S-Allyl-L-Cysteine Affects Cell Proliferation and Expression of H2S-Synthetizing Enzymes in MCF-7 and MDA-MB-231 Adenocarcinoma Cell Lines
Abstract
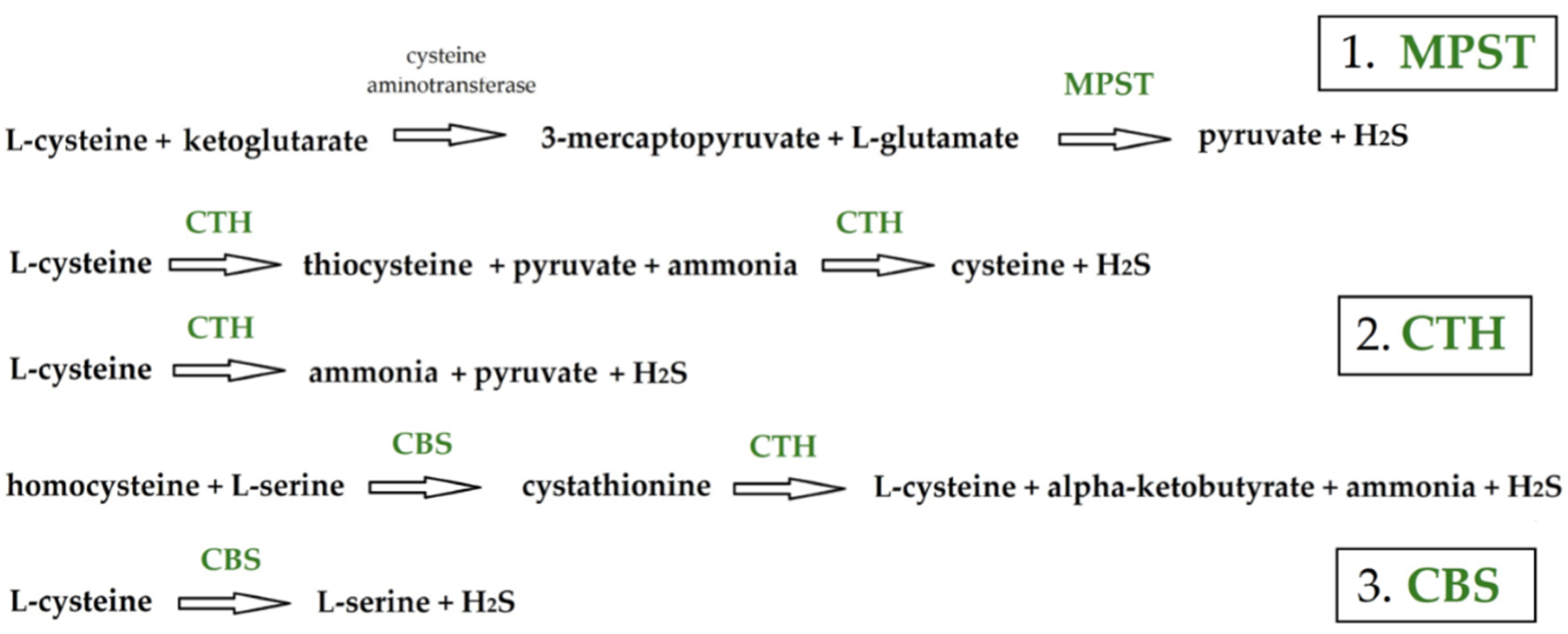
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Cell Membrane Integrity by the LDH Test
2.3. Cell Viability by the MTS Test
2.4. Gene Expression at the Protein Level by Western Blot Analysis
2.5. Statistical Analysis
3. Results
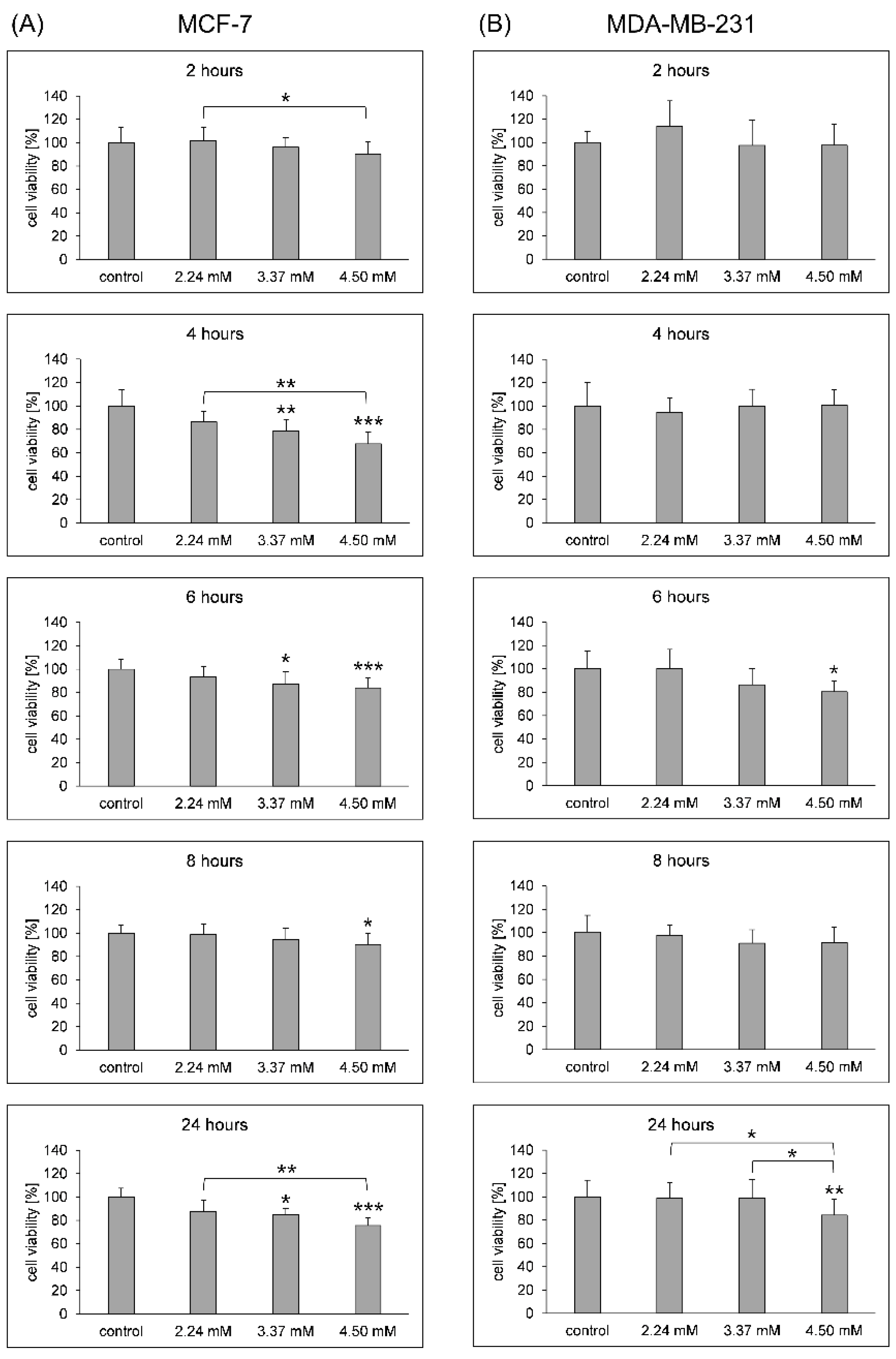
3.1. Cell Membrane Integrity and Viability
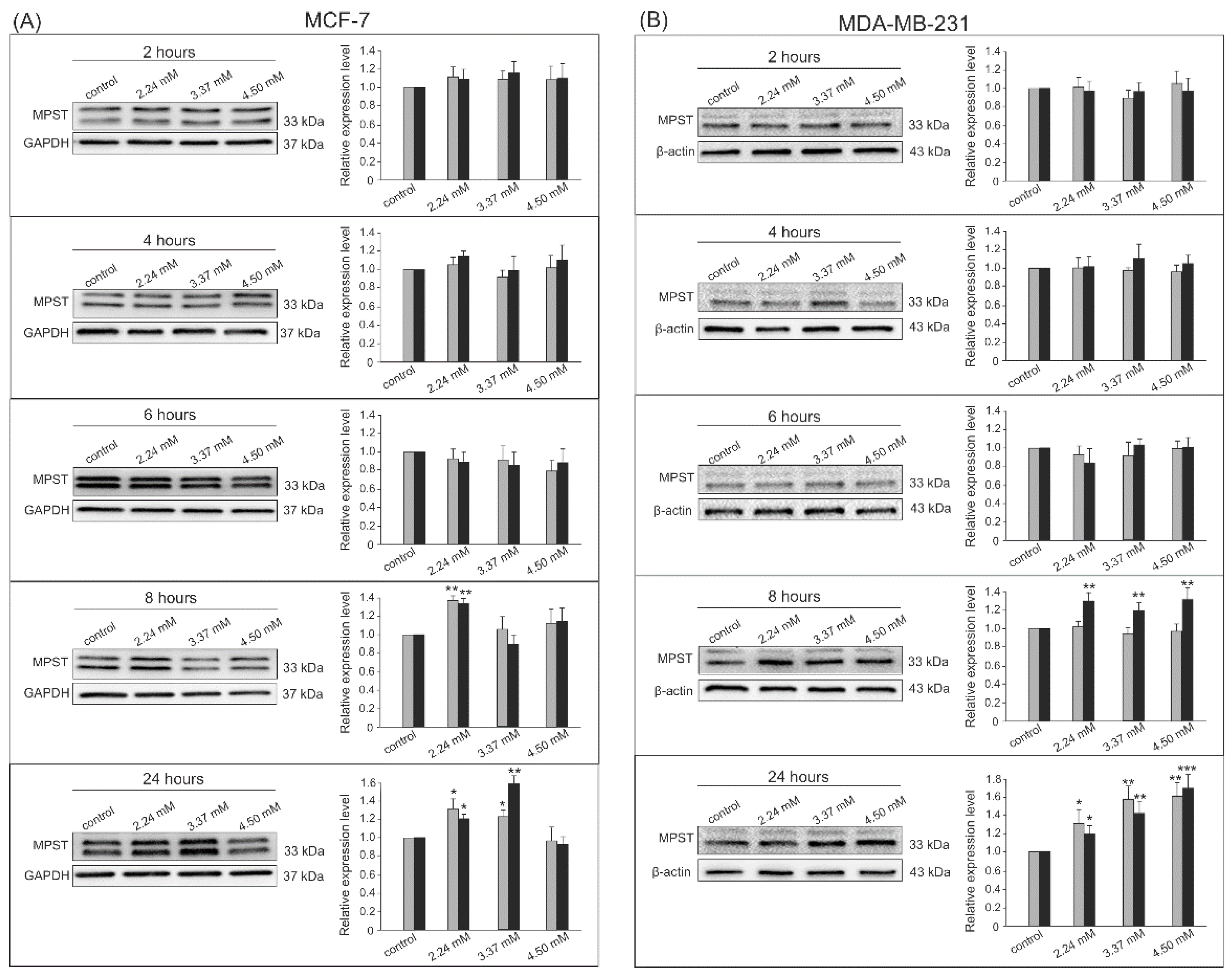
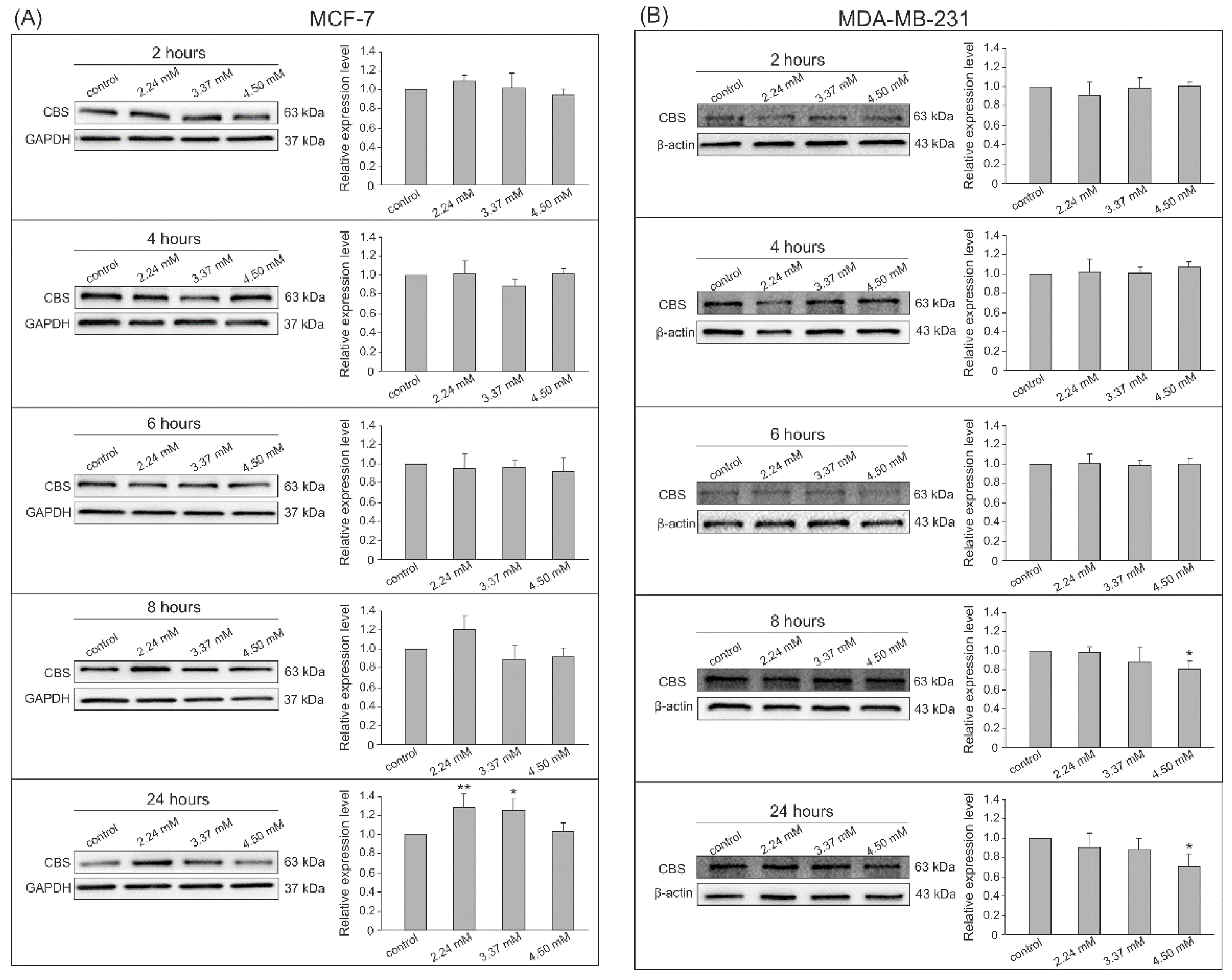
3.2. Gene Expression by Western Blot Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Smolarz, B.; Nowak, A.Z.; Romanowicz, H. Breast Cancer-Epidemiology, Classification, Pathogenesis and Treatment (Review of Literature). Cancers 2022, 14, 2569. [Google Scholar] [CrossRef]
- Cailleau, R.; Olivé, M.; Cruciger, Q.V. Long-term human breast carcinoma cell lines of metastatic origin: Preliminary characterization. In Vitro 1978, 14, 911–915. [Google Scholar] [CrossRef]
- Liu, H.; Zang, C.; Fenner, M.H.; Possinger, K.; Elstner, E. PPARgamma ligands and ATRA inhibit the invasion of human breast cancer cells in vitro. Breast Cancer Res Treat. 2003, 79, 63–74. [Google Scholar] [CrossRef]
- Chavez, K.J.; Garimella, S.V.; Lipkowitz, S. Triple negative breast cancer cell lines: One tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010, 32, 35–48. [Google Scholar] [CrossRef]
- Soule, H.D.; Vazguez, J.; Long, A.; Albert, S.; Brennan, M. A human cell line from a pleural effusion derived from a breast carcinoma. J. Natl. Cancer Inst. 1973, 51, 1409–1416. [Google Scholar] [CrossRef]
- Hasan, M.; Browne, E.; Guarinoni, L.; Darveau, T.; Hilton, K.; Witt-Enderby, P.A. Novel Melatonin, Estrogen, and Progesterone Hormone Therapy Demonstrates Anti-Cancer Actions in MCF-7 and MDA-MB-231 Breast Cancer Cells. Breast Cancer 2020, 14, 1178223420924634. [Google Scholar] [CrossRef]
