Lactobacillus crispatus CCFM1339 Inhibits Vaginal Epithelial Barrier Injury Induced by Gardnerella vaginalis in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain Preparation
2.2. Cell Culture
2.3. Cell Epithelial Barrier Integrity Detection
2.4. Cell Permeability Analysis
2.5. Scratch Assay
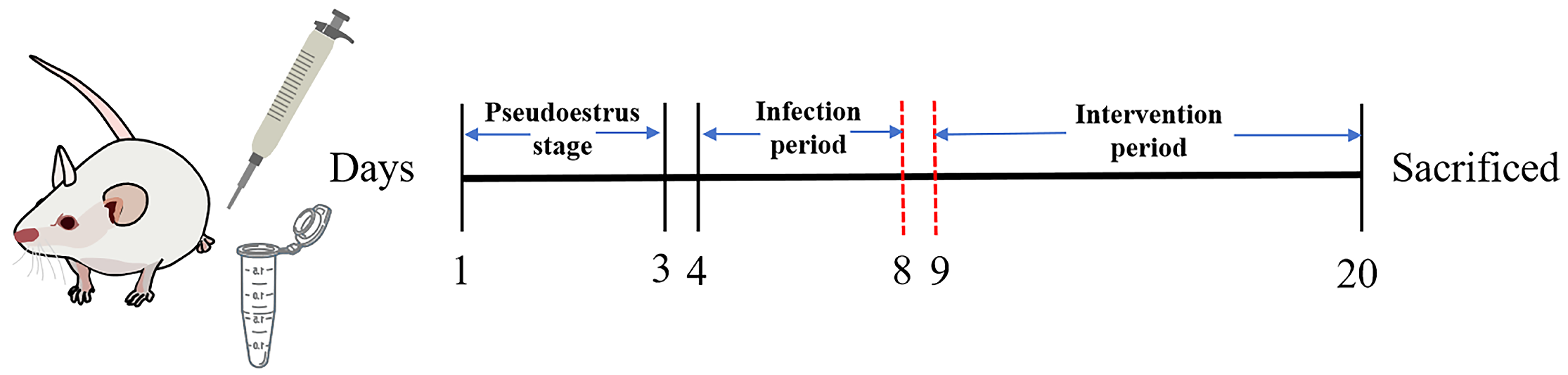
2.6. Animal Experiment
2.7. Histopathological Observation
2.8. ELISA
2.9. cDNA Generation and qPCR
2.10. Vaginal Microbiota Analysis
2.11. Statistical Analysis
3. Results
3.1. Screening for Lactobacillus Strains Capable of Enhancing the Vaginal Epithelial Barrier
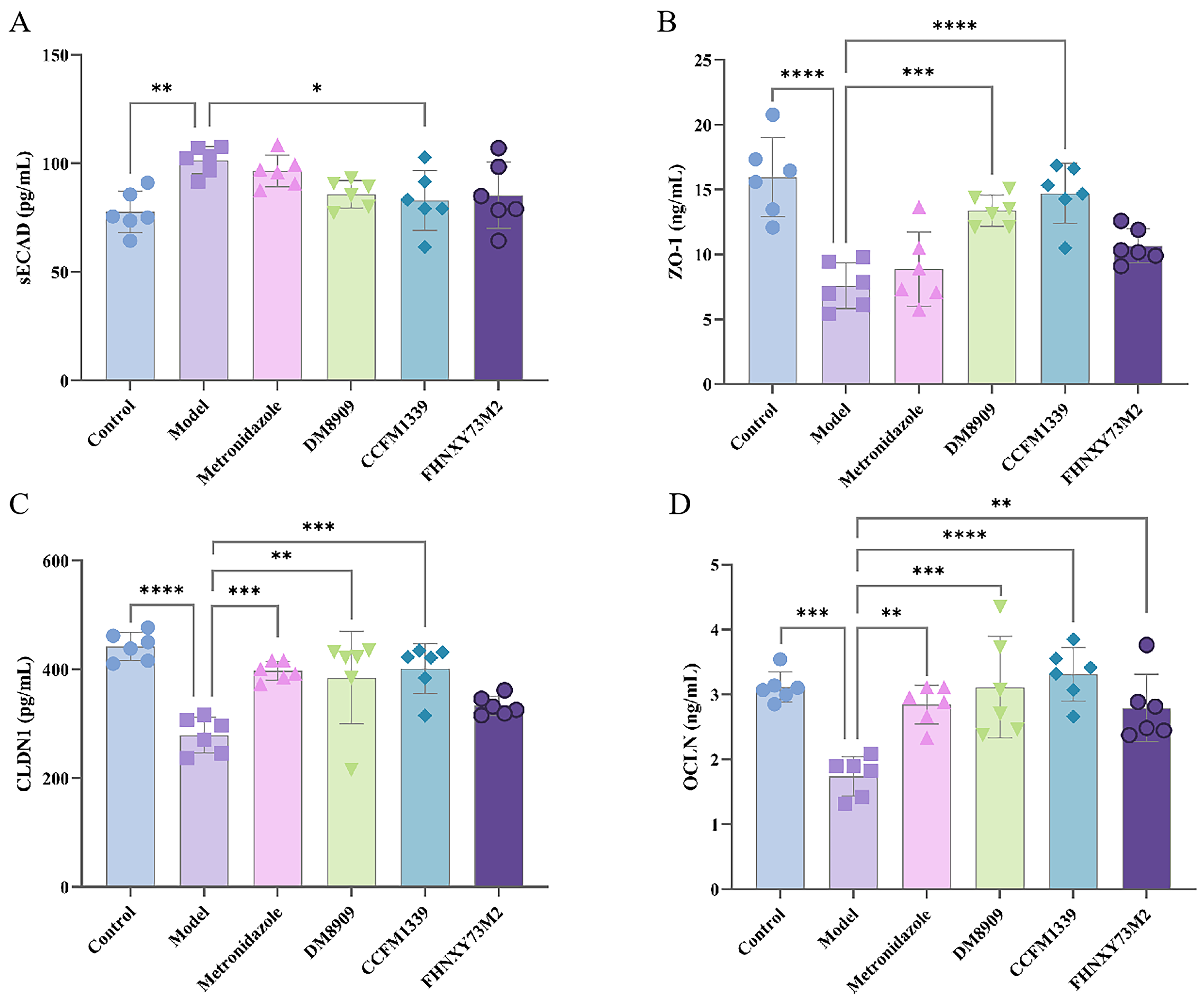
3.2. Effects of L. crispatus CCFM1339 on Mechanical Barrier
3.3. Effects of L. crispatus CCFM1339 on Inflammatory Barrier
3.4. Effects of L. crispatus CCFM1339 on Anti-Infection Index
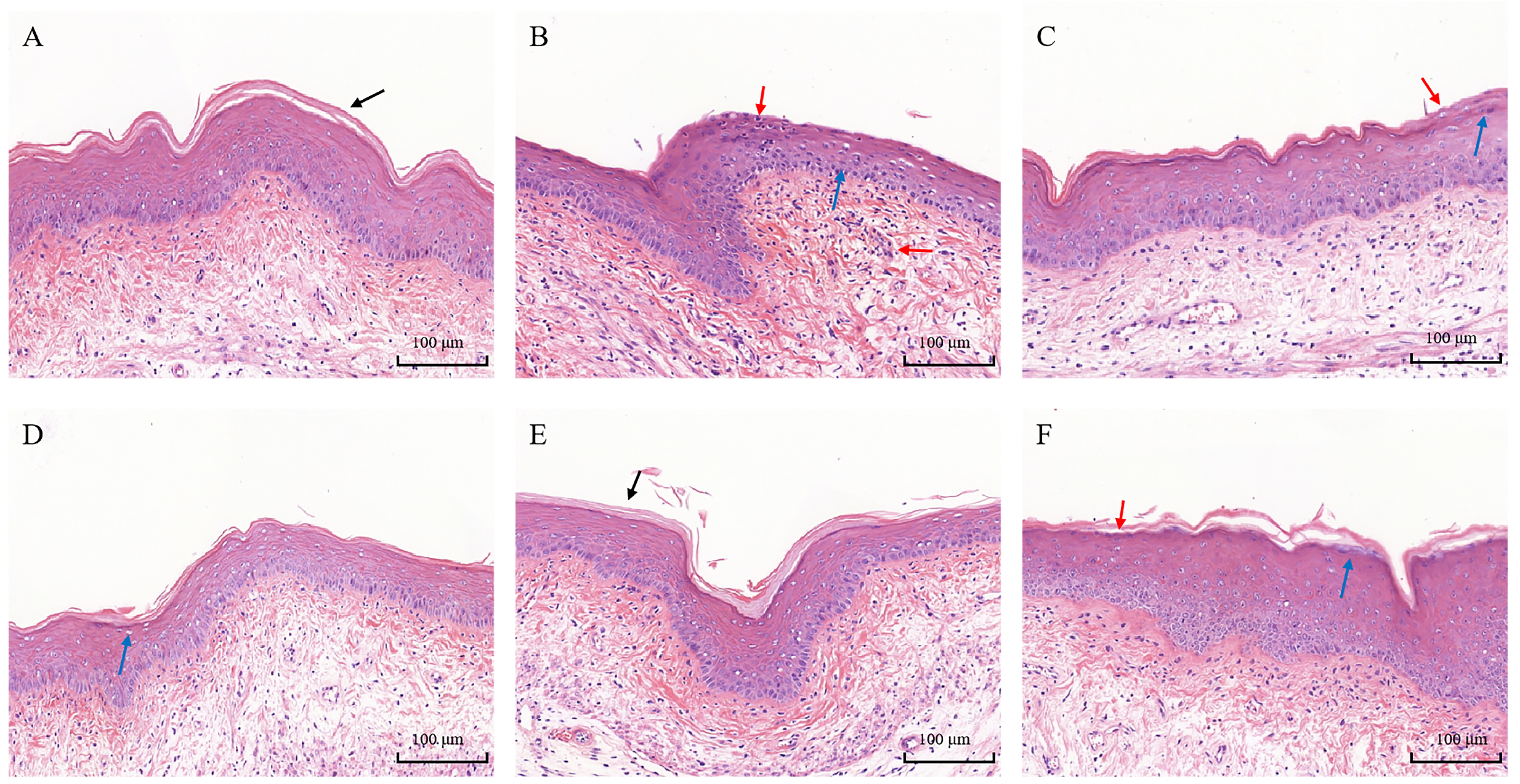
3.5. Histopathological Analysis
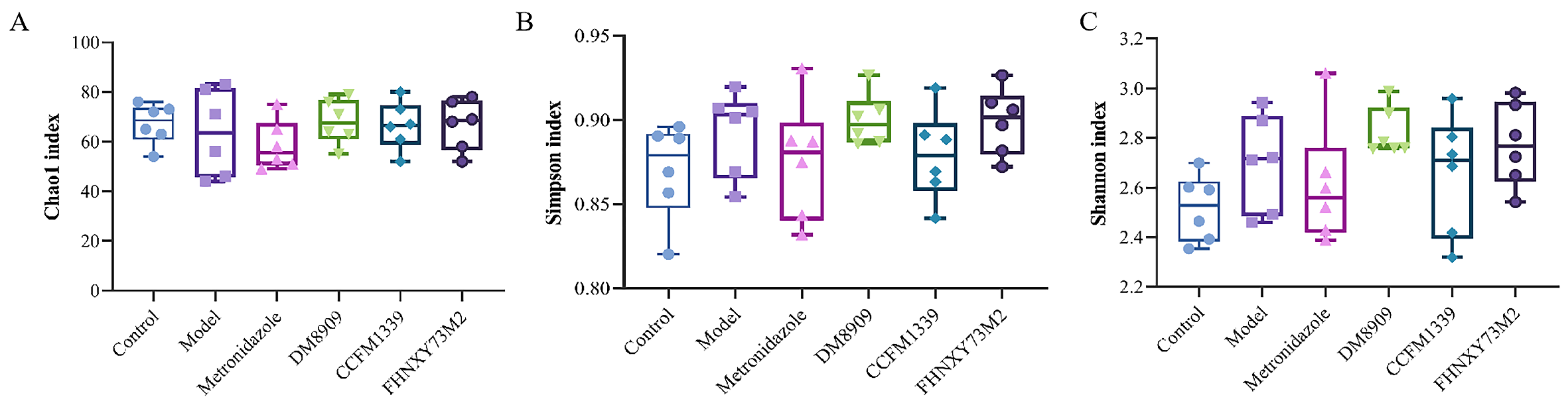
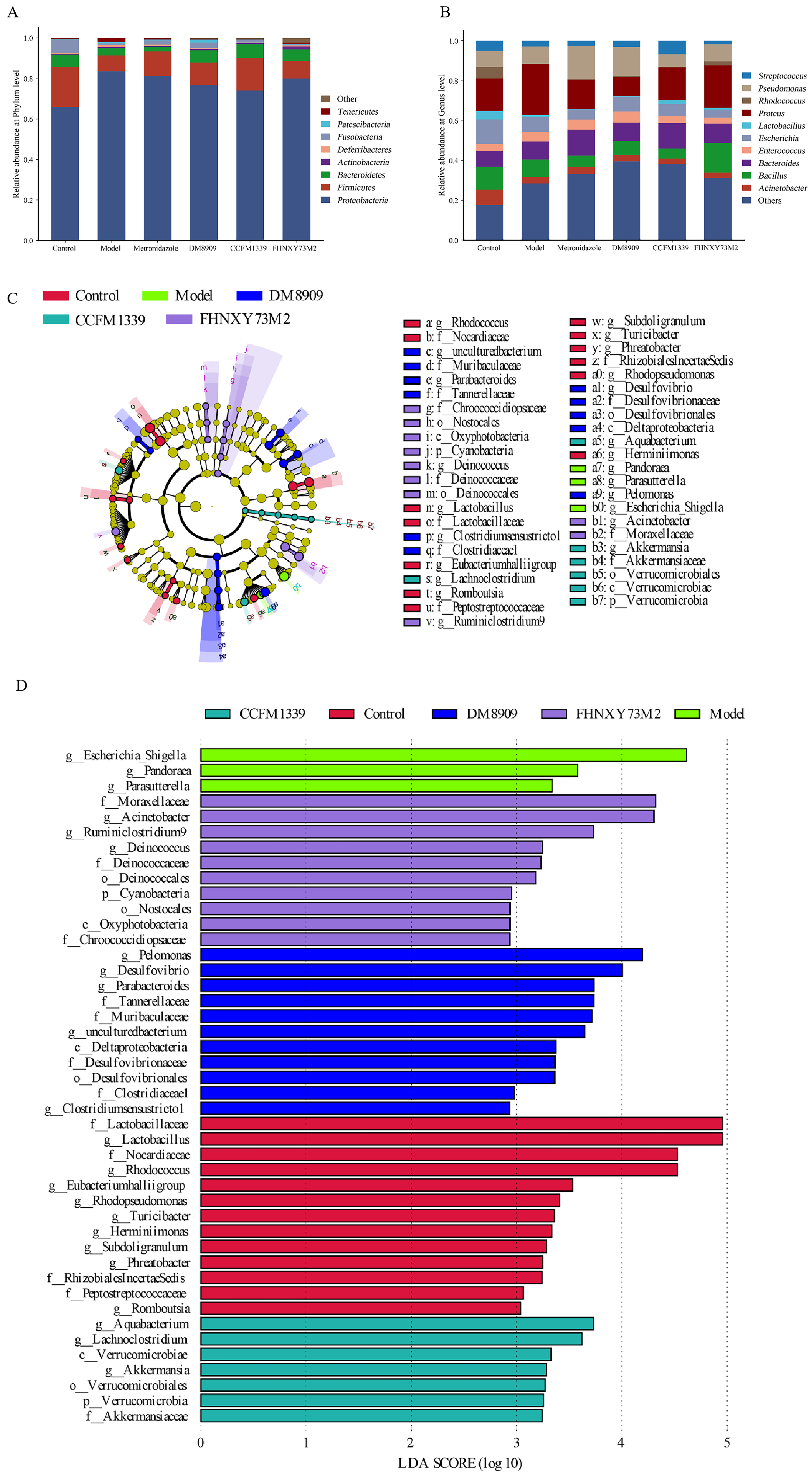
3.6. Microbiological Analysis of Mouse Vagina
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farage, M.; Maibach, H. Lifetime changes in the vulva and vagina. Arch. Gynecol. Obstet. 2006, 273, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4680–4687. [Google Scholar] [CrossRef]
- Andersch-Björkman, Y.; Thomsson, K.A.; Larsson, J.M.H.; Ekerhovd, E.; Hansson, G.C. Large scale identification of proteins, mucins, and their o-glycosylation in the endocervical mucus during the menstrual cycle. Mol. Cell. Proteom. 2007, 6, 708–716. [Google Scholar] [CrossRef]
- Radtke, A.L.; Quayle, A.J.; Herbst-Kralovetz, M.M. Microbial products alter the expression of membrane-associated mucin and antimicrobial peptides in a three-dimensional human endocervical epithelial cell model. Biol. Reprod. 2012, 87, 132–141. [Google Scholar] [CrossRef]
- Gipson, I.K.; Moccia, R.; Spurr-Michaud, S.; Argueso, P.; Gargiulo, A.R.; Offner, G.D.; Keutmann, H.T. The amount of muc5b mucin in cervical mucus peaks at midcycle. J. Clin. Endocrinol. Metab. 2001, 86, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Filardo, S.; Pietro, M.D.; Tranquilli, G.; Latino, M.A.; Recine, N.; Porpora, M.G.; Sessa, R. Selected immunological mediators and cervical microbial signatures in women with chlamydia trachomatis infection. MSystems 2019, 4, e00094-19. [Google Scholar] [CrossRef] [PubMed]
