Fluorescence-Polarization-Based Assaying of Lysozyme with Chitooligosaccharide Tracers
Abstract
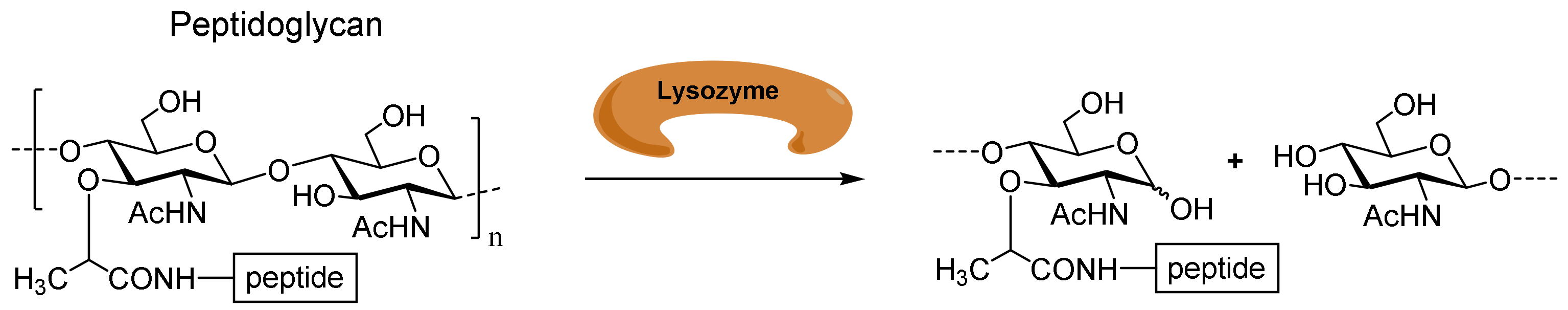
:1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
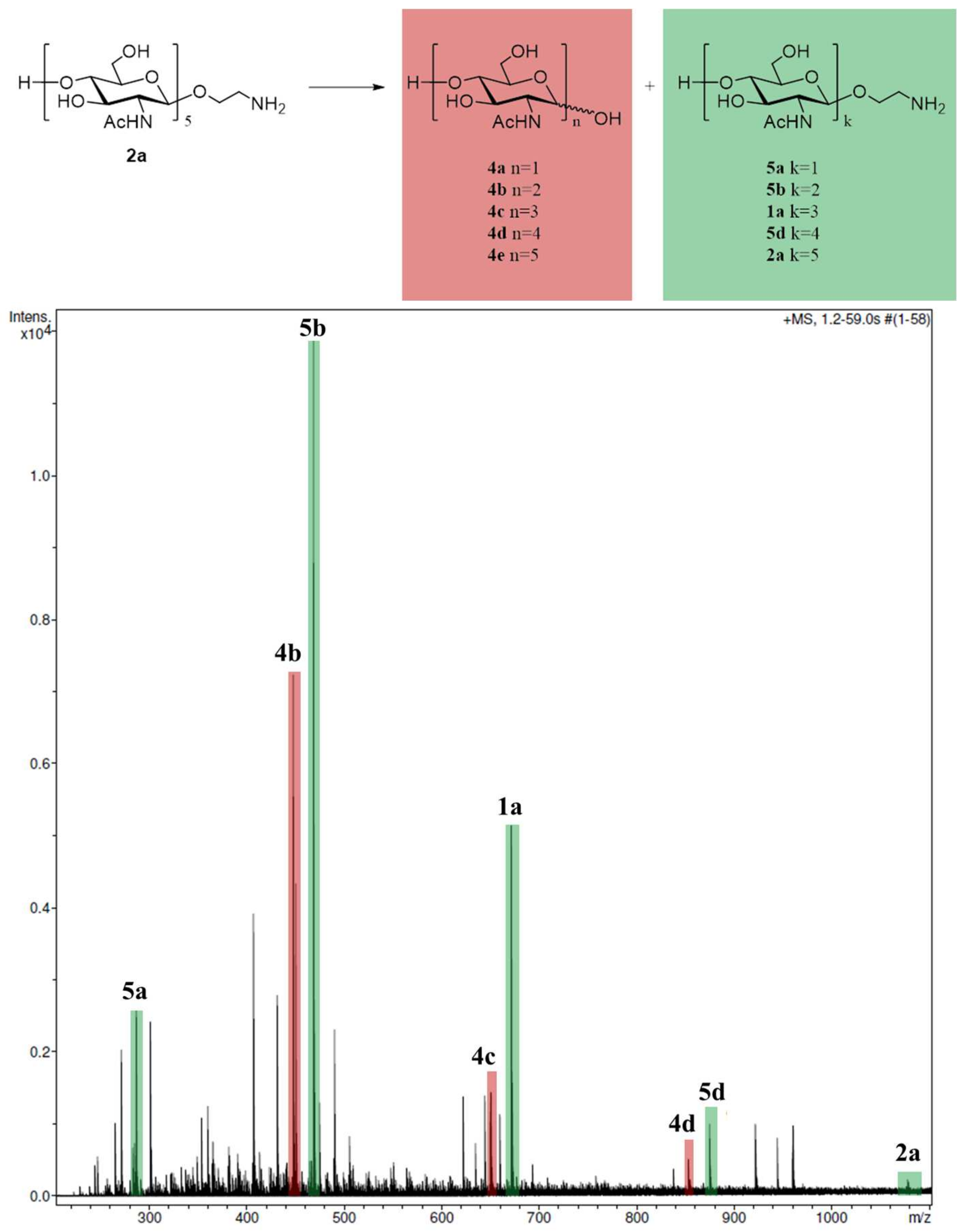
2.2. Enzymatic Reaction of Oligosaccharide 2a with Lysozyme
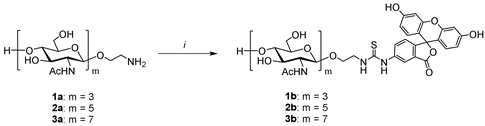
2.3. Synthesis of Fluorescein-Labeled Chitooligosaccharides (Tracers)
2.3.1. Synthesis of Trisaccharide Tracer 1b (Typical Procedure)
2.3.2. Synthesis of Pentasaccharide Tracer 2b
2.3.3. Synthesis of Heptasaccharide Tracer 3b
2.4. Fluorescence Polarization Assay
2.5. Plotting the Calibration Curves
2.6. Lysozyme Quantification in Human Tears
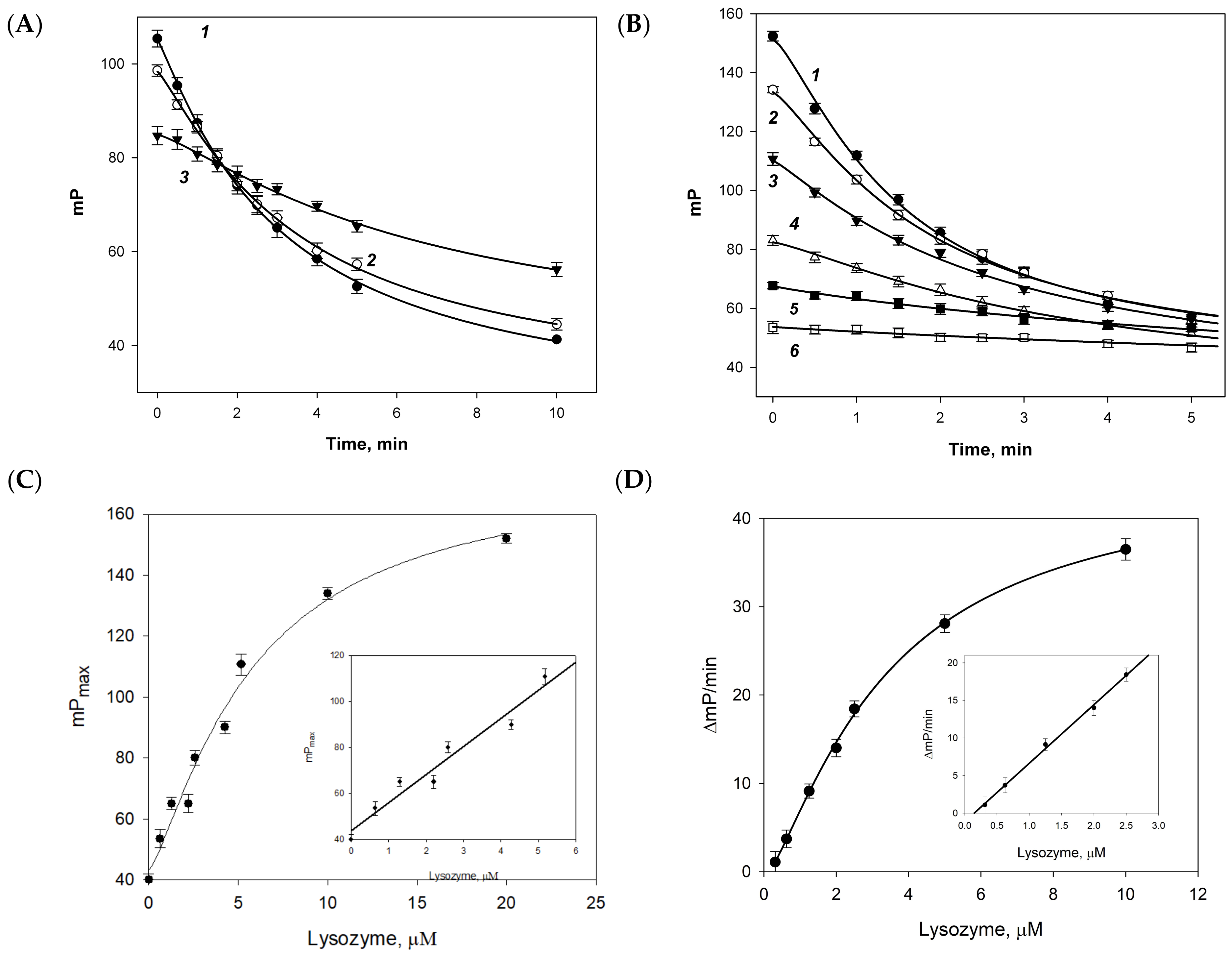
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bowman, A.L.; Grant, I.M.; Mulholland, A.J. QM/MM Simulations Predict a Covalent Intermediate in the Hen Egg White Lysozyme Reaction with Its Natural Substrate. Chem. Commun. 2008, 37, 4425–4427. [Google Scholar] [CrossRef] [PubMed]
- Hadfield, A.T.; Harvey, D.J.; Archer, D.B.; MacKenzie, D.A.; Jeenes, D.J.; Radford, S.E.; Lowe, G.; Dobson, C.M.; Johnson, L.N. Crystal Structure of the Mutant D52S Hen Egg White Lysozyme with an Oligosaccharide Product. J. Mol. Biol. 1994, 243, 856–872. [Google Scholar] [CrossRef] [PubMed]
- Held, J.; van Smaalen, S. The Active Site of Hen Egg-White Lysozyme: Flexibility and Chemical Bonding. Acta Crystallogr. D Biol. Crystallogr. 2014, 70, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Vocadlo, D.J.; Davies, G.J.; Laine, R.; Withers, S.G. Catalysis by Hen Egg-White Lysozyme Proceeds via a Covalent Intermediate. Nature 2001, 412, 835–838. [Google Scholar] [CrossRef]
- Levashov, P.A.; Matolygina, D.A.; Ovchinnikova, E.D.; Adamova, I.Y.; Gasanova, D.A.; Smirnov, S.A.; Nelyub, V.A.; Belogurova, N.G.; Tishkov, V.I.; Eremeev, N.L.; et al. The Bacteriolytic Activity of Native and Covalently Immobilized Lysozyme against Gram-Positive and Gram-Negative Bacteria Is Differentially Affected by Charged Amino Acids and Glycine. FEBS Open Bio 2019, 9, 510–518. [Google Scholar] [CrossRef]
