Exosomes as Promising Therapeutic Tools for Regenerative Endodontic Therapy
Abstract
:1. Introduction
2. Biological Characteristics of Exosomes
3. The Role and Mechanism of Exosomes in Regulating Pulp–Dentin Complex Regeneration
3.1. Exosomes in Cellular Proliferation and Migration
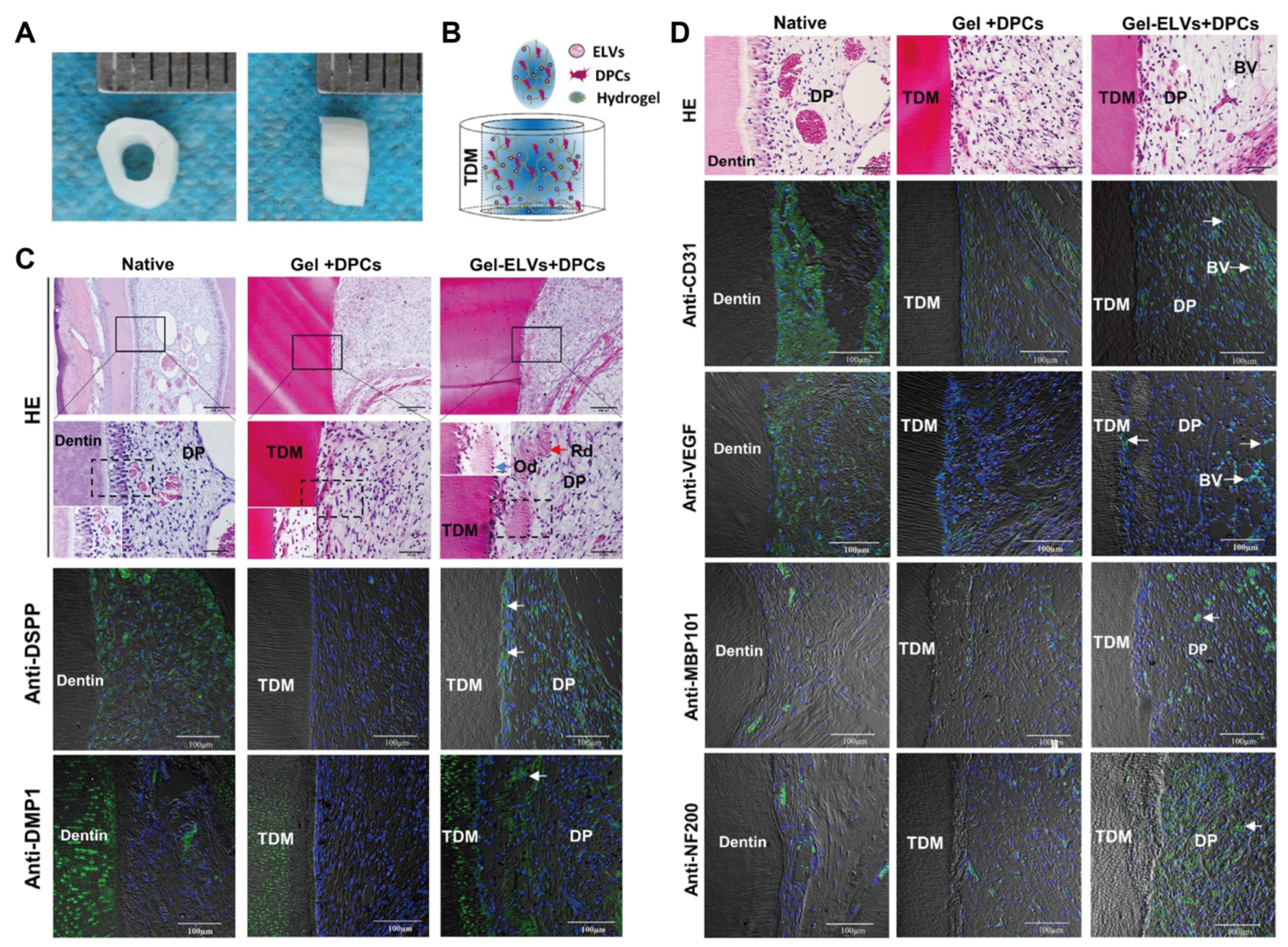
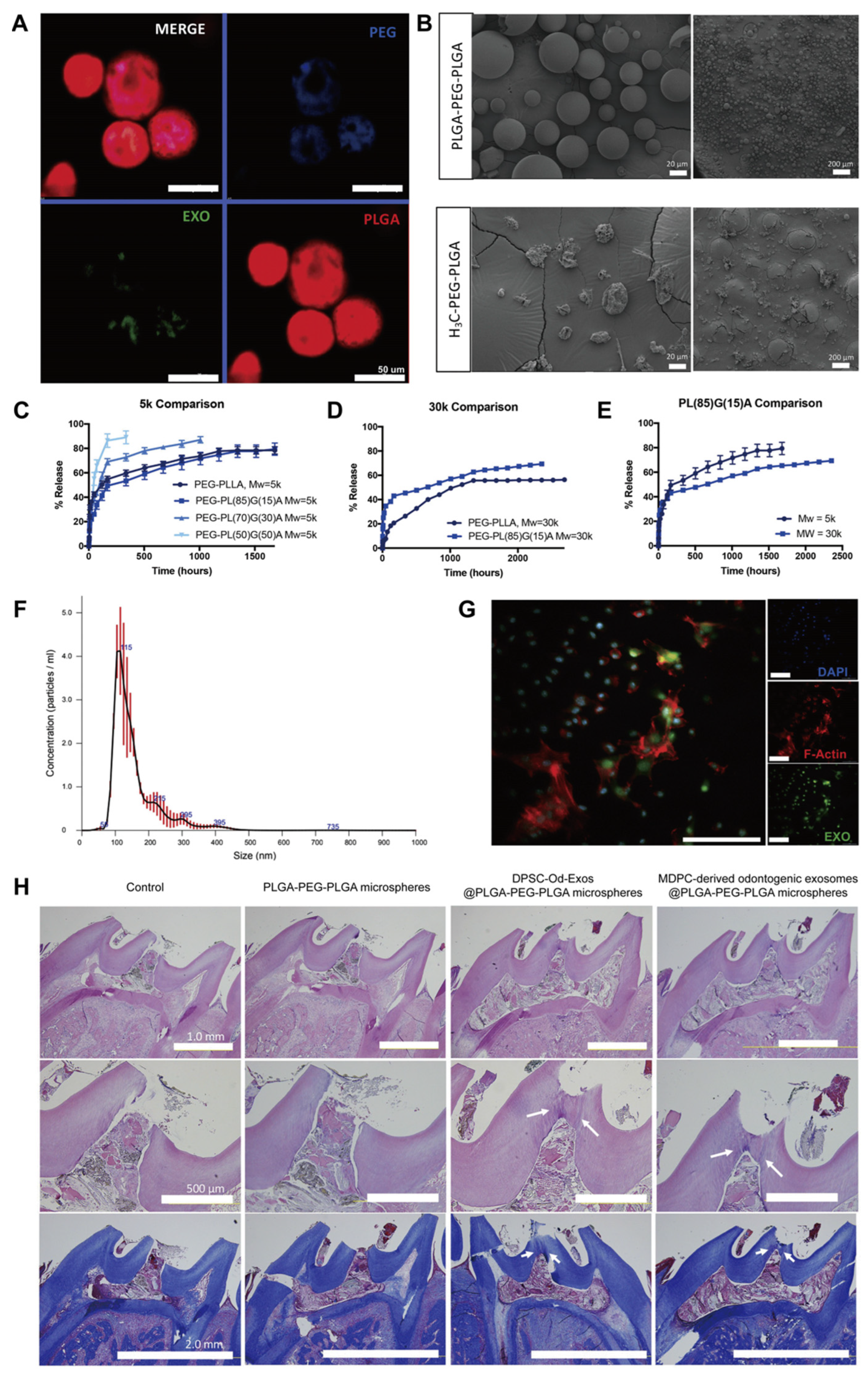
3.2. Exosomes in Odontogenesis
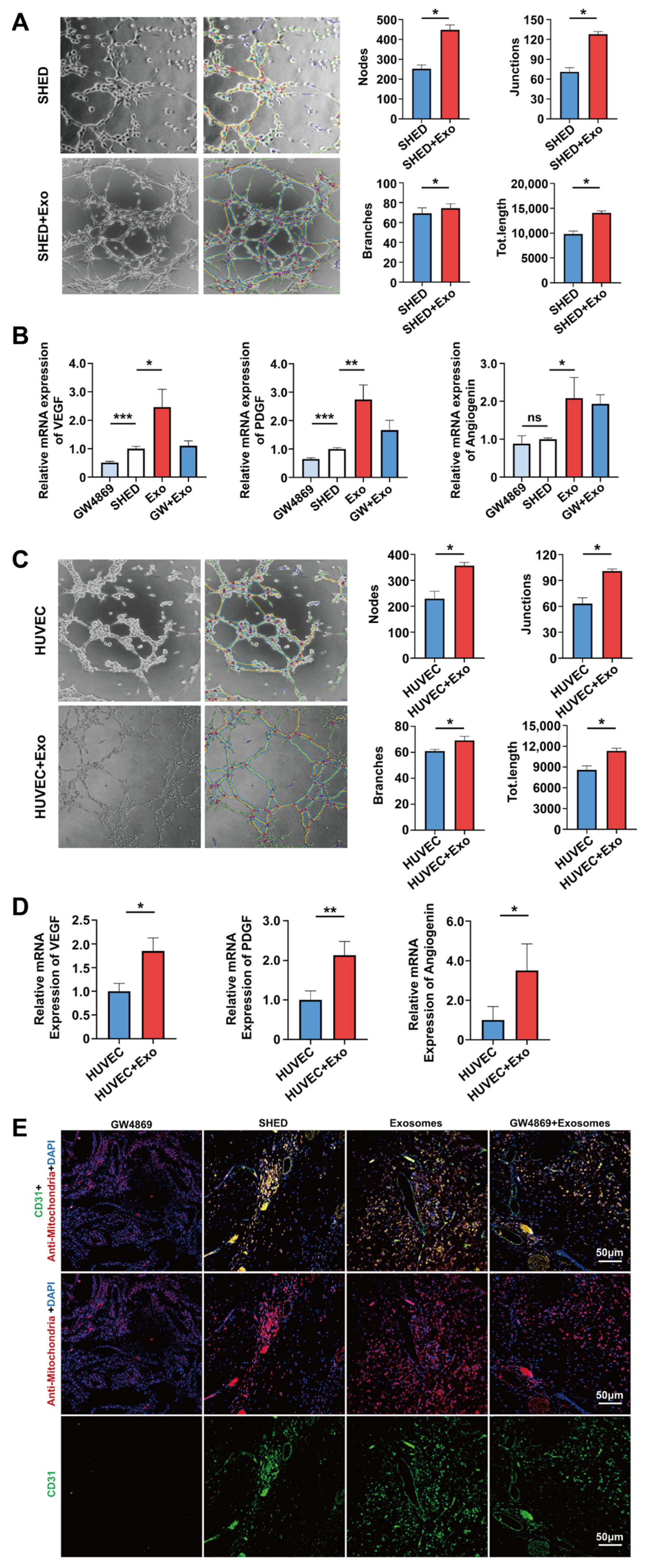
3.3. Exosomes in Angiogenesis
3.4. Exosomes in Neurogenesis
4. Influencing Factors of Exosomes Regulating Pulp–Dentin Complex Regeneration
4.1. Types of Parent Cells
4.2. Culture Environment of Parental Cells
4.3. Exosome Concentration
4.4. Application Environment of Exosomes
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Ricucci, D.; Siqueira, J.F., Jr.; Abdelsayed, R.A.; Lio, S.G.; Rôças, I.N. Bacterial invasion of pulp blood vessels in teeth with symptomatic irreversible pulpitis. J. Endod. 2021, 47, 1854–1864. [Google Scholar] [CrossRef]
- Lee, A.H.; Cheung, G.S.; Wong, M.C. Long-term outcome of primary non-surgical root canal treatment. Clin. Oral. Investig. 2012, 16, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Ricucci, D.; Siqueira, J.F., Jr.; Li, Y.; Tay, F.R. Vital pulp therapy: Histopathology and histobacteriology-based guidelines to treat teeth with deep caries and pulp exposure. J. Dent. 2019, 86, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Cohenca, N.; Paranjpe, A.; Berg, J. Vital pulp therapy. Dent. Clin. N. Am. 2013, 57, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Brouwer, F.; Schwendicke, A.; Paris, S. Different materials for direct pulp capping: Systematic review and meta-analysis and trial sequential analysis. Clin. Oral. Investig. 2016, 20, 1121–1132. [Google Scholar] [CrossRef]
- Ghoddusi, J.; Forghani, M.; Parisay, I. New approaches in vital pulp therapy in permanent teeth. Iran. Endod. J. 2014, 9, 15–22. [Google Scholar] [CrossRef]
- Cox, C.F.; Suzuki, S. Re-evaluating pulp protection: Calcium hydroxide liners vs. cohesive hybridization. J. Am. Dent. Assoc. 1994, 125, 823–831. [Google Scholar] [CrossRef]
- Asgary, S.; Eghbal, M.J.; Parirokh, M.; Ghanavati, F.; Rahimi, H. A comparative study of histologic response to different pulp capping materials and a novel endodontic cement. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2008, 106, 609–614. [Google Scholar] [CrossRef]
- Giuroiu, C.L.; Căruntu, I.D.; Lozneanu, L.; Melian, A.; Vataman, M.; Andrian, S. Dental pulp: Correspondences and contradictions between clinical and histological diagnosis. Biomed. Res. Int. 2015, 2015, 960321. [Google Scholar] [CrossRef]
- Glickman, G.N. AAE consensus conference on diagnostic terminology: Background and perspectives. J. Endod. 2009, 35, 1619–1620. [Google Scholar] [CrossRef]
- Fonzar, F.; Fonzar, A.; Buttolo, P.; Worthington, H.V.; Esposito, M. The prognosis of root canal therapy: A 10-year retrospective cohort study on 411 patients with 1175 endodontically treated teeth. Eur. J. Oral Implantol. 2009, 2, 201–208. [Google Scholar]
- Imura, N.; Pinheiro, E.T.; Gomes, B.P.; Zaia, A.A.; Ferraz, C.C.R.; Souza-Filho, F.J. The outcome of endodontic treatment: A retrospective study of 2000 cases performed by a specialist. J. Endod. 2007, 33, 1278–1282. [Google Scholar] [CrossRef]
- Shi, X.; Mao, J.; Liu, Y. Pulp stem cells derived from human permanent and deciduous teeth: Biological characteristics and therapeutic applications. Stem Cells Transl. Med. 2020, 9, 445–464. [Google Scholar] [CrossRef]
- Xie, Z.; Shen, Z.; Zhan, P.; Yang, J.; Huang, Q.; Huang, S.; Chen, L.; Lin, Z. Functional dental pulp regeneration: Basic research and clinical translation. Int. J. Mol. Sci. 2021, 22, 8991. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.; Yamaza, T.; Shea, L.D.; Djouad, F.; Kuhn, N.Z.; Tuan, R.S.; Shi, S. Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng. Part A 2010, 16, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Xuan, K.; Li, B.; Guo, H.; Sun, W.; Kou, X.; He, X.; Zhang, Y.; Sun, J.; Liu, A.; Liao, L.; et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci. Transl. Med. 2018, 10, eaaf3227. [Google Scholar] [CrossRef] [PubMed]