- Hearps, A.; Tyssen, D.; Srbinovski, D.; Bayigga, L.; Diaz, D.; Aldunate, M.; Cone, R.; Gugasyan, R.; Anderson, D.; Tachedjian, G. Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with hiv acquisition. Mucosal Immunol. 2017, 10, 1480–1490. [Google Scholar] [CrossRef]
- Delgado-Diaz, D.J.; Jesaveluk, B.; Hayward, J.A.; Tyssen, D.; Alisoltani, A.; Potgieter, M.; Bell, L.; Ross, E.; Iranzadeh, A.; Allali, I.; et al. Lactic acid from vaginal microbiota enhances cervicovaginal epithelial barrier integrity by promoting tight junction protein expression. Microbiome 2022, 10, 141. [Google Scholar] [CrossRef]
- Vornhagen, J.; Armistead, B.; Santana-Ufret, V.; Gendrin, C.; Merillat, S.; Coleman, M.; Quach, P.; Boldenow, E.; Alishetti, V.; Leonhard-Melief, C.; et al. Group b streptococcus exploits vaginal epithelial exfoliation for ascending infection. J. Clin. Investig. 2018, 128, 1985–1999. [Google Scholar] [CrossRef]
- Zevin, A.S.; Xie, I.Y.; Birse, K.; Arnold, K.; Romas, L.; Westmacott, G.; Novak, R.M.; McCorrister, S.; McKinnon, L.R.; Cohen, C.R.; et al. Microbiome composition and function drives wound-healing impairment in the female genital tract. PLoS Pathog. 2016, 12, e1005889. [Google Scholar] [CrossRef]
- Cauci, S.; Culhane, J.F.; Santolo, M.D.; McCollum, K. Among pregnant women with bacterial vaginosis, the hydrolytic enzymes sialidase and prolidase are positively associated with interleukin-1β. Am. J. Obstet. Gynecol. 2008, 198, 132e1. [Google Scholar] [CrossRef]
- d’Enfert, C.; Kaune, A.-K.; Alaban, L.-R.; Chakraborty, S.; Cole, N.; Delavy, M.; Kosmala, D.; Marsaux, B.; Frois-Martins, R.; Morelli, M.; et al. The impact of the fungus-host-microbiota interplay upon candida albicans infections: Current knowledge and new perspectives. FEMS Microbiol. Rev. 2021, 45, fuaa060. [Google Scholar]
- Mitra, A.; MacIntyre, D.A.; Marchesi, J.R.; Lee, Y.S.; Bennett, P.R.; Kyrgiou, M. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: What do we know and where are we going next? Microbiome 2016, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Tomas, M.; Oliveira, A.P.; Simoes, S.; Oliveira, J.M.; Oliveira, R.P. Bacterial vaginosis: Standard treatments and alternative strategies. Int. J. Pharm. 2020, 587, 119659. [Google Scholar] [CrossRef] [PubMed]
