Abstract
Cholesterol is an essential molecule of life, and its synthesis can be inhibited by both genetic and nongenetic mechanisms. Hundreds of chemicals that we are exposed to in our daily lives can alter sterol biosynthesis. These also encompass various classes of FDA-approved medications, including (but not limited to) commonly used antipsychotic, antidepressant, antifungal, and cardiovascular medications. These medications can interfere with various enzymes of the post-lanosterol biosynthetic pathway, giving rise to complex biochemical changes throughout the body. The consequences of these short- and long-term homeostatic disruptions are mostly unknown. We performed a comprehensive review of the literature and built a catalogue of chemical agents capable of inhibiting post-lanosterol biosynthesis. This process identified significant gaps in existing knowledge, which fall into two main areas: mechanisms by which sterol biosynthesis is altered and consequences that arise from the inhibitions of the different steps in the sterol biosynthesis pathway. The outcome of our review also reinforced that sterol inhibition is an often-overlooked mechanism that can result in adverse consequences and that there is a need to develop new safety guidelines for the use of (novel and already approved) medications with sterol biosynthesis inhibiting side effects, especially during pregnancy.
1. Introduction
Cholesterol is an essential molecule of life, acting as a structural component of all cell membranes as well as an essential regulator of membrane fluidity and cell signaling [1,2,3,4]. The sterol biosynthetic pathway intermediates also serve as precursors for many bioactive molecules, including neurosteroids such as vitamin D [5,6,7]. Pathogenic variants in the sterol biosynthesis pathway that completely eliminate cholesterol production are lethal, while pathogenic variants resulting in significantly diminished cholesterol synthesis give rise to specific clinical syndromes [8,9,10,11]. These syndromes affect the entire body, with systemic dysmorphologies, altered brain development, and subsequent intellectual disability [8].
Cholesterol synthesis can be also inhibited by nongenetic mechanisms, however [12]. Hundreds of chemicals that we are exposed to in our daily lives can alter sterol biosynthesis [13]. Importantly, these include various classes of FDA-approved medications, including (but not limited to) commonly used antipsychotic, antidepressant, antifungal, and cardiovascular medications [14,15,16,17,18,19,20,21]. They can interfere with various enzymes of the post-lanosterol biosynthetic pathway, giving rise to complex biochemical changes throughout the body [22,23].
Cholesterol biosynthesis inhibition can be both beneficial and detrimental, and this depends on the context in which the inhibition occurs [24]. Targeted inhibition by statins is beneficial and saves lives [25], while inhibition as a side effect of psychotropic medications might have unwanted consequences [26]. The impact of these side effects will greatly depend on the stage of life [27,28]. The most robust sterol biosynthesis occurs during the fetal and early postnatal period [29], and developmental interference with this process can lead to lasting consequences that would not be seen in adult exposure [30]. Specifically, the results of a recent review of human clinical data suggested that sterol-inhibiting medications should be considered as teratogens [31].
Chemical inhibition of sterol biosynthesis is a greatly understudied area and is rarely appreciated by researchers or clinicians. To highlight this issue, we performed a comprehensive review of the literature, focusing on the sterol-inhibiting effects and side effects of chemical compounds and prescription medications. The outcome of this review strongly reinforces the notion that sterol inhibition is an often-overlooked mechanism that can result in adverse consequences, and identifies a number of significant gaps in knowledge that should be addressed in future studies.
2. Materials and Methods
The literature databases EMBASE and MEDLINE were searched using the search strategies described in Supplemental Table S1. Briefly, we sought out studies involving any of the enzymes involved in the Bloch and/or Kandutsch–Russell pathway and any type of medication intervention. We excluded studies that only modified the sterol pathways through genetically altered animals or cells. The titles and abstracts were each reviewed by two authors (E.S.P., Z.K.) to determine relevance for inclusion. If there was ambiguity or disagreement regarding relevance for inclusion at the title/abstract review step, the manuscript was moved along to the full-text review step.
3. Results
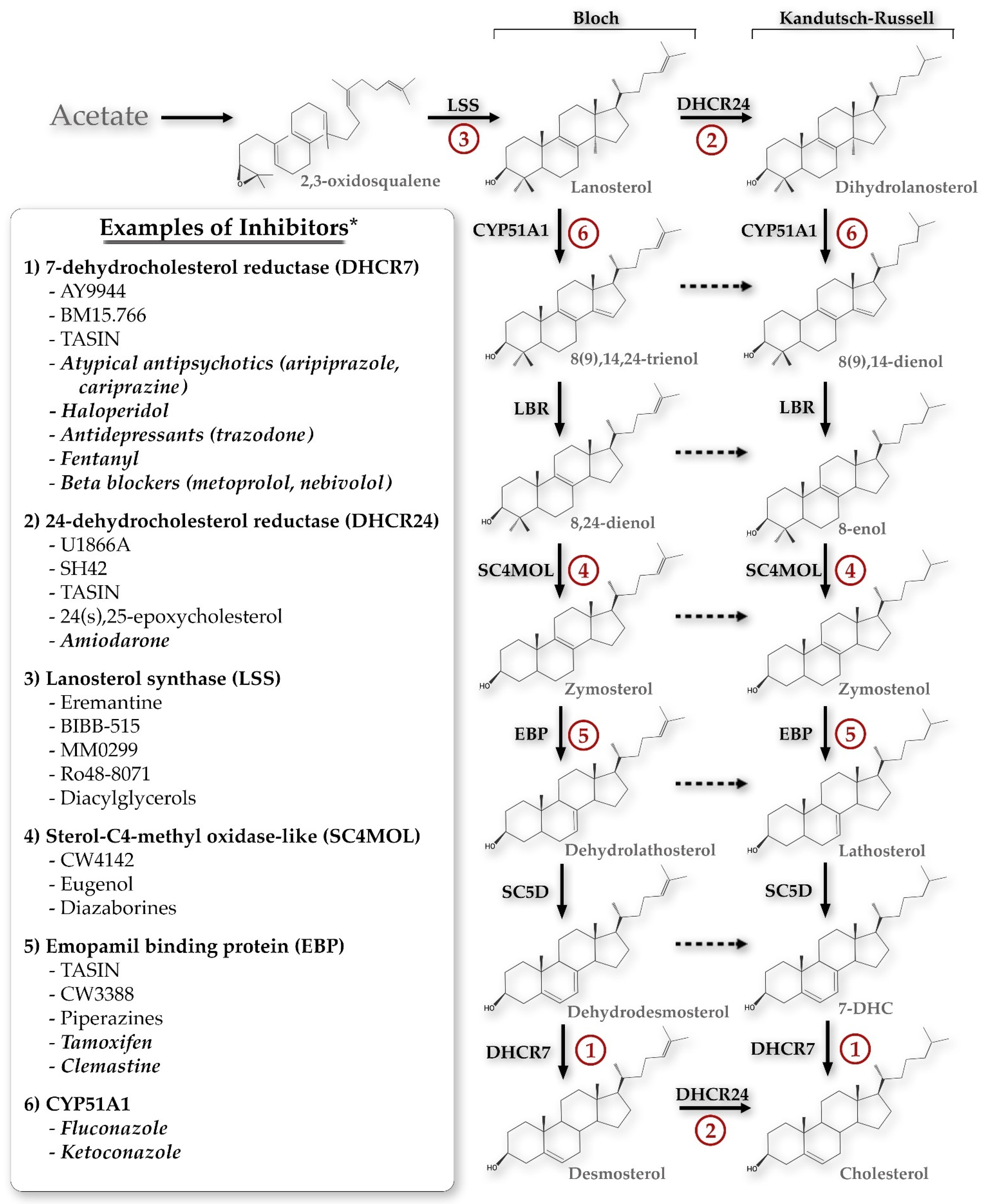
The initial search returned 518 manuscripts. After initial title and abstract review, 333 manuscripts were excluded, resulting in a total of 185 manuscripts for full text review. Additional relevant manuscripts were identified through review of the reference lists of the included full text review manuscripts. Figure 1 summarizes the inhibitors of each of the key enzymes in the Bloch and Kandutsch–Russell sterol pathways. Additionally, each of those enzymes will be discussed in depth below.
Figure 1.
The primary sterol synthesis pathways discussed in this review, demonstrating potential chemical inhibitors for each of the key enzyme steps. * This list is not exhaustive but is meant to demonstrate the breadth of compounds potentially inhibiting each step, including both specific inhibitors and FDA-approved medications (the latter denoted by italics). CYP51A1, cytochrome P450 family 51 subfamily A member 1; LBR, lamin B receptor; SC5D, sterol-C5-desaturase; 7-DHC, 7-dehydrocholesterol; TASIN, truncated adenomatous polyposis coli selective inhibitors.
3.1. Chemicals That Alter 7-Dehydrocholesterol Reductase (DHCR7) Activity
The Dhcr7 gene codes for the DHCR7 protein, which is an enzyme responsible for converting 7-dehydrocholesterol (7-DHC) to cholesterol [32,33,34,35]. Mutations in this gene can result in a rare autosomal recessive disorder known as Smith–Lemli–Opitz syndrome (SLOS: OMIM 270400 [36]). The phenotype of this disease can encompass microcephaly with agenesis of corpus callosum, convulsions, micrognathia, upward turned nose, syndactyly, and/or polydactyly [9,37]. Heterozygous carriers of a Dhcr7 mutation will have a slight accumulation of 7-DHC [38], but these smaller changes appear to have no influence on vitality. Several investigators have sought to model DHCR7 inhibition through administration of chemicals that either specifically target DHCR7 production (as in the cases of AY9944 and BM15766) or result in DHCR7 inhibition as an off-target effect. These different chemicals and the data around their use and efficacy are described below.
3.1.1. AY9944
AY9944 is a potent inhibitor of DHCR7 that can mimic the SLOS phenotype [39,40], likely through a combination of direct DHCR7 inhibition in addition to alterations in sonic hedgehog (Shh) signaling, since Shh activation requires covalent linkage to cholesterol [41]. The exact mechanism and effects of changes in Shh signaling are still under investigation [42]. Delivery of AY9944 can model SLOS either through administration during pregnancy to affect the developing fetus or as chronic postnatal administration. Studies assessing administration during pregnancy have found accumulation of 7-DHC, 8-DHC, and trienols in the embryos of the treated dams [43] as well as teratogenicity in rats, reproducing some of the clinical phenotype of SLOS [42].
Chronic AY9944 administration to rats is also used as a model of SLOS and causes progressive irreversible retinal dysfunction and degeneration [44,45]. Xu et al. [46] identified five 7-DHC-derived oxysterols (3β,5α-dihydroxycholest-7-en-6-one (DHCEO), 4α- and 4β-hydroxy-7-DHC, 24-hydroxy-7-DHC, and 7-ketocholesterol) as well as signs of altered bile acid metabolism, membrane dynamics, amino acid catabolism, urea cycle, polyamine synthesis, glucose utilization, and antioxidant mobilization in retinas from AY9944-treated rats. Levels of the branched chain amino acids (BCAAs) were significantly lower in treated retinas compared to controls, while BCAA catabolites were higher, suggesting increased BCAA breakdown. Pipecolate, a proapoptotic catabolite of lysine degradation, was also elevated 3–4-fold in brain and retina and >5-fold in the serum of treated animals compared to controls [46]. Although prolonged administration of AY9944 results in significant elevations of 7-DHC, the 8-DHC concentrations and ratio of 8-DHC:7-DHC do not reach the levels of those seen in humans with SLOS [47].
Although prolonged DHCR7 alterations tend to result in negative outcomes, mimicking SLOS, short-term alterations during periods of stress may be protective. For example, Dhcr7 mRNA has been found to be upregulated in the leukocytes of those with sepsis who have poor outcome, while inhibiting DHCR7 with AY9944 in zebrafish exposed to LPS decreased lethality [48]. The exact mechanism of this protection is still being evaluated, but the benefits of short-term AY9944 appear to be consistent across several disease models. AY9944 suppresses lipid peroxidation and ferroptosis in human hepatocellular carcinoma Huh-7 cells [49], inhibits vesicular stomatitis virus (VSV) infection in a cell culture model [50], and promotes clearance of zika and other viruses in vitro and in vivo through an interferon-dependent mechanism [51]. These effects can be further supplemented by preventing 7-DHC peroxidation with vitamins E and C (plus sodium selenite) [46].
3.1.2. BM15.766
Another specific competitive inhibitor of DHCR7 is the piperazine derivative BM15.766 [52,53,54], which has been shown to increase 7-DHC and 8-DHC in both the liver and testis [55]. Similar to AY9944, BM15.766 is also teratogenic in rats and replicates some of the SLOS phenotype. Near-term fetuses exposed to maternal administration of BM15.766 between gestational day 1 and 11 demonstrated facial malformations and brain anomalies along the holoprosencephaly spectrum, including pituitary agenesis associated with maternal decreased cholesterol and increased 7-DHC concentrations [56]. Similarly, chronic postnatal administration of BM15.766 to rats starting at the time of weaning resulted in learning deficits that were partially recovered by adding 2% cholesterol to the feeding regimen [54], which not only recovers low cholesterol concentrations but also reduces plasma 7-DHC after BM15.766 treatment [57].
