Beyond Natriuretic Peptides: Unveiling the Power of Emerging Biomarkers in Heart Failure
Abstract
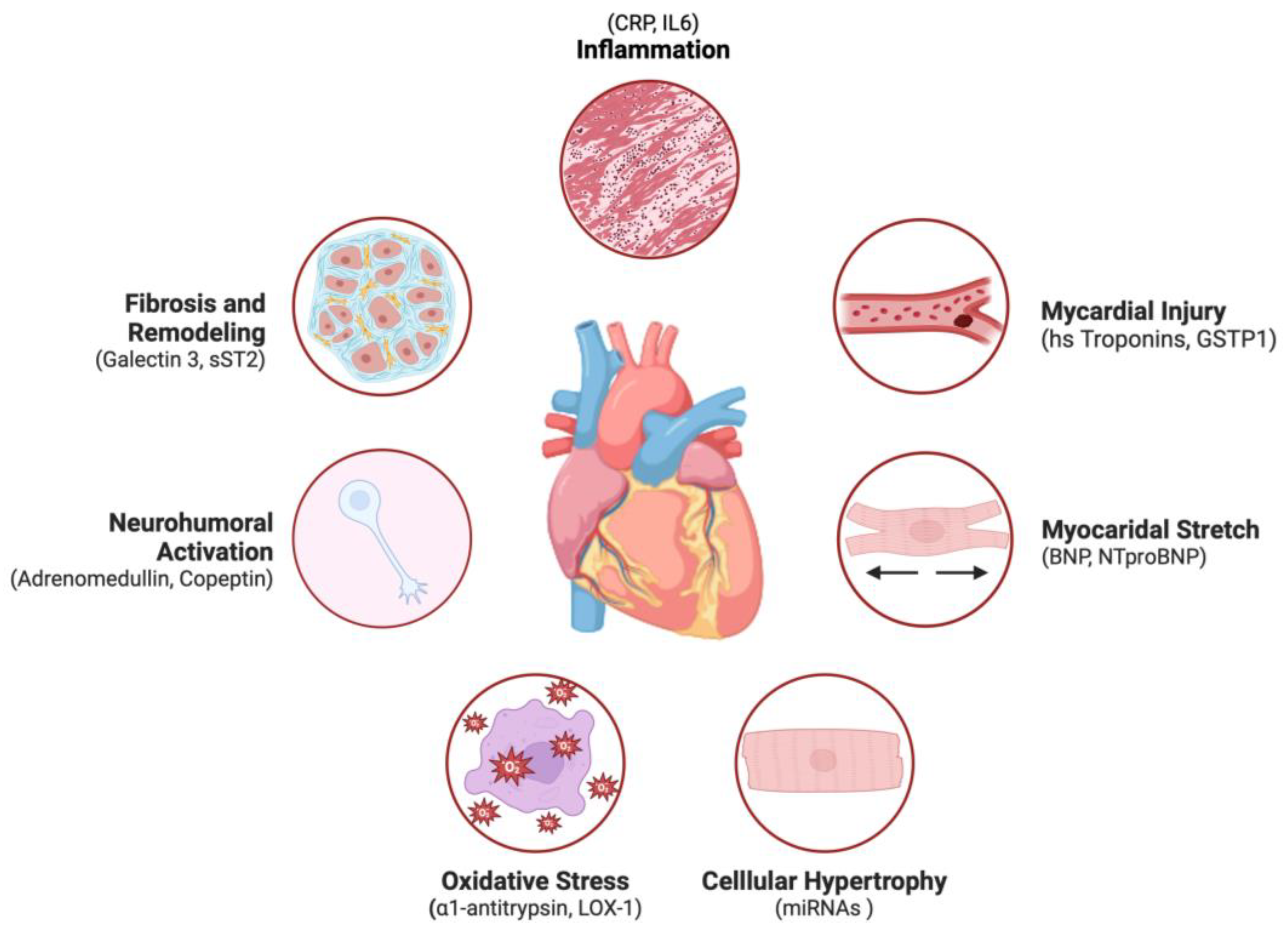
1. Introduction
2. Biomarkers in Heart Failure: An Overview
2.1. Definition and Classification of Biomarkers
2.2. Neurohormonal Activation Biomarkers
2.2.1. Norepinephrine
2.2.2. Chromogranin A and B
2.2.3. Plasma Renin Activity (PRA)
2.2.4. Adrenomedullin (ADM)
2.2.5. Copeptin
2.2.6. Endothelin-1 (ET-1)
2.2.7. Urocortin-1
2.3. Biomarkers of Cardiac Damage
2.3.1. Cardiac Troponins
2.3.2. Heart-Type Fatty-Acid-Binding Protein (H-FABP)
2.3.3. Glutathione Transferase P1 (GSTP1)
2.4. Markers of Myocardial Fibrosis
2.4.1. Galectin-3
2.4.2. Soluble Isoform of Suppression of Tumorigenicity 2
2.4.3. Growth Differentiation Factor-15
2.4.4. Wnt-β Catenin
2.4.5. Non-Coding RNAs
2.5. Biomarkers of Inflammation
The Role of Insulin
3. Multi-Marker Testing
4. The Advent of Omics in HF
4.1. Genomics
4.2. Epigenomics
4.3. Transcriptomics
4.4. Proteomics
4.5. Metabolomics
5. Shifting to Therapeutic Targets
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ibrahim, N.E.; Januzzi, J.L. Established and Emerging Roles of Biomarkers in Heart Failure. Circ. Res. 2018, 123, 614–629. [Google Scholar] [CrossRef]
- Dattilo, G.; Laterra, G.; Licordari, R.; Parisi, F.; Pistelli, L.; Colarusso, L.; Zappia, L.; Vaccaro, V.; Demurtas, E.; Allegra, M.; et al. The Long-Term Benefit of Sacubitril/Valsartan in Patients with HFrEF: A 5-Year Follow-Up Study in a Real World Population. J. Clin. Med. 2023, 12, 6247. [Google Scholar] [CrossRef]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2018, 379, 1007–1016. [Google Scholar] [CrossRef]
- Dumeny, L.; Vardeny, O.; Edelmann, F.; Pieske, B.; Duarte, J.D.; Cavallari, L.H. NR3C2 Genotype Is Associated with Response to Spironolactone in Diastolic Heart Failure Patients from the Aldo-DHF Trial. Pharmacotherapy 2021, 41, 978–987. [Google Scholar] [CrossRef]
- Pellicori, P.; Ferreira, J.P.; Mariottoni, B.; Brunner-La Rocca, H.; Ahmed, F.Z.; Verdonschot, J.; Collier, T.; Cuthbert, J.J.; Petutschnigg, J.; Mujaj, B.; et al. Effects of Spironolactone on Serum Markers of Fibrosis in People at High Risk of Developing Heart Failure: Rationale, Design and Baseline Characteristics of a Proof-of-concept, Randomised, Precision-medicine, Prevention Trial. The Heart OMics in AGing (HOMAGE) Trial. Eur. J. Heart Fail. 2020, 22, 1711–1723. [Google Scholar] [CrossRef]
- Napoli, C.; Benincasa, G.; Donatelli, F.; Ambrosio, G. Precision Medicine in Distinct Heart Failure Phenotypes: Focus on Clinical Epigenetics. Am. Heart J. 2020, 224, 113–128. [Google Scholar] [CrossRef]
- Seferović, P.M.; Fragasso, G.; Petrie, M.; Mullens, W.; Ferrari, R.; Thum, T.; Bauersachs, J.; Anker, S.D.; Ray, R.; Çavuşoğlu, Y.; et al. Sodium–Glucose Co-Transporter 2 Inhibitors in Heart Failure: Beyond Glycaemic Control. A Position Paper of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1495–1503. [Google Scholar] [CrossRef]
- Biasucci, L.M.; Maino, A.; Grimaldi, M.C.; Cappannoli, L.; Aspromonte, N. Novel Biomarkers in Heart Failure: New Insight in Pathophysiology and Clinical Perspective. J. Clin. Med. 2021, 10, 2771. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.G.; Gaggin, H.K. Leveraging Biomarkers for Precision Medicine in Heart Failure. J. Card. Fail. 2023, 29, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Nakao, M.; Anzai, T. Risk Stratification Towards Precision Medicine in Heart Failure—Current Progress and Future Perspectives. Circ. J. 2021, 85, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Wettersten, N. Biomarkers in Acute Heart Failure: Diagnosis, Prognosis, and Treatment. Int. J. Heart Fail. 2021, 3, 81. [Google Scholar] [CrossRef] [PubMed]
- Sarhene, M.; Wang, Y.; Wei, J.; Huang, Y.; Li, M.; Li, L.; Acheampong, E.; Zhengcan, Z.; Xiaoyan, Q.; Yunsheng, X.; et al. Biomarkers in Heart Failure: The Past, Current and Future. Heart Fail. Rev. 2019, 24, 867–903. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.E.; Berezin, A.A. Biomarkers in Heart Failure: From Research to Clinical Practice. Ann. Lab. Med. 2023, 43, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, R.; Lee, R.T. Directions from Hecate: Towards a Multi-Marker Approach for Heart Failure Assessment. Eur. J. Heart Fail. 2011, 13, 691–693. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 145, e137–e161. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Ullah, A.; Sajid, S.; Qureshi, M.; Kamran, M.; Anwaar, M.A.; Naseem, M.A.; Zaman, M.U.; Mahmood, F.; Rehman, A.; Shehryar, A.; et al. Novel Biomarkers and the Multiple-Marker Approach in Early Detection, Prognosis, and Risk Stratification of Cardiac Diseases: A Narrative Review. Cureus 2023, 15, e42081. [Google Scholar] [CrossRef]
