Bioactive Molecules from the Exoskeleton of Procambarus clarkii: Reducing Capacity, Radical Scavenger, and Antitumor and Anti-Inflammatory Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Exoskeleton Flour Production
2.3. Chitosan Extraction and FT-IR Characterization
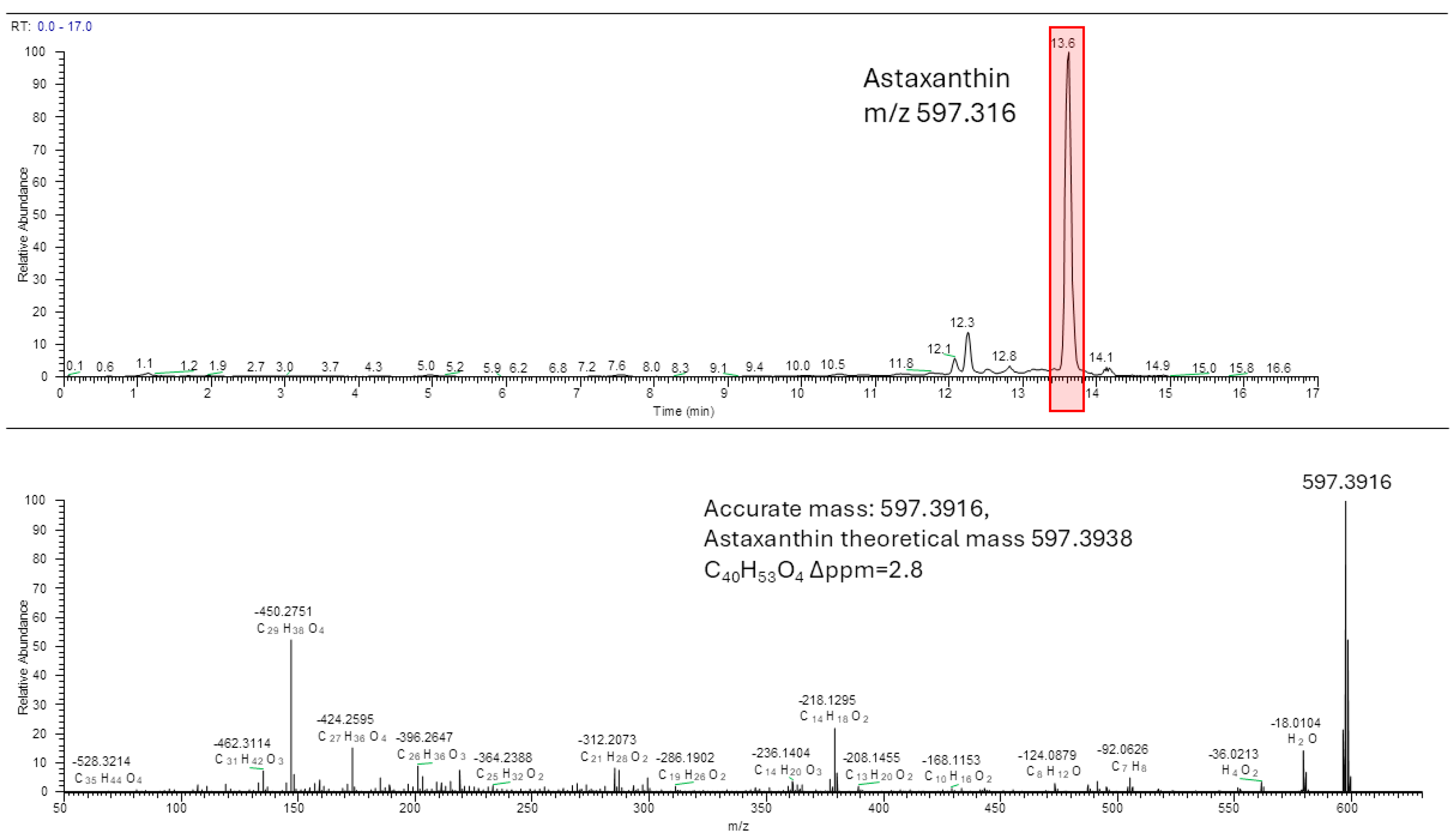
2.4. Astaxanthin Ultrasound-Assisted Extraction
Astaxanthin Determination Through HPLC-HRMS
2.5. Extraction of Phenolic Compounds and Total Phenol Content (TPC)
Determination of Phenolic Compounds Through HPLC-HESI-MS
| Precursor Ion (m/z) [M-H]− | Product Ion (m/z) | Collision Energy (V) | RF Lens (V) | LOD (µg/L) | |
|---|---|---|---|---|---|
| Gallic Acid | 169 | 79 | 24 | 101 | 30 |
| 169 | 125 | 14 | 101 | ||
| Vanillic Acid | 177 | 123 | 20 | 105 | 32 |
| 177 | 152 | 20 | 105 | ||
| Ferulic Acid | 193 | 134 | 15 | 99 | 22 |
| 193 | 178 | 13 | 99 | ||
| Chlorogenic Acid | 353 | 179 | 45 | 180 | 26 |
| 353 | 191 | 45 | 180 | ||
| Catechin | 289 | 203 | 20 | 147 | 28 |
| 289 | 245 | 15 | 147 | ||
| Mandelic Acid | 151 | 77 | 18 | 65 | 26 |
| 151 | 107 | 10 | 65 | ||
| Gentisic Acid | 153 | 108 | 22 | 90 | 24 |
| 153 | 109 | 14 | 90 | ||
| Syringic Acid | 197 | 153 | 12 | 100 | 23 |
| 197 | 182 | 14 | 100 | ||
| Caffeic Acid | 179 | 107 | 25 | 101 | 26 |
| 179 | 135 | 16 | 103 | ||
| Trans-OH-Cynnamic | 163 | 93 | 31 | 90 | 27 |
| 163 | 119 | 14 | 90 | ||
| Rutin | 609 | 271 | 60 | 299 | 26 |
| 609 | 300 | 38 | 299 | ||
| Resveratrol | 227 | 143 | 27 | 156 | 25 |
| 227 | 185 | 20 | 156 | ||
| Apigenin-7Glu | 433 | 269 | 20 | 123 | 24 |
| 433 | 271 | 20 | 123 | ||
| Quercetin | 301 | 151 | 18 | 166 | 25 |
| 301 | 179 | 21 | 166 | ||
| Kaempferol | 285 | 202 | 20 | 195 | 26 |
| 285 | 239 | 29 | 195 | ||
| Hydroxytyrosol | 153 | 95 | 21 | 97 | 25 |
| 153 | 123 | 14 | 97 | ||
| Coumaric Acid | 163 | 93 | 31 | 91 | 27 |
| 163 | 119 | 13 | 91 | ||
| Luteolin | 285 | 133 | 35 | 187 | 26 |
| 285 | 175 | 26 | 187 | ||
| Apigenin | 269 | 117 | 35 | 178 | 25 |
| 269 | 151 | 25 | 178 |
2.6. Reducing Capacity and Radical Scavenging Test
2.6.1. Sample Preparation
2.6.2. Reducing Capacity Test
2.6.3. Radical Scavenging Activity Assay
2.7. In Vitro Antitumor and Anti-Inflammatory Activity
2.8. Statistical Analysis
3. Results
3.1. Exoskeleton Yield and Humidity
3.2. Chitosan Extraction and Characterization
3.3. Astaxanthin Identification and Quantification
3.4. Polyphenol Compound Identification and Quantification
| Bio-Phenol | Calibration Equation | mg/100 mL |
|---|---|---|
| Mandelic Acid | Y = −25181.1 + 488.34X | 1.5 |
| Trans-OH-Cynnamic Acid | Y = −121.915 + 6685.59X | 0.2 |
| Ferulic Acid | Y = 1614 + 1181X | 0.12 |
| Rutin | Y = −61,324.9 + 2962.87X | 0.1 |
| Coumaric Acid | Y = −10,089 + 6127X | 0.5 |
| Gentisic | Y = −58,051.4 + 3441.27X | <LOQ |
| Luteolin | Y = −1577 + 10979X | <LOQ |
3.5. Reducing and Radical Scavenging Capacity
| FRAP mg AAE/g | DPPH• µmol TE/g | ABTS•+ µmol TE/g | |
|---|---|---|---|
| Chitosan | 3.27 ± 0.14 c | 2.22 ± 0.07 c | 3.82 ± 0.13 c |
| Astaxanthin extract | 4.27 ± 0.24 b | 7.91 ± 0.19 b | 9.76 ± 0.14 b |
| Phenolic extract | 1.21 ± 0.03 a | 4.93 ± 0.07 a | 6.89 ± 0.14 a |
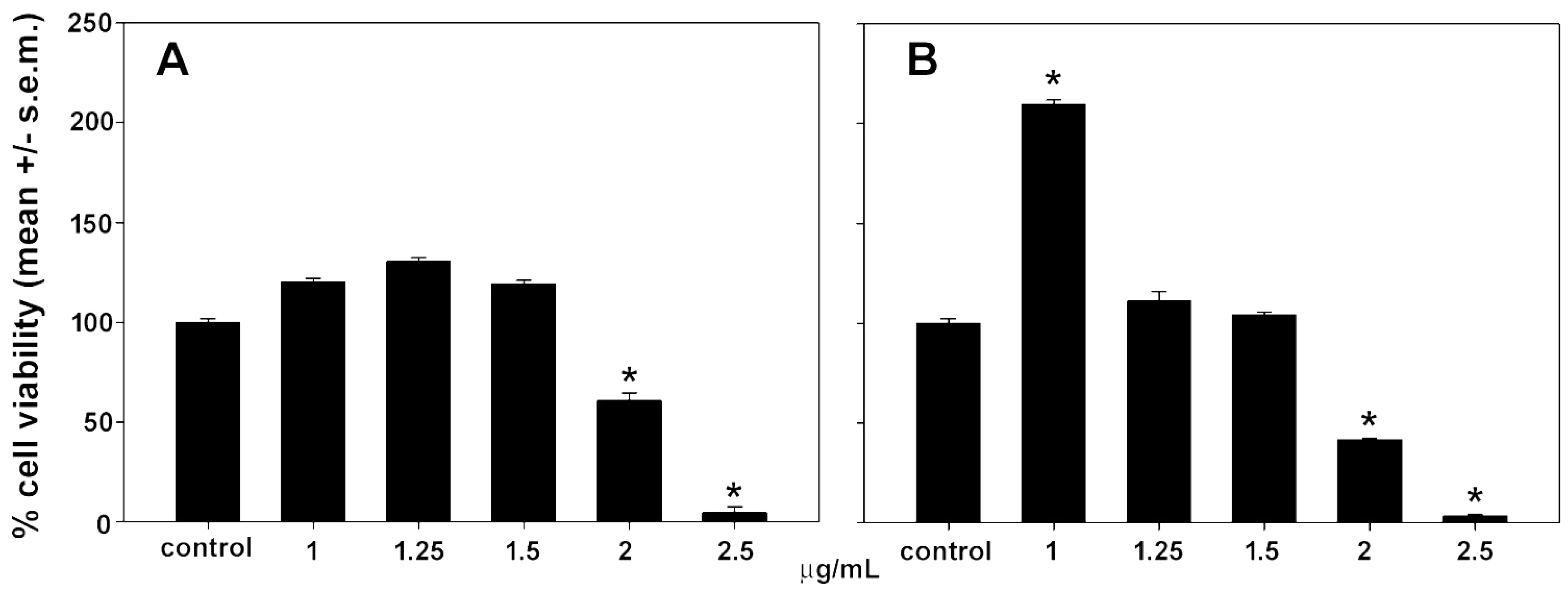
3.6. In Vitro Antitumoral Activity
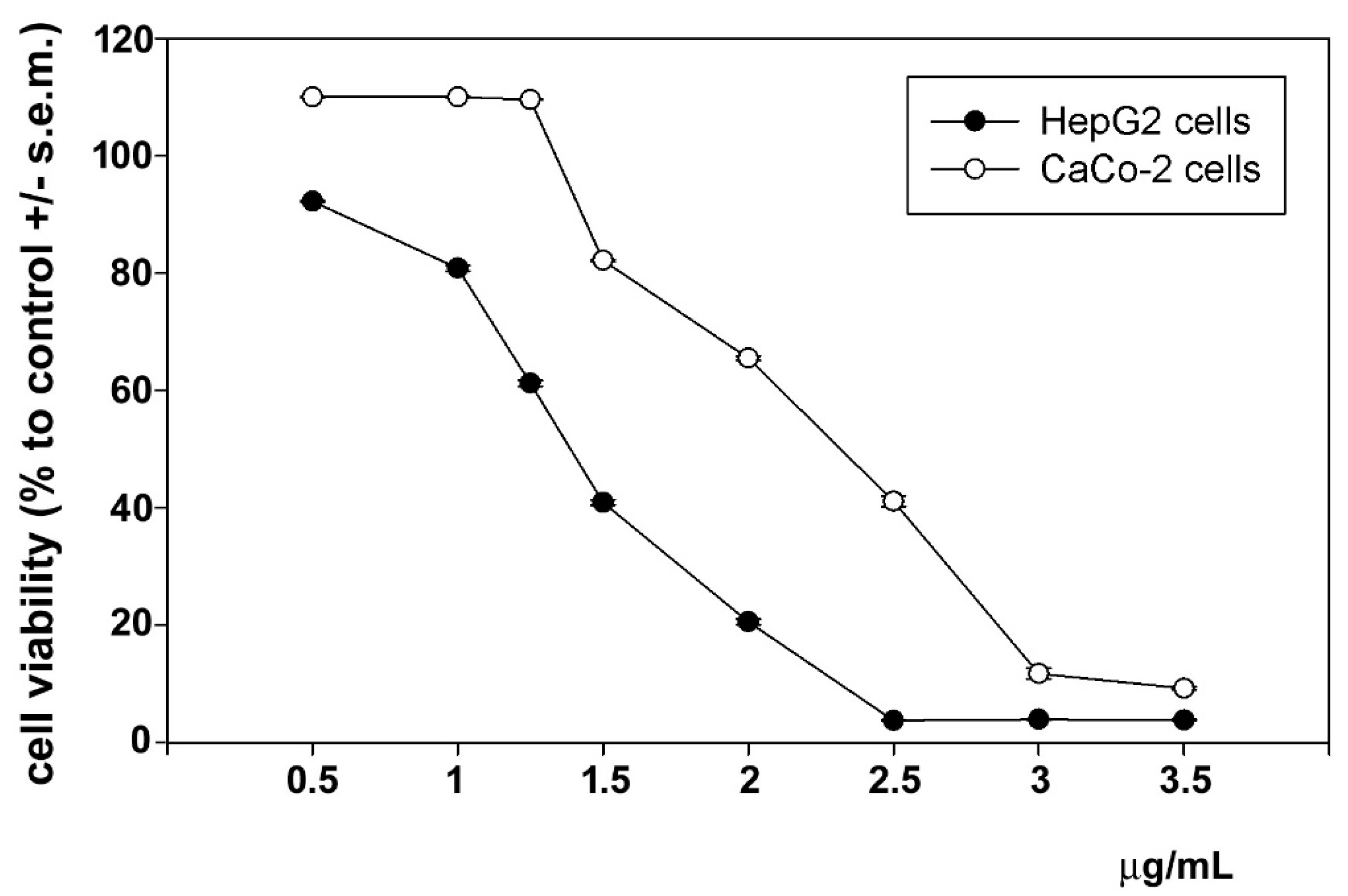
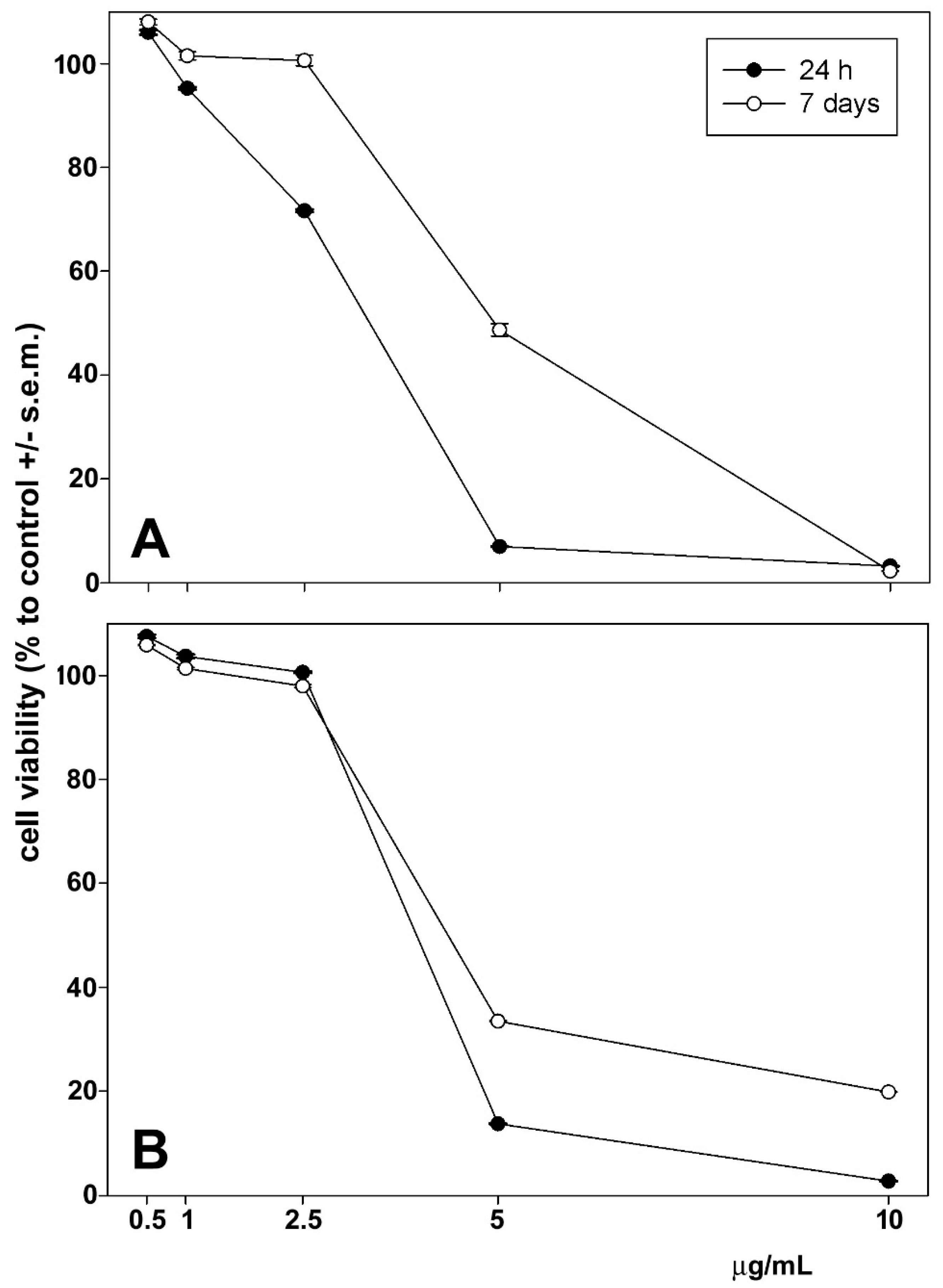
3.7. In Vitro Anti-Inflammatory Activity
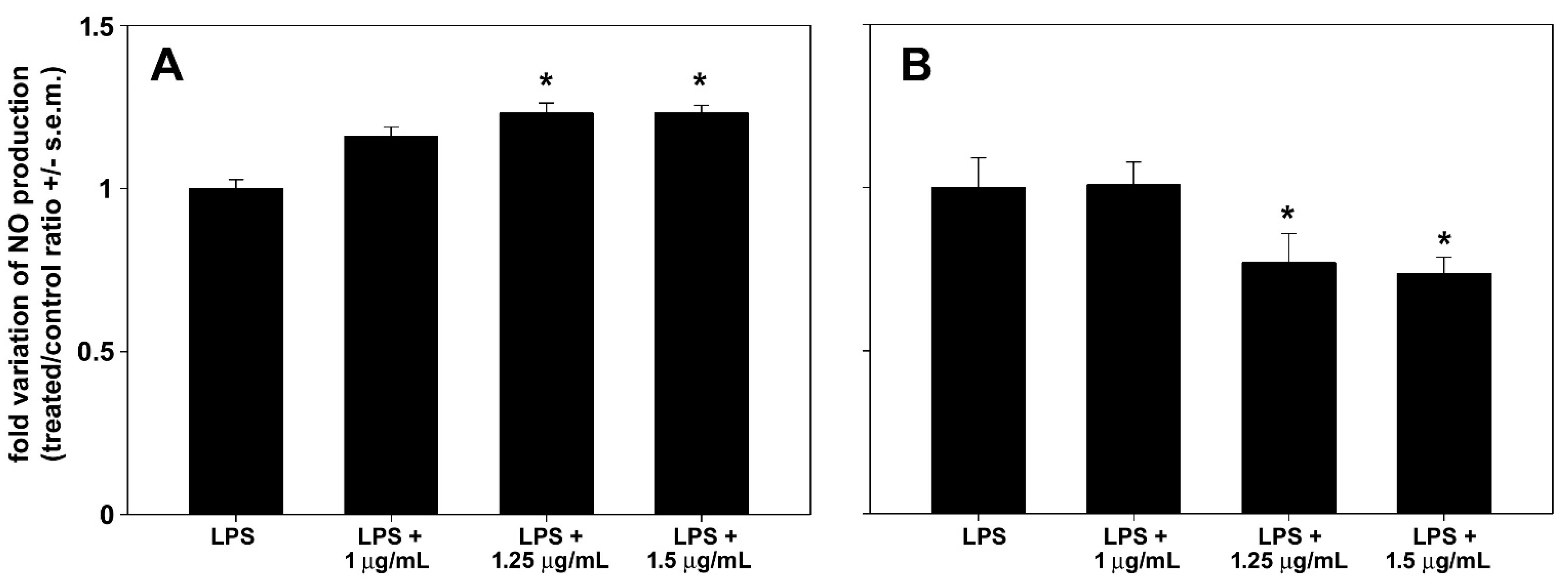
