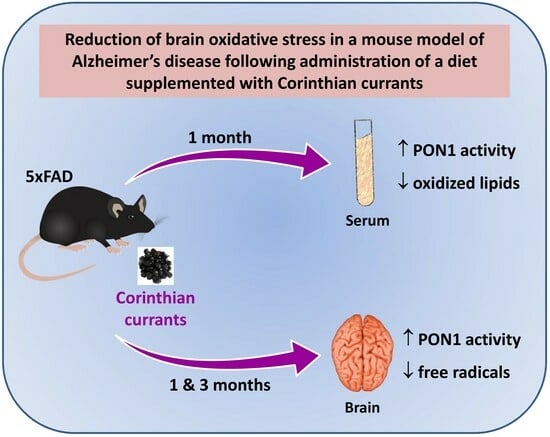
Corinthian Currants Promote the Expression of Paraoxonase-1 and Enhance the Antioxidant Status in Serum and Brain of 5xFAD Mouse Model of Alzheimer’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation of Currant Paste and Extract
2.3. Animal Study Protocol
2.4. Preparation of Cortical Homogenates
2.5. Measurement of apoA-I Levels in Serum
2.6. Measurement of Paraoxonase Activity of PON1 in Serum
2.7. Measurement of Arylesterase Activity of PON1 in Serum and Cortical Homogenates
2.8. Measurement of Malondialdehyde (MDA) Levels in Serum
2.9. Measurement of Free Radical Levels in Cortical Homogenates
2.10. Culture of Cells and Transfection Procedure
2.11. In Vitro Activation of Recombinant PON1 by Currant Extract
2.12. Detection of Cellular PON1
2.13. Cell Viability Assay
2.14. Statistical Analysis
3. Results
3.1. Effect of Age, Sex, and Currant Diet on PON1 Activity and Lipid Peroxidation Product Levels in Serum of 5xFAD Mice
3.2. Effect of Age, Sex, and Currant Diet on PON1 Activity and Free Radical Levels in the Brain of 5xFAD Mice
3.3. Effect of Currant Extract on the Activity of PON1 Secreted from Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, D.M., 3rd; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of neurodegenerative diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef] [PubMed]
- Perluigi, M.; Di Domenico, F.; Butterfield, D.A. Oxidative damage in neurodegeneration: Roles in the pathogenesis and progression of Alzheimer disease. Physiol. Rev. 2024, 104, 103–197. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Guo, J.; Ye, X.Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef]
- Arslan, J.; Jamshed, H.; Qureshi, H. Early Detection and Prevention of Alzheimer’s Disease: Role of Oxidative Markers and Natural Antioxidants. Front. Aging Neurosci. 2020, 12, 231. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Valacchi, G.; Tisato, V.; Zuliani, G.; Marsillach, J. Evaluating the link between Paraoxonase-1 levels and Alzheimer’s disease development. Minerva Med. 2019, 110, 238–250. [Google Scholar] [CrossRef]
- Gaidukov, L.; Tawfik, D.S. High affinity, stability, and lactonase activity of serum paraoxonase PON1 anchored on HDL with ApoA-I. Biochemistry 2005, 44, 11843–11854. [Google Scholar] [CrossRef]
- Furlong, C.E.; Marsillach, J.; Jarvik, G.P.; Costa, L.G. Paraoxonases-1, -2 and -3: What are their functions? Chem. Biol. Interact. 2016, 259, 51–62. [Google Scholar] [CrossRef]
- Navab, M.; Ananthramaiah, G.M.; Reddy, S.T.; Van Lenten, B.J.; Ansell, B.J.; Fonarow, G.C.; Vahabzadeh, K.; Hama, S.; Hough, G.; Kamranpour, N.; et al. The oxidation hypothesis of atherogenesis: The role of oxidized phospholipids and HDL. J. Lipid Res. 2004, 45, 993–1007. [Google Scholar] [CrossRef]
- Zuin, M.; Rosta, V.; Trentini, A.; Bosi, C.; Zuliani, G.; Cervellati, C. Paraoxonase 1 activity in patients with Alzheimer disease: Systematic review and meta-analysis. Chem. Biol. Interact. 2023, 382, 110601. [Google Scholar] [CrossRef]
- Leduc, V.; Legault, V.; Dea, D.; Poirier, J. Normalization of gene expression using SYBR green qPCR: A case for paraoxonase 1 and 2 in Alzheimer’s disease brains. J. Neurosci. Methods 2011, 200, 14–19. [Google Scholar] [CrossRef]
- Marsillach, J.; Mackness, B.; Mackness, M.; Riu, F.; Beltran, R.; Joven, J.; Camps, J. Immunohistochemical analysis of paraoxonases-1, 2, and 3 expression in normal mouse tissues. Free. Radic. Biol. Med. 2008, 45, 146–157. [Google Scholar] [CrossRef]