- Heczko, P.B.; Tomusiak, A.; Adamski, P.; Jakimiuk, A.J.; Stefański, G.; Mikołajczyk-Cichońska, A.; Suda-Szczurek, M.; Strus, M. Supplementation of standard antibiotic therapy with oral probiotics for bacterial vaginosis and aerobic vaginitis: A randomised, double-blind, placebo-controlled trial. BMC Women’s Health 2015, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Ehrström, S.; Daroczy, K.; Rylander, E.; Samuelsson, C.; Johannesson, U.; Anzén, B.; Påhlson, C. Lactic acid bacteria colonization and clinical outcome after probiotic supplementation in conventionally treated bacterial vaginosis and vulvovaginal candidiasis. Microbes Infect. 2010, 12, 691–699. [Google Scholar] [CrossRef]
- Armstrong, E.; Hemmerling, A.; Miller, S.; Burke, K.E.; Newmann, S.J.; Morris, S.R.; Reno, H.; Huibner, S.; Kulikova, M.; Nagelkerke, N.; et al. Sustained effect of lactin-v (lactobacillus crispatus ctv-05) on genital immunology following standard bacterial vaginosis treatment: Results from a randomised, placebo-controlled trial. Lancet Microbe 2022, 3, e435–e442. [Google Scholar] [CrossRef]
- Nold, C.; Anton, L.; Brown, A.; Elovitz, M. Inflammation promotes a cytokine response and disrupts the cervical epithelial barrier: A possible mechanism of premature cervical remodeling and preterm birth. Am. J. Obstet. Gynecol. 2012, 206, 208e1. [Google Scholar] [CrossRef]
- Takada, K.; Komine-Aizawa, S.; Hirohata, N.; Trinh, Q.D.; Nishina, A.; Kimura, H.; Hayakawa, S. Poly i: C induces collective migration of hacat keratinocytes via il-8. BMC Immunol. 2017, 18, 19. [Google Scholar] [CrossRef]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, W.; Jiang, Y.; Lessing, D.J.; Chu, W. Synthetic bacterial consortia transplantation for the treatment of gardnerella vaginalis-induced bacterial vaginosis in mice. Microbiome 2023, 11, 54. [Google Scholar] [CrossRef]