3.1.3. Other DHCR7 Inhibitors
Several in silico and high-throughput in vitro studies have sought to uncover additional chemicals that result in elevated 7-DHC through the inhibition of DHCR7. One such study used the Distributed Structure-Searchable Toxicity (DSSTox) Database Network developed by Environmental Protection Agency to screen environmental molecules that display structures similar to AY9944 and identified the widely used disinfectant benzalkonium chlorides as a potent inhibitor in the cell culture system [19]. When administered in vitro to mouse neurospheres cultured from embryonic neuronal progenitor cells, benzalkonium chloride inhibits DHCR7; however, when administered to pregnant mice, its actions are much more nonspecific, inhibiting multiple sterols (cholesterol, dehydro-cholesterol, zymosterol, desmosterol, lanosterol, lathosterol) in the brain of postnatal day 0 pups [19,20,21].
Other studies have used high-throughput in vitro screening to evaluate over 5000 currently used chemicals to evaluate those that resulted in the off-target effect of 7-DHC elevation. The list of chemicals that resulted in the highest magnitude of 7-DHC change include several antipsychotics and antidepressants as well as the beta-blocker metoprolol [15,16]. Several of these chemicals will be discussed in greater detail below.
Although much of the data on the effects of antipsychotics on 7-DHC have focused on the atypical antipsychotics cariprazine and aripiprazole, the first-generation antipsychotic haloperidol also shows similar effects. Haloperidol treatment of primary neurons and astrocytes inhibited DHCR7 as well as other enzymes in the post-lanosterol pathway [58]. Similarly, in vivo treatment of adult rats resulted in a dose-dependent increase in the brain concentrations of 7-DHC [59].
Cariprazine, an atypical antipsychotic, is one of the most potent 7-DHC-elevating medications, demonstrating similar increases in 7-DHC compared to AY9944 when administered to Neuro2a or human fibroblast cells [60]. Its effects have been tested in several different models, showing similar significant elevations of 7-DHC in vitro in Neuro2a cells infected by VSV [50] as well as in multiple organs, including the fetal brain in vivo after maternal in utero exposure [61].
Aripiprazole, another atypical antipsychotic, has been associated with increased 7-DHC and 8-DHC in human blood samples [59]. Both cariprazine and aripiprazole significantly altered cholesterol biosynthesis precursor profiles in highly proliferative NPCs and early post-mitotic neurons differentiated from human-induced pluripotent stem cells [62]. Additionally, aripiprazole increased 7-DHC in airway and bronchial epithelial cells and potentiated ozone-induced cytokine release in a donor sex-specific manner [63]. Both cariprazine and aripiprazole share the common metabolite 2,3-DCPP which is a potent inhibitor of DHCR7 as well, likely due to the shared dichlorophyenil-piperazine substructure in aripiprazole, cariprazine, and 2,3-DCPP [60].
Trazodone, a commonly prescribed antidepressant, is also a potent DHCR7 inhibitor. Trazodone inhibition of DHCR7 increases 7-DHC and 7-DHD and decreases desmosterol, though its effects on decreasing cholesterol have not been consistently demonstrated. Where the other medications discussed above (haloperidol, cariprazine, aripiprazole) have more complex sterol disruption profiles—with many inhibiting other enzymes at higher doses and increasing zymosterol, zymostenol, lathosterol, DHL, 8-DHD, and 8-DHC—trazodone is mostly a DHCR7 inhibitor [58]. Similar to aripiprazole, trazodone has also been associated with increased 7-DHC and 8-DHC in human blood samples [59,64], and the presence of trazodone in perimortem toxicology screens was strongly associated with observed differences in 7-DHC and desmosterol concentrations in postmortem brain samples [65]. These human studies are further supported by many studies where the levels of 7-DHC were measured after in vitro [50] or in vivo [58,59,64,66,67] exposure to trazodone.
Several other medication classes have also been found to inhibit DHCR7, including in utero exposure to fentanyl [68]. After early fentanyl exposure during pregnancy, a series of ten patients demonstrated clinical phenotypes mimicking SLOS, including microcephaly, bilateral 2,3-toe syndactyly, single palmar creases, and cleft palate. Initial screening showed elevated 7-DHC and 8-DHC, and after one month, follow-up showed normal sterol values. No genetic cause was identified. This report is unique because it is the first to raise concern potentially correlating in utero exposure to fentanyl to neonatal 7-DHC concentrations.
Additionally, administration of the beta-blocker metoprolol to pregnant mice also inhibits DHCR7 in the brains of newborn pups, increasing 7-DHC and decreasing desmosterol [69]. The DHCR7 inhibition by metoprolol is thought to be part of the mechanism regarding why it has some inhibitory effect on VSV infection in cell culture [50]. The newer beta-blocker nebivolol is 10 times more potent in inhibiting DHCR7 in both HepG2 and Neuro2a cell cultures than metoprolol [69].
Lastly, truncated adenomatous polyposis coli selective inhibitors (TASIN), LK-980, and UV radiation may alter DHCR7 activity. TASINs were found to inhibit EBP, DHCR7, and DHCR24, leading to colorectal cancer cell death [70]. LK-980 ((4-Phenethylpiperazin-1-yl)-1-(pyridine-3-yl) ethanol) was synthesized and tested in HepG2 cells and was a specific inhibitor of DHCR7 with partial inhibition of other enzymes [71]. UV radiation decreases DHCR7 protein level in skin cells (primarily keratinocytes), allowing more 7-DHC to be converted to vitamin D [72]. Keratinocytes have an active sterol synthesis pathway and both DHCR7 and DHCR24 are important in normal keratinocytes physiology [73].
3.2. Chemicals That Alter 24-Dehydrocholesterol Reductase (DHCR24) Activity
The Dhcr24 gene codes for the DHCR24 protein, which is a nearly ubiquitous enzyme in sterol synthesis, as it not only catalyzes the conversion of desmosterol to cholesterol but can also convert several of the sterol intermediates in the Bloch pathway over to the corresponding intermediates in the Kandutsch–Russell pathway [74]. DHCR24 deficiency is the underlying mechanism for desmosterolosis (OMIM 602398 [75]), a genetic syndrome characterized by elevated desmosterol concentrations and multiple congenital anomalies [76,77].
DHCR24 is also known as SELective Alzheimer’s Disease INdicator-1 (seladin-1) as it was shown to be downregulated in Alzheimer’s disease (AD) [78]. In addition to AD, however, DHCR24 is also altered in oncogenic and oxidative stress [79], hepatitis C virus (HCV) infections [80], and the development of foam cells [81]. Specifically, Dhcr24 inhibition and the accumulation of desmosterol might have antiapoptotic activity [82] and anti-inflammatory effects [81] and may decrease hepatitis C viral infection [80,83]. DHCR24 is also a key enzyme in bone development, as DHCR24 KO metatarsal cultures have poor growth due to the absence of proliferating chondrocytes in the growth plate and abnormal hypertrophy of prehypertrophic chondrocytes [84]. Ultimately, the role of DHCR24 in AD remains controversial, but it has also been postulated to be a possible medication target for HCV infections [80] and arteriosclerosis [85].
Desmosterol is endogenous agonist for the liver X receptor (LXR) which is a master regulator of lipid metabolism with potential role in inflammation, atherosclerosis, cancer, diabetes mellitus, multiple sclerosis, nonalcoholic steatohepatitis, and viral infections [86]. Many studies have shown that lipid and cholesterol metabolism are critical in the development and progression of different types of tumors, and the overexpression of cholesterol synthesis genes is associated with resistance to conventional antitumor pharmaceuticals [87,88]. As such, many ongoing research studies are exploring the combination of inhibiting sterol enzymes such as DHCR24 with other antitumor treatments.
3.2.1. U18666A
Molecular dynamics simulations of DHCR24, desmosterol, FAD, and U18666A showed that U18666A interacts with flavin adenine dinucleotide (FAD) by forming three hydrogen bonds with the Lys292, Lys367, and Gly438 of DHCR24. U18666A induces secondary structural changes in the interaction of DHCR24, FAD, and desmosterol, thereby blocking DHCR24 activity through an allosteric-inhibiting mechanism [89]. Administration of U18666A has been shown to reproduce the effects of DHCR24 knockout in bone development [84], and has been tested in various systems, as described throughout the rest of this section.
Given the early association with AD, it is not surprising that much of the research using U18666A to date has assessed changes in brain sterol synthesis. For example, intracerebral injection of rats with U18666A for 14–21 days led to decreased cholesterol and increased desmosterol, amyloid beta accumulation, decreased neuron-specific enolase, decreased p-Akt and p-GSK-3beta, and Morris water maze cognitive impairment [90]. Additionally, in a permanent middle cerebral artery occlusion model, DHCR24-HET and WT mice treated with U18666A both had increased ischemic lesions at 48 h after injury [91]. The authors concluded that the DHCR24 enzyme has a neuroprotective role against cell death after ischemia, which was most likely through altered association of other proteins with the lipid rafts. In a cell culture system, U18666A also prevented the cellular accumulation of disease-associated isoforms of prion proteins, though this finding was not reproduced in mice [92].
As noted above, DHCR24 is thought to play a significant role in cancer, so U18666A has also been tested in cancer models. As an example, simultaneous treatment of melanoma cell lines with FASN and DHCR24 inhibitors (PLX4032 and U1866A) increased number of apoptotic cells [93]. Additionally, DHCR24 is coexpressed with parvalbumin in cochlear hair cells in rat organs of Corti and is upregulated in response to cisplatin-induced injury. The inhibition of DHCR24 with U18666A increased the sensitivity of hair cells to cisplatin-induced toxicity [94]. One infectious risk factor for the development of various cancers is chronic HCV infection. HCV in human hepatocytes induces expression of DHCR24 in vitro, and a similar effect was seen in human hepatocytes of chimeric mouse liver. Both treatment with U18666A and DHCR24 siRNA suppressed HCV infection in cell lines [80].
3.2.2. SH42
In 2017, Müller et al. sought to synthesize a novel Dhcr24 inhibitor using the structures of several known sterol inhibitors (e.g., U18666A, MGI-21, etc.) as a basis. Of the novel compounds that were developed and tested in mice, compound 27 (coded as SH-42) was chosen due to its selectivity, activity, and lack of cytotoxicity [95]. A subsequent study demonstrated that SH42 led to accumulation of desmosterol, increased biosynthesis of polyunsaturated fatty acids, and production of anti-inflammatory mediators [96,97]. The study concluded that DHCR24 is involved in inflammation because desmosterol influences PUFA synthesis—PUFAs are substrates for production of lipid mediators in onset and offset of inflammation—in macrophages through LXR binding.
3.2.3. Other DHCR24 Inhibitors
Screening a collection of FDA-approved medications identified 49 compounds that inhibit DHCR24 and elevate desmosterol levels in vitro in Neuro2a cells, including the tyrosine kinase inhibitors imatinib, ponatinib, and masitinib [15]. An in silico screening used the DrugBank database and molecular dynamics simulation analysis which provided four potential DHCR24 inhibitor candidates: irbesartan, risperidone, tolvaptan, and conivaptan. All four significantly lowered cholesterol in HepG2 cells [98]. Both the types of cells used and the methods of sterol detection were different between these two studies, which are likely the reasons for the lack of overlap in candidate chemicals between the two studies.
Additionally, other studies have demonstrated changes to DHCR24 with chemicals that were not discovered in either of the two screening studies above. The most studied of these has been amiodarone, which was shown to inhibit both EBP and DHCR24, as confirmed by sterol measurements in liver and kidney cell lines [18,99]. Further supporting the in vitro studies, patient serum samples containing detectable amounts of amiodarone also had elevated levels of the sterol precursors zymosterol, 8-DHC, and desmosterol [99]. A separate clinical study performed sterol analysis in 236 cardiac patients (126 with and 110 without amiodarone treatment) and showed that amiodarone administration was accompanied by a robust increase in serum desmosterol levels independently of gender, age, body mass index, cardiac and other diseases, and the use of statins [18]. The patient samples taken before and after initiation of amiodarone therapy showed a systematic increase in desmosterol upon amiodarone administration, suggesting a direct causal link between amiodarone administration and desmosterol accumulation.
Post-transcriptional DHCR24 enzyme activity regulation can also be accomplished through phosphorylation [100] or feedback inhibition from oxysterols [101,102]. Specifically, phosphorylation of the residues T110, Y299, and Y507 are key in the regulation of DHCR24 activity. Inhibition of protein kinase C also greatly decreases DHCR24 activity, though not through the phosphorylation of the known residue targets [100]. Additionally, the oxysterol 24(S),25-epoxycholesterol (24,25EC), which is structurally similar to desmosterol, directly inhibits DHCR24 activity [101]. This oxysterol inhibited enzymatic activity of DHCR24 without affecting its protein level and resulted in increased level of desmosterol and decreased levels of cholesterol [102].