- Clarke, R.; Jones, B.C.; Sevigny, C.M.; Hilakivi-Clarke, L.A.; Sengupta, S. Experimental models of endocrine responsive breast cancer: Strengths, limitations, and use. Cancer Drug Resist. 2021, 4, 762–783. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; González-Sarrías, A. Dietary Bioactive Compounds and Breast Cancer. Int. J. Mol. Sci. 2023, 24, 9731. [Google Scholar] [CrossRef]
- Cirino, G.; Szabo, C.; Papapetropoulos, A. Physiological roles of hydrogen sulfide in mammalian cells, tissues, and organs. Physiol. Rev. 2023, 103, 31–276. [Google Scholar] [CrossRef]
- Magli, E.; Perissutti, E.; Santagada, V.; Caliendo, G.; Corvino, A.; Esposito, G.; Esposito, G.; Fiorino, F.; Migliaccio, M.; Scognamiglio, A.; et al. H2S Donors and Their Use in Medicinal Chemistry. Biomolecules 2021, 11, 1899. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, H.; Jin, L.; Lin, S. Anticarcinogenic Effects of Isothiocyanates on Hepatocellular Carcinoma. Int. J. Mol. Sci. 2022, 23, 13834. [Google Scholar] [CrossRef]
- Sundaram, M.K.; Rani, P.; Haque, S.; Akhter, N.; Khan, S.; Ahmad, S.; Hussain, A. Dietary isothiocyanates inhibit cancer progression by modulation of epigenome. Semin. Cancer Biol. 2022, 83, 353–376. [Google Scholar] [CrossRef]
- Jurkowska, H.; Wróbel, M.; Szlęzak, D.; Jasek-Gajda, E. New aspects of antiproliferative activity of 4-hydroxybenzyl isothiocyanate, a natural H2S-donor. Amino Acids 2018, 50, 699–709. [Google Scholar] [CrossRef]
- Zaorska, E.; Tomasova, L.; Koszelewski, D.; Ostaszewski, R.; Ufnal, M. Hydrogen Sulfide in Pharmacotherapy, Beyond the Hydrogen Sulfide-Donors. Biomolecules 2020, 10, 323. [Google Scholar] [CrossRef]
- Kosuge, Y. Neuroprotective mechanisms of S-allyl-L-cysteine in neurological disease. Exp. Ther. Med. 2020, 19, 1565–1569. [Google Scholar] [CrossRef]
- Lu, X.; Li, N.; Qiao, X.; Qiu, Z.; Liu, P. Composition analysis and antioxidant properties of black garlic extract. J. Food Drug Anal. 2017, 25, 340–349. [Google Scholar] [CrossRef]
- Orozco-Morales, M.; Hernández-Pedro, N.Y.; Barrios-Bernal, P.; Arrieta, O.; Ruiz-Godoy, L.M.; Aschner, M.; Santamaría, A.; Colín-González, A.L. S-allylcysteine induces cytotoxic effects in two human lung cancer cell lines via induction of oxidative damage, downregulation of Nrf2 and NF-κB, and apoptosis. Anticancer Drugs 2021, 32, 117–126. [Google Scholar] [CrossRef]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef]