- Sierra, L.-J.; Brown, A.G.; Barilá, G.O.; Anton, L.; Barnum, C.E.; Shetye, S.S.; Soslowsky, L.J.; Elovitz, M.A. Colonization of the cervicovaginal space with gardnerella vaginalis leads to local inflammation and cervical remodeling in pregnant mice. PLoS ONE 2018, 13, e0191524. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.-M.; Kang, G.-D.; Jeong, J.-J.; Choi, H.S.; Kim, D.-H. Neomangiferin modulates the th17/treg balance and ameliorates colitis in mice. Phytomedicine 2016, 23, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.M.; Ma, N.; Mitchell, A.J.; Wu, M.C.; Valint, D.; Proll, S.; Reed, S.D.; Guthrie, K.A.; Lacroix, A.Z.; Larson, J.C.; et al. Association between postmenopausal vulvovaginal discomfort, vaginal microbiota, and mucosal inflammation. Am. J. Obstet. Gynecol. 2021, 225, 159.e1. [Google Scholar] [CrossRef] [PubMed]
- Lewis, W.G.; Robinson, L.S.; Gilbert, N.M.; Perry, J.C.; Lewis, A.L. Degradation, foraging, and depletion of mucus sialoglycans by the vagina-adapted actinobacterium gardnerella vaginalis. J. Biol. Chem. 2013, 288, 12067–12079. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gong, H.; Liu, L.; Ramos-Solis, N.; Seye, C.I.; Derbigny, W.A. Tlr3 deficiency exacerbates the loss of epithelial barrier function during genital tract chlamydia muridarum infection. PLoS ONE 2019, 14, e0207422. [Google Scholar] [CrossRef] [PubMed]
- Dusio, G.F.; Cardani, D.; Zanobbio, L.; Mantovani, M.; Luchini, P.; Battini, L.; Galli, V.; Diana, A.; Balsari, A.; Rumio, C. Stimulation of tlrs by lmw-ha induces self-defense mechanisms in vaginal epithelium. Immunol. Cell Biol. 2011, 89, 630–639. [Google Scholar] [CrossRef]
- Blaskewicz, C.D.; Pudney, J.; Anderson, D.J. Structure and function of intercellular junctions in human cervical and vaginal mucosal epithelia. Biol. Reprod. 2011, 85, 97–104. [Google Scholar] [CrossRef]
- Genc, M.R.; Witkin, S.S.; Delaney, M.L.; Paraskevas, L.-R.; Tuomala, R.E.; Norwitz, E.R.; Onderdonk, A.B. A disproportionate increase in il-1β over il-1ra in the cervicovaginal secretions of pregnant women with alteredvaginal microflora correlates with preterm birth. Am. J. Obstet. Gynecol. 2004, 190, 1191–1197. [Google Scholar] [CrossRef]
- Schwecht, I.; Nazli, A.; Gill, B.; Kaushic, C. Lactic acid enhances vaginal epithelial barrier integrity and ameliorates inflammatory effects of dysbiotic short chain fatty acids and hiv-1. Sci. Rep. 2023, 13, 20065. [Google Scholar] [CrossRef]
