Composition and Function of Neutrophil Extracellular Traps
Abstract
:1. Introduction
2. Types and Formation Mechanisms of NETs
2.1. Suicidal NETosis
2.2. Vital NETosis
2.3. Mitochondrial NETosis
3. Composition of NETs
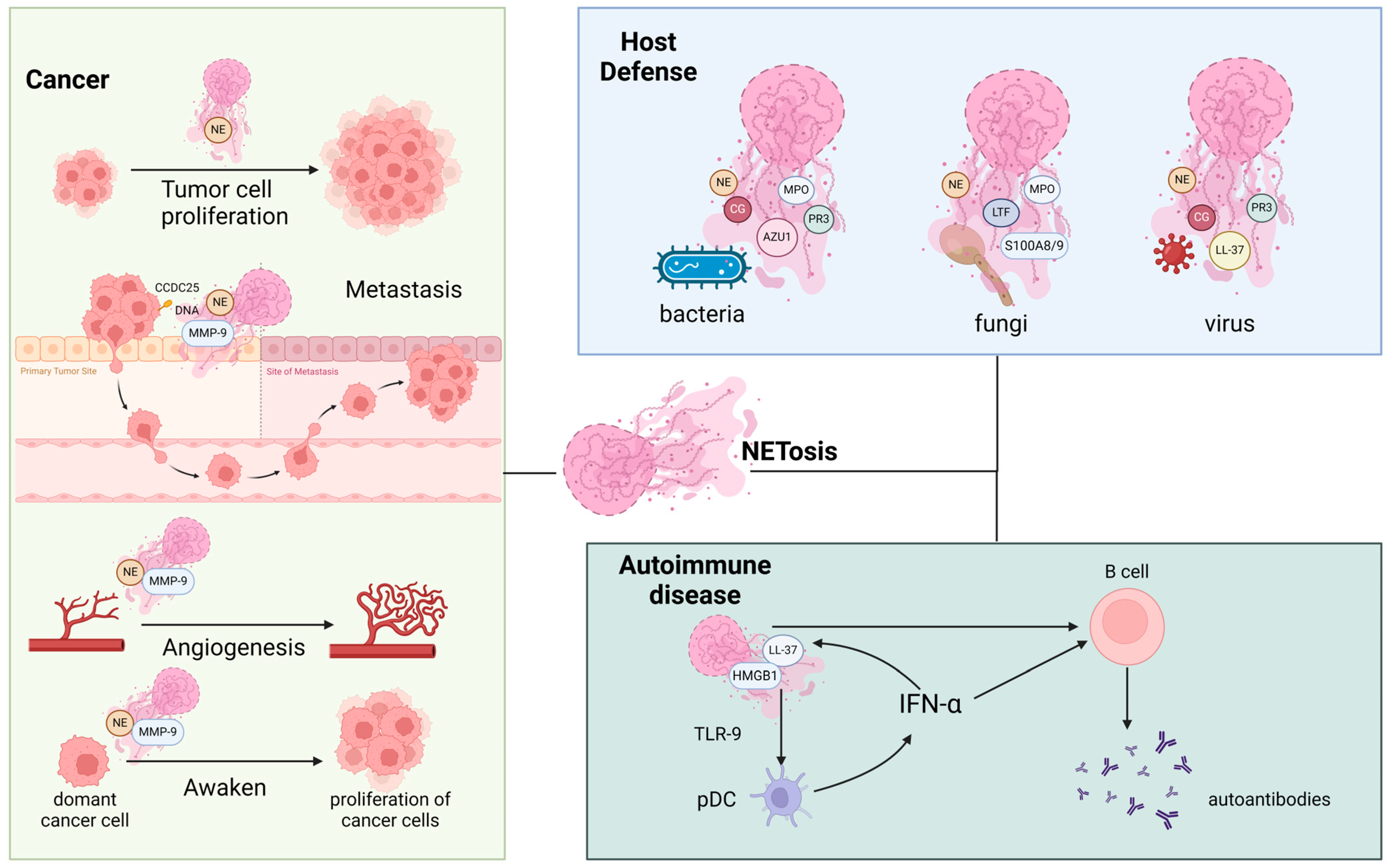
4. Roles of NET Components in Various Diseases
4.1. The Roles of NET Components in Host Defense against Invasive Pathogens
4.1.1. NETs in Bacterial Defense
4.1.2. NETs in Fungal Defense
4.1.3. NETs in Viral Defense
4.2. The Roles of NET Components in Cancer
4.3. The Roles of NET Components in Autoimmune Diseases
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Borregaard, N. Neutrophils, from marrow to microbes. Immunity 2010, 33, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Yipp, B.G.; Kubes, P. NETosis: How vital is it? Blood 2013, 122, 2784–2794. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.; Mihalache, C.; Kozlowski, E.; Schmid, I.; Simon, H.U. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009, 16, 1438–1444. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Berends, E.T.M.; Chan, R.; Schwab, E.; Roy, S.; Sen, C.K.; Torres, V.J.; Wozniak, D.J. Staphylococcus aureus biofilms release leukocidins to elicit extracellular trap formation and evade neutrophil-mediated killing. Proc. Natl. Acad. Sci. USA 2018, 115, 7416–7421. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Pilsczek, F.H.; Salina, D.; Poon, K.K.; Fahey, C.; Yipp, B.G.; Sibley, C.D.; Robbins, S.M.; Green, F.H.; Surette, M.G.; Sugai, M.; et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 2010, 185, 7413–7425. [Google Scholar] [CrossRef]
- Yang, H.; Biermann, M.H.; Brauner, J.M.; Liu, Y.; Zhao, Y.; Herrmann, M. New Insights into Neutrophil Extracellular Traps: Mechanisms of Formation and Role in Inflammation. Front. Immunol. 2016, 7, 302. [Google Scholar] [CrossRef]
- Urban, C.F.; Reichard, U.; Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006, 8, 668–676. [Google Scholar] [CrossRef]
- Saitoh, T.; Komano, J.; Saitoh, Y.; Misawa, T.; Takahama, M.; Kozaki, T.; Uehata, T.; Iwasaki, H.; Omori, H.; Yamaoka, S.; et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe 2012, 12, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Hakkim, A.; Fuchs, T.A.; Martinez, N.E.; Hess, S.; Prinz, H.; Zychlinsky, A.; Waldmann, H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 2011, 7, 75–77. [Google Scholar] [CrossRef]
- Denning, N.L.; Aziz, M.; Gurien, S.D.; Wang, P. DAMPs and NETs in Sepsis. Front. Immunol. 2019, 10, 2536. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Stadler, S.; Correll, S.; Li, P.; Wang, D.; Hayama, R.; Leonelli, L.; Han, H.; Grigoryev, S.A.; et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J. Cell Biol. 2009, 184, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef]
- Thiam, H.R.; Wong, S.L.; Qiu, R.; Kittisopikul, M.; Vahabikashi, A.; Goldman, A.E.; Goldman, R.D.; Wagner, D.D.; Waterman, C.M. NETosis proceeds by cytoskeleton and endomembrane disassembly and PAD4-mediated chromatin decondensation and nuclear envelope rupture. Proc. Natl. Acad. Sci. USA 2020, 117, 7326–7337. [Google Scholar] [CrossRef] [PubMed]
- Sollberger, G.; Choidas, A.; Burn, G.L.; Habenberger, P.; Di Lucrezia, R.; Kordes, S.; Menninger, S.; Eickhoff, J.; Nussbaumer, P.; Klebl, B.; et al. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci. Immunol. 2018, 3, eaar6689. [Google Scholar] [CrossRef]
- Kambara, H.; Liu, F.; Zhang, X.; Liu, P.; Bajrami, B.; Teng, Y.; Zhao, L.; Zhou, S.; Yu, H.; Zhou, W.; et al. Gasdermin D Exerts Anti-inflammatory Effects by Promoting Neutrophil Death. Cell Rep. 2018, 22, 2924–2936. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef]
- Yipp, B.G.; Petri, B.; Salina, D.; Jenne, C.N.; Scott, B.N.; Zbytnuik, L.D.; Pittman, K.; Asaduzzaman, M.; Wu, K.; Meijndert, H.C.; et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 2012, 18, 1386–1393. [Google Scholar] [CrossRef]
