lncRNA Profiling of Exosomes and Its Communication Role in Regulating Silica-Stimulated Macrophage Apoptosis and Fibroblast Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Exosome Isolation
2.3. RNA Quantification and Qualification
2.4. Library Preparation for lncRNA Sequencing
2.5. Differentially Expressed lncRNAs Identification
2.6. LncRNA Target Gene Prediction and Functional Annotation
2.7. Cell Culture and Treatment
2.8. TUNEL Analysis
2.9. Western Blot
2.10. Real-Time Quantitative PCR (RT-qPCR) for lncRNA Measurement
2.11. Immunofluorescence
2.12. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Silicosis Patients and Healthy Controls
3.2. Identification of Differentially Expressed lncRNAs in Peripheral Blood Serum Exosomes of Silicosis Patients
3.3. Identification of Differentially Expressed lncRNAs Based on OPLS-DA
3.4. Prediction and Functional Annotation of lncRNA Targets
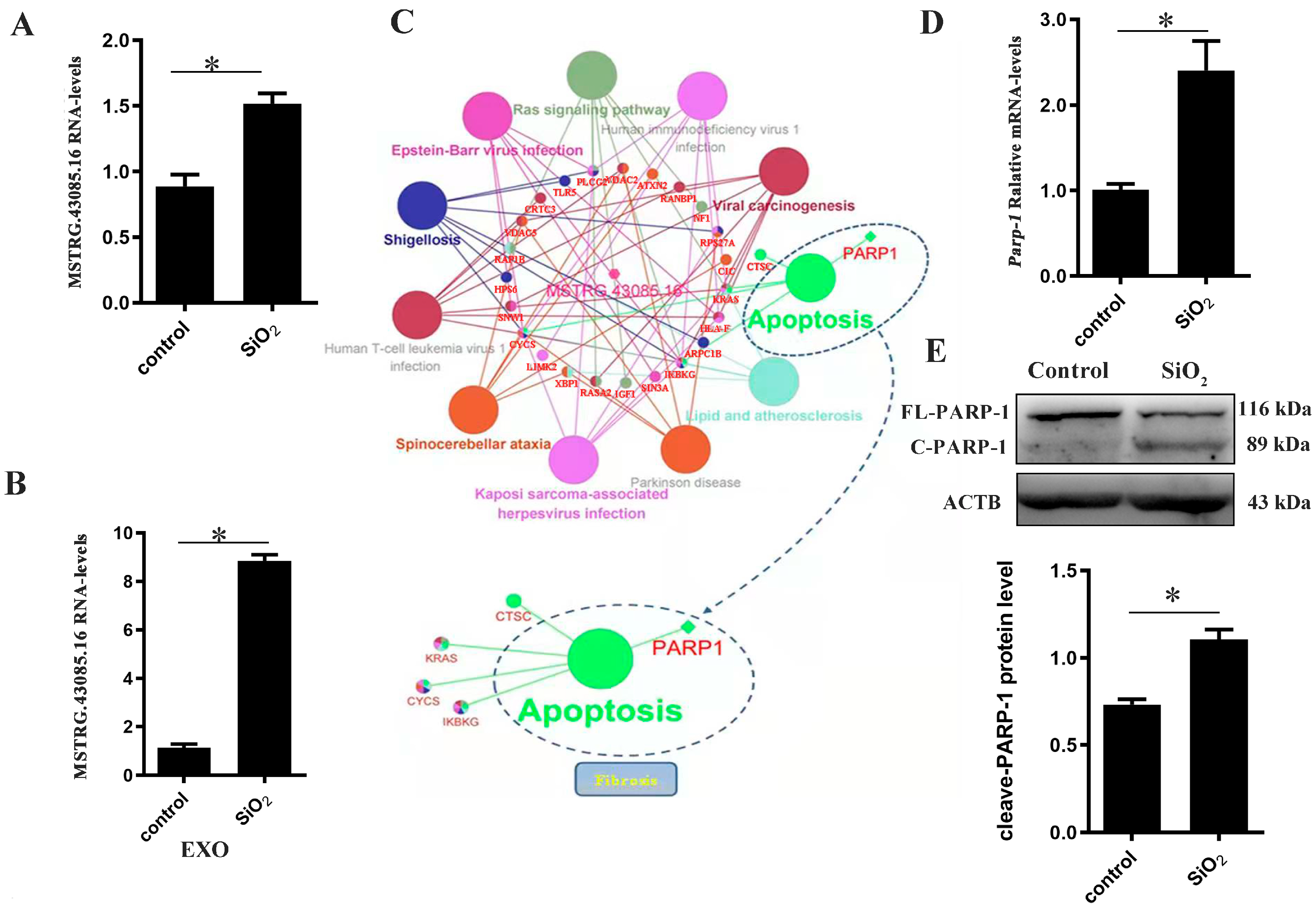
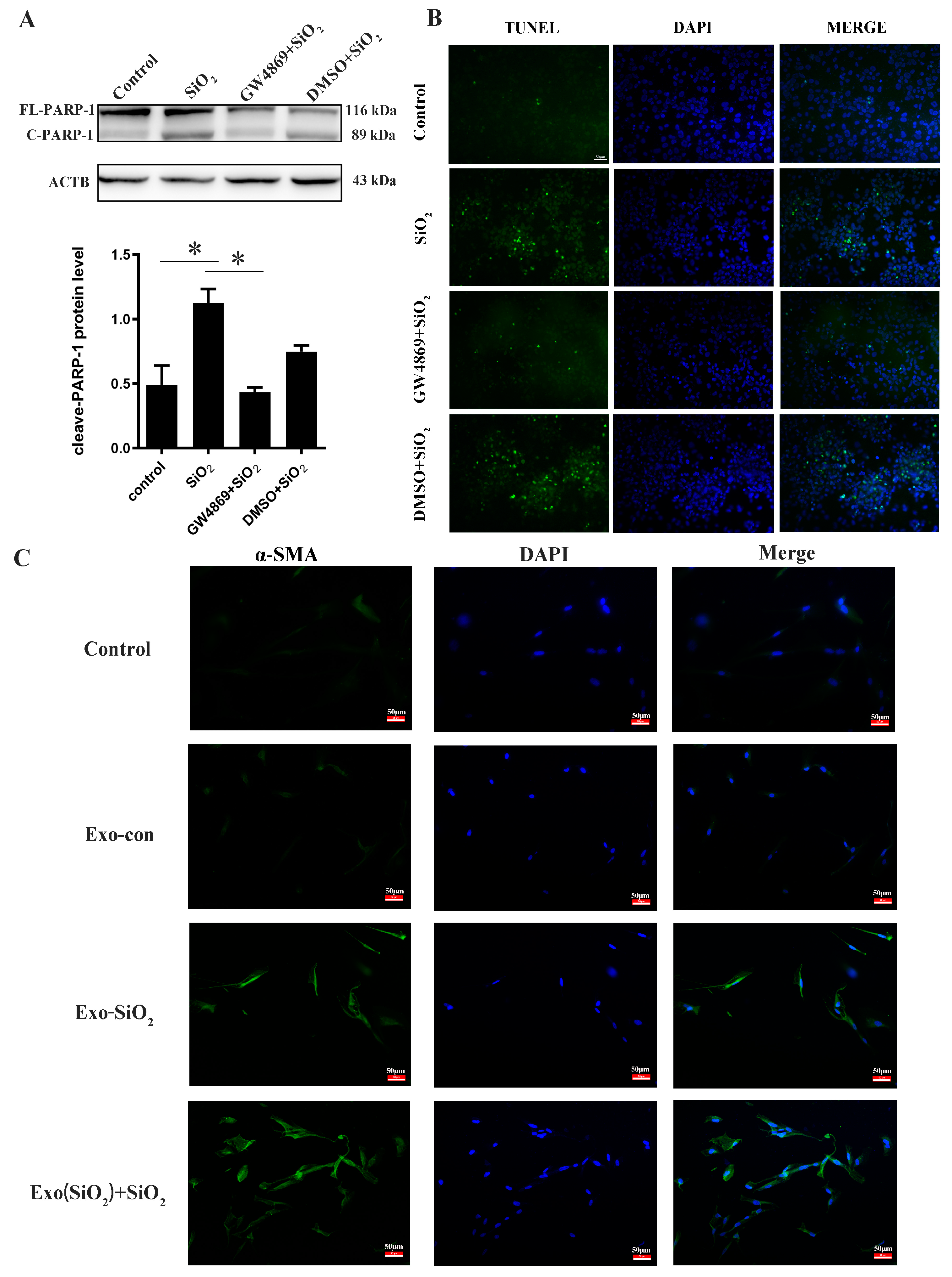
3.5. The Validation of lncRNA MSTRG.43085.16-PARP1 Regulatory Mechanism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leung, C.C.; Tak Sun Yu, I.; Chen, W. Silicosis. Lancet 2012, 379, 2008–2018. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Xing, X.; Xi, S.; Jing, H.; Yuan, J.; Fu, Z.; Zhao, H. Trends in Global, Regional and National Incidence of Pneumoconiosis Caused by Different Aetiologies: An Analysis from the Global Burden of Disease Study 2017. Occup. Environ. Med. 2020, 77, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.L.; Mazurek, J.M. Trends in Pneumoconiosis Deaths—United States, 1999–2018. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 693–698. [Google Scholar] [CrossRef]
