Cellular and Molecular Effects of Microgravity on the Immune System: A Focus on Bioactive Lipids
Abstract
1. Introduction
2. Effects of Microgravity on Inflammation
3. Bioactive Lipids and Their Involvement in Microgravity
3.1. Metabolism and Signaling of Eicosanoids, and Their Involvement in Immune Response
Eicosanoids in Microgravity
3.2. Metabolism and Signaling of Endocannabinoids, and Their Involvement in Immune Response
Endocannabinoids in Microgravity
3.3. Additional Bioactive Lipids in Microgravity
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hughes-Fulford, M. To Infinity … and beyond! Human Spaceflight and Life Science. FASEB J. 2011, 25, 2858–2864. [Google Scholar] [CrossRef] [PubMed]
- Cogoli, A. Signal Transduction in T Lymphocytes in Microgravity. Gravit. Space Biol. Bull. 1997, 10, 5–16. [Google Scholar] [PubMed]
- Cogoli, A.; Bechler, B.; Cogoli-Greuter, M.; Criswell, S.B.; Joller, H.; Joller, P.; Hunzinger, E.; Müller, O. Mitogenic Signal Transduction in T Lymphocytes in Microgravity. J. Leukoc. Biol. 1993, 53, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Cogoli, A.; Tschopp, A.; Fuchs-Bislin, P. Cell Sensitivity to Gravity. Science 1984, 225, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Engelke, K.; Ebert, R.; Müller-Deubert, S.; Rudert, M.; Ziouti, F.; Jundt, F.; Felsenberg, D.; Jakob, F. Interactions between Muscle and Bone—Where Physics Meets Biology. Biomolecules 2020, 10, 432. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wang, H.; Liu, Z.; Lin, L.; Wang, L.; Xie, M.; Li, D.; Zhang, J.; Zhang, R. Endoplasmic Reticulum Stress-Dependent Activation of iNOS/NO-NF-κB Signaling and NLRP3 Inflammasome Contributes to Endothelial Inflammation and Apoptosis Associated with Microgravity. FASEB J. 2020, 34, 10835–10849. [Google Scholar] [CrossRef] [PubMed]
- La Barbera, G.; Capriotti, A.L.; Michelini, E.; Piovesana, S.; Calabretta, M.M.; Zenezini Chiozzi, R.; Roda, A.; Laganà, A. Proteomic Analysis and Bioluminescent Reporter Gene Assays to Investigate Effects of Simulated Microgravity on Caco-2 Cells. Proteomics 2017, 17, 1700081. [Google Scholar] [CrossRef] [PubMed]
- Ludtka, C.; Moore, E.; Allen, J.B. The Effects of Simulated Microgravity on Macrophage Phenotype. Biomedicines 2021, 9, 1205. [Google Scholar] [CrossRef] [PubMed]
- Leuti, A.; Fazio, D.; Fava, M.; Piccoli, A.; Oddi, S.; Maccarrone, M. Bioactive Lipids, Inflammation and Chronic Diseases. Adv. Drug Deliv. Rev. 2020, 159, 133–169. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in Inflammation: Emergence of the pro-Resolving Superfamily of Mediators. J. Clin. Invest. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A Guiding Map for Inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Leuti, A.; Maccarrone, M. Bioactive Lipids and Chronic Inflammation: Managing the Fire Within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Panigrahy, D.; Gilligan, M.M.; Serhan, C.N.; Kashfi, K. Resolution of Inflammation: An Organizing Principle in Biology and Medicine. Pharmacol. Ther. 2021, 227, 107879. [Google Scholar] [CrossRef] [PubMed]