- McIlroy, D.J.; Jarnicki, A.G.; Au, G.G.; Lott, N.; Smith, D.W.; Hansbro, P.M.; Balogh, Z.J. Mitochondrial DNA neutrophil extracellular traps are formed after trauma and subsequent surgery. J. Crit. Care 2014, 29, e1131–e1135. [Google Scholar] [CrossRef]
- Dunham-Snary, K.J.; Surewaard, B.G.; Mewburn, J.D.; Bentley, R.E.; Martin, A.Y.; Jones, O.; Al-Qazazi, R.; Lima, P.A.; Kubes, P.; Archer, S.L. Mitochondria in human neutrophils mediate killing of Staphylococcus aureus. Redox Biol. 2022, 49, 102225. [Google Scholar] [CrossRef]
- Lood, C.; Blanco, L.P.; Purmalek, M.M.; Carmona-Rivera, C.; De Ravin, S.S.; Smith, C.K.; Malech, H.L.; Ledbetter, J.A.; Elkon, K.B.; Kaplan, M.J. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016, 22, 146–153. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Z.; Li, L.; Zhang, Z.; Jin, X.; Wu, P.; Sun, S.; Pan, J.; Su, K.; Jia, F.; et al. Aged neutrophils form mitochondria-dependent vital NETs to promote breast cancer lung metastasis. J. Immunother. Cancer 2021, 9, e002875. [Google Scholar] [CrossRef]
- Amini, P.; Stojkov, D.; Felser, A.; Jackson, C.B.; Courage, C.; Schaller, A.; Gelman, L.; Soriano, M.E.; Nuoffer, J.M.; Scorrano, L.; et al. Neutrophil extracellular trap formation requires OPA1-dependent glycolytic ATP production. Nat. Commun. 2018, 9, 2958. [Google Scholar] [CrossRef]
- Dwyer, M.; Shan, Q.; D’Ortona, S.; Maurer, R.; Mitchell, R.; Olesen, H.; Thiel, S.; Huebner, J.; Gadjeva, M. Cystic fibrosis sputum DNA has NETosis characteristics and neutrophil extracellular trap release is regulated by macrophage migration-inhibitory factor. J. Innate Immun. 2014, 6, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Urban, C.F.; Ermert, D.; Schmid, M.; Abu-Abed, U.; Goosmann, C.; Nacken, W.; Brinkmann, V.; Jungblut, P.R.; Zychlinsky, A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009, 5, e1000639. [Google Scholar] [CrossRef]
- O’Donoghue, A.J.; Jin, Y.; Knudsen, G.M.; Perera, N.C.; Jenne, D.E.; Murphy, J.E.; Craik, C.S.; Hermiston, T.W. Global substrate profiling of proteases in human neutrophil extracellular traps reveals consensus motif predominantly contributed by elastase. PLoS ONE 2013, 8, e75141. [Google Scholar] [CrossRef] [PubMed]
- Petretto, A.; Bruschi, M.; Pratesi, F.; Croia, C.; Candiano, G.; Ghiggeri, G.; Migliorini, P. Neutrophil extracellular traps (NET) induced by different stimuli: A comparative proteomic analysis. PLoS ONE 2019, 14, e0218946. [Google Scholar] [CrossRef]
- Chapman, E.A.; Lyon, M.; Simpson, D.; Mason, D.; Beynon, R.J.; Moots, R.J.; Wright, H.L. Caught in a Trap? Proteomic Analysis of Neutrophil Extracellular Traps in Rheumatoid Arthritis and Systemic Lupus Erythematosus. Front. Immunol. 2019, 10, 423. [Google Scholar] [CrossRef]
- Mulcahy, H.; Charron-Mazenod, L.; Lewenza, S. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2008, 4, e1000213. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, G.; Twig, G.; Shor, D.B.; Furer, A.; Sherer, Y.; Mozes, O.; Komisar, O.; Slonimsky, E.; Klang, E.; Lotan, E.; et al. A volcanic explosion of autoantibodies in systemic lupus erythematosus: A diversity of 180 different antibodies found in SLE patients. Autoimmun. Rev. 2015, 14, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Q.; Zhang, X.; Liu, X.; Zhou, B.; Chen, J.; Huang, D.; Li, J.; Li, H.; Chen, F.; et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature 2020, 583, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Doolin, T.; Amir, H.M.; Duong, L.; Rosenzweig, R.; Urban, L.A.; Bosch, M.; Pol, A.; Gross, S.P.; Siryaporn, A. Mammalian histones facilitate antimicrobial synergy by disrupting the bacterial proton gradient and chromosome organization. Nat. Commun. 2020, 11, 3888. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Xin, L.; Beverley, S.M.; Carlsen, E.D.; Popov, V.; Chang, K.P.; Wang, M.; Soong, L. Differential microbicidal effects of human histone proteins H2A and H2B on Leishmania promastigotes and amastigotes. Infect. Immun. 2011, 79, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Huang, C.M.; Nakatsuji, T.; Thiboutot, D.; Kang, S.A.; Monestier, M.; Gallo, R.L. Histone H4 is a major component of the antimicrobial action of human sebocytes. J. Investig. Dermatol. 2009, 129, 2489–2496. [Google Scholar] [CrossRef]