- Yang, H.; Wu, Q.Y.; Li, M.Y.; Lao, C.S.; Zhang, Y.J. Pulmonary Toxicity in Rats Caused by Exposure to Intratracheal Instillation of SiO2 Nanoparticles. Biomed. Environ. Sci. 2017, 30, 264–279. [Google Scholar]
- Wang, J.; Zhou, F.; Mei, H.; Wang, Y.; Ma, H.; Shi, L.; Huang, A.; Zhang, T.; Lin, Z.; Wu, G. Pharmacological Targeting of Bet Proteins Attenuates Radiation-Induced Lung Fibrosis. Sci. Rep. 2018, 8, 998. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Li, C.; Lu, Y.; Lei, X.; Zhang, Y.; Li, S.; Liu, F.; Chen, Y.; Weng, D.; Chen, J. Dioscin Alleviates Crystalline Silica-Induced Pulmonary Inflammation and Fibrosis through Promoting Alveolar Macrophage Autophagy. Theranostics 2019, 9, 1878–1892. [Google Scholar] [CrossRef]
- Tan, S.; Chen, S. Macrophage Autophagy and Silicosis: Current Perspective and Latest Insights. Int. J. Mol. Sci. 2021, 22, 453. [Google Scholar] [CrossRef]
- Chen, S.; Tan, S.; Yang, S.; Chen, G.; Zhu, L.; Sun, Z.; Li, H.; Yao, S. Nicotine Induces Apoptosis through Exacerbation of Blocked Alveolar Macrophage Autophagic Degradation in Silicosis. Toxicol. Lett. 2020, 334, 94–101. [Google Scholar] [CrossRef]
- Li, S.; Xu, H.; Yi, X.; Niu, S.; Zhang, Q.; Xu, D.; Zhang, L.; Wei, Z.; Gao, X.; Cai, W.; et al. Ac-Sdkp Increases Alpha-Tat 1 and Promotes the Apoptosis in Lung Fibroblasts and Epithelial Cells Double-Stimulated with Tgf-Beta1 and Silica. Toxicol. Appl. Pharmacol. 2019, 369, 17–29. [Google Scholar]
- Wei, Z.; Xu, H.; Zhang, Y.; Yi, X.; Yang, X.; Chen, Y.; Mao, N.; Li, S.; Xu, D.; Li, S.; et al. Rho Gdp Dissociation Inhibitor Alpha Silencing Attenuates Silicosis by Inhibiting Rhoa/Rho Kinase Signalling. Exp. Cell Res. 2019, 380, 131–140. [Google Scholar] [CrossRef]