- de Cara, S.P.H.M.; Origassa, C.S.T.; de Sá Silva, F.; Moreira, M.S.N.A.; de Almeida, D.C.; Pedroni, A.C.F.; Carvalho, G.L.; Cury, D.P.; Câmara, N.O.S.; Marques, M.M. Angiogenic properties of dental pulp stem cells conditioned medium on endothelial cells in vitro and in rodent orthotopic dental pulp regeneration. Heliyon. 2019, 5, e01560. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Hayashi, Y.; Iohara, K.; Osako, Y.; Hirose, Y.; Nakashima, M. Trophic effects and regenerative potential of mobilized mesenchymal stem cells from bone marrow and adipose tissue as alternative cell sources for pulp/dentin regeneration. Cell Transplant. 2015, 24, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhao, Y.; Fang, T.J.; Ge, L. Effect of the soluble factors released by dental apical papilla-derived stem cells on the osteo/odontogenic, angiogenic, and neurogenic differentiation of dental pulp cells. Stem Cells Dev. 2020, 29, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Damania, A.; Jaiman, D.; Teotia, A.K.; Kumar, A. Mesenchymal stromal cell-derived exosome-rich fractionated secretome confers a hepatoprotective effect in liver injury. Stem Cell Res. Ther. 2018, 9, 31. [Google Scholar] [CrossRef]
- Kang, T.; Jones, T.M.; Naddell, C.; Bacanamwo, M.; Calvert, J.W.; Thompson, W.E.; Bond, V.C.; Chen, Y.E.; Liu, D. Adipose-derived stem cells induce angiogenesis via microvesicle transport of miRNA-31. Stem Cells Transl. Med. 2016, 5, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Miyaki, S.; Ishitobi, H.; Matsuyama, S.; Nakasa, T.; Kamei, N.; Akimoto, T.; Higashi, Y.; Ochi, M. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015, 589, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Narayanan, R.; Alapati, S.; Ravindran, S. Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration. Biomaterials 2016, 111, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Y.; Jia, S.; Chen, H.; Duan, Y.; Li, X.; Wang, S.; Wang, T.; Lyu, Y.; Chen, G.; et al. Exosome-like vesicles derived from Hertwig’s epithelial root sheath cells promote the regeneration of dentin-pulp tissue. Theranostics. 2020, 10, 5914–5931. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, D.A.; Mansour, A.M.; Saeed, M.A.; Zaher, A.R.; Grawish, M.E. Stem cell-derived exosomes for dentin-pulp complex regeneration: A mini-review. Restor. Dent. Endod. 2023, 48, e20. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell. 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during re-ticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, Y.; Li, H.J. Advances in mesenchymal stem cell exosomes: A review. Stem Cell Res. Ther. 2021, 12, 71. [Google Scholar] [CrossRef]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal stem cell-derived exosomes: Applications in regenerative medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science. 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Wei, H.; Chen, Q.; Lin, L.; Sha, C.; Li, T.; Liu, Y.; Yin, X.; Xu, Y.; Chen, L.; Gao, W.; et al. Regulation of exosome production and cargo sorting. Int. J. Biol. Sci. 2021, 17, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Livshits, M.A.; Khomyakova, E.; Evtushenko, E.G.; Lazarev, V.N.; Kulemin, N.A.; Semina, S.E.; Generozov, E.V.; Govorun, V.M. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci. Rep. 2015, 5, 17319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J. Extracell Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Sahoo, S.; Losordo, D.W. Exosomes and cardiac repair after myocardial infarction. Circ. Res. 2014, 114, 333–344. [Google Scholar] [CrossRef]
- Vicencio, J.M.; Yellon, D.M.; Sivaraman, V.; Das, D.; Boi-Doku, C.; Arjun, S.; Zheng, Y.; Riquelme, J.A.; Kearney, J.; Sharma, V.; et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J. Am. Coll. Cardiol. 2015, 65, 1525–1536. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Buller, B.; Chopp, M. Exosomes—Beyond stem cells for restorative therapy in stroke and neurological injury. Nat. Rev. Neurol. 2019, 15, 193–203. [Google Scholar] [CrossRef]
- Zagrean, A.M.; Hermann, D.M.; Opris, I.; Zagrean, L.; Popa-Wagner, A. Multicellular crosstalk between exosomes and the neurovascular unit after cerebral ischemia. therapeutic implications. Front. Neurosci. 2018, 12, 811. [Google Scholar] [CrossRef]