- Nicogossian, A.E.; Huntoon, C.L.; Pool, S.L. Space Physiology and Medicine; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1989; ISBN 978-0-8121-1162-0. [Google Scholar]
- Taylor, G.R.; Neale, L.S.; Dardano, J.R. Immunological Analyses of U.S. Space Shuttle Crewmembers. Aviat. Space Environ. Med. 1986, 57, 213–217. [Google Scholar] [PubMed]
- Grove, D.S.; Pishak, S.A.; Mastro, A.M. The Effect of a 10-Day Space Flight on the Function, Phenotype, and Adhesion Molecule Expression of Splenocytes and Lymph Node Lymphocytes. Exp. Cell Res. 1995, 219, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenberg, M.; Pippia, P.; Meloni, M.A.; Cossu, G.; Cogoli-Greuter, M.; Cogoli, A. Microgravity Simulations with Human Lymphocytes in the Free Fall Machine and in the Random Positioning Machine. J. Gravit. Physiol. 1998, 5, P23–P26. [Google Scholar] [PubMed]
- Kaur, I.; Simons, E.R.; Castro, V.A.; Ott, C.M.; Pierson, D.L. Changes in Monocyte Functions of Astronauts. Brain Behav. Immun. 2005, 19, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, B.B.; Penkala, J.E.; Vens, C.; Huls, H.; Cubbage, M.; Sams, C.F. T Cell Activation Responses Are Differentially Regulated during Clinorotation and in Spaceflight. FASEB J. 1999, 13, 2071–2082. [Google Scholar] [CrossRef]
- Tschopp, A.; Cogoli, A. Low Gravity Lowers Immunity to Disease. New Sci. 1984, 103, 36. [Google Scholar]
- Schwarzenberg, M.; Pippia, P.; Meloni, M.A.; Cossu, G.; Cogoli-Greuter, M.; Cogoli, A. Signal Transduction in T Lymphocytes--a Comparison of the Data from Space, the Free Fall Machine and the Random Positioning Machine. Adv. Space Res. 1999, 24, 793–800. [Google Scholar] [CrossRef]
- Crucian, B.E.; Cubbage, M.L.; Sams, C.F. Altered Cytokine Production by Specific Human Peripheral Blood Cell Subsets Immediately Following Space Flight. J. Interferon Cytokine Res. 2000, 20, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Buravkova, L.B.; Rykova, M.P.; Grigorieva, V.; Antropova, E.N. Cell Interactions in Microgravity: Cytotoxic Effects of Natural Killer Cells in Vitro. J. Gravit. Physiol. 2004, 11, P177–P180. [Google Scholar] [PubMed]
- Tauber, S.; Hauschild, S.; Paulsen, K.; Gutewort, A.; Raig, C.; Hürlimann, E.; Biskup, J.; Philpot, C.; Lier, H.; Engelmann, F.; et al. Signal Transduction in Primary Human T Lymphocytes in Altered Gravity during Parabolic Flight and Clinostat Experiments. Cell Physiol. Biochem. 2015, 35, 1034–1051. [Google Scholar] [CrossRef]
- Pippia, P.; Sciola, L.; Cogoli-Greuter, M.; Meloni, M.A.; Spano, A.; Cogoli, A. Activation Signals of T Lymphocytes in Microgravity. J. Biotechnol. 1996, 47, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Wang, C.; Feng, M.; Zhao, Y. Microgravity Inhibits Resting T Cell Immunity in an Exposure Time-Dependent Manner. Int. J. Med. Sci. 2014, 11, 87–96. [Google Scholar] [CrossRef]