- Colombo, M.; Moita, C.; van Niel, G.; Kowal, J.; Vigneron, J.; Benaroch, P.; Manel, N.; Moita, L.F.; Théry, C.; Raposo, G. Analysis of Escrt Functions in Exosome Biogenesis, Composition and Secretion Highlights the Heterogeneity of Extracellular Vesicles. J. Cell Sci. 2013, 126 Pt 24, 5553–5565. [Google Scholar] [CrossRef]
- Kourembanas, S. Exosomes: Vehicles of Intercellular Signaling, Biomarkers, and Vectors of Cell Therapy. Annu. Rev. Physiol. 2015, 77, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, Y.; Wang, H.; Zhu, Z.; Xiao, Z. Visualizing of the Cellular Uptake and Intracellular Trafficking of Exosomes by Live-Cell Microscopy. J. Cell. Biochem. 2010, 111, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Xu, L.; Xu, X.; Qin, Z.; Zhou, X.; Xiao, Y.; Liang, Y.; Xia, J. Exosome-Mediated Delivery of Gene Vectors for Gene Therapy. Nanoscale 2021, 13, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, B.; Zhang, A.; Hassounah, F.; Seow, Y.; Wood, M.; Ma, F.; Klein, J.D.; Price, S.R.; Wang, X.H. Exosome-Mediated Mir-29 Transfer Reduces Muscle Atrophy and Kidney Fibrosis in Mice. Mol. Ther. 2019, 27, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Li, J.; Huang, B.; Liu, J.; Chen, X.; Chen, X.-M.; Xu, Y.-M.; Huang, L.-F.; Wang, X.-Z. Exosomes: Novel Biomarkers for Clinical Diagnosis. Sci. World J. 2015, 2015, 657086. [Google Scholar] [CrossRef] [PubMed]
- Kubo, H. Extracellular Vesicles in Lung Disease. Chest 2018, 153, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, J.; Xu, B.; Liu, Y.; Liu, Z. Reduced Exosome Mir-425 and Mir-744 in the Plasma Represents the Progression of Fibrosis and Heart Failure. Kaohsiung J. Med. Sci. 2018, 34, 626–633. [Google Scholar] [CrossRef]
