Abstract
The regulation of plant biomass degradation by fungi is critical to the carbon cycle, and applications in bioproducts and biocontrol. Trichoderma harzianum is an important plant biomass degrader, enzyme producer, and biocontrol agent, but few putative major transcriptional regulators have been deleted in this species. The T. harzianum ortholog of the transcriptional activator XYR1/XlnR/XLR-1 was deleted, and the mutant strains were analyzed through growth profiling, enzymatic activities, and transcriptomics on cellulose. From plate cultures, the Δxyr1 mutant had reduced growth on D-xylose, xylan, and cellulose, and from shake-flask cultures with cellulose, the Δxyr1 mutant had ~90% lower β-glucosidase activity, and no detectable β-xylosidase or cellulase activity. The comparison of the transcriptomes from 18 h shake-flask cultures on D-fructose, without a carbon source, and cellulose, showed major effects of XYR1 deletion whereby the Δxyr1 mutant on cellulose was transcriptionally most similar to the cultures without a carbon source. The cellulose induced 43 plant biomass-degrading CAZymes including xylanases as well as cellulases, and most of these had massively lower expression in the Δxyr1 mutant. The expression of a subset of carbon catabolic enzymes, other transcription factors, and sugar transporters was also lower in the Δxyr1 mutant on cellulose. In summary, T. harzianum XYR1 is the master regulator of cellulases and xylanases, as well as regulating carbon catabolic enzymes.
1. Introduction
The Trichoderma genus includes important biocontrol agents and plant growth-promoting filamentous fungi, as well as saprotrophic fungi that are critical to nutrient cycles [1]. Trichoderma is found in diverse habitats such as soil, water, and the rhizosphere of plants [2]. The efficient degradation of plant biomass by Trichoderma species relies on the production of a diverse range of plant biomass-degrading enzymes, such as cellulases and hemicellulases, and requires a gene regulation system that can tightly control the expression of the relevant genes to optimize enzyme production under appropriate conditions [3,4]. Trichoderma species are known to be highly efficient producers of plant biomass-degrading enzymes such as cellulases, making them a valuable source for industrial applications such as plant biomass conversion and biofuel production. In particular, cellulase enzymes play a crucial role in the breakdown of cellulose, one of the most abundant polysaccharides on Earth. Trichoderma harzianum is one of the most important Trichoderma species, and is recognized for its significant potential in various biotechnological applications, such as plant disease biocontrol, plant growth promotion [5], and plant biomass-degrading enzyme production [6].
The transcriptional regulation of plant biomass-degrading enzymes in filamentous fungi is a complex process involving a range of activating and repressing transcription factors that are signaled to via a range of environmental signals, with inducing sugars derived from plant biomass being the most prominent environmental signals [3,4,7]. Within ascomycete fungi, there are lineage-specific transcription factors as well as more taxonomically broad conserved transcription factors, and a further layer of complexity is added by the variation in the range of enzymes activated by orthologous transcription factors. E.g., XYR1/XlnR/XLR-1 is one of the major transcriptional activators of plant biomass-degrading enzymes, and can activate cellulase and xylanase gene expression in fungi such as Aspergillus niger [8] and Trichoderma reesei [9], and XYR1/XlnR can in effect act as a master regulator. In contrast, in another ascomycete, Neurospora crassa, the activation is limited mainly to xylanase gene expression [10]. As well as XYR1/XlnR/XLR-1, other transcriptional activators of (hemi-)cellulose expression include ACE2 [11], ACE3 [12], and CLR-2 [13]. The transcription factor XPP1 has been identified as a xylanase but not a cellulase regulator in T. reesei [14]. Other regulators activate a narrower range of enzyme activities, such as the arabinose- and galactose-related ARA1 transcription factor [15]. As well as activators, there can be repressors that can be either wide-domain-acting, such as the carbon catabolite repressor CRE1/CreA [16], or plant biomass-degrading enzyme-specific transcriptional repressors such as ACE1 [17] and RCE1 [18]. As well as transcription factors, the regulation of xylanase and cellulase expression is related to other factors such as how in T. reesei, the packaging of chromatin is an important factor [19].
Within the Trichoderma genus, there are only three species where xyr1 has been deleted, and these are T. reesei (Section Longibrachiatum), T. atroviride (Section Trichoderma), and T. cf. guizhouense (Harzianum/Virens clade). In T. reesei, XYR1 is a transcription factor that acts as a master regulator, controlling the expression of cellulases and hemicellulases in response to the presence of cellulose and hemicellulose substrates. In T. reesei QM9414, the deletion of xyr1 led to a lower expression of cellulases and xylanases on crude plant biomass substrates [20] and Avicel cellulose [21]. Similarly, in T. reesei RUT-C30, the deletion of xyr1 led to a lower expression of most cellulases and hemicellulases, and many sugar transporters, when cultured on a mixture of Avicel cellulose and wheat bran [22]. In the T. reesei RUT-C30 Δxyr1 mutant, other genes had an increased expression related to a suggested starvation stress response due to the inability of the Δxyr1 mutant to obtain carbon from the complex plant biomass substrates [22]. As well as regulating the plant biomass-degrading enzymes, T. reesei XYR1 can also regulate genes involved in the catabolism of D-xylose, as evidenced by the dramatically reduced growth of the T. reesei QM9414 Δxyr1 mutant on D-xylose [9]. In T. reesei QM9414, XYR1 is also involved in co-regulation with other transcription factors; e.g., ARA1 and XYR1 co-regulate the transcriptional response to L-arabinose [15]. In the Trichoderma cf. guizhouense strain NJAU4742, XYR1 appeared to be the main regulator of cellulases and xylanases, although the induction pattern of cellulases and xylanases varied depending on whether Avicel cellulose or xylan was used as the inducer [23]. Although extensive research has elucidated the role of xyr1 in cellulase and hemicellulase production in T. reesei, and more recently in T. cf. guizhouense, there are no reports of the regulon of XYR1 in the Trichoderma atroviride P1 strain Δxyr1 mutant, as the study focused on other aspects of XYR1 function [24]. There are also no reports of the deletion of xyr1 in T. harzianum.
Previously, xyr1 was overexpressed in the T. harzianum strain P49P11 under the control of a constitutive promoter [25]. In the T. harzianum xyr1 overexpressing strain, there was ~50% higher cellulase, xylanase, and β-glucosidase activities, and a higher expression of cellulases and xylanases was measured in cultures with sugar cane bagasse [25]. The higher levels of gene expression and activities in the T. harzianum P49P11 xyr1 overexpressing strain suggested that XYR1 in T. harzianum could be co-regulating the expression of cellulases and xylanases, but an xyr1 deletion mutant would be required to investigate this more conclusively. A further recent analysis of a set of wild type T. harzianum strains suggested an extensive xyr1 co-expression gene network, which is potentially regulated by XYR1 [26]. In our study, we deleted xyr1 in T. harzianum and analyzed growth patterns, enzymatic activities, and transcriptional responses to cellulose, to understand the function of T. harzianum XYR1.
2. Materials and Methods
2.1. Strains, Media, and General Growth Conditions
Escherichia coli DH5α was utilized for routine cloning, and the strain Trichoderma harzianum CBS 226.95 was used as the parental strain for gene knockout. All T. harzianum plate cultures were incubated at 28 °C on PDA for sporulation, or minimal medium with 15 g/L agar during the transformation work. The composition of the minimal medium (MM) used was 5 g/L (NH4)2SO4, 15 g/L KH2PO4, 0.6 g/L MgSO4, 0.6 g/L CaCl2, and 2 mL/L Vishniac solution 500X stock, and adjusted to pH 5.5 with KH2PO4 or K2HPO4 [27].
2.2. Construction of xyr1 Deletion T. harzianum Strains
PEG-mediated protoplast transformation of T. harzianum was performed based on the protocol described previously [28]. Table S1 lists the primer sequences used in the generation of the deletion fragment, the screening of transformants, and the confirmation of deletion mutants, and Figure 1 illustrates the binding sites of these primers. The hygromycin resistance gene cassette was amplified from the pcDNA1 plasmid, and combined with ~2000 bp upstream and downstream flanks of the xyr1 CDS (Triha1_1126), and the pCE-Zero vector using the recombination-based ClonExpress Ultra One Step cloning kit (Vazyme, Nanjing, China). The deletion fragment was amplified from deletion plasmids, and 1 µg was used to transform protoplasts, and 120 µg/mL hygromycin was used to select for transformants. After PCR-based screening of transformants, single-spore purification, and culturing on and off hygromycin selection to confirm the stability of strains was performed. The strain spore suspensions were stored at −80 °C in 30% glycerol. Two independent xyr1 deletion mutants were generated (Δxyr1-2 and Δxyr1-63). The gene modifications in the recombinant strains were confirmed using PCR and the sequencing of the PCR products at Sangon Biotech, Shanghai, China.
