High Glucose Promotes and Aggravates the Senescence and Dysfunction of Vascular Endothelial Cells in Women with Hyperglycemia in Pregnancy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Isolation of Endothelial Cells and Experimental Design
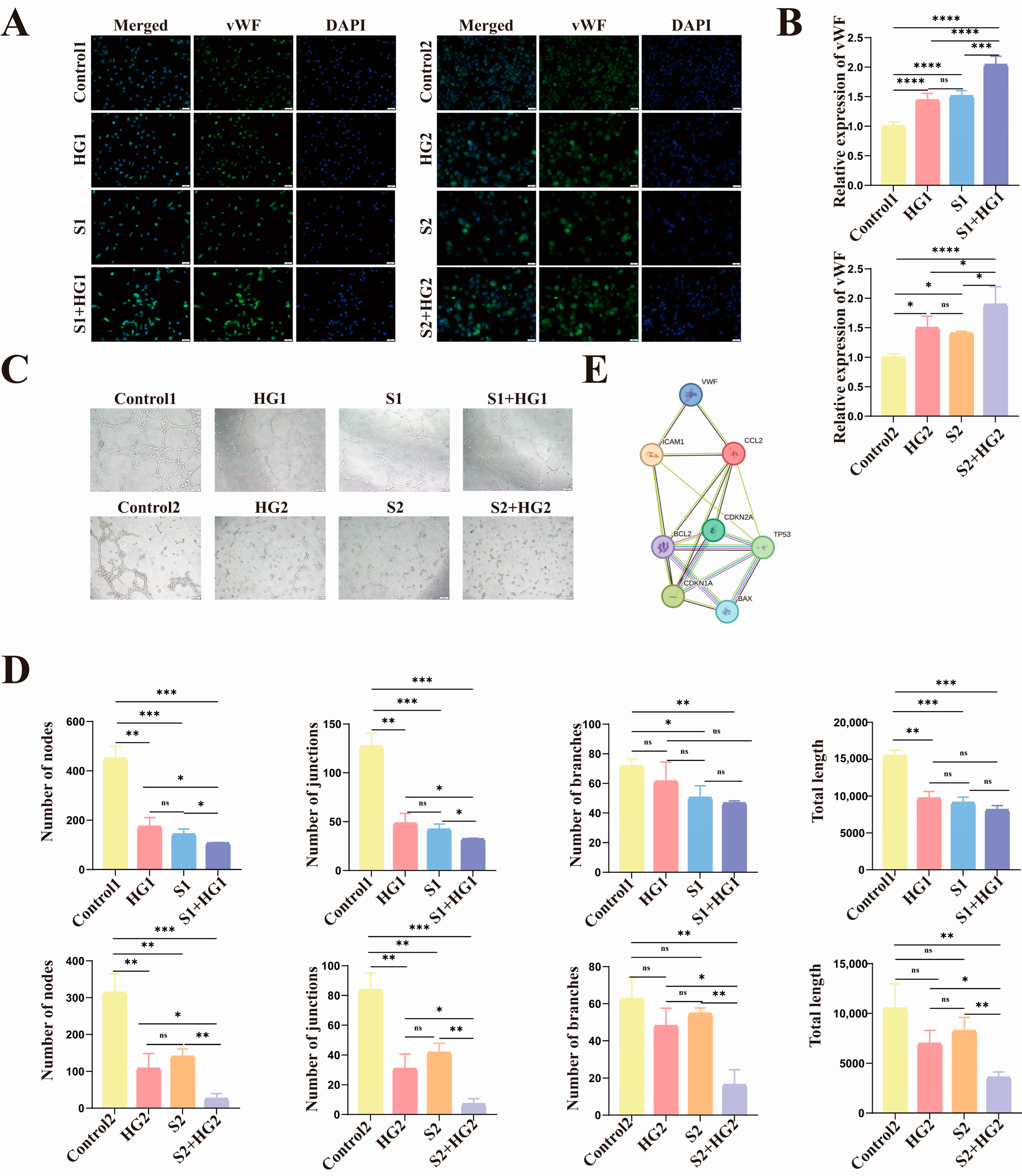
2.3. Immunofluorescent Detection of vWF
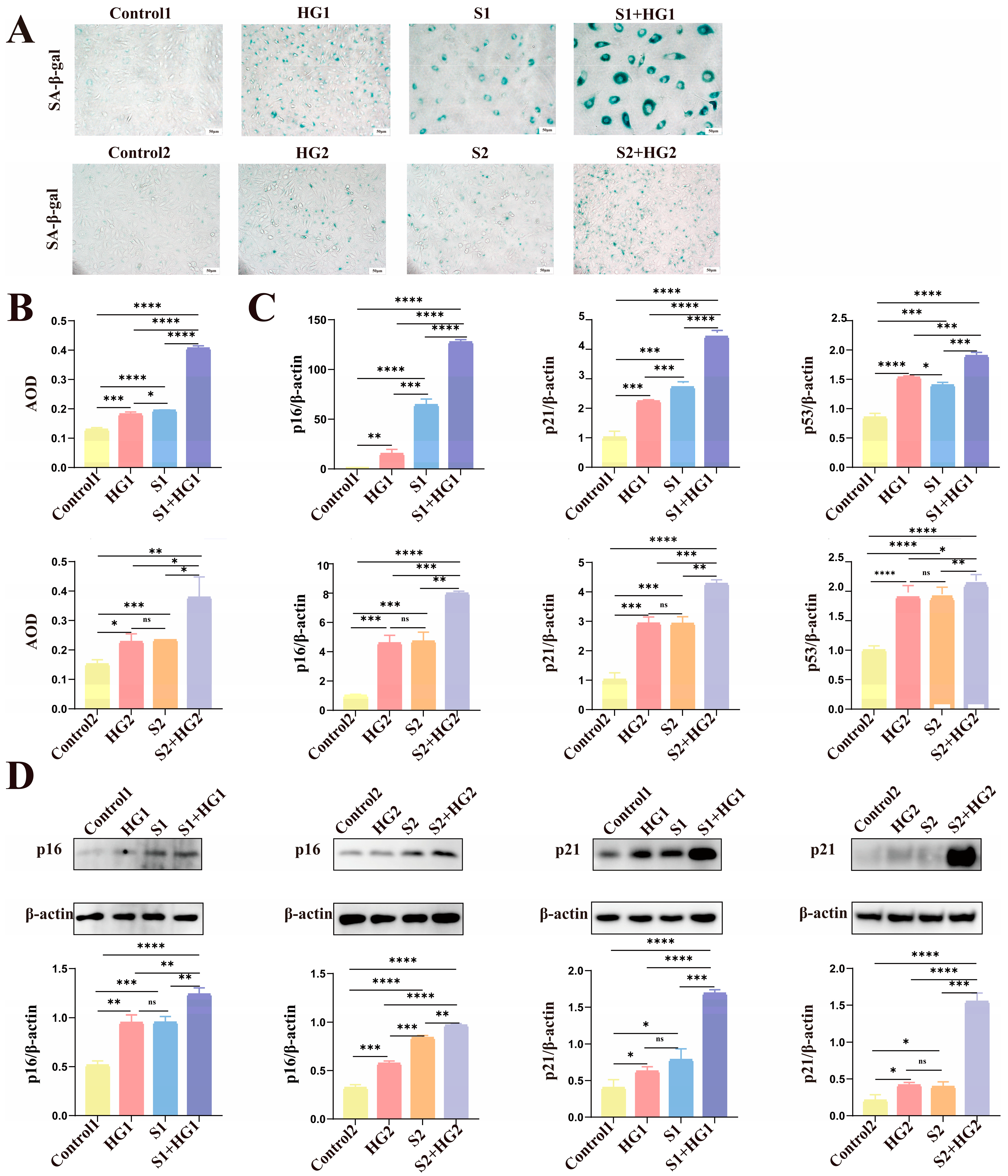
2.4. SA-β-Gal Staining
2.5. Extraction of RNA and Real-Time Quantitative PCR
2.6. Western Blot
2.7. The Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. The CCK8 Viability Assay
2.9. Tube Formation Assay/Matrigel Assay
2.10. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Study Participants
3.2. Excessive Senescence of HUVECs in HIP Pregnant Women
3.3. High Glucose Promotes Excessive Senescence in HUVECs
3.4. High Glucose Induces Anti-Apoptotic Characteristics of Senescent HUVECs with Lower Proliferative Activity and Tube-Forming Capacity
3.5. HIP Women’s Umbilical Vein Endothelial Cells Have Anti-Apoptotic Characteristics as Well as Markedly Reduced Proliferative Activity and Tube-Forming Capacity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: A World Health Organization Guideline. Diabetes Res. Clin. Pract. 2014, 103, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Hod, M.; Kapur, A.; Sacks, D.A.; Hadar, E.; Agarwal, M.; Di Renzo, G.C.; Cabero Roura, L.; Mcintyre, H.D.; Morris, J.L.; Divakar, H. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care. Int. J. Gynaecol. Obstet. 2015, 131 (Suppl. S3), S173–S211. [Google Scholar] [CrossRef] [PubMed]
- Magliano, D.J.; Boyko, E.J. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Gude, N.M.; Roberts, C.T.; Kalionis, B.; King, R.G. Growth and function of the normal human placenta. Thromb. Res. 2004, 114, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Clyne, A.M. Endothelial response to glucose: Dysfunction, metabolism, and transport. Biochem. Soc. Trans. 2021, 49, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, C.; Eltit, F.; Santibanez, J.F.; Gatica, S.; Cabello-Verrugio, C.; Simon, F. Endothelial dysfunction in pregnancy metabolic disorders. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2020, 1866, 165414. [Google Scholar] [CrossRef]