As mentioned in the DHCR7 section, TASINs target EBP, DHCR7, and DHCR24 enzymes in human colonic epithelial cells and carcinogenic HCEC [70,103]. These findings were further supported by a study to develop small molecules toxic to colorectal cancer cells with cancer-causing mutations, in which the researchers identified several different TASIN compounds. They noted that one specific TASIN, TASIN-1, inhibited EBP, DHCR7, and DHCR24, but in their study, the toxic effects of TASIN were exclusively dependent on EBP inhibition in their particular DLD-1 cell line [70].
Several other chemicals have been shown in single studies to potentially inhibit DHCR24 but have not yet been reproduced. These include studies on the phytochemicals in Cannabis sativa [104] and the chemical genkwadaphnin, extracted from the flower buds of Daphne genkwa [105]. The synthetic gamma secretase modulator E2012 also inhibited DHCR24 and led to development of cataract in rats, with increased desmosterol and decreases cholesterol in lens, liver, and plasma [106]. LK-980 (4-Phenethylpiperazin-1-yl)-1-(pyridine-3-yl)ethanol) primarily inhibited DHCR7 in HepG2 cells, but also inhibited DHCR14, DHCR24, and SC5D to a lesser extent [71]. Lastly, metabolic and culture conditions may be important for DHCR24 expression, as high glucose added to medium was shown to decrease Dhcr24 mRNA in one specific cell type [107]; however, it is unclear if this mRNA change translates into changes in sterol concentrations.
3.3. Chemicals That Alter Lanosterol Synthase (LSS) Activity
The primary function of LSS is to convert 2,3-oxidosqualene into lanosterol [108,109]. LSS mutations have been associated with cataracts (OMIM 600909 [110]), hypotrichosis (OMIM 618275 [111]) in affected children, and a phenotype referred to as alopecia-intellectual disability syndrome-4 (OMIM 618840 [112]) [113,114]. Although it is a less-studied entity, chemical-induced modeling of LSS deficiency may be particularly relevant because genetic models to date have either relied on additional mutations such as Fdft1 to produce the phenotype [115] or are lethal to the embryos [116]. Additionally, LSS inhibitors have clinical relevance as potential antifungal and/or cholesterol-lowering compounds.
Several studies have screened and prioritized potential LSS inhibitory compounds, including extracts from foods, in different model systems [117,118,119]. These include the experimental compound BIBB-515, which induced 24(S),25 epoxycholesterol and resulted in decreased human rhinovirus replication [120] and sensitized chronic lymphocytic leukemia cells to chemotherapeutics [121]. Diverting sterols into the “shunt” pathway starting with 24(S),25 epoxycholesterol by MM0299 administration also inhibited growth of glioma stem-like cells [122]. Lastly, essential oils behaved similar to the positive LSS inhibitor control Ro 48-8071 in decreasing intracellular lipid levels and cholesterol synthesis and were, thus, hypothesized to function as natural compounds against atherosclerogenesis [123].
As mentioned above, LSS inhibitors have also been shown to have antifungal and antiprotozoan properties, including epohelmins A and B [124]. Due to their focus on microorganisms, many of the studies assessing LSS inhibition are solely in vitro analyses. Although many of these studies suggested LSS inhibition, it should be noted that the concentrations of chemical administered were widely variable: from 500 μM eremanthine [119] to screening several compounds at 20–50 μM [118] and 0.25–0.5 μM diacylglycerols [125].
3.4. Chemicals That Alter Sterol-C4-Methyl Oxidase-like (SC4MOL) Activity
SC4MOL deficiency (OMIM 616834 [126]) can result in microcephaly, congenital cataracts, and psoriasiform dermatitis [127]. SC4MOL is highly conserved, and several of the studies targeting its activity have, therefore, been studied in fungal species either to test their antifungal effects or as a model species. In either case, further validation in mammalian cells or animal models would be required before conclusions can be formed about their potential for modeling SC4MOL deficiency.
Seeking additional effective antifungal therapies, one group demonstrated that administering diazaborines to Saccharomyces cerevisiae leads to accumulation of ERG25 substrate, 4,4-dimethylzymosterol, and depletion of zymosterol and ergosterol [128]. Similarly, eugenol—one of the main ingredients in Syzygium aromaticum (cloves) and an inhibitor of SC4MOL—may inhibit mycelia in Rhizoctonia solani, a cause of rice sheath blight. Eugenol downregulated expression of C-4 methyl sterol oxidase, inhibited synthesis of ergosterol, and led to increased oxidative stress [129].
Similar to LSS inhibition, inhibiting SC4MOL may aid in sensitizing cancer cells to chemotherapy. Although they used siRNA and shRNA rather than medication treatment, Sukhanova et al. demonstrated that SC4MOL inactivation sensitized A431, SCC61, SCC68, and PC9 tumor cells to erlotinib, an EGFR kinase inhibitor [130]. The mechanistic theory for this is that specific sterol metabolites (specifically FF-MAS and T-MAS) interfere with endosomal trafficking of EGRF and limit EGFR stability.
Overall, inhibiting enzymes in the cholesterol synthesis pathways tends to result in cellular dysfunction and/or death. One exception to this, that will be explored further in the section below, is that genetic inhibition of SC4MOL increases formation of oligodendrocytes in mouse oligodendrocyte precursor cell culture [131]. This group used the small molecule inhibitor CW4142 which led to increased accumulation of 8,9-unsaturated sterols.
3.5. Chemicals That Alter Emopamil-Binding Protein (EBP) Activity
Mutations in the EBP gene [132] have been associated with two different primary phenotypes. The first is X-linked dominant chondrodysplasia punctata (OMIM 302960 [133]), in which affected females demonstrate elevated levels of 8-dehydrocholesterol and 8(9)-cholestenol, skeletal dysplasia, and hyperkeratotic skin changes [134]. An X-linked recessive mutation has also been documented in males and has been termed male EBP disorder with neurologic defects (MEND, OMIM 300960 [135]). MEND results in a highly variable phenotype that may include intellectual disabilities, spine and digit abnormalities, cataracts, and/or dermatologic changes.
Long et al. described the crystal structure of EBP, demonstrating a fold involving five transmembrane helix that created a membrane cavity binding site that can accommodate multiple different pharmacological ligands [136]. The compounds that bind with EBP tend to have a positively charged amine group. Several molecules have been identified that have moderate or high affinity for EBP, including tamoxifen, clomiphene, amiodarone, haloperidol, and opipramol [137]. Novel chemicals have also been developed, such as the 2- or 2,6-disubstituted (CH3, CH 3O, Cl, F) cis- and trans-4-(4-aryl)cyclohexyl-1-(2-pyridyl) piperazines, that also inhibit EBP [138]. The most selective 2,6-dimethoxy derivative (cis-33) demonstrated the highest potency (EC 50 = 12.9 μM) and efficacy (70%) in inhibiting proliferation of the human prostate cancer PC-3 cell line. Lastly, in addition to inhibition of DHCR7 and DHCR24, TASINs have also been shown to inhibit EBP [70]. In fact, while TASINs inhibit DHCR7 and DHCR24 in DLD1 and HT29 cell lines, only antitumor effects of TASINs—and especially those of TASIN-1 [139]—appear to act through modulation of EBP expression and not DHCR7 or DHCR24 [70].
As noted in the SC4MOL section, inhibition of some of the sterol synthesis enzymes has been associated with enhanced oligodendrocyte formation and myelination. This effect has been most consistently documented with EBP inhibition. One screening study of a structurally diverse library of 10,000 small molecules found that many of those molecules that effectively promoted oligodendrocyte formation also inhibited the cholesterol synthesis enzymes CYP51, TM7SF2, or EBP [140]. These molecules included the potent EBP-inhibitor, CW3388, which enhanced oligodendrocyte formation in mouse cell cultures.
The mechanism by which EBP inhibition promotes oligodendrocytes is not yet fully known, but the process appears to rely on the accumulation of 8,9-unsaturated sterols [141,142]. In the first days in vitro, oligodendrocyte precursor cell maturation involves high levels of accumulation of zymostenol and zymosterol [143], and inhibition of EBP using peripheral injection of tamoxifen and clemastine in a mouse model of multiple sclerosis resulted in significantly improved myelin repair [143]. Further screening demonstrated that inhibiting delta (14)-sterol reductase with the chemical U-73343 provided similar enhancement of oligodendrocyte formation, also likely through the accumulation of 8,9-unsaturated sterols [144]. The pro-oligodendrocyte effect does not fully rely on 8,9-unsaturated sterols, however, as similar effects were also seen with LSS inhibition and exogenous administration of 24,25-epoxycholesterol [145]. Overall, these studies demonstrate promise for sterol inhibition in enhancing myelination, but future studies will need to attempt to answer the question of how to provide promyelination without suffering the negative effects of sterol inhibition described throughout the rest of this review.
3.6. Chemicals That Alter Cytochrome P450 Family 51 Subfamily A Member 1 (CYP51A1) Activity
CYP51A1 is involved in the removal of the 14⍺-lanosterol methyl group in the conversion of lanosterol to 4,4-dimethylcholesta-8(9),14,24-trien-3β-ol, and is, therefore, also referred to as lanosterol 14⍺-demethylase [146]. Although a few studies have sought to alter CYP51A1 in mammalian cholesterol synthesis [147], the vast majority of CYP51A1 research has focused on its vital role in the synthesis of ergosterol in nonhuman species such as fungi [148,149]. Specifically, azole CYP51A1 inhibitors have been developed and are widely used in medicine and agriculture to treat fungal infections [150,151]. The accumulation of these chemicals in soil and waterways could pose risks to the environment [152] and is thought to disrupt sex hormone synthesis and signaling involved in fetal development [153] and pubertal maturation [154]. A full discussion of the mechanisms of these risks is outside the scope of this current review, however.
3.7. Chemicals That Alter Sterol-C5-Desaturase (SC5D) Activity
SC5D deficiency can result in lathosterolosis, a clinical syndrome with a complex phenotype involving multiple congenital anomalies, intellectual disabilities, and liver failure [155]. Other than the observation that SC5D is a minor target of the chemical LK-980 [71], very little is known about how many or which medications may affect SC5D activity.
4. Discussion
Cholesterol is an important and incredibly complex small molecule—a Janus-like chemical that is simultaneously both life-sustaining and potentially life-threatening [156,157,158,159,160]. Cholesterol and its intermediates are essential structural components of all cell membranes, serve as precursors for hundreds of bioactive compounds, regulate cell division and membrane raft assembly, and serve as molecular underpinnings for all specialized functional processes of the body [161,162,163,164]. Due to the highly conserved biosynthesis pathways across vertebrate species, we can study its biosynthesis in animal models with both genetic and chemical tools [165]. While the biochemical pathways of sterol biosynthesis have been studied for decades (and we have a reasonably good understanding of them), the consequences of sterol biosynthesis disruption by xenobiotic agents are extremely understudied. In particular, the study of the adverse consequences of various chemicals on the fetal sterol biosynthesis continues to be full of opportunities to address critical missing knowledge.
There are hundreds of chemicals that can inhibit various parts of the sterol biosynthesis pathways. Many of these chemicals are FDA-approved medications that are prescribed hundreds of millions of times each year in the USA alone. Through the review of the literature, we identified many areas with gaps of knowledge that could and should be addressed in future studies. These gaps can be largely divided into two main areas: mechanisms by which sterol biosynthesis is altered and consequences that arise from the inhibitions of the various steps of the sterol biosynthesis pathway.
Currently, we have very limited data of the mechanisms by which the various prescription medications and nonprescription chemicals inhibit the sterol biosynthetic processes. For example, do the various chemicals act as direct inhibitors of enzymes through competitive binding for their substrates, or do they influence enzyme levels via secondary transcriptional mechanisms? Additionally, the mechanistic experiments have historically focused on the effects on modulating cholesterol levels, with much less attention paid to the biological roles of the accumulating or decreasing precursor or metabolite levels. Yet, we know that many of the precursors and metabolites have important biological activities that can be beneficial (e.g., decreasing viral load [50,51]) or detrimental (e.g., disrupting critical patterning through the sonic hedgehog pathway [166]). These precursors and metabolites modulate immune function, growth, synapse formation, and many other developmental and homeostatic processes.