- Nagahara, N. Multiple role of 3-mercaptopyruvate sulfurtransferase: Antioxidative function, H2S and polysulfide production and possible SOx production. Br. J. Pharmacol. 2018, 175, 577–589. [Google Scholar] [CrossRef]
- Murphy, B.; Bhattacharya, R.; Mukherjee, P. Hydrogen sulfide signaling in mitochondria and disease. FASEB J. 2019, 33, 13098–13125. [Google Scholar] [CrossRef]
- Yang, J.; Minkler, P.; Grove, D.; Wang, R.; Willard, B.; Dweik, R.; Hine, C. Non-enzymatic hydrogen sulfide production from cysteine in blood is catalyzed by iron and vitamin B6. Commun. Biol. 2019, 2, 194. [Google Scholar] [CrossRef]
- Gong, Z.; Ye, H.; Huo, Y.; Wang, L.; Huang, Y.; Huang, M.; Yuan, X. S-allyl-cysteine attenuates carbon tetrachloride-induced liver fibrosis in rats by targeting STAT3/SMAD3 pathway. Am. J. Transl. Res. 2018, 10, 1337–1346. [Google Scholar]
- Ide, M.; Ohnishi, T.; Toyoshima, M.; Balan, S.; Maekawa, M.; Shimamoto-Mitsuyama, C.; Iwayama, Y.; Ohba, H.; Watanabe, A.; Ishii, T.; et al. Excess hydrogen sulfide and polysulfides production underlies a schizophrenia pathophysiology. EMBO Mol. Med. 2019, 11, e10695. [Google Scholar] [CrossRef]
- Bronowicka-Adamska, P.; Bentke, A.; Lasota, M.; Wróbel, M. Effect of S-Allyl-L-Cysteine on MCF-7 Cell Line 3-Mercaptopyruvate Sulfurtransferase/Sulfane Sulfur System, Viability and Apoptosis. Int. J. Mol. Sci. 2020, 21, 1090. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the Lactate Dehydrogenase Assay. Cold Spring Harb. Protoc. 2018, 2018, 465–468. [Google Scholar] [CrossRef]
- Cory, A.H.; Owen, T.C.; Barltrop, J.A.; Cory, J.G. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991, 3, 207–212. [Google Scholar] [CrossRef]
- Khabar, K.S.; al-Zoghaibi, F.; Dzimiri, M.; Taha, M.; Al-Tuwaijri, A.; al-Ahdal, M.N. MTS interferon assay: A simplified cellular dehydrogenase assay for interferon activity using a water-soluble tetrazolium salt. J. Interferon Cytokine Res. 1996, 16, 31–33. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Kaszuba, K.; Bentke, A.; Krawczyk, A.; Szlęzak, D.; Wróbel, M. A study on the cytotoxic effect of fluoride on the human fibroblast cell line HS27. Fluoride 2020, 53, 124–135. [Google Scholar]