- Ragland, S.A.; Criss, A.K. From Bacterial Killing to Immune Modulation: Recent Insights into the Functions of Lysozyme. PLoS Pathog. 2017, 13, e1006512. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, L.; Eckert, T.; Burg-Roderfeld, M.; Rojas-Macias, M.A.; Lütteke, T.; Krylov, V.B.; Argunov, D.A.; Datta, A.; Markart, P.; et al. Lysozyme’s Lectin-like Characteristics Facilitates Its Immune Defense Function. Q. Rev. Biophys. 2017, 50, e9. [Google Scholar] [CrossRef]
- Sava, G.; Benetti, A.; Ceschia, V.; Pacor, S. Lysozyme and Cancer: Role of Exogenous Lysozyme as Anticancer Agent. Anticancer Res. 1989, 9, 583–591. [Google Scholar]
- Jiang, L.; Li, Y.; Wang, L.; Guo, J.; Liu, W.; Meng, G.; Zhang, L.; Li, M.; Cong, L.; Sun, M. Recent Insights into the Prognostic and Therapeutic Applications of Lysozymes. Front. Pharmacol. 2021, 12, 767642. [Google Scholar] [CrossRef]
- Vidal, M.-L.; Gautron, J.; Nys, Y. Development of an ELISA for Quantifying Lysozyme in Hen Egg White. J. Agric. Food Chem. 2005, 53, 2379–2385. [Google Scholar] [CrossRef]
- Li, J.; He, X.-W.; Wu, Y.-L.; Li, W.-Y.; Zhang, Y.-K. Determination of Lysozyme at the Nanogram Level by a Resonance Light-Scattering Technique with Functionalized CdTe Nanoparticles. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2007, 23, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Berezin, I.V.; Klyosov, A.A.; Rabinowitch, M.L. Kinetics of Enzymatic Reactions in Heterogeneous Systems. I. The Kinetic Regularities for the Bacterial Cell Micrococcus lysodeikticus Digestion by Lysozyme. Russ. J. Bioorganic Chem. 1976, 2, 680–688. [Google Scholar]
- Hultmark, D.; Steiner, H.; Rasmuson, T.; Boman, H.G. Insect Immunity. Purification and Properties of Three Inducible Bactericidal Proteins from Hemolymph of Immunized Pupae of Hyalophora cecropia. Eur. J. Biochem. 1980, 106, 7–16. [Google Scholar] [CrossRef]