4. Discussion
4.1. Bioactive Molecules’ Characterization and Antitumoral and Anti-Inflammatory Activity
4.2. Reducing and Radical Scavenging Capacities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ngasotter, S.; Xavier, K.A.M.; Meitei, M.M.; Waikhom, D.; Madhulika; Pathak, J.; Singh, S.K. Crustacean Shell Waste Derived Chitin and Chitin Nanomaterials for Application in Agriculture, Food, and Health—A Review. Carbohydr. Polym. Technol. Appl. 2023, 6, 100349. [Google Scholar] [CrossRef]
- Taher, F.A.; Ibrahim, S.A.; El-Aziz, A.A.; Abou El-Nour, M.F.; El-Sheikh, M.A.; El-Husseiny, N.; Mohamed, M.M. Anti-Proliferative Effect of Chitosan Nanoparticles (Extracted from Crayfish Procambarus clarkii, Crustacea: Cambaridae) against MDA-MB-231 and SK-BR-3 Human Breast Cancer Cell Lines. Int. J. Biol. Macromol. 2019, 126, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.M.; Morad, M.Y.; Hamdi, S.A.H.; Fol, M.F. Biocontrol Potential of Chitosan Extracted from Procambarus clarkii (Crustacea: Cambaridae) against Eobania vermiculata Snails (Muller 1774) in Egypt. Egypt. J. Biol. Pest Control 2022, 32, 32. [Google Scholar] [CrossRef]
- El-Naggar, M.M.; Abou-Elmagd, W.S.I.; Suloma, A.; El-Shabaka, H.A.; Khalil, M.T.; Abd El-Rahman, F.A. Optimization and Physicochemical Characterization of Chitosan and Chitosan Nanoparticles Extracted from the Crayfish Procambarus clarkii Wastes. J. Shellfish Res. 2019, 38, 385. [Google Scholar] [CrossRef]
- Synowiecki, J.; Al-Khateeb, N.A.A.Q. The Recovery of Protein Hydrolysate during Enzymatic Isolation of Chitin from Shrimp Crangon crangon Processing Discards. Food Chem. 2000, 68, 147–152. [Google Scholar] [CrossRef]
- Al-Manhel, A.J.; Al-Hilphy, A.R.S.; Niamah, A.K. Extraction of Chitosan, Characterisation and Its Use for Water Purification. J. Saudi Soc. Agric. Sci. 2018, 17, 186–190. [Google Scholar] [CrossRef]
- Xing, K.; Zhu, X.; Peng, X.; Qin, S. Chitosan Antimicrobial and Eliciting Properties for Pest Control in Agriculture: A Review. Agron. Sustain. Dev. 2015, 35, 569–588. [Google Scholar] [CrossRef]
- Kumar, P.T.S.; Raj, N.M.; Praveen, G.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. In Vitro and In Vivo Evaluation of Microporous Chitosan Hydrogel/Nanofibrin Composite Bandage for Skin Tissue Regeneration. Tissue Eng. Part A 2013, 19, 380–392. [Google Scholar] [CrossRef]
- Wang, C.-H.; Cherng, J.-H.; Liu, C.-C.; Fang, T.-J.; Hong, Z.-J.; Chang, S.-J.; Fan, G.-Y.; Hsu, S.-D. Procoagulant and Antimicrobial Effects of Chitosan in Wound Healing. Int. J. Mol. Sci. 2021, 22, 7067. [Google Scholar] [CrossRef]
- M. Ways, T.; Lau, W.; Khutoryanskiy, V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Drug Release Study of the Chitosan-Based Nanoparticles. Heliyon 2022, 8, e08674. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural Modification, Biological Activity and Application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef] [PubMed]
- Mauro, M.; Pinto, P.; Settanni, L.; Puccio, V.; Vazzana, M.; Hornsby, B.L.; Fabbrizio, A.; Di Stefano, V.; Barone, G.; Arizza, V. Chitosan Film Functionalized with Grape Seed Oil—Preliminary Evaluation of Antimicrobial Activity. Sustainability 2022, 14, 5410. [Google Scholar] [CrossRef]