- Bentke-Imiolek, A.; Kaszuba, K.; Bronowicka-Adamska, P.; Czopik, B.; Zarzecka, J.; Wróbel, M. The Cytotoxicity of OptiBond Solo Plus and Its Effect on Sulfur Enzymes Expression in Human Fibroblast Cell Line Hs27. Coatings 2022, 12, 382. [Google Scholar] [CrossRef]
- Bronowicka-Adamska, P.; Bentke, A.; Wróbel, M. Hydrogen sulfide generation from l-cysteine in the human glioblastoma-astrocytoma U-87 MG and neuroblastoma SHSY5Y cell lines. Acta Biochim. Pol. 2017, 64, 171–176. [Google Scholar] [CrossRef]
- Bronowicka-Adamska, P.; Kaczor-Kamińska, M.; Wróbel, M.; Bentke-Imiolek, A. Differences in nonoxidative sulfur metabolism between normal human breast MCF-12A and adenocarcinoma MCF-7 cell lines. Anal. Biochem. 2024, 687, 115434. [Google Scholar] [CrossRef]
- Szlęzak, D.; Hutsch, T.; Ufnal, M.; Wróbel, M. Heart and kidney H2S production is reduced in hypertensive and older rats. Biochimie 2022, 199, 130–138. [Google Scholar] [CrossRef]
- Nagahara, N. Catalytic site cysteines of thiol enzyme: Sulfurtransferases. J. Amino Acids 2011, 2011, 709404. [Google Scholar] [CrossRef]
- Khosravi-Shahi, P.; Cabezón-Gutiérrez, L.; Aparicio Salcedo, M.I. State of art of advanced triple negative breast cancer. Breast J. 2019, 25, 967–970. [Google Scholar] [CrossRef]
- Huang, Z.; Yu, P.; Tang, J. Characterization of Triple-Negative Breast Cancer MDA-MB-231 Cell Spheroid Model. OncoTargets Ther. 2020, 13, 5395–5405. [Google Scholar] [CrossRef]
- Al-Momany, B.; Hammad, H.; Ahram, M. Dihydrotestosterone Induces Chemo-Resistance of Triple-Negative Breast MDA-MB-231 Cancer Cells Towards Doxorubicin Independent of ABCG2 and miR-328-3p. Curr. Mol. Pharmacol. 2021, 14, 860–870. [Google Scholar] [CrossRef]
- Lee, H.; To, N.B.; Kim, M.; Nguyen, Y.T.; Cho, S.K.; Choi, H.K. Metabolic and lipidomic characterization of radioresistant MDA-MB-231 human breast cancer cells to investigate potential therapeutic targets. J. Pharm. Biomed. Anal. 2022, 208, 114449. [Google Scholar] [CrossRef]