- Joo, H.-M.; Hyun, Y.-J.; Myoung, K.-S.; Ahn, Y.-T.; Lee, J.-H.; Huh, C.-S.; Han, M.J.; Kim, D.-H. Lactobacillus johnsonii hy7042 ameliorates gardnerella vaginalis-induced vaginosis by killing gardnerella vaginalis and inhibiting nf-κb activation. Int. Immunopharmacol. 2011, 11, 1758–1765. [Google Scholar] [CrossRef]
- Kammala, A.K.; Mosebarger, A.; Radnaa, E.; Rowlinson, E.; Vora, N.; Fortunato, S.J.; Sharma, S.; Safarzadeh, M.; Menon, R. Extracellular vesicles-mediated recombinant il-10 protects against ascending infection-associated preterm birth by reducing fetal inflammatory response. Front. Immunol. 2023, 14, 1196453. [Google Scholar] [CrossRef]
- Shen, H.; Goldberg, E.; Saltzman, W.M. Gene expression and mucosal immune responses after vaginal dna immunization in mice using a controlled delivery matrix. J. Control. Release 2003, 86, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Kawamura, M.; Ueno, M.; Hiraishi, K.; Adachi, M.; Serizawa, T.; Akashi, M.; Baba, M. Mucosal immunization with inactivated hiv-1-capturing nanospheres induces a significant hiv-1-specific vaginal antibody response in mice. J. Med. Virol. 2003, 69, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Breedveld, A.C.; Schuster, H.J.; van Houdt, R.; Painter, R.C.; Mebius, R.E.; der Veer, C.v.; Bruisten, S.M.; Savelkoul, P.H.; Egmond, M.v. Enhanced iga coating of bacteria in women with lactobacillus crispatus-dominated vaginal microbiota. Microbiome 2022, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Noda-Nicolau, N.M.; Bastos, L.B.; Bolpetti, A.N.; Pinto, G.V.S.; Marcolino, L.D.; Marconi, C.; Ferreira, C.S.T.; Polettini, J.; Vieira, E.P.; Silva, M.G.D. Cervicovaginal levels of human β-defensin 1, 2, 3, and 4 of reproductive-aged women with chlamydia trachomatis infection. J. Low. Genit. Tract Dis. 2017, 21, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, A.; Bruyere, D.; Roncarati, P.; Peixoto, P.; Hervouet, E.; Cobraiville, G.; Taminiau, B.; Masson, M.; Gallego, C.; Mazzucchelli, G.; et al. Hpv infection alters vaginal microbiome through down-regulating host mucosal innate peptides used by lactobacilli as amino acid sources. Nat. Commun. 