- Hoeksema, M.; Tripathi, S.; White, M.; Qi, L.; Taubenberger, J.; van Eijk, M.; Haagsman, H.; Hartshorn, K.L. Arginine-rich histones have strong antiviral activity for influenza A viruses. Innate Immun. 2015, 21, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Yang, J.; Zou, J.; Bi, Z.; He, C.; Lei, H.; He, X.; Li, X.; Alu, A.; Ren, W.; et al. Histones released by NETosis enhance the infectivity of SARS-CoV-2 by bridging the spike protein subunit 2 and sialic acid on host cells. Cell Mol. Immunol. 2022, 19, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Karkowska-Kuleta, J.; Smolarz, M.; Seweryn-Ozog, K.; Satala, D.; Zawrotniak, M.; Wronowska, E.; Bochenska, O.; Kozik, A.; Nobbs, A.H.; Gogol, M.; et al. Proteinous Components of Neutrophil Extracellular Traps Are Arrested by the Cell Wall Proteins of Candida albicans during Fungal Infection, and Can Be Used in the Host Invasion. Cells 2021, 10, 2736. [Google Scholar] [CrossRef]
- Lande, R.; Ganguly, D.; Facchinetti, V.; Frasca, L.; Conrad, C.; Gregorio, J.; Meller, S.; Chamilos, G.; Sebasigari, R.; Riccieri, V.; et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 2011, 3, 73ra19. [Google Scholar] [CrossRef]
- Tripathi, S.; Verma, A.; Kim, E.J.; White, M.R.; Hartshorn, K.L. LL-37 modulates human neutrophil responses to influenza A virus. J. Leukoc. Biol. 2014, 96, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Weinrauch, Y.; Drujan, D.; Shapiro, S.D.; Weiss, J.; Zychlinsky, A. Neutrophil elastase targets virulence factors of enterobacteria. Nature 2002, 417, 91–94. [Google Scholar] [CrossRef]
- Houghton, A.M.; Rzymkiewicz, D.M.; Ji, H.; Gregory, A.D.; Egea, E.E.; Metz, H.E.; Stolz, D.B.; Land, S.R.; Marconcini, L.A.; Kliment, C.R.; et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat. Med. 2010, 16, 219–223. [Google Scholar] [CrossRef]
- Futamata, E.; Masuda, S.; Nishibata, Y.; Tanaka, S.; Tomaru, U.; Ishizu, A. Vanishing Immunoglobulins: The Formation of Pauci-Immune Lesions in Myeloperoxidase-Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Nephron 2018, 138, 328–330. [Google Scholar] [CrossRef]
- Lopes, B.R.P.; da Silva, G.S.; de Lima Menezes, G.; de Oliveira, J.; Watanabe, A.S.A.; Porto, B.N.; da Silva, R.A.; Toledo, K.A. Serine proteases in neutrophil extracellular traps exhibit anti-Respiratory Syncytial Virus activity. Int. Immunopharmacol. 2022, 106, 108573. [Google Scholar] [CrossRef] [PubMed]
- Daigo, K.; Hamakubo, T. Host-protective effect of circulating pentraxin 3 (PTX3) and complex formation with neutrophil extracellular traps. Front. Immunol. 2012, 3, 378. [Google Scholar] [CrossRef]
- Khandagale, A.; Lazzaretto, B.; Carlsson, G.; Sundin, M.; Shafeeq, S.; Römling, U.; Fadeel, B. JAGN1 is required for fungal killing in neutrophil extracellular traps: Implications for severe congenital neutropenia. J. Leukoc. Biol. 2018, 104, 1199–1213. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Krumbholz, M.; Schönermarck, U.; Back, W.; Gross, W.L.; Werb, Z.; Gröne, H.J.; Brinkmann, V.; Jenne, D.E. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 2009, 15, 623–625. [Google Scholar] [CrossRef]
- Averhoff, P.; Kolbe, M.; Zychlinsky, A.; Weinrauch, Y. Single residue determines the specificity of neutrophil elastase for Shigella virulence factors. J. Mol. Biol. 2008, 377, 1053–1066. [Google Scholar] [CrossRef]
- Schlievert, P.M.; Kilgore, S.H.; Beck, L.A.; Yoshida, T.; Klingelhutz, A.J.; Leung, D.Y.M. Host Cationic Antimicrobial Molecules Inhibit S. aureus Exotoxin Production. mSphere 2023, 8, e0057622. [Google Scholar] [CrossRef]
- Braian, C.; Hogea, V.; Stendahl, O. Mycobacterium tuberculosis- induced neutrophil extracellular traps activate human macrophages. J. Innate Immun. 2013, 5, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.D.; Nascimento, M.T.; Decote-Ricardo, D.; Côrte-Real, S.; Morrot, A.; Heise, N.; Nunes, M.P.; Previato, J.O.; Mendonça-Previato, L.; DosReis, G.A.; et al. Capsular polysaccharides from Cryptococcus neoformans modulate production of neutrophil extracellular traps (NETs) by human neutrophils. Sci. Rep. 2015, 5, 8008. [Google Scholar] [CrossRef]