- Chen, H.; Gan, X.; Li, Y.; Gu, J.; Liu, Y.; Deng, Y.; Wang, X.; Hong, Y.; Hu, Y.; Su, L.; et al. Nlrp12- and Nlrc4-Mediated Corneal Epithelial Pyroptosis Is Driven by Gsdmd Cleavage Accompanied by Il-33 Processing in Dry Eye. Ocul. Surf. 2020, 18, 783–794. [Google Scholar] [CrossRef]
- Wang, D.; Hao, C.; Zhang, L.; Zhang, J.; Liu, S.; Li, Y.; Qu, Y.; Zhao, Y.; Huang, R.; Wei, J.; et al. Exosomal Mir-125a-5p Derived from Silica-Exposed Macrophages Induces Fibroblast Transdifferentiation. Ecotoxicol. Environ. Saf. 2020, 192, 110253. [Google Scholar] [CrossRef]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding Rnas. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Barres, C.; Blanc, L.; Bette-Bobillo, P.; André, S.; Mamoun, R.; Gabius, H.-J.; Vidal, M. Galectin-5 Is Bound onto the Surface of Rat Reticulocyte Exosomes and Modulates Vesicle Uptake by Macrophages. Blood 2010, 115, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Skog, J.; Wurdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Sena-Esteves, M.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma Microvesicles Transport Rna and Proteins That Promote Tumour Growth and Provide Diagnostic Biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Cai, X.; Qian, Q.; Peng, W.; Yu, J.; Zhang, X.; Tian, L.; Wang, C. Lncrna Zeb1-As1 Promotes Pulmonary Fibrosis through Zeb1-Mediated Epithelial-Mesenchymal Transition by Competitively Binding Mir-141-3p. Cell Death Dis. 2019, 10, 129. [Google Scholar] [CrossRef]
- Zhang, K.; Han, X.; Zhang, Z.; Zheng, L.; Hu, Z.; Yao, Q.; Cui, H.; Shu, G.; Si, M.; Li, C.; et al. The Liver-Enriched Lnc-Lfar1 Promotes Liver Fibrosis by Activating Tgfbeta and Notch Pathways. Nat. Commun. 2017, 8, 144. [Google Scholar] [CrossRef]