2022, 13, 1076. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Casanova, J.-L.; Puel, A. Mucocutaneous il-17 immunity in mice and humans: Host defense vs. excessive inflammation. Mucosal Immunol. 2018, 11, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.-E.; Jeong, J.-J.; Choi, S.-Y.; Kim, H.; Han, M.J.; Kim, D.-H. Lactobacillus rhamnosus hn001 and lactobacillus acidophilus gla-14 attenuate gardnerella vaginalis-infected bacterial vaginosis in mice. Nutrients 2017, 9, 531. [Google Scholar] [CrossRef]
- Ziklo, N.; Huston, W.M.; Taing, K.; Timms, P. High expression of ido1 and tgf-β1 during recurrence and post infection clearance with chlamydia trachomatis, are independent of host ifn-γ response. BMC Infect. Dis. 2019, 19, 218. [Google Scholar] [CrossRef]
- Borges, S.; Silva, J.; Teixeira, P. The role of lactobacilli and probiotics in maintaining vaginal health. Arch. Gynecol. Obstet. 2014, 289, 479–489. [Google Scholar] [CrossRef]
- Zuend, C.F.; Lamont, A.; Noel-Romas, L.; Knodel, S.; Birse, K.; Kratzer, K.; McQueen, P.; Perner, M.; Ayele, H.; Mutch, S.; et al. Increased genital mucosal cytokines in canadian women associate with higher antigen-presenting cells, inflammatory metabolites, epithelial barrier disruption, and the depletion of l. crispatus. Microbiome 2023, 11, 159. [Google Scholar]
- Berard, A.R.; Brubaker, D.K.; Birse, K.; Lamont, A.; Mackelprang, R.D.; Noël-Romas, L.; Perner, M.; Hou, X.; Irungu, E.; Mugo, N.; et al. Vaginal epithelial dysfunction is mediated by the microbiome, metabolome, and mtor signaling. Cell Rep. 2023, 42, 5. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.J.; Marathe, J.; Pudney, J. The structure of the human vaginal stratum corneum and its role in immune defense. Am. J. Reprod. Immunol. 2014, 71, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Anton, L.; Ferguson, B.; Friedman, E.S.; Gerson, K.D.; Brown, A.G.; Elovitz, M.A. Gardnerella vaginalis alters cervicovaginal epithelial cell function through microbe-specific immune responses. Microbiome 2022, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Onderdonk, A.B.; Delaney, M.L.; Fichorova, R.N. The