- Nikawa, H.; Samaranayake, L.P.; Tenovuo, J.; Pang, K.M.; Hamada, T. The fungicidal effect of human lactoferrin on Candida albicans and Candida krusei. Arch. Oral. Biol. 1993, 38, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Gazendam, R.P.; van Hamme, J.L.; Tool, A.T.; Hoogenboezem, M.; van den Berg, J.M.; Prins, J.M.; Vitkov, L.; van de Veerdonk, F.L.; van den Berg, T.K.; Roos, D.; et al. Human Neutrophils Use Different Mechanisms to Kill Aspergillus fumigatus Conidia and Hyphae: Evidence from Phagocyte Defects. J. Immunol. 2016, 196, 1272–1283. [Google Scholar] [CrossRef]
- Albrengues, J.; Shields, M.A.; Ng, D.; Park, C.G.; Ambrico, A.; Poindexter, M.E.; Upadhyay, P.; Uyeminami, D.L.; Pommier, A.; Küttner, V.; et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 2018, 361, eaao4227. [Google Scholar] [CrossRef]
- Bruschi, M.; Petretto, A.; Santucci, L.; Vaglio, A.; Pratesi, F.; Migliorini, P.; Bertelli, R.; Lavarello, C.; Bartolucci, M.; Candiano, G.; et al. Neutrophil Extracellular Traps protein composition is specific for patients with Lupus nephritis and includes methyl-oxidized αenolase (methionine sulfoxide 93). Sci. Rep. 2019, 9, 7934. [Google Scholar] [CrossRef]
- Whittall-García, L.P.; Torres-Ruiz, J.; Zentella-Dehesa, A.; Tapia-Rodríguez, M.; Alcocer-Varela, J.; Mendez-Huerta, N.; Gómez-Martín, D. Neutrophil extracellular traps are a source of extracellular HMGB1 in lupus nephritis: Associations with clinical and histopathological features. Lupus 2019, 28, 1549–1557. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, Y.; Yin, S.W.; Gao, X.J.; Shi, W.W.; Wang, Y.; Huang, X.; Wang, L.; Zou, L.Y.; Zhao, J.H.; et al. Neutrophil extracellular trap formation is associated with autophagy-related signalling in ANCA-associated vasculitis. Clin. Exp. Immunol. 2015, 180, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Guimarães-Costa, A.B.; Nascimento, M.T.; Froment, G.S.; Soares, R.P.; Morgado, F.N.; Conceição-Silva, F.; Saraiva, E.M. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc. Natl. Acad. Sci. USA 2009, 106, 6748–6753. [Google Scholar] [CrossRef]
- Grinberg, N.; Elazar, S.; Rosenshine, I.; Shpigel, N.Y. Beta-hydroxybutyrate abrogates formation of bovine neutrophil extracellular traps and bactericidal activity against mammary pathogenic Escherichia coli. Infect. Immun. 2008, 76, 2802–2807. [Google Scholar] [CrossRef]
- Halverson, T.W.; Wilton, M.; Poon, K.K.; Petri, B.; Lewenza, S. DNA is an antimicrobial component of neutrophil extracellular traps. PLoS Pathog. 2015, 11, e1004593. [Google Scholar] [CrossRef]
- Hirsch, J.G. Bactericidal action of histone. J. Exp. Med. 1958, 108, 925–944. [Google Scholar] [CrossRef]
- Ekaney, M.L.; Otto, G.P.; Sossdorf, M.; Sponholz, C.; Boehringer, M.; Loesche, W.; Rittirsch, D.; Wilharm, A.; Kurzai, O.; Bauer, M.; et al. Impact of plasma histones in human sepsis and their contribution to cellular injury and inflammation. Crit. Care 2014, 18, 543. [Google Scholar] [CrossRef]
- Allam, R.; Kumar, S.V.; Darisipudi, M.N.; Anders, H.J. Extracellular histones in tissue injury and inflammation. J. Mol. Med. 2014, 92, 465–472. [Google Scholar] [CrossRef]
- Daigo, K.; Takamatsu, Y.; Hamakubo, T. The Protective Effect against Extracellular Histones Afforded by Long-Pentraxin PTX3 as a Regulator of NETs. Front. Immunol. 2016, 7, 344. [Google Scholar] [CrossRef]
- Azzouz, L.; Cherry, A.; Riedl, M.; Khan, M.; Pluthero, F.G.; Kahr, W.H.A.; Palaniyar, N.; Licht, C. Relative antibacterial functions of complement and NETs: NETs trap and complement effectively kills bacteria. Mol. Immunol. 2018, 97, 71–81. [Google Scholar] [CrossRef]