- Hamdi, S.; Elsayed, N.; Algayar, M.; Ishak, V.; Ahmed, M.; Ahmed, S.; Kamal, M.; Abd El-Ghany, M. Biological Extraction, HPLC Quantification and Medical Applications of Astaxanthin Extracted from Crawfish “Procambarus clarkii” Exoskeleton By-Product. Biology 2022, 11, 1215. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Negro-Balmaseda, J.J.; Mínguez-Mosquera, M.I.; Cascajo-Almenara, M.V.; Garrido-Fernández, J. Astaxanthin from Crayfish (Procambarus clarkii) as a Pigmentary Ingredient in the Feed of Laying Hens. Grasas Aceites 2008, 59, 139–145. [Google Scholar] [CrossRef]
- Pu, J.; Bankston, J.D.; Sathivel, S. Production of Microencapsulated Crawfish (Procambarus clarkii) Astaxanthin in Oil by Spray Drying Technology. Dry. Technol. 2011, 29, 1150–1160. [Google Scholar] [CrossRef]
- Ambati, R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A Novel Potential Treatment for Oxidative Stress and Inflammation in Cardiovascular Disease. Am. J. Cardiol. 2008, 101, S58–S68. [Google Scholar] [CrossRef] [PubMed]
- Oehlenschläger, J.; Ostermeyer, U. Feed additives for influencing the color of fish and crustaceans. In Handbook on Natural Pigments in Food and Beverages; Woodhead Publishing: Duxford, UK; Cambridge, MA, USA; Kidlington, UK, 2024; pp. 351–367. [Google Scholar] [CrossRef]
- Commission Implementing Regulation. Commission Implementing Regulation (EU) 2015/1415 of 20 August 2015 on the authorization of astaxanthin as a feed additive for fish, crustaceans and ornamental fish. Off. J. Eur. Union L 2015, 220, 7–10. [Google Scholar]
- Onodenalore, A.C.; Hossain, A.; Banoub, J.; Shahidi, F. Unique Heterocyclic Phenolic Compounds from Shrimp (Pandalus borealis) and Beyond. Food. Prod. Process. Nutr. 2024, 6, 29. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Lacikova, L.; Jancova, M.; Muselik, J.; Masterova, I.; Grancai, D.; Fickova, M. Antiproliferative, Cytotoxic, Antioxidant Activity and Polyphenols Contents in Leaves of Four Staphylea L. Species. Molecules 2009, 14, 3259–3267. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Importance of Insoluble-Bound Phenolics to the Antioxidant Potential Is Dictated by Source Material. Antioxidants 2023, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Pereira, U.C.; Chagas Barros, R.G.; Santana Andrade, J.K.; De Oliveira, C.S.; Gualberto, N.C.; Narain, N. Effect of In Vitro Gastrointestinal Digestion on Bioaccessibility of Phenolic Compounds and Antioxidant Capacity of Crustaceans Residues with Potential Antidiabetic Impact. LWT 2020, 133, 110004. [Google Scholar] [CrossRef]
- Loureiro, T.G.; Anastácio, P.M.S.G.; Araujo, P.B.; Souty-Grosset, C.; Almerão, M.P. Red Swamp Crayfish: Biology, Ecology and Invasion—An Overview. Nauplius 2015, 23, 1–19. [Google Scholar] [CrossRef]
- Souty-Grosset, C.; Anastácio, P.M.; Aquiloni, L.; Banha, F.; Choquer, J.; Chucholl, C.; Tricarico, E. The Red Swamp Crayfish Procambarus clarkii in Europe: Impacts on Aquatic Ecosystems and Human Well-Being. Limnologica 2016, 58, 78–93. [Google Scholar] [CrossRef]
- Savoca, D.; Vazzana, M.; Arizza, V.; Maccotta, A.; Orecchio, S.; Longo, F.; Giudice, V.; D’Oca, G.; Messina, S.; Marrone, F.; et al. Contamination Profiles of Selected Pollutants in Procambarus clarkii Non-Edible Portions Highlight Their Potential Exploitation Applications. JoX 2024, 14, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, F.; Acquistapace, P. Invasive Crayfish in Europe: The Impact of Procambarus clarkii on the Littoral Community of a Mediterranean Lake. Freshw. Biol. 2007, 52, 1249–1259. [Google Scholar] [CrossRef]