- Cohn, J.N.; Levine, T.B.; Olivari, M.T.; Garberg, V.; Lura, D.; Francis, G.S.; Simon, A.B.; Rector, T. Plasma Norepinephrine as a Guide to Prognosis in Patients with Chronic Congestive Heart Failure. N. Engl. J. Med. 1984, 311, 819–823. [Google Scholar] [CrossRef]
- Francis, G.S.; Cohn, J.N.; Johnson, G.; Rector, T.S.; Goldman, S.; Simon, A. Plasma Norepinephrine, Plasma Renin Activity, and Congestive Heart Failure. Relations to Survival and the Effects of Therapy in V-HeFT II. The V-HeFT VA Cooperative Studies Group. Circulation 1993, 87, VI40–VI48. [Google Scholar] [PubMed]
- Ceconi, C. Chromogranin A in Heart Failure. A Novel Neurohumoral Factor and a Predictor for Mortality. Eur. Heart J. 2002, 23, 967–974. [Google Scholar] [CrossRef]
- Røsjø, H.; Husberg, C.; Dahl, M.B.; Stridsberg, M.; Sjaastad, I.; Finsen, A.V.; Carlson, C.R.; Øie, E.; Omland, T.; Christensen, G. Chromogranin B in Heart Failure: A Putative Cardiac Biomarker Expressed in the Failing Myocardium. Circ. Heart Fail. 2010, 3, 503–511. [Google Scholar] [CrossRef]
- Vergaro, G.; Emdin, M.; Iervasi, A.; Zyw, L.; Gabutti, A.; Poletti, R.; Mammini, C.; Giannoni, A.; Fontana, M.; Passino, C. Prognostic Value of Plasma Renin Activity in Heart Failure. Am. J. Cardiol. 2011, 108, 246–251. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Cheema, B.; Cleveland, E.; Sankar, K.; Subacius, H.; Fonarow, G.C.; Solomon, S.D.; Lewis, E.F.; Greene, S.J.; Maggioni, A.P.; et al. Plasma Renin Activity, Response to Aliskiren, and Clinical Outcomes in Patients Hospitalized for Heart Failure: The ASTRONAUT Trial. Eur. J. Heart Fail. 2018, 20, 677–686. [Google Scholar] [CrossRef]
- Voors, A.A.; Kremer, D.; Geven, C.; Ter Maaten, J.M.; Struck, J.; Bergmann, A.; Pickkers, P.; Metra, M.; Mebazaa, A.; Düngen, H.; et al. Adrenomedullin in Heart Failure: Pathophysiology and Therapeutic Application. Eur. J. Heart Fail. 2019, 21, 163–171. [Google Scholar] [CrossRef]
- Maisel, A.; Mueller, C.; Nowak, R.; Peacock, W.F.; Landsberg, J.W.; Ponikowski, P.; Mockel, M.; Hogan, C.; Wu, A.H.B.; Richards, M.; et al. Mid-Region Pro-Hormone Markers for Diagnosis and Prognosis in Acute Dyspnea. J. Am. Coll. Cardiol. 2010, 55, 2062–2076. [Google Scholar] [CrossRef]
- Shah, R.V.; Truong, Q.A.; Gaggin, H.K.; Pfannkuche, J.; Hartmann, O.; Januzzi, J.L. Mid-Regional pro-Atrial Natriuretic Peptide and pro-Adrenomedullin Testing for the Diagnostic and Prognostic Evaluation of Patients with Acute Dyspnoea. Eur. Heart J. 2012, 33, 2197–2205. [Google Scholar] [CrossRef]
- Chatterjee, K. Neurohormonal Activation in Congestive Heart Failure and the Role of Vasopressin. Am. J. Cardiol. 2005, 95, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Maisel, A.; Xue, Y.; Shah, K.; Mueller, C.; Nowak, R.; Peacock, W.F.; Ponikowski, P.; Mockel, M.; Hogan, C.; Wu, A.H.B.; et al. Increased 90-Day Mortality in Patients with Acute Heart Failure with Elevated Copeptin: Secondary Results From the Biomarkers in Acute Heart Failure (BACH) Study. Circ. Heart Fail. 2011, 4, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wang, R.; Yan, L.; Lin, M.; Liu, X.; You, T. Copeptin in Heart Failure: Review and Meta-Analysis. Clin. Chim. Acta 2017, 475, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-L.; Xie, S.; Qiao, X.; An, Y.-M.; Zhang, Y.; Li, L.; Guo, X.-B.; Zhang, F.-C.; Wu, L.-L. Plasma Endothelin-1-Related Peptides as the Prognostic Biomarkers for Heart Failure: A PRISMA-Compliant Meta-Analysis. Medicine 2017, 96, e9342. [Google Scholar] [CrossRef]
- Perez, A.L.; Grodin, J.L.; Wu, Y.; Hernandez, A.F.; Butler, J.; Metra, M.; Felker, G.M.; Voors, A.A.; McMurray, J.J.; Armstrong, P.W.; et al. Increased Mortality with Elevated Plasma Endothelin-1 in Acute Heart Failure: An ASCEND-HF Biomarker Substudy. Eur. J. Heart Fail. 2016, 18, 290–297. [Google Scholar] [CrossRef]
- Gaggin, H.K.; Truong, Q.A.; Gandhi, P.U.; Motiwala, S.R.; Belcher, A.M.; Weiner, R.B.; Baggish, A.L.; Januzzi, J.L. Systematic Evaluation of Endothelin 1 Measurement Relative to Traditional and Modern Biomarkers for Clinical Assessment and Prognosis in Patients with Chronic Systolic Heart Failure. Am. J. Clin. Pathol. 2017, 147, 461–472. [Google Scholar] [CrossRef]
- Rademaker, M.T.; Richards, A.M. Urocortins: Actions in Health and Heart Failure. Clin. Chim. Acta 2017, 474, 76–87. [Google Scholar] [CrossRef]
- Van Kimmenade, R.R.J.; Januzzi, J.L. Emerging Biomarkers in Heart Failure. Clin. Chem. 2012, 58, 127–138. [Google Scholar] [CrossRef]
- Berezin, A.E. Circulating Biomarkers in Heart Failure. In Heart Failure: From Research to Clinical Practice; Islam, M.S., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2018; Volume 1067, pp. 89–108. ISBN 978-3-319-78279-9. [Google Scholar]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; ESC Scientific Document Group. Fourth Universal Definition of Myocardial Infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Latini, R.; Masson, S.; Anand, I.S.; Missov, E.; Carlson, M.; Vago, T.; Angelici, L.; Barlera, S.; Parrinello, G.; Maggioni, A.P.; et al. Prognostic Value of Very Low Plasma Concentrations of Troponin T in Patients with Stable Chronic Heart Failure. Circulation 2007, 116, 1242–1249. [Google Scholar] [CrossRef]