- Shi, R.; Jin, Y.; Hu, W.; Lian, W.; Cao, C.; Han, S.; Zhao, S.; Yuan, H.; Yang, X.; Shi, J.; et al. Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128-3p/SIRT1-mediated autophagy. Am. J. Physiol. Cell Physiol. 2020, 318, C848–C856. [Google Scholar] [CrossRef]
- Tan, C.Y.; Lai, R.C.; Wong, W.; Dan, Y.Y.; Lim, S.-K.; Ho, H.K. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res. Ther. 2014, 5, 76. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, W.; Tan, Y.; Zou, S.; Zhang, H.; Mao, F.; Gong, A.; Qian, H.; Xu, W. hucMSC exosome-derived GPX1 is required for the eecovery of hepatic oxidant injury. Mol. Ther. 2017, 25, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chu, W.C.; Lai, R.C.; Lim, S.K.; Hui, J.H.P.; Toh, W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 2016, 24, 2135–2140. [Google Scholar] [CrossRef]
- Furuta, T.; Miyaki, S.; Ishitobi, H.; Ogura, T.; Kato, Y.; Kamei, N.; Miyado, K.; Higashi, Y.; Ochi, M. Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. Stem Cells Transl. Med. 2016, 5, 1620–1630. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Li, H.; Chen, C.; Hu, B.; Niu, X.; Li, Q.; Zhao, B.; Xie, Z.; Wang, Y. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res. Ther. 2016, 7, 136. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.-C.; Yuan, T.; Zhang, Y.-L.; Yin, W.-J.; Guo, S.-C.; Zhang, C.-Q. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 2017, 7, 180–195. [Google Scholar] [CrossRef]
- Shi, X.; Jiang, N.; Mao, J.; Luo, D.; Liu, Y. Mesenchymal stem cell-derived exosomes for organ development and cell-free therapy. Nano Select. 2021, 2, 1291–1325. [Google Scholar] [CrossRef]
- Zou, J.; Mao, J.; Shi, X. Influencing factors of pulp-dentin complex regeneration and related biological strategies. J. Zhejiang Univ. (Med. Sci.) 2022, 51, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Chen, H.; Gao, B. Potential therapeutic effects of exosomes in regenerative endodontics. Arch. Oral. Biol. 2020, 120, 104946. [Google Scholar] [CrossRef]
- Ivica, A.; Ghayor, C.; Zehnder, M.; Valdec, S.; Weber, F.E. Pulp-derived exosomes in a fibrin-based regenerative root filling material. J. Clin. Med. 2020, 9, 491. [Google Scholar] [CrossRef]
- Li, Z.; Liang, Y.; Pan, K.; Li, H.; Yu, M.; Guo, W.; Chen, G.; Tian, W. Schwann cells secrete extracellular vesicles to promote and maintain the proliferation and multipotency of hDPCs. Cell Prolif. 2017, 50, e12353. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ju, Y.; Liu, S.; Fu, Y.; Zhao, S. Exosomes derived from lipopolysaccharide-preconditioned human dental pulp stem cells regulate Schwann cell migration and differentiation. Connect. Tissue Res. 2021, 62, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Abdik, H.; Avsar Abdik, E.; Hızlı Deniz, A.A.; Taşlı, P.N.; Şahin, F. A novel virtue in stem cell research: Exosomes and their role in differentiation. Adv. Exp. Med. Biol. 2019, 1144, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhong, Y.; Kong, Y.; Chen, Y.; Feng, J.; Zheng, J. Lineage-specific exosomes promote the odontogenic differentiation of human dental pulp stem cells (DPSCs) through TGFβ1/smads signaling pathway via transfer of microRNAs. Stem Cell Res. Ther. 2019, 10, 170. [Google Scholar] [CrossRef]
- Swanson, W.B.; Gong, T.; Zhang, Z.; Eberle, M.; Niemann, D.; Dong, R.; Rambhia, K.J.; Ma, P.X. Controlled release of odontogenic exosomes from a biodegradable vehicle mediates dentinogenesis as a novel biomimetic pulp capping therapy. J. Control Release. 2020, 324, 679–694. [Google Scholar] [CrossRef]
- Zhuang, X.; Ji, L.; Jiang, H.; Liu, Y.; Liu, X.; Bi, J.; Zhao, W.; Ding, Z.; Chen, X. Exosomes derived from stem cells from the apical papilla promote dentine-pulp complex regeneration by inducing specific dentinogenesis. Stem Cells Int. 2020, 2020, 5816723. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xian, X.; Lin, X.; Huang, L.; Liang, A.; Jiang, H.; Gong, Q. Hypoxia alters the proteome profile and enhances the angiogenic potential of dental pulp stem cell-derived exosomes. Biomolecules. 2022, 12, 575. [Google Scholar] [CrossRef]