- Chucholl, C.; Wendler, F. Positive Selection of Beautiful Invaders: Long-Term Persistence and Bio-Invasion Risk of Freshwater Crayfish in the Pet Trade. Biol. Invasions 2017, 19, 197–208. [Google Scholar] [CrossRef]
- Lo Parrino, E.; Ficetola, G.F.; Manenti, R.; Falaschi, M. Thirty Years of Invasion: The Distribution of the Invasive Crayfish Procambarus clarkii in Italy. Biogeographia 2019, 35, 43–50. [Google Scholar] [CrossRef]
- Oficialdegui, F.J.; Sánchez, M.I.; Clavero, M. One Century Away from Home: How the Red Swamp Crayfish Took over the World. Rev. Fish Biol. Fisheries 2020, 30, 121–135. [Google Scholar] [CrossRef]
- Alcorlo, P.; Geiger, W.; Otero, M. Reproductive Biology and Life Cycle of the Invasive Crayfish Procambarus clarkii (Crustacea: Decapoda) in Diverse Aquatic Habitats of South-Western Spain: Implications for Population Control. Fundam. Appl. Limnol. 2009, 173, 197–212. [Google Scholar] [CrossRef]
- Kerby, J.L.; Riley, S.P.D.; Kats, L.B.; Wilson, P. Barriers and Flow as Limiting Factors in the Spread of an Invasive Crayfish (Procambarus clarkii) in Southern California Streams. Biol. Conserv. 2005, 126, 402–409. [Google Scholar] [CrossRef]
- Cruz, M.J.; Rebelo, R. Colonization of Freshwater Habitats by an Introduced Crayfish, Procambarus clarkii, in Southwest Iberian Peninsula. Hydrobiologia 2007, 575, 191–201. [Google Scholar] [CrossRef]
- REGULATION, C. Commission Implementing Regulation (EU) 2016/1141 of 13 July 2016 adopting a list of invasive alien species of Union concern pursuant to Regulation (EU) No 1143/2014 of the European Parliament and of the Council. Off. J. Eur. Union L 2016, 189, 4–8. [Google Scholar]
- Barbaresi, S.; Gherardi, F. The Invasion of the Alien Crayfish Procambarus clarkii in Europe, with Particular Reference to Italy. Biol. Invasions 2000, 2, 259–264. [Google Scholar] [CrossRef]
- Elkhodary, G.; Beltagy, D.; Samak, N.; Abdul- Aziz, K.; Mona, M. Assessment of Antioxidant, Antimicrobial and Anticancer Activities of Carotenoid Extracted from Erugosquilla Massavensis and Procambarus clarkii Exoskeletons. Egypt. J. Cancer Biomed. Res. 2017, 1, 49–58. [Google Scholar] [CrossRef]
- Hamdi, S.; Fol, M. Procambrus clarkii (Crustacea: Cambaridae) a Biological Control Agent against Four Intermediate Host Snails in Egypt. Egypt. J. Exp. Biol. (Zool.) 2017, 13, 193–199. [Google Scholar] [CrossRef]
- Punginelli, D.; Catania, V.; Vazzana, M.; Mauro, M.; Spinello, A.; Barone, G.; Barberi, G.; Fiorica, C.; Vitale, M.; Cunsolo, V.; et al. A Novel Peptide with Antifungal Activity from Red Swamp Crayfish Procambarus clarkii. Antibiotics 2022, 11, 1792. [Google Scholar] [CrossRef] [PubMed]
- Naselli-Flores, L.; Barone, R.; Mosello, R. Eutrophication Control by Lime Addition: A Preliminary Approach in Sicilian Reservoirs. Hydrobiologia 2003, 504, 297–303. [Google Scholar] [CrossRef]
- Hosney, A.; Ullah, S.; Barčauskaitė, K. A Review of the Chemical Extraction of Chitosan from Shrimp Wastes and Prediction of Factors Affecting Chitosan Yield by Using an Artificial Neural Network. Mar. Drugs 2022, 20, 675. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An Infrared Investigation in Relation with Chitin and Chitosan Characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Hu, J.; Lu, W.; Lv, M.; Wang, Y.; Ding, R.; Wang, L. Extraction and Purification of Astaxanthin from Shrimp Shells and the Effects of Different Treatments on Its Content. Rev. Bras. Farmacogn. 2019, 29, 24–29. [Google Scholar] [CrossRef]