- Aimo, A.; Januzzi, J.L.; Vergaro, G.; Ripoli, A.; Latini, R.; Masson, S.; Magnoli, M.; Anand, I.S.; Cohn, J.N.; Tavazzi, L.; et al. Prognostic Value of High-Sensitivity Troponin T in Chronic Heart Failure: An Individual Patient Data Meta-Analysis. Circulation 2018, 137, 286–297. [Google Scholar] [CrossRef]
- Wettersten, N.; Maisel, A. Veterans Affairs San Diego Healthcare System, La Jolla, CA, USA Role of Cardiac Troponin Levels in Acute Heart Failure. Card. Fail. Rev. 2015, 1, 102. [Google Scholar] [CrossRef]
- Xue, Y.; Clopton, P.; Peacock, W.F.; Maisel, A.S. Serial Changes in High-sensitive Troponin I Predict Outcome in Patients with Decompensated Heart Failure. Eur. J. Heart Fail. 2011, 13, 37–42. [Google Scholar] [CrossRef]
- Pascual-Figal, D.A.; Casas, T.; Ordonez-LLanos, J.; Manzano-Fernández, S.; Bonaque, J.C.; Boronat, M.; Muñoz-Esparza, C.; Valdés, M.; Januzzi, J.L. Highly Sensitive Troponin T for Risk Stratification of Acutely Destabilized Heart Failure. Am. Heart J. 2012, 163, 1002–1010. [Google Scholar] [CrossRef]
- Packer, M.; Januzzi, J.L.; Ferreira, J.P.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Brueckmann, M.; Jamal, W.; Cotton, D.; et al. Concentration-dependent Clinical and Prognostic Importance of High-sensitivity Cardiac Troponin T in Heart Failure and a Reduced Ejection Fraction and the Influence of Empagliflozin: The EMPEROR-Reduced Trial. Eur. J. Heart Fail. 2021, 23, 1529–1538. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Chmurzyńska, A. The Multigene Family of Fatty Acid-Binding Proteins (FABPs): Function, Structure and Polymorphism. J. Appl. Genet. 2006, 47, 39–48. [Google Scholar] [CrossRef]
- O’Donoghue, M.; De Lemos, J.A.; Morrow, D.A.; Murphy, S.A.; Buros, J.L.; Cannon, C.P.; Sabatine, M.S. Prognostic Utility of Heart-Type Fatty Acid Binding Protein in Patients with Acute Coronary Syndromes. Circulation 2006, 114, 550–557. [Google Scholar] [CrossRef]
- Setsuta, K.; Seino, Y.; Ogawa, T.; Arao, M.; Miyatake, Y.; Takano, T. Use of Cytosolic and Myofibril Markers in the Detection of Ongoing Myocardial Damage in Patients with Chronic Heart Failure. Am. J. Med. 2002, 113, 717–722. [Google Scholar] [CrossRef]
- Kitai, T.; Kim, Y.-H.; Kiefer, K.; Morales, R.; Borowski, A.G.; Grodin, J.L.; Tang, W.H.W. Circulating Intestinal Fatty Acid-Binding Protein (I-FABP) Levels in Acute Decompensated Heart Failure. Clin. Biochem. 2017, 50, 491–495. [Google Scholar] [CrossRef]
- Qian, H.-Y.; Huang, J.; Yang, Y.-J.; Yang, Y.-M.; Li, Z.-Z.; Zhang, J.-M. Heart-Type Fatty Acid Binding Protein in the Assessment of Acute Pulmonary Embolism. Am. J. Med. Sci. 2016, 352, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Simeunovic, D.; Odanovic, N.; Pljesa-Ercegovac, M.; Radic, T.; Radovanovic, S.; Coric, V.; Milinkovic, I.; Matic, M.; Djukic, T.; Ristic, A.; et al. Glutathione Transferase P1 Polymorphism Might Be a Risk Determinant in Heart Failure. Dis. Markers 2019, 2019, 6984845. [Google Scholar] [CrossRef] [PubMed]
- Andrukhova, O.; Salama, M.; Rosenhek, R.; Gmeiner, M.; Perkmann, T.; Steindl, J.; Aharinejad, S. Serum Glutathione S-Transferase P1 1 in Prediction of Cardiac Function. J. Card. Fail. 2012, 18, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Bošnjak, I.; Selthofer-Relatić, K.; Včev, A. Prognostic Value of Galectin-3 in Patients with Heart Failure. Dis. Markers 2015, 2015, 690205. [Google Scholar] [CrossRef] [PubMed]
- Henderson, N.C.; Mackinnon, A.C.; Farnworth, S.L.; Kipari, T.; Haslett, C.; Iredale, J.P.; Liu, F.-T.; Hughes, J.; Sethi, T. Galectin-3 Expression and Secretion Links Macrophages to the Promotion of Renal Fibrosis. Am. J. Pathol. 2008, 172, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Reifenberg, K.; Lehr, H.-A.; Torzewski, M.; Steige, G.; Wiese, E.; Küpper, I.; Becker, C.; Ott, S.; Nusser, P.; Yamamura, K.-I.; et al. Interferon-γ Induces Chronic Active Myocarditis and Cardiomyopathy in Transgenic Mice. Am. J. Pathol. 2007, 171, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Meijers, W.C.; Januzzi, J.L.; deFilippi, C.; Adourian, A.S.; Shah, S.J.; Van Veldhuisen, D.J.; De Boer, R.A. Elevated Plasma Galectin-3 Is Associated with near-Term Rehospitalization in Heart Failure: A Pooled Analysis of 3 Clinical Trials. Am. Heart J. 2014, 167, 853–860.e4. [Google Scholar] [CrossRef] [PubMed]