- Zhang, Z.; Shuai, Y.; Zhou, F.; Yin, J.; Hu, J.; Guo, S.; Wang, Y.; Liu, W. PDLSCs regulate angiogenesis of periodontal ligaments via VEGF transferred by exosomes in periodontitis. Int. J. Med. Sci. 2020, 17, 558–567, Erratum in Int. J. Med. Sci. 2022, 19, 833. [Google Scholar] [CrossRef]
- Xian, X.; Gong, Q.; Li, C.; Guo, B.; Jiang, H. Exosomes with highly angiogenic potential for possible use in pulp regeneration. J. Endod. 2018, 44, 751–758. [Google Scholar] [CrossRef]
- Wu, M.; Liu, X.; Li, Z.; Huang, X.; Guo, H.; Guo, X.; Yang, X.; Li, B.; Xuan, K.; Jin, Y. SHED aggregate exosomes shuttled miR-26a promote angiogenesis in pulp regeneration via TGF-β/SMAD2/3 signalling. Cell Prolif. 2021, 54, e13074. [Google Scholar] [CrossRef]
- Chai, Y.; Jiang, X.; Ito, Y.; Bringas, P.; Han, J.; Rowitch, D.H.; Soriano, P.; McMahon, A.P.; Sucov, H.M. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 2000, 127, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; He, Y.; Wang, X.; Key, B.; Lee, B.H.; Li, H.; Ye, Q. Potential roles of dental pulp stem cells in neural regeneration and repair. Stem Cells Int. 2018, 2018, 1731289. [Google Scholar] [CrossRef]
- Venugopal, C.; Rai, K.S.; Pinnelli, V.B.; Kutty, B.M.; Dhanushkodi, A. Neuroprotection by human dental pulp mesenchymal stem cells: From billions to nano. Curr. Gene Ther. 2018, 18, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Nguyen, P.D.; Shanti, R.M.; Shi, S.; Shakoori, P.; Zhang, Q.; Le, A.D. Gingiva-derived mesenchymal stem cell extracellular vesicles activate schwann cell repair phenotype and promote nerve regeneration. Tissue Eng. Part A 2019, 25, 887–900. [Google Scholar] [CrossRef]
- Jarmalavičiūtė, A.; Tunaitis, V.; Pivoraitė, U.; Venalis, A.; Pivoriūnas, A. Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine-induced apoptosis. Cytotherapy 2015, 17, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.; Kumar, S.; Padmanabhan, P.; Gulyas, B.; Wan, A.C.; Rajendran, V.M. Lineage-specific exosomes could override extracellular matrix mediated human mesenchymal stem cell differentiation. Biomaterials 2018, 182, 312–322. [Google Scholar] [CrossRef]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem cell properties of human dental pulp stem cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef]
- Grottkau, B.E.; Purudappa, P.P.; Lin, Y.F. Multilineage differentiation of dental pulp stem cells from green fluorescent protein transgenic mice. Int. J. Oral. Sci. 2010, 2, 21–27. [Google Scholar] [CrossRef]
- Kim, S.; Shin, S.J.; Song, Y.; Kim, E. In vivo experiments with dental pulp stem cells for pulp-dentin complex regeneration. Mediators Inflamm. 2015, 2015, 409347. [Google Scholar] [CrossRef]
- Martinez Saez, D.; Sasaki, R.T.; Neves, A.D.; Da Silva, M.C. Stem cells from human exfoliated deciduous teeth: A growing literature. Cells Tissues Organs. 2016, 202, 269–280. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, W.; Liu, A.; Wu, M.; Shuai, Y.; Li, B.; Huang, X.; Liu, X.; Yang, X.; Guo, X.; et al. SHED promote angiogenesis in stem cell-mediated dental pulp regeneration. Biochem. Biophys. Res. Commun. 2020, 529, 1158–1164. [Google Scholar] [CrossRef]
- Yuan, X.; Yuan, Z.; Wang, Y.; Wan, Z.; Wang, X.; Yu, S.; Han, J.; Huang, J.; Xiong, C.; Ge, L.; et al. Vascularized pulp regeneration via injecting simvastatin functionalized GelMA cryogel microspheres loaded with stem cells from human exfoliated deciduous teeth. Mater. Today Bio. 2022, 13, 100209. [Google Scholar] [CrossRef]
- Vu, H.T.; Han, M.-R.; Lee, J.-H.; Kim, J.-S.; Shin, J.-S.; Yoon, J.-Y.; Park, J.-H.; Dashnyam, K.; Knowles, J.C.; Lee, H.-H.; et al. Investigating the effects of conditioned media from stem cells of human exfoliated deciduous teeth on dental pulp stem cells. Biomedicines 2022, 10, 906. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.-M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.-Y.; Shi, S.; et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef] [PubMed]
- Na, S.; Zhang, H.; Huang, F.; Wang, W.; Ding, Y.; Li, D.; Jin, Y. Regeneration of dental pulp/dentine complex with a three-dimensional and scaffold-free stem-cell sheet-derived pellet. J. Tissue Eng. Regen. Med. 2016, 10, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Bi, F.; Guo, W.H. Advances in research on Hertwig’s epithelial root sheath cells. J. Kunming Med. Univ. 2022, 43, 159–166. [Google Scholar] [CrossRef]