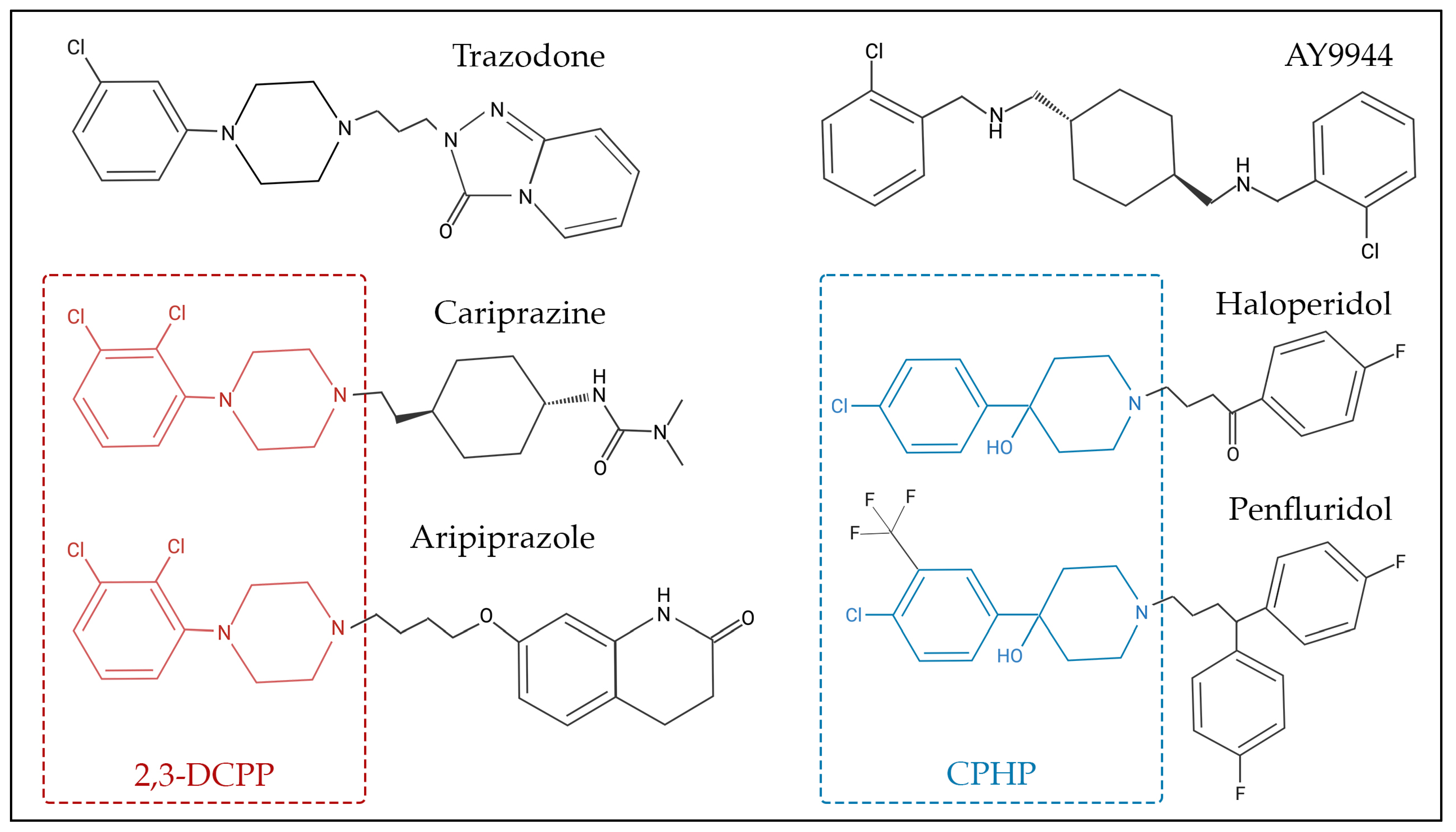
Many sterol biosynthesis inhibiting chemicals share a degree of structural similarities (Figure 2), which could be responsible for the inhibition of sterol biosynthesis enzymes, particularly DHCR7. For example, 2,3-dichlorophenyl piperazine (2,3-DCPP) is a substructure and metabolite of both aripiprazole and cariprazine, and by itself it is a DHCR7-inhibiting compound [60]. In addition, 4-chlorophenyl-4-hydroxypiperidine (CPHP) is a structural component of both haloperidol and penfluridol, two antipsychotics that also elevate 7-DHC. Although 2,3-DCPP and CPHP are distinct chemical structures, a side-by-side comparison between the structures of 2,3-DCPP and CPHP shows that both have terminal modified phenyl rings linked to either a piperidine or piperazine ring. Such relationships between sterol biosynthesis inhibition and common chemical substructures should be further investigated by computational approaches that involve systematic fragmentation of molecules (e.g., molBLOCKS tool), followed by enrichment analysis [167]. The identification of these sterol-biosynthesis-inhibiting substructures will be important for knowledge-based drug development, especially for evaluation of medications that can be safely used during pregnancy.
Figure 2.
Chemical structure of selected 7-dehydrocholesterol reductase (DHCR7) inhibitors. Some of these compounds share chemical substructure similarities, while others lack a common structure. Examples of similar structures include 2,3-dichlorophenyl piperazine (2,3-DCPP)—denoted in red—which is shared by aripiprazole and cariprazine, while 4-chlorophenyl-4-hydroxypiperidine (CPHP)—denoted in blue—is common for haloperidol and penfluridol. AY9944 and trazodone are examples of chemicals that share similar, though not identical, structures to other DHCR7-inhibiting chemicals.
Human studies clearly indicate that post-lanosterol biosynthesis inhibitors act as teratogens [31], yet these unwanted consequences of sterol inhibition are still mostly unknown. This is particularly throughout the critical time windows of exposure—such as during fetal development—that give rise to the most consequential disruptions of development. Similarly, we do not yet understand the vulnerability of the different tissues and what metabolite level thresholds result in toxicity and long-lasting phenotypical changes. In addition, due to the all-encompassing metabolic role of this pathway, phenotypes will likely vary significantly in which organs and biological processes are involved, impairing the ability to developed focused, targeted phenotypic detection.
Outside of teratogenicity, however, there is a paucity of data directly linking the sterol effects of the medications in this review to their clinical effects. For example, the central nervous system side effects of beta-blockers have always been assumed to be brain adrenergic receptor-mediated (and related to their main mechanism of action), although there is very sparse experimental evidence that proves this assumption [168]. Untangling mechanisms of both the main action and side effects is an extremely challenging task, especially if different mechanisms of action occur simultaneously. A recent study evaluated interactions between antipsychotics and medications used in the treatment of cardiovascular disease (CVD) [26]. The highest number of interactions was recorded among beta-blockers and antipsychotics [169]. Although this study only examined cardiovascular side effects, it is notable that the combinations that reported the most common adverse outcomes included the medications identified above as having 7-DHC elevating side effects—including metoprolol and nebivolol. Despite these existing concerns, however, beta-blockers are still often prescribed in addition to standard antipsychotic regimes to treat CVD or extrapyramidal symptoms such as akathisia [169,170].
The highest toxic intermediates will most certainly give rise to complex dysmorphologies and intellectual disabilities. Are lower levels of the same intermediates safe, however, or do they give rise to more nuanced changes (without dysmorphology) that manifest later in life as mild to moderate functional impartments such as autism or learning challenges? For example, the grave disruption of post-lanosterol biosynthesis in SLOS gives rise to both congenital malformations and intellectual disability, with 75% of patients on the autism spectrum [171,172,173]. What remains unknown is whether a lesser magnitude of 7-DHC elevation could lead to only a milder behavioral phenotype without dysmorphology. To add additional complexity, there are several highly oxidizable molecules in this pathway (such as 7-DHD and 8-DHC) that could alter the oxidative milieu in acute periods of stress, yet we have not yet even identified the oxysterols that arise by their autooxidation [174]. Additionally, polypharmacy is a significant challenge throughout clinical medicine [175], and we lack adequate knowledge around the summative or potentiating effects of simultaneously utilizing multiple medications with sterol-biosynthesis-inhibiting side effects. Lastly, many psychotropic medications inhibit the post-lanosterol pathway, but can we safely assume that sterol inhibition is merely a side effect of the medications, or could the sterol biosynthesis effects actually contribute to their therapeutic efficacy?
The method of addressing all these remaining questions will depend on the various in vivo and in vitro models described above to thoroughly investigate sterol inhibition. Luckily, there are a plethora of advanced analytical methods by which the meaningful results can be obtained, but the outcome of these experiments (and the reproducibility of the findings) will greatly depend on the quality of the experimental design. Investigators should pay great attention to the dose of the inhibitor, duration and frequency of exposure, developmental age, genotype, assay strengths and limitations, statistical approaches, power of studies, and many other factors. Additionally, biochemists and bench researchers should work to partner with practicing clinicians to both increase the translatability of the research being performed and to help clinicians develop a greater appreciation of the spectrum of commonly used prescription medications that affect sterol biosynthesis, with yet-unknown long-term clinical consequences. A study that can provide a good cautionary example in this context is the newly identified fetal fentanyl syndrome, that presumably arises from DHCR7 inhibition [176].
5. Conclusions
Hundreds of chemicals can inhibit sterol biosynthesis, acting through different enzymes and various molecular mechanisms. This area remains greatly understudied both in the context of mechanistic molecular events and consequences on human health. We must develop models and procedures to study these processes and extensively utilize them to develop safety guidelines in this area, especially during pregnancy. Novel chemicals (and particularly medications awaiting FDA approval) should undergo a comprehensive battery of testing for sterol-inhibiting effects, and a significant number of already approved prescription medications should be re-evaluated in the same context.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biom14040410/s1, Table S1: Search strings used for literature review.
Author Contributions
Conceptualization, Z.K., K.M. and E.S.P.; data curation, Z.K. and E.S.P.; writing—original draft preparation, Z.K., K.M. and E.S.P.; writing—review and editing, Z.K., K.M. and E.S.P.; visualization, Z.K., K.M. and E.S.P.; supervision, Z.K. and K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the National Institute of Mental Health, grant number R01 MH110636.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable, as no new data were created for this manuscript.
