Lingering Effects of Early Institutional Rearing and Cytomegalovirus Infection on the Natural Killer Cell Repertoire of Adopted Adolescents
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Familial Resources
2.3. General Health
2.4. Blood Collection
2.5. Immune Measures
2.5.1. Complete Blood Count
2.5.2. Immunophenotyping
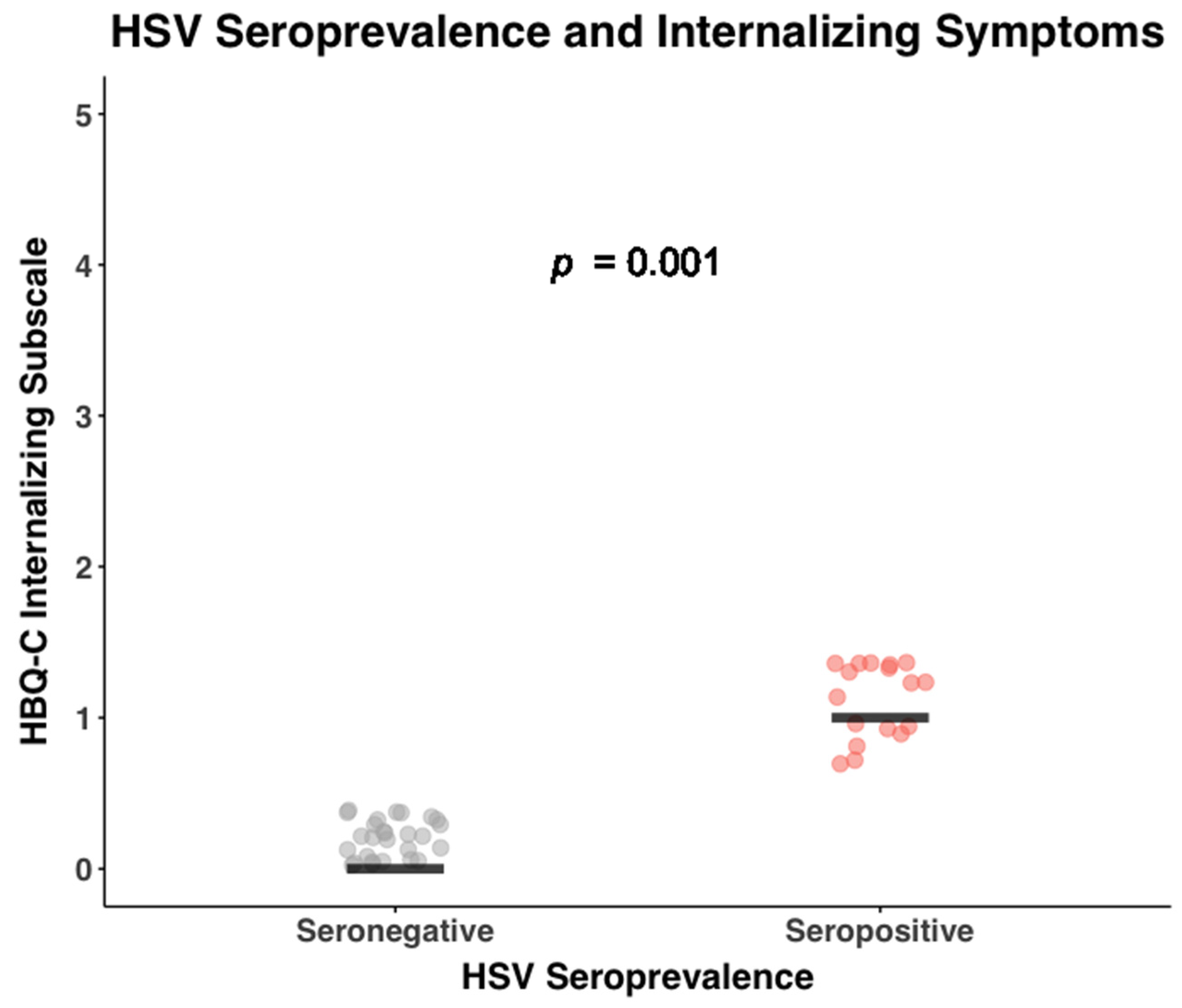
2.5.3. Cytomegalovirus (CMV) and Herpes Simplex Virus (HSV) Antibody
2.5.4. Cytokine Levels and Cell Culture Stimulation
2.5.5. C-Reactive Protein (CRP)
2.6. Data Analytic Plan
- Sample description: For the demographic and general health variables, participants from the two rearing conditions were compared with independent t-tests for continuous variables. The chi-squared test was used to evaluate differences in categorical variables, including that the representation of females and males was equivalent in both conditions.