- Cooper, D.; Pellis, N.R. Suppressed PHA Activation of T Lymphocytes in Simulated Microgravity Is Restored by Direct Activation of Protein Kinase C. J. Leukoc. Biol. 1998, 63, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Crucian, B.E.; Stowe, R.P.; Pierson, D.L.; Sams, C.F. Immune System Dysregulation Following Short- vs Long-Duration Spaceflight. Aviat. Space Environ. Med. 2008, 79, 835–843. [Google Scholar] [CrossRef]
- Taylor, G.R.; Dardano, J.R. Human Cellular Immune Responsiveness Following Space Flight. Aviat. Space Environ. Med. 1983, 54, S55–S59. [Google Scholar]
- Stervbo, U.; Roch, T.; Westhoff, T.H.; Gayova, L.; Kurchenko, A.; Seibert, F.S.; Babel, N. Repeated Changes to the Gravitational Field Negatively Affect the Serum Concentration of Select Growth Factors and Cytokines. Front. Physiol. 2019, 10, 402. [Google Scholar] [CrossRef]
- Leuti, A.; Fava, M.; Pellegrini, N.; Forte, G.; Fanti, F.; Della Valle, F.; De Dominicis, N.; Sergi, M.; Maccarrone, M. Simulated Microgravity Affects Pro-Resolving Properties of Primary Human Monocytes. Cells 2024, 13, 100. [Google Scholar] [CrossRef]
- Meloni, M.A.; Galleri, G.; Pani, G.; Saba, A.; Pippia, P.; Cogoli-Greuter, M. Space Flight Affects Motility and Cytoskeletal Structures in Human Monocyte Cell Line J-111. Cytoskeleton 2011, 68, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Tian, H.; Wang, P.; Li, L.; Zhang, Z.; Zhang, J.; Zhao, Y. Spaceflight and Simulated Microgravity Suppresses Macrophage Development via Altered RAS/ERK/NFκB and Metabolic Pathways. Cell Mol. Immunol. 2021, 18, 1489–1502. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, H.; Luo, H.; Zhu, L.; Zhao, Y.; Tian, H.; Wang, R.; Shang, P.; Zhao, Y. Microgravity Activates P38 MAPK-C/EBPβ Pathway to Regulate the Expression of Arginase and Inflammatory Cytokines in Macrophages. Inflamm. Res. 2015, 64, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Guéguinou, N.; Jeandel, J.; Kaminski, S.; Baatout, S.; Ghislin, S.; Frippiat, J.-P. Modulation of Iberian Ribbed Newt Complement Component C3 by Stressors Similar to Those Encountered during a Stay Onboard the International Space Station. Int. J. Mol. Sci. 2019, 20, 1579. [Google Scholar] [CrossRef] [PubMed]
- Talas, M.; Batkai, L.; Stoger, I.; Nagy, K.; Hiros, L.; Konstantinova, I.; Rykova, M.; Mozgovaya, I.; Guseva, O.; Kozharinov, V. Results of Space Experiment Program “Interferon”. Acta Astronaut. 1984, 11, 379–386. [Google Scholar] [CrossRef]
- Nwanaji-Enwerem, J.C.; Nwanaji-Enwerem, U.; Laan, L.V.D.; Galazka, J.M.; Redeker, N.S.; Cardenas, A. A Longitudinal Epigenetic Aging and Leukocyte Analysis of Simulated Space Travel: The Mars-500 Mission. Cell Rep. 2020, 33, 108406. [Google Scholar] [CrossRef] [PubMed]