Acknowledgments
We would like to thank Cindy Schmidt, the medical librarian at the Leon S. McGoogan Health Science Library at the University of Nebraska Medical Center, who helped us in the literature search for this project, including developing the search string.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Björkhem, I.; Meaney, S. Brain cholesterol: Long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Dietschy, J.M.; Turley, S.D. Cholesterol metabolism in the brain. Curr. Opin. Lipidol. 2001, 12, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Saher, G. Cholesterol Metabolism in Aging and Age-Related Disorders. Annu. Rev. Neurosci. 2023, 46, 59–78. [Google Scholar] [CrossRef] [PubMed]
- Gliozzi, M.; Musolino, V.; Bosco, F.; Scicchitano, M.; Scarano, F.; Nucera, S.; Zito, M.C.; Ruga, S.; Carresi, C.; Macrì, R.; et al. Cholesterol homeostasis: Researching a dialogue between the brain and peripheral tissues. Pharmacol. Res. 2021, 163, 105215. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, W.J.; Wang, Y. Oxysterols as lipid mediators: Their biosynthetic genes, enzymes and metabolites. Prostaglandins Other Lipid Mediat. 2020, 147, 106381. [Google Scholar] [CrossRef]
- Wang, Y.; Sousa, K.M.; Bodin, K.; Theofilopoulos, S.; Sacchetti, P.; Hornshaw, M.; Woffendin, G.; Karu, K.; Sjövall, J.; Arenas, E.; et al. Targeted lipidomic analysis of oxysterols in the embryonic central nervous system. Mol. Biosyst. 2009, 5, 529–541. [Google Scholar] [CrossRef]
- Porter, F.D.; Herman, G.E. Malformation syndromes caused by disorders of cholesterol synthesis. J. Lipid Res. 2011, 52, 6–34. [Google Scholar] [CrossRef] [PubMed]
- Porter, F.D. Smith-Lemli-Opitz syndrome: Pathogenesis, diagnosis and management. Eur. J. Hum. Genet. 2008, 16, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Porter, F.D. Cholesterol precursors and facial clefting. J. Clin. Investig. 2006, 116, 2322–2325. [Google Scholar] [CrossRef]
- Cocciadiferro, D.; Mazza, T.; Vecchio, D.; Biagini, T.; Petrizzelli, F.; Agolini, E.; Villani, A.; Minervino, D.; Martinelli, D.; Rizzo, C.; et al. Exploiting in silico structural analysis to introduce emerging genotype-phenotype correlations in DHCR24-related sterol biosynthesis disorder: A case study. Front. Genet. 2023, 14, 1307934. [Google Scholar] [CrossRef] [PubMed]
- Korade, Z.; Heffer, M.; Mirnics, K. Medication effects on developmental sterol biosynthesis. Mol. Psychiatry 2022, 27, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Tonini, C.; Segatto, M.; Gagliardi, S.; Bertoli, S.; Leone, A.; Barberio, L.; Mandalà, M.; Pallottini, V. Maternal Dietary Exposure to Low-Dose Bisphenol A Affects Metabolic and Signaling Pathways in the Brain of Rat Fetuses. Nutrients 2020, 12, 1448. [Google Scholar] [CrossRef]
- Wages, P.A.; Joshi, P.; Tallman, K.A.; Kim, H.H.; Bowman, A.B.; Porter, N.A. Screening ToxCast™ for Chemicals That Affect Cholesterol Biosynthesis: Studies in Cell Culture and Human Induced Pluripotent Stem Cell-Derived Neuroprogenitors. Environ. Health Perspect. 2020, 128, 17014. [Google Scholar] [CrossRef] [PubMed]
- Wages, P.A.; Kim, H.-Y.H.; Korade, Z.; Porter, N.A. Identification and characterization of prescription drugs that change levels of 7-dehydrocholesterol and desmosterol. J. Lipid Res. 2018, 59, 1916–1926. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Korade, Z.; Tallman, K.A.; Liu, W.; Weaver, C.D.; Mirnics, K.; Porter, N.A. Inhibitors of 7-Dehydrocholesterol Reductase: Screening of a Collection of Pharmacologically Active Compounds in Neuro2a Cells. Chem. Res. Toxicol. 2016, 29, 892–900. [Google Scholar] [CrossRef]
- Simonen, P.; Lehtonen, J.; Lampi, A.M.; Piironen, V.; Stenman, U.H.; Kupari, M.; Gylling, H. Desmosterol accumulation in users of amiodarone. J. Intern. Med. 2018, 283, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Simonen, P.; Li, S.; Chua, N.K.; Lampi, A.M.; Piironen, V.; Lommi, J.; Sinisalo, J.; Brown, A.J.; Ikonen, E.; Gylling, H. Amiodarone disrupts cholesterol biosynthesis pathway and causes accumulation of circulating desmosterol by inhibiting 24-dehydrocholesterol reductase. J. Intern. Med. (GBR) 2020, 288, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Herron, J.; Reese, R.C.; Tallman, K.A.; Narayanaswamy, R.; Porter, N.A.; Xu, L. Identification of Environmental Quaternary Ammonium Compounds as Direct Inhibitors of Cholesterol Biosynthesis. Toxicol. Sci. Off. J. Soc. Toxicol. 2016, 151, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Herron, J.M.; Hines, K.M.; Tomita, H.; Seguin, R.P.; Cui, J.Y.; Xu, L. Multiomics Investigation Reveals Benzalkonium Chloride Disinfectants Alter Sterol and Lipid Homeostasis in the Mouse Neonatal Brain. Toxicol. Sci. 2019, 171, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Herron, J.M.; Tomita, H.; White, C.C.; Kavanagh, T.J.; Xu, L. Benzalkonium Chloride Disinfectants Induce Apoptosis, Inhibit Proliferation, and Activate the Integrated Stress Response in a 3-D In Vitro Model of Neurodevelopment. Chem. Res. Toxicol. 2021, 34, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Simonen, P.; Lommi, J.; Lemström, K.; Tolva, J.; Sinisalo, J.; Gylling, H. Amiodarone accumulates two cholesterol precursors in myocardium: A controlled clinical study. J. Intern. Med. 2023, 294, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Hrubec, T.C.; Seguin, R.P.; Xu, L.; Cortopassi, G.A.; Datta, S.; Hanlon, A.L.; Lozano, A.J.; McDonald, V.A.; Healy, C.A.; Anderson, T.C.; et al. Altered toxicological endpoints in humans from common quaternary ammonium compound disinfectant exposure. Toxicol. Rep. 2021, 8, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.G.; Pfrieger, F.; Dotti, C.G. Cholesterol in brain disease: Sometimes determinant and frequently implicated. EMBO Rep. 2014, 15, 1036–1052. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A.R.; Lavie, C.J.; Milani, R.V.; OʼKeefe, J. The effects of statins on prevention of stroke and dementia: A review. J. Cardiopulm. Rehabil. Prev. 2012, 32, 240–249. [Google Scholar] [CrossRef]
- Siwek, M.; Woroń, J.; Gorostowicz, A.; Wordliczek, J. Adverse effects of interactions between antipsychotics and medications used in the treatment of cardiovascular disorders. Pharmacol. Rep. 2020, 72, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Kidnapillai, S.; Bortolasci, C.C.; Panizzutti, B.; Spolding, B.; Connor, T.; Bonifacio, K.; Sanigorski, A.; Dean, O.M.; Crowley, T.; Jamain, S.; et al. Drugs used in the treatment of bipolar disorder and their effects on cholesterol biosynthesis—A possible therapeutic mechanism. World J. Biol. Psychiatry 2019, 20, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Kaler, J.; Ray, S.D. The Benefits Outweigh the Risks of Treating Hypercholesterolemia: The Statin Dilemma. Cureus 2023, 15, e33648. [Google Scholar] [CrossRef]
- Dietschy, J.M.; Turley, S.D. Thematic review series: Brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 2004, 45, 1375–1397. [Google Scholar] [CrossRef] [PubMed]
- Tierney, E.; Bukelis, I.; Thompson, R.E.; Ahmed, K.; Aneja, A.; Kratz, L.; Kelley, R.I. Abnormalities of cholesterol metabolism in autism spectrum disorders. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006, 141b, 666–668. [Google Scholar] [CrossRef]
- Boland, M.R.; Tatonetti, N.P. Investigation of 7-dehydrocholesterol reductase pathway to elucidate off-target prenatal effects of pharmaceuticals: A systematic review. Pharmacogenom. J. 2016, 16, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Moebius, F.F.; Fitzky, B.U.; Lee, J.N.; Paik, Y.K.; Glossmann, H. Molecular cloning and expression of the human delta7-sterol reductase. Proc. Natl. Acad. Sci. USA 1998, 95, 1899–1902. [Google Scholar] [CrossRef] [PubMed]
- Fitzky, B.U.; Witsch-Baumgartner, M.; Erdel, M.; Lee, J.N.; Paik, Y.K.; Glossmann, H.; Utermann, G.; Moebius, F.F. Mutations in the Delta7-sterol reductase gene in patients with the Smith-Lemli-Opitz syndrome. Proc. Natl. Acad. Sci. USA 1998, 95, 8181–8186. [Google Scholar] [CrossRef] [PubMed]
- Wassif, C.A.; Maslen, C.; Kachilele-Linjewile, S.; Lin, D.; Linck, L.M.; Connor, W.E.; Steiner, R.D.; Porter, F.D. Mutations in the human sterol delta7-reductase gene at 11q12-13 cause Smith-Lemli-Opitz syndrome. Am. J. Hum. Genet. 1998, 63, 55–62. [Google Scholar] [CrossRef]
- Waterham, H.R.; Wijburg, F.A.; Hennekam, R.C.; Vreken, P.; Poll-The, B.T.; Dorland, L.; Duran, M.; Jira, P.E.; Smeitink, J.A.; Wevers, R.A.; et al. Smith-Lemli-Opitz syndrome is caused by mutations in the 7-dehydrocholesterol reductase gene. Am. J. Hum. Genet. 1998, 63, 329–338. [Google Scholar] [CrossRef]
- Online Mendelian Inheritance in Man (OMIM). Johns Hopkins University, Baltimore, MD. 270400: 1/12/24. Available online: https://omim.org (accessed on 20 February 2024).
- Kelley, R.I.; Hennekam, R.C. The Smith-Lemli-Opitz syndrome. J. Med. Genet. 2000, 37, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.L.; Iben, J.; Simpson, C.L.; Thurm, A.; Swedo, S.; Tierney, E.; Bailey-Wilson, J.E.; Biesecker, L.G.; Porter, F.D.; Wassif, C.A. Determination of the allelic frequency in Smith-Lemli-Opitz syndrome by analysis of massively parallel sequencing data sets. Clin. Genet. 2015, 87, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Fliesler, S.J.; Peachey, N.S.; Richards, M.J.; Nagel, B.A.; Vaughan, D.K. Retinal degeneration in a rodent model of Smith-Lemli-Opitz syndrome: Electrophysiologic, biochemical, and morphologic features. Arch. Ophthalmol. 2004, 122, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, W.; Sheflin, L.G.; Fliesler, S.J.; Porter, N.A. Novel oxysterols observed in tissues and fluids of AY9944-treated rats: A model for Smith-Lemli-Opitz syndrome. J. Lipid Res. 2011, 52, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Myers, B.R.; Neahring, L.; Zhang, Y.; Roberts, K.J.; Beachy, P.A. Rapid, direct activity assays for Smoothened reveal Hedgehog pathway regulation by membrane cholesterol and extracellular sodium. Proc. Natl. Acad. Sci. USA 2017, 114, e11141–e11150. [Google Scholar] [CrossRef] [PubMed]
- Roux, C.; Wolf, C.; Mulliez, N.; Gaoua, W.; Cormier, V.; Chevy, F.; Citadelle, D. Role of cholesterol in embryonic development. Am. J. Clin. Nutr. 2000, 71, 1270S–1279S. [Google Scholar] [CrossRef]
- Kolf-Clauw, M.; Chevy, F.; Wolf, C.; Siliart, B.; Citadelle, D.; Roux, C. Inhibition of 7-dehydrocholesterol reductase by the teratogen AY9944: A rat model for Smith-Lemli-Opitz syndrome. Teratology 1996, 54, 115–125. [Google Scholar] [CrossRef]
- Fliesler, S.J.; Xu, L. Oxysterols and Retinal Degeneration in a Rat Model of Smith-Lemli-Opitz Syndrome: Implications for an Improved Therapeutic Intervention. Molecules 2018, 23, 2720. [Google Scholar] [CrossRef]
- Fliesler, S.J.; Peachey, N.S.; Herron, J.; Hines, K.M.; Weinstock, N.I.; Ramachandra Rao, S.; Xu, L. Prevention of Retinal Degeneration in a Rat Model of Smith-Lemli-Opitz Syndrome. Sci. Rep. 2018, 8, 1286. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Sheflin, L.G.; Porter, N.A.; Fliesler, S.J. 7-Dehydrocholesterol-derived oxysterols and retinal degeneration in a rat model of Smith-Lemli-Opitz syndrome. Biochim. Biophys. Acta 2012, 1821, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.; Chevy, F.; Pham, J.; Kolf-Clauw, M.; Citadelle, D.; Mulliez, N.; Roux, C. Changes in serum sterols of rats treated with 7-dehydrocholesterol-delta 7-reductase inhibitors: Comparison to levels in humans with Smith-Lemli-Opitz syndrome. J. Lipid Res. 1996, 37, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Guirgis, F.W.; Jacob, V.; Wu, D.; Henson, M.; Daly-Crews, K.; Hopson, C.; Black, L.P.; DeVos, E.L.; Sulaiman, D.; Labilloy, G.; et al. DHCR7 Expression Predicts Poor Outcomes and Mortality from Sepsis. Res. Sq. 2023. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Karasawa, T.; Komada, T.; Matsumura, T.; Baatarjav, C.; Ito, J.; Nakagawa, K.; Yamamuro, D.; Ishibashi, S.; Miura, K.; et al. DHCR7 as a novel regulator of ferroptosis in hepatocytes. bioRxiv 2022. [Google Scholar] [CrossRef]