- Wein, L.; Loi, S. Mechanisms of resistance of chemotherapy in early-stage triple negative breast cancer (TNBC). Breast 2017, 34, S27–S30. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, S.; Wang, R.; Wang, X. Identification of molecular subtypes and a six-gene risk model related to cuproptosis for triple negative breast cancer. Front. Genet. 2022, 13, 1022236. [Google Scholar] [CrossRef] [PubMed]
- Gapter, L.A.; Yuin, O.Z.; Ng, K.Y. S-Allylcysteine reduces breast tumor cell adhesion and invasion. Biochem. Biophys. Res. Commun. 2008, 367, 446–451. [Google Scholar] [CrossRef]
- Luo, J.; Li, Y.N.; Wang, F.; Zhang, W.M.; Geng, X. S-adenosylmethionine inhibits the growth of cancer cells by reversing the hypomethylation status of c-myc and H-ras in human gastric cancer and colon cancer. Int. J. Biol. Sci. 2010, 6, 784–795. [Google Scholar] [CrossRef]
- Jurkowska, H.; Wróbel, M. Inhibition of Human Neuroblastoma Cell Proliferation by N-acetyl-L-cysteine as a Result of Increased Sulfane Sulfur Level. Anticancer Res. 2018, 38, 5109–5113. [Google Scholar] [CrossRef]
- Welch, C.; Wuarin, L.; Sidell, N. Antiproliferative effect of the garlic compound S-allyl cysteine on human neuroblastoma cells in vitro. Cancer Lett. 1992, 63, 211–219. [Google Scholar] [CrossRef]
- Chu, Q.; Ling, M.T.; Feng, H.; Cheung, H.W.; Tsao, S.W.; Wang, X.; Wong, Y.C. A novel anticancer effect of garlic derivatives: Inhibition of cancer cell invasion through restoration of E-cadherin expression. Carcinogenesis 2006, 27, 2180–2189. [Google Scholar] [CrossRef]
- Ng, K.T.; Guo, D.Y.; Cheng, Q.; Geng, W.; Ling, C.C.; Li, C.X.; Liu, X.B.; Ma, Y.Y.; Lo, C.M.; Poon, R.T.; et al. A garlic derivative, S-allylcysteine (SAC), suppresses proliferation and metastasis of hepatocellular carcinoma. PLoS ONE 2012, 7, e31655. [Google Scholar] [CrossRef]
- Cave, D.D.; Desiderio, V.; Mosca, L.; Ilisso, C.P.; Mele, L.; Caraglia, M.; Cacciapuoti, G.; Porcelli, M. S-Adenosylmethionine-mediated apoptosis is potentiated by autophagy inhibition induced by chloroquine in human breast cancer cells. J. Cell. Physiol. 2018, 33, 1370–1383. [Google Scholar] [CrossRef]