- Chen, L.; Brigstock, D.R. Integrins and Heparan Sulfate Proteoglycans on Hepatic Stellate Cells (Hsc) Are Novel Receptors for Hsc-Derived Exosomes. FEBS Lett. 2016, 590, 4263–4274. [Google Scholar] [CrossRef]
- Zhang, Q.; Ban, J.; Chang, S.; Qu, H.; Chen, J.; Liu, F. The Aggravate Role of Exosomal Circrna11:120406118|12040782 on Macrophage Pyroptosis through Mir-30b-5p/Nlrp3 Axis in Silica-Induced Lung Fibrosis. Int. Immunopharmacol. 2023, 114, 109476. [Google Scholar] [CrossRef]
- Yang, G.; Yang, Y.; Liu, Y.; Liu, X. Regulation of Alveolar Macrophage Death in Pulmonary Fibrosis: A Review. Apoptosis 2023, 28, 1505–1519. [Google Scholar] [CrossRef]
- Kraus, A.J.; Brink, B.G.; Siegel, T.N. Efficient and Specific Oligo-Based Depletion of Rrna. Sci. Rep. 2019, 9, 12281. [Google Scholar] [CrossRef]
- Zhou, Y.; Cras-Méneur, C.; Ohsugi, M.; Stormo, G.D.; Permutt, M.A. A Global Approach to Identify Differentially Expressed Genes in Cdna (Two-Color) Microarray Experiments. Bioinformatics 2007, 23, 2073–2079. [Google Scholar] [CrossRef]
- Troyanskaya, O.G.; Garber, M.E.; Brown, P.O.; Botstein, D.; Altman, R.B. Nonparametric Methods for Identifying Differentially Expressed Genes in Microarray Data. Bioinformatics 2002, 18, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Shao, F.; Duan, W.; Zhao, Y.; Chen, F. Variance Component Testing for Identifying Differentially Expressed Genes in Rna-Seq Data. PeerJ 2017, 5, e3797. [Google Scholar] [CrossRef][Green Version]
- Yumba-Mpanga, A.; Struck-Lewicka, W.; Wawrzyniak, R.; Markuszewski, M.; Roslan, M.; Kaliszan, R.; Markuszewski, M.J. Metabolomic Heterogeneity of Urogenital Tract Cancers Analyzed by Complementary Chromatographic Techniques Coupled with Mass Spectrometry. Curr. Med. Chem. 2019, 26, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hao, C.; Yao, S.; Tang, R.; Guo, W.; Cong, H.; Li, J.; Bao, L.; Wang, D.; Li, Y.; et al. Exosomal Mirna Profiling to Identify Nanoparticle Phagocytic Mechanisms. Small 2018, 14, e1704008. [Google Scholar] [CrossRef] [PubMed]
