Gut Microbiota Modulation: A Novel Strategy for Rheumatoid Arthritis Therapy
Abstract
:1. Introduction
2. The Connection Between Gut Microbiota Dysbiosis, Immune System Dysregulation and RA Progression
3. Mechanisms That Link Gut Microbiota Dysbiosis and RA Pathogenesis
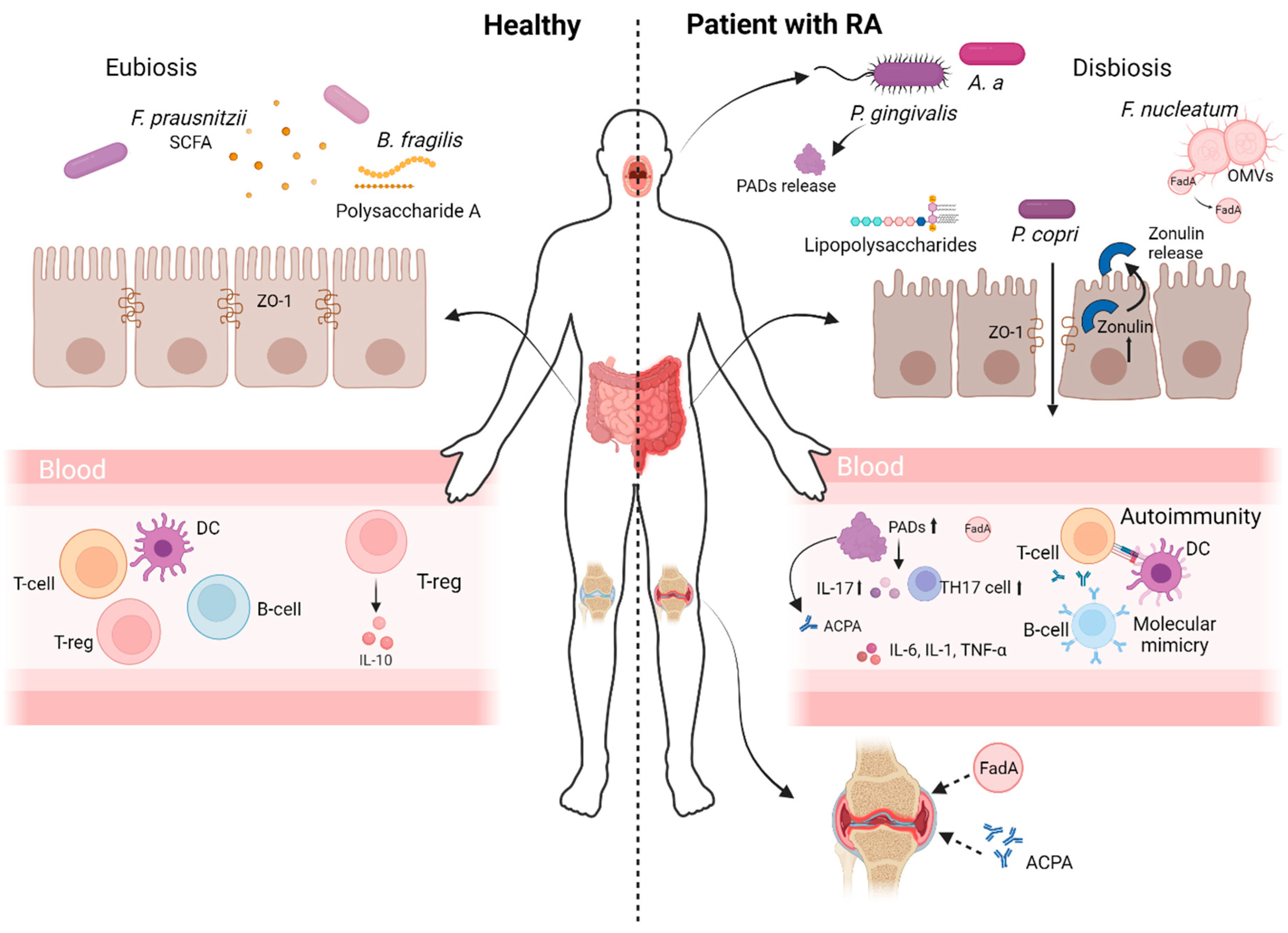
3.1. Bacterial Translocation
3.2. Molecular Mimicry
3.3. Bacterial Metabolites
3.3.1. Metabolites That May Cause Citrullination
3.3.2. Production of Immunomodulatory Metabolites
4. RA Management by Manipulating the Gut Microbiota
4.1. Treatment of RA with Probiotics and Prebiotics
4.2. Diet and Lifestyle That Influence the Gut Microbiome in Treating RA
4.3. Traditional Chinese Medicine (TCM) for RA
4.4. Faecal Microbiota Transplantation (FMT) for RA
5. Conclusions and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Cooles, F.A.; Isaacs, J.D. Pathophysiology of rheumatoid arthritis. Curr. Opin. Rheumatol. 2011, 23, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Di Matteo, A.; Bathon, J.M.; Emery, P. Rheumatoid arthritis. Lancet 2023, 402, 2019–2033. [Google Scholar] [CrossRef] [PubMed]
- Scherer, H.U.; Häupl, T.; Burmester, G.R. The etiology of rheumatoid arthritis. J. Autoimmun. 2020, 110, 102400. [Google Scholar] [CrossRef] [PubMed]
- van der Woude, D.; Alemayehu, W.G.; Verduijn, W.; de Vries, R.R.; Houwing-Duistermaat, J.J.; Huizinga, T.W.; Toes, R.E. Gene-environment interaction influences the reactivity of autoantibodies to citrullinated antigens in rheumatoid arthritis. Nat. Genet. 2010, 42, 814–816. [Google Scholar] [CrossRef]
- van der Helm-van Mil, A.H.M.; Wesoly, J.Z.; Huizinga, T.W.J. Understanding the genetic contribution to rheumatoid arthritis. Curr. Opin. Rheumatol. 2005, 17, 299–304. [Google Scholar] [CrossRef]
- Klareskog, L.; Padyukov, L.; Lorentzen, J.; Alfredsson, L. Mechanisms of Disease: Genetic susceptibility and environmental triggers in the development of rheumatoid arthritis. Nat. Clin. Pract. Rheumatol. 2006, 2, 425–433. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Anzaghe, M.; Schülke, S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells 2020, 9, 880. [Google Scholar] [CrossRef]
- Källberg, H.; Ding, B.; Padyukov, L.; Bengtsson, C.; Rönnelid, J.; Klareskog, L.; Alfredsson, L.; EIRA Study Group. Smoking is a major preventable risk factor for rheumatoid arthritis: Estimations of risks after various exposures to cigarette smoke. Ann. Rheum. Dis. 2011, 70, 508–511. [Google Scholar] [CrossRef]
- Maranini, B.; Bortoluzzi, A.; Silvagni, E.; Govoni, M. Focus on Sex and Gender: What We Need to Know in the Management of Rheumatoid Arthritis. J. Pers. Med. 2022, 12, 499. [Google Scholar] [CrossRef]
- Intriago, M.; Maldonado, G.; Cárdenas, J.; Ríos, C. Clinical Characteristics in Patients with Rheumatoid Arthritis: Differences between Genders. Sci. World J. 2019, 2019, 1–6. [Google Scholar] [CrossRef]
- Zandman-Goddard, G.; Peeva, E.; Shoenfeld, Y. Gender and autoimmunity. Autoimmun. Rev. 2007, 6, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Ortona, E.; Pierdominici, M.; Maselli, A.; Veroni, C.; Aloisi, F.; Shoenfeld, Y. Sex-based differences in autoimmune diseases. Ann. Ist. Super. Sanità 2016, 52, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Straub, R.H. The Complex Role of Estrogens in Inflammation. Endocr. Rev. 2007, 28, 521–574. [Google Scholar] [CrossRef] [PubMed]
- Prieto, G.A.; Rosenstein, Y. Oestradiol potentiates the suppressive function of human CD4+ CD25+ regulatory T cells by promoting their proliferation. Immunology 2006, 118, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Yap, H.-Y.; Tee, S.Z.-Y.; Wong, M.M.-T.; Chow, S.-K.; Peh, S.-C.; Teow, S.-Y. Pathogenic Role of Immune Cells in Rheumatoid Arthritis: Implications in Clinical Treatment and Biomarker Development. Cells 2018, 7, 161. [Google Scholar] [CrossRef]
- Weyand, C.M.; Goronzy, J.J. Ectopic Germinal Center Formation in Rheumatoid Synovitis. Ann. N. Y Acad. Sci. 2003, 987, 140–149. [Google Scholar] [CrossRef]
- Kondo, Y.; Yokosawa, M.; Kaneko, S.; Furuyama, K.; Segawa, S.; Tsuboi, H.; Matsumoto, I.; Sumida, T. Review: Transcriptional Regulation of CD4+ T Cell Differentiation in Experimentally Induced Arthritis and Rheumatoid Arthritis. Arthritis Rheumatol. 2018, 70, 653–661. [Google Scholar] [CrossRef]
- Wu, F.; Gao, J.; Kang, J.; Wang, X.; Niu, Q.; Liu, J.; Zhang, L. B Cells in Rheumatoid Arthritis: Pathogenic Mechanisms and Treatment Prospects. Front. Immunol. 2021, 12, 750753. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef]
- Chemin, K.; Gerstner, C.; Malmström, V. Effector Functions of CD4+ T Cells at the Site of Local Autoimmune Inflammation—Lessons From Rheumatoid Arthritis. Front. Immunol. 2019, 10, 353. [Google Scholar] [CrossRef]
- Cascão, R.; Moura, R.A.; Perpétuo, I.; Canhão, H.; Vieira-Sousa, E.; Mourão, A.F.; Rodrigues, A.M.; Polido-Pereira, J.; Queiroz, M.V.; Rosário, H.S.; et al. Identification of a cytokine network sustaining neutrophil and Th17 activation in untreated early rheumatoid arthritis. Arthritis Res. Ther. 2010, 12, R196. [Google Scholar] [CrossRef] [PubMed]
- Danks, L.; Komatsu, N.; Guerrini, M.M.; Sawa, S.; Armaka, M.; Kollias, G.; Nakashima, T.; Takayanagi, H. RANKL expressed on synovial fibroblasts is primarily responsible for bone erosions during joint inflammation. Ann. Rheum. Dis. 2016, 75, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Shiba, N.; Hiraoka, K. New Insights into the Role of Synovial Fibroblasts Leading to Joint Destruction in Rheumatoid Arthritis. Int. J. Mol. Sci. 2023, 24, 5173. [Google Scholar] [CrossRef] [PubMed]