- Infanger, M.; Kossmehl, P.; Shakibaei, M.; Baatout, S.; Witzing, A.; Grosse, J.; Bauer, J.; Cogoli, A.; Faramarzi, S.; Derradji, H.; et al. Induction of Three-Dimensional Assembly and Increase in Apoptosis of Human Endothelial Cells by Simulated Microgravity: Impact of Vascular Endothelial Growth Factor. Apoptosis 2006, 11, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Morbidelli, L.; Monici, M.; Marziliano, N.; Cogoli, A.; Fusi, F.; Waltenberger, J.; Ziche, M. Simulated Hypogravity Impairs the Angiogenic Response of Endothelium by Up-Regulating Apoptotic Signals. Biochem. Biophys. Res. Commun. 2005, 334, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, C.; Sun, S.; Zhang, C.; Lü, D.; Chen, Q.; Long, M. Microgravity-Induced Alterations of Inflammation-Related Mechanotransduction in Endothelial Cells on Board SJ-10 Satellite. Front. Physiol. 2018, 9, 1025. [Google Scholar] [CrossRef]
- Xia, M.; Nguyen, T.P.; Bao, J.-X.; Meng, N.; Boini, K.M.; Li, P.-L. Microgravity-Induced Activation of Nlrp3 Inflammasomes in Mouse Vascular Endothelial Cells. FASEB J. 2016, 30, 1204–1209. [Google Scholar] [CrossRef]
- Battista, N.; Di Tommaso, M.; Bari, M.; Maccarrone, M. The Endocannabinoid System: An Overview. Front. Behav. Neurosci. 2012, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Norris, P.C. Eicosanoid Storm in Infection and Inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.K.; Calder, P.C. Omega-6 Fatty Acids and Inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism Pathways of Arachidonic Acids: Mechanisms and Potential Therapeutic Targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, K.; Yang, L.; Liu, R.; Chu, Y.; Qin, X.; Yang, P.; Yu, H. Lipid Metabolism in Inflammation-Related Diseases. Analyst 2018, 143, 4526–4536. [Google Scholar] [CrossRef]
- Gusach, A.; Luginina, A.; Marin, E.; Brouillette, R.L.; Besserer-Offroy, É.; Longpré, J.-M.; Ishchenko, A.; Popov, P.; Patel, N.; Fujimoto, T.; et al. Structural Basis of Ligand Selectivity and Disease Mutations in Cysteinyl Leukotriene Receptors. Nat. Commun. 2019, 10, 5573. [Google Scholar] [CrossRef] [PubMed]
- Arita, M.; Ohira, T.; Sun, Y.-P.; Elangovan, S.; Chiang, N.; Serhan, C.N. Resolvin E1 Selectively Interacts with Leukotriene B4 Receptor BLT1 and ChemR23 to Regulate Inflammation1. J. Immunol. 2007, 178, 3912–3917. [Google Scholar] [CrossRef]
- Maccarrone, M.; Bab, I.; Bíró, T.; Cabral, G.A.; Dey, S.K.; Marzo, V.D.; Konje, J.C.; Kunos, G.; Mechoulam, R.; Pacher, P.; et al. Endocannabinoid Signaling at the Periphery: 50 Years after THC. Trends Pharmacol. Sci. 2015, 36, 277–296. [Google Scholar] [CrossRef]
- Yao, C.; Narumiya, S. Prostaglandin-cytokine Crosstalk in Chronic Inflammation. Br. J. Pharmacol. 2019, 176, 337–354. [Google Scholar] [CrossRef]
- Montinari, M.R.; Minelli, S.; De Caterina, R. The First 3500 years of Aspirin History from Its Roots—A Concise Summary. Vasc. Pharmacol 2019, 113, 1–8. [Google Scholar] [CrossRef]
- Maccarrone, M.; Finazzi-Agrò, A. Microgravity Increases the Affinity of Lipoxygenases for Free Fatty Acids. FEBS Lett. 2001, 489, 283. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Putti, S.; Finazzi Agro, A. Altered Gravity Modulates 5-Lipoxygenase in Human Erythroleukemia K562 Cells. J. Gravit. Physiol. 1998, 5, P97–P98. [Google Scholar] [PubMed]