- Korade, Z.; Tallman, K.A.; Kim, H.Y.H.; Balog, M.; Genaro-Mattos, T.C.; Pattnaik, A.; Mirnics, K.; Pattnaik, A.K.; Porter, N.A. Dose-Response Effects of 7-Dehydrocholesterol Reductase Inhibitors on Sterol Profiles and Vesicular Stomatitis Virus Replication. ACS Pharm. Transl. Sci. 2022, 5, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Li, W.; Zheng, X.; Qi, L.; Wang, H.; Zhang, C.; Wan, X.; Zheng, Y.; Zhong, R.; Zhou, X.; et al. Targeting 7-Dehydrocholesterol Reductase Integrates Cholesterol Metabolism and IRF3 Activation to Eliminate Infection. Immunity 2020, 52, 109–122.e106. [Google Scholar] [CrossRef] [PubMed]
- Aufenanger, J.; Pill, J.; Schmidt, F.H.; Stegmeier, K. The effects of BM 15.766, an inhibitor of 7-dehydrocholesterol delta 7-reductase, on cholesterol biosynthesis in primary rat hepatocytes. Biochem. Pharmacol. 1986, 35, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Salen, G.; Shefer, S.; Ness, G.C.; Chen, T.S.; Zhao, Z.; Tint, G.S. Reproducing abnormal cholesterol biosynthesis as seen in the Smith-Lemli-Opitz syndrome by inhibiting the conversion of 7-dehydrocholesterol to cholesterol in rats. J. Clin. Investig. 1995, 95, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Servatius, R.J.; Shefer, S.; Tint, G.S.; O’Brien, W.T.; Batta, A.K.; Salen, G. Relationship between abnormal cholesterol synthesis and retarded learning in rats. Metabolism 1998, 47, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Lindenthal, B.; Bertsch, T.; Fassbender, K.; Stroick, M.; Kühl, S.; Lütjohann, D.; Von Bergmann, K. Influence of simvastatin, pravastatin, and BM 15.766 on neutral sterols in liver and testis of guinea pigs. Metab. Clin. Exp. 2002, 51, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Kolf-Clauw, M.; Chevy, F.; Siliart, B.; Wolf, C.; Mulliez, N.; Roux, C. Cholesterol biosynthesis inhibited by BM15.766 induces holoprosencephaly in the rat. Teratology 1997, 56, 188–200. [Google Scholar] [CrossRef]
- Honda, A.; Shefer, S.; Salen, G.; Xu, G.; Batta, A.K.; Tint, G.S.; Honda, M.; Chen, T.C.; Holick, M.F. Regulation of the last two enzymatic reactions in cholesterol biosynthesis in rats: Effects of BM 15.766, cholesterol, cholic acid, lovastatin, and their combinations. Hepatology 1996, 24, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Tallman, K.A.; Allen, L.B.; Klingelsmith, K.B.; Anderson, A.; Genaro-Mattos, T.C.; Mirnics, K.; Porter, N.A.; Korade, Z. Prescription Medications Alter Neuronal and Glial Cholesterol Synthesis. ACS Chem. Neurosci. 2021, 12, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Korade, Ž.; Liu, W.; Warren, E.B.; Armstrong, K.; Porter, N.A.; Konradi, C. Effect of psychotropic drug treatment on sterol metabolism. Schizophr. Res. 2017, 187, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Genaro-Mattos, T.C.; Tallman, K.A.; Allen, L.B.; Anderson, A.; Mirnics, K.; Korade, Z.; Porter, N.A. Dichlorophenyl piperazines, including a recently-approved atypical antipsychotic, are potent inhibitors of DHCR7, the last enzyme in cholesterol biosynthesis. Toxicol. Appl. Pharmacol. 2018, 349, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Genaro-Mattos, T.C.; Anderson, A.; Allen, L.B.; Tallman, K.A.; Porter, N.A.; Korade, Z.; Mirnics, K. Maternal cariprazine exposure inhibits embryonic and postnatal brain cholesterol biosynthesis. Mol. Psychiatry 2020, 25, 2685–2694. [Google Scholar] [CrossRef]
- Kim, J.; Neely, M.D.; Kim, H.Y.; Tallman, K.; Porter, N. 2,3-Dichlorophenylpiperazine (DCPP)-derived Antipsychotics Obstruct Cholesterol Biosynthesis in hiPSC-derived Neural Precursors and Early Postmitotic Neurons. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Speen, A.M.; Hoffman, J.R.; Kim, H.-Y.H.; Escobar, Y.N.; Nipp, G.E.; Rebuli, M.E.; Porter, N.A.; Jaspers, I. Small Molecule Antipsychotic Aripiprazole Potentiates Ozone-Induced Inflammation in Airway Epithelium. Chem. Res. Toxicol. 2019, 32, 1997–2005. [Google Scholar] [CrossRef] [PubMed]
- Korade, Z.; Allen, L.B.; Anderson, A.; Tallman, K.A.; Genaro-Mattos, T.C.; Porter, N.A.; Mirnics, K. Trazodone effects on developing brain. Transl. Psychiatry 2021, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Cenik, B.; Palka, J.M.; Thompson, B.M.; McDonald, J.G.; Tamminga, C.A.; Cenik, C.; Brown, E.S. Desmosterol and 7-dehydrocholesterol concentrations in post mortem brains of depressed people: The role of trazodone. Transl. Psychiatry 2022, 12, 139. [Google Scholar] [CrossRef] [PubMed]
- Balog, M.; Anderson, A.; Genaro-Mattos, T.C.; Korade, Z.; Mirnics, K. Individual and simultaneous treatment with antipsychotic aripiprazole and antidepressant trazodone inhibit sterol biosynthesis in the adult brain. J. Lipid Res. 2022, 63, 100249. [Google Scholar] [CrossRef] [PubMed]
- Balog, M.; Anderson, A.C.; Heffer, M.; Korade, Z.; Mirnics, K. Effects of Psychotropic Medication on Somatic Sterol Biosynthesis of Adult Mice. Biomolecules 2022, 12, 1535. [Google Scholar] [CrossRef]
- Wadman, E.; Fernandes, E.; Muss, C.; Powell-Hamilton, N.; Wojcik, M.H.; Madden, J.A.; Carreon, C.K.; Clark, R.D.; Stenftenagel, A.; Chikalard, K.; et al. A novel syndrome associated with prenatal fentanyl exposure. Genet. Med. Open 2023, 1, 100834. [Google Scholar] [CrossRef]
- Allen, L.B.; Mirnics, K. Metoprolol Inhibits Developmental Brain Sterol Biosynthesis in Mice. Biomolecules 2022, 12, 1211. [Google Scholar] [CrossRef] [PubMed]
- Theodoropoulos, P.C.; Wang, W.; Budhipramono, A.; Thompson, B.M.; Madhusudhan, N.; Mitsche, M.A.; McDonald, J.G.; De Brabander, J.K.; Nijhawan, D. A Medicinal Chemistry-Driven Approach Identified the Sterol Isomerase EBP as the Molecular Target of TASIN Colorectal Cancer Toxins. J. Am. Chem. Soc. 2020, 142, 6128–6138. [Google Scholar] [CrossRef] [PubMed]
- Ačimovič, J.; Korošec, T.; Seliškar, M.; Bjorkhem, I.; Monostory, K.; Szabo, P.; Pascussi, J.M.; Belič, A.; Urleb, U.; Kocjan, D.; et al. Inhibition of human sterol Δ7-reductase and other postlanosterol enzymes by LK-980, a novel inhibitor of cholesterol synthesis. Drug Metab. Dispos. 2011, 39, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.H.; Lee, Y.; Kim, M.-K.; Lee, D.H.; Chung, J.H. UV increases skin-derived 1α,25-dihydroxyvitamin D 3 production, leading to MMP-1 expression by altering the balance of vitamin D and cholesterol synthesis from 7-dehydrocholesterol. J. Steroid Biochem. Mol. Biol. 2019, 195, 105449. [Google Scholar] [CrossRef] [PubMed]
- Minner, F.; Ruban, E.; Mathay, C.; Herin, M.; Poumay, Y. Involvement of DHCR7 and DHCR24 in the physiology of epidermal keratinocytes. J. Investig. Dermatol. 2009, 129, S53. [Google Scholar] [CrossRef]
- Waterham, H.R.; Koster, J.; Romeijn, G.J.; Hennekam, R.C.; Vreken, P.; Andersson, H.C.; FitzPatrick, D.R.; Kelley, R.I.; Wanders, R.J. Mutations in the 3beta-hydroxysterol Delta24-reductase gene cause desmosterolosis, an autosomal recessive disorder of cholesterol biosynthesis. Am. J. Hum. Genet. 2001, 69, 685–694. [Google Scholar] [CrossRef]
- Online Mendelian Inheritance in Man (OMIM). Johns Hopkins University, Baltimore, MD. 602398: 7/15/21. Available online: https://omim.org (accessed on 20 February 2024).
- Zolotushko, J.; Flusser, H.; Markus, B.; Shelef, I.; Langer, Y.; Heverin, M.; Björkhem, I.; Sivan, S.; Birk, O.S. The desmosterolosis phenotype: Spasticity, microcephaly and micrognathia with agenesis of corpus callosum and loss of white matter. Eur. J. Hum. Genet. 2011, 19, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, C.P.; Koster, J.; Katsonis, P.; Kratz, L.; Shchelochkov, O.A.; Scaglia, F.; Kelley, R.I.; Lichtarge, O.; Waterham, H.R.; Shinawi, M. Desmosterolosis-phenotypic and molecular characterization of a third case and review of the literature. Am. J. Med. Genet. A 2011, 155a, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Greeve, I.; Hermans-Borgmeyer, I.; Brellinger, C.; Kasper, D.; Gomez-Isla, T.; Behl, C.; Levkau, B.; Nitsch, R.M. The human DIMINUTO/DWARF1 homolog seladin-1 confers resistance to Alzheimer’s disease-associated neurodegeneration and oxidative stress. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 7345–7352. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Miloslavskaya, I.; Demontis, S.; Maestro, R.; Galaktionov, K. Regulation of cellular response to oncogenic and oxidative stress by Seladin-1. Nature 2004, 432, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Tsukiyama-Kohara, K.; Hayashi, M.; Hirata, Y.; Satoh, M.; Tokunaga, Y.; Tateno, C.; Hayashi, Y.; Hishima, T.; Funata, N.; et al. Augmentation of DHCR24 expression by hepatitis C virus infection facilitates viral replication in hepatocytes. J. Hepatol. 2011, 55, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Spann, N.J.; Garmire, L.X.; McDonald, J.G.; Myers, D.S.; Milne, S.B.; Shibata, N.; Reichart, D.; Fox, J.N.; Shaked, I.; Heudobler, D.; et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell 2012, 151, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Kambe, F.; Cao, X.; Kozaki, Y.; Kaji, T.; Ishii, T.; Seo, H. 3β-Hydroxysteroid-Δ24 Reductase Is a Hydrogen Peroxide Scavenger, Protecting Cells from Oxidative Stress-Induced Apoptosis. Endocrinology 2008, 149, 3267–3273. [Google Scholar] [CrossRef]
- Rodgers, M.A.; Villareal, V.A.; Schaefer, E.A.; Peng, L.F.; Corey, K.E.; Chung, R.T.; Yang, P.L. Lipid metabolite profiling identifies desmosterol metabolism as a new antiviral target for hepatitis C virus. J. Am. Chem. Soc. 2012, 134, 6896–6899. [Google Scholar] [CrossRef]
- Mirza, R.; Qiao, S.; Tateyama, K.; Miyamoto, T.; Xiuli, L.; Seo, H. 3β-Hydroxysterol-Delta24 reductase plays an important role in long bone growth by protecting chondrocytes from reactive oxygen species. J. Bone Miner. Metab. 2012, 30, 144–153. [Google Scholar] [CrossRef]
- Spann, N.J.; Glass, C.K. Sterols and oxysterols in immune cell function. Nat. Immunol. 2013, 14, 893–900. [Google Scholar] [CrossRef]
- Muller, C.; Hank, E.; Giera, M.; Bracher, F. Dehydrocholesterol Reductase 24 (DHCR24): Medicinal Chemistry, Pharmacology and Novel Therapeutic Options. Curr. Med. Chem. 2022, 29, 4005–4025. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Zhu, X.L.; Liu, F.; Xu, Q.Y.; Ge, Q.L.; Jiang, S.H.; Yang, X.M.; Li, J.; Wang, Y.H.; Wu, Q.K.; et al. Cholesterol Synthetase DHCR24 Induced by Insulin Aggravates Cancer Invasion and Progesterone Resistance in Endometrial Carcinoma. Sci. Rep. 2017, 7, 41404. [Google Scholar] [CrossRef]
- Lee, G.T.; Ha, Y.S.; Jung, Y.S.; Moon, S.K.; Kang, H.W.; Lee, O.J.; Joung, J.Y.; Choi, Y.H.; Yun, S.J.; Kim, W.J.; et al. DHCR24 is an independent predictor of progression in patients with non-muscle-invasive urothelial carcinoma, and its functional role is involved in the aggressive properties of urothelial carcinoma cells. Ann. Surg. Oncol. 2014, 21 (Suppl. S4), S538–S545. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Chen, X.; Sun, D.; Xu, B.; Zhao, L.; Shi, X.; Liu, H.; Gao, B.; Lu, X. The mechanism of the effect of U18666a on blocking the activity of 3β-hydroxysterol Δ-24-reductase (DHCR24): Molecular dynamics simulation study and free energy analysis. J. Mol. Model. 2016, 22, 46. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Yang, B.; Wang, H.; Lu, C.; Chang, A.K.; Huang, X.; Zhang, X.; Lu, Z.; Lu, X.; et al. Suppression of neuronal cholesterol biosynthesis impairs brain functions through insulin-like growth factor I-Akt signaling. Int. J. Biol. Sci. 2021, 17, 3702–3716. [Google Scholar] [CrossRef]