- Marahleh, A.; Kitaura, H.; Ohori, F.; Kishikawa, A.; Ogawa, S.; Shen, W.R.; Qi, J.; Noguchi, T.; Nara, Y.; Mizoguchi, I. TNF-α Directly Enhances Osteocyte RANKL Expression and Promotes Osteoclast Formation. Front. Immunol. 2019, 10, 2925. [Google Scholar] [CrossRef]
- Paleolog, E.M. Angiogenesis in rheumatoid arthritis. Arthritis Res. 2002, 4, S81. [Google Scholar] [CrossRef]
- Elshabrawy, H.A.; Chen, Z.; Volin, M.V.; Ravella, S.; Virupannavar, S.; Shahrara, S. The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis 2015, 18, 433–448. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.; Breedveld, F.C.; Dougados, M.; Emery, P.; Gaujoux-Viala, C.; Gorter, S.; Knevel, R.; Nam, J.; Schoels, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann. Rheum. Dis. 2010, 69, 964–975. [Google Scholar] [CrossRef]
- Silverstein, F.E.; Faich, G.; Goldstein, J.L.; Simon, L.S.; Pincus, T.; Whelton, A.; Makuch, R.; Eisen, G.; Agrawal, N.M.; Stenson, W.F.; et al. Gastrointestinal Toxicity With Celecoxib vs Nonsteroidal Anti-inflammatory Drugs for Osteoarthritis and Rheumatoid Arthritis. JAMA 2000, 284, 1247. [Google Scholar] [CrossRef]
- Moura, M.P.S.D.G.; Lopes, L.C.; Silva, M.T.; Barberato-Filho, S.; Motta, R.H.L.; Bergamaschi, C.d.C. Use of steroid and nonsteroidal anti-inflammatories in the treatment of rheumatoid arthritis. Medicine 2018, 97, e12658. [Google Scholar] [CrossRef]
- Szostak, B.; Machaj, F.; Rosik, J.; Pawlik, A. Using pharmacogenetics to predict methotrexate response in rheumatoid arthritis patients. Expert Opin. Drug Metab. Toxicol. 2020, 16, 617–626. [Google Scholar] [CrossRef]
- Taylor, P.C. Clinical efficacy of launched JAK inhibitors in rheumatoid arthritis. Rheumatology 2019, 58, i17–i26. [Google Scholar] [CrossRef] [PubMed]
- Winthrop, K.L. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat. Rev. Rheumatol. 2017, 13, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Manova, M.; Savova, A.; Vasileva, M.; Terezova, S.; Kamusheva, M.; Grekova, D.; Petkova, V.; Petrova, G. Comparative Price Analysis of Biological Products for Treatment of Rheumatoid Arthritis. Front. Pharmacol. 2018, 9, 1070. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewé, R.B.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 2023, 82, 3–18. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Williams, K.L.; Enslow, R.; Suresh, S.; Beaton, C.; Hodge, M.; Brooks, A.E. Using the Microbiome as a Regenerative Medicine Strategy for Autoimmune Diseases. Biomedicines 2023, 11, 1582. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Van Hul, M.; Cani, P.D.; Petitfils, C.; De Vos, W.M.; Tilg, H.; El-Omar, E.M. What defines a healthy gut microbiome? Gut 2024, 73, 1893–1908. [Google Scholar] [CrossRef]
- Bodkhe, R.; Balakrishnan, B.; Taneja, V. The role of microbiome in rheumatoid arthritis treatment. Ther. Adv. Musculoskelet. Dis. 2019, 11, 1759720X1984463. [Google Scholar] [CrossRef]
- Xu, X.; Wang, M.; Wang, Z.; Chen, Q.; Chen, X.; Xu, Y.; Dai, M.; Wu, B.; Li, Y. The bridge of the gut–joint axis: Gut microbial metabolites in rheumatoid arthritis. Front. Immunol. 2022, 13, 1007610. [Google Scholar] [CrossRef]
- Brusca, S.B.; Abramson, S.B.; Scher, J.U. Microbiome and mucosal inflammation as extra-articular triggers for rheumatoid arthritis and autoimmunity. Curr. Opin. Rheumatol. 2014, 26, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Dorożyńska, I.; Majewska-Szczepanik, M.; Marcińska, K.; Szczepanik, M. Partial depletion of natural gut flora by antibiotic aggravates collagen induced arthritis (CIA) in mice. Pharmacol. Rep. 2014, 66, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Breyner, N.M.; Michon, C.; de Sousa, C.S.; Vilas Boas, P.B.; Chain, F.; Azevedo, V.A.; Langella, P.; Chatel, J.M. Microbial Anti-Inflammatory Molecule (MAM) from Faecalibacterium prausnitzii Shows a Protective Effect on DNBS and DSS-Induced Colitis Model in Mice through Inhibition of NF-κB Pathway. Front. Microbiol. 2017, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Lee, A.R.; Kim, H.; Jhun, J.; Lee, S.Y.; Choi, J.W.; Jeong, Y.; Park, M.S.; Ji, G.E.; Cho, M.L.; et al. Faecalibacterium prausnitzii alleviates inflammatory arthritis and regulates IL-17 production, short chain fatty acids, and the intestinal microbial flora in experimental mouse model for rheumatoid arthritis. Arthritis Res. Ther. 2023, 25, 130. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, B.; Zhang, J.; Li, W.; Mou, F.; Wang, H.; Zou, Q.; Zhong, B.; Wu, L.; Wei, H.; et al. Role of the Gut Microbiome in Modulating Arthritis Progression in Mice. Sci. Rep. 2016, 6, 30594. [Google Scholar] [CrossRef]
- Rogier, R.; Evans-Marin, H.; Manasson, J.; van der Kraan, P.M.; Walgreen, B.; Helsen, M.M.; van den Bersselaar, L.A.; van de Loo, F.A.; Van Lent, P.L.; Abramson, S.B.; et al. Alteration of the intestinal microbiome characterizes preclinical inflammatory arthritis in mice and its modulation attenuates established arthritis. Sci. Rep. 2017, 7, 15613. [Google Scholar] [CrossRef]
- Maeda, Y.; Kurakawa, T.; Umemoto, E.; Motooka, D.; Ito, Y.; Gotoh, K.; Hirota, K.; Matsushita, M.; Furuta, Y.; Narazaki, M.; et al. Dysbiosis Contributes to Arthritis Development via Activation of Autoreactive T Cells in the Intestine. Arthritis Rheumatol. 2016, 68, 2646–2661. [Google Scholar] [CrossRef]
- Chen, J.; Wright, K.; Davis, J.M.; Jeraldo, P.; Marietta, E.V.; Murray, J.; Nelson, H.; Matteson, E.L.; Taneja, V. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016, 8, 43. [Google Scholar] [CrossRef]