- Gasperi, V.; Rapino, C.; Battista, N.; Bari, M.; Mastrangelo, N.; Angeletti, S.; Dainese, E.; Maccarrone, M. A Functional Interplay between 5-Lipoxygenase and μ-Calpain Affects Survival and Cytokine Profile of Human Jurkat T Lymphocyte Exposed to Simulated Microgravity. BioMed Res. Int. 2014, 2014, e782390. [Google Scholar] [CrossRef] [PubMed]
- Eun, J.C.; Moore, E.E.; Banerjee, A.; Kelher, M.R.; Khan, S.Y.; Elzi, D.J.; McLaughlin, N.J.D.; Silliman, C.C. Leukotriene B4 and Its Metabolites Prime the Neutrophil Oxidase and Induce Proinflammatory Activation of Human Pulmonary Microvascular Endothelial Cells. Shock 2011, 35, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Battista, N.; Meloni, M.A.; Bari, M.; Mastrangelo, N.; Galleri, G.; Rapino, C.; Dainese, E.; Agrò, A.F.; Pippia, P.; Maccarrone, M. 5-Lipoxygenase-Dependent Apoptosis of Human Lymphocytes in the International Space Station: Data from the ROALD Experiment. FASEB J. 2012, 26, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, M.; Liu, Y.; Wang, C.; Takahashi, K.; Naruse, K. Meta-Analysis-Assisted Detection of Gravity-Sensitive Genes in Human Vascular Endothelial Cells. Front. Cell Dev. Biol. 2021, 9, 689662. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hu, B.; Lv, X.; Zhu, S.; Zhen, G.; Wan, M.; Jain, A.; Gao, B.; Chai, Y.; Yang, M.; et al. Prostaglandin E2 Mediates Sensory Nerve Regulation of Bone Homeostasis. Nat. Commun. 2019, 10, 181. [Google Scholar] [CrossRef]
- Hu, B.; Lv, X.; Chen, H.; Xue, P.; Gao, B.; Wang, X.; Zhen, G.; Crane, J.L.; Pan, D.; Liu, S.; et al. Sensory Nerves Regulate Mesenchymal Stromal Cell Lineage Commitment by Tuning Sympathetic Tones. J. Clin. Invest. 2020, 130, 3483–3498. [Google Scholar] [CrossRef]
- Guo, Q.; Chen, N.; Patel, K.; Wan, M.; Zheng, J.; Cao, X. Unloading-Induced Skeletal Interoception Alters Hypothalamic Signaling to Promote Bone Loss and Fat Metabolism. Adv. Sci. 2023, 10, e2305042. [Google Scholar] [CrossRef]
- Belyavskaya, N.A. Free and Membrane-Bound Calcium in Microgravity and Microgravity Effects at the Membrane Level. Adv. Space Res. 1996, 17, 169–177. [Google Scholar] [CrossRef]
- Sun, Z.; Cao, X.; Zhang, Z.; Hu, Z.; Zhang, L.; Wang, H.; Zhou, H.; Li, D.; Zhang, S.; Xie, M. Simulated Microgravity Inhibits L-Type Calcium Channel Currents Partially by the up-Regulation of miR-103 in MC3T3-E1 Osteoblasts. Sci. Rep. 2015, 5, 8077. [Google Scholar] [CrossRef] [PubMed]
- Holowka, D.; Calloway, N.; Cohen, R.; Gadi, D.; Lee, J.; Smith, N.; Baird, B. Roles for Ca2+ Mobilization and Its Regulation in Mast Cell Functions. Front. Immunol. 2012, 3, 20565. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Di Marzo, V.; Gertsch, J.; Grether, U.; Howlett, A.C.; Hua, T.; Makriyannis, A.; Piomelli, D.; Ueda, N.; van der Stelt, M. Goods and Bads of the Endocannabinoid System as a Therapeutic Target: Lessons Learned after 30 Years. Pharmacol. Rev. 2023, 75, 885–958. [Google Scholar] [CrossRef] [PubMed]