- Passafiume, R.; Perrone, A.; Sortino, G.; Gianguzzi, G.; Saletta, F.; Gentile, C.; Farina, V. Chemical–Physical Characteristics, Polyphenolic Content and Total Antioxidant Activity of Three Italian-Grown Pomegranate Cultivars. NFS J. 2019, 16, 9–14. [Google Scholar] [CrossRef]
- Attanzio, A.; D’Anneo, A.; Pappalardo, F.; Bonina, F.P.; Livrea, M.A.; Allegra, M.; Tesoriere, L. Phenolic Composition of Hydrophilic Extract of Manna from Sicilian Fraxinus angustifolia Vahl and Its Reducing, Antioxidant and Anti-Inflammatory Activity In Vitro. Antioxidants 2019, 8, 494. [Google Scholar] [CrossRef]
- Indelicato, S.; Houmanat, K.; Bongiorno, D.; Ejjilani, A.; Hssaini, L.; Razouk, R.; Charafi, J.; Ennahli, S.; Hanine, H. Freeze Dried Pomegranate Juices of Moroccan Fruits: Main Representative Phenolic Compounds. J. Sci. Food. Agric. 2023, 103, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Gautam, S.; Sharma, A. Physical, Biochemical and Antioxidant Properties of Some Indian Honeys. Food Chem. 2010, 118, 391–397. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Luparello, C.; Branni, R.; Abruscato, G.; Lazzara, V.; Drahos, L.; Arizza, V.; Mauro, M.; Di Stefano, V.; Vazzana, M. Cytotoxic Capability and the Associated Proteomic Profile of Cell-Free Coelomic Fluid Extracts from the Edible Sea Cucumber Holothuria tubulosa on HepG2 Liver Cancer Cells. EXCLI J. 2022, 21, 722–743. [Google Scholar] [CrossRef]
- Faraone, F.P.; Giacalone, G.; Canale, D.E.; D’Angelo, S.; Favaccio, G.; Garozzo, V.; Giancontieri, G.L.; Isgrò, C.; Melfi, R.; Morello, B.; et al. Tracking the Invasion of the Red Swamp Crayfish Procambarus clarkii (Girard, 1852) (Decapoda Cambaridae) in Sicily: A “Citizen Science” Approach. BG 2017, 32, 25–29. [Google Scholar] [CrossRef]
- Di Leo, C.; Faraone, F.P.; Lo Valvo, M. A New Record of the Red Swamp Crayfish, Procambarus clarkii (Girard, 1852) (Crustacea Cambaridae), in Sicily, Italy. Biodivers. J. 2014, 5, 425–428. [Google Scholar]
- Vecchioni, L.; Faraone, F.P.; Stoch, F.; Arculeo, M.; Marrone, F. Diversity and Distribution of the Inland Water Decapods of Sicily (Crustacea, Malacostraca). Diversity 2022, 14, 246. [Google Scholar] [CrossRef]
- Gortari, M.C.; Hours, R.A. Biotechnological Processes for Chitin Recovery out of Crustacean Waste: A Mini-Review. Electron. J. Biotechnol. 2013, 16. [Google Scholar] [CrossRef]
- Yi, K.; Miao, S.; Yang, B.; Li, S.; Lu, Y. Harnessing the Potential of Chitosan and Its Derivatives for Enhanced Functionalities in Food Applications. Foods 2024, 13, 439. [Google Scholar] [CrossRef]
- Omar, B.A.; Elmasry, R.; Eita, A.; Soliman, M.M.; El-Tahan, A.M.; Sitohy, M. Upgrading the Preparation of High-Quality Chitosan from Procambarus clarkii Wastes over the Traditional Isolation of Shrimp Chitosan. Saudi J. Biol. Sci. 2022, 29, 911–919. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Wenling, C.; Duohui, J.; Jiamou, L.; Yandao, G.; Nanming, Z.; Xiufang, Z. Effects of the Degree of Deacetylation on the Physicochemical Properties and Schwann Cell Affinity of Chitosan Films. J. Biomater. Appl. 2005, 20, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Šimat, V.; Rathod, N.; Čagalj, M.; Hamed, I.; Generalić Mekinić, I. Astaxanthin from Crustaceans and Their Byproducts: A Bioactive Metabolite Candidate for Therapeutic Application. Mar. Drugs 2022, 20, 206. [Google Scholar] [CrossRef]