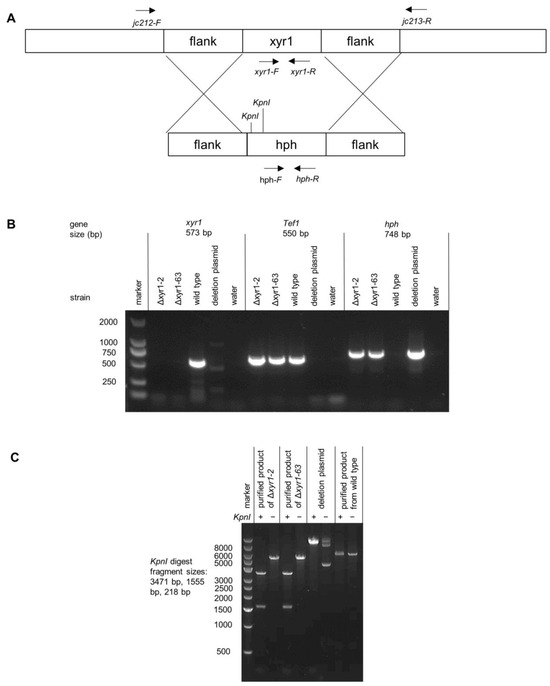
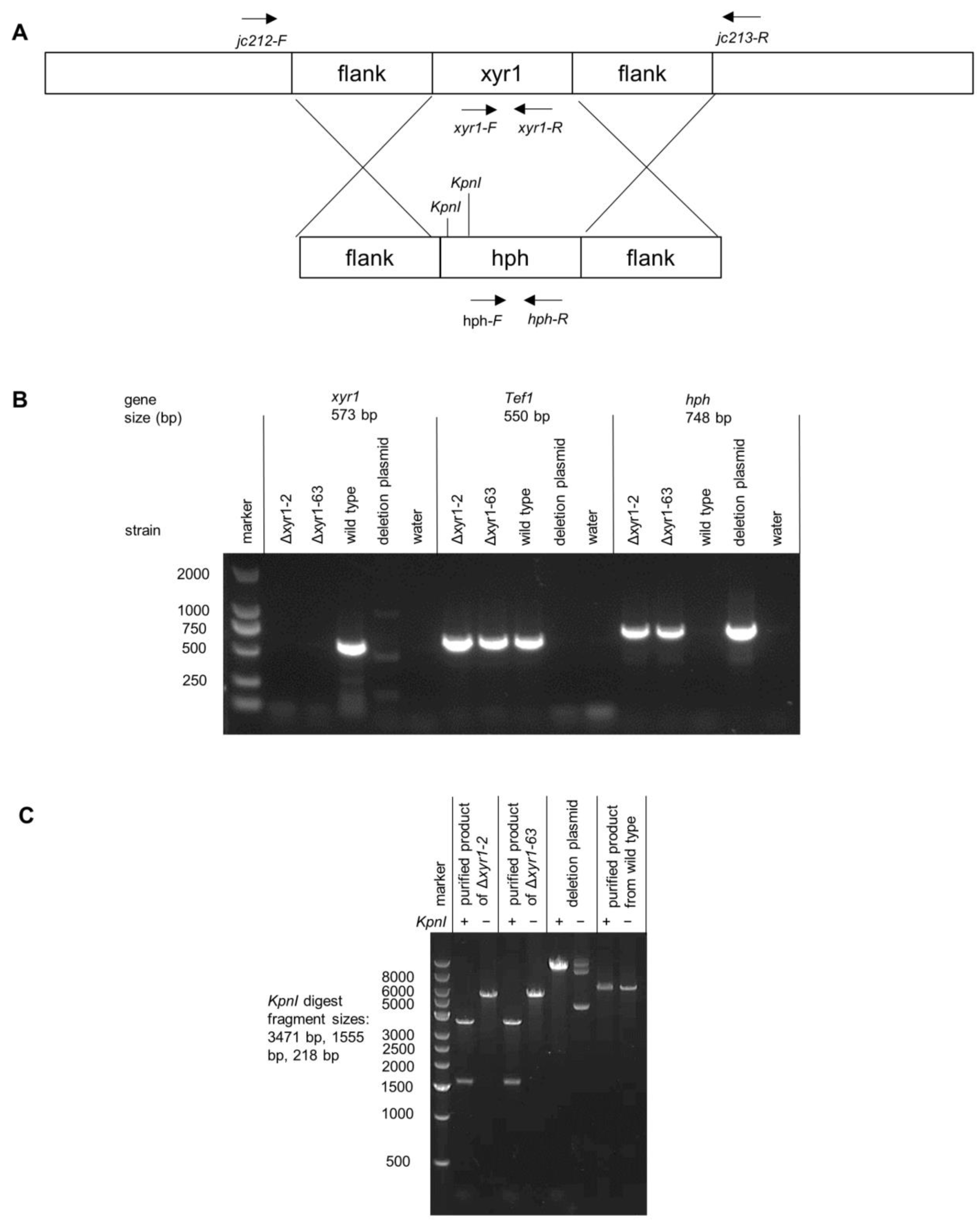
Figure 1.
PCR and restriction digest verification of T. harzianum Δxyr1 mutants. (A) Schematic diagram of xyr1 locus and deletion cassette highlighting the location with black arrows of primer binding and restriction digest sites, (B) agarose gel images demonstrating the absence of the xyr1 sequence in the mutants, and (C) gel image from the restriction digest of the PCR product that spans across the xyr1 locus to verify integration at the xyr1 locus. It should be noted that the PCR products from wild type and Δxyr1 loci were too similar in size to distinguish, and instead, restriction digests of the products were used.
2.3. Growth Profiling Analysis of T. harzianum Δxyr1 Mutants
The growth profiling was performed in triplicate in 9 cm Petri dishes on MM with 1.5% Ultra-pure agarose (Invitrogen, Carlsbad, CA, USA) with one of the following carbon sources: 25 mM D-glucose (Cat. no. G6172, Macklin, Shanghai, China), D-fructose (Cat. no. D809612, Macklin), D-xylose (Cat. no. XBO998, Sangon Biotech, Shanghai, China), L-arabinose (Cat. no. L824031, Macklin), cellobiose (Cat. no. C6182, Macklin), 1% w/v microcrystalline cellulose from cotton linters (Cat. no. 435236, Sigma, Shanghai, China), or 1% w/v xylan (from corn cob) (Cat. no. X823251, Macklin), or without an added carbon source. For growth profiling plate cultures, the plates were inoculated with a ~4 mm2 agar plug from the growing edge of the T. harzianum colony from a PDA plate, and then incubated for at least four days at 28 °C with 12 h light and 12 h dark. After 48 h, the colony diameters were measured, and after both 48 h and 96 h, photos of the colonies were taken. Table S3 contains the colony diameter measurements from the two repeat experiments. Two independent deletion strains were tested for growth on the carbon sources to confirm the reliability of attributing the observed phenotypes to the deleted gene. We selected one of the deletion strains to use in further studies.
2.4. Shake-Flask Cultures for Enzymatic and Transcriptomic Analyses
To inoculate pre-cultures, spores were harvested from a PDA plate using a 0.05% tween 20 solution, filtered with a nappy gauze, and inoculated into a 250 mL flask containing 50 mL MM supplemented with 1% w/v D-fructose and 0.1% w/v peptone to give a 1 × 104/mL spore final concentration. The pre-cultures were incubated at 28 °C and 200 RPM for 36 h in the dark. For transfer, the mycelia were collected using a 38 µm nylon net filter and washed twice with ~200 mL MM solution. Then, 1 g wet weight of mycelia was transferred into 250 mL flasks containing 50 mL of MM with either 25 mM D-fructose, 1% w/v cellulose (microcrystalline from cotton linters), or without an added carbon source, and incubated for up to 36 h at 28 °C with 200 RPM in the dark. Liquid samples from the cultures were collected at various time-points for enzyme assays and PAGE gel analysis. The liquid samples from the cultures were centrifuged, and the supernatants were flash-frozen in liquid nitrogen and stored at −20 °C. For transcriptomic analysis, mycelia were collected from cultures from the 18 h time-point, flash-frozen in liquid nitrogen, and stored at −80 °C. To take account of variability from different spore plates, separate spore plates were used to inoculate each of the three sets of replicate pre-cultures, and each set of replicate pre-cultures was transferred to their respective replicate cultures with various carbon sources.
2.5. Enzyme Activity and PAGE Gel Analysis of the T. harzianum Δxyr1 Mutant
p-nitrophenol (pNP) enzymatic activity assays were performed in 96-well plates using a total volume of 100 μL containing 20 mM Tris-HCl pH 7.5, 2.5 mM of the p-nitrophenol-linked substrate, and culture supernatants. A range of concentrations of p-nitrophenol standard solution (0.5 to 25 nmol/100 µL) were prepared to determine the concentration of p-nitrophenol released. The absorbance was measured at 405 nm using a spectrophotometric plate reader. Enzyme activity units are defined as the amount of nmol pNP released by 1 μL of culture supernatant in 1 h. β-glucosidase activity was detected using p-nitrophenyl-β-D-glucopyranoside, and β-xylosidase activity was detected using p-nitrophenyl-β-D-xylopyranoside (both from Macklin, Shanghai, China). The p-nitrophenol linked substrates were prepared as 50 mM stock solutions in water. Two technical replicates and three biological replicates were set up in the experiment. In a time-course analysis of the enzyme activities, aliquots from 0 h, 12 h, 24 h, and 36 h, from D-fructose, no carbon, and cellulose cultures were analyzed.
Cellulase activity assays were used to measure the breakdown of cellulose by enzymes from the culture supernatants. The assays were performed in 2 mL tubes using a total volume of 750 μL containing 50 mM sodium acetate pH 4.5, 10 mg (1.3% w/v) cellulose (microcrystalline from cotton linters) with 100 μL culture supernatant from separate cellulose shake-flask cultures from 18 h or 36 h, and 0.02% w/v NaN3 to prevent microbial growth. The reactions were shaken horizontally at 37 °C with 200 RPM for up to 72 h. At 0 h, 6 h, 24 h, and 72 h, aliquots of 70 µL were taken from the reactions and were heated at 99 °C for 5 min to inactivate the enzymes. The glucose concentration was measured using a D-Glucose Assay Kit (GOPOD Format) (Megazyme, Bray, Ireland) by measuring the absorbance at 490 nm using a spectrophotometric plate reader.
For PAGE gel analysis, the culture supernatants were concentrated using 10 kDa MWCO diafiltration columns (Millipore, Cork, Ireland) to 10–20 times the original concentration. The protein samples were mixed with 5X loading buffer containing DTT (Cat. no P0015L, Beyotime, Shanghai, China) and heated at 95 °C for 5 min. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed using a 10% (w/v) polyacrylamide gel prepared according to Ultrafast SDS-PAGE Gel Preparation Kit (Zomanbio, Beijing, China). A pre-stained protein ladder (Solarbio, Beijing, China) was also run on the PAGE gels. The PAGE gel was run using a Mini-PROTEAN Tetra Cell System (Bio-Rad, Hercules, CA, USA) with running buffer containing 0.1% w/v SDS, 0.303% w/v Tris base, and 1.44% w/v glycine. The PAGE gels were silver-stained according to the Fast Silver Stain Kit (Beyotime).
2.6. RNA Extraction, Sequencing, and Analysis of the T. harzianum Δxyr1 Mutant
Total RNA was extracted using the Magnetic Tissue/Cell/Blood Total RNA Kit (Tiangen Biotech, Beijing, China) according to the manufacturer’s instructions. RNA purity and quantity were evaluated using a NanoDrop spectrophotometer (Thermo Scientific, Waltham, MA, USA), and RNA integrity was evaluated using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA).
For the library preparation for transcriptome sequencing, a total amount of 1.5 µg RNA per sample was used as input material for the RNA sample preparations. Sequencing libraries were generated using NEBNext® Ultra™ RNA Library Prep Kit for Illumina® (NEB, Ipswich, MA, USA) following the manufacturer’s recommendations, and index codes were added to attribute sequences to each sample. Briefly, mRNA was purified from total RNA using poly-T oligo-attached magnetic beads. Fragmentation was carried out using divalent cations under elevated temperature in NEBNext First Strand Synthesis Reaction Buffer (5X). To select cDNA fragments of preferentially 200–250 bp in length, the library fragments were purified with the AMPure XP system (Beckman Coulter, Brea, California, USA). Then, 3 μL USER Enzyme (NEB, USA) was used with size-selected, adaptor-ligated cDNA at 37 °C for 15 min, followed by 5 min at 95 °C, before PCR was performed with Phusion High-Fidelity DNA polymerase, Universal PCR primers, and Index (X) Primer. The purified PCR products (AMPure XP system) and library quality was assessed on the Agilent Bioanalyzer 2100 system. The library preparations were sequenced on an Illumina NovaSeq 6000 platform by Beijing Allwegene Technology (Beijing, China), and paired-end 150 bp reads were generated. About ~40 M raw reads for each sample were generated. The RNA-Seq reads from this project were submitted to the GEO database (GEO accession GSE252008).