- Chiang, H.I.; Li, J.R.; Liu, C.C.; Liu, P.Y.; Chen, H.H.; Chen, Y.M.; Lan, J.L.; Chen, D.Y. An Association of Gut Microbiota with Different Phenotypes in Chinese Patients with Rheumatoid Arthritis. J. Clin. Med. 2019, 8, 1770. [Google Scholar] [CrossRef]
- Kishikawa, T.; Maeda, Y.; Nii, T.; Motooka, D.; Matsumoto, Y.; Matsushita, M.; Matsuoka, H.; Yoshimura, M.; Kawada, S.; Teshigawara, S.; et al. Metagenome-wide association study of gut microbiome revealed novel aetiology of rheumatoid arthritis in the Japanese population. Ann. Rheum. Dis. 2020, 79, 103–111. [Google Scholar] [CrossRef]
- Ruiz-Limon, P.; Mena-Vázquez, N.; Moreno-Indias, I.; Manrique-Arija, S.; Lisbona-Montanez, J.M.; Cano-Garcia, L.; Tinahones, F.J. and Fernández-Nebro, A. Collinsella is associated with cumulative inflammatory burden in an established rheumatoid arthritis cohort. Biomed. Pharmacother. 2022, 153, 113518. [Google Scholar] [CrossRef] [PubMed]
- Mena-Vázquez, N.; Ruiz-Limón, P.; Moreno-Indias, I.; Manrique-Arija, S.; Tinahones, F.J.; Fernández-Nebro, A. Expansion of Rare and Harmful Lineages is Associated with Established Rheumatoid Arthritis. J. Clin. Med. 2020, 9, 1044. [Google Scholar] [CrossRef] [PubMed]
- Gul’neva, M.I.; Noskov, S.M. Colonic microbial biocenosis in rheumatoid arthritis. Klin Med. 2011, 89, 45–48. [Google Scholar]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B.; et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife 2013, 2, e01202. [Google Scholar] [CrossRef]
- Rooney, C.M.; Mankia, K.; Mitra, S.; Moura, I.B.; Emery, P.; Wilcox, M.H. Perturbations of the gut microbiome in anti-CCP positive individuals at risk of developing rheumatoid arthritis. Rheumatology 2021, 60, 3380–3387. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Jia, H.; Feng, Q.; Wang, D.; Liang, D.; Wu, X.; Li, J.; Tang, L.; Li, Y.; et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015, 21, 895–905. [Google Scholar] [CrossRef]
- Hamamoto, Y.; Ouhara, K.; Munenaga, S.; Shoji, M.; Ozawa, T.; Hisatsune, J.; Kado, I.; Kajiya, M.; Matsuda, S.; Kawai, T.; et al. Effect of Porphyromonas gingivalis infection on gut dysbiosis and resultant arthritis exacerbation in mouse model. Arthritis Res. Ther. 2020, 22, 249. [Google Scholar] [CrossRef]
- Arimatsu, K.; Yamada, H.; Miyazawa, H.; Minagawa, T.; Nakajima, M.; Ryder, M.I.; Gotoh, K.; Motooka, D.; Nakamura, S.; Iida, T.; et al. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci. Rep. 2014, 4, 4828. [Google Scholar] [CrossRef]
- Yamazaki, K. Oral-gut axis as a novel biological mechanism linking periodontal disease and systemic diseases: A review. Jpn. Dent. Sci. Rev. 2023, 59, 273–280. [Google Scholar] [CrossRef]
- Firestein, G.S.; McInnes, I.B. Immunopathogenesis of Rheumatoid Arthritis. Immunity 2017, 46, 183–196. [Google Scholar] [CrossRef]
- Tajik, N.; Frech, M.; Schulz, O.; Schälter, F.; Lucas, S.; Azizov, V.; Dürholz, K.; Steffen, F.; Omata, Y.; Rings, A.; et al. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat. Commun. 2020, 11, 1995. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Chairez, M.F.; Cid-Gallegos, M.S.; Jiménez-Martínez, C.; Prieto-Contreras, L.F.; Bollain-y-Goytia de-la-Rosa, J.J. The role of microbiota on rheumatoid arthritis onset. Int. J. Rheum. Dis. 2024, 27, e15122. [Google Scholar] [CrossRef] [PubMed]
- Brandl, C.; Bucci, L.; Schett, G.; Zaiss, M.M. Crossing the barriers: Revisiting the gut feeling in rheumatoid arthritis. Eur. J. Immunol. 2021, 51, 798–810. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, B.; Li, S.; Yang, L.; Zhu, D.; Wang, Y.; Wang, H.; Wang, T.; Shi, B.; Gai, Z.; et al. Detection and characterization of bacterial nucleic acids in culture-negative synovial tissue and fluid samples from rheumatoid arthritis or osteoarthritis patients. Sci. Rep. 2018, 8, 14305. [Google Scholar] [CrossRef]
- Chriswell, M.E.; Lefferts, A.R.; Clay, M.R.; Hsu, A.R.; Seifert, J.; Feser, M.L.; Rims, C.; Bloom, M.S.; Bemis, E.A.; Liu, S.; et al. Clonal IgA and IgG autoantibodies from individuals at risk for rheumatoid arthritis identify an arthritogenic strain of Subdoligranulum. Sci. Transl. Med. 2022, 14, eabn5166. [Google Scholar] [CrossRef]
- Rashid, T.; Ebringer, A. Autoimmunity in Rheumatic Diseases Is Induced by Microbial Infections via Crossreactivity or Molecular Mimicry. Autoimmune Dis. 2012, 2012, 539282. [Google Scholar] [CrossRef]
- Blank, M.; Barzilai, O.; Shoenfeld, Y. Molecular mimicry and auto-immunity. Clin. Rev. Allergy Immunol. 2007, 32, 111–118. [Google Scholar] [CrossRef]
- Alpizar-Rodriguez, D.; Lesker, T.R.; Gronow, A.; Gilbert, B.; Raemy, E.; Lamacchia, C.; Gabay, C.; Finckh, A.; Strowig, T. Prevotella copri in individuals at risk for rheumatoid arthritis. Ann. Rheum. Dis. 2019, 78, 590–593. [Google Scholar] [CrossRef]
- Pianta, A.; Arvikar, S.; Strle, K.; Drouin, E.E.; Wang, Q.; Costello, C.E.; Steere, A.C. Evidence of the Immune Relevance of Prevotella copri, a Gut Microbe, in Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 2017, 69, 964–975. [Google Scholar] [CrossRef]
- Pianta, A.; Arvikar, S.L.; Strle, K.; Drouin, E.E.; Wang, Q.; Costello, C.E.; Steere, A.C. Two rheumatoid arthritis–specific autoantigens correlate microbial immunity with autoimmune responses in joints. J. Clin. Investig. 2017, 127, 2946–2956. [Google Scholar] [CrossRef]
- Pianta, A.; Chiumento, G.; Ramsden, K.; Wang, Q.; Strle, K.; Arvikar, S.; Costello, C.E.; Steere, A.C. Identification of Novel, Immunogenic HLA–DR-Presented Prevotella copri Peptides in Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 2021, 73, 2200–2205. [Google Scholar] [CrossRef] [PubMed]