- Abbasi Gamasaee, N.; Radmansouri, M.; Ghiasvand, S.; Shahriari, F.; Zare Marzouni, H.; Aryan, H.; Jangholi, E.; Javidi, M.A. Hypericin Induces Apoptosis in MDA-MB-175-VII Cells in Lower Dose Compared to MDA-MB-231. Arch. Iran. Med. 2018, 21, 387–392. [Google Scholar]
- Liu, Y.P.; Lei, J.; Yin, M.M.; Chen, Y. Organoantimony (III) Derivative Induces Necroptosis in Human Breast Cancer MDA-MB-231 Cells. Anticancer Agents Med. Chem. 2022, 22, 2448–2457. [Google Scholar] [CrossRef]
- Şenol, H.; Tulay, P.; Ergören, M.Ç.; Hanoğlu, A.; Çalış, İ.; Mocan, G. Cytotoxic Effects of Verbascoside on MCF-7 and MDA-MB-231. Turk. J. Pharm. Sci. 2021, 18, 637–644. [Google Scholar] [CrossRef]
- Ulukaya, E.; Ari, F.; Dimas, K.; Ikitimur, E.I.; Guney, E.; Yilmaz, V.T. Anti-cancer activity of a novel palladium(II) complex on human breast cancer cells in vitro and in vivo. Eur. J. Med. Chem. 2011, 46, 4957–4963. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, A.; Wiczk, A.; Kaczyńska, A.; Antosiewicz, J.; Herman-Antosiewicz, A. Sulforaphane inhibits growth of phenotypically different breast cancer cells. Eur. J. Nutr. 2013, 52, 1949–1958. [Google Scholar] [CrossRef]
- Aumeeruddy, M.Z.; Mahomoodally, M.F. Combating breast cancer using combination therapy with 3 phytochemicals: Piperine, sulforaphane, and thymoquinone. Cancer 2019, 125, 1600–1611. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, W.; Hao, M. Phenethyl isothiocyanate reduces breast cancer stem cell-like properties by epigenetic reactivation of CDH1. Oncol. Rep. 2021, 45, 337–348. [Google Scholar] [CrossRef]
- Usukhbayar, N.; Uesugi, S.; Kimura, K.I. 3,6-Epidioxy-1,10-bisaboladiene and sulfasalazine synergistically induce ferroptosis-like cell death in human breast cancer cell lines. Biosci. Biotechnol. Biochem. 2023, 87, 1336–1344. [Google Scholar] [CrossRef]
- Schröder, M.; Yusein-Myashkova, S.; Petrova, M.; Dobrikov, G.; Kamenova-Nacheva, M.; Todorova, J.; Pasheva, E.; Ugrinova, I. The Effect of a Ferrocene Containing Camphor Sulfonamide DK-164 on Breast Cancer Cell Lines. Anticancer Agents Med. Chem. 2019, 19, 1874–1886. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, K.; Lin, G.; Zhao, Z. Antitumor mechanisms of S-allyl mercaptocysteine for breast cancer therapy. BMC Complement. Altern. Med. 2014, 14, 270. [Google Scholar] [CrossRef]