Raw data (raw reads) of fastq format were first processed through in-house Perl scripts. In this step, clean data (clean reads) were obtained by removing reads containing adapter sequences, reads containing poly-N, and low-quality reads from raw data. At the same time, Q20, Q30, GC-content, and the sequence duplication level of the clean data were calculated. All downstream analyses were based on clean data with high quality. These clean reads were then mapped to the reference genome sequence using STAR aligner (v2.5.2b) [29]. Only reads with a perfect match or one mismatch were further analyzed and annotated based on the reference genome for the T. harzianum CBS 226.95 strain from NCBI at https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_003025095.1/ (accessed on 13 November 2023) [30]. The percentage of uniquely mapped reads was at least 95% in all samples. HTSeq (v 0.5.4) [31] was used to count the read numbers mapped to each gene. Gene expression levels were estimated by fragments per kilobase of transcript per million fragments mapped (FPKM). Differential expression analysis was performed using the DESeq2 (1.14.1) [32]. The general criteria for a gene to be considered differentially expressed were >2-fold change in expression and Padj < 0.05 from DESeq2 analysis, and an FPKM > 1 in one condition. The PCA was performed using FactoMineR [33] in the R statistical environment. Hierarchical clustering was performed using the log2 FPKM values in the R statistical environment, using the gplots package with the Euclidian distance and complete linkage options selected. The Venn diagrams were generated using the EVenn tool at http://www.ehbio.com/test/venn/#/ (accessed on 13 November 2023) [34].
For gene annotations of the T. harzianum Triha1 genes, the annotations from JGI Mycosm were used [35]. For the assignment of the plant biomass-degrading subset of CAZymes and activities, the annotations from [30] were used. For the assignments of T. harzianum orthologs of transcription factors related to CAZyme regulation, and the assignment of orthologs of carbon metabolism pathway genes, the annotations of T. reesei QM6a Trire2 genes from [20] were assigned to the T. harzianum reciprocal BLAST best hit. The reciprocal BLAST best hit analysis of Trire2 and Triha1 proteins was performed in Galaxy using BLAST Reciprocal Best Hits (Galaxy Version 0.3.0) [36,37] using the UseGalaxy.eu server [38]. Table S2 lists the annotations used in the analysis.
3. Results
3.1. T. harzianum xyr1 Deletion Mutants Generated Using Hygromycin Resistance Cassette
The T. harzianum CBS 226.95 is one of the most widely studied T. harzianum strains; it is considered the reference strain for the species, and was used here for the deletion of xyr1. A linear fragment containing a hygromycin resistance cassette flanked by sequences upstream and downstream of T. harzianum xyr1 (Triha1_1126) was used for the deletion of xyr1. Two independent deletion mutants were purified through single-spore isolation after the initial screen of the transformants (Δxyr1-2 and Δxyr1-63). Two approaches demonstrated that the mutants had the xyr1 gene deleted. Firstly, no PCR product for the xyr1 coding sequence was obtained from the deletion mutants from the attempted amplification using xyr1 coding sequence primers, as Figure 1B demonstrates. The primers for xyr1 amplified well from the wild type gDNA, and also, the gDNA from the mutant was of good quality because there was a PCR product amplified using primers for tef1 (translation elongation factor 1-alpha) and hph (hygromycin phosphotransferase) (Figure 1B). Secondly, another set of primers was used that bound to the DNA outside of the sequences flanking the resistance cassette to verify the recombination of the deletion cassette at the xyr1 locus (see Figure 1A for the location of these primers). The difference in the size of the products amplified from the wild type and Δxyr1 loci was too small to easily distinguish on an agarose gel, and instead, restriction digests were used to distinguish the two PCR products. The KpnI restriction enzyme digested twice in the product amplified from the Δxyr1 locus and did not digest in the product amplified from the wild type locus, thus confirming the recombination of the hygromycin resistance cassette at the xyr1 locus in both Δxyr1 strains (Figure 1C).
3.2. The T. harzianum Δxyr1 Mutant Showed Reduced Growth on a Subset of Carbon Sources
For plate growth profiling, two independent T. harzianum Δxyr1 mutant strains (Δxyr1-2 and Δxyr1-63) were grown on eight different carbon sources, including monosaccharides, a disaccharide, and polysaccharides, and compared with the parental wild-type strain (Figure 2). The growth was compared based on the colony diameter and the density of the colony appearance after 48 h, and based on the density of the colony appearance and apparent sporulation levels at 96 h. As expected for a deletion of xyr1, there were no clear, consistent differences between the two Δxyr1 mutants and wild type on D-glucose, D-fructose, and without an added carbon source, in terms of colony diameter, colony density, or levels of visible sporulation (Figure 2 and Figure S1).
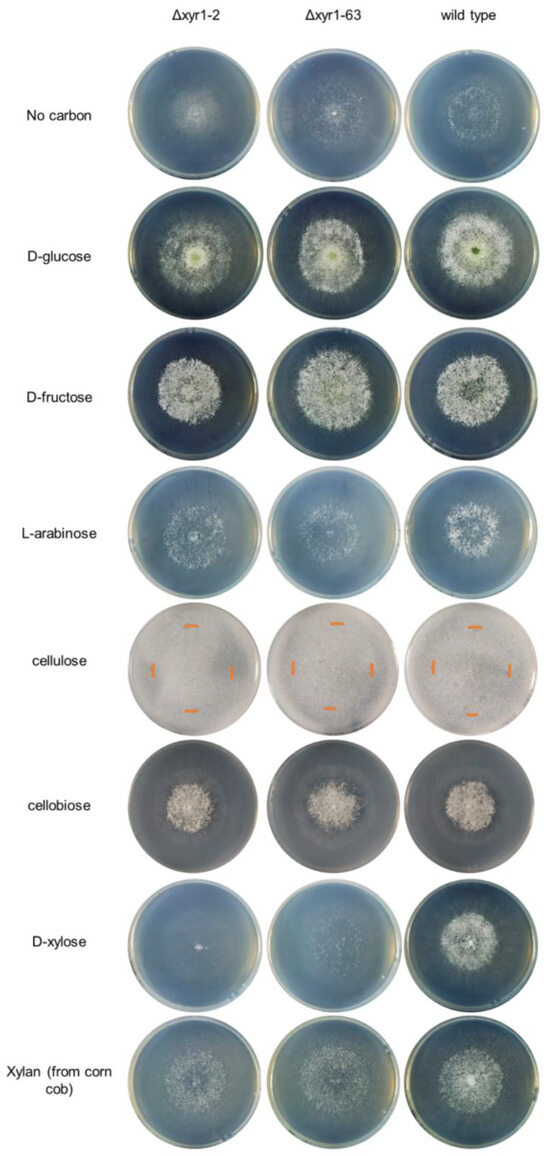
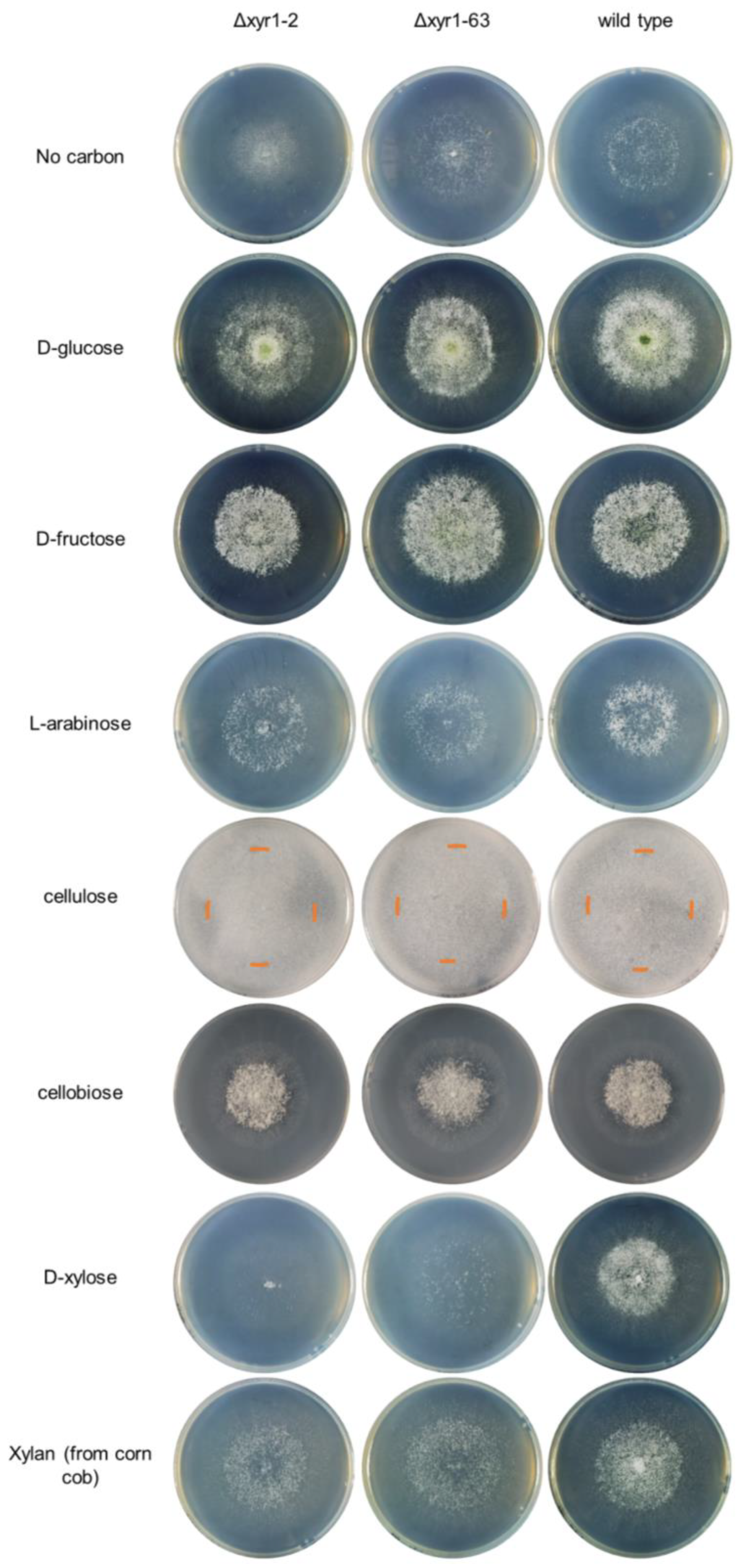
Figure 2.
Growth profiling analysis of T. harzianum Δxyr1 deletion mutants. The edge of the colony on the cellulose cultures is marked with an orange-colored line on the plate images. The growth profiling experiment was repeated twice with similar results from both repeats. Table S3 contains the colony diameter measurements from the two repeat experiments.
Cellulose and xylan are two key components of plant biomass, and growth on these polymers, and sugars that compose these polymers (i.e., cellobiose from cellulose, and D-xylose and L-arabinose from xylan) can indicate the role of T. harzianum XYR1 in the degradation and catabolism of plant biomass-related polysaccharides. At 48 h and 96 h on cellulose, the Δxyr1 mutant mycelia appeared less dense than the wild type, and there was less sporulation in the Δxyr1 mutants at 96 h, although there was also no significant decrease in the radial growth at 48 h (Table S3). On cellobiose, there was also no clearly visible decrease in the growth of the Δxyr1 mutants compared to the wild type. At 48 h on D-xylose, there was a significant reduction (p < 0.05) of 15–25% in the colony diameter of both Δxyr1 mutants, along with a less dense mutant colony appearance (Figure 2). At the 96 h time-point, as well as a less dense colony appearance, there was much less sporulation visible on the Δxyr1 mutant cultures on D-xylose (Figure S1). The appearance of the Δxyr1 mutant colonies on D-xylose was similar to their appearance of the cultures without an added carbon source, suggesting that the Δxyr1 mutants cannot catabolize D-xylose for any level of growth. On L-arabinose, there appeared to be a small reduction in sporulation levels at the 96 h time-point in the Δxyr1 mutants compared to the wild type, but there were no clear, consistent reductions in colony diameter or density in the Δxyr1 mutants; however, it needs to be stated that the wild type grew relatively poorly on L-arabinose compared to the other monosaccharides tested. On xylan from corn cob, there was also a less dense colony appearance at 48 h but not to the same extent as on D-xylose, and there were no significant reductions in colony diameter compared to the wild type. At the 96 h time-point, the mutant colonies grown on xylan were also less dense than the wild type, and less sporulation was visible.