- Huang, C.H.; Ma, Y.Y.; Ren, L.; Cai, Q.; Chen, R.; Fu, Q. Conditioned media and exosomes from rat adipose-derived mesenchymal stem cells enhance bone regeneration: A study in vitro. Chin. J. Stomatol. Res. (Electron. Ed.) 2018, 12, 101–109. [Google Scholar]
- Song, Z.J.; Huang, C.H.; Ren, L.; Cai, Q.; Chen, R.; Fu, Q. The effect of murine adipose-derived stem cells exosomes on hypoxia induced osteocytes apoptosis. Chin. J. Stomatol. Res. (Electron. Ed.) 2017, 11, 157–163. [Google Scholar]
- Zhao, H.; Shang, Q.; Pan, Z.; Bai, Y.; Li, Z.; Zhang, H.; Zhang, Q.; Guo, C.; Zhang, L.; Wang, Q. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes 2018, 67, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Bao, L.; Lin, T.; Lu, Y.; Wu, Y. Proliferation and odontogenic differentiation of human umbilical cord mesenchymal stem cells and human dental pulp cells co-cultured in hydrogel. Arch. Oral. Biol. 2020, 109, 104582. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Zhang, Y.; Zhang, H.; Liu, W.; Zhang, N.; Zhang, X.; Zhou, G.; Wu, L.; Hua, K.; et al. Exosomes derived from human umbilical cord mesenchymal stem cells accelerate growth of VK2 vaginal epithelial cells through MicroRNAs in vitro. Hum. Reprod. 2019, 34, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, Y.; Guo, Q.; Gao, Q.; Ding, Y.; Wang, H.; Xu, W.; Yu, B.; Wang, M.; Zhao, Y.; et al. Exosomes derived from human umbilical cord mesenchymal stem cells promote fibroblast-to-myofibroblast differentiation in inflammatory environments and benefit cardioprotective effects. Stem Cells Dev. 2019, 28, 799–811. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Y.; Chen, L.; Ye, L.; Cui, J.; Sun, Q.; Li, K.; Li, Z.; Liu, L. Human umbilical cord mesenchymal stem cells: A new therapeutic option for tooth regeneration. Stem Cells Int. 2015, 2015, 549432. [Google Scholar] [CrossRef]
- Brizuela, C.; Meza, G.; Urrejola, D.; Quezada, M.; Concha, G.; Ramírez, V.; Angelopoulos, I.; Cadiz, M.; Tapia-Limonchi, R.; Khoury, M. Cell-based regenerative endodontics for treatment of periapical lesions: A randomized, controlled phase I/II clinical trial. J. Dent. Res. 2020, 99, 523–529. [Google Scholar] [CrossRef]
- Huang, X.; Qiu, W.; Pan, Y.; Li, J.; Chen, Z.; Zhang, K.; Luo, Y.; Wu, B.; Xu, W. Exosomes from LPS-stimulated hDPSCs activated the angiogenic potential of HUVECs in vitro. Stem Cells Int. 2021, 2021, 6685307. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Tang, J.; Dou, L.; Yang, S.; Sun, Y.; Mao, H.; Yang, D. Dental pulp stem cell-derived exosomes regulate anti-inflammatory and osteogenesis in periodontal ligament stem cells and promote the repair of experimental periodontitis in rats. Int. J. Nanomed. 2023, 18, 4683–4703. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Ye, Y.; He, S.; Song, J. SHED-derived conditioned exosomes enhance the osteogenic differentiation of PDLSCs via Wnt and BMP signaling in vitro. Differentiation 2020, 111, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Faruqu, F.N.; Zhou, S.; Sami, N.; Gheidari, F.; Lu, H.; Al-Jamal, K.T. Three-dimensional culture of dental pulp pluripotent-like stem cells (DPPSCs) enhances Nanog expression and provides a serum-free condition for exosome isolation. FASEB Bioadv. 2020, 2, 419–433. [Google Scholar] [CrossRef]
- Zhang, Q. The Study of Exosomes Derived from Aggregates of Stem Cells from Human Exfoliated Deciduous Teeth on Dental Pulp Regeneration. PhD Dissertation, Air Force Medical University, Xi’an, China, 2019. [Google Scholar] [CrossRef]
- Liu, D.; Shi, B.; Zhou, W.; Tao, G. Exosomes from hypoxia-conditioned apical papilla stem cells accelerate angiogenesis in vitro through Notch/JAG1/VEGF signaling. Tissue Cell. 2023, 84, 102197. [Google Scholar] [CrossRef]