- Miró, Ò.; González De La Presa, B.; Herrero-Puente, P.; Fernández Bonifacio, R.; Möckel, M.; Mueller, C.; Casals, G.; Sandalinas, S.; Llorens, P.; Martín-Sánchez, F.J.; et al. The GALA Study: Relationship between Galectin-3 Serum Levels and Short- and Long-Term Outcomes of Patients with Acute Heart Failure. Biomarkers 2017, 22, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Gehlken, C.; Suthahar, N.; Meijers, W.C.; De Boer, R.A. Galectin-3 in Heart Failure. Heart Fail. Clin. 2018, 14, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Januzzi, J.L.; Bayes-Genis, A.; Vergaro, G.; Sciarrone, P.; Passino, C.; Emdin, M. Clinical and Prognostic Significance of sST2 in Heart Failure. J. Am. Coll. Cardiol. 2019, 74, 2193–2203. [Google Scholar] [CrossRef]
- Aimo, A.; Vergaro, G.; Ripoli, A.; Bayes-Genis, A.; Pascual Figal, D.A.; De Boer, R.A.; Lassus, J.; Mebazaa, A.; Gayat, E.; Breidthardt, T.; et al. Meta-Analysis of Soluble Suppression of Tumorigenicity-2 and Prognosis in Acute Heart Failure. JACC Heart Fail. 2017, 5, 287–296. [Google Scholar] [CrossRef]
- Boisot, S.; Beede, J.; Isakson, S.; Chiu, A.; Clopton, P.; Januzzi, J.; Maisel, A.S.; Fitzgerald, R.L. Serial Sampling of ST2 Predicts 90-Day Mortality Following Destabilized Heart Failure. J. Card. Fail. 2008, 14, 732–738. [Google Scholar] [CrossRef]
- Van Vark, L.C.; Lesman-Leegte, I.; Baart, S.J.; Postmus, D.; Pinto, Y.M.; Orsel, J.G.; Westenbrink, B.D.; Brunner-la Rocca, H.P.; Van Miltenburg, A.J.M.; Boersma, E.; et al. Prognostic Value of Serial ST2 Measurements in Patients with Acute Heart Failure. J. Am. Coll. Cardiol. 2017, 70, 2378–2388. [Google Scholar] [CrossRef]
- Emdin, M.; Aimo, A.; Vergaro, G.; Bayes-Genis, A.; Lupón, J.; Latini, R.; Meessen, J.; Anand, I.S.; Cohn, J.N.; Gravning, J.; et al. sST2 Predicts Outcome in Chronic Heart Failure Beyond NT−proBNP and High-Sensitivity Troponin T. J. Am. Coll. Cardiol. 2018, 72, 2309–2320. [Google Scholar] [CrossRef]
- Huang, A.; Qi, X.; Hou, W.; Qi, Y.; Zhao, N.; Liu, K. Prognostic Value of sST2 and NT-proBNP at Admission in Heart Failure with Preserved, Mid-Ranged and Reduced Ejection Fraction. Acta Cardiol. 2018, 73, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.E. Diabetes Mellitus Related Biomarker: The Predictive Role of Growth-Differentiation Factor-15. Diabetes Metab. Syndr. 2016, 10, S154–S157. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Dogon, G.; Zeller, M.; Cottin, Y.; Vergely, C. GDF15 and Cardiac Cells: Current Concepts and New Insights. Int. J. Mol. Sci. 2021, 22, 8889. [Google Scholar] [CrossRef]
- Chow, S.L.; Maisel, A.S.; Anand, I.; Bozkurt, B.; De Boer, R.A.; Felker, G.M.; Fonarow, G.C.; Greenberg, B.; Januzzi, J.L.; Kiernan, M.S.; et al. Role of Biomarkers for the Prevention, Assessment, and Management of Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2017, 135, e1054–e1091. [Google Scholar] [CrossRef] [PubMed]
- Cotter, G.; Voors, A.A.; Prescott, M.F.; Felker, G.M.; Filippatos, G.; Greenberg, B.H.; Pang, P.S.; Ponikowski, P.; Milo, O.; Hua, T.A.; et al. Growth Differentiation Factor 15 (GDF-15) in Patients Admitted for Acute Heart Failure: Results from the RELAX-AHF Study. Eur. J. Heart Fail. 2015, 17, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Kempf, T.; Von Haehling, S.; Peter, T.; Allhoff, T.; Cicoira, M.; Doehner, W.; Ponikowski, P.; Filippatos, G.S.; Rozentryt, P.; Drexler, H.; et al. Prognostic Utility of Growth Differentiation Factor-15 in Patients with Chronic Heart Failure. J. Am. Coll. Cardiol. 2007, 50, 1054–1060. [Google Scholar] [CrossRef]
- Anand, I.S.; Kempf, T.; Rector, T.S.; Tapken, H.; Allhoff, T.; Jantzen, F.; Kuskowski, M.; Cohn, J.N.; Drexler, H.; Wollert, K.C. Serial Measurement of Growth-Differentiation Factor-15 in Heart Failure: Relation to Disease Severity and Prognosis in the Valsartan Heart Failure Trial. Circulation 2010, 122, 1387–1395. [Google Scholar] [CrossRef]
- Ni, B.; Sun, M.; Zhao, J.; Wang, J.; Cao, Z. The Role of β-Catenin in Cardiac Diseases. Front. Pharmacol. 2023, 14, 1157043. [Google Scholar] [CrossRef]
- Procopio, M.C.; Lauro, R.; Nasso, C.; Carerj, S.; Squadrito, F.; Bitto, A.; Di Bella, G.; Micari, A.; Irrera, N.; Costa, F. Role of Adenosine and Purinergic Receptors in Myocardial Infarction: Focus on Different Signal Transduction Pathways. Biomedicines 2021, 9, 204. [Google Scholar] [CrossRef] [PubMed]
- Lofrumento, F.; Irrera, N.; Licordari, R.; Perfetti, S.; Nasso, E.; Liotta, P.; Isgrò, G.; Garcia-Ruiz, V.; Squadrito, F.; Carerj, S.; et al. Off-Target Effects of P2Y12 Receptor Inhibitors: Focus on Early Myocardial Fibrosis Modulation. IJMS 2023, 24, 17546. [Google Scholar] [CrossRef]
- Yousefi, F.; Shabaninejad, Z.; Vakili, S.; Derakhshan, M.; Movahedpour, A.; Dabiri, H.; Ghasemi, Y.; Mahjoubin-Tehran, M.; Nikoozadeh, A.; Savardashtaki, A.; et al. TGF-β and WNT Signaling Pathways in Cardiac Fibrosis: Non-Coding RNAs Come into Focus. Cell Commun. Signal 2020, 18, 87. [Google Scholar] [CrossRef]
- Kumarswamy, R.; Thum, T. Non-Coding RNAs in Cardiac Remodeling and Heart Failure. Circ. Res. 2013, 113, 676–689. [Google Scholar] [CrossRef]