The reduction in growth on the polysaccharides suggested a reduction in enzymatic activity in the Δxyr1 mutant, and the enzymatic activities related to the degradation of cellulose and xylan polysaccharides were measured. As both T. harzianum Δxyr1 mutants showed the same growth trends on the different carbon sources, only one of the mutants (Δxyr1-2) was used for subsequent analyses.
3.3. Enzyme Activity and Protein Secretion Show Major Decreases in the T. harzianum Δxyr1 Mutant
We analyzed the enzyme activity of the T. harzianum Δxyr1-2 mutant, as compared to the T. harzianum wild-type strain by measuring β-glucosidase, β-xylosidase, and cellulase activities from culture supernatants at various time-points from no carbon, D-fructose, and cellulose shake-flask cultures (Figure 3).
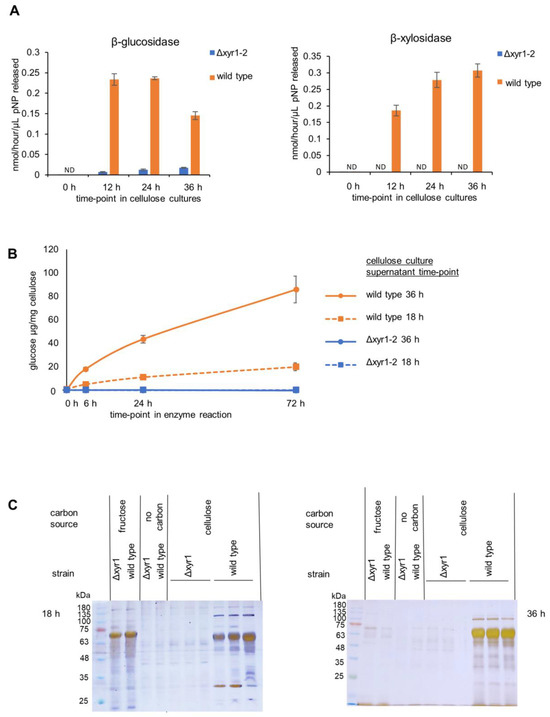
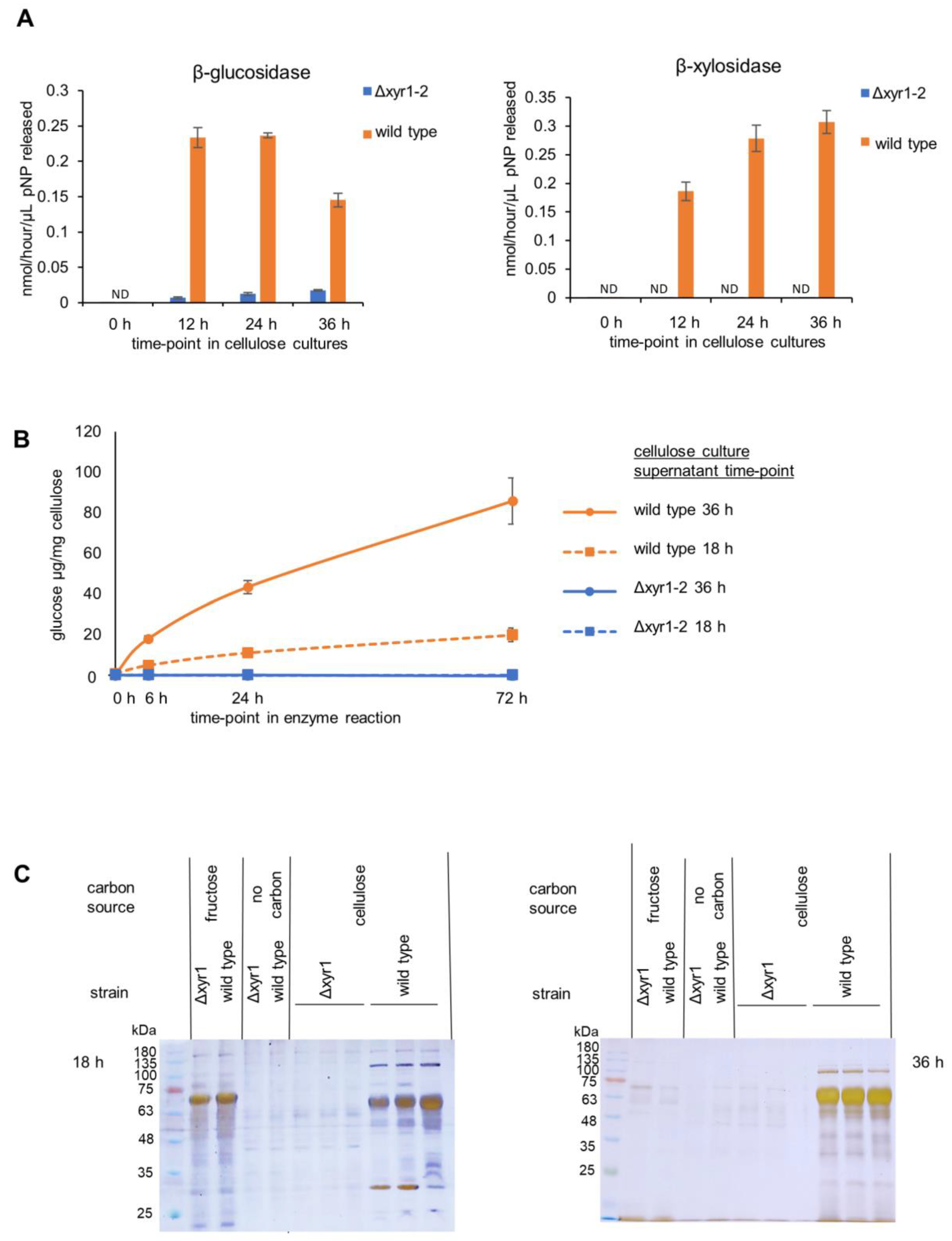
Figure 3.
Enzyme activity and PAGE gel analysis of the T. harzianum Δxyr1 mutant. (A) β-glucosidase and β-xylosidase enzyme activities from the time-course of wild-type and Δxyr1 mutant cellulose cultures, (B), cellulase activity from wild-type (orange-colored lines) and Δxyr1 mutant (blue-colored lines) 18 h (dashed lines) and 36 h (solid lines) cellulose-induced cultures, and (C) silver-stained PAGE gel analysis of supernatants from wild-type and Δxyr1 mutant 18 h and 36 h cellulose cultures. The cellulase activity assay was repeated twice with the same trends in both repeats. ND = not detected.
The presence of cellulose clearly induced β-glucosidase and β-xylosidase activities, with activities detected at 12 h after the transfer of mycelia from the T. harzianum D-fructose pre-cultures to shake-flasks containing cellulose (Figure 3), whereas there was little or no β-glucosidase and β-xylosidase activity detected at the same time-points after transfer to D-fructose cultures or cultures without a carbon source. At 12 h in the cellulose wild-type cultures, the β-glucosidase activity and β-xylosidase activity were detected at ~0.2 nmol/h/µL, and were maintained at a similar level at 24 h and 36 h. In contrast, the T. harzianum Δxyr1-2 β-glucosidase activity was ~90% lower than the wild type, and β-xylosidase was not detected in the Δxyr1-2 mutant. Clearly, the transcriptional activator XYR1 is crucial for β-xylosidase and β-glucosidase production in T. harzianum cellulose cultures. From the activity of β-glucosidase and β-xylosidase, 18 h was selected as the time-point for the RNA-Seq. The 18 h time-point was analyzed for cellulase activity and PAGE gel analysis alongside the 36 h time-point samples.
Cellulase activity was measured from T. harzianum wild type and Δxyr1-2 18 h and 36 h cellulose-induced cultures. The amount of glucose released from the cellulose was measured at three time-points in the enzyme assay (6 h, 24 h, and 72 h). The same volumetric amount of culture supernatants were used from the 18 h and 36 h cultures, and there was higher cellulase activity from the 36 h compared to the 18 h cultures. Approximately 10% cellulose was converted to glucose using the enzymes from the 36 h wild-type culture. In stark contrast, no cellulase activity was detected from the Δxyr1-2 mutant cultures compared to the wild type from neither the 18 h nor the 36 h cultures. The detection limit of the glucose measurement assay was 33.3 µg/mL in the enzyme reaction or equivalent to the conversion of 0.25% of the cellulose to glucose. Even though more cellulase activity was detected from the 36 h wild-type cultures compared to the 18 h wild-type cultures, no cellulase activity was detected from either of the Δxyr1-2 mutant culture time-points. The lack of glucose detection did not change over hydrolysis time, highlighting that cellulase activity from the Δxyr1-2 mutant culture was very low. The large decrease in activity between the wild type and the Δxyr1-2 mutant from 18 h and 36 h suggests that XYR1 is the major regulator of cellulase activity when cellulose is the inducer and carbon source.
The secreted proteins from the 18 h and 36 h T. harzianum wild-type and Δxyr1-2 mutant cultures were analyzed using PAGE gel (Figure 3). In the protein samples from wild-type cultures, the banding patterns clearly showed an induction of protein production due to the presence of cellulose, as several bands were present in the supernatant from the wild-type cellulose cultures that were absent from the supernatants from the D-fructose and no carbon control cultures. In stark contrast to the wild-type cellulose cultures, almost no protein bands were detected from the Δxyr1-2 mutant control cultures even though sensitive silver staining was used. The absence from the Δxyr1-2 mutant supernatants of many of the protein bands visible in the wild-type cellulose culture supernatants is consistent with the low level or lack of β-glucosidase, β-xylosidase, and cellulase enzymatic activities detected from the Δxyr1-2 mutant.
3.4. Overview RNA-Seq Analysis of the T. harzianum Δxyr1 Mutant
To investigate cellulose-induced genes that were regulated by T. harzianum XYR1, transcriptomics was used. At 18 h after transfer to shake-flask cultures, the transcriptome on the wild type and the Δxyr1-2 mutant growing on cellulose was compared, and also compared to D-fructose control cultures, which identified which genes were induced by cellulose. The transcriptome analysis of a further control without a carbon source was compared to show whether the stress condition of the absence of a carbon source may have also regulated genes. Notably, when mycelial samples from the 18 h shake-flask cultures were collected, fewer mycelia were visible in the cellulose cultures of the Δxyr1-2 mutant compared to the wild-type cellulose cultures.