- Jiang, Z.-L.; Huang, G.-X. Resonance Scattering Spectra of Micrococcus lysodeikticus and Its Application to Assay of Lysozyme Activity. Clin. Chim. Acta Int. J. Clin. Chem. 2007, 376, 136–141. [Google Scholar] [CrossRef]
- Rohrbach, F.; Karadeniz, H.; Erdem, A.; Famulok, M.; Mayer, G. Label-Free Impedimetric Aptasensor for Lysozyme Detection Based on Carbon Nanotube-Modified Screen-Printed Electrodes. Anal. Biochem. 2012, 421, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Niamnont, N.; Jurat, N.; Sessomboon, N.; Boonkitpatarakul, K.; Sukwattanasinitt, M. Salicylicylphenylacetylene Fluorophore Mixed with Graphene Oxide for Selective Lysozyme Detection. J. Lumin. 2018, 196, 201–206. [Google Scholar] [CrossRef]
- Senturk, H.; Eksin, E.; Işık, Ö.; İlaslan, Z.; Mısırlı, F.; Erdem, A. Impedimetric Aptasensor for Lysozyme Detection Based on Carbon Nanofibres Enriched Screen-Printed Electrodes. Electrochim. Acta 2021, 377, 138078. [Google Scholar] [CrossRef]
- Ren, X.; Yang, L.; Li, Y.; Cheshari, E.C.; Li, X. The Integration of Molecular Imprinting and Surface-Enhanced Raman Scattering for Highly Sensitive Detection of Lysozyme Biomarker Aided by Density Functional Theory. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2020, 228, 117764. [Google Scholar] [CrossRef]
- Hendrickson, O.D.; Taranova, N.A.; Zherdev, A.V.; Dzantiev, B.B.; Eremin, S.A. Eremin Fluorescence Polarization-Based Bioassays: New Horizons. Sensors 2020, 20, 7132. [Google Scholar] [CrossRef]
- Prystay, L.; Gosselin, M.; Banks, P. Determination of Equilibrium Dissociation Constants in Fluorescence Polarization. J. Biomol. Screen. 2001, 6, 141–150. [Google Scholar] [CrossRef]
- Qi, J.; Oppenheimer, M.; Sobrado, P. Fluorescence Polarization Binding Assay for Aspergillus fumigatus Virulence Factor UDP-Galactopyranose Mutase. Enzyme Res. 2011, 2011, 513905. [Google Scholar] [CrossRef]
- Krylov, V.B.; Petruk, M.I.; Karimova, M.P.; Mukhametova, L.I.; Matveev, A.L.; Tikunova, N.V.; Eremin, S.A.; Nifantiev, N.E. Potential of Fluorescence Polarization Immunoassay for the Detection of Aspergillus fumigatus Galactomannan. Russ. Chem. Bull. 2019, 68, 2365–2369. [Google Scholar] [CrossRef]