- Callis, T.E.; Pandya, K.; Seok, H.Y.; Tang, R.-H.; Tatsuguchi, M.; Huang, Z.-P.; Chen, J.-F.; Deng, Z.; Gunn, B.; Shumate, J.; et al. MicroRNA-208a Is a Regulator of Cardiac Hypertrophy and Conduction in Mice. J. Clin. Investig. 2009, 119, 2772–2786. [Google Scholar] [CrossRef]
- Wang, J.-X.; Jiao, J.-Q.; Li, Q.; Long, B.; Wang, K.; Liu, J.-P.; Li, Y.-R.; Li, P.-F. miR-499 Regulates Mitochondrial Dynamics by Targeting Calcineurin and Dynamin-Related Protein-1. Nat. Med. 2011, 17, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiao, L.; Sun, L.; Li, Y.; Gao, Y.; Xu, C.; Shao, Y.; Li, M.; Li, C.; Lu, Y.; et al. LncRNA ZFAS1 as a SERCA2a Inhibitor to Cause Intracellular Ca2+ Overload and Contractile Dysfunction in a Mouse Model of Myocardial Infarction. Circ. Res. 2018, 122, 1354–1368. [Google Scholar] [CrossRef]
- Yang, L.; Deng, J.; Ma, W.; Qiao, A.; Xu, S.; Yu, Y.; Boriboun, C.; Kang, X.; Han, D.; Ernst, P.; et al. Ablation of lncRNA Miat Attenuates Pathological Hypertrophy and Heart Failure. Theranostics 2021, 11, 7995–8007. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhang, Z.; Zheng, L.; Wei, W.; Chen, Z. Long Non-Coding RNAs in the Pathogenesis of Heart Failure: A Literature Review. Front. Cardiovasc. Med. 2022, 9, 950284. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, K.F.; Pothineni, N.V.K.; Rutland, J.; Ding, Z.; Mehta, J.L. Immunity, Inflammation, and Oxidative Stress in Heart Failure: Emerging Molecular Targets. Cardiovasc. Drugs Ther. 2017, 31, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Elster, S.K.; Braunwald, E.; Wood, H.F. A Study of C-Reactive Protein in the Serum of Patients with Congestive Heart Failure. Am. Heart J. 1956, 51, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Castiglione, V.; Borrelli, C.; Saccaro, L.F.; Franzini, M.; Masi, S.; Emdin, M.; Giannoni, A. Oxidative Stress and Inflammation in the Evolution of Heart Failure: From Pathophysiology to Therapeutic Strategies. Eur. J. Prev. Cardiol. 2020, 27, 494–510. [Google Scholar] [CrossRef]
- Vasan, R.S.; Sullivan, L.M.; Roubenoff, R.; Dinarello, C.A.; Harris, T.; Benjamin, E.J.; Sawyer, D.B.; Levy, D.; Wilson, P.W.F.; D’Agostino, R.B. Inflammatory Markers and Risk of Heart Failure in Elderly Subjects without Prior Myocardial Infarction: The Framingham Heart Study. Circulation 2003, 107, 1486–1491. [Google Scholar] [CrossRef]
- Cesari, M.; Penninx, B.W.J.H.; Newman, A.B.; Kritchevsky, S.B.; Nicklas, B.J.; Sutton-Tyrrell, K.; Rubin, S.M.; Ding, J.; Simonsick, E.M.; Harris, T.B.; et al. Inflammatory Markers and Onset of Cardiovascular Events: Results From the Health ABC Study. Circulation 2003, 108, 2317–2322. [Google Scholar] [CrossRef] [PubMed]
- Núñez, J.; Bayés-Genís, A.; Revuelta-López, E.; Ter Maaten, J.M.; Miñana, G.; Barallat, J.; Cserkóová, A.; Bodi, V.; Fernández-Cisnal, A.; Núñez, E.; et al. Clinical Role of CA125 in Worsening Heart Failure. JACC Heart Fail. 2020, 8, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Lubrano, V.; Balzan, S. Role of Oxidative Stress-Related Biomarkers in Heart Failure: Galectin 3, A1-Antitrypsin and LOX-1: New Therapeutic Perspective? Mol. Cell Biochem. 2020, 464, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Sasso, F.C.; Carbonara, O.; Cozzolino, D.; Rambaldi, P.; Mansi, L.; Torella, D.; Gentile, S.; Turco, S.; Torella, R.; Salvatore, T. Effects of Insulin-Glucose Infusion on Left Ventricular Function at Rest and during Dynamic Exercise in Healthy Subjects and Noninsulin Dependent Diabetic Patients. J. Am. Coll. Cardiol. 2000, 36, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Galiero, R.; Caturano, A.; Vetrano, E.; Rinaldi, L.; Coviello, F.; Di Martino, A.; Albanese, G.; Marfella, R.; Sardu, C.; et al. Effects of Metformin in Heart Failure: From Pathophysiological Rationale to Clinical Evidence. Biomolecules 2021, 11, 1834. [Google Scholar] [CrossRef]
- Ge, Z.; Li, A.; McNamara, J.; Dos Remedios, C.; Lal, S. Pathogenesis and Pathophysiology of Heart Failure with Reduced Ejection Fraction: Translation to Human Studies. Heart Fail. Rev. 2019, 24, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Ky, B.; French, B.; Levy, W.C.; Sweitzer, N.K.; Fang, J.C.; Wu, A.H.B.; Goldberg, L.R.; Jessup, M.; Cappola, T.P. Multiple Biomarkers for Risk Prediction in Chronic Heart Failure. Circ. Heart Fail. 2012, 5, 183–190. [Google Scholar] [CrossRef]
- Demissei, B.G.; Cotter, G.; Prescott, M.F.; Felker, G.M.; Filippatos, G.; Greenberg, B.H.; Pang, P.S.; Ponikowski, P.; Severin, T.M.; Wang, Y.; et al. A Multimarker Multi-Time Point-Based Risk Stratification Strategy in Acute Heart Failure: Results from the RELAX-AHF Trial. Eur. J. Heart Fail. 2017, 19, 1001–1010. [Google Scholar] [CrossRef]