- Hai, T.C.; Van Man, P. Optimization of ultrasound-assisted extraction of astaxanthin from black tiger shrimp (Penaeus monodon) shells using deep eutectic solvent and ethanol as a co-solvent. LWT 2024, 212, 116965. [Google Scholar] [CrossRef]
- Panagiotakopoulos, I.; Karantonis, H.C.; Kartelias, I.G.; Nasopoulou, C. Ultrasonic-Assisted Extraction of Astaxanthin from Shrimp By-Products Using Vegetable Oils. Mar. Drugs 2023, 21, 467. [Google Scholar] [CrossRef] [PubMed]
- Denga, M.; Qu, Y.; Wu, T.; Na, Y.; Liang, N.; Zhao, L. Amino acid-based natural deep eutectic solvent combined with ultrasonic extraction: Green extraction of astaxanthin from shrimp shells. Biomass Convers. Biorefinery 2024, 14, 24631–24640. [Google Scholar] [CrossRef]
- Babin, A.; Motreuil, S.; Teixeira, M.; Bauer, A.; Rigaud, T.; Moreau, J.; Moret, Y. Origin of the Natural Variation in the Storage of Dietary Carotenoids in Freshwater Amphipod Crustaceans. PLoS ONE 2020, 15, e0231247. [Google Scholar] [CrossRef] [PubMed]
- Yanar, Y.; Çelik, M.; Yanar, M. Seasonal Changes in Total Carotenoid Contents of Wild Marine Shrimps (Penaeus semisulcatus and Metapenaeus monoceros) Inhabiting the Eastern Mediterranean. Food Chem. 2004, 88, 267–269. [Google Scholar] [CrossRef]
- Messina, C.M.; Manuguerra, S.; Renda, G.; Santulli, A. Biotechnological Applications for the Sustainable Use of Marine By-Products: In Vitro Antioxidant and Pro-Apoptotic Effects of Astaxanthin Extracted with Supercritical CO2 from Parapeneus longirostris. Mar. Biotechnol. 2019, 21, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.; Cesi, V.; Prete, E.; Negroni, A.; Palone, F.; Cucchiara, S.; Oliva, S.; Leter, B.; Stronati, L. Krill Oil Reduces Intestinal Inflammation by Improving Epithelial Integrity and Impairing Adherent-Invasive Escherichia coli Pathogenicity. Dig. Liver Dis. 2016, 48, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Yang, J. Copper-Induced Oxidative Damage to the Prophenoloxidase-Activating System in the Freshwater Crayfish Procambarus clarkii. Fish Shellfish Immunol. 2016, 52, 221–229. [Google Scholar] [CrossRef]
- Wei, K.; Wei, Y.; Song, C. The Response of Phenoloxidase to Cadmium-Disturbed Hepatopancreatic Immune-Related Molecules in Freshwater Crayfish Procambarus clarkii. Fish Shellfish Immunol. 2020, 99, 190–198. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Benjakul, S.; Ahmad, M.; Arfat, Y.A.; Panichayupakaranant, P. Undesirable Enzymatic Browning in Crustaceans: Causative Effects and Its Inhibition by Phenolic Compounds. Crit. Rev. Food Sci. Nutr. 2015, 55, 1992–2003. [Google Scholar] [CrossRef] [PubMed]
- Maia, M.L.; Grosso, C.; Barroso, M.F.; Silva, A.; Delerue-Matos, C.; Domingues, V.F. Bioactive Compounds of Shrimp Shell Waste from Palaemon serratus and Palaemon varians from Portuguese Coast. Antioxidants 2023, 12, 435. [Google Scholar] [CrossRef]
- Świsłocka, R.; Świderski, G.; Nasiłowska, J.; Sokołowska, B.; Wojtczak, A.; Lewandowski, W. Research on the Electron Structure and Antimicrobial Properties of Mandelic Acid and Its Alkali Metal Salts. Int. J. Mol. Sci. 2023, 24, 3078. [Google Scholar] [CrossRef]