- Yang, Q.; Li, F.; Jia, G.; Liu, R. Aged black garlic extract inhibits the growth of estrogen receptor-positive breast cancer cells by downregulating MCL-1 expression through the ROS-JNK pathway. PLoS ONE 2023, 18, e0286454. [Google Scholar] [CrossRef]
- Vijayan, S.; Loganathan, C.; Sakayanathan, P.; Thayumanavan, P. Synthesis and Characterization of Plumbagin S-Allyl Cysteine Ester: Determination of Anticancer Activity In Silico and In Vitro. Appl. Biochem. Biotechnol. 2022, 194, 5827–5847. [Google Scholar] [CrossRef]
- Nagahara, N.; Wróbel, M. H2S, Polysulfides, and Enzymes: Physiological and Pathological Aspects. Biomolecules 2020, 10, 640. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Sarna, L.K.; Siow, Y.L.; O, K. High-fat diet stimulates hepatic cystathionine β-synthase and cystathionine γ-lyase expression. Can. J. Physiol. Pharmacol. 2013, 91, 913–919. [Google Scholar] [CrossRef]
- Wang, L.; Shi, H.; Liu, Y.; Zhang, W.; Duan, X.; Li, M.; Shi, X.; Wang, T. Cystathionine-γ-lyase promotes the metastasis of breast cancer via the VEGF signaling pathway. Int. J. Oncol. 2019, 55, 473–487. [Google Scholar] [CrossRef]
- Sen, S.; Kawahara, B.; Gupta, D.; Tsai, R.; Khachatryan, M.; Roy-Chowdhuri, S.; Bose, S.; Yoon, A.; Faull, K.; Farias-Eisner, R.; et al. Role of cystathionine β-synthase in human breast Cancer. Free Radic. Biol. Med. 2015, 86, 228–238. [Google Scholar] [CrossRef]
- Hellmich, M.R.; Szabo, C. Hydrogen Sulfide and Cancer. In Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2015; Volume 230, pp. 233–241. [Google Scholar] [CrossRef]
- Erdélyi, K.; Ditrói, T.; Johansson, H.J.; Czikora, Á.; Balog, N.; Silwal-Pandit, L.; Ida, T.; Olasz, J.; Hajdú, D.; Mátrai, Z.; et al. Reprogrammed transsulfuration promotes basal-like breast tumor progression via realigning cellular cysteine persulfidation. Proc. Natl. Acad. Sci. USA 2021, 118, e2100050118. [Google Scholar] [CrossRef]
- Zuhra, K.; Augsburger, F.; Majtan, T.; Szabo, C. Cystathionine-β-Synthase: Molecular Regulation and Pharmacological Inhibition. Biomolecules 2020, 10, 697. [Google Scholar] [CrossRef]