- Di Fulvio, P.; Pandolfi, A.; Formoso, G.; Di Silvestre, S.; Di Tomo, P.; Giardinelli, A.; De Marco, A.; Di Pietro, N.; Taraborrelli, M.; Sancilio, S.; et al. Features of endothelial dysfunction in umbilical cord vessels of women with gestational diabetes. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ni, X.; Huang, X.; Yao, J.; He, Q.; Wang, K.; Duan, T. Potential Role of Hyperglycemia in Fetoplacental Endothelial Dysfunction in Gestational Diabetes Mellitus. Cell. Physiol. Biochem. 2016, 39, 1317–1328. [Google Scholar] [CrossRef]
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef]
- Lip, G.Y.; Blann, A. von Willebrand factor: A marker of endothelial dysfunction in vascular disorders? Cardiovasc. Res. 1997, 34, 255–265. [Google Scholar] [CrossRef]
- Papadopoulou, C.; Corrigall, V.; Taylor, P.R.; Poston, R.N. The role of the chemokines MCP-1, GRO-α, IL-8 and their receptors in the adhesion of monocytic cells to human atherosclerotic plaques. Cytokine 2008, 43, 181–186. [Google Scholar] [CrossRef]
- Kornacki, J.; Gutaj, P.; Kalantarova, A.; Sibiak, R.; Jankowski, M.; Wender-Ozegowska, E. Endothelial Dysfunction in Pregnancy Complications. Biomedicines 2021, 9, 1756. [Google Scholar] [CrossRef]
- Calcinotto, A.; Kohli, J.; Zagato, E.; Pellegrini, L.; Demaria, M.; Alimonti, A. Cellular Senescence: Aging, Cancer, and Injury. Physiol. Rev. 2019, 99, 1047–1078. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef] [PubMed]
- Birch, J.; Gil, J. Senescence and the SASP: Many therapeutic avenues. Genes Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Di Tomo, P.; Alessio, N.; Falone, S.; Pietrangelo, L.; Lanuti, P.; Cordone, V.; Santini, S.J.; Di Pietrantonio, N.; Marchisio, M.; Protasi, F.; et al. Endothelial cells from umbilical cord of women affected by gestational diabetes: A suitable in vitro model to study mechanisms of early vascular senescence in diabetes. FASEB J. 2021, 35, e21662. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Ma, C.; Fan, G.; Liu, H.; Lin, X.; Li, J.; Li, N.; Wang, S.; Zeng, M.; Zhang, Y.; et al. SIRT3 protects endothelial cells from high glucose-induced senescence and dysfunction via the p53 pathway. Life Sci. 2021, 264, 118724. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Liu, Z.; Wu, A.; Khan, A.H.; Zhu, Y.; Ding, S.; Li, X.; Zhao, Y.; Dai, X.; Zhou, J.; et al. Hyperglycemia Promotes Endothelial Cell Senescence through AQR/PLAU Signaling Axis. Int. J. Mol. Sci. 2022, 23, 2879. [Google Scholar] [CrossRef]
- Chandel, S.; Kumaragurubaran, R.; Giri, H.; Dixit, M. Isolation and Culture of Human Umbilical Vein Endothelial Cells (HUVECs). Methods Mol. Biol. 2024, 2711, 147–162. [Google Scholar]
- Wang, Z.; Wei, D.; Xiao, H. Methods of cellular senescence induction using oxidative stress. Methods Mol. Biol. 2013, 1048, 135–144. [Google Scholar]
- Trinidad, F.R.J.; Ruiz, M.A.; Batlló, N.S.; Vea Badenes, À.; Gibert, J.B.; Cañellas, A.V.; Moreno, M.R.; Rofastes, X.F.; Tenas, M.S.; Dantas, A.P.; et al. Linking In Vitro Models of Endothelial Dysfunction with Cell Senescence. Life 2021, 11, 1323. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.; Halicka, D.; Brodsky, S.; Avram, A.; Eskander, J.; Bloomgarden, N.A.; Darzynkiewicz, Z.; Goligorsky, M.S. Contribution of p16INK4a and p21CIP1 pathways to induction of premature senescence of human endothelial cells: Permissive role of p53. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1575–H1586. [Google Scholar] [CrossRef] [PubMed]
- Mijit, M.; Caracciolo, V.; Melillo, A.; Amicarelli, F.; Giordano, A. Role of p53 in the Regulation of Cellular Senescence. Biomolecules 2020, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Tho, L.M.; Xu, N.; Gillespie, D.A. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv. Cancer Res. 2010, 108, 73–112. [Google Scholar] [PubMed]