- Pua, H.H.; Happ, H.C.; Gray, C.J.; Mar, D.J.; Chiou, N.-T.; Hesse, L.E.; Ansel, K.M. Increased Hematopoietic Extracellular Rnas and Vesicles in the Lung During Allergic Airway Responses. Cell Rep. 2019, 26, 933–944.e4. [Google Scholar] [CrossRef] [PubMed]
- Dentinger, B.T.M.; Margaritescu, S.; Moncalvo, J. Rapid and Reliable High-Throughput Methods of DNA Extraction for Use in Barcoding and Molecular Systematics of Mushrooms. Mol. Ecol. Resour. 2010, 10, 628–633. [Google Scholar] [CrossRef]
- Kang, J.-Y.; Mun, D.; Kim, H.; Yun, N.; Joung, B. Serum Exosomal Long Noncoding Rnas as a Diagnostic Biomarker for Atrial Fibrillation. Heart Rhythm. 2022, 19, 1450–1458. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, B.; Zhou, Z.; Huang, H.; Yu, C.; Wang, X.; Xu, C.; Gao, Y.; Chen, S. Extracellular Vesicles-Transmitted Long Non-Coding Rna Mtus2-5 Promotes Proliferation and Vascularization of Human Vascular Endothelial Cells in Patients with Budd–Chiari Syndrome. J. Cell. Mol. Med. 2023, 27, 3431–3442. [Google Scholar] [CrossRef]
- Huang, R.; Yu, T.; Li, Y.; Hu, J. Upregulated Has-Mir-4516 as a Potential Biomarker for Early Diagnosis of Dust-Induced Pulmonary Fibrosis in Patients with Pneumoconiosis. Toxicol. Res. Camb. 2018, 7, 415–422. [Google Scholar] [CrossRef]
- Maru, B.; Messikommer, A.; Huang, L.; Seipel, K.; Kovecses, O.; Valk, P.J.; Theocharides, A.P.; Mercier, F.E.; Pabst, T.; McKeague, M.; et al. Parp-1 Improves Leukemia Outcomes by Inducing Parthanatos During Chemotherapy. Cell Rep. Med. 2023, 4, 101191. [Google Scholar] [CrossRef]