- Scipioni, L.; Ciaramellano, F.; Carnicelli, V.; Leuti, A.; Lizzi, A.R.; De Dominicis, N.; Oddi, S.; Maccarrone, M. Microglial Endocannabinoid Signalling in AD. Cells 2022, 11, 1237. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Borroto-Escuela, D.; Angelats, E.; Etayo, Í.; Reyes-Resina, I.; Pulido-Salgado, M.; Rodríguez-Pérez, A.I.; Canela, E.I.; Saura, J.; Lanciego, J.L.; et al. Receptor-Heteromer Mediated Regulation of Endocannabinoid Signaling in Activated Microglia. Role of CB1 and CB2 Receptors and Relevance for Alzheimer’s Disease and Levodopa-Induced Dyskinesia. Brain Behav. Immun. 2018, 67, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Oddi, S.; Scipioni, L.; Maccarrone, M. Endocannabinoid System and Adult Neurogenesis: A Focused Review. Curr. Opin. Pharmacol. 2020, 50, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Leuti, A.; Smoum, R.; Mechoulam, R.; Maccarrone, M. Bioactive Lipids ALIAmides Differentially Modulate Inflammatory Responses of Distinct Subsets of Primary Human T Lymphocytes. FASEB J. 2018, 32, 5716–5723. [Google Scholar] [CrossRef]
- D’Amico, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. ALIAmides Update: Palmitoylethanolamide and Its Formulations on Management of Peripheral Neuropathic Pain. Int. J. Mol. Sci. 2020, 21, 5330. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Battistini, L.; Maccarrone, M. Endocannabinoid Signalling in Innate and Adaptive Immunity. Immunology 2015, 144, 352–364. [Google Scholar] [CrossRef]
- Buchheim, J.-I.; Matzel, S.; Rykova, M.; Vassilieva, G.; Ponomarev, S.; Nichiporuk, I.; Hörl, M.; Moser, D.; Biere, K.; Feuerecker, M.; et al. Stress Related Shift Toward Inflammaging in Cosmonauts After Long-Duration Space Flight. Front. Physiol. 2019, 10, 380818. [Google Scholar] [CrossRef]
- Sardinha, J.; Kelly, M.E.M.; Zhou, J.; Lehmann, C. Experimental Cannabinoid 2 Receptor-Mediated Immune Modulation in Sepsis. Mediat. Inflamm. 2014, 2014, 978678. [Google Scholar] [CrossRef] [PubMed]
- Gambacurta, A.; Merlini, G.; Ruggiero, C.; Diedenhofen, G.; Battista, N.; Bari, M.; Balsamo, M.; Piccirillo, S.; Valentini, G.; Mascetti, G.; et al. Human Osteogenic Differentiation in Space: Proteomic and Epigenetic Clues to Better Understand Osteoporosis. Sci. Rep. 2019, 9, 8343. [Google Scholar] [CrossRef] [PubMed]
- Battista, N.; Di Tommaso, M.; Norfini, A.; Passerai, M.; Chiurchiù, V.; Maccarrone, M.; Bari, M. Altered Anandamide Metabolism in Microgravity: The “RESLEM” Experiment. Front. Physiol. 2018, 9, 256. [Google Scholar] [CrossRef]
- Strewe, C.; Feuerecker, M.; Nichiporuk, I.; Kaufmann, I.; Hauer, D.; Morukov, B.; Schelling, G.; Choukèr, A. Effects of Parabolic Flight and Spaceflight on the Endocannabinoid System in Humans. Rev. Neurosci. 2012, 23, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Choukèr, A.; Kaufmann, I.; Kreth, S.; Hauer, D.; Feuerecker, M.; Thieme, D.; Vogeser, M.; Thiel, M.; Schelling, G. Motion Sickness, Stress and the Endocannabinoid System. PLoS ONE 2010, 5, e10752. [Google Scholar] [CrossRef]