- Zhang, X.; Rodriguez-Niño, A.; Pastene, D.O.; Pallavi, P.; van den Born, J.; Bakker, S.J.L.; Krämer, B.K.; Yard, B.A. Methylglyoxal induces p53 activation and inhibits mTORC1 in human umbilical vein endothelial cells. Sci. Rep. 2021, 11, 8004. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.D.; Pastene, D.O.; Breedijk, A.; Rodriguez, A.; Hofmann, B.B.; Sticht, C.; von Ochsenstein, E.; Allgayer, H.; van den Born, J.; Bakker, S.; et al. Methylglyoxal down-regulates the expression of cell cycle associated genes and activates the p53 pathway in human umbilical vein endothelial cells. Sci. Rep. 2019, 9, 1152. [Google Scholar] [CrossRef] [PubMed]
- Tousian, H.; Razavi, B.M.; Hosseinzadeh, H. Alpha-mangostin decreased cellular senescence in human umbilical vein endothelial cells. DARU J. Pharm. Sci. 2020, 28, 45–55. [Google Scholar] [CrossRef]
- Yokoyama, M.; Shimizu, I.; Nagasawa, A.; Yoshida, Y.; Katsuumi, G.; Wakasugi, T.; Hayashi, Y.; Ikegami, R.; Suda, M.; Ota, Y.; et al. p53 plays a crucial role in endothelial dysfunction associated with hyperglycemia and ischemia. J. Mol. Cell. Cardiol. 2019, 129, 105–117. [Google Scholar] [CrossRef]
- Varma, S.; Lal, B.K.; Zheng, R.; Breslin, J.W.; Saito, S.; Pappas, P.J.; Hobson, R.W.; Durán, W.N.; Whetzel, A.M.; Bolick, D.T.; et al. Hyperglycemia alters PI3k and Akt signaling and leads to endothelial cell proliferative dysfunction. Am. J. Physiol.-Heart Circ. Physiol. 2005, 289, H1744–H1751. [Google Scholar] [CrossRef]
- Lin, F.; Yang, Y.; Wei, S.; Huang, X.; Peng, Z.; Ke, X.; Zeng, Z.; Song, Y. Hydrogen Sulfide Protects Against High Glucose-Induced Human Umbilical Vein Endothelial Cell Injury Through Activating PI3K/Akt/eNOS Pathway. Drug Des. Dev. Ther. 2020, 14, 621–633. [Google Scholar] [CrossRef]
- Liu, Y.-P.; Shao, S.-J.; Guo, H.-D. Schwann cells apoptosis is induced by high glucose in diabetic peripheral neuropathy. Life Sci. 2020, 248, 117459. [Google Scholar] [CrossRef]
- Soto-Gamez, A.; Quax, W.J.; Demaria, M. Regulation of Survival Networks in Senescent Cells: From Mechanisms to Interventions. J. Mol. Biol. 2019, 431, 2629–2643. [Google Scholar] [CrossRef] [PubMed]
