 |
| Gastroesophageal Reflux % | 2.9 |
| Hypothyroidism % | 2.9 |
| Psychiatric Disorders % | 2.9 |
| Pharmacological Therapy | % |
|---|---|
| Beta blockers | 52.9 |
| Statins | 38.2 |
| Alpha blockers | 17.6 |
| Anticoagulants | 17.6 |
| Diuretics | 17.6 |
| Insulin | 11.8 |
| Metformin | 11.8 |
| Proton Pump Inhibitors | 8.8 |
| Allopurinol | 5.9 |
| Hormonal analogues | 2.9 |
| Benzodiazepines | 2.9 |
| Vitamin B9 | 2.9 |
| Parameters | Time 0 (n = 30) | Time 24 (n = 30) | p Value * |
|---|---|---|---|
| WBC (cells × 103/L) | 6.7 ± 1.7 | 7.3 ± 3.3 | 0.4116 |
| RBC (cells/mcL) | 4.7 ± 0.5 | 4.6 ± 0.5 | 0.2886 |
| Hb (g/dL) | 14.6 ± 1.2 | 14.6 ± 1.5 | 0.8140 |
| Hct (%) | 43.1 ± 3.4 | 42.6 ± 3.7 | 0.6335 |
| Plt (cells/mcL) | 224.6 ± 56.8 | 233.3 ± 54.3 | 0.5577 |
| Neut % | 61.5 ± 7.5 | 63.5 ± 8.5 | 0.3552 |
| Lymph % | 28.1± 6.6 | 27.5 ± 8.2 | 0.7830 |
| Mono % | 7.3 ± 1.7 | 6.3 ± 1.3 | 0.0113 * |
| Eos % | 2.3 ± 1.9 | 2.0 ± 1.4 | 0.5458 |
| Baso % | 0.8 ± 0.4 | 0.5 ± 0.3 | 0.0013 ** |
| BUN (mg/dL) | 41.5 ± 10.8 | 40.9 ± 11.0 | 0.8385 |
| Glu (mg/dL) | 100.1 ± 11.9 | 89.2 ± 18.7 | 0.0220 * |
| TB (mg/dL) | 0.8 ± 0.4 | 0.8 ± 0.3 | 0.5801 |
| TG (mg/dL) | 123.7 ± 58.2 | 100.1 ± 43.6 | 0.0837 |
| GGT (UI/L) | 27.2 ± 6.8 | 27.6 ± 16.4 | 0.9067 |
| AST/SGOT (U/L) | 29.4 ± 18.3 | 27.4 ± 13.8 | 0.6316 |
| ALT/SGPT (U/L) | 30.0 ± 20.2 | 30.8 ± 21.1 | 0.8958 |
| Cr (mg/dL) | 1.0 ± 0.2 | 1.0 ± 0.4 | 0.8160 |
| eGFR (mL/min) | 74.6 ± 17.3 | 80.6 ± 23.0 | 0.2801 |
| TC (mg/dL) | 189.7 ± 36.7 | 174.0 ± 19.9 | 0.0393 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belardi, R.; Pacifici, F.; Cosio, T.; Lambiase, S.; Shumak, R.G.; Artosi, F.; Rivieccio, A.; Cavalloro, D.; Dellambra, E.; Bianchi, L.; et al. Role of Nicotinamide in the Pathogenesis of Actinic Keratosis: Implications for NAD+/SIRT1 Pathway. Biomolecules 2024, 14, 1512. https://doi.org/10.3390/biom14121512
Belardi R, Pacifici F, Cosio T, Lambiase S, Shumak RG, Artosi F, Rivieccio A, Cavalloro D, Dellambra E, Bianchi L, et al. Role of Nicotinamide in the Pathogenesis of Actinic Keratosis: Implications for NAD+/SIRT1 Pathway. Biomolecules. 2024; 14(12):1512. https://doi.org/10.3390/biom14121512
Chicago/Turabian StyleBelardi, Riccardo, Francesca Pacifici, Terenzio Cosio, Sara Lambiase, Ruslana Gaeta Shumak, Fabio Artosi, Antonia Rivieccio, Danilo Cavalloro, Elena Dellambra, Luca Bianchi, and et al. 2024. "Role of Nicotinamide in the Pathogenesis of Actinic Keratosis: Implications for NAD+/SIRT1 Pathway" Biomolecules 14, no. 12: 1512. https://doi.org/10.3390/biom14121512
APA StyleBelardi, R., Pacifici, F., Cosio, T., Lambiase, S., Shumak, R. G., Artosi, F., Rivieccio, A., Cavalloro, D., Dellambra, E., Bianchi, L., Della-Morte, D., & Campione, E. (2024). Role of Nicotinamide in the Pathogenesis of Actinic Keratosis: Implications for NAD+/SIRT1 Pathway. Biomolecules, 14(12), 1512. https://doi.org/10.3390/biom14121512