- Bruns, S.; Kniemeyer, O.; Hasenberg, M.; Aimanianda, V.; Nietzsche, S.; Thywissen, A.; Jeron, A.; Latgé, J.P.; Brakhage, A.A.; Gunzer, M. Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathog. 2010, 6, e1000873. [Google Scholar] [CrossRef]
- Branzk, N.; Lubojemska, A.; Hardison, S.E.; Wang, Q.; Gutierrez, M.G.; Brown, G.D.; Papayannopoulos, V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014, 15, 1017–1025. [Google Scholar] [CrossRef]
- Kondori, N.; Baltzer, L.; Dolphin, G.T.; Mattsby-Baltzer, I. Fungicidal activity of human lactoferrin-derived peptides based on the antimicrobial αβ region. Int. J. Antimicrob. Agents 2011, 37, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, W.; Takase, M.; Wakabayashi, H.; Kawase, K.; Tomita, M. Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. J. Appl. Bacteriol. 1992, 73, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Okubo, K.; Kamiya, M.; Urano, Y.; Nishi, H.; Herter, J.M.; Mayadas, T.; Hirohama, D.; Suzuki, K.; Kawakami, H.; Tanaka, M.; et al. Lactoferrin Suppresses Neutrophil Extracellular Traps Release in Inflammation. EBioMedicine 2016, 10, 204–215. [Google Scholar] [CrossRef]
- Zhou, Y.; Deng, S.; Du, C.; Zhang, L.; Li, L.; Liu, Y.; Wang, Y.; Zhang, Y.; Zhu, L. Leukotriene B4-induced neutrophil extracellular traps impede the clearance of Pneumocystis. Eur. J. Immunol. 2024, e2350779. [Google Scholar] [CrossRef]
- Narasaraju, T.; Yang, E.; Samy, R.P.; Ng, H.H.; Poh, W.P.; Liew, A.A.; Phoon, M.C.; van Rooijen, N.; Chow, V.T. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am. J. Pathol. 2011, 179, 199–210. [Google Scholar] [CrossRef]
- McNamara, P.S.; Ritson, P.; Selby, A.; Hart, C.A.; Smyth, R.L. Bronchoalveolar lavage cellularity in infants with severe respiratory syncytial virus bronchiolitis. Arch. Dis. Child 2003, 88, 922–926. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, R.; Shen, Y.; Zhao, Y.; Zhao, Z.; Fan, T.; Yang, X.; Wang, L.; Zhang, W.; Chen, C.; et al. High Levels of Circulating Cell-free DNA Are Associated with a Poor Prognosis in Patients with Severe Fever with Thrombocytopenia Syndrome. Clin. Infect. Dis. 2020, 70, 1941–1949. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, L.; Zhang, Y.; Pu, L.; Liu, J.; Li, X.; Chen, Z.; Hao, Y.; Wang, B.; Han, J.; et al. High Level of Neutrophil Extracellular Traps Correlates with Poor Prognosis of Severe Influenza A Infection. J. Infect. Dis. 2018, 217, 428–437. [Google Scholar] [CrossRef]
- Zhang, N.; Zhu, L.; Zhang, Y.; Zhou, C.; Song, R.; Yang, X.; Huang, L.; Xiong, S.; Huang, X.; Xu, F.; et al. Circulating Rather Than Alveolar Extracellular Deoxyribonucleic Acid Levels Predict Outcomes in Influenza. J. Infect. Dis. 2020, 222, 1145–1154. [Google Scholar] [CrossRef]
- Delgado-Rizo, V.; Martínez-Guzmán, M.A.; Iñiguez-Gutierrez, L.; García-Orozco, A.; Alvarado-Navarro, A.; Fafutis-Morris, M. Neutrophil Extracellular Traps and Its Implications in Inflammation: An Overview. Front. Immunol. 2017, 8, 81. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef]
- Souza, P.S.S.; Barbosa, L.V.; Diniz, L.F.A.; da Silva, G.S.; Lopes, B.R.P.; Souza, P.M.R.; de Araujo, G.C.; Pessoa, D.; de Oliveira, J.; Souza, F.P.; et al. Neutrophil extracellular traps possess anti-human respiratory syncytial virus activity: Possible interaction with the viral F protein. Virus Res. 2018, 251, 68–77. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Sun, J.; Li, K. The Formation of NETs and Their Mechanism of Promoting Tumor Metastasis. J. Oncol. 2023, 2023, 7022337. [Google Scholar] [CrossRef]
- Cools-Lartigue, J.; Spicer, J.; Najmeh, S.; Ferri, L. Neutrophil extracellular traps in cancer progression. Cell. Mol. Life Sci. 2014, 71, 4179–4194. [Google Scholar] [CrossRef]
- Okamoto, M.; Mizuno, R.; Kawada, K.; Itatani, Y.; Kiyasu, Y.; Hanada, K.; Hirata, W.; Nishikawa, Y.; Masui, H.; Sugimoto, N.; et al. Neutrophil Extracellular Traps Promote Metastases of Colorectal Cancers through Activation of ERK Signaling by Releasing Neutrophil Elastase. Int. J. Mol. Sci. 2023, 24, 1118. [Google Scholar] [CrossRef]