- James, N.G.; Jameson, D.M. Steady-State Fluorescence Polarization/Anisotropy for the Study of Protein Interactions. Methods Mol. Biol. 2014, 1076, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Rohe, A.; Henze, C.; Erdmann, F.; Sippl, W.; Schmidt, M. A Fluorescence Anisotropy-Based Myt1 Kinase Binding Assay. Assay Drug Dev. Technol. 2014, 12, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Pirruccello, M.; Nandez, R.; Idevall-Hagren, O.; Alcazar-Roman, A.; Abriola, L.; Berwick, S.A.; Lucast, L.; Morel, D.; De Camilli, P. Identification of Inhibitors of Inositol 5-Phosphatases through Multiple Screening Strategies. ACS Chem. Biol. 2014, 9, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Vickers, C.J.; González-Páez, G.E.; Umotoy, J.C.; Cayanan-Garrett, C.; Brown, S.J.; Wolan, D.W. Small-Molecule Procaspase Activators Identified Using Fluorescence Polarization. ChemBioChem 2013, 14, 1419–1422. [Google Scholar] [CrossRef] [PubMed]
- Baughman, B.M.; Jake Slavish, P.; DuBois, R.M.; Boyd, V.A.; White, S.W.; Webb, T.R. Identification of Influenza Endonuclease Inhibitors Using a Novel Fluorescence Polarization Assay. ACS Chem. Biol. 2012, 7, 526–534. [Google Scholar] [CrossRef]
- Haus, P.; Korbus, M.; Schröder, M.; Meyer-Almes, F.-J. Identification of Selective Class II Histone Deacetylase Inhibitors Using a Novel Dual-Parameter Binding Assay Based on Fluorescence Anisotropy and Lifetime. J. Biomol. Screen. 2011, 16, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Hauser, C.; Wodtke, R.; Löser, R.; Pietsch, M. A Fluorescence Anisotropy-Based Assay for Determining the Activity of Tissue Transglutaminase. Amino Acids 2017, 49, 567–583. [Google Scholar] [CrossRef]
- An, J.; Kim, S.Y.; Yang, E.G.; Chung, H.S. A Fluorescence-Polarization-Based Lipopolysaccharide-Caspase-4 Interaction Assay for the Development of Inhibitors. Molecules 2022, 27, 2458. [Google Scholar] [CrossRef]