- Castellazzi, M.; Trentini, A.; Romani, A.; Valacchi, G.; Bellini, T.; Bonaccorsi, G.; Fainardi, E.; Cavicchio, C.; Passaro, A.; Zuliani, G.; et al. Decreased arylesterase activity of paraoxonase-1 (PON-1) might be a common denominator of neuroinflammatory and neurodegenerative diseases. Int. J. Biochem. Cell Biol. 2016, 81, 356–363. [Google Scholar] [CrossRef]
- Salazar, J.G.; Marsillach, J.; Reverte, I.; Mackness, B.; Mackness, M.; Joven, J.; Camps, J.; Colomina, M.T. Paraoxonase-1 and -3 Protein Expression in the Brain of the Tg2576 Mouse Model of Alzheimer’s Disease. Antioxidants 2021, 10, 339. [Google Scholar] [CrossRef]
- Cassidy, L.; Fernandez, F.; Johnson, J.B.; Naiker, M.; Owoola, A.G.; Broszczak, D.A. Oxidative stress in Alzheimer’s disease: A review on emergent natural polyphenolic therapeutics. Complement. Ther. Med. 2020, 49, 102294. [Google Scholar] [CrossRef]
- Venigalla, M.; Sonego, S.; Gyengesi, E.; Sharman, M.J.; Munch, G. Novel promising therapeutics against chronic neuroinflammation and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 2016, 95, 63–74. [Google Scholar] [CrossRef]
- Costa, L.G.; Giordano, G.; Furlong, C.E. Pharmacological and dietary modulators of paraoxonase 1 (PON1) activity and expression: The hunt goes on. Biochem. Pharmacol. 2011, 81, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.; Del Bo, C.; Porrini, M.; Ciappellano, S.; Riso, P. Role of polyphenols and polyphenol-rich foods in the modulation of PON1 activity and expression. J. Nutr. Biochem. 2017, 48, 1–8. [Google Scholar] [CrossRef]
- Vinson, J.A.; Su, X.; Zubik, L.; Bose, P. Phenol antioxidant quantity and quality in foods: Fruits. J. Agric. Food Chem. 2001, 49, 5315–5321. [Google Scholar] [CrossRef]
- Olmo-Cunillera, A.; Escobar-Avello, D.; Perez, A.J.; Marhuenda-Munoz, M.; Lamuela-Raventos, R.M.; Vallverdu-Queralt, A. Is Eating Raisins Healthy? Nutrients 2019, 12, 54. [Google Scholar] [CrossRef]
- Chiou, A.; Karathanos, V.T.; Mylona, A.; Salta, F.N.; Preventi, F.; Andrikopoulos, N.K. Currants (Vitis vinifera L.) content of simple phenolics and antioxidant activity. Food Chem. 2007, 102, 516–522. [Google Scholar] [CrossRef]
- Mountaki, C.; Dafnis, I.; Panagopoulou, E.A.; Vasilakopoulou, P.B.; Karvelas, M.; Chiou, A.; Karathanos, V.T.; Chroni, A. Mechanistic insight into the capacity of natural polar phenolic compounds to abolish Alzheimer’s disease-associated pathogenic effects of apoE4 forms. Free. Radic. Biol. Med. 2021, 171, 284–301. [Google Scholar] [CrossRef]
- Dafnis, I.; Mountaki, C.; Fanarioti, E.; Mastellos, D.C.; Karvelas, M.; Karathanos, V.T.; Tzinia, A.; Dermon, C.R.; Chroni, A. Temporal Pattern of Neuroinflammation Associated with a Low Glycemic Index Diet in the 5xFAD Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2022, 59, 7303–7322. [Google Scholar] [CrossRef] [PubMed]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef] [PubMed]
- Jawhar, S.; Trawicka, A.; Jenneckens, C.; Bayer, T.A.; Wirths, O. Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal Abeta aggregation in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol. Aging 2012, 33, 196.e29–196.e40. [Google Scholar] [CrossRef]
- Lazou, A.; Nikolidaki, E.; Karathanos, V.; Zogzas, N. Thermal properties of Corinthian currant pastes as affected by storage. J. Food Process. Preserv. 2020, 44, e14755. [Google Scholar] [CrossRef]
- Kountouri, A.M.; Gioxari, A.; Karvela, E.; Kaliora, A.C.; Karvelas, M.; Karathanos, V.T. Chemopreventive properties of raisins originating from Greece in colon cancer cells. Food Funct. 2013, 4, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Nikolidaki, E.K.; Chiou, A.; Christea, M.; Gkegka, A.P.; Karvelas, M.; Karathanos, V.T. Sun dried Corinthian currant (Vitis vinifera L., var. Apyrena) simple sugar profile and macronutrient characterization. Food Chem. 2017, 221, 365–372. [Google Scholar] [CrossRef]