- Van Heemst, J.; Jansen, D.T.; Polydorides, S.; Moustakas, A.K.; Bax, M.; Feitsma, A.L.; Bontrop-Elferink, D.G.; Baarse, M.; Van Der Woude, D.; Wolbink, G.J.; et al. Crossreactivity to vinculin and microbes provides a molecular basis for HLA-based protection against rheumatoid arthritis. Nat. Commun. 2015, 6, 6681. [Google Scholar] [CrossRef] [PubMed]
- Bennike, T.B.; Ellingsen, T.; Glerup, H.; Bonderup, O.K.; Carlsen, T.G.; Meyer, M.K.; Bøgsted, M.; Christiansen, G.; Birkelund, S.; Andersen, V.; et al. Proteome Analysis of Rheumatoid Arthritis Gut Mucosa. J. Proteome Res. 2017, 16, 346–354. [Google Scholar] [CrossRef]
- Schmidt, T.S.; Hayward, M.R.; Coelho, L.P.; Li, S.S.; Costea, P.I.; Voigt, A.Y.; Wirbel, J.; Maistrenko, O.M.; Alves, R.J.; Bergsten, E.; et al. Extensive transmission of microbes along the gastrointestinal tract. Elife 2019, 8, e42693. [Google Scholar] [CrossRef] [PubMed]
- Iwauchi, M.; Horigome, A.; Ishikawa, K.; Mikuni, A.; Nakano, M.; Xiao, J.Z.; Odamaki, T.; Hironaka, S. Relationship between oral and gut microbiota in elderly people. Immun. Inflamm. Dis. 2019, 7, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, S.; Takeshita, T.; Asakawa, M.; Shibata, Y.; Takeuchi, K.; Yamanaka, W.; Yamashita, Y. Relative abundance of total subgingival plaque-specific bacteria in salivary microbiota reflects the overall periodontal condition in patients with periodontitis. PLoS ONE 2017, 12, e0174782. [Google Scholar] [CrossRef]
- Schwenzer, A.; Quirke, A.M.; Marzeda, A.M.; Wong, A.; Montgomery, A.B.; Sayles, H.R.; Eick, S.; Gawron, K.; Chomyszyn-Gajewska, M.; Łazarz-Bartyzel, K.; et al. Association of Distinct Fine Specificities of Anti−Citrullinated Peptide Antibodies With Elevated Immune Responses to Prevotella intermedia in a Subgroup of Patients With Rheumatoid Arthritis and Periodontitis. Arthritis Rheumatol. 2017, 69, 2303–2313. [Google Scholar] [CrossRef]
- Esberg, A.; Johansson, L.; Johansson, I.; Dahlqvist, S.R. Oral Microbiota Identifies Patients in Early Onset Rheumatoid Arthritis. Microorganisms 2021, 9, 1657. [Google Scholar] [CrossRef]
- Rosenstein, E.D.; Greenwald, R.A.; Kushner, L.J.; Weissmann, G. Hypothesis: The Humoral Immune Response to Oral Bacteria Provides a Stimulus for the Development of Rheumatoid Arthritis. Inflammation 2004, 28, 311–318. [Google Scholar] [CrossRef]
- Möller, B.; Kollert, F.; Sculean, A.; Villiger, P.M. Infectious Triggers in Periodontitis and the Gut in Rheumatoid Arthritis (RA): A Complex Story About Association and Causality. Front. Immunol. 2020, 11, 1108. [Google Scholar] [CrossRef]
- Fuggle, N.R.; Smith, T.O.; Kaul, A.; Sofat, N. Hand to Mouth: A Systematic Review and Meta-Analysis of the Association between Rheumatoid Arthritis and Periodontitis. Front. Immunol. 2016, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Bañuelos, E.; Mukherjee, A.; Darrah, E.; Andrade, F. Rheumatoid Arthritis-Associated Mechanisms of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. J. Clin. Med. 2019, 8, 1309. [Google Scholar] [CrossRef] [PubMed]
- Gabarrini, G.; de Smit, M.; Westra, J.; Brouwer, E.; Vissink, A.; Zhou, K.; Rossen, J.W.A.; Stobernack, T.; van Dijl, J.M.; Jan van Winkelhoff, A. The peptidylarginine deiminase gene is a conserved feature of Porphyromonas gingivalis. Sci. Rep. 2015, 5, 13936. [Google Scholar] [CrossRef]
- Wegner, N.; Wait, R.; Sroka, A.; Eick, S.; Nguyen, K.A.; Lundberg, K.; Kinloch, A.; Culshaw, S.; Potempa, J.; Venables, P.J. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and α-enolase: Implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 2010, 62, 2662–2672. [Google Scholar] [CrossRef]
- Konig, M.F.; Abusleme, L.; Reinholdt, J.; Palmer, R.J.; Teles, R.P.; Sampson, K.; Rosen, A.; Nigrovic, P.A.; Sokolove, J.; Giles, J.T.; et al. Aggregatibacter actinomycetemcomitans–induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci. Transl. Med. 2016, 8, 369ra176. [Google Scholar] [CrossRef]
- Brewer, R.C.; Lanz, T.V.; Hale, C.R.; Sepich-Poore, G.D.; Martino, C.; Swafford, A.D.; Carroll, T.S.; Kongpachith, S.; Blum, L.K.; Elliott, S.E.; et al. Oral mucosal breaks trigger anti-citrullinated bacterial and human protein antibody responses in rheumatoid arthritis. Sci. Transl. Med. 2023, 15, eabq8476. [Google Scholar] [CrossRef]
- Bright, R.; Thiele, G.M.; Manavis, J.; Mikuls, T.R.; Payne, J.B.; Bartold, P.M. Gingival tissue, an extrasynovial source of malondialdehyde-acetaldehyde adducts, citrullinated and carbamylated proteins. J. Periodontal Res. 2018, 53, 139–143. [Google Scholar] [CrossRef]
- Thiele, G.M.; Duryee, M.J.; Anderson, D.R.; Klassen, L.W.; Mohring, S.M.; Young, K.A.; Benissan-Messan, D.; Sayles, H.; Dusad, A.; Hunter, C.D.; et al. Malondialdehyde-Acetaldehyde Adducts and Anti–Malondialdehyde-Acetaldehyde Antibodies in Rheumatoid Arthritis. Arthritis Rheumatol. 2015, 67, 645–655. [Google Scholar] [CrossRef]
- Shacter, E. Quantification and Significance of Protein Oxidation in Biological Samples*. Drug Metab. Rev. 2000, 32, 307–326. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Tuma, D.J. Role of malondialdehyde-acetaldehyde adducts in liver injury1, 2. Free Radic. Biol. Med. 2002, 32, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Willis, M.S.; Klassen, L.W.; Tuma, D.J.; Thiele, G.M. Malondialdehyde–acetaldehyde-haptenated protein induces cell death by induction of necrosis and apoptosis in immune cells. Int. Immunopharmacol. 2002, 2, 519–535. [Google Scholar] [CrossRef] [PubMed]