- Kalmode, H.P.; Podsiadly, I.; Kabra, A.; Boulton, A.; Reddy, P.; Gao, Y.; Li, C.; Bushweller, J.H. Small-Molecule Inhibitors of the MLL1 CXXC Domain, an Epigenetic Reader of DNA Methylation. ACS Med. Chem. Lett. 2022, 13, 1363–1369. [Google Scholar] [CrossRef]
- Schade, S.Z.; Jolley, M.E.; Sarauer, B.J.; Simonson, L.G. BODIPY-Alpha-Casein, a pH-Independent Protein Substrate for Protease Assays Using Fluorescence Polarization. Anal. Biochem. 1996, 243, 1–7. [Google Scholar] [CrossRef]
- Cupp-Enyard, C. Use of the Protease Fluorescent Detection Kit to Determine Protease Activity. J. Vis. Exp. 2009, 30, e1514. [Google Scholar] [CrossRef]
- Kinoshita, K.; Maeda, H.; Hinuma, Y. Fluorescence Polarization Assay of Plasmin, Plasminogen, and Plasminogen Activator. Anal. Biochem. 1980, 104, 15–22. [Google Scholar] [CrossRef]
- Glukhikh, A.S.; Shevelkova, A.N.; Eremin, S.A.; Sinitsyn, A.P. Use of the Method of Fluorescence Polarization to Determine the Degree of Order in the Action of Amylases. Biochem. Mosc. 1991, 56, 873–877. [Google Scholar]
- Maeda, H. A New Lysozyme Assay Based on Fluorescence Polarization or Fluorescence Intensity Utilizing a Fluorescent Peptidoglycan Substrate. J. Biochem. 1980, 88, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Ueda, K.; Maeda, H.; Kambara, T. Determination of Lysozyme Activity by Fluorescence Polarization in Rheumatoid Synovial Fluids and Release of Lysozyme from Polymorphonuclear Leukocytes by Chemotactic Factors. J. Immunol. Methods 1987, 103, 221–227. [Google Scholar] [CrossRef]
- Nawaz, N.; Wen, S.; Wang, F.; Nawaz, S.; Raza, J.; Iftikhar, M.; Usman, M. Lysozyme and Its Application as Antibacterial Agent in Food Industry. Molecules 2022, 27, 6305. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Goossens, E.; Eeckhaut, V.; Calvo, E.P.; Lopez-Ulibarri, R.; Eising, I.; Klausen, M.; Debunne, N.; De Spiegeleer, B.; Ducatelle, R.; et al. Dietary Muramidase Degrades Bacterial Peptidoglycan to NOD-Activating Muramyl Dipeptides and Reduces Duodenal Inflammation in Broiler Chickens. Br. J. Nutr. 2021, 126, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Bi, B.; An, Q.; Kong, L.; Wang, Q.; Tao, L.; Zhang, X. Effect of Dietary Inclusion of Lysozyme on Growth Performance and Plasma Biochemical Parameters of Rainbow Trout (Oncorhynchus mykiss). Aquac. Nutr. 2012, 18, 332–339. [Google Scholar] [CrossRef]