- Ara-Somohano, C.; Bonadona, A.; Carpentier, F.; Pavese, P.; Vesin, A.; Hamidfar-Roy, R.; Minet, C.; Vanzetto, G.; Schwebel, C.; Timsit, J.-F. Evaluation of Eight Biomarkers to Predict Short-Term Mortality in Patients with Acute Severe Dyspnea. Minerva Anestesiol. 2017, 83, 824–835. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Orlenko, A.; Zhao, L.; Basso, M.D.; Cvijic, M.E.; Li, Z.; Spires, T.E.; Yarde, M.; Wang, Z.; Seiffert, D.A.; et al. Multiple Plasma Biomarkers for Risk Stratification in Patients with Heart Failure and Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2020, 75, 1281–1295. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Richards, A.M.; Maisel, A.S.; Mueller, C.; Ky, B. Multimarker Testing with ST2 in Chronic Heart Failure. Am. J. Cardiol. 2015, 115, 76B–80B. [Google Scholar] [CrossRef] [PubMed]
- Bayes-Genis, A.; Núñez, J. Step by Step Toward Biomarker-Based Precision Medicine in Heart Failure. Clin. Chem. 2019, 65, 1187–1189. [Google Scholar] [CrossRef] [PubMed]
- McGarrah, R.W.; Crown, S.B.; Zhang, G.-F.; Shah, S.H.; Newgard, C.B. Cardiovascular Metabolomics. Circ. Res. 2018, 122, 1238–1258. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Dai, D.L.Y.; Ioannou, K.; Chen, V.; Lam, K.K.; Hollander, Z.; Wilson-McManus, J.E.; Assadian, S.; Toma, M.; Ng, R.; et al. Ensembling Electrical and Proteogenomics Biomarkers for Improved Prediction of Cardiac-Related 3-Month Hospitalizations: A Pilot Study. Can. J. Cardiol. 2019, 35, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Sethi, Y.; Patel, N.; Kaka, N.; Kaiwan, O.; Kar, J.; Moinuddin, A.; Goel, A.; Chopra, H.; Cavalu, S. Precision Medicine and the Future of Cardiovascular Diseases: A Clinically Oriented Comprehensive Review. JCM 2023, 12, 1799. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.A. Designer Science and the “Omic” Revolution. Nat. Biotechnol. 2000, 18, 127. [Google Scholar] [CrossRef] [PubMed]
- Apple, F.S.; Cullen, L.; Felker, G.M.; Ginsburg, G.; Morrow, D. Cardiovascular Disease: Impact of Biomarkers, Proteomics, and Genomics. Clin. Chem. 2017, 63, 1–4. [Google Scholar] [CrossRef]
- Edwards, A.V.G.; White, M.Y.; Cordwell, S.J. The Role of Proteomics in Clinical Cardiovascular Biomarker Discovery. Mol. Cell Proteom. 2008, 7, 1824–1837. [Google Scholar] [CrossRef]
- Domínguez, F.; Cuenca, S.; Bilińska, Z.; Toro, R.; Villard, E.; Barriales-Villa, R.; Ochoa, J.P.; Asselbergs, F.; Sammani, A.; Franaszczyk, M.; et al. Dilated Cardiomyopathy Due to BLC2-Associated Athanogene 3 (BAG3) Mutations. J. Am. Coll. Cardiol. 2018, 72, 2471–2481. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Henry, A.; Roselli, C.; Lin, H.; Sveinbjörnsson, G.; Fatemifar, G.; Hedman, Å.K.; Wilk, J.B.; Morley, M.P.; Chaffin, M.D.; et al. Genome-Wide Association and Mendelian Randomisation Analysis Provide Insights into the Pathogenesis of Heart Failure. Nat. Commun. 2020, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Kessler, T.; Vilne, B.; Schunkert, H. The Impact of Genome-Wide Association Studies on the Pathophysiology and Therapy of Cardiovascular Disease. EMBO Mol. Med. 2016, 8, 688–701. [Google Scholar] [CrossRef] [PubMed]
- McPherson, R.; Tybjaerg-Hansen, A. Genetics of Coronary Artery Disease. Circ. Res. 2016, 118, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xu, W.-W.; Hu, S.-J. Heart Failure: Advanced Development in Genetics and Epigenetics. Biomed. Res. Int. 2015, 2015, 352734. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Grimaldi, V.; De Pascale, M.R.; Sommese, L.; Infante, T.; Soricelli, A. Novel Epigenetic-Based Therapies Useful in Cardiovascular Medicine. World J. Cardiol. 2016, 8, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Movassagh, M.; Choy, M.-K.; Knowles, D.A.; Cordeddu, L.; Haider, S.; Down, T.; Siggens, L.; Vujic, A.; Simeoni, I.; Penkett, C.; et al. Distinct Epigenomic Features in End-Stage Failing Human Hearts. Circulation 2011, 124, 2411–2422. [Google Scholar] [CrossRef]
- Haas, J.; Frese, K.S.; Park, Y.J.; Keller, A.; Vogel, B.; Lindroth, A.M.; Weichenhan, D.; Franke, J.; Fischer, S.; Bauer, A.; et al. Alterations in Cardiac DNA Methylation in Human Dilated Cardiomyopathy. EMBO Mol. Med. 2013, 5, 413–429. [Google Scholar] [CrossRef]
- Meder, B.; Haas, J.; Sedaghat-Hamedani, F.; Kayvanpour, E.; Frese, K.; Lai, A.; Nietsch, R.; Scheiner, C.; Mester, S.; Bordalo, D.M.; et al. Epigenome-Wide Association Study Identifies Cardiac Gene Patterning and a Novel Class of Biomarkers for Heart Failure. Circulation 2017, 136, 1528–1544. [Google Scholar] [CrossRef]
- Gora, M.; Kiliszek, M.; Burzynska, B. Will Global Transcriptome Analysis Allow the Detection of Novel Prognostic Markers in Coronary Artery Disease and Heart Failure? Curr. Genom. 2013, 14, 388–396. [Google Scholar] [CrossRef]
- Costa, V.; Aprile, M.; Esposito, R.; Ciccodicola, A. RNA-Seq and Human Complex Diseases: Recent Accomplishments and Future Perspectives. Eur. J. Hum. Genet. 2013, 21, 134–142. [Google Scholar] [CrossRef]