- Park, S.Y.; Nam, J.S. The force awakens: Metastatic dormant cancer cells. Exp. Mol. Med. 2020, 52, 569–581. [Google Scholar] [CrossRef]
- Shibue, T.; Brooks, M.W.; Weinberg, R.A. An integrin-linked machinery of cytoskeletal regulation that enables experimental tumor initiation and metastatic colonization. Cancer Cell 2013, 24, 481–498. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, L.; Li, X.; Zhuo, W. Neutrophil Extracellular Traps in Tumor Metastasis: Pathological Functions and Clinical Applications. Cancers 2021, 13, 2832. [Google Scholar] [CrossRef]
- Wada, Y.; Yoshida, K.; Tsutani, Y.; Shigematsu, H.; Oeda, M.; Sanada, Y.; Suzuki, T.; Mizuiri, H.; Hamai, Y.; Tanabe, K.; et al. Neutrophil elastase induces cell proliferation and migration by the release of TGF-alpha, PDGF and VEGF in esophageal cell lines. Oncol. Rep. 2007, 17, 161–167. [Google Scholar]
- Shabani, F.; Farasat, A.; Mahdavi, M.; Gheibi, N. Calprotectin (S100A8/S100A9): A key protein between inflammation and cancer. Inflamm. Res. 2018, 67, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Block, H.; Rossaint, J.; Zarbock, A. The Fatal Circle of NETs and NET-Associated DAMPs Contributing to Organ Dysfunction. Cells 2022, 11, 1919. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, J.M.; Le Goff, B.; Neel, A.; Maugars, Y.; Hamidou, M. NETosis: At the crossroads of rheumatoid arthritis, lupus, and vasculitis. Jt. Bone Spine 2017, 84, 255–262. [Google Scholar] [CrossRef]
- Darrah, E.; Andrade, F. NETs: The missing link between cell death and systemic autoimmune diseases? Front. Immunol. 2012, 3, 428. [Google Scholar] [CrossRef]
- Schellekens, G.A.; de Jong, B.A.; van den Hoogen, F.H.; van de Putte, L.B.; van Venrooij, W.J. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J. Clin. Investig. 1998, 101, 273–281. [Google Scholar] [CrossRef]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 2013, 5, 178ra140. [Google Scholar] [CrossRef]
- Wang, W.; Peng, W.; Ning, X. Increased levels of neutrophil extracellular trap remnants in the serum of patients with rheumatoid arthritis. Int. J. Rheum. Dis. 2018, 21, 415–421. [Google Scholar] [CrossRef]
- Ciesielski, O.; Biesiekierska, M.; Panthu, B.; Soszyński, M.; Pirola, L.; Balcerczyk, A. Citrullination in the pathology of inflammatory and autoimmune disorders: Recent advances and future perspectives. Cell. Mol. Life Sci. 2022, 79, 94. [Google Scholar] [CrossRef] [PubMed]
- Foulquier, C.; Sebbag, M.; Clavel, C.; Chapuy-Regaud, S.; Al Badine, R.; Méchin, M.C.; Vincent, C.; Nachat, R.; Yamada, M.; Takahara, H.; et al. Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum. 2007, 56, 3541–3553. [Google Scholar] [CrossRef] [PubMed]
- Herteman, N.; Vargas, A.; Lavoie, J.P. Characterization of Circulating Low-Density Neutrophils Intrinsic Properties in Healthy and Asthmatic Horses. Sci. Rep. 2017, 7, 7743. [Google Scholar] [CrossRef]
- Smith, C.K.; Kaplan, M.J. The role of neutrophils in the pathogenesis of systemic lupus erythematosus. Curr. Opin. Rheumatol. 2015, 27, 448–453. [Google Scholar] [CrossRef]
- Garcia-Romo, G.S.; Caielli, S.; Vega, B.; Connolly, J.; Allantaz, F.; Xu, Z.; Punaro, M.; Baisch, J.; Guiducci, C.; Coffman, R.L.; et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 2011, 3, 73ra20. [Google Scholar] [CrossRef]
- Martinelli, S.; Urosevic, M.; Daryadel, A.; Oberholzer, P.A.; Baumann, C.; Fey, M.F.; Dummer, R.; Simon, H.U.; Yousefi, S. Induction of genes mediating interferon-dependent extracellular trap formation during neutrophil differentiation. J. Biol. Chem. 2004, 279, 44123–44132. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Herrmann, M.; Muñoz, L.E. Clearance Deficiency and Cell Death Pathways: A Model for the Pathogenesis of SLE. Front. Immunol. 2016, 7, 35. [Google Scholar] [CrossRef]
- Jennette, J.C.; Falk, R.J.; Hu, P.; Xiao, H. Pathogenesis of antineutrophil cytoplasmic autoantibody-associated small-vessel vasculitis. Annu. Rev. Pathol. 2013, 8, 139–160. [Google Scholar] [CrossRef]