- Dedemadi, A.-G.; Gkolfinopoulou, C.; Nikoleri, D.; Nikoloudaki, M.; Ruhanen, H.; Holopainen, M.; Kakela, R.; Christopoulou, G.; Bournazos, S.; Constantoulakis, P.; et al. Improvement of high-density lipoprotein atheroprotective properties in patients with systemic lupus erythematosus after belimumab treatment. Rheumatology 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Yagi, K. Simple assay for the level of total lipid peroxides in serum or plasma. Methods Mol. Biol. 1998, 108, 101–106. [Google Scholar] [CrossRef]
- da Costa, E.S.L.D.; Pereira, P.; Regner, G.G.; Boaretto, F.B.M.; Hoffmann, C.; Pfluger, P.; da Silva, L.L.; Steffens, L.R.; Moras, A.M.; Moura, D.J.; et al. DNA damage and oxidative stress induced by seizures are decreased by anticonvulsant and neuroprotective effects of lobeline, a candidate to treat alcoholism. Metab. Brain Dis. 2018, 33, 53–61. [Google Scholar] [CrossRef]
- Abramoff, M.D.; Magelhaes, P.J.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Link, J.C.; Chen, X.; Prien, C.; Borja, M.S.; Hammerson, B.; Oda, M.N.; Arnold, A.P.; Reue, K. Increased high-density lipoprotein cholesterol levels in mice with XX versus XY sex chromosomes. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1778–1786. [Google Scholar] [CrossRef]
- Wehner, J.M.; Murphy-Erdosh, C.; Smolen, A.; Smolen, T.N. Genetic variation in paraoxonase activity and sensitivity to diisopropylphosphofluoridate in inbred mice. Pharmacol. Biochem. Behav. 1987, 28, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Sangiovanni, E.; Fumagalli, M.; Colombo, E.; Frigerio, G.; Colombo, F.; Peres de Sousa, L.; Altindisli, A.; Restani, P.; Dell’Agli, M. Evaluation of the Anti-Inflammatory Activity of Raisins (Vitis vinifera L.) in Human Gastric Epithelial Cells: A Comparative Study. Int. J. Mol. Sci. 2016, 17, 1156. [Google Scholar] [CrossRef]
- Cosme, P.; Rodriguez, A.B.; Espino, J.; Garrido, M. Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef]
- Connelly, P.W.; Maguire, G.F.; Picardo, C.M.; Teiber, J.F.; Draganov, D. Development of an immunoblot assay with infrared fluorescence to quantify paraoxonase 1 in serum and plasma. J. Lipid Res. 2008, 49, 245–250. [Google Scholar] [CrossRef]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef]
- Bradley, M.A.; Markesbery, W.R.; Lovell, M.A. Increased levels of 4-hydroxynonenal and acrolein in the brain in preclinical Alzheimer disease. Free. Radic. Biol. Med. 2010, 48, 1570–1576. [Google Scholar] [CrossRef]
- Resende, R.; Moreira, P.I.; Proenca, T.; Deshpande, A.; Busciglio, J.; Pereira, C.; Oliveira, C.R. Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free. Radic. Biol. Med. 2008, 44, 2051–2057. [Google Scholar] [CrossRef]
- Zhu, X.; Smith, M.A.; Honda, K.; Aliev, G.; Moreira, P.I.; Nunomura, A.; Casadesus, G.; Harris, P.L.; Siedlak, S.L.; Perry, G. Vascular oxidative stress in Alzheimer disease. J. Neurol. Sci. 2007, 257, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Massaad, C.A. Neuronal and vascular oxidative stress in Alzheimer’s disease. Curr. Neuropharmacol. 2011, 9, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Gol, M.; Ghorbanian, D.; Soltanpour, N.; Faraji, J.; Pourghasem, M. Protective effect of raisin (currant) against spatial memory impairment and oxidative stress in Alzheimer disease model. Nutr. Neurosci. 