- Masini, M.A.; Bonetto, V.; Manfredi, M.; Pastò, A.; Barberis, E.; Timo, S.; Vanella, V.V.; Robotti, E.; Masetto, F.; Andreoli, F.; et al. Prolonged Exposure to Simulated Microgravity Promotes Stemness Impairing Morphological, Metabolic and Migratory Profile of Pancreatic Cancer Cells: A Comprehensive Proteomic, Lipidomic and Transcriptomic Analysis. Cell Mol. Life Sci. 2022, 79, 226. [Google Scholar] [CrossRef]
- Nguyen, H.P.; Tran, P.H.; Kim, K.-S.; Yang, S.-G. The Effects of Real and Simulated Microgravity on Cellular Mitochondrial Function. npj Micrograv. 2021, 7, 1–11. [Google Scholar] [CrossRef]
- Li, B.-B.; Chen, Z.-Y.; Jiang, N.; Guo, S.; Yang, J.-Q.; Chai, S.-B.; Yan, H.-F.; Sun, P.-M.; Hu, G.; Zhang, T.; et al. Simulated Microgravity Significantly Altered Metabolism in Epidermal Stem Cells. Vitr. Cell. Dev. Biol. Anim. 2020, 56, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Çelen, İ.; Jayasinghe, A.; Doh, J.H.; Sabanayagam, C.R. Transcriptomic Signature of the Simulated Microgravity Response in Caenorhabditis Elegans and Comparison to Spaceflight Experiments. Cells 2023, 12, 270. [Google Scholar] [CrossRef]
- Manis, C.; Murgia, A.; Manca, A.; Pantaleo, A.; Cao, G.; Caboni, P. Untargeted Lipidomics of Erythrocytes under Simulated Microgravity Conditions. Int. J. Mol. Sci. 2023, 24, 4379. [Google Scholar] [CrossRef]
- Su, Y.-T.; Cheng, Y.-P.; Zhang, X.; Xie, X.-P.; Chang, Y.-M.; Bao, J.-X. Acid Sphingomyelinase/Ceramide Mediates Structural Remodeling of Cerebral Artery and Small Mesenteric Artery in Simulated Weightless Rats. Life Sci. 2020, 243, 117253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ouyang, F.; Meng, X.; Lodhi, A.F.; Kakar, M.U.; Li, B.; Feng, L. Screening of Lipids for Long-Term Brain Irradiation Effect Based on Non-Targeted Lipidomics. Acta Astronaut. 2023, 212, 556–567. [Google Scholar] [CrossRef]
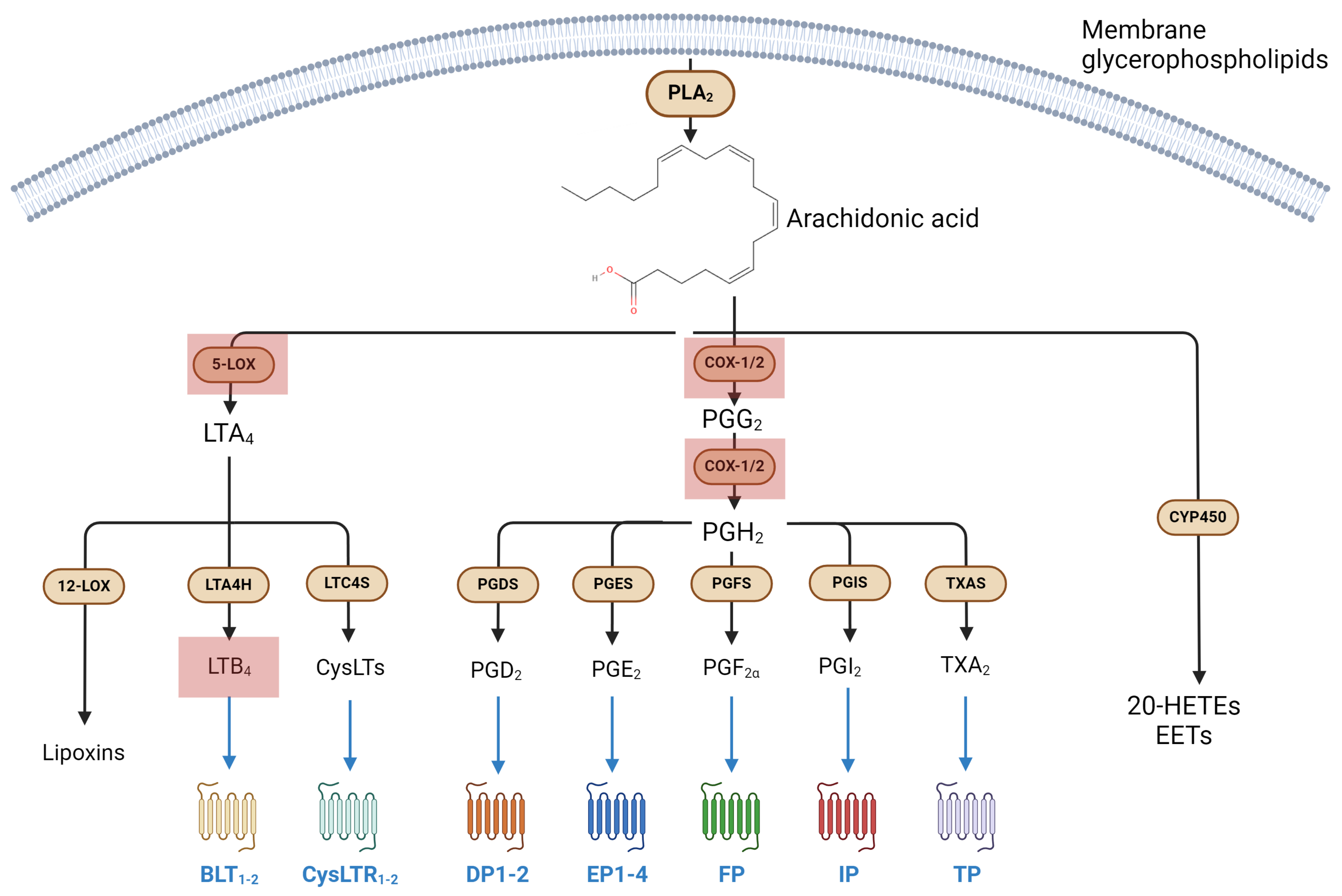
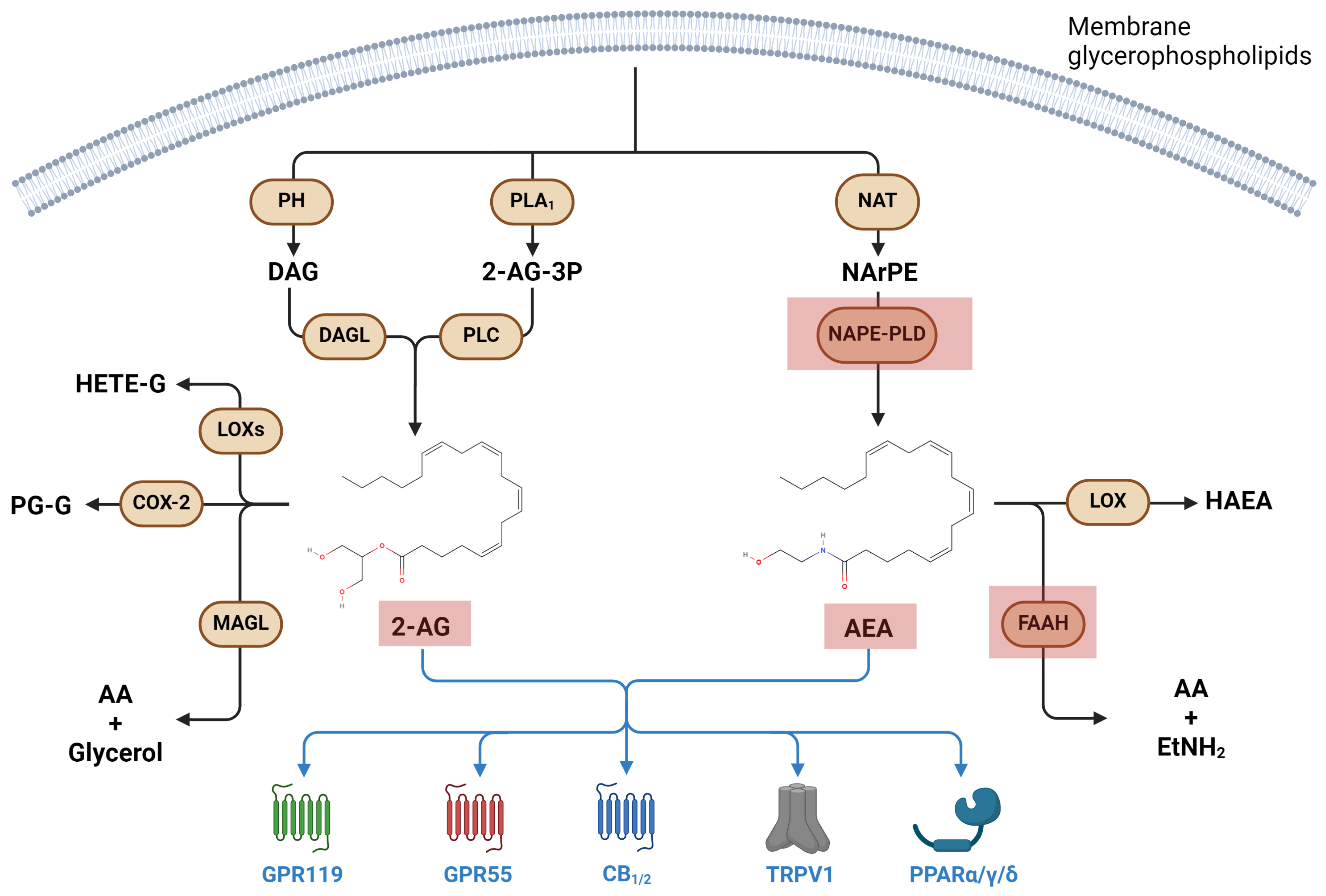
| Cellular/Molecular Target | Effect after Exposure to Microgravity | Method | Reference |
|---|---|---|---|
| Neutrophils | Increased numbers | ISS Space Shuttle flights | [71] [29] |
| Parabolic flights (30 parabolas with a duration of 20 s of microgravity) | [30] | ||
| Eosinophils | Reduced numbers | Space Shuttle flights | [29] |
| NK cells | Increased numbers | ISS | [71] [23] [36] |
| Reduced INF-γ and perforin production and activity. | |||
| B cells | Increased numbers | ISS | [71] |
| Reduced numbers | Parabolic flights (30 parabolas with a duration of 20 s of microgravity) | [30] | |
| T cells | Decreased activation and proliferation | Clinorotation Space Shuttle flights | [22] [3] [25] [19] [27] |
| ISS | [23] | ||
| Post flight immune assessment | [28] | ||
| Parabolic flights (30 parabolas with a duration of 20 s of microgravity) | [30] | ||