- Rutgers, A.; Sanders, J.S.; Stegeman, C.A.; Kallenberg, C.G. Pauci-immune necrotizing glomerulonephritis. Rheum. Dis. Clin. N. Am. 2010, 36, 559–572. [Google Scholar] [CrossRef]
| NET Component (Protein IDs) | Stimulus | Cellular Localization | Related Diseases or Pathogens | Reference |
|---|---|---|---|---|
| DNA | Spontaneous, PMA, LPS, A23187 | nucleus | P. aeruginosa | Mulcahy et al., 2008 Mulcahy, Charron-Mazenod, and Lewenza [31] |
| SLE | Yaniv et al., 2015 Yaniv, Twig, Shor, Furer, Sherer, Mozes, Komisar, Slonimsky, Klang, Lotan, Welt, Marai, Shina, Amital, and Shoenfeld [32] | |||
| liver metastases | Yaniv et al., 2015 Yang, Liu, Zhang, Liu, Zhou, Chen, Huang, Li, Li, Chen, Liu, Xing, Chen, Su, and Song [33] | |||
| Histone H2A (Q99878) | Spontaneous, PMA, LPS, A23187 | nucleus | E. coli and S. aureus | Doolin et al., 2020 Doolin, Amir, Duong, Rosenzweig, Urban, Bosch, Pol, Gross, and Siryaporn [34] |
| Leishmania spp. | Wang et al., 2011 Wang, Chen, Xin, Beverley, Carlsen, Popov, Chang, Wang, and Soong [35] | |||
| Histones H2B (Q16778) | Spontaneous, PMA, LPS, A23187 | nucleus | Leishmania spp. | Wang et al., 2011 Wang, Chen, Xin, Beverley, Carlsen, Popov, Chang, Wang, and Soong [35] |
| Histone H4 (P62805) | Spontaneous, PMA, LPS, A23187 | nucleus | S. aureus and Propionibacterium acnes | Lee et al., 2009 Lee, Huang, Nakatsuji, Thiboutot, Kang, Monestier, and Gallo [36] |
| IAV | Hoeksema et al., 2015 Hoeksema, Tripathi, White, Qi, Taubenberger, van Eijk, Haagsman, and Hartshorn [37] | |||
| SARS-CoV-2 | Hong et al., 2022 Hong, Yang, Zou, Bi, He, Lei, He, Li, Alu, Ren, Wang, Jiang, Zhong, Jia, Yang, Yu, Huang, Yang, Zhou, Zhao, Kuang, Wang, Wang, Chen, Luo, Zhang, Lu, Chen, Que, He, Sun, Wang, Shen, Lu, Zhao, Yang, Yang, Wang, Li, Song, Dai, Chen, Geng, Gou, Chen, Dong, Peng, Huang, Qian, Cheng, Fan, Wei, Su, Tong, Lu, Peng, and Wei [38] | |||
| LL-37 (J3KNB4) | Spontaneous, PMA, LPS, A23187 | extracellular | E. coli and S. aureus | Doolin et al., 2020 Doolin, Amir, Duong, Rosenzweig, Urban, Bosch, Pol, Gross, and Siryaporn [34] |
| C. albicans | Karkowska-Kuleta et al., 2021 Karkowska-Kuleta, Smolarz, Seweryn-Ozog, Satala, Zawrotniak, Wronowska, Bochenska, Kozik, Nobbs, Gogol, and Rapala-Kozik [39] | |||
| SLE | Lande et al., 2011 Lande, Ganguly, Facchinetti, Frasca, Conrad, Gregorio, Meller, Chamilos, Sebasigari, Riccieri, Bassett, Amuro, Fukuhara, Ito, Liu, and Gilliet [40] | |||
| IAV | Tripathi et al., 2014 Tripathi, Verma, Kim, White, and Hartshorn [41] | |||
| neutrophil elastase (P08246) | PMA, LPS, A23187 | organelle | Shigella or Yersinia | Weinrauch et al., 2002 Weinrauch, Drujan, Shapiro, Weiss, and Zychlinsky [42] |
| C. albicans | Karkowska-Kuleta et al., 2021 Karkowska-Kuleta, Smolarz, Seweryn-Ozog, Satala, Zawrotniak, Wronowska, Bochenska, Kozik, Nobbs, Gogol, and Rapala-Kozik [39] | |||
| lung adenocarcinomas | Houghton et al., 2010 Houghton, Rzymkiewicz, Ji, Gregory, Egea, Metz, Stolz, Land, Marconcini, Kliment, Jenkins, Beaulieu, Mouded, Frank, Wong, and Shapiro [43] | |||
| AVV | Futamata et al., 2018 Futamata, Masuda, Nishibata, Tanaka, Tomaru, and Ishizu [44] | |||
| RSV | Lopes et al., 2022 Lopes, da Silva, de Lima Menezes, de Oliveira, Watanabe, Porto, da Silva, and Toledo [45] | |||
| Myeloperoxidase (P05164-2) | Spontaneous, PMA, LPS, A23187 | organelle | bacteria | Daigo et al., 2012 Daigo and Hamakubo [46] |
| C. albicans | Khandagale et al., 2018 Khandagale, Lazzaretto, Carlsson, Sundin, Shafeeq, Römling, and Fadeel [47] | |||
| AVV | Kessenbrock et al., 2009 Kessenbrock, Krumbholz, Schönermarck, Back, Gross, Werb, Gröne, Brinkmann, and Jenne [48] | |||
| HIV-1 | Saitoh et al., 2012 Saitoh, Komano, Saitoh, Misawa, Takahama, Kozaki, Uehata, Iwasaki, Omori, Yamaoka, Yamamoto, and Akira [11] | |||
| Azurocidin (P20160) | Spontaneous, PMA, LPS, A23187 | cytoplasm | host defense | Daigo et al., 2012 Daigo and Hamakubo [46] |
| Cathepsin G (P08311) | Spontaneous, PMA, LPS, A23187 | nucleus, membrane, cytoplasm, extracellular | host defense | Averhoff et al., 2008 Averhoff, Kolbe, Zychlinsky, and Weinrauch [49] |