- Kudose, S.; Cossey, L.N.; Canetta, P.A.; Sekulic, M.; Vanbeek, C.A.; Huls, F.B.; Gupta, I.; Bu, L.; Alexander, M.P.; Cornell, L.D.; et al. Clinicopathologic Spectrum of Lysozyme-Associated Nephropathy. Kidney Int. Rep. 2023, 8, 1585–1595. [Google Scholar] [CrossRef]
- Helal, R.; Melzig, M.F. Determination of Lysozyme Activity by a Fluorescence Technique in Comparison with the Classical Turbidity Assay. Pharm.-Int. J. Pharm. Sci. 2008, 63, 415–419. [Google Scholar]
- Aggarwal, A.N.; Agarwal, R.; Dhooria, S.; Prasad, K.T.; Sehgal, I.S.; Muthu, V. Pleural Fluid Lysozyme as a Diagnostic Biomarker of Pleural Tuberculosis: A Systematic Review and Meta-Analysis. Lung India Off. Organ Indian Chest Soc. 2022, 39, 428–436. [Google Scholar] [CrossRef]
- Daniel, M.P.; Gaikwad, V.; Verghese, M.; Abraham, R.; Kapoor, R. Serum Lysozyme (Muramidase) Levels in Intra-Abdominal Abscesses: An Experimental Study. Indian J. Surg. 2015, 77, 117–119. [Google Scholar] [CrossRef]
- Zhong, W.; Kuntz, D.A.; Ember, B.; Singh, H.; Moremen, K.W.; Rose, D.R.; Boons, G.-J. Probing the Substrate Specificity of Golgi Alpha-Mannosidase II by Use of Synthetic Oligosaccharides and a Catalytic Nucleophile Mutant. J. Am. Chem. Soc. 2008, 130, 8975–8983. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Ustyuzhanina, N.E.; Kusaykin, M.I.; Krylov, V.B.; Shashkov, A.S.; Dmitrenok, A.S.; Usoltseva, R.V.; Zueva, A.O.; Nifantiev, N.E.; Zvyagintseva, T.N. Expression and Biochemical Characterization and Substrate Specificity of the Fucoidanase from Formosa algae. Glycobiology 2017, 27, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Ayers, J.D.; Lowary, T.L.; Morehouse, C.B.; Besra, G.S. Synthetic Arabinofuranosyl Oligosaccharides as Mycobacterial