- Al Sabaani, N. Kaempferol Protects against Hydrogen Peroxide-Induced Retinal Pigment Epithelium Cell Inflammation and Apoptosis by Activation of Sirt1 and Inhibition of Parp1. J. Ocul. Pharmacol. Ther. 2020, 36, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Savelyev, N.V.; Shepelev, N.M.; Lavrik, O.I.; Rubtsova, M.P.; Dontsova, O.A. Parp1 Regulates the Biogenesis and Activity of Telomerase Complex through Modification of H/Aca-Proteins. Front. Cell Dev. Biol. 2021, 9, 621134. [Google Scholar] [CrossRef] [PubMed]
- Katabami, M.; Kinoshita, I.; Ariga, S.; Shimizu, Y.; Dosaka-Akita, H. Crystalline Silica-Exposed Human Lung Epithelial Cells Presented Enhanced Anchorage-Independent Growth with Upregulated Expression of Brd4 and Ezh2 in Autocrine and Paracrine Manners. PLoS ONE 2023, 18, e0285354. [Google Scholar] [CrossRef] [PubMed]
- Ban, J.; Liu, F.; Zhang, Q.; Chang, S.; Zeng, X.; Chen, J. Macrophage-Derived Exosomal Lncrna Mstrg.91634.7 Inhibits Fibroblasts Activation by Targeting Pink1 in Silica-Induced Lung Fibrosis. Toxicol. Lett. 2023, 372, 36–44. [Google Scholar] [CrossRef]
- Ban, J.; Zhang, Q.; Chang, S.; Qu, H.; Liu, F. The Therapeutic Effect of Exosomal Lncrna Mstrg.91634.7 on Mitochondrial Dysfunction During Sio2-Induced Lung Fibrosis. Int. Immunopharmacol. 2023, 121, 110508. [Google Scholar] [CrossRef]
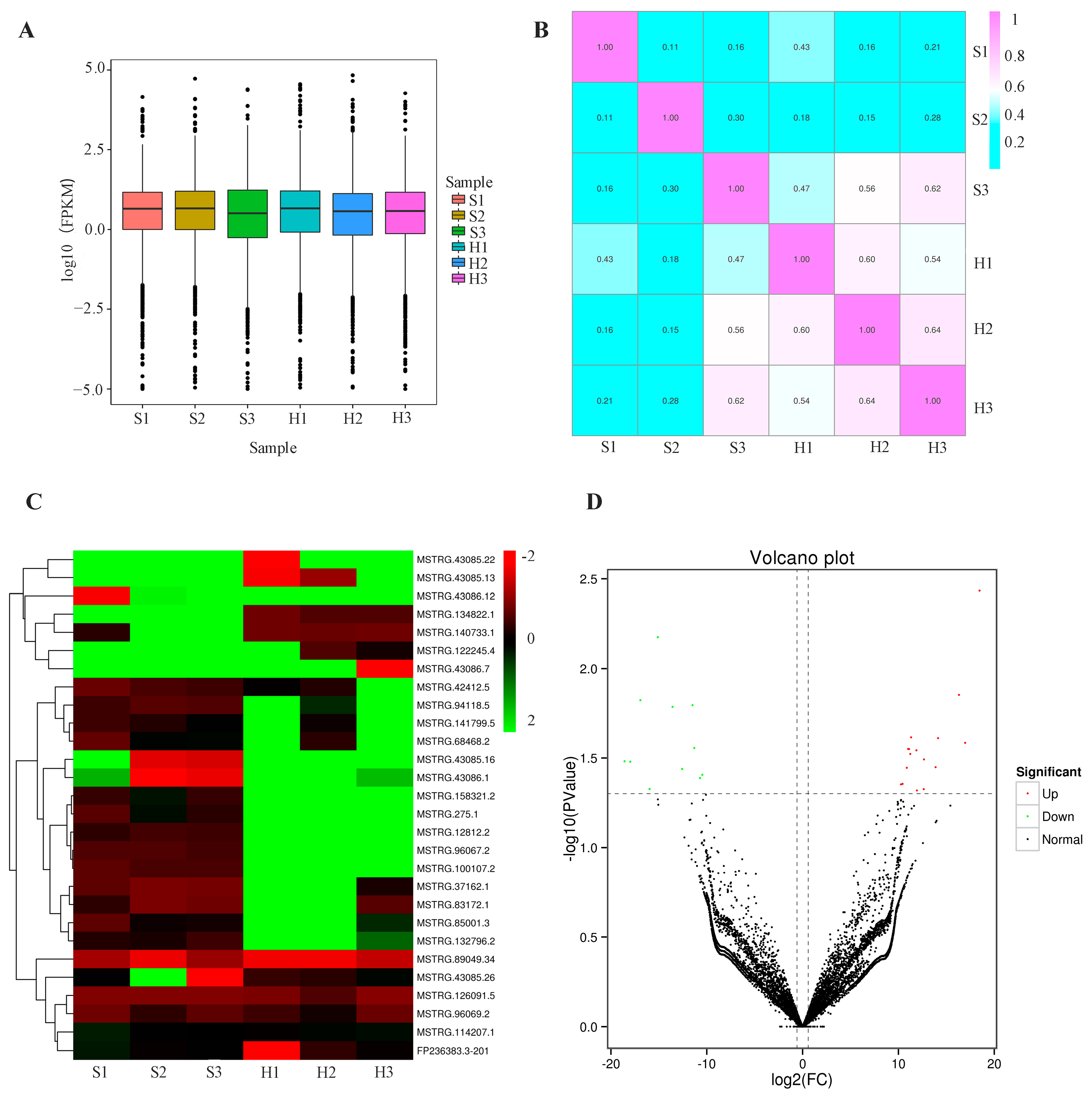
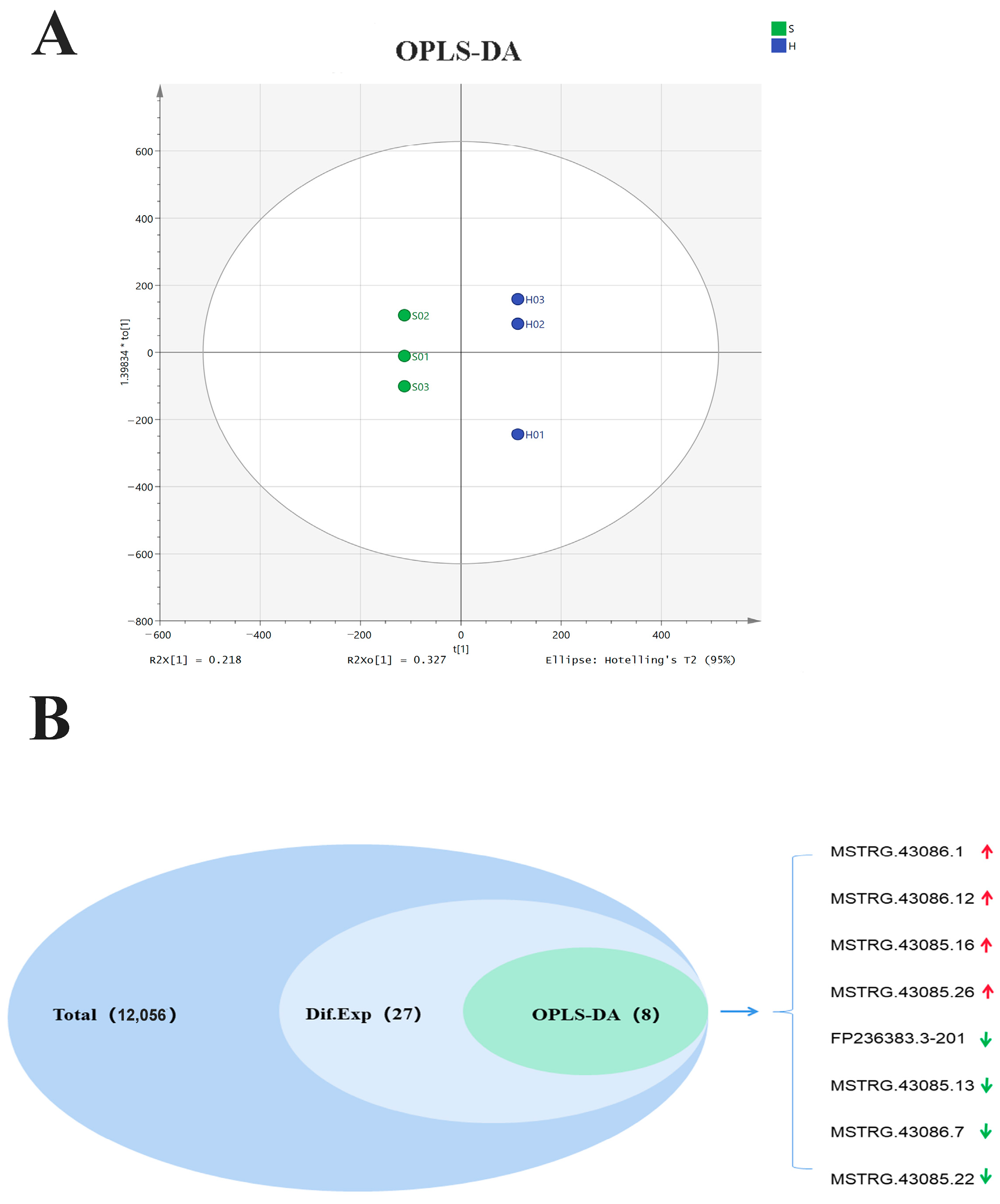
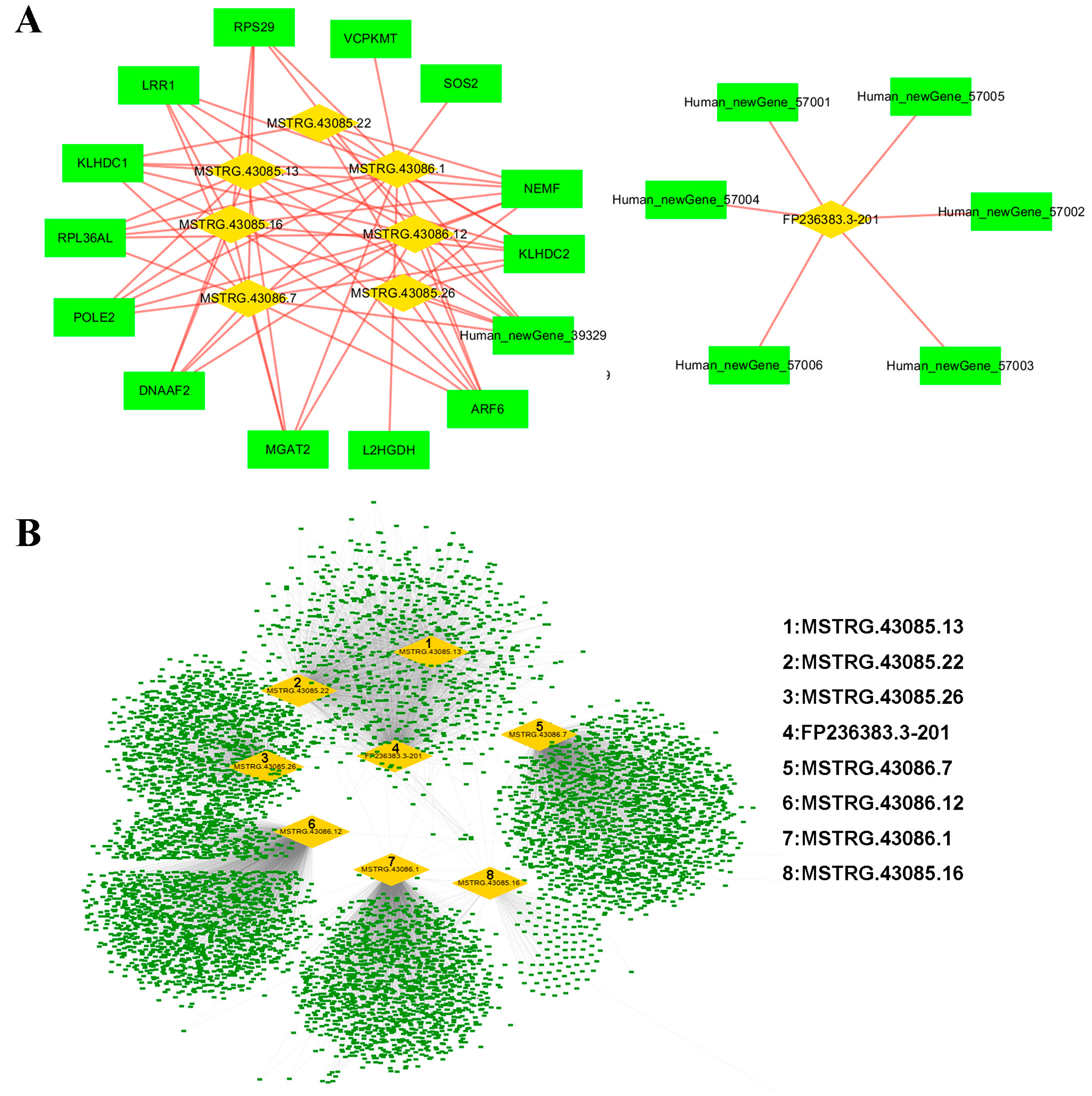
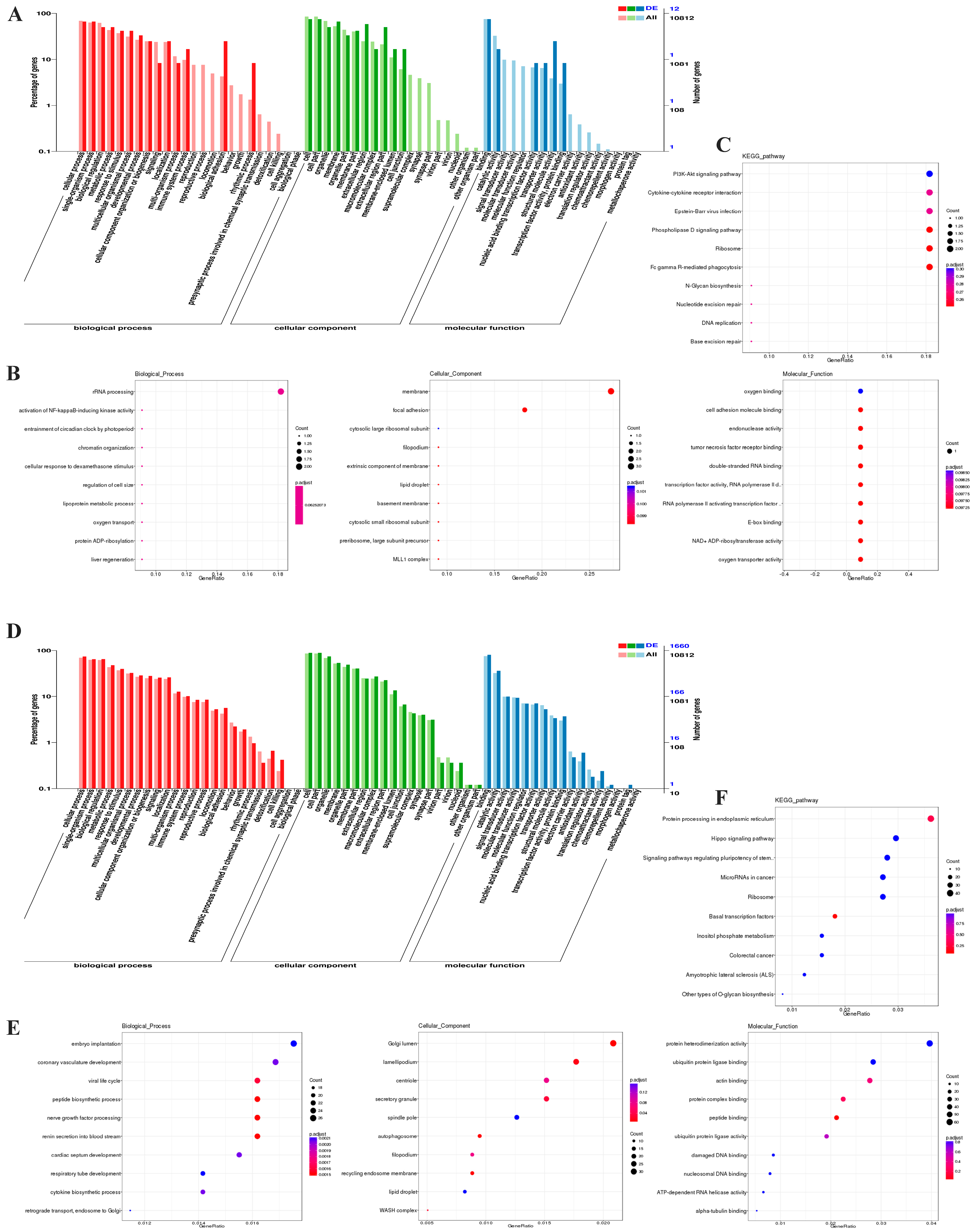
| Variables | Patient | Control |
|---|---|---|
| N | 20 | 29 |
| Age a | 54.7 ± 2.4 | 54.7 ± 2.6 |
| Accumulated time of exposure silica particle (years) b | 26.2 ± 4.3 | 32.3 ± 7.3 |