- Thiele, G.M.; Tuma, D.J.; Willis, M.S.; Miller, J.A.; McDonald, T.L.; Sorrell, M.F.; Klassen, L.W. Soluble proteins modified with acetaldehyde and malondialdehyde are immunogenic in the absence of adjuvant. Alcohol. Clin. Exp. Res. 1998, 22, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Tuma, D.J.; Kearley, M.L.; Thiele, G.M.; Worrall, S.; Haver, A.; Klassen, L.W.; Sorrell, M.F. Elucidation of Reaction Scheme Describing Malondialdehyde−Acetaldehyde−Protein Adduct Formation. Chem. Res. Toxicol. 2001, 14, 822–832. [Google Scholar] [CrossRef]
- Lee, J.A.; Mikuls, T.R.; Sayles, H.R.; Thiele, G.M.; Duryee, M.J.; Payne, J.B. Associations between periodontitis and serum anti-malondialdehyde–acetaldehyde antibody concentrations in rheumatoid arthritis: A case-control study. J. Periodontol. 2024, 95, 929–941. [Google Scholar] [CrossRef]
- Ansaldo, E.; Farley, T.K.; Belkaid, Y. Control of Immunity by the Microbiota. Annu. Rev. Immunol. 2021, 39, 449–479. [Google Scholar] [CrossRef]
- Ceccarelli, F.; Orrù, G.; Pilloni, A.; Bartosiewicz, I.; Perricone, C.; Martino, E.; Lucchetti, R.; Fais, S.; Vomero, M.; Olivieri, M.; et al. Porphyromonas gingivalis in the tongue biofilm is associated with clinical outcome in rheumatoid arthritis patients. Clin. Exp. Immunol. 2018, 194, 244–252. [Google Scholar] [CrossRef]
- Sato, K.; Takahashi, N.; Kato, T.; Matsuda, Y.; Yokoji, M.; Yamada, M.; Nakajima, T.; Kondo, N.; Endo, N.; Yamamoto, R.; et al. Aggravation of collagen-induced arthritis by orally administered Porphyromonas gingivalis through modulation of the gut microbiota and gut immune system. Sci. Rep. 2017, 7, 6955. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, W.; Dai, K.; Liu, N.; Wang, J.; Lu, X.; Ma, J.; Zhang, M.; Xu, M.; Long, X.; et al. Inflammatory response of gut, spleen, and liver in mice induced by orally administered Porphyromonas gingivalis. J. Oral Microbiol. 2022, 14, 2088936. [Google Scholar] [CrossRef]
- An, L.; Wirth, U.; Koch, D.; Schirren, M.; Drefs, M.; Koliogiannis, D.; Nieß, H.; Andrassy, J.; Guba, M.; Bazhin, A.V.; et al. The Role of Gut-Derived Lipopolysaccharides and the Intestinal Barrier in Fatty Liver Diseases. J. Gastrointest. Surg. 2022, 26, 671–683. [Google Scholar] [CrossRef]
- Hong, M.; Li, Z.; Liu, H.; Zheng, S.; Zhang, F.; Zhu, J.; Shi, H.; Ye, H.; Chou, Z.; Gao, L.; et al. Fusobacterium nucleatum aggravates rheumatoid arthritis through FadA-containing outer membrane vesicles. Cell Host Microbe 2023, 31, 798–810.e7. [Google Scholar] [CrossRef] [PubMed]
- Seymour, B.J.; Allen, B.E.; Kuhn, K.A. Microbial Mechanisms of Rheumatoid Arthritis Pathogenesis. Curr. Rheumatol. Rep. 2024, 26, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Moulin, D.; Millard, M.; Taïeb, M.; Michaudel, C.; Aucouturier, A.; Lefèvre, A.; Bermúdez-Humarán, L.G.; Langella, P.; Sereme, Y.; Wanherdrick, K.; et al. Counteracting tryptophan metabolism alterations as a new therapeutic strategy for rheumatoid arthritis. Ann. Rheum. Dis. 2024, 83, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Seymour, B.J.; Trent, B.; Allen, B.E.; Berlinberg, A.J.; Tangchittsumran, J.; Jubair, W.K.; Chriswell, M.E.; Liu, S.; Ornelas, A.; Stahly, A.; et al. Microbiota-dependent indole production stimulates the development of collagen-induced arthritis in mice. J. Clin. Investig. 2023, 134, 4. [Google Scholar] [CrossRef] [PubMed]
- Golpour, F.; Abbasi-Alaei, M.; Babaei, F.; Mirzababaei, M.; Parvardeh, S.; Mohammadi, G.; Nassiri-Asl, M. Short chain fatty acids, a possible treatment option for autoimmune diseases. Biomed. Pharmacother. 2023, 163, 114763. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Assimakopoulos, S.F.; Triantos, C.; Thomopoulos, K.; Fligou, F.; Maroulis, I.; Marangos, M.; Gogos, C.A. Gut-origin sepsis in the critically ill patient: Pathophysiology and treatment. Infection 2018, 46, 751–760. [Google Scholar] [CrossRef]
- Siddiqui, M.T.; Cresci, G.A. The Immunomodulatory Functions of Butyrate. J. Inflamm. Res. 2021, 14, 6025–6041. [Google Scholar] [CrossRef]
- Lucas, S.; Omata, Y.; Hofmann, J.; Böttcher, M.; Iljazovic, A.; Sarter, K.; Albrecht, O.; Schulz, O.; Krishnacoumar, B.; Krönke, G.; et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018, 9, 55. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Larraufie, P.; Jarry, A.; Béguet-Crespel, F.; Marinelli, L.; Ledue, F.; Reimann, F.; Blottière, H.M.; Lapaque, N. Butyrate Produced by Commensal Bacteria Down-Regulates Indolamine 2,3-Dioxygenase 1 (IDO-1) Expression via a Dual Mechanism in Human Intestinal Epithelial Cells. Front. Immunol. 2018, 9, 2838. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, X.; Zheng, Y.; Zhang, M.; Fei, W.; Sun, D.; Zhao, M.; Ye, Y.; Zheng, C. Short-chain fatty acids regulate B cells differentiation via the FFA2 receptor to alleviate rheumatoid arthritis. Br. J. Pharmacol. 2022, 179, 4315–4329. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Kwon, J.E.; Lee, S.H.; Kim, E.K.; Ryu, J.G.; Jung, K.A.; Choi, J.W.; Park, M.J.; Moon, Y.M.; Park, S.H.; et al. Attenuation of Rheumatoid Inflammation by Sodium Butyrate Through Reciprocal Targeting of HDAC2 in Osteoclasts and HDAC8 in T Cells. Front. Immunol. 2018, 9, 1525. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dai, J.; Zhao, C.; Hu, T.; Zhao, G.; Wang, Q.; Zhang, L. Gut Subdoligranulum variabile ameliorates rheumatoid arthritis by promoting TSG-6 synthesis from joint cells. Front. Immunol. 2024, 15, 1418717. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA 2010, 107, 12204–12209. [Google Scholar] [CrossRef]
- Chasov, V.; Zmievskaya, E.; Ganeeva, I.; Gilyazova, E.; Davletshin, D.; Filimonova, M.; Valiullina, A.; Kudriaeva, A.; Bulatov, E. Systemic lupus erythematosus therapeutic strategy: From immunotherapy to gut microbiota modulation. J. Biomed. Res. 2024, 38, 1. [Google Scholar] [CrossRef]