In the PCA analysis of the expression of all genes, there was a clear separation of the expression of the wild-type cultures on cellulose compared to the Δxyr1-2 mutant on cellulose (Figure 4A). Interestingly, the transcriptomes of the Δxyr1-2 mutant on cellulose clustered with all of the no carbon cultures from either the wild type or the Δxyr1-2 mutant cultured without a carbon source. This clustering pattern suggested the major effects of the deletion of xyr1 on the physiology and ability of the T. harzianum Δxyr1-2 mutant to grow on cellulose, to the extent that the transcriptomes are similar to that of T. harzianum cultured in the absence of a carbon source. The PCA analysis showed that the major effect of the deletion of xyr1 was when cultured with cellulose, whereas the wild-type and Δxyr1-2 culture replicates growing on D-fructose clustered among each other, and the wild-type and Δxyr1-2 culture replicates on no carbon also clustered among each other (Figure 4A). There was also a separation in the clusters of wild type cultured with cellulose and wild type cultured with D-fructose, albeit the distance for one of the replicates was less than between other groups but showed a clear effect of cellulose on the transcriptome of T. harzianum.
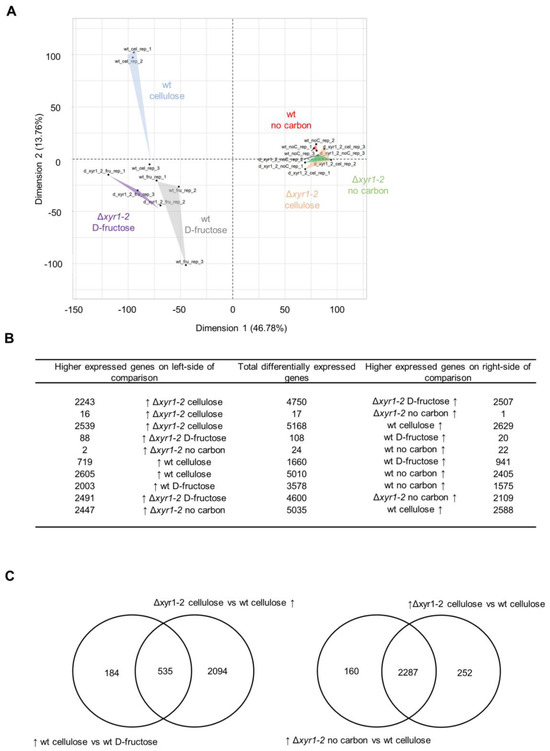
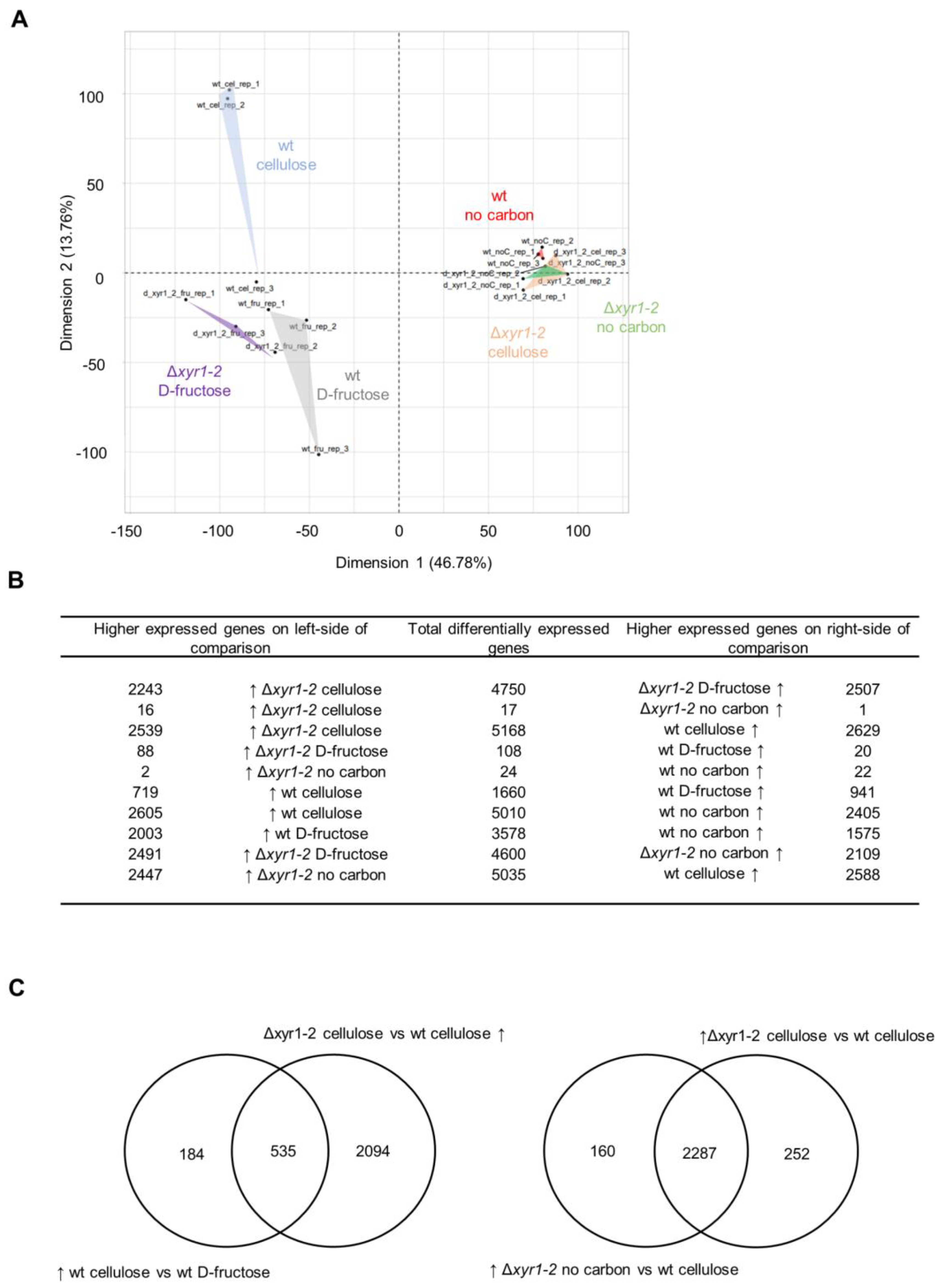
Figure 4.
Overview RNA-Seq analysis of the T. harzianum Δxyr1 mutant. (A) Principal component analysis of the global transcriptomes of the T. harzianum wild type and Δxyr1-2 mutant 18 h after transfer to shake-flask cultures of D-fructose, no carbon, or cellulose. (B) Summary of the number of differentially expressed genes, and (C) Venn diagrams showing overlap in genes differentially expressed in particular comparisons. The arrow symbol ↑ indicates higher expressed genes in a particular condition in a comparison.
The patterns in the PCA were also reflected in the number of DE genes (Figure 4B). In the comparison of the wild type and the Δxyr1-2 mutant cultured on cellulose, there were 5168 genes differentially expressed, with similar numbers of genes with a lower and with a higher expression compared to the mutant. There were 1660 genes differentially expressed in the comparison of the wild type on cellulose and the wild type on D-fructose, with 719 genes higher expressed on cellulose compared to the D-fructose cultures. Of these 719 genes with a higher expression on cellulose in the wild type, ~75% had a lower expression in the Δxyr1-2 mutant, and these genes were considered to be directly or indirectly regulated by XYR1 (Figure 4C). Very few genes were differentially expressed in the comparisons of the wild-type culture on no carbon, the Δxyr1-2 culture on cellulose, and the Δxyr1-2 culture on no carbon, which was consistent with the clustering pattern of the PCA. Also, there were very few genes differentially expressed in the comparison of the wild-type and Δxyr1-2 cultures on D-fructose, which supports the lack of background or other mutations besides Δxyr1 in the mutant, and is in line with the lack of differences in the growth profiling in plate cultures containing D-fructose.
3.5. RNA-Seq Analysis of the T. harzianum Δxyr1 Mutant Shows the Regulation of Xylanases as well as Cellulases
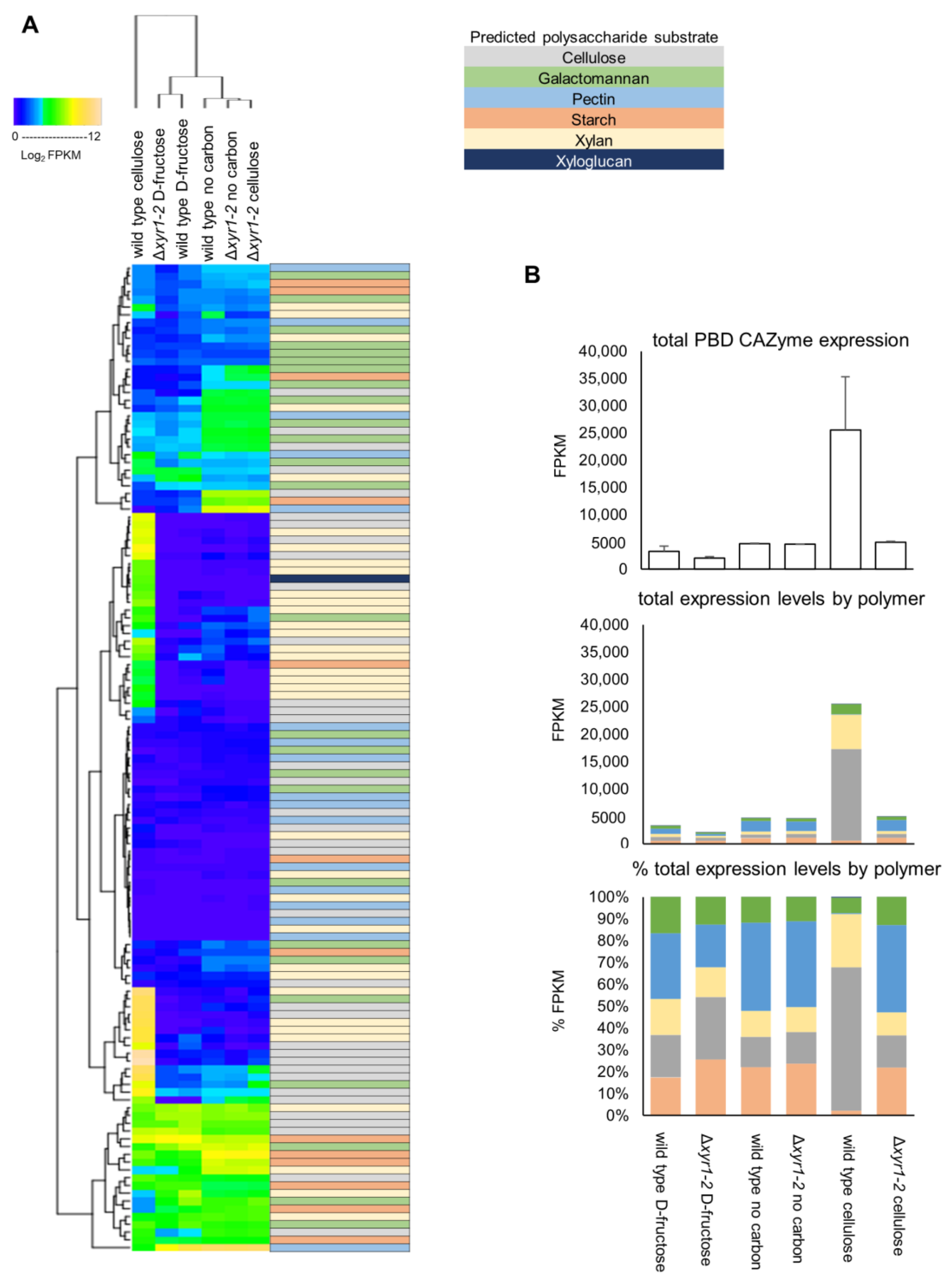
The T. harzianum genome encodes for 128 plant biomass-degrading (PBD) CAZymes, and the summing of the total expression of the PBD CAZymes showed that there was an ~8-fold induction of these PBD CAZymes in the wild type on cellulose compared to the D-fructose cultures, to a level of expression that was 2.6% of the total expression. In the Δxyr1-2 mutant on cellulose compared to the wild type on cellulose, there was a huge 81% reduction in the total expression of PBD CAZymes (Figure 5).