- Ounzain, S.; Micheletti, R.; Beckmann, T.; Schroen, B.; Alexanian, M.; Pezzuto, I.; Crippa, S.; Nemir, M.; Sarre, A.; Johnson, R.; et al. Genome-Wide Profiling of the Cardiac Transcriptome after Myocardial Infarction Identifies Novel Heart-Specific Long Non-Coding RNAs. Eur. Heart J. 2015, 36, 353a–368a. [Google Scholar] [CrossRef]
- Schiano, C.; Costa, V.; Aprile, M.; Grimaldi, V.; Maiello, C.; Esposito, R.; Soricelli, A.; Colantuoni, V.; Donatelli, F.; Ciccodicola, A.; et al. Heart Failure: Pilot Transcriptomic Analysis of Cardiac Tissue by RNA-Sequencing. Cardiol. J. 2017, 24, 539–553. [Google Scholar] [CrossRef]
- Toma, M.; Mak, G.J.; Chen, V.; Hollander, Z.; Shannon, C.P.; Lam, K.K.Y.; Ng, R.T.; Tebbutt, S.J.; Wilson-McManus, J.E.; Ignaszewski, A.; et al. Differentiating Heart Failure Phenotypes Using Sex-Specific Transcriptomic and Proteomic Biomarker Panels. ESC Heart Fail. 2017, 4, 301–311. [Google Scholar] [CrossRef]
- Mebazaa, A.; Vanpoucke, G.; Thomas, G.; Verleysen, K.; Cohen-Solal, A.; Vanderheyden, M.; Bartunek, J.; Mueller, C.; Launay, J.-M.; Van Landuyt, N.; et al. Unbiased Plasma Proteomics for Novel Diagnostic Biomarkers in Cardiovascular Disease: Identification of Quiescin Q6 as a Candidate Biomarker of Acutely Decompensated Heart Failure. Eur. Heart J. 2012, 33, 2317–2324. [Google Scholar] [CrossRef]
- Raphael, R.; Purushotham, D.; Gastonguay, C.; Chesnik, M.A.; Kwok, W.-M.; Wu, H.-E.; Shah, S.J.; Mirza, S.P.; Strande, J.L. Combining Patient Proteomics and in Vitro Cardiomyocyte Phenotype Testing to Identify Potential Mediators of Heart Failure with Preserved Ejection Fraction. J. Transl. Med. 2016, 14, 18. [Google Scholar] [CrossRef]
- DeAguero, J.L.; McKown, E.N.; Zhang, L.; Keirsey, J.; Fischer, E.G.; Samedi, V.G.; Canan, B.D.; Kilic, A.; Janssen, P.M.L.; Delfín, D.A. Altered Protein Levels in the Isolated Extracellular Matrix of Failing Human Hearts with Dilated Cardiomyopathy. Cardiovasc. Pathol. 2017, 26, 12–20. [Google Scholar] [CrossRef]
- Stenemo, M.; Nowak, C.; Byberg, L.; Sundström, J.; Giedraitis, V.; Lind, L.; Ingelsson, E.; Fall, T.; Ärnlöv, J. Circulating Proteins as Predictors of Incident Heart Failure in the Elderly. Eur. J. Heart Fail. 2018, 20, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Arab, S.; Gramolini, A.O.; Ping, P.; Kislinger, T.; Stanley, B.; van Eyk, J.; Ouzounian, M.; MacLennan, D.H.; Emili, A.; Liu, P.P. Cardiovascular Proteomics: Tools to Develop Novel Biomarkers and Potential Applications. J. Am. Coll. Cardiol. 2006, 48, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Andrès, N.; Rossignol, P.; Iraqi, W.; Fay, R.; Nuée, J.; Ghio, S.; Cleland, J.G.F.; Zannad, F.; Lacolley, P. Association of Galectin-3 and Fibrosis Markers with Long-term Cardiovascular Outcomes in Patients with Heart Failure, Left Ventricular Dysfunction, and Dyssynchrony: Insights from the CARE-HF (Cardiac Resynchronization in Heart Failure) Trial. Eur. J. Heart Fail. 2012, 14, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Núñez, J.; Llàcer, P.; García-Blas, S.; Bonanad, C.; Ventura, S.; Núñez, J.M.; Sánchez, R.; Fácila, L.; De La Espriella, R.; Vaquer, J.M.; et al. CA125-Guided Diuretic Treatment Versus Usual Care in Patients with Acute Heart Failure and Renal Dysfunction. Am. J. Med. 2020, 133, 370–380.e4. [Google Scholar] [CrossRef]
- Gaggin, H.K.; Motiwala, S.; Bhardwaj, A.; Parks, K.A.; Januzzi, J.L. Soluble Concentrations of the Interleukin Receptor Family Member ST2 and β-Blocker Therapy in Chronic Heart Failure. Circ Heart Fail. 2013, 6, 1206–1213. [Google Scholar] [CrossRef]
- Cheng, S.; Shah, S.H.; Corwin, E.J.; Fiehn, O.; Fitzgerald, R.L.; Gerszten, R.E.; Illig, T.; Rhee, E.P.; Srinivas, P.R.; Wang, T.J.; et al. Potential Impact and Study Considerations of Metabolomics in Cardiovascular Health and Disease: A Scientific Statement From the American Heart Association. Circ. Cardiovasc. Genet. 2017, 10, e000032. [Google Scholar] [CrossRef]
- Hunter, W.G.; Kelly, J.P.; McGarrah, R.W.; Khouri, M.G.; Craig, D.; Haynes, C.; Ilkayeva, O.; Stevens, R.D.; Bain, J.R.; Muehlbauer, M.J.; et al. Metabolomic Profiling Identifies Novel Circulating Biomarkers of Mitochondrial Dysfunction Differentially Elevated in Heart Failure with Preserved Versus Reduced Ejection Fraction: Evidence for Shared Metabolic Impairments in Clinical Heart Failure. J. Am. Heart Assoc. 2016, 5, e003190. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Kelly, J.P.; McGarrah, R.W.; Hellkamp, A.S.; Fiuzat, M.; Testani, J.M.; Wang, T.S.; Verma, A.; Samsky, M.D.; Donahue, M.P.; et al. Prognostic Implications of Long-Chain Acylcarnitines in Heart Failure and Reversibility with Mechanical Circulatory Support. J. Am. Coll. Cardiol. 2016, 67, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.L.; Tang, W.H.W. Metabolic Biomarkers in Heart Failure. Heart Fail. Clin. 2018, 14, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Shen, A.; Huang, Y.; Su, L.; Lai, W.; Wang, P.; Xie, Z.; Xie, Z.; Zeng, Q.; Ren, H.; et al. 1H-NMR-Based Metabolic Analysis of Human Serum Reveals Novel Markers of Myocardial Energy Expenditure in Heart Failure Patients. PLoS ONE 2014, 9, e88102. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Z.; Chen, J.; Zhao, H.; Luo, L.; Chen, C.; Xu, X.; Zhang, W.; Gao, K.; Li, B.; et al. Metabolomic Identification of Diagnostic Plasma Biomarkers in Humans with Chronic Heart Failure. Mol. BioSyst. 2013, 9, 2618. [Google Scholar] [CrossRef] [PubMed]