- Berthelot, J.-M.; Lioté, F.; Sibilia, J. Methotrexate also improves rheumatoid arthritis through correction of microbiota dysbiosis. Jt. Bone Spine 2023, 90, 105602. [Google Scholar] [CrossRef]
- Bixio, R.; Bertelle, D.; Bertoldo, E.; Morciano, A.; Rossini, M. The potential pathogenic role of gut microbiota in rheumatic diseases: A human-centred narrative review. Intern. Emerg. Med. 2024, 19, 891–900. [Google Scholar] [CrossRef]
- Kumar, V.; Rana, A.; Jagtap, P.; Dhewa, T.; Taneja, N.K. Prebiotics, Probiotics and Synbiotics. In Nutritional Science and Technology: Concept to Application; Scrivener Publishing: Beverly, MA, USA, 2023; pp. 21–62. [Google Scholar] [CrossRef]
- Yang, Y.; Hong, Q.; Zhang, X.; Liu, Z. Rheumatoid arthritis and the intestinal microbiome: Probiotics as a potential therapy. Front. Immunol. 2024, 15, 1331486. [Google Scholar] [CrossRef]
- Stoidis, C.N.; Misiakos, E.P.; Patapis, P.; Fotiadis, C.I.; Spyropoulos, B.G. Potential benefits of pro- and prebiotics on intestinal mucosal immunity and intestinal barrier in short bowel syndrome. Nutr. Res. Rev. 2011, 24, 21–30. [Google Scholar] [CrossRef]
- Aqaeinezhad Rudbane, S.M.; Rahmdel, S.; Abdollahzadeh, S.M.; Zare, M.; Bazrafshan, A.; Mazloomi, S.M. The efficacy of probiotic supplementation in rheumatoid arthritis: A meta-analysis of randomized, controlled trials. Inflammopharmacology 2018, 26, 67–76. [Google Scholar] [CrossRef]
- Shan, L.; Chelliah, R.; Rahman, S.M.E.; Hwan, O.D. Unraveling the gut microbiota’s role in Rheumatoid arthritis: Dietary pathways to modulation and therapeutic potential. Crit. Rev. Food Sci. Nutr. 2024, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Alipour, B.; Homayouni-Rad, A.; Vaghef-Mehrabany, E.; Sharif, S.K.; Vaghef-Mehrabany, L.; Asghari-Jafarabadi, M.; Nakhjavani, M.R.; Mohtadi-Nia, J. Effects of Lactobacillus casei supplementation on disease activity and inflammatory cytokines in rheumatoid arthritis patients: A randomized double-blind clinical trial. Int. J. Rheum. Dis. 2014, 17, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Amdekar, S.; Singh, V.; Singh, R.; Sharma, P.; Keshav, P.; Kumar, A. Lactobacillus casei reduces the Inflammatory Joint Damage Associated with Collagen-Induced Arthritis (CIA) by Reducing the Pro-Inflammatory Cytokines. J. Clin. Immunol. 2011, 31, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Yang, B.; Ross, R.P.; Stanton, C.; Shi, G.; Zhao, J.; Zhang, H.; Chen, W. Protective effects of Bifidobacterium adolescentis on collagen-induced arthritis in rats depend on timing of administration. Food Funct. 2020, 11, 4499–4511. [Google Scholar] [CrossRef]
- Jeong, Y.; Jhun, J.; Lee, S.Y.; Na, H.S.; Choi, J.; Cho, K.H.; Lee, S.Y.; Lee, A.R.; Park, S.J.; You, H.J.; et al. Therapeutic Potential of a Novel Bifidobacterium Identified Through Microbiome Profiling of RA Patients With Different RF Levels. Front. Immunol. 2021, 12, 736196. [Google Scholar] [CrossRef]
- Zamani, B.; Golkar, H.R.; Farshbaf, S.; Emadi-Baygi, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akhavan, R.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z. Clinical and metabolic response to probiotic supplementation in patients with rheumatoid arthritis: A randomized, double-blind, placebo-controlled trial. Int. J. Rheum. Dis. 2016, 19, 869–879. [Google Scholar] [CrossRef]
- Marietta, E.V.; Murray, J.A.; Luckey, D.H.; Jeraldo, P.R.; Lamba, A.; Patel, R.; Luthra, H.S.; Mangalam, A.; Taneja, V. Suppression of Inflammatory Arthritis by Human Gut-Derived Prevotella histicola in Humanized Mice. Arthritis Rheumatol. 2016, 68, 2878–2888. [Google Scholar] [CrossRef]
- Cao, J.; Yu, Z.; Liu, W.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Probiotic characteristics of Bacillus coagulans and associated implications for human health and diseases. J. Funct. Foods 2020, 64, 103643. [Google Scholar] [CrossRef]
- Mandel, D.R.; Eichas, K.; Holmes, J. Bacillus coagulans: A viable adjunct therapy for relieving symptoms of rheumatoid arthritis according to a randomized, controlled trial. BMC Complement. Altern. Med. 2010, 10, 1. [Google Scholar] [CrossRef]
- Kang, Y.; Cai, Y.; Zhang, X.; Kong, X.; Su, J. Altered gut microbiota in RA: Implications for treatment. Z Rheumatol. 2017, 76, 451–457. [Google Scholar] [CrossRef]
- Abhari, K.; Shekarforoush, S.S.; Hosseinzadeh, S.; Nazifi, S.; Sajedianfard, J.; Eskandari, M.H. The effects of orally administered Bacillus coagulans and inulin on prevention and progression of rheumatoid arthritis in rats. Food Nutr. Res. 2016, 60, 30876. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chun, S.H.; Cheon, Y.H.; Kim, M.; Kim, H.O.; Lee, H.; Hong, S.T.; Park, S.J.; Park, M.S.; Suh, Y.S.; et al. Peptoniphilus gorbachii alleviates collagen-induced arthritis in mice by improving intestinal homeostasis and immune regulation. Front. Immunol. 2024, 14, 1286387. [Google Scholar] [CrossRef] [PubMed]
- Mitsou, E.K.; Kakali, A.; Antonopoulou, S.; Mountzouris, K.C.; Yannakoulia, M.; Panagiotakos, D.B.; Kyriacou, A. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br. J. Nutr. 2017, 117, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Häger, J.; Bang, H.; Hagen, M.; Frech, M.; Träger, P.; Sokolova, M.V.; Steffen, U.; Tascilar, K.; Sarter, K.; Schett, G.; et al. The Role of Dietary Fiber in Rheumatoid Arthritis Patients: A Feasibility Study. Nutrients 2019, 11, 2392. [Google Scholar] [CrossRef] [PubMed]
- De Santis, S.; Cavalcanti, E.; Mastronardi, M.; Jirillo, E.; Chieppa, M. Nutritional Keys for Intestinal Barrier Modulation. Front. Immunol. 2015, 6, 612. [Google Scholar] [CrossRef]