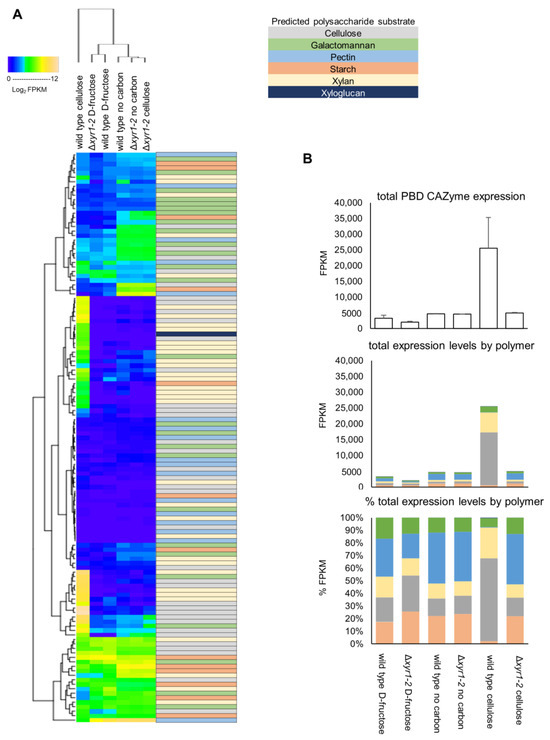
Figure 5.
(A) Heatmap of the expression patterns of plant biomass-degrading (PBD) CAZy. The log2 FPKM values were clustered using hierarchical clustering. The predicted polysaccharide substrate that the PBD CAZyme acts on is color-coded. (B) Totals of PBD CAZy expression, and proportions attributed to activities towards particular polysaccharides present in plant biomass. Error bars on graphs represent standard errors (n = 3). See Figure S2 for a high-resolution image of the heatmap where the gene IDs and annotations for each of the rows of the heatmap are visible.
There were 50 PBD CAZymes that had a lower expression in the Δxyr1-2 mutant on cellulose compared to the wild type on cellulose. Of these PBD CAZymes, 43 were induced by cellulose (i.e., significantly higher expressed in the wild-type cultures on cellulose compared to D-fructose). There were ~30 PBD CAZymes whose expression in the Δxyr1-2 mutant on cellulose was reduced to a similar level to the wild type or the Δxyr1-2 mutant on D-fructose, suggesting that the induction on cellulose of these PBD CAZymes was completely regulated by XYR1. Twenty-one PBD CAZymes had a higher expression in the Δxyr1-2 mutant on cellulose compared to the wild type on cellulose, and this may partly be explained by a carbon starvation-type response as 18 of these had a higher expression in the Δxyr1-2 mutant on no carbon compared to the wild type growing on cellulose. The hierarchical clustering in Figure 5A gives an overview of the expression pattern changes in PBD CAZymes.
There are 36 PBD CAZy genes in T. harzianum that are predicted to have an activity involved in the degradation of cellulose, the polymer present in the shake-flask cultures used for RNA-Seq analysis. The total expression of PBD CAZy cellulases in the wild-type cultures increased ~20-fold from ~600 FPKM on D-fructose to ~16,700 FPKM on cellulose, and the total expression was only ~700 FPKM in the Δxyr1-2 mutant on cellulose (Figure 5). Of the 14 endo-β-1,4-glucanases, 6 were induced by the presence of cellulose, and all of these had a lower expression in the Δxyr1-2 mutant on cellulose compared to the expression in the wild type on cellulose. There are two cellobiohydrolases in T. harzianum, and both appear to be completely regulated by XYR1 because their expression was <5 FPKM in the Δxyr1-2 mutant on cellulose compared to ~5000 FPKM in the wild type on cellulose. There are 16 T. harzianum genes predicted as β-glucosidases, and 8 were induced by the presence of cellulose, and 7 had a lower expression in the Δxyr1-2 mutant on cellulose. Another two of the T. harzianum β-glucosidases (GH3 family members Triha1_504610 and Triha1_96503) did not have a lower expression in the Δxyr1-2 mutant and appeared to be constitutively expressed as they were also expressed at similar levels on the D-fructose and no carbon cultures. There are three AA9 family LPMOs in T. harzianum, and two of these appeared to be completely regulated by XYR1 with an expression of ~1000 FPKM in the wild-type cellulose cultures, and almost zero in the Δxyr1-2 mutant on cellulose, while the third AA9 family LPMO was not expressed in any of the wild-type or mutant cultures.
T. harzianum contains 35 genes predicted to be active on xylan, including main-chain acting endo-xylanases and β-xylosidases, and side-chain acting α-arabinofuranosidases and α-glucuronidases, and esterases. The total expression of PBD CAZy predicted to be active on xylan in the wild-type cultures increased 12-fold from ~500 FPKM on D-fructose to ~6000 FPKM on cellulose, and the total expression was only ~500 FPKM in the Δxyr1-2 mutant on cellulose. Of the nine T. harzianum endo-xylanases (from GH10, GH11, and GH30_7 families), eight were expressed >1 FPKM, and all eight of these were induced in wild-type cellulose cultures, and appeared to be almost completely regulated by XYR1 as the expression level in the Δxyr1-2 mutant on cellulose was <5 FPKM, which was similar to the expression levels for the genes in the D-fructose and no carbon cultures. Of the six predicted β-xylosidases in T. harzianum, three were induced in cellulose cultures, and all three had a lower expression in the Δxyr1-2 mutant on cellulose, indicating that these were also regulated by XYR1. Also, nine of the predicted xylan side-chain acting enzymes were induced in cellulose cultures and all nine had a lower expression in the Δxyr1-2 mutant. T. harzianum also contains PBD CAZymes that act on other polysaccharides such as pectin, xyloglucan, and mannan, and subsets of these enzymes were also induced by cellulose in wild-type cultures, and had a lower expression in the Δxyr1-2 mutant, indicating that they are also regulated by XYR1 (Figure 5 and Table S2).
3.6. Expression Changes in the Δxyr1 Mutant beyond Plant Biomass-Degrading CAZymes
A list of putative T. harzianum carbon catabolic enzymes was made from the reciprocal best BLAST hits of carbon catabolic enzymes annotated in T. reesei [20] (Table S2). Of the putative carbon catabolic enzymes with a higher expression in wild-type cellulose cultures, these included most of the genes that could function on a pentose catabolic pathway (T. harzianum orthologs of lad1, xdh1, xki1, and xyl1), and a D-galactose-oxido-reductive pathway (T. harzianum orthologs of lad1, xdh1, xyl1, and lxr4). In the Δxyr1-2 mutant on cellulose, the xyl1 and xki1 orthologs had a significantly lower expression compared to the wild type on cellulose, while xdh1 was not significantly different, and lad1 had a higher expression in the Δxyr1-2 mutant on cellulose. The higher expression of lad1 in the Δxyr1-2 mutant on cellulose may be related to carbon starvation because the lad1 expression was also higher in the no carbon wild type compared to D-fructose wild type cultures.
The expression of other transcription factors when xyr1 is deleted can indicate whether the changes in expression are likely directly related to xyr1 deletion or indirectly due to changes in the expression of other transcription factors, and probably the genes regulated by those other transcription factors. T. harzianum xyr1 was induced ~5-fold in the cellulose wild-type cultures compared to D-fructose control, and as expected, the expression was not detected from the Δxyr1-2 mutant samples. A list of putative T. harzianum carbon utilization-related transcription factors was made from reciprocal best BLAST hits from the transcription factor orthologs in T. reesei that consisted of xpp1, cre1, mcmA, ace1, bglR, clbr2, ara1, ace3, amyR, gaaR, malR, rhaR, gaaX, ace2, rce1, clbr3, and clr2 (Table S2). As well as xyr1, the T. harzianum orthologs of the two cellulase activators ace3 and clr2 were higher expressed in the wild type cellulose compared to D-fructose cultures, and both ace3 and clr2 had a lower expression in the Δxyr1-2 mutant on cellulose. Although xpp1, cre1, and amyR transcription factors did not have a higher expression on wild type cellulose compared to D-fructose cultures, all three of these transcription factors had a lower expression in the Δxyr1-2 mutant on cellulose compared to wild type on cellulose. The transcription factors rce1 and clbr3 had a higher expression in the Δxyr1-2 mutant on cellulose compared to the wild type on cellulose. The other ten transcription factor orthologs analyzed (mcmA, ace1, bglR, clbr2, ara1, gaaR, malR, rhaR, gaaX, and ace2) were all expressed > 1 FPKM, but none were higher expressed on wild type cellulose compared to D-fructose cultures nor differentially expressed between the wild-type and Δxyr1-2 mutant cellulose cultures (Table S2).
Sugar transport is another key component of plant biomass utilization, and there are 90 T. harzianum genes annotated with the Pfam domain for sugar (and other) transporter (PF00083) (Table S2). Of these predicted transporters, 11 were higher expressed on the cellulose compared to D-fructose wild-type cultures, and 9 of these were lower expressed on Δxyr1-2 compared to wild-type cellulose cultures. There were five other sugar transporters with a lower expression in the Δxyr1-2 mutant compared to wild type on cellulose that were not induced by the presence of cellulose in the wild-type cultures. There were 28 sugar transporters that had a higher expression in the Δxyr1-2 mutant compared to wild type on cellulose, and the higher expression of about half of these may be related to carbon starvation conditions as the expression of 13/28 was higher also in the wild-type no carbon condition compared to the wild-type D-fructose condition (Table S2).
4. Discussion
Here, we have demonstrated how the T. harzianum transcriptional activator XYR1 affects growth and enzymatic activities, regulates a wide range of plant biomass-degrading CAZymes, and appears to be the major regulator for the degradation of cellulose, with profound adverse stress effects in Δxyr1 deletion mutants cultured with cellulose.