- Desmoulin, F.; Galinier, M.; Trouillet, C.; Berry, M.; Delmas, C.; Turkieh, A.; Massabuau, P.; Taegtmeyer, H.; Smih, F.; Rouet, P. Metabonomics Analysis of Plasma Reveals the Lactate to Cholesterol Ratio as an Independent Prognostic Factor of Short-Term Mortality in Acute Heart Failure. PLoS ONE 2013, 8, e60737. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.L.; Almeida, J.R.; Resende, L.M.; Martins, W.; Henriques, F.A.F.A.; Baldasso, P.A.; Soares, A.M.; Taranto, A.G.; Resende, R.R.; Marangoni, S.; et al. Isolation and Characterization of a Natriuretic Peptide from Crotalus Oreganus Abyssus (Grand Canyon Rattlesnake) and Its Effects on Systemic Blood Pressure and Nitrite Levels. Int. J. Pept. Res. Ther. 2011, 17, 165–173. [Google Scholar] [CrossRef]
- Kolur, V.; Vastrad, B.; Vastrad, C.; Kotturshetti, S.; Tengli, A. Identification of Candidate Biomarkers and Therapeutic Agents for Heart Failure by Bioinformatics Analysis. BMC Cardiovasc. Disord. 2021, 21, 329. [Google Scholar] [CrossRef] [PubMed]

| Category | Examples | Key Features |
|---|---|---|
| Natriuretic Peptides (NPs) | B-type natriuretic peptide (BNP), N-terminal pro–B-type natriuretic peptide (NT-proBNP), Atrial natriuretic peptide (ANP) | Reflect cardiac stress; crucial for diagnosing, staging, prognostication, and management of HF |
| Neurohormonal Activation Biomarkers | Norepinephrine, Chromogranin, Plasma renin activity (PRA), Adrenomedullin (ADM), Mid-regional pro-ADM (MR-proADM) | Indicate severity and predict outcomes in HF; include hormones and enzymes related to the body’s stress response |
| Cardiac Damage Biomarkers | Cardiac Troponins (TnT and TnI) | Indicate myocardial injury; useful prognostic stratification in acute and chronic HF |
| Markers of Myocardial Remodeling, Inflammation, and Oxidative Stress | sST2, Galectin-3, GDF 15 | Predict mortality and hospitalization risks; reflect structural changes, inflammation, and oxidative stress in the heart |
| Biomarker | Advantages | Limitations | Guideline Recommendations |
|---|---|---|---|
| B-type Natriuretic Peptide (BNP) and N-terminal proBNP (NT-proBNP) | Sensitive indicators Reflect hemodynamic changes Prognostic value | Influenced by age, renal function, and obesity Variability in cut-off values | Diagnosis: ESC I A ACC/AHA IIa C Prognosis: ACC/AHA at admission I A and at discharge IIa B Screening: ACC/AHA IIa B |
| MR-proANP | Stable measure of ANP activity Additive diagnostic value over BNP or NT-proBNP | The role in clinical practice is still evolving | Diagnosis: ESC I A |
| Cardiac Troponins | Indicate myocardial injury High specificity | Elevation can be caused by other cardiac and non-cardiac conditions | Diagnosis: ESC I C Prognosis: ACC/AHA at admission I A |
| Galectin-3 Soluble ST2 (sST2) | Reflects fibrosis and inflammation Predictor of remodeling and progression | Elevated in other conditions like renal failure | Prognosis: ACC/AHA at admission IIb B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Licordari, R.; Correale, M.; Bonanno, S.; Beltrami, M.; Ciccarelli, M.; Micari, A.; Palazzuoli, A.; Dattilo, G. Beyond Natriuretic Peptides: Unveiling the Power of Emerging Biomarkers in Heart Failure. Biomolecules 2024, 14, 309. https://doi.org/10.3390/biom14030309
Licordari R, Correale M, Bonanno S, Beltrami M, Ciccarelli M, Micari A, Palazzuoli A, Dattilo G. Beyond Natriuretic Peptides: Unveiling the Power of Emerging Biomarkers in Heart Failure. Biomolecules. 2024; 14(3):309. https://doi.org/10.3390/biom14030309
Chicago/Turabian StyleLicordari, Roberto, Michele Correale, Salvatore Bonanno, Matteo Beltrami, Michele Ciccarelli, Antonio Micari, Alberto Palazzuoli, and Giuseppe Dattilo. 2024. "Beyond Natriuretic Peptides: Unveiling the Power of Emerging Biomarkers in Heart Failure" Biomolecules 14, no. 3: 309. https://doi.org/10.3390/biom14030309
APA StyleLicordari, R., Correale, M., Bonanno, S., Beltrami, M., Ciccarelli, M., Micari, A., Palazzuoli, A., & Dattilo, G. (2024). Beyond Natriuretic Peptides: Unveiling the Power of Emerging Biomarkers in Heart Failure. Biomolecules, 14(3), 309. https://doi.org/10.3390/biom14030309