- Fasano, A. Zonulin and Its Regulation of Intestinal Barrier Function: The Biological Door to Inflammation, Autoimmunity, and Cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef]
- Guerreiro, C.S.; Calado, Â.; Sousa, J.; Fonseca, J.E. Diet, Microbiota, and Gut Permeability—The Unknown Triad in Rheumatoid Arthritis. Front. Med. 2018, 5, 349. [Google Scholar] [CrossRef]
- Mu, Q.; Kirby, J.; Reilly, C.M.; Luo, X.M. Leaky Gut As a Danger Signal for Autoimmune Diseases. Front. Immunol. 2017, 8, 598. [Google Scholar] [CrossRef]
- Liu, G.; Gu, K.; Wang, F.; Jia, G.; Zhao, H.; Chen, X.; Wu, C.; Zhang, R.; Tian, G.; Cai, J.; et al. Tryptophan Ameliorates Barrier Integrity and Alleviates the Inflammatory Response to Enterotoxigenic Escherichia coli K88 Through the CaSR/Rac1/PLC-γ1 Signaling Pathway in Porcine Intestinal Epithelial Cells. Front. Immunol. 2021, 12, 748497. [Google Scholar] [CrossRef]
- Shariatpanahi, Z.V.; Eslamian, G.; Ardehali, S.H.; Baghestani, A.-R. Effects of Early Enteral Glutamine Supplementation on Intestinal Permeability in Critically Ill Patients. Indian J. Crit. Care Med. 2019, 23, 356–362. [Google Scholar] [CrossRef]
- Finamore, A.; Massimi, M.; Conti Devirgiliis, L.; Mengheri, E. Zinc Deficiency Induces Membrane Barrier Damage and Increases Neutrophil Transmigration in Caco-2 Cells1. J. Nutr. 2008, 138, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhen, S.; Xu, H.; Sun, S.; Wang, Z.; Li, M.; Zou, L.; Zhang, Y.; Zhao, Y.; Cui, Y.; et al. Vitamin C alleviates rheumatoid arthritis by modulating gut microbiota balance. Biosci. Trends 2024, 18, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Graf, D.; Di Cagno, R.; Fåk, F.; Flint, H.J.; Nyman, M.; Saarela, M.; Watzl, B. Contribution of diet to the composition of the human gut microbiota. Microb. Ecol. Health Dis. 2015, 26, 26164. [Google Scholar] [CrossRef]
- Damms-Machado, A.; Louis, S.; Schnitzer, A.; Volynets, V.; Rings, A.; Basrai, M.; Bischoff, S.C. Gut permeability is related to body weight, fatty liver disease, and insulin resistance in obese individuals undergoing weight reduction. Am. J. Clin. Nutr. 2017, 105, 127–135. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Lu, B.; Solomon, D.H.; Costenbader, K.H.; Karlson, E.W. Alcohol Consumption and Risk of Incident Rheumatoid Arthritis in Women: A Prospective Study. Arthritis Rheumatol. 2014, 66, 1998–2005. [Google Scholar] [CrossRef]
- Källberg, H.; Jacobsen, S.; Bengtsson, C.; Pedersen, M.; Padyukov, L.; Garred, P.; Frisch, M.; Karlson, E.W.; Klareskog, L.; Alfredsson, L. Alcohol consumption is associated with decreased risk of rheumatoid arthritis: Results from two Scandinavian case–control studies. Ann. Rheum. Dis. 2009, 68, 222–227. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, Z.; Pan, P.; Zhao, Y.; Zhou, M.; Liu, L.; Zhai, Y.; Wang, H.; Xu, L.; Mei, D.; et al. Low-dose ethanol consumption inhibits neutrophil extracellular traps formation to alleviate rheumatoid arthritis. Commun. Biol. 2023, 6, 1088. [Google Scholar] [CrossRef]
- Fan, J.; Zhou, Y.; Meng, R.; Tang, J.; Zhu, J.; Aldrich, M.C.; Cox, N.J.; Zhu, Y.; Li, Y.; Zhou, D. Cross-talks between gut microbiota and tobacco smoking: A two-sample Mendelian randomization study. BMC Med. 2023, 21, 163. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liu, M.; Cheng, Y.; Wang, X.; Wang, W. Prevention and treatment of rheumatoid arthritis through traditional Chinese medicine: Role of the gut microbiota. Front. Immunol. 2023, 14, 1233994. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Ma, S.; Xu, X.; Zhang, B.; Cai, Q. Effects of rhizome of Atractylodes koreana (Nakai) Kitam on intestinal flora and metabolites in rats with rheumatoid arthritis. J. Ethnopharmacol. 2021, 281, 114026. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Tao, Y.; Fang, Y.; Lian, X.; Zhang, Q.; Xia, Y.; Wei, Z.; Dai, Y. The gut microbiota modulator berberine ameliorates collagen-induced arthritis in rats by facilitating the generation of butyrate and adjusting the intestinal hypoxia and nitrate supply. FASEB J. 2019, 33, 12311–12323. [Google Scholar] [CrossRef]
- Cheng, X.; Pi, Z.; Zheng, Z.; Liu, S.; Song, F.; Liu, Z. Combined 16S rRNA gene sequencing and metabolomics to investigate the protective effects of Wu-tou decoction on rheumatoid arthritis in rats. J. Chromatogr. B 2022, 1199, 123249. [Google Scholar] [CrossRef]
- Peng, J.; Lu, X.; Xie, K.; Xu, Y.; He, R.; Guo, L.; Han, Y.; Wu, S.; Dong, X.; Lu, Y.; et al. Dynamic Alterations in the Gut Microbiota of Collagen-Induced Arthritis Rats Following the Prolonged Administration of Total Glucosides of Paeony. Front. Cell Infect. Microbiol. 2019, 9, 204. [Google Scholar] [CrossRef]
- Hu, Q.; Wu, C.; Yu, J.; Luo, J.; Peng, X. Angelica sinensis polysaccharide improves rheumatoid arthritis by modifying the expression of intestinal Cldn5, Slit3 and Rgs18 through gut microbiota. Int. J. Biol. Macromol. 2022, 209, 153–161. [Google Scholar] [CrossRef]
- Zeng, W.; Shen, J.; Bo, T.; Peng, L.; Xu, H.; Nasser, M.I.; Zhuang, Q.; Zhao, M. Cutting Edge: Probiotics and Fecal Microbiota Transplantation in Immunomodulation. J. Immunol. Res. 2019, 2019, 1–17. [Google Scholar] [CrossRef]
- Yang, R.; Chen, Z.; Cai, J. Fecal microbiota transplantation: Emerging applications in autoimmune diseases. J. Autoimmun. 2023, 141, 103038. [Google Scholar] [CrossRef]
- Surawicz, C.M.; Brandt, L.J.; Binion, D.G.; Ananthakrishnan, A.N.; Curry, S.R.; Gilligan, P.H.; McFarland, L.V.; Mellow, M.; Zuckerbraun, B.S. Guidelines for Diagnosis, Treatment, and Prevention of Clostridium difficile Infections. Am. J. Gastroenterol. 2013, 108, 478–498. [Google Scholar] [CrossRef]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109.e6. [Google Scholar] [CrossRef] [PubMed]