The reduced growth of the T. harzianum Δxyr1 mutants on D-xylose is similar to the reduced growth of the T. reesei QM9414 Δxyr1 mutant on D-xylose [9,20]. Notably, the growth appears a lot less for the T. reesei QM9414 Δxyr1 mutant compared to the T. harzianum Δxyr1 mutants, and this is likely due to the greater ability of T. harzianum to grow on agarose as a sole carbon source. The T. harzianum Δxyr1 mutant is the second species within the Trichoderma genus, where reduced growth is demonstrated on D-xylose. Although there are Δxyr1 mutants in T. atroviride [24] and T. cf. guizhouense [23], to our knowledge, there are no tests of growth on D-xylose in these studies. The reduced growth of the two Trichoderma species Δxyr1 mutants on D-xylose is similar to the reduced growth on D-xylose in Fusarium graminearum Δxyr1 and Magnaporthe oryzae Δxlr1, but in stark contrast to two Aspergilli species, where in A. nidulans and A. niger ΔxlnR mutants, there is no reduction in growth on D-xylose [39]. A role for T. harzianum XYR1 in regulating D-xylose catabolism is supported by the lower expression of two genes (xyl1 and xki1) putatively from the pentose catabolic pathway in the Δxyr1-2 cultures on cellulose compared to wild-type cultures on cellulose (Table S2). There was clearly reduced growth on corn cob xylan in the Δxyr1 mutants. This could be explained by the reduced ability of the Δxyr1 mutants to grow on xylose, which is one of the main components of xylan, and also the lower expression of xylan-degrading enzymes such as β-xylosidases and endo-xylanases which the RNA-Seq data from the cellulose cultures showed were regulated by XYR1. Xylan extracted from corn cobs was shown to have a sugar composition that, as well as xylose, included arabinose and glucose [40], and the presence of these other sugars may account for some of the growth of the Δxyr1 mutants on corn cob xylan.
There was no clear reduction in the growth of the Δxyr1 mutants on cellobiose (Figure 2 and Figure S1), and this was surprising because the RNA-Seq data from the cellulose cultures showed that several β-glucosidases, that hydrolyze cellobiose to glucose, were regulated by XYR1, but there were also at least two other β-glucosidases that were expressed and not regulated by XYR1 (Table S2), and β-glucosidase activity was detected from the Δxyr1 culture supernatants (Figure 3). Perhaps, the β-glucosidase activity from these other non-XYR1-regulated β-glucosidases is sufficient to hydrolyze the cellobiose for growth in the plate cultures. Although cellobiose was not used as a carbon source in the shake-flask cultures, it is interesting to speculate on what might be the enzymatic and secretory response of the wild-type and Δxyr1 strains to cellobiose. There are two studies suggesting that cellobiose is a relatively poor inducer of cellulolytic activities in T. harzianum. In the T. harzianum FJ1 strain cultured with cellobiose compared to cellulose, the levels of cellulase and xylanase, but not β-glucosidase, activities were much lower [41]. In the T. harzianum KUC1716 strain, the authors stated that the endoglucanase activity was low when cellobiose was used as the inducer compared to cellulose [42]. It is also possible that when cellobiose is used as the inducer, there could be some carbon catabolite repression effects due to an excess of glucose released from cellobiose. In any event, as the cellulolytic activity is likely relatively lower when cellobiose is the inducer, the effect of the deletion of xyr1 is also likely to be less compared to when other carbon sources such as cellulose are used as the inducer of cellulolytic activity. On the cellulose plate cultures, the reduced growth of the Δxyr1 mutants is likely explained by the hugely reduced expression of endo-cellulases and cellobiohydrolases in the Δxyr1-2 mutant in cellulose shake-flask cultures (Figure 5), as well as the inability of the culture supernatants from the Δxyr1-2 mutant cellulose cultures to release any detectable glucose from the cellulose in the cellulase enzymatic assays (Figure 3B).
In an analysis of XYR1/XlnR/XLR-1 mutants in different species, one of the key differences between species is whether cellulases, xylanases, or both are regulated by XYR1/XlnR/XLR-1. Recently, in Myceliophthora thermophila, the deletion of xlr1 was shown to reduce mainly xylanase gene expression when xylose or xylan was the inducer, while cellulase gene expression was not altered [43], similar to N. crassa XLR-1 [10]. T. harzianum XYR1 appears to regulate both cellulase and xylanase genes based on the large reductions in expression of most cellulase and xylanase genes in the Δxyr1-2 mutant in the cellulose shake-flask cultures (Figure 5), and the reduced growth on both xylan and cellulose plate cultures (Figure 2 and Figure S1). In T. cf. guizhouense NJAU4742, when Avicel cellulose was used as the inducer, both cellulases and xylanases were induced, and both sets of genes had a much lower expression in the T. cf. guizhouense Δxyr1 mutant [23]. The expression pattern in the T. harzianum Δxyr1 mutant on cellulose appears consistent with the T. cf. guizhouense Δxyr1 mutant results. The analysis of C-catabolic enzymes and growth on various carbon sources was not analyzed with the T. cf. guizhouense Δxyr1 mutant, and therefore is not possible to compare with the data from our T. harzianum Δxyr1 mutant. The induction of xylan-degrading enzymes and D-xylose catabolic enzymes by cellulose may be somewhat surprising, but breakdown products of cellulose, such as cellobiose, may also lead to the activation of xylan-degrading enzymes. Alternatively, the microcrystalline cellulose derived from cotton linters used in the cultures may contain small amounts of xylan or xylose, which could contribute to the induction of the xylan-degrading enzymes.
Another key factor in comparing xyr1 mutants in different species and carbon sources is the extent to which XYR1 controls the regulation of the degradation of a particular substrate. One of the remarkable results was how the transcriptomes of the T. harzianum Δxyr1 mutant on cellulose clustered with transcriptomes of the cultures without an added carbon source (Figure 4). This pattern suggests that T. harzianum XYR1 almost completely controls the degradation of cellulose in shake-flask cultures. There were similar suggestions of stress responses in T. reesei RUT-C30 Δxyr1 analysis whereby starvation stress-related genes had a higher expression in the T. reesei RUT-C30 Δxyr1 mutant on lignocellulose-related substrates [22].
Previously, xyr1 was overexpressed in the T. harzianum strain P49P11 [25], and it is useful to compare the trends in the expression analysis of selected genes in the xyr1 overexpression mutant with the expression of those genes in the RNA-Seq dataset with the Δxyr1-2 mutant. The gene IDs were not listed in that study but using the primer sequences from that study [25], the Triha1 gene IDs were found using the PrimerBLAST software at NCBI whereby the software works by searching a database of Triha1 sequences for matches with the primer sequences. The following six genes all had a higher expression in the xyr1 overexpression strain in the sugarcane bagasse cultures: cbh1 (Triha1_7497), CE5 (Triha1_118643), cre1 (Triha1_502975), EGIII (Triha1_91916), GH10 (Triha1_91773), and GH11 (Triha1_115099). The trend from the xyr1 overexpression strain was the inverse of the trend in the Δxyr1-2 deletion mutant cultures on cellulose, whereby all seven of these genes had a significantly lower expression (2FC, Padj<0.05, FPKM>1) compared to the wild-type cultures on cellulose. It was not possible to correlate the fold changes in expression because for cbh1 (Triha1_7497), CE5 (Triha1_118643), EGIII (Triha1_91916), GH10 (Triha1_91773), and GH11 (Triha1_115099), the expression was close to zero FPKM in the Δxyr1-2 mutant culture on cellulose (Table S2). Also, the higher expression of cre1 in the xyr1 overexpression mutant [25] possibly led to some repression effects, which may confound attempts to correlate the expression levels in the datasets from the overexpression and deletion mutants.
The T. harzianum Δxyr1 deletion mutant is a useful resource for future investigations on the regulation of transcriptional responses to crude plant biomass substrates, and investigating which sugars are signaling via XYR1. Also, investigating the role of XYR1 in induced resistance in plants has potential for strain improvement in T. harzianum for biocontrol applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biom14020148/s1, Table S1. List of primer sequences used in the study for the generation of the deletion fragment for xyr1 deletion, for screening transformants, and confirming the integration of the deletion cassette at the xyr1 locus (EXCEL file). Table S2. Gene annotations for Trichoderma harzianum (Triha1), and RNA-Seq dataset for wild-type and Δxyr1-2 mutant cultures on D-fructose (fru), without a carbon source (noC), and microcrystalline cellulose (cel). (EXCEL file). Table S3. Colony diameters from 48 h from the growth profiling of T. harzianum Δxyr1 mutants on various carbon sources from (A) first repeat and (B) second repeat. A t-test was used to indicate significant differences between mutant and control (EXCEL file). Figure S1. Images from 96 h from the plate-culture growth profiling analysis of the Trichoderma harzianum Δxyr1 mutant. The growth profiling experiment was repeated twice with similar results from both repeats. Figure S2. High-resolution image of the heatmap of the expression patterns of plant biomass-degrading (PBD) CAZy where the gene IDs and annotations for each of the rows of the heatmap are readable (PDF file). Figure S3. Uncropped agarose or PAGE gel images used in figures (PPT file).
Author Contributions
L.W. (Lunji Wang): conceptualization; project administration; writing—review and editing; supervision. Y.Z.: investigation; validation; writing—original draft; visualization. S.C.: investigation; supervision. X.W.: investigation. W.M.A.: investigation. T.T.: investigation. Y.C.: investigation. J.Z.: investigation. S.D.: data curation; writing—review and editing; methodology. M.J.: supervision; writing—review and editing; conceptualization. P.F.: resources; writing—review and editing; methodology. D.Z.: writing—review and editing; supervision; conceptualization. I.S.D.: conceptualization; writing—review and editing; resources. L.W. (Lihui Wei): conceptualization; funding acquisition; project administration; supervision. P.D.: conceptualization; funding acquisition; project administration; writing—review and editing; supervision; methodology. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the China Agriculture Research System of MOF and MARA (CARS-24-C-01), and the Jiangsu Coast Land Resources Development Co., Ltd.’s 2022 Saline-alkali Land Management and Soil Fertility Enhancement Science and Technology “Open Competition Mechanism to Select the Best Candidates” Project (2022YHTDJB03).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The RNA-Seq data from this study were deposited in the NCBI GEO database with the accession number GSE252008.
Acknowledgments
Assistance from Allwegene Company for next-generation sequencing is acknowledged.
Conflicts of Interest
Pengxiao Fu has a potential perceived financial conflict of interest as he is an employee of a commercial company called Jiangsu Coastal Ecological Science and Technology Development Co., Ltd., Nanjing, China. The other authors do not declare a conflict of interest.