| RSV | Lopes et al., 2022 Lopes, da Silva, de Lima Menezes, de Oliveira, Watanabe, Porto, da Silva, and Toledo [45] | |||
| Myeloblastin (PR3) (P24158) | PMA, LPS, A23187 | membrane, extracellular | host defense | Averhoff et al., 2008 Averhoff, Kolbe, Zychlinsky, and Weinrauch [49] |
| AVV | Kessenbrock et al., 2009 Kessenbrock, Krumbholz, Schönermarck, Back, Gross, Werb, Gröne, Brinkmann, and Jenne [48] | |||
| RSV | Lopes et al., 2022 Lopes, da Silva, de Lima Menezes, de Oliveira, Watanabe, Porto, da Silva, and Toledo [45] | |||
| Neutrophil defensing (P59665) | PMA | extracellular | S. aureus | Schlievert et al., 2023 Schlievert, Kilgore, Beck, Yoshida, Klingelhutz, and Leung [50] |
| SLE | Lande et al., 2011 Lande, Ganguly, Facchinetti, Frasca, Conrad, Gregorio, Meller, Chamilos, Sebasigari, Riccieri, Bassett, Amuro, Fukuhara, Ito, Liu, and Gilliet [40] | |||
| IAV | Tripathi et al., 2014 Tripathi, Verma, Kim, White, and Hartshorn [41] | |||
| HIV-1 | Saitoh et al., 2012 Saitoh, Komano, Saitoh, Misawa, Takahama, Kozaki, Uehata, Iwasaki, Omori, Yamaoka, Yamamoto, and Akira [11] | |||
| Heat shock protein 72 (P08107) | Spontaneous, PMA, LPS, A23187 | nucleus, cytoplasm, extracellular | M. tuberculosis | Braian et al., 2013, Braian, Hogea and Stendahl [51] |
| Interstitial collagenase (P03956) | - | extracellular | fungi | Rocha et al., 2015 Rocha, Nascimento, Decote-Ricardo, Côrte-Real, Morrot, Heise, Nunes, Previato, Mendonça-Previato, DosReis, Saraiva, and Freire-de-Lima [52] |
| Lactotransferrin (P02788) | Spontaneous, PMA, LPS, A23187 | cytoplasm, extracellular | Candida species’ | Nikawa et al., 1993 Nikawa, Samaranayake, Tenovuo, Pang, and Hamada [53] |
| Calprotectin S100A8(P05109) S100A9(P06702) | Spontaneous, PMA, LPS, A23187 | membrane, cytoplasm, extracellular | C. albicans | Urban et al., 2006 Urban, Reichard, Brinkmann, and Zychlinsky [10] |
| A. fumigatus | Gazendam et al., 2016 Gazendam, van Hamme, Tool, Hoogenboezem, van den Berg, Prins, Vitkov, van de Veerdonk, van den Berg, Roos, and Kuijpers [54] | |||
| matrix metalloproteinase 9 (P14780) | LPS | extracellular | cancer | Albrengues et al., 2018 Albrengues, Shields, Ng, Park, Ambrico, Poindexter, Upadhyay, Uyeminami, Pommier, Küttner, Bružas, Maiorino, Bautista, Carmona, Gimotty, Fearon, Chang, Lyons, Pinkerton, Trotman, Goldberg, Yeh, and Egeblad [55] |
| α-enolase (P06733) | Spontaneous, PMA, LPS | cytoplasm | SLE | Bruschi et al., 2019 Bruschi, Petretto, Santucci, Vaglio, Pratesi, Migliorini, Bertelli, Lavarello, Bartolucci, Candiano, Prunotto, and Ghiggeri [56] |
| Annexin A1 (P04083) | Spontaneous, PMA, LPS, A23187 | cytoplasm | SLE | Bruschi et al., 2019 Bruschi, Petretto, Santucci, Vaglio, Pratesi, Migliorini, Bertelli, Lavarello, Bartolucci, Candiano, Prunotto, and Ghiggeri [56] |
| High-mobility group box 1 (P09429) | Spontaneous, PMA, LPS, A23187 | nucleus, cytoplasm | SLE | Whittall-García et al., 2019 Whittall-García, Torres-Ruiz, Zentella-Dehesa, Tapia-Rodríguez, Alcocer-Varela, Mendez-Huerta, and Gómez-Martín [57] |
| lysosomal membrane protein-2 (P13473) | - | membrane | AVV | Tang et al., 2015 Tang, Zhang, Yin, Gao, Shi, Wang, Huang, Wang, Zou, Zhao, Huang, Shan, Gounni, Wu, and Zhang [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Du, C.; Zhang, Y.; Zhu, L. Composition and Function of Neutrophil Extracellular Traps. Biomolecules 2024, 14, 416. https://doi.org/10.3390/biom14040416
Wang Y, Du C, Zhang Y, Zhu L. Composition and Function of Neutrophil Extracellular Traps. Biomolecules. 2024; 14(4):416. https://doi.org/10.3390/biom14040416
Chicago/Turabian StyleWang, Yijie, Chunjing Du, Yue Zhang, and Liuluan Zhu. 2024. "Composition and Function of Neutrophil Extracellular Traps" Biomolecules 14, no. 4: 416. https://doi.org/10.3390/biom14040416
APA StyleWang, Y., Du, C., Zhang, Y., & Zhu, L. (2024). Composition and Function of Neutrophil Extracellular Traps. Biomolecules, 14(4), 416. https://doi.org/10.3390/biom14040416