| Monocytes | Increased numbers | ISS | [71] [18] [32] |
| Reduced expression of both CD62L and HLA; reduced IL-6, TNFα and IL-10 after LPS stimulation; reduced phagocytosis, oxidative burst and degranulation | |||
| Cytoskeletal alterations | |||
| Hematopoietic Stem Cells | Alteration of Ras/Erk/NF-κB pathway, alteration of complement system activation and alteration of cytokine production. | RCCS (10−2 g) RCCS (10−2–10−3 g) | [33] [35] |
| Endothelial cells | Increased apoptosis, down-regulation of adhesion molecules and enhancement of the Nlrp3-dependent inflammatory cascade. | 3D clinostat SCCS | [40] [39] [38] [41] |
| Treg lymphocytes | Reduced numbers | ISS | [71] |
| COX-2 | Increased activity in K562 cells in hypergravity. Reduced activity in K562 under microgravity | Centrifugation at 22 g for 12 h | [53] |
| Clinorotation at 1 g for 12 h | [53] | ||
| 5-LOX | Reduced activity in K562 cells under microgravity | Clinorotation at 1 g for 12 h | [53] |
| Increased activity in hypergravity | Centrifugation at 22 g for 12 h | [53] | |
| Increased activity in PBMCs under authentic microgravity | ISS (ROALD 10−4–10−5 g) | [42] | |
| NAPE | Time-dependent up regulation after 48 h of microgravity aboard the ISS | ISS (RESLEM 10−4–10−5 g) | [74] |
| FAAH | Time-dependent down-regulation after 48 h of microgravity aboard the ISS | ISS (RESLEM 10−4–10−5 g) | [74] |
| AEA | Reduced levels in volunteers experiencing motion sickness | Parabolic flight | [76] |
| 2-AG | Reduced levels in volunteers experiencing motion sickness | Parabolic flight | [76] [75] |
| LTB4 | Increased synthesis | RCCS (7.2 rpm) | [54] |
| Anti-inflammatory cytokines | Reduced production | ISS (IMMUNO) | [71] |
| Pro-inflammatory cytokines | Increased production | ISS (IMMUNO) | [71] |
| Anti-apoptotic cytokines | Reduced production | RCCS (7.2 rpm) | [54] |
| Pro-apoptotic cytokines | Increased production | RCCS (7.2 rpm) | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fava, M.; De Dominicis, N.; Forte, G.; Bari, M.; Leuti, A.; Maccarrone, M. Cellular and Molecular Effects of Microgravity on the Immune System: A Focus on Bioactive Lipids. Biomolecules 2024, 14, 446. https://doi.org/10.3390/biom14040446
Fava M, De Dominicis N, Forte G, Bari M, Leuti A, Maccarrone M. Cellular and Molecular Effects of Microgravity on the Immune System: A Focus on Bioactive Lipids. Biomolecules. 2024; 14(4):446. https://doi.org/10.3390/biom14040446
Chicago/Turabian StyleFava, Marina, Noemi De Dominicis, Giulia Forte, Monica Bari, Alessandro Leuti, and Mauro Maccarrone. 2024. "Cellular and Molecular Effects of Microgravity on the Immune System: A Focus on Bioactive Lipids" Biomolecules 14, no. 4: 446. https://doi.org/10.3390/biom14040446
APA StyleFava, M., De Dominicis, N., Forte, G., Bari, M., Leuti, A., & Maccarrone, M. (2024). Cellular and Molecular Effects of Microgravity on the Immune System: A Focus on Bioactive Lipids. Biomolecules, 14(4), 446. https://doi.org/10.3390/biom14040446