| Stage I | 20 | — |
| Gene ID | Phenotype | p-Value | log2FC a | Regulated |
|---|---|---|---|---|
| MSTRG.43086.12 | Predicted | 0.026033861 | 16.96111063 | up |
| MSTRG.43085.16 | Predicted | 0.014004392 | 16.31897018 | up |
| MSTRG.43086.7 | Predicted | 0.033170095 | −17.98422883 | down |
| MSTRG.43086.1 | Predicted | 0.003682449 | 18.46426007 | up |
| MSTRG.43085.22 | Predicted | 0.033005847 | −18.57420519 | down |
| MSTRG.43085.26 | Predicted | 0.032206632 | 12.66049827 | up |
| MSTRG.43085.13 | Predicted | 0.014999992 | −16.92435452 | down |
| FP236383.3-201 | Predicted | 0.006676377 | −15.10674163 | down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ban, J.; Chang, S.; Ma, P.; Wang, X.; Liu, F. lncRNA Profiling of Exosomes and Its Communication Role in Regulating Silica-Stimulated Macrophage Apoptosis and Fibroblast Activation. Biomolecules 2024, 14, 146. https://doi.org/10.3390/biom14020146
Ban J, Chang S, Ma P, Wang X, Liu F. lncRNA Profiling of Exosomes and Its Communication Role in Regulating Silica-Stimulated Macrophage Apoptosis and Fibroblast Activation. Biomolecules. 2024; 14(2):146. https://doi.org/10.3390/biom14020146
Chicago/Turabian StyleBan, Jiaqi, Shuai Chang, Pengwei Ma, Xin Wang, and Fangwei Liu. 2024. "lncRNA Profiling of Exosomes and Its Communication Role in Regulating Silica-Stimulated Macrophage Apoptosis and Fibroblast Activation" Biomolecules 14, no. 2: 146. https://doi.org/10.3390/biom14020146
APA StyleBan, J., Chang, S., Ma, P., Wang, X., & Liu, F. (2024). lncRNA Profiling of Exosomes and Its Communication Role in Regulating Silica-Stimulated Macrophage Apoptosis and Fibroblast Activation. Biomolecules, 14(2), 146. https://doi.org/10.3390/biom14020146