- Hernández-Jiménez, M.; Martínez-López, D.; Gabandé-Rodríguez, E.; Martín-Segura, A.; Lizasoain, I.; Ledesma, M.D.; Dotti, C.G.; Moro, M.A. Seladin-1/DHCR24 is neuroprotective by associating EAAT2 glutamate transporter to lipid rafts in experimental stroke. Stroke J. Cereb. Circ. 2016, 47, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, K.; Nakamura, Y.; Nishijima, M.; Yamakawa, Y. Prevention of prion propagation by dehydrocholesterol reductase inhibitors in cultured cells and a therapeutic trial in mice. Biol. Pharm. Bull. 2007, 30, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Stamatakos, S.; Beretta, G.L.; Vergani, E.; Dugo, M.; Corno, C.; Corna, E.; Tinelli, S.; Frigerio, S.; Ciusani, E.; Rodolfo, M.; et al. Deregulated FASN expression in BRAF inhibitor-resistant melanoma cells unveils new targets for drug combinations. Cancers 2021, 13, 2284. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.Y.; Chang, H.M.; Wang, J.; Qi, M.H.; Wang, W.L.; Qiu, Y.; Liang, K.; Chen, F.Q.; Zha, D.J.; Qiu, J.H. Inhibition of DHCR24 increases the cisplatin-induced damage to cochlear hair cells in vitro. Neurosci. Lett. 2019, 706, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Hemmers, S.; Bartl, N.; Plodek, A.; Körner, A.; Mirakaj, V.; Giera, M.; Bracher, F. New chemotype of selective and potent inhibitors of human delta 24-dehydrocholesterol reductase. Eur. J. Med. Chem. 2017, 140, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Körner, A.; Zhou, E.; Müller, C.; Mohammed, Y.; Herceg, S.; Bracher, F.; Rensen, P.C.N.; Wang, Y.; Mirakaj, V.; Giera, M. Inhibition of Δ24-dehydrocholesterol reductase activates pro-resolving lipid mediator biosynthesis and inflammation resolution. Proc. Natl. Acad. Sci. USA 2019, 116, 20623–20634. [Google Scholar] [CrossRef]
- Zhou, E.; Ge, X.; Nakashima, H.; Li, R.; van der Zande, H.J.P.; Liu, C.; Li, Z.; Müller, C.; Bracher, F.; Mohammed, Y.; et al. Inhibition of DHCR24 activates LXRα to ameliorate hepatic steatosis and inflammation. EMBO Mol. Med. 2023, 15, e16845. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, Z.; Li, Y.; Liu, T.; Zhao, L.; Gao, T.; Lu, X.; Gao, B. Virtual Screening of Novel 24-Dehydroxysterol Reductase (DHCR24) Inhibitors and the Biological Evaluation of Irbesartan in Cholesterol-Lowering Effect. Molecules 2023, 28, 2634. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.B.; Genaro-Mattos, T.C.; Anderson, A.; Porter, N.A.; Mirnics, K.; Korade, Z. Amiodarone Alters Cholesterol Biosynthesis through Tissue-Dependent Inhibition of Emopamil Binding Protein and Dehydrocholesterol Reductase 24. ACS Chem. Neurosci. 2020, 11, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Luu, W.; Zerenturk, E.J.; Kristiana, I.; Bucknall, M.P.; Sharpe, L.J.; Brown, A.J. Signaling regulates activity of DHCR24, the final enzyme in cholesterol synthesis. J. Lipid Res. 2014, 55, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Zerenturk, E.; Sharpe, L.J.; Kristiana, I.; Brown, A.J. DHCR24: Unexpected new directions for a terminal step in cholesterol synthesis. FASEB J. 2013, 27, 822.2. [Google Scholar] [CrossRef]
- Zerenturk, E.J.; Kristiana, I.; Gill, S.; Brown, A.J. The endogenous regulator 24(S),25-epoxycholesterol inhibits cholesterol synthesis at DHCR24 (Seladin-1). Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2012, 1821, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Phull, M.S.; Jadav, S.S.; Gundla, R.; Mainkar, P.S. A perspective on medicinal chemistry approaches towards adenomatous polyposis coli and Wnt signal based colorectal cancer inhibitors. Eur. J. Med. Chem. 2021, 212, 113149. [Google Scholar] [CrossRef] [PubMed]
- Taiwo, O.A.; Dosumu, O.A.; Oni, E.O.; Akomolafe, V.O.; Elazab, S.T.; Qusti, S.; Alshammari, E.M.; Batiha, G.E.S.; Ojo, O.A. Preclinical prediction of phytochemicals identified from cannabis as novel inhibitors of TEX 11, DHCR24, and CatSper 1 in fertility drug design. Inform. Med. Unlocked 2021, 26, 100747. [Google Scholar] [CrossRef]
- Wu, J.; Guo, L.; Qiu, X.; Ren, Y.; Li, F.; Cui, W.; Song, S. Genkwadaphnin inhibits growth and invasion in hepatocellular carcinoma by blocking DHCR24-mediated cholesterol biosynthesis and lipid rafts formation. Br. J. Cancer 2020, 123, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Nakano-Ito, K.; Fujikawa, Y.; Hihara, T.; Shinjo, H.; Kotani, S.; Suganuma, A.; Aoki, T.; Tsukidate, K. E2012-induced cataract and its predictive biomarkers. Toxicol. Sci. 2014, 137, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Giannini, S.; Benvenuti, S.; Luciani, P.; Manuelli, C.; Cellai, I.; Deledda, C.; Pezzatini, A.; Vannelli, G.B.; Maneschi, E.; Rotella, C.M.; et al. Intermittent high glucose concentrations reduce neuronal precursor survival by altering the IGF system: The involvement of the neuroprotective factor DHCR24 (Seladin-1). J. Endocrinol. 2008, 198, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.H.; Matsuda, S.P.; Liu, D.R.; Corey, E.J. Molecular cloning of the human gene encoding lanosterol synthase from a liver cDNA library. Biochem. Biophys. Res. Commun. 1995, 213, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Roessler, E.; Mittaz, L.; Du, Y.; Scott, H.S.; Chang, J.; Rossier, C.; Guipponi, M.; Matsuda, S.P.; Muenke, M.; Antonarakis, S.E. Structure of the human Lanosterol synthase gene and its analysis as a candidate for holoprosencephaly (HPE1). Hum. Genet. 1999, 105, 489–495. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Online Mendelian Inheritance in Man (OMIM). Johns Hopkins University, Baltimore, MD. 600909: 2/10/22. Available online: https://omim.org (accessed on 20 February 2024).
- Online Mendelian Inheritance in Man (OMIM). Johns Hopkins University, Baltimore, MD. 618275: 12/27/21. Available online: https://omim.org (accessed on 20 February 2024).
- Online Mendelian Inheritance in Man (OMIM). Johns Hopkins University, Baltimore, MD. 618840: 1/2/24. Available online: https://omim.org (accessed on 20 February 2024).
- Zhao, L.; Chen, X.J.; Zhu, J.; Xi, Y.B.; Yang, X.; Hu, L.D.; Ouyang, H.; Patel, S.H.; Jin, X.; Lin, D.; et al. Lanosterol reverses protein aggregation in cataracts. Nature 2015, 523, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.T.; Tafazzoli, A.; Mattern, M.; Sivalingam, S.; Wolf, S.; Rupp, A.; Thiele, H.; Altmüller, J.; Nürnberg, P.; Ellwanger, J.; et al. Bi-allelic Mutations in LSS, Encoding Lanosterol Synthase, Cause Autosomal-Recessive Hypotrichosis Simplex. Am. J. Hum. Genet. 2018, 103, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Li, G.; Abe, I.; Nakayama, J.; Guo, Z.; Sawashita, J.; Ugawa, T.; Nishizono, S.; Serikawa, T.; Higuchi, K.; et al. Lanosterol synthase mutations cause cholesterol deficiency-associated cataracts in the Shumiya cataract rat. J. Clin. Investig. 2006, 116, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, M.E.; Flenniken, A.M.; Ji, X.; Teboul, L.; Wong, M.D.; White, J.K.; Meehan, T.F.; Weninger, W.J.; Westerberg, H.; Adissu, H.; et al. High-throughput discovery of novel developmental phenotypes. Nature 2016, 537, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Karunagaran, S.; Kavitha, R.; Vadivelu, M.; Lee, K.W.; Meganathan, C. Insight mechanism of the selective lanosterol synthase inhibitor: Molecular modeling, docking and density functional theory approaches. Curr. Comput.-Aided Drug Des. 2017, 13, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Wallace, I.M.; Urbanus, M.L.; Luciani, G.M.; Burns, A.R.; Han, M.K.L.; Wang, H.; Arora, K.; Heisler, L.E.; Proctor, M.; St Onge, R.P.; et al. Compound prioritization methods increase rates of chemical probe discovery in model organisms. Chem. Biol. 2011, 18, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Sakano, Y.; Shimizu, K.; Shibuya, M.; Ebizuka, Y.; Goda, Y. Constituents of Laurus nobilis L. inhibit recombinant human lanosterol synthase. J. Nat. Med. 2006, 60, 78–81. [Google Scholar] [CrossRef]
- McCrae, C.; Dzgoev, A.; Ståhlman, M.; Horndahl, J.; Svärd, R.; Große, A.; Großkopf, T.; Skujat, M.-A.; Williams, N.; Schubert, S.; et al. Lanosterol Synthase Regulates Human Rhinovirus Replication in Human Bronchial Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2018, 59, 713–722. [Google Scholar] [CrossRef]
- Bobba, R.K.; Benakanakere, I.; Bearelly, S.; Arya, M.; Sleigtholm, R.; Freter, C. Mevalonate pathway inhibitors in the treatment of B-cell chronic lymphocytic leukemia. J. Clin. Oncol. 2012, 30, 6561. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Wang, W.; Sternisha, A.C.; Corley, C.D.; Wang, H.Y.L.; Wang, X.; Ortiz, F.; Lim, S.K.; Abdullah, K.G.; Parada, L.F.; et al. Selective and brain-penetrant lanosterol synthase inhibitors target glioma stem-like cells by inducing 24(S),25-epoxycholesterol production. Cell Chem. Biol. 2023, 30, 214–229.e218. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.; Otero, C.; Dumrauf, B.; Rodenak-Kladniew, B.; Montero-Villegas, S.; Viña, S.; García De Bravo, M.; Crespo, R. Effect of essential oils from introduced and local plants on cholesterol metabolism in hepatic and foam cells. A search for natural antiatherogenic compounds. Biocell 2019, 43, 99. [Google Scholar]
- Sakano, Y.; Shibuya, M.; Yamaguchi, Y.; Masuma, R.; Tomoda, H.; Omura, S.; Ebizuka, Y. Epohelmins A and B, novel lanosterol synthase inhibitors from a fungal strain FKI-0929. J. Antibiot. 2004, 57, 564–568. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tanaka, R.; Sakano, Y.; Nagatsu, A.; Shibuya, M.; Ebizuka, Y.; Goda, Y. Synthesis of digalactosyl diacylglycerols and their structure-inhibitory activity on human lanosterol synthase. Bioorg. Med. Chem. Lett. 2005, 15, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Online Mendelian Inheritance in Man (OMIM). Johns Hopkins University, Baltimore, MD. 616834: 1/13/22. Available online: https://omim.org (accessed on 20 February 2024).
- He, M.; Kratz, L.E.; Michel, J.J.; Vallejo, A.N.; Ferris, L.; Kelley, R.I.; Hoover, J.J.; Jukic, D.; Gibson, K.M.; Wolfe, L.A.; et al. Mutations in the human SC4MOL gene encoding a methyl sterol oxidase cause psoriasiform dermatitis, microcephaly, and developmental delay. J. Clin. Investig. 2011, 121, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Steere, L.; Zhang, Y.-K.; McGregor, C.; Hahne, C.; Zhou, Y.; Liu, C.; Cai, Y.; Zhou, H.; Chen, X.; et al. Inhibiting C-4 Methyl Sterol Oxidase with Novel Diazaborines to Target Fungal Plant Pathogens. ACS Chem. Biol. 2022, 17, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Q.; Wu, X.; Jiang, M.; Jin, H.; Tao, K.; Hou, T. Unraveling the polypharmacology of a natural antifungal product, eugenol, against Rhizoctonia solani. Pest. Manag. Sci. 2021, 77, 3469–3483. [Google Scholar] [CrossRef] [PubMed]
- Sukhanova, A.; Gorin, A.; Serebriiskii, I.G.; Gabitova, L.; Zheng, H.; Restifo, D.; Egleston, B.L.; Cunningham, D.; Bagnyukova, T.; Liu, H.; et al. Targeting C4-demethylating genes in the cholesterol pathway sensitizes cancer cells to EGF receptor inhibitors via increased EGF receptor degradation. Cancer Discov. 2013, 3, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Pleshinger, M.J.; Friedrich, R.M.; Hubler, Z.; Rivera-León, A.M.; Gao, F.; Yan, D.; Sax, J.L.; Srinivasan, R.; Bederman, I.; Shick, H.E.; et al. Inhibition of SC4MOL and HSD17B7 shifts cellular sterol composition and promotes oligodendrocyte formation. RSC Chem. Biol. 2022, 3, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Braverman, N.; Lin, P.; Moebius, F.F.; Obie, C.; Moser, A.; Glossmann, H.; Wilcox, W.R.; Rimoin, D.L.; Smith, M.; Kratz, L.; et al. Mutations in the gene encoding 3 beta-hydroxysteroid-delta 8, delta 7-isomerase cause X-linked dominant Conradi-Hünermann syndrome. Nat. Genet. 1999, 22, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Online Mendelian Inheritance in Man (OMIM). Johns Hopkins University, Baltimore, MD. 302960: 3/2/22. Available online: https://omim.org (accessed on 20 February 2024).