human microbiome during bacterial vaginosis. Clin. Microbiol. Rev. 2016, 29, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Bennett, P.R.; Lee, Y.S.; Kundu, S.; Teoh, T.; Adan, M.; Ahmed, S.; Brown, R.G.; David, A.L.; Lewis, H.V.; et al. Microbial-driven preterm labour involves crosstalk between the innate and adaptive immune response. Nat. Commun. 2022, 13, 975. [Google Scholar] [CrossRef]
- Hedge, S.R.; Barrientes, F.; Desmond, R.A.; Schwebke, J.R. Local and systemic cytokine levels in relation to changes in vaginal flora. J. Infect. Dis. 2006, 193, 556–562. [Google Scholar] [CrossRef]
- Li, Z.; Palaniyandi, S.; Zeng, R.; Tuo, W.; Roopenian, D.C.; Zhu, X. Transfer of igg in the female genital tract by mhc class i-related neonatal fc receptor (fcrn) confers protective immunity to vaginal infection. Proc. Natl. Acad. Sci. USA 2011, 108, 4388–4393. [Google Scholar] [CrossRef]
- VanBenschoten, H.M.; Woodrow, K.A. Vaginal delivery of vaccines. Adv. Drug Deliv. Rev. 2021, 178, 113956. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Kannan, A.; Nunn, K.L.; Murphy, M.A.; Subramani, D.B.; Moench, T.; Cone, R.; Lai, S.K. Igg in cervicovaginal mucus traps hsv and prevents vaginal herpes infections. Mucosal Immunol. 2014, 7, 1036–1044. [Google Scholar] [CrossRef]
- Murphy, K.; Gromisch, M.; Srinivasan, S.; Wang, T.; Wood, L.; Proll, S.; Liu, C.; Fiedler, T.; Valint, D.; Fredricks, D.N.; et al. Iga coating of vaginal bacteria is reduced in the setting of bacterial vaginosis (bv) and preferentially targets bv-associated species. Infect. Immun. 2023, 1, e00373. [Google Scholar] [CrossRef] [PubMed]
- Feinen, B.; Jerse, A.E.; Gaffen, S.L.; Russell, M.W. Critical role of th17 responses in a murine model of neisseria gonorrhoeae genital infection. Mucosal Immunol. 2010, 3, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Bradley, F.; Birse, K.; Hasselrot, K.; Noël-Romas, L.; Introini, A.; Wefer, H.; Seifert, M.; Engstrand, L.; Tjernlund, A.; Broliden, K.; et al. The vaginal microbiome amplifies sex hormone-associated cyclic changes in cervicovaginal inflammation and epithelial barrier disruption. Am. J. Reprod. Immunol. 2018, 80, e12863. [Google Scholar] [CrossRef] [PubMed]