References
- Kubicek, C.P.; Steindorff, A.S.; Chenthamara, K.; Manganiello, G.; Henrissat, B.; Zhang, J.; Cai, F.; Kopchinskiy, A.G.; Kubicek, E.M.; Kuo, A.; et al. Evolution and comparative genomics of the most common Trichoderma species. BMC Genom. 2019, 20, 485. [Google Scholar] [CrossRef]
- Samuels, G.J. Trichoderma: Systematics, the sexual state, and ecology. Phytopathology 2006, 96, 195–206. [Google Scholar] [CrossRef]
- Benocci, T.; Aguilar-Pontes, M.V.; Zhou, M.; Seiboth, B.; de Vries, R.P. Regulators of plant biomass degradation in ascomycetous fungi. Biotechnol. Biofuels 2017, 10, 152. [Google Scholar] [CrossRef]
- Kowalczyk, J.E.; Daly, P. Transcriptional Regulation: How Saprobic Fungi Tune the Production of Plant Cell Wall Degrading Enzymes. In Encyclopedia of Mycology; Zaragoza, Ó., Casadevall, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2, pp. 528–535. [Google Scholar]
- Xiao, Z.; Zhao, Q.; Li, W.; Gao, L.; Liu, G. Strain improvement of Trichoderma harzianum for enhanced biocontrol capacity: Strategies and prospects. Front. Microbiol. 2023, 14, 1146210. [Google Scholar] [CrossRef]
- Ferreira Filho, J.A.; Horta, M.A.C.; Beloti, L.L.; dos Santos, C.A.; de Souza, A.P. Carbohydrate-active enzymes in Trichoderma harzianum: A bioinformatic analysis bioprospecting for key enzymes for the biofuels industry. BMC Genom. 2017, 18, 779. [Google Scholar] [CrossRef] [PubMed]
- Amore, A.; Giacobbe, S.; Faraco, V. Regulation of Cellulase and Hemicellulase Gene Expression in Fungi. Curr. Genom. 2013, 14, 230–249. [Google Scholar] [CrossRef] [PubMed]
- de Souza, W.R.; Maitan-Alfenas, G.P.; de Gouvêa, P.F.; Brown, N.A.; Savoldi, M.; Battaglia, E.; Goldman, M.H.S.; de Vries, R.P.; Goldman, G.H. The influence of Aspergillus niger transcription factors AraR and XlnR in the gene expression during growth in D-xylose, L-arabinose and steam-exploded sugarcane bagasse. Fungal Genet. Biol. 2013, 60, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Stricker, A.R.; Grosstessner-Hain, K.; Würleitner, E.; Mach, R.L. Xyr1 (Xylanase Regulator 1) Regulates both the Hydrolytic Enzyme System and D-Xylose Metabolism in Hypocrea jecorina. Eukaryot. Cell 2006, 5, 2128–2137. [Google Scholar] [CrossRef]
- Sun, J.; Tian, C.; Diamond, S.; Glass, N.L. Deciphering Transcriptional Regulatory Mechanisms Associated with Hemicellulose Degradation in Neurospora crassa. Eukaryot. Cell 2012, 11, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Aro, N.; Saloheimo, A.; Ilmén, M.; Penttilä, M. ACEII, a Novel Transcriptional Activator Involved in Regulation of Cellulase and Xylanase Genes of Trichoderma reesei. J. Biol. Chem. 2001, 276, 24309–24314. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Wu, C.; Liu, P.; Wang, W.; Wei, D. The transcription factor ACE3 controls cellulase activities and lactose metabolism via two additional regulators in the fungus Trichoderma reesei. J. Biol. Chem. 2019, 294, 18435–18450. [Google Scholar] [CrossRef]
- Coradetti, S.T.; Craig, J.P.; Xiong, Y.; Shock, T.; Tian, C.; Glass, N.L. Conserved and essential transcription factors for cellulase gene expression in ascomycete fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 7397–7402. [Google Scholar] [CrossRef]
- Derntl, C.; Rassinger, A.; Srebotnik, E.; Mach, R.L.; Mach-Aigner, A.R. Xpp1 regulates the expression of xylanases, but not of cellulases in Trichoderma reesei. Biotechnol. Biofuels 2015, 8, 112. [Google Scholar] [CrossRef]
- Benocci, T.; Aguilar-Pontes, M.V.; Kun, R.S.; Seiboth, B.; de Vries, R.P.; Daly, P. ARA1 regulates not only L-arabinose but also D-galactose catabolism in Trichoderma reesei. FEBS Lett. 2018, 592, 60–70. [Google Scholar] [CrossRef]
- Portnoy, T.; Margeot, A.; Linke, R.; Atanasova, L.; Fekete, E.; Sandor, E.; Hartl, L.; Karaffa, L.; Druzhinina, I.; Seiboth, B.; et al. The CRE1 carbon catabolite repressor of the fungus Trichoderma reesei: A master regulator of carbon assimilation. BMC Genom. 2011, 12, 269. [Google Scholar] [CrossRef]
- Aro, N.; Ilmén, M.; Saloheimo, A.; Penttilä, M. ACEI of Trichoderma reesei is a repressor of cellulase and xylanase expression. Appl Environ. Microbiol. 2003, 69, 56–65. [Google Scholar] [CrossRef]
- Cao, Y.; Zheng, F.; Wang, L.; Zhao, G.; Chen, G.; Zhang, W.; Liu, W. Rce1, a novel transcriptional repressor, regulates cellulase gene expression by antagonizing the transactivator Xyr1 in Trichoderma reesei. Mol. Microbiol. 2017, 105, 65–83. [Google Scholar] [CrossRef]
- Mello-de-Sousa, T.M.; Rassinger, A.; Pucher, M.E.; dos Santos Castro, L.; Persinoti, G.F.; Silva-Rocha, R.; Poças-Fonseca, M.J.; Mach, R.L.; Nascimento Silva, R.; Mach-Aigner, A.R. The impact of chromatin remodelling on cellulase expression in Trichoderma reesei. BMC Genom. 2015, 16, 588. [Google Scholar] [CrossRef]
- Benocci, T.; Aguilar Pontes, M.V.; Kun, R.S.; Lubbers, R.J.M.; Lail, K.; Wang, M.; Lipzen, A.; Ng, V.; Grigoriev, I.V.; Seiboth, B.; et al. Deletion of either the regulatory gene ara1 or metabolic gene xki1 in Trichoderma reesei leads to increased CAZyme gene expression on crude plant biomass. Biotechnol. Biofuels 2019, 12, 81. [Google Scholar] [CrossRef]
- Dos Santos, C.L.; Paula, R.G.; Antonieto, A.C.; Persinoti, G.F.; Silva-Rocha, R.; Silva, R.N. Understanding the Role of the Master Regulator XYR1 in Trichoderma reesei by Global Transcriptional Analysis. Front. Microbiol. 2016, 7, 175. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, L.; Zhang, L.; Zou, G.; Liu, R.; Jiang, Y.; Zhou, Z. RNA Sequencing Reveals Xyr1 as a Transcription Factor Regulating Gene Expression beyond Carbohydrate Metabolism. BioMed Res. Int. 2016, 2016, 4841756. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, J.; Guo, C.; Xu, H.; Wang, W.; Yang, M.; Shen, Q.; Zhang, R.; Miao, Y. Exploring the multi-level regulation of lignocellulases in the filamentous fungus Trichoderma guizhouense NJAU4742 from an omics perspective. Microb. Cell Fact. 2022, 21, 144. [Google Scholar] [CrossRef]
- Reithner, B.; Mach-Aigner, A.R.; Herrera-Estrella, A.; Mach, R.L. Trichoderma atroviride Transcriptional Regulator Xyr1 Supports the Induction of Systemic Resistance in Plants. Appl. Environ. Microbiol. 2014, 80, 5274–5281. [Google Scholar] [CrossRef]
- da Silva Delabona, P.; Rodrigues, G.N.; Zubieta, M.P.; Ramoni, J.; Codima, C.A.; Lima, D.J.; Farinas, C.S.; da Cruz Pradella, J.G.; Seiboth, B. The relation between xyr1 overexpression in Trichoderma harzianum and sugarcane bagasse saccharification performance. J. Biotechnol. 2017, 246, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Rosolen, R.R.; Aono, A.H.; Almeida, D.A.; Ferreira Filho, J.A.; Horta, M.A.C.; De Souza, A.P. Network Analysis Reveals Different Cellulose Degradation Strategies Across Trichoderma harzianum Strains Associated with XYR1 and CRE1. Front. Genet. 2022, 13, 807243. [Google Scholar] [CrossRef] [PubMed]
- Penttilä, M.; Nevalainen, H.; Rättö, M.; Salminen, E.; Knowles, J. A versatile transformation system for the cellulolytic filamentous fungus Trichoderma reesei. Gene 1987, 61, 155–164. [Google Scholar] [CrossRef]
- Cai, F.; Kubicek, C.P.; Druzhinina, I.S. Genetic Transformation of Trichoderma spp. In Biofuels and Biodiesel; Basu, C., Ed.; Springer: New York, NY, USA, 2021; pp. 171–185. [Google Scholar]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Chenthamara, K.; Zhang, J.; Atanasova, L.; Yang, D.; Miao, Y.; Rahimi, M.J.; Grujic, M.; Cai, F.; Pourmehdi, S.; et al. Massive lateral transfer of genes encoding plant cell wall-degrading enzymes to the mycoparasitic fungus Trichoderma from its plant-associated hosts. PLoS Genet. 2018, 14, e1007322. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 18. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, H.; Liu, Y.; Liu, Y.X.; Huang, L. EVenn: Easy to create repeatable and editable Venn diagrams and Venn networks online. J. Genet. Genom. 2021, 48, 863–866. [Google Scholar] [CrossRef]
- Grigoriev, I.V.; Nikitin, R.; Haridas, S.; Kuo, A.; Ohm, R.; Otillar, R.; Riley, R.; Salamov, A.; Zhao, X.; Korzeniewski, F.; et al. MycoCosm portal: Gearing up for 1000 fungal genomes. Nucleic Acids Res. 2014, 42, D699–D704. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Cock, P.J.; Chilton, J.M.; Grüning, B.; Johnson, J.E.; Soranzo, N. NCBI BLAST+ integrated into Galaxy. Gigascience 2015, 4, 39. [Google Scholar] [CrossRef]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Čech, M.; Chilton, J.; Clements, D.; Coraor, N.; Grüning, B.A.; et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef]
- Klaubauf, S.; Narang, H.M.; Post, H.; Zhou, M.; Brunner, K.; Mach-Aigner, A.R.; Mach, R.L.; Heck, A.J.R.; Altelaar, A.F.M.; de Vries, R.P. Similar is not the same: Differences in the function of the (hemi-)cellulolytic regulator XlnR (Xlr1/Xyr1) in filamentous fungi. Fungal Genet. Biol. 2014, 72, 73–81. [Google Scholar] [CrossRef]
- Melo-Silveira, R.F.; Fidelis, G.P.; Costa, M.S.; Telles, C.B.; Dantas-Santos, N.; de Oliveira Elias, S.; Ribeiro, V.B.; Barth, A.L.; Macedo, A.J.; Leite, E.L.; et al. In vitro antioxidant, anticoagulant and antimicrobial activity and in inhibition of cancer cell proliferation by xylan extracted from corn cobs. Int. J. Mol. Sci. 2012, 13, 409–426. [Google Scholar] [CrossRef]
- Kim, K.C.; Yoo, S.S.; Oh, Y.A.; Kim, S.J. Isolation and characteristics of Trichoderma harzianum FJ1 producing cellulases and xylanase. J. Microbiol. Biotechnol. 2003, 13, 1–8. [Google Scholar]
- Lee, H.; Lee, Y.M.; Heo, Y.M.; Lee, A.; Hong, J.-H.; Jang, S.; Min, M.; Lee, J.; Kim, J.; Kim, G.-H.; et al. Optimization of Endoglucanase Production by Trichoderma harzianum KUC1716 and Enzymatic Hydrolysis of Lignocellulosic Biomass. BioResources 2015, 10, 7466–7476. [Google Scholar] [CrossRef]
- dos Santos Gomes, A.C.; Falkoski, D.; Battaglia, E.; Peng, M.; Nicolau de Almeida, M.; Coconi Linares, N.; Meijnen, J.-P.; Visser, J.; de Vries, R.P. Myceliophthora thermophila Xyr1 is predominantly involved in xylan degradation and xylose catabolism. Biotechnol. Biofuels 2019, 12, 220. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).