Arabinosyltransferase Substrates. Bioorg. Med. Chem. Lett. 1998, 8, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Sethi, M.K.; Buettner, F.F.R.; Krylov, V.B.; Takeuchi, H.; Nifantiev, N.E.; Haltiwanger, R.S.; Gerardy-Schahn, R.; Bakker, H. Identification of Glycosyltransferase 8 Family Members as Xylosyltransferases Acting on O-Glucosylated Notch Epidermal Growth Factor Repeats. J. Biol. Chem. 2010, 285, 1582–1586. [Google Scholar] [CrossRef]
- Sethi, M.K.; Buettner, F.F.R.; Ashikov, A.; Krylov, V.B.; Takeuchi, H.; Nifantiev, N.E.; Haltiwanger, R.S.; Gerardy-Schahn, R.; Bakker, H. Molecular Cloning of a Xylosyltransferase That Transfers the Second Xylose to O-Glucosylated Epidermal Growth Factor Repeats of Notch. J. Biol. Chem. 2012, 287, 2739–2748. [Google Scholar] [CrossRef] [PubMed]
- Yudina, O.N.; Tsvetkov, Y.E.; Nifantiev, N.E. Synthesis of 2-Aminoethyl Glycosides of Chitooligosaccharides. Russ. Chem. Bull. 2015, 64, 2932–2941. [Google Scholar] [CrossRef]
- Song, H.; Inaka, K.; Maenaka, K.; Matsushima, M. Structural Changes of Active Site Cleft and Different Saccharide Binding Modes in Human Lysozyme Co-Crystallized with Hexa-N-Acetyl-Chitohexaose at pH 4.0. J. Mol. Biol. 1994, 244, 522–540. [Google Scholar] [CrossRef] [PubMed]
- Powning, R.F.; Irzykiewicz, H. The Effect of Lysozyme on Chitin Oligosaccharides. Biochim. Biophys. Acta 1966, 124, 218–220. [Google Scholar] [CrossRef]
- Holler, E.; Rupley, J.A.; Hess, G.P. Productive and Unproductive Lysozyme-Chitosaccharide Complexes. Kinetic Investigations. Biochemistry 1975, 14, 2377–2385. [Google Scholar] [CrossRef] [PubMed]
- Roman, D.L.; Ostafe, V.; Isvoran, A. Deeper inside the Specificity of Lysozyme When Degrading Chitosan. A Structural Bioinformatics Study. J. Mol. Graph. Model. 2020, 100, 107676. [Google Scholar] [CrossRef] [PubMed]



 | ||||
| Entry | Substrate | Product | Conditions * (i) | Yield, % |
| 1 | 1a | 1b | FITC (1.2 eq), Na2CO3 (3 eq), DMF, H2O, 60 °C | 96 |
| 2 | 2a | 2b | FITC (1.2 eq), Na2CO3 (3 eq), DMF, H2O, 60 °C | 83 |