- Sadi, G.; Kaya, A.; Yalcin, H.A.; Emsen, B.; Kocabas, A.; Kartal, D.I.; Altay, A. Wild Edible Mushrooms from Turkey as Possible Anticancer Agents on HepG2 Cells Together with Their Antioxidant and Antimicrobial Properties. Int. J. Med. Mushrooms 2016, 18, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Janicke, B.; Hegardt, C.; Krogh, M.; Önning, G.; Åkesson, B.; Cirenajwis, H.M.; Oredsson, S.M. The Antiproliferative Effect of Dietary Fiber Phenolic Compounds Ferulic Acid and p -Coumaric Acid on the Cell Cycle of Caco-2 Cells. Nutr. Cancer 2011, 63, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Karakurt, S.; Abuşoğlu, G.; Arituluk, Z.C. Comparison of Anticarcinogenic Properties of Viburnum Opulus and Its Active Compound P-Coumaric Acid on Human Colorectal Carcinoma. Turk. J. Biol. 2020, 44, 252–263. [Google Scholar] [CrossRef]
- Wang, J.; Lai, X.; Yuan, D.; Liu, Y.; Wang, J.; Liang, Y. Effects of Ferulic Acid, a Major Component of Rice Bran, on Proliferation, Apoptosis, and Autophagy of HepG2 Cells. Food Res. Int. 2022, 161, 111816. [Google Scholar] [CrossRef]
- Kim, M.; Yoon, W.-J.; Kim, S. Phytochemical Compositions of Immature Wheat Bran, and Its Antioxidant Capacity, Cell Growth Inhibition, and Apoptosis Induction through Tumor Suppressor Gene. Molecules 2016, 21, 1292. [Google Scholar] [CrossRef] [PubMed]
- Janda, E.; Martino, C.; Riillo, C.; Parafati, M.; Lascala, A.; Mollace, V.; Boutin, J.A. Apigenin and Luteolin Regulate Autophagy by Targeting NRH-Quinone Oxidoreductase 2 in Liver Cells. Antioxidants 2021, 10, 776. [Google Scholar] [CrossRef]
- Ben Sghaier, M.; Pagano, A.; Mousslim, M.; Ammari, Y.; Kovacic, H.; Luis, J. Rutin Inhibits Proliferation, Attenuates Superoxide Production and Decreases Adhesion and Migration of Human Cancerous Cells. Biomed. Pharmacother. 2016, 84, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.-H.; Tsai, M.-C.; Wang, C.-C.; Wu, S.-W.; Chang, Y.-J.; Wu, C.-H.; Wang, C.-J. Mulberry Leaf Polyphenol Extract and Rutin Induces Autophagy Regulated by P53 in Human Hepatoma HepG2 Cells. Pharmaceuticals 2021, 14, 1310. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Zheng, B.; Li, T.; Liu, R.H. Quantification of Phytochemicals, Cellular Antioxidant Activities and Antiproliferative Activities of Raw and Roasted American Pistachios (Pistacia vera L.). Nutrients 2022, 14, 3002. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Wu, C.; Fan, G.; Li, T.; Dou, J.; Zhu, J.; Li, C.; Kou, X. Jujube Peel Polyphenols Synergistically Inhibit Lipopolysaccharide-Induced Inflammation through Multiple Signaling Pathways in RAW 264.7 Cells. Food Chem. Toxicol. 2022, 164, 113062. [Google Scholar] [CrossRef]
- Park, C.M.; Song, Y.-S. Luteolin and Luteolin-7- O -Glucoside Inhibit Lipopolysaccharide-Induced Inflammatory Responses through Modulation of NF-κB/AP-1/PI3K-Akt Signaling Cascades in RAW 264.7 Cells. Nutr. Res. Pract. 2013, 7, 423. [Google Scholar] [CrossRef]
- Lampiasi, N.; Montana, G. The Molecular Events behind Ferulic Acid Mediated Modulation of IL-6 Expression in LPS-Activated Raw 264.7 Cells. Immunobiology 2016, 221, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Choi, W.; Yoo, S.H.; Nam, S.-W.; Shin, P.-G.; Kim, K.K.; Kim, G.-D. Modulation of Inflammatory Pathways and Adipogenesis by the Action of Gentisic Acid in RAW 264.7 and 3T3-L1 Cell Lines. J. Microbiol. Biotechnol. 2021, 31, 1079–1087. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Yang, M.; Liu, M. Rutin Prevents Inflammation Induced by Lipopolysaccharide in RAW 264.7 Cells via Conquering the TLR4-MyD88-TRAF6-NF-κB Signalling Pathway. J. Pharm. Pharmacol. 2021, 73, 110–117. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary Phenolics: Chemistry, Bioavailability and Effects on Health. Nat. Prod. Rep. 2009, 26, 1001. [Google Scholar] [CrossRef] [PubMed]