- Duval, H.; Johnson, N.; Li, J.; Evans, A.; Chen, S.; Licence, D.; Skepper, J.; Charnock-Jones, D.S.; Smith, S.; Print, C. Vascular development is disrupted by endothelial cell-specific expression of the anti-apoptotic protein Bcl-2. Angiogenesis 2007, 10, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Klimova, B.; Novotny, M.; Kuca, K. Anti-Aging Drugs—Prospect of Longer Life? Curr. Med. Chem. 2018, 25, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.S.; Gubbi, S.; Barzilai, N. Benefits of Metformin in Attenuating the Hallmarks of Aging. Cell Metab. 2020, 32, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gan, D.; Lin, S.; Zhong, Y.; Chen, M.; Zou, X.; Shao, Z.; Xiao, G. Metformin in aging and aging-related diseases: Clinical applications and relevant mechanisms. Theranostics 2022, 12, 2722–2740. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Zhou, M.; Chen, C.; Wu, X.; Wang, X. Role of AMPK mediated pathways in autophagy and aging. Biochimie 2022, 195, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Pafundi, P.C.; Galiero, R.; Rinaldi, L.; Caturano, A.; Vetrano, E.; Aprea, C.; Albanese, G.; Di Martino, A.; Ricozzi, C.; et al. Can Metformin Exert as an Active Drug on Endothelial Dysfunction in Diabetic Subjects? Biomedicines 2020, 9, 3. [Google Scholar] [CrossRef]
- Kurauti, M.A.; Soares, G.M.; Marmentini, C.; Bronczek, G.A.; Branco, R.C.S.; Boschero, A.C. Insulin and aging. Vitam Horm. 2021, 115, 185–219. [Google Scholar]
- González, M.; Rojas, S.; Avila, P.; Cabrera, L.; Villalobos, R.; Palma, C.; Aguayo, C.; Peña, E.; Gallardo, V.; Guzmán-Gutiérrez, E.; et al. Insulin Reverses D-Glucose–Increased Nitric Oxide and Reactive Oxygen Species Generation in Human Umbilical Vein Endothelial Cells. PLoS ONE 2015, 10, e0122398. [Google Scholar] [CrossRef]
- Scifres, C.M.; Parks, W.T.; Feghali, M.; Caritis, S.N.; Catov, J.M. Placental maternal vascular malperfusion and adverse pregnancy outcomes in gestational diabetes mellitus. Placenta 2017, 49, 10–15. [Google Scholar] [CrossRef]
- Gathiram, P.; Moodley, J. Pre-eclampsia: Its pathogenesis and pathophysiolgy. Cardiovasc. J. Afr. 2016, 27, 71–78. [Google Scholar] [CrossRef]