| 3 | 3a | 3b | FITC (1.2 eq), Na2CO3 (3 eq), DMF, H2O, 60 °C | 0 |
| 4 | 3a | 3b | FITC (1.3 eq), Na2CO3 (7.5 eq), DMSO, H2O, 60 °C | 17 |
| Compound | Chemical Formula | Calc. | Found |
|---|---|---|---|
| 4a | C8H15NO6 | 244.0792 [M+Na+] | not found |
| 4b | C16H28N2O11 | 447.1585 [M+Na+] | 447.1581 [M+Na+] |
| 4c | C24H41N3O16 | 650.2379 [M+Na+] | 650.2379 [M+Na+] |
| 4d | C32H54N4O21 | 853.3173 [M+Na+] | 853.3172 [M+Na+] |
| 4e | C40H67N5O26 | 1056.3966 [M+Na+] | not found |
| 5a | C10H20N2O6 | 287.1214 [M+Na+] | 287.1291 [M+Na+] |
| 5b | C18H33N3O11 | 468.2188 [M+H+] | 468.2184 [M+H+] |
| 1a | C26H46N4O16 | 671.2982 [M+H+] | 671.2981 [M+H+] |
| 5d | C34H59N5O21 | 874.3775 [M+H+] | 874.3771 [M+H+] |
| 2a | C42H72N6O26 | 1077.4569 [M+H+] | 1077.4544 [M+H+] |
| Sample |
ELISA, Commercial Kit, μM |
Binding Activity Assay, Based on mPmax, μM | Enzymatic Activity Assay, Based on ΔmP/min, μM |
|---|---|---|---|
| (Reference Assay) | (This Study) | ||
| 1 | 79.3 ± 12.4 | 56.6 ± 8.3 | 17.2 ± 1.4 |
| 2 | 100.0 ± 14.5 | 77.9 ± 10.3 | 24.1 ± 0.7 |
| 3 | 84.8 ± 3.4 | 64.8 ± 7.6 | 37.2 ± 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukhametova, L.I.; Zherdev, D.O.; Kuznetsov, A.N.; Yudina, O.N.; Tsvetkov, Y.E.; Eremin, S.A.; Krylov, V.B.; Nifantiev, N.E. Fluorescence-Polarization-Based Assaying of Lysozyme with Chitooligosaccharide Tracers. Biomolecules 2024, 14, 170. https://doi.org/10.3390/biom14020170
Mukhametova LI, Zherdev DO, Kuznetsov AN, Yudina ON, Tsvetkov YE, Eremin SA, Krylov VB, Nifantiev NE. Fluorescence-Polarization-Based Assaying of Lysozyme with Chitooligosaccharide Tracers. Biomolecules. 2024; 14(2):170. https://doi.org/10.3390/biom14020170
Chicago/Turabian StyleMukhametova, Liliya I., Dmitry O. Zherdev, Anton N. Kuznetsov, Olga N. Yudina, Yury E. Tsvetkov, Sergei A. Eremin, Vadim B. Krylov, and Nikolay E. Nifantiev. 2024. "Fluorescence-Polarization-Based Assaying of Lysozyme with Chitooligosaccharide Tracers" Biomolecules 14, no. 2: 170. https://doi.org/10.3390/biom14020170