- Herman, G.E.; Kelley, R.I.; Pureza, V.; Smith, D.; Kopacz, K.; Pitt, J.; Sutphen, R.; Sheffield, L.J.; Metzenberg, A.B. Characterization of mutations in 22 females with X-linked dominant chondrodysplasia punctata (Happle syndrome). Genet. Med. Off. J. Am. Coll. Med. Genet. 2002, 4, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Online Mendelian Inheritance in Man (OMIM). Johns Hopkins University, Baltimore, MD. 300960: 2/6/23. Available online: https://omim.org (accessed on 20 February 2024).
- Long, T.; Hassan, A.; Thompson, B.M.; McDonald, J.G.; Wang, J.; Li, X. Structural basis for human sterol isomerase in cholesterol biosynthesis and multidrug recognition. Nat. Commun. 2019, 10, 2452. [Google Scholar] [CrossRef]
- Moebius, F.F.; Reiter, R.J.; Bermoser, K.; Glossmann, H.; Cho, S.Y.; Paik, Y.K. Pharmacological analysis of sterol delta8-delta7 isomerase proteins with [3H]ifenprodil. Mol. Pharmacol. 1998, 54, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Berardi, F.; Abate, C.; Ferorelli, S.; De Robertis, A.F.; Leopoldo, M.; Colabufo, N.A.; Niso, M.; Perrone, R. Novel 4-(4-aryl)cyclohexyl-1-(2-pyridyl)piperazines as Δ8–Δ7 sterol isomerase (emopamil binding protein) selective ligands with antiproliferative activity. J. Med. Chem. 2008, 51, 7523–7531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Theodoropoulos, P.C.; Eskiocak, U.; Wang, W.; Moon, Y.-A.; Posner, B.; Williams, N.S.; Wright, W.E.; Kim, S.B.; Nijhawan, D. Selective targeting of mutant adenomatous polyposis coli (APC) in colorectal cancer. Sci. Transl. Med. 2016, 8, 361ra140. [Google Scholar] [CrossRef]
- Allimuthu, D.; Hubler, Z.; Najm, F.J.; Tang, H.; Bederman, I.; Seibel, W.; Tesar, P.J.; Adams, D.J. Diverse Chemical Scaffolds Enhance Oligodendrocyte Formation by Inhibiting CYP51, TM7SF2, or EBP. Cell Chem. Biol. 2019, 26, 593–599.e594. [Google Scholar] [CrossRef]
- Hubler, Z.; Allimuthu, D.; Bederman, I.; Elitt, M.S.; Madhavan, M.; Allan, K.C.; Shick, H.E.; Garrison, E.; Karl, M.T.; Factor, D.C.; et al. Accumulation of 8,9-unsaturated sterols drives oligodendrocyte formation and remyelination. Nature 2018, 560, 372–376. [Google Scholar] [CrossRef]
- Sax, J.L.; Hershman, S.N.; Hubler, Z.; Allimuthu, D.; Elitt, M.S.; Bederman, I.; Adams, D.J. Enhancers of Human and Rodent Oligodendrocyte Formation Predominantly Induce Cholesterol Precursor Accumulation. ACS Chem. Biol. 2022, 17, 2188–2200. [Google Scholar] [CrossRef]
- Sheng, G.; Wang, D.; Giera, S.; Pan, Q.; Farley, J.; Radzwill, K.; Garron, C.; Byrne, A.; Merriman, G.; Samad, T.; et al. Emopamil binding protein inhibition as a remyelination therapy for multiple sclerosis. Mult. Scler. J. 2019, 25, 911. [Google Scholar] [CrossRef]
- Sax, J.L.; Hubler, Z.; Allimuthu, D.; Adams, D.J. Screening Reveals Sterol Derivatives with Pro-Differentiation, Pro-Survival, or Potent Cytotoxic Effects on Oligodendrocyte Progenitor Cells. ACS Chem. Biol. 2021, 16, 1288–1297. [Google Scholar] [CrossRef]
- Hubler, Z.; Friedrich, R.M.; Sax, J.L.; Allimuthu, D.; Gao, F.; Rivera-León, A.M.; Pleshinger, M.J.; Bederman, I.; Adams, D.J. Modulation of lanosterol synthase drives 24,25-epoxysterol synthesis and oligodendrocyte formation. Cell Chem. Biol. 2021, 28, 866–875.e865. [Google Scholar] [CrossRef]
- Lamb, D.C.; Kelly, D.E.; Kelly, S.L. Molecular diversity of sterol 14alpha-demethylase substrates in plants, fungi and humans. FEBS Lett. 1998, 425, 263–265. [Google Scholar] [CrossRef]
- Korade, Z.; Kim, H.Y.; Tallman, K.A.; Liu, W.; Koczok, K.; Balogh, I.; Xu, L.; Mirnics, K.; Porter, N.A. The Effect of Small Molecules on Sterol Homeostasis: Measuring 7-Dehydrocholesterol in Dhcr7-Deficient Neuro2a Cells and Human Fibroblasts. J. Med. Chem. 2016, 59, 1102–1115. [Google Scholar] [CrossRef]
- Hankins, E.G.; Gillespie, J.R.; Aikenhead, K.; Buckner, F.S. Upregulation of sterol C14-demethylase expression in Trypanosoma cruzi treated with sterol biosynthesis inhibitors. Mol. Biochem. Parasitol. 2005, 144, 68–75. [Google Scholar] [CrossRef]
- Parker, J.E.; Merkamm, M.; Manning, N.J.; Pompon, D.; Kelly, S.L.; Kelly, D.E. Differential azole antifungal efficacies contrasted using a Saccharomyces cerevisiae strain humanized for sterol 14 alpha-demethylase at the homologous locus. Antimicrob. Agents Chemother. 2008, 52, 3597–3603. [Google Scholar] [CrossRef]
- Lamb, D.C.; Kelly, D.E.; Waterman, M.R.; Stromstedt, M.; Rozman, D.; Kelly, S.L. Characteristics of the heterologously expressed human lanosterol 14alpha-demethylase (other names: P45014DM, CYP51, P45051) and inhibition of the purified human and Candida albicans CYP51 with azole antifungal agents. Yeast 1999, 15, 755–763. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Lv, Q.; Yan, L.; Wang, Y.; Jiang, Y. The Fungal CYP51s: Their Functions, Structures, Related Drug Resistance, and Inhibitors. Front. Microbiol. 2019, 10, 691. [Google Scholar] [CrossRef]
- Chen, Z.F.; Ying, G.G. Occurrence, fate and ecological risk of five typical azole fungicides as therapeutic and personal care products in the environment: A review. Environ. Int. 2015, 84, 142–153. [Google Scholar] [CrossRef]
- Mogensen, D.M.; Pihl, M.B.; Skakkebæk, N.E.; Andersen, H.R.; Juul, A.; Kyhl, H.B.; Swan, S.; Kristensen, D.M.; Andersen, M.S.; Lind, D.V.; et al. Prenatal exposure to antifungal medication may change anogenital distance in male offspring: A preliminary study. Environ. Health 2017, 16, 68. [Google Scholar] [CrossRef]
- Schoelwer, M.; Eugster, E.A. Treatment of Peripheral Precocious Puberty. Endocr. Dev. 2016, 29, 230–239. [Google Scholar] [CrossRef]
- Brunetti-Pierri, N.; Corso, G.; Rossi, M.; Ferrari, P.; Balli, F.; Rivasi, F.; Annunziata, I.; Ballabio, A.; Russo, A.D.; Andria, G.; et al. Lathosterolosis, a novel multiple-malformation/mental retardation syndrome due to deficiency of 3beta-hydroxysteroid-delta5-desaturase. Am. J. Hum. Genet. 2002, 71, 952–958. [Google Scholar] [CrossRef]
- Schade, D.S.; Shey, L.; Eaton, R.P. Cholesterol Review: A Metabolically Important Molecule. Endocr. Pract. 2020, 26, 1514–1523. [Google Scholar] [CrossRef]
- Sharpe, L.J.; Brown, A.J. Controlling cholesterol synthesis beyond 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR). J. Biol. Chem. 2013, 288, 18707–18715. [Google Scholar] [CrossRef]
- Wood, W.G.; Igbavboa, U.; Eckert, G.; Müller, W. Cholesterol-a Janus-faced molecule in the central nervous system. In Handbook of Neurochemistry and Molecular Neurobiology: Neural Membranes and Transport; Springer: Boston, MA, USA, 2007; pp. 151–170. [Google Scholar]
- Brown, M.S.; Radhakrishnan, A.; Goldstein, J.L. Retrospective on Cholesterol Homeostasis: The Central Role of Scap. Annu. Rev. Biochem. 2018, 87, 783–807. [Google Scholar] [CrossRef]
- Benachenhou, S.; Laroui, A.; Dionne, O.; Rojas, D.; Toupin, A.; Çaku, A. Cholesterol alterations in fragile X syndrome, autism spectrum disorders and other neurodevelopmental disorders. Int. Rev. Neurobiol. 2023, 173, 115–139. [Google Scholar] [CrossRef]
- Ikonen, E.; Zhou, X. Cholesterol transport between cellular membranes: A balancing act between interconnected lipid fluxes. Dev. Cell 2021, 56, 1430–1436. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Wang, Y. Sterols, Oxysterols, and Accessible Cholesterol: Signalling for Homeostasis, in Immunity and during Development. Front. Physiol. 2021, 12, 723224. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Wang, Y. Cholesterol metabolism: From lipidomics to immunology. J. Lipid Res. 2022, 63, 100165. [Google Scholar] [CrossRef]
- Simons, K.; Ehehalt, R. Cholesterol, lipid rafts, and disease. J. Clin. Investig. 2002, 110, 597–603. [Google Scholar] [CrossRef]
- Ačimovič, J.; Goyal, S.; Košir, R.; Goličnik, M.; Perše, M.; Belič, A.; Urlep, Ž.; Guengerich, F.P.; Rozman, D. Cytochrome P450 metabolism of the post-lanosterol intermediates explains enigmas of cholesterol synthesis. Sci. Rep. 2016, 6, 28462. [Google Scholar] [CrossRef]
- Xu, S.; Tang, C. Cholesterol and Hedgehog Signaling: Mutual Regulation and Beyond. Front. Cell Dev. Biol. 2022, 10, 774291. [Google Scholar] [CrossRef]
- Ghersi, D.; Genaro-Mattos, T.C. Identifying Molecular Fragments That Drive 7-Dehydrocholesterol Elevation. ACS Pharm. Transl. Sci. 2022, 5, 3–7. [Google Scholar] [CrossRef]
- McAinsh, J.; Cruickshank, J.M. Beta-blockers and central nervous system side effects. Pharmacol. Ther. 1990, 46, 163–197. [Google Scholar] [CrossRef]
- Shek, E.; Bardhan, S.; Cheine, M.V.; Ahonen, J.; Wahlbeck, K. Beta-blocker supplementation of standard drug treatment for schizophrenia. Schizophr. Bull. 2010, 36, 1079–1080. [Google Scholar] [CrossRef]
- Miller, C.H.; Fleischhacker, W.W. Managing antipsychotic-induced acute and chronic akathisia. Drug Saf. 2000, 22, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Stransky, A.; Tierney, E. Cognitive and behavioral aspects of Smith-Lemli-Opitz syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 2012, 160c, 295–300. [Google Scholar] [CrossRef]
- Thurm, A.; Tierney, E.; Farmer, C.; Albert, P.; Joseph, L.; Swedo, S.; Bianconi, S.; Bukelis, I.; Wheeler, C.; Sarphare, G.; et al. Development, behavior, and biomarker characterization of Smith-Lemli-Opitz syndrome: An update. J. Neurodev. Disord. 2016, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Tierney, E.; Nwokoro, N.A.; Kelley, R.I. Behavioral phenotype of RSH/Smith-Lemli-Opitz syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 2000, 6, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Lamberson, C.R.; Muchalski, H.; McDuffee, K.B.; Tallman, K.A.; Xu, L.; Porter, N.A. Propagation rate constants for the peroxidation of sterols on the biosynthetic pathway to cholesterol. Chem. Phys. Lipids 2017, 207, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Genaro-Mattos, T.C.; Klingelsmith, K.B.; Allen, L.B.; Anderson, A.; Tallman, K.A.; Porter, N.A.; Korade, Z.; Mirnics, K. Sterol Biosynthesis Inhibition in Pregnant Women Taking Prescription Medications. ACS Pharm. Transl. Sci. 2021, 4, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Chikalard, K.; Stenftenagel, A.; Kimonis, V. Smith-Lemli-Opitz Phenotype with Transiently Abnormal Sterols in the Setting of Perinatal Fentanyl Exposure, a Case Report. J. Investig. Med. 2023, 71, 327. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).