- Dorayappan, K.D.P.; Wanner, R.; Wallbillich, J.J.; Saini, U.; Zingarelli, R.; Suarez, A.A.; Cohn, D.E.; Selvendiran, K. Hypoxia-induced exosomes contribute to a more aggressive and chemoresistant ovarian cancer phenotype: A novel mechanism linking STAT3/Rab proteins. Oncogene 2018, 37, 3806–3821. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Rong, Y.; Qian, D.; Chen, J.; Zhou, Z.; Luo, Y.; Jiang, D.; Cheng, L.; Zhao, S.; et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020, 103, 196–212. [Google Scholar] [CrossRef]
- Liu, P.; Qin, L.; Liu, C.; Mi, J.; Zhang, Q.; Wang, S.; Zhuang, D.; Xu, Q.; Chen, W.; Guo, J.; et al. Exosomes derived from hypoxia-conditioned stem cells of human deciduous exfoliated teeth enhance angiogenesis via the transfer of let-7f-5p and miR-210-3p. Front. Cell Dev. Biol. 2022, 10, 879877. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ren, J.; Bai, Y.; Pei, X.; Han, Y. Exosomes from hypoxia-treated human adipose-derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF-R. Int. J. Biochem. Cell Biol. 2019, 109, 59–68, Erratum in Int. J. Biochem. Cell Biol. 2020, 126, 105805. [Google Scholar] [CrossRef] [PubMed]
- McGettrick, A.F.; O’Neill, L.A.J. The role of HIF in immunity and inflammation. Cell Metab. 2020, 32, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-King, H.; García, N.A.; Ontoria-Oviedo, I.; Ciria, M.; Montero, J.A.; Sepúlveda, P. Hypoxia inducible factor-1alpha potentiates jagged 1-mediated angiogenesis by mesenchymal stem cell-derived exosomes. Stem Cells. 2017, 35, 1747–1759. [Google Scholar] [CrossRef] [PubMed]
- Padilla, F.; Puts, R.; Vico, L.; Raum, K. Stimulation of bone repair with ultrasound: A review of the possible mechanic effects. Ultrasonics 2014, 54, 1125–1145. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.L.; Smith, N.B.; Bailey, M.R.; Czarnota, G.J.; Hynynen, K.; Makin, I.R.S. Overview of therapeutic ultrasound applications and safety considerations. J. Ultrasound Med. 2012, 31, 623–634. [Google Scholar] [CrossRef]
- He, Y.-F.; Wang, X.-L.; Deng, S.-P.; Wang, Y.-L.; Huang, Q.-Q.; Lin, S.; Lyu, G.-R. Latest progress in low-intensity pulsed ultrasound for studying exosomes derived from stem/progenitor cells. Front Endocrinol. 2023, 14, 1286900. [Google Scholar] [CrossRef]
- Chen, D.; Xiang, M.; Gong, Y.; Xu, L.; Zhang, T.; He, Y.; Zhou, M.; Xin, L.; Li, J.; Song, J. LIPUS promotes FOXO1 accumulation by downregulating miR-182 to enhance osteogenic differentiation in hPDLCs. Biochimie 2019, 165, 219–228, Erratum in Biochimie 2022, 194, 51–54. [Google Scholar] [CrossRef]
- Costa, V.; Carina, V.; Conigliaro, A.; Raimondi, L.; De Luca, A.; Bellavia, D.; Salamanna, F.; Setti, S.; Alessandro, R.; Fini, M.; et al. MiR-31-5p is a LIPUS-mechanosensitive microRNA that targets HIF-1α signaling and cytoskeletal proteins. Int. J. Mol. Sci. 2019, 20, 1569. [Google Scholar] [CrossRef]
- Li, X.; Zhong, Y.; Zhou, W.; Song, Y.; Li, W.; Jin, Q.; Gao, T.; Zhang, L.; Xie, M. Low-intensity pulsed ultrasound (LIPUS) enhances the anti-inflammatory effects of bone marrow mesenchymal stem cells (BMSCs)-derived extracellular vesicles. Cell Mol. Biol. Lett. 2023, 28, 9. [Google Scholar] [CrossRef]
- Zhu, M.Y. Effect and the Possible Mechanism of LIPUS-Stimulated Human Dental Follicle Stem Cell-Derived Exosomes on the Proliferation and Differentiation of hDFSCs. Master’s Thesis, Chongqing Medical University, Chongqing, China, 2020. [Google Scholar] [CrossRef]
- Al-Daghreer, S.; Doschak, M.; Sloan, A.J.; Major, P.W.; Heo, G.; Scurtescu, C.; Tsui, Y.Y.; El-Bialy, T. Long term effect of low intensity pulsed ultrasound on a human tooth slice organ culture. Arch. Oral. Biol. 2012, 57, 760–768. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Yang, Z.; Lu, K.; Zuo, J.; Zhou, Z. Effect of low-intensity pulsed ultrasound on a rat model of dentin-dental pulp injury and repair. Ultrasound Med. Biol. 2017, 43, 163–175. [Google Scholar] [CrossRef]
- Zuo, J.; Zhen, J.; Wang, F.; Li, Y.; Zhou, Z. Effect of Low-Intensity Pulsed Ultrasound on the Expression of Calcium Ion Transport-Related Proteins during Tertiary Dentin For-mation. Ultrasound Med. Biol. 2018, 44, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Asnaashari, M.; Safavi, N. Application of low level lasers in dentistry (endodontic). J. Lasers Med. Sci. 2013, 4, 57–66. [Google Scholar] [PubMed]