- De Groot, P.; Nikolic, T.; Pellegrini, S.; Sordi, V.; Imangaliyev, S.; Rampanelli, E.; Hanssen, N.; Attaye, I.; Bakker, G.; Duinkerken, G.; et al. Faecal microbiota transplantation halts progression of human new-onset type 1 diabetes in a randomised controlled trial. Gut 2021, 70, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhu, Z.; Lin, X.; Li, H.; Wen, C.; Bao, J.; He, Z. Gut microbiota mediated the therapeutic efficacies and the side effects of prednisone in the treatment of MRL/lpr mice. Arthritis Res. Ther. 2021, 23, 240. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.M.; Gaudreau, M.-C.; Al-Gadban, M.M.; Gudi, R.; Vasu, C. Impact of dietary deviation on disease progression and gut microbiome composition in lupus-prone SNF1 mice. Clin. Exp. Immunol. 2015, 181, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Tiburcio, Á.Z. Improvement in a Patient with Active Systemic Lupus Erythematosous Treated with Transplant of Intestinal Microbiota. Gastroenterol. Med. Res. 2019, 3, 3. [Google Scholar] [CrossRef]
- Huang, C.; Yi, P.; Zhu, M.; Zhou, W.; Zhang, B.; Yi, X.; Long, H.; Zhang, G.; Wu, H.; Tsokos, G.C.; et al. Safety and efficacy of fecal microbiota transplantation for treatment of systemic lupus erythematosus: An EXPLORER trial. J. Autoimmun. 2022, 130, 102844. [Google Scholar] [CrossRef]
- Zeng, J.; Peng, L.; Zheng, W.; Huang, F.; Zhang, N.; Wu, D.; Yang, Y. Fecal microbiota transplantation for rheumatoid arthritis: A case report. Clin. Case Rep. 2021, 9, 906–909. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Kelly, C.R.; Mullish, B.H.; Allegretti, J.R.; Kassam, Z.; Putignani, L.; Fischer, M.; Keller, J.J.; Costello, S.P.; et al. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut 2019, 68, 2111–2121. [Google Scholar] [CrossRef]

| Object of Study | Healthy Control | Bacteria | Cytokine Levels | Additional Info | Ref. | |
|---|---|---|---|---|---|---|
| Increased | Decreased | |||||
| Murine models | ||||||
| CIA mice | + | Desulfovibrio Prevotella Parabacteroides Odoribacter Acetatifactor Blautia Coprococcus Ruminococcus | Enterorhabdus Myroides Rikenella Brochothrix Lactococcu Streptococcus | Antibiotic treatment worsened arthritis and increased levels of IL-6, IFN-γ and IL-17 | [45] | |
| CIA mice | Ruminococcaceae Lachnospiraceae Desulfovibrinocaceae | S24-7 Bacteroidaceae | IL-17 ↑ GM-CSF ↑ TNF-α ↑ IL-6 ↑ IL-8 ↑ | Elimination of the gut microbiota by antibiotics during established arthritis reduced gut Th17 cells and attenuated arthritis | [46] | |
| Human study | ||||||
| RA patients | + | Actinobacteria Collinsella Eggerthella Turicibacter Streptococcus | Faecalibacterium | IL-17A ↑ | Decreased bacterial diversity | [48] |
| RA patients with positive anti-CCP antibodies | + | Verrucomicrobiae Blautia Akkermansia Clostridiales | Lower diversity index of gut microbiota | [49] | ||
| High TNF-α and IL-17A levels | Enterobacteriaceae Klebsiella | Bifidobacterium | TNF-α ↑ IL-17A ↑ | |||
| ACPA-positive patients | Blautia Akkermansia Clostridiales | |||||
| RA patients | Prevotella denticola | [50] | ||||
| RA patients | + | Collinsella Bifidobacterium Enterococcus Sedimentibacter | Dorea Sarcina | IL-1β ↑ IL-6 ↑ TNF-α ↑ | A number of metabolic pathways, including fatty acid biosynthesis and glycosaminoglycan degradation, were enriched in the case–control study | [51] |
| RA patients | + | Collinsella aerofaciens Sedimentibacter Enterococcus | Sarcina 02d06 Porphyromonas Dorea formicigenerans | IL-17 ↑ IL-17A ↑ | Create an inflammatory imbalance and induce Th17 or Th1 responses | [52] |
| RA patients | Enterococci Clostridia Enterobacteria Staphylococci | Lactobacteria Bifidobacteria Bacteroids Lactopositive colibacteria | [53] | |||
| Patients up to 6 months after the diagnosis | + | Prevotella | Bacteroides | IL-17 ↑ | Molecular mimicry—molecular structures of a Prevotella copri resemble those of the host | [54] |
| Newly diagnosed RA with anti-CCP antibodies | + | Lachnospiraceae Helicobacteraceae Ruminococcaceae Erysipelotrichaceae Bifidobacteriaceae | [55] | |||
| Faecal, dental and salivary samples from RA patients | + | Lactobacillus salivarius | Haemophilus spp. | [56] | ||
| Both RF and ACPA positive | Haemophilus spp. | Similar findings were observed in their dental and salivary samples | ||||
| Higher inflammatory protein levels | Lactobacillus salivarius | Haemophilus spp. | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chasov, V.; Gilyazova, E.; Ganeeva, I.; Zmievskaya, E.; Davletshin, D.; Valiullina, A.; Bulatov, E. Gut Microbiota Modulation: A Novel Strategy for Rheumatoid Arthritis Therapy. Biomolecules 2024, 14, 1653. https://doi.org/10.3390/biom14121653
Chasov V, Gilyazova E, Ganeeva I, Zmievskaya E, Davletshin D, Valiullina A, Bulatov E. Gut Microbiota Modulation: A Novel Strategy for Rheumatoid Arthritis Therapy. Biomolecules. 2024; 14(12):1653. https://doi.org/10.3390/biom14121653
Chicago/Turabian StyleChasov, Vitaly, Elvina Gilyazova, Irina Ganeeva, Ekaterina Zmievskaya, Damir Davletshin, Aygul Valiullina, and Emil Bulatov. 2024. "Gut Microbiota Modulation: A Novel Strategy for Rheumatoid Arthritis Therapy" Biomolecules 14, no. 12: 1653. https://doi.org/10.3390/biom14121653
APA StyleChasov, V., Gilyazova, E., Ganeeva, I., Zmievskaya, E., Davletshin, D., Valiullina, A., & Bulatov, E. (2024). Gut Microbiota Modulation: A Novel Strategy for Rheumatoid Arthritis Therapy. Biomolecules, 14(12), 1653. https://doi.org/10.3390/biom14121653