2019, 22, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Garige, M.; Gong, M.; Varatharajalu, R.; Lakshman, M.R. Quercetin up-regulates paraoxonase 1 gene expression via sterol regulatory element binding protein 2 that translocates from the endoplasmic reticulum to the nucleus where it specifically interacts with sterol responsive element-like sequence in paraoxonase 1 promoter in HuH7 liver cells. Metabolism 2010, 59, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Gouedard, C.; Barouki, R.; Morel, Y. Induction of the paraoxonase-1 gene expression by resveratrol. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2378–2383. [Google Scholar] [CrossRef] [PubMed]
- Zagayko, A.L.; Kravchenko, G.B.; Krasilnikova, O.A.; Ogai, Y.O. Grape polyphenols increase the activity of HDL enzymes in old and obese rats. Oxidative Med. Cell. Longev. 2013, 2013, 593761. [Google Scholar] [CrossRef]
- Figueira, I.; Garcia, G.; Pimpao, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavares, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci. Rep. 2017, 7, 11456. [Google Scholar] [CrossRef]
- Figueira, I.; Menezes, R.; Macedo, D.; Costa, I.; Dos Santos, C.N. Polyphenols Beyond Barriers: A Glimpse into the Brain. Curr. Neuropharmacol. 2017, 15, 562–594. [Google Scholar] [CrossRef] [PubMed]
- Vasilakopoulou, P.B.; Fanarioti, E.; Tsarouchi, M.; Kokotou, M.G.; Dermon, C.R.; Karathanos, V.T.; Chiou, A. Polar phenol detection in rat brain: Development and validation of a versatile UHPLC-MS method and application on the brain tissues of Corinthian currant (Vitis vinifera L. var. Apyrena) fed rats. Food Chem. 2022, 390, 133131. [Google Scholar] [CrossRef]
- Fischer, R.; Maier, O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: Role of TNF. Oxidative Med. Cell. Longev. 2015, 2015, 610813. [Google Scholar] [CrossRef]
- Prasad, K.N. Oxidative stress and pro-inflammatory cytokines may act as one of the signals for regulating microRNAs expression in Alzheimer’s disease. Mech. Ageing Dev. 2017, 162, 63–71. [Google Scholar] [CrossRef] [PubMed]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lymperopoulos, D.; Dedemadi, A.-G.; Voulgari, M.-L.; Georgiou, E.; Dafnis, I.; Mountaki, C.; Panagopoulou, E.A.; Karvelas, M.; Chiou, A.; Karathanos, V.T.; et al. Corinthian Currants Promote the Expression of Paraoxonase-1 and Enhance the Antioxidant Status in Serum and Brain of 5xFAD Mouse Model of Alzheimer’s Disease. Biomolecules 2024, 14, 426. https://doi.org/10.3390/biom14040426
Lymperopoulos D, Dedemadi A-G, Voulgari M-L, Georgiou E, Dafnis I, Mountaki C, Panagopoulou EA, Karvelas M, Chiou A, Karathanos VT, et al. Corinthian Currants Promote the Expression of Paraoxonase-1 and Enhance the Antioxidant Status in Serum and Brain of 5xFAD Mouse Model of Alzheimer’s Disease. Biomolecules. 2024; 14(4):426. https://doi.org/10.3390/biom14040426
Chicago/Turabian StyleLymperopoulos, Dimitris, Anastasia-Georgia Dedemadi, Maria-Lydia Voulgari, Eirini Georgiou, Ioannis Dafnis, Christina Mountaki, Eirini A. Panagopoulou, Michalis Karvelas, Antonia Chiou, Vaios T. Karathanos, and et al. 2024. "Corinthian Currants Promote the Expression of Paraoxonase-1 and Enhance the Antioxidant Status in Serum and Brain of 5xFAD Mouse Model of Alzheimer’s Disease" Biomolecules 14, no. 4: 426. https://doi.org/10.3390/biom14040426
APA StyleLymperopoulos, D., Dedemadi, A.-G., Voulgari, M.-L., Georgiou, E., Dafnis, I., Mountaki, C., Panagopoulou, E. A., Karvelas, M., Chiou, A., Karathanos, V. T., & Chroni, A. (2024). Corinthian Currants Promote the Expression of Paraoxonase-1 and Enhance the Antioxidant Status in Serum and Brain of 5xFAD Mouse Model of Alzheimer’s Disease. Biomolecules, 14(4), 426. https://doi.org/10.3390/biom14040426