- Gutiérrez, D.; Rouabhia, M.; Ortiz, J.; Gaviria, D.; Alfonso, C.; Muñoz, A.; Inostroza, C. Low-level laser irradiation promotes proliferation and differentiation on apical papilla stem cells. J. Lasers Med. Sci. 2021, 12, e75. [Google Scholar] [CrossRef]
- Tabatabaei, F.S.; Torshabi, M.; Mojahedi Nasab, M.; Khosraviani, K.; Khojasteh, A. Effect of low-level diode laser on proliferation and osteogenic differentiation of dental pulp stem cells. Laser Phys. 2015, 25, 095602. [Google Scholar] [CrossRef]
- Ginani, F.; Soares, D.M.; de Oliveira Rocha, H.A.; de Souza, L.B.; Barboza, C.A.G. Low-level laser irradiation induces in vitro proliferation of stem cells from human exfoliated deciduous teeth. Lasers Med. Sci. 2018, 33, 95–102. [Google Scholar] [CrossRef]
- Paschalidou, M.; Athanasiadou, E.; Arapostathis, K.; Kotsanos, N.; Koidis, P.T.; Bakopoulou, A.; Theocharidou, A. Biological effects of low-level laser irradiation (LLLI) on stem cells from human exfoliated deciduous teeth (SHED). Clin. Oral. Investig. 2020, 24, 167–180. [Google Scholar] [CrossRef]
- Marques, M.M.; Diniz, I.M.; de Cara, S.P.; Pedroni, A.C.F.; Abe, G.L.; D’Almeida-Couto, R.S.; Lima, P.L.V.; Tedesco, T.K.; Moreira, M.S. Photobiomodulation of dental derived mesenchymal stem cells: A systematic review. Photomed. Laser Surg. 2016, 34, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Staffoli, S.; Romeo, U.; Amorim, R.N.S.; Migliau, G.; Palaia, G.; Resende, L.; Polimeni, A. The effects of low level laser irradiation on proliferation of human dental pulp: A narrative review. Clin. Ter. 2017, 168, e320–e326. [Google Scholar] [CrossRef]
- Ferreira, L.S.; Diniz, I.M.A.; Maranduba, C.M.S.; Miyagi, S.P.H.; Rodrigues, M.F.S.D.; Moura-Netto, C.; Marques, M.M. Short-term evaluation of photobiomodulation therapy on the proliferation and undifferentiated status of dental pulp stem cells. Lasers Med. Sci. 2019, 34, 659–666. [Google Scholar] [CrossRef]
- Moreira, M.S.; Sarra, G.; Carvalho, G.L.; Gonçalves, F.; Caballero-Flores, H.V.; Pedroni, A.C.F.; Lascala, C.A.; Catalani, L.H.; Marques, M.M. Physical and biological properties of a chitosan hydrogel scaffold associated to photobiomodulation therapy for dental pulp regeneration: An in vitro and in vivo study. Biomed. Res. Int. 2021, 2021, 6684667. [Google Scholar] [CrossRef]
- Dai, M.; Yu, M.; Zhang, Y.; Tian, W. Exosome-like vesicles derived from adipose tissue provide biochemical cues for adipose tissue regeneration. Tissue Eng. Part A 2017, 23, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Teo, K.Y.W.; Zhang, S.; Lai, R.C.; Rosa, V.; Tong, H.J.; Duggal, M.S.; Lim, S.K.; Toh, W.S. Mesenchymal stromal cell exosomes enhance dental pulp cell functions and promote pulp-dentin regeneration. Biomater. Biosyst. 2023, 11, 100078. [Google Scholar] [CrossRef] [PubMed]
- Namjoynik, A.; Islam, M.A.; Islam, M. Evaluating the efficacy of human dental pulp stem cells and scaffold combination for bone regeneration in animal models: A systematic review and meta-analysis. Stem Cell Res. Ther. 2023, 14, 132. [Google Scholar] [CrossRef] [PubMed]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart hydrogels in tissue engineering and regenerative medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Moussa, D.G.; Aparicio, C. Present and future of tissue engineering scaffolds for dentin-pulp complex regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Huang, Y.; Liu, H.; Tang, Z.; Chen, Y.; Huang, Z.; Xu, S.; Du, J.; Jia, B. Hydrogels for the treatment of oral and maxillofacial diseases: Current research, challenges, and future directions. Biomater. Sci. 2022, 10, 6413–6446. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Liu, Y.; Jiang, L.; Wang, X.; Chen, Y.; Li, L.; Song, M.; Zhang, H.; Zhang, Y.S.; Zhang, X. Injectable decellularized dental pulp matrix-functionalized hydrogel microspheres for endodontic regeneration. Acta Biomater. 2023, 156, 37–48. [Google Scholar] [CrossRef]
- Khayat, A.; Monteiro, N.; Smith, E.E.; Pagni, S.; Zhang, W.; Khademhosseini, A.; Yelick, P. GelMA-encapsulated hDPSCs and HUVECs for dental pulp regeneration. J. Dent. Res. 2017, 96, 192–199. [Google Scholar] [CrossRef]