- Ahmadifar, E.; Yousefi, M.; Karimi, M.; Fadaei Raieni, R.; Dadar, M.; Yilmaz, S.; Dawood, M.A.O.; Abdel-Latif, H.M.R. Benefits of Dietary Polyphenols and Polyphenol-Rich Additives to Aquatic Animal Health: An Overview. Rev. Fish. Sci. Aquac. 2021, 29, 478–511. [Google Scholar] [CrossRef]
- Sun, T.; Qin, Y.; Xie, J.; Xue, B.; Zhu, Y.; Wu, J.; Bian, X.; Li, X. Antioxidant Activity of Oligochitosan Maillard Reaction Products Using Oligochitosan as the Amino or Carbonyl Groups Donors. Int. J. Food Prop. 2018, 21, 1964–1971. [Google Scholar] [CrossRef]
- Pu, S.; Li, J.; Sun, L.; Zhong, L.; Ma, Q. An In Vitro Comparison of the Antioxidant Activities of Chitosan and Green Synthesized Gold Nanoparticles. Carbohyd. Polym. 2019, 211, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus Astaxanthin: Applications for Human Health and Nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Olszowy, M. What Is Responsible for Antioxidant Properties of Polyphenolic Compounds from Plants? Plant Physiol. Biochem. 2019, 144, 135–143. [Google Scholar] [CrossRef]
- Makkey, A.; Ibrahim Mahmoud, M.; Ibrahim, S.; Desouky, M.; Abouzied, A.; Mohamed, S. Population Dynamic Parameters, Economic Evaluation of Crayfish (Procambarus clarkii) and Value Added of Its Shells from River Nile, Egypt. Egypt. J. Aquac. 2023, 12, 1–25. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longo, F.; Di Gaudio, F.; Attanzio, A.; Marretta, L.; Luparello, C.; Indelicato, S.; Bongiorno, D.; Barone, G.; Tesoriere, L.; Giardina, I.C.; et al. Bioactive Molecules from the Exoskeleton of Procambarus clarkii: Reducing Capacity, Radical Scavenger, and Antitumor and Anti-Inflammatory Activities. Biomolecules 2024, 14, 1635. https://doi.org/10.3390/biom14121635
Longo F, Di Gaudio F, Attanzio A, Marretta L, Luparello C, Indelicato S, Bongiorno D, Barone G, Tesoriere L, Giardina IC, et al. Bioactive Molecules from the Exoskeleton of Procambarus clarkii: Reducing Capacity, Radical Scavenger, and Antitumor and Anti-Inflammatory Activities. Biomolecules. 2024; 14(12):1635. https://doi.org/10.3390/biom14121635
Chicago/Turabian StyleLongo, Francesco, Francesca Di Gaudio, Alessandro Attanzio, Laura Marretta, Claudio Luparello, Serena Indelicato, David Bongiorno, Giampaolo Barone, Luisa Tesoriere, Ilenia Concetta Giardina, and et al. 2024. "Bioactive Molecules from the Exoskeleton of Procambarus clarkii: Reducing Capacity, Radical Scavenger, and Antitumor and Anti-Inflammatory Activities" Biomolecules 14, no. 12: 1635. https://doi.org/10.3390/biom14121635
APA StyleLongo, F., Di Gaudio, F., Attanzio, A., Marretta, L., Luparello, C., Indelicato, S., Bongiorno, D., Barone, G., Tesoriere, L., Giardina, I. C., Abruscato, G., Perlotti, M., Hornsby, L. B., Arizza, V., Vazzana, M., Marrone, F., Vizzini, A., Martino, C., Savoca, D., ... Mauro, M. (2024). Bioactive Molecules from the Exoskeleton of Procambarus clarkii: Reducing Capacity, Radical Scavenger, and Antitumor and Anti-Inflammatory Activities. Biomolecules, 14(12), 1635. https://doi.org/10.3390/biom14121635