- France, M.; Alizadeh, M.; Brown, S.; Ma, B.; Ravel, J. Towards a deeper understanding of the vaginal microbiota. Nat. Microbiol. 2022, 7, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Anton, L.; Sierra, L.-J.; DeVine, A.; Barila, G.; Heiser, L.; Brown, A.G.; Elovitz, M.A. Common cervicovaginal microbial supernatants alter cervical epithelial function: Mechanisms by which lactobacillus crispatus contributes to cervical health. Front. Microbiol. 2018, 9, 2181. [Google Scholar] [CrossRef]
- Chen, T.; Xia, C.; Hu, H.; Wang, H.; Tan, B.; Tian, P.; Zhao, X.; Wang, L.; Han, Y.; Deng, K.-Y.; et al. Dysbiosis of the rat vagina is efficiently rescued by vaginal microbiota transplantation or probiotic combination. Int. J. Antimicrob. Agents 2021, 57, 106277. [Google Scholar] [CrossRef]




| Gene | Primer Sequence (5′-3′) |
|---|---|
| GAPDH | Forward: 5′-TGAGTGGCAAAGTGGAGAT-3′ |
| Reverse: 5′-TTTGCCGTGAGTGGAGTCAT-3′ | |
| IL-17 | Forward: 5′-TGAGTGGCAAAGTGGAGAT-3′ |
| Reverse: 5′-CTTTCCCTCCGCATTGACAC-3′ | |
| Foxp3 | Forward: 5′-CCCATCCCCAGGAGTCTT-3′ |
| Reverse: 5′-ACCATGACTAGGGGCACTGTA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Lin, R.; Mao, B.; Tang, X.; Zhao, J.; Zhang, Q.; Cui, S. Lactobacillus crispatus CCFM1339 Inhibits Vaginal Epithelial Barrier Injury Induced by Gardnerella vaginalis in Mice. Biomolecules 2024, 14, 240. https://doi.org/10.3390/biom14020240
Huang X, Lin R, Mao B, Tang X, Zhao J, Zhang Q, Cui S. Lactobacillus crispatus CCFM1339 Inhibits Vaginal Epithelial Barrier Injury Induced by Gardnerella vaginalis in Mice. Biomolecules. 2024; 14(2):240. https://doi.org/10.3390/biom14020240
Chicago/Turabian StyleHuang, Xiaoyan, Rumeng Lin, Bingyong Mao, Xin Tang, Jianxin Zhao, Qiuxiang Zhang, and Shumao Cui. 2024. "Lactobacillus crispatus CCFM1339 Inhibits Vaginal Epithelial Barrier Injury Induced by Gardnerella vaginalis in Mice" Biomolecules 14, no. 2: 240. https://doi.org/10.3390/biom14020240
APA StyleHuang, X., Lin, R., Mao, B., Tang, X., Zhao, J., Zhang, Q., & Cui, S. (2024). Lactobacillus crispatus CCFM1339 Inhibits Vaginal Epithelial Barrier Injury Induced by Gardnerella vaginalis in Mice. Biomolecules, 14(2), 240. https://doi.org/10.3390/biom14020240