- Khattak, S.; Wahid, F.; Liu, L.P.; Jia, S.-R.; Chu, L.-Q.; Xie, Y.-Y.; Li, Z.-X.; Zhong, C. Applications of cellulose and chitin/chitosan derivatives and composites as antibacterial materials: Current state and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zou, J.; Cai, M.; Wang, Y.; Mao, J.; Shi, X. Applicatoin of chitosan-based hydrogel in oral tissue engineering. J. Cent. South Univ. (Med. Sci.) 2023, 48, 138–147. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, X.; Chen, X.; Wei, Y.; Du, W.; Wang, Y.; Liu, L.; Zhao, W.; Han, Z.; Kong, D.; et al. Enhanced therapeutic effects of mesenchymal stem cell-derived exosomes with an injectable hydrogel for hindlimb ischemia treatment. ACS Appl. Mater. Interfaces 2018, 10, 30081–30091. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Kuang, S.; Zhang, Y.; Yang, M.; Qin, W.; Shi, X.; Lin, Z. Chitosan hydrogel incorporated with dental pulp stem cell-derived exosomes alleviates periodontitis in mice via a macrophage-dependent mechanism. Bioact. Mater. 2020, 5, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xing, R.; Bai, S.; Yan, X. Recent advances of self-assembling peptide-based hydrogels for biomedical applications. Soft Matter. 2019, 15, 1704–1715. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Tian, L.; Shamirzaei-Jeshvaghani, E.; Dehghani, L.; Ramakrishna, S. Structural properties of scaffolds: Crucial parameters towards stem cells differentiation. World J. Stem Cells. 2015, 7, 728–744. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.M.Z.; Patel, U.; Ahmed, I. Development of microspheres for biomedical applications: A review. Prog. Biomater. 2015, 4, 1–19. [Google Scholar] [CrossRef]
- Bi, Y.G.; Lin, Z.T.; Deng, S.T. Fabrication and characterization of hydroxyapatite/sodium alginate/chitosan composite microspheres for drug delivery and bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 576–583. [Google Scholar] [CrossRef]
- Li, X.; Chen, D.; Xie, S. Current progress and prospects of organic nanoparticles against bacterial biofilm. Adv. Colloid. Interface Sci. 2021, 294, 102475. [Google Scholar] [CrossRef]
- Hussein, H.; Kishen, A. Antibiofilm and immune response of engineered bioactive nanoparticles for endodontic disinfection. J. Clin. Med. 2020, 9, 730. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.; Kishen, A. Proteomic profiling reveals engineered chitosan nanoparticles mediated cellular crosstalk and immunomodulation for therapeutic application in apical periodontitis. Bioact. Mater. 2021, 11, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q. Progress in the diagnosis and treatment strategies of caries-derived dental pulp diseases. Chin. J. Stomatol. 2021, 56, 16–21. [Google Scholar] [CrossRef]
- Zou, J.; Xia, H.; Jiang, Q.; Su, Z.; Wen, S.; Liang, Z.; Ouyang, Y.; Liu, J.; Zhang, Z.; Chen, D.; et al. Exosomes derived from odontogenic stem cells: Its role in the dentin-pulp complex. Regen. Ther. 2023, 24, 135–146. [Google Scholar] [CrossRef]
- Zarà, M.; Amadio, P.; Campodonico, J.; Sandrini, L.; Barbieri, S.S. Exosomes in cardiovascular diseases. Diagnostics 2020, 10, 943. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.T.; Ma, Y.; Yang, B.; Tian, W.D. Exosomes-based strategies for dental pulp regeneration. Zhonghua Kou Qiang Yi Xue Za Zhi 2021, 56, 709–714. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, Q.; Wang, Y.; Jiang, N.; Wang, Y.; Wang, R.; Hu, X.; Mao, J.; Shi, X. Exosomes as Promising Therapeutic Tools for Regenerative Endodontic Therapy. Biomolecules 2024, 14, 330. https://doi.org/10.3390/biom14030330
Kong Q, Wang Y, Jiang N, Wang Y, Wang R, Hu X, Mao J, Shi X. Exosomes as Promising Therapeutic Tools for Regenerative Endodontic Therapy. Biomolecules. 2024; 14(3):330. https://doi.org/10.3390/biom14030330
Chicago/Turabian StyleKong, Qingyue, Yujie Wang, Nan Jiang, Yifan Wang, Rui Wang, Xiaohan Hu, Jing Mao, and Xin Shi. 2024. "Exosomes as Promising Therapeutic Tools for Regenerative Endodontic Therapy" Biomolecules 14, no. 3: 330. https://doi.org/10.3390/biom14030330
APA StyleKong, Q., Wang, Y., Jiang, N., Wang, Y., Wang, R., Hu, X., Mao, J., & Shi, X. (2024). Exosomes as Promising Therapeutic Tools for Regenerative Endodontic Therapy. Biomolecules, 14(3), 330. https://doi.org/10.3390/biom14030330