| Name | Forward Primer, 5′-3′ | Reverse Primer, 5′-3′ |
|---|---|---|
| β-actin | TGGCACCCAGCACAATGAA | CTAAGTCATAGTCCGCCTAGAAGCA |
| p16 | CATGGAGCCTTCGGCTGAC | GCGCTGCCCATCATCATG |
| p21 | TGTCACTGTCTTGTACCCTTG | GGCGTTTGGAGTGGTAGAA |
| p53 | GTACCACCATCCACTACAACTAC | CACAAACACGCACCTCAAAG |
| BCL2 | TGTGGATGACTGAGTACCTGAACC | CAGCCAGGAGAAATCAAACAGAGG |
| BAX | AAGCGACTGATGTCCCTGTCTC | GATGGTGAGTGAGGCGGTGAG |
| vWf | ACCTTGGTCACATCTTCACATTCAC | GTCATTGGCTCCGTTCTCATCAC |
| CCL2 | CAGCCAGATGCAATCAATGCC | TGGAATCCTGAACCCACTTCT |
| ICAM-1 | ACCTATGGCAACGACTCCTTCTC | GTGTCTCCTGGCTCTGGTTCC |
| Index | Control Group (n = 6) | HIP Group (n = 9) | p Value |
|---|---|---|---|
| Age (year) | 30.17 ± 1.72 | 31.00 ± 4.56 | 0.6785 |
| Gestational age (weeks) | 39.02 ± 0.84 | 39.29 ± 1.53 | 0.7108 |
| Pre-pregnancy BMI (kg/m2) | 25.97 ± 2.27 | 27.43 ± 4.23 | 0.4551 |
| Pregnancy weight gain/kg | 14.30 ± 3.88 | 12.33 ± 3.01 | 0.2887 |
| FPG/(mmol/L) | 4.45 ± 0.23 | 5.00 ± 0.41 | 0.0019 |
| 1hPG/(mmol/L) | 6.87 ± 1.47 | 9.70 ± 1.16 | 0.0011 |
| 2hPG/(mmol/L) | 6.07 ± 0.79 | 8.49 ± 1.42 | 0.0022 |
| Glycated albumin (%) | 10.80 ± 1.07 | 12.99 ± 1.33 | 0.0051 |
| Glycosylated hemoglobin (%) | 5.22 ± 0.15 | 5.36 ± 0.42 | 0.4392 |
| Newborn body mass (g) | 3678.00 ± 513.32 | 3387.00 ± 573.17 | 0.3443 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Li, M.; Li, H. High Glucose Promotes and Aggravates the Senescence and Dysfunction of Vascular Endothelial Cells in Women with Hyperglycemia in Pregnancy. Biomolecules 2024, 14, 329. https://doi.org/10.3390/biom14030329
Zheng L, Li M, Li H. High Glucose Promotes and Aggravates the Senescence and Dysfunction of Vascular Endothelial Cells in Women with Hyperglycemia in Pregnancy. Biomolecules. 2024; 14(3):329. https://doi.org/10.3390/biom14030329
Chicago/Turabian StyleZheng, Lin, Mingqing Li, and Huaping Li. 2024. "High Glucose Promotes and Aggravates the Senescence and Dysfunction of Vascular Endothelial Cells in Women with Hyperglycemia in Pregnancy" Biomolecules 14, no. 3: 329. https://doi.org/10.3390/biom14030329