- Jiang, Q.Y.; Li, J.M.; Jing, M.R.; Zhang, Y.X.; Zhang, Q.Q.; Cai, C.B.; Wang, D.; Qi, H.W.; Li, T.; Li, Y.Z.; et al. Cystathionine β-Synthase Regulates the Proliferation, Migration, and Invasion of Thyroid Carcinoma Cells. Oxidative Med. Cell. Longev. 2022, 2022, 8678363. [Google Scholar] [CrossRef]
- Czikora, Á.; Erdélyi, K.; Ditrói, T.; Szántó, N.; Jurányi, E.P.; Szanyi, S.; Tóvári, J.; Strausz, T.; Nagy, P. Cystathionine β-synthase overexpression drives metastatic dissemination in pancreatic ductal adenocarcinoma via inducing epithelial-to-mesenchymal transformation of cancer cells. Redox Biol. 2022, 57, 102505. [Google Scholar] [CrossRef]
- Nandi, S.S.; Mishra, P.K. H2S and homocysteine control a novel feedback regulation of cystathionine beta synthase and cystathionine gamma lyase in cardiomyocytes. Sci. Rep. 2017, 7, 3639. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bentke-Imiolek, A.; Szlęzak, D.; Zarzycka, M.; Wróbel, M.; Bronowicka-Adamska, P. S-Allyl-L-Cysteine Affects Cell Proliferation and Expression of H2S-Synthetizing Enzymes in MCF-7 and MDA-MB-231 Adenocarcinoma Cell Lines. Biomolecules 2024, 14, 188. https://doi.org/10.3390/biom14020188
Bentke-Imiolek A, Szlęzak D, Zarzycka M, Wróbel M, Bronowicka-Adamska P. S-Allyl-L-Cysteine Affects Cell Proliferation and Expression of H2S-Synthetizing Enzymes in MCF-7 and MDA-MB-231 Adenocarcinoma Cell Lines. Biomolecules. 2024; 14(2):188. https://doi.org/10.3390/biom14020188
Chicago/Turabian StyleBentke-Imiolek, Anna, Dominika Szlęzak, Marta Zarzycka, Maria Wróbel, and Patrycja Bronowicka-Adamska. 2024. "S-Allyl-L-Cysteine Affects Cell Proliferation and Expression of H2S-Synthetizing Enzymes in MCF-7 and MDA-MB-231 Adenocarcinoma Cell Lines" Biomolecules 14, no. 2: 188. https://doi.org/10.3390/biom14020188
APA StyleBentke-Imiolek, A., Szlęzak, D., Zarzycka, M., Wróbel, M., & Bronowicka-Adamska, P. (2024). S-Allyl-L-Cysteine Affects Cell Proliferation and Expression of H2S-Synthetizing Enzymes in MCF-7 and MDA-MB-231 Adenocarcinoma Cell Lines. Biomolecules, 14(2), 188. https://doi.org/10.3390/biom14020188