- NK cell subsets in PI and NA adolescents: Path models were used to assess the percent of NK cells expressing CD56 at high and low density (i.e., CD56bright and CD56dim) in the blood of PI and NA adolescents. Then, differences in the percent of NK cells expressing both CD56 and CD16 surface markers were examined (i.e., CD56+CD16− or CD56+CD16+). Finally, the co-expression of NKG2C and CD57 was evaluated for each of the 5 primary NK cell subsets. All analyses took sex of the participant, time of blood collection across the morning, and participant age into consideration. The Benjamini-Hochberg (BH) procedure was used to correct for multiple comparisons. Cohen’s F2 was also calculated to interpret the effect sizes of significant differences between the two rearing conditions, where F2 values of ≥0.02, ≥0.15, and ≥0.35 reflect small, medium, and large effect sizes, respectively.
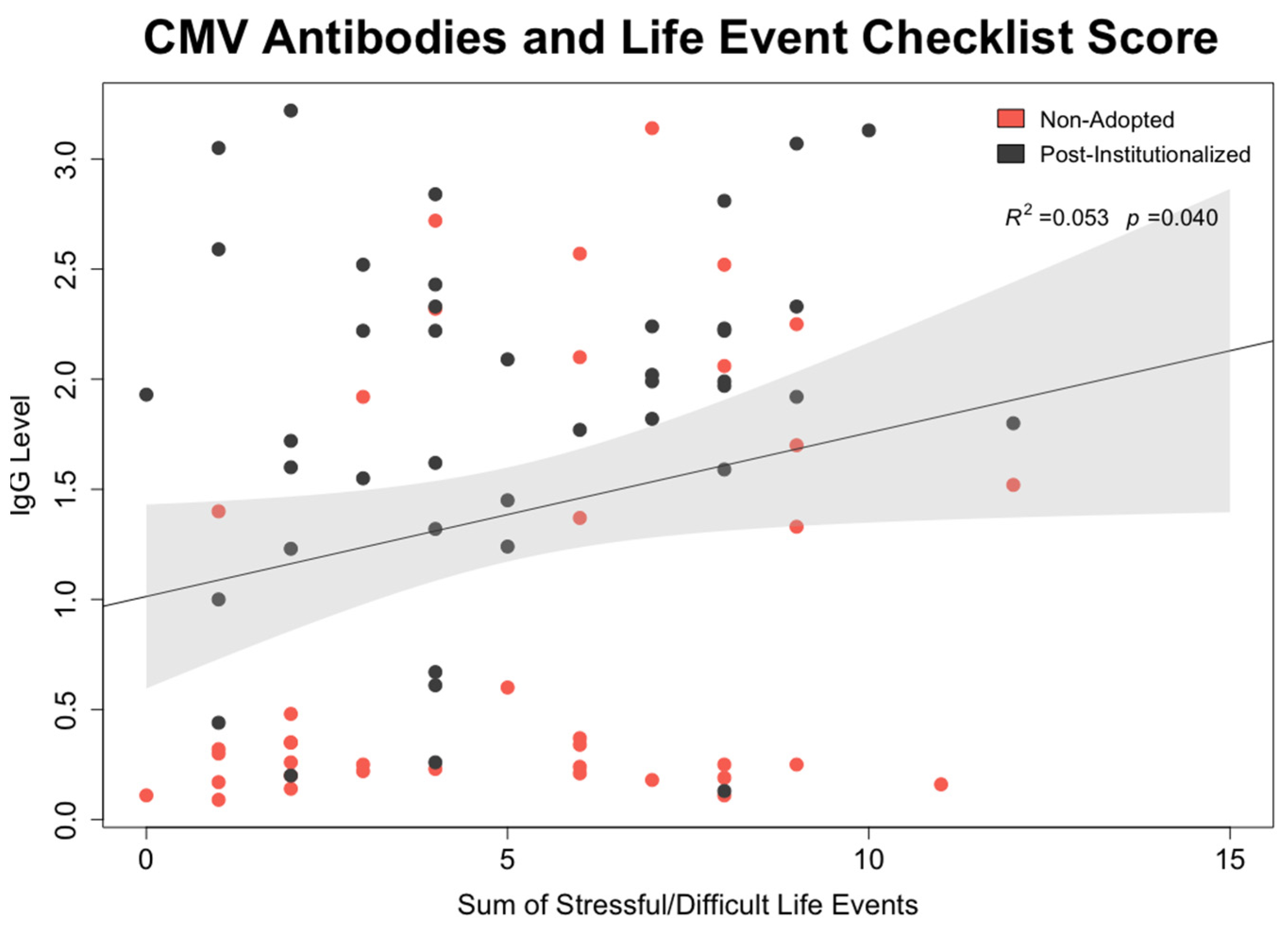
- CMV mediation of effect of early rearing on NK cell: To test whether CMV seropositivity was statistically associated with differences in NK cell percentages and expression of surface membrane molecules, path models were generated in which each NK cell lineage was simultaneously regressed on the participant’s early rearing condition and CMV antibody level. In the same model, CMV antibody titer was regressed on the two rearing conditions. Mediation was tested using the model indirect command in Mplus. Calculated estimates and accompanying confidence intervals were based on 10,000 bootstrapped replications. All models considered sex of the participant, time of blood collection during the morning, and participant age at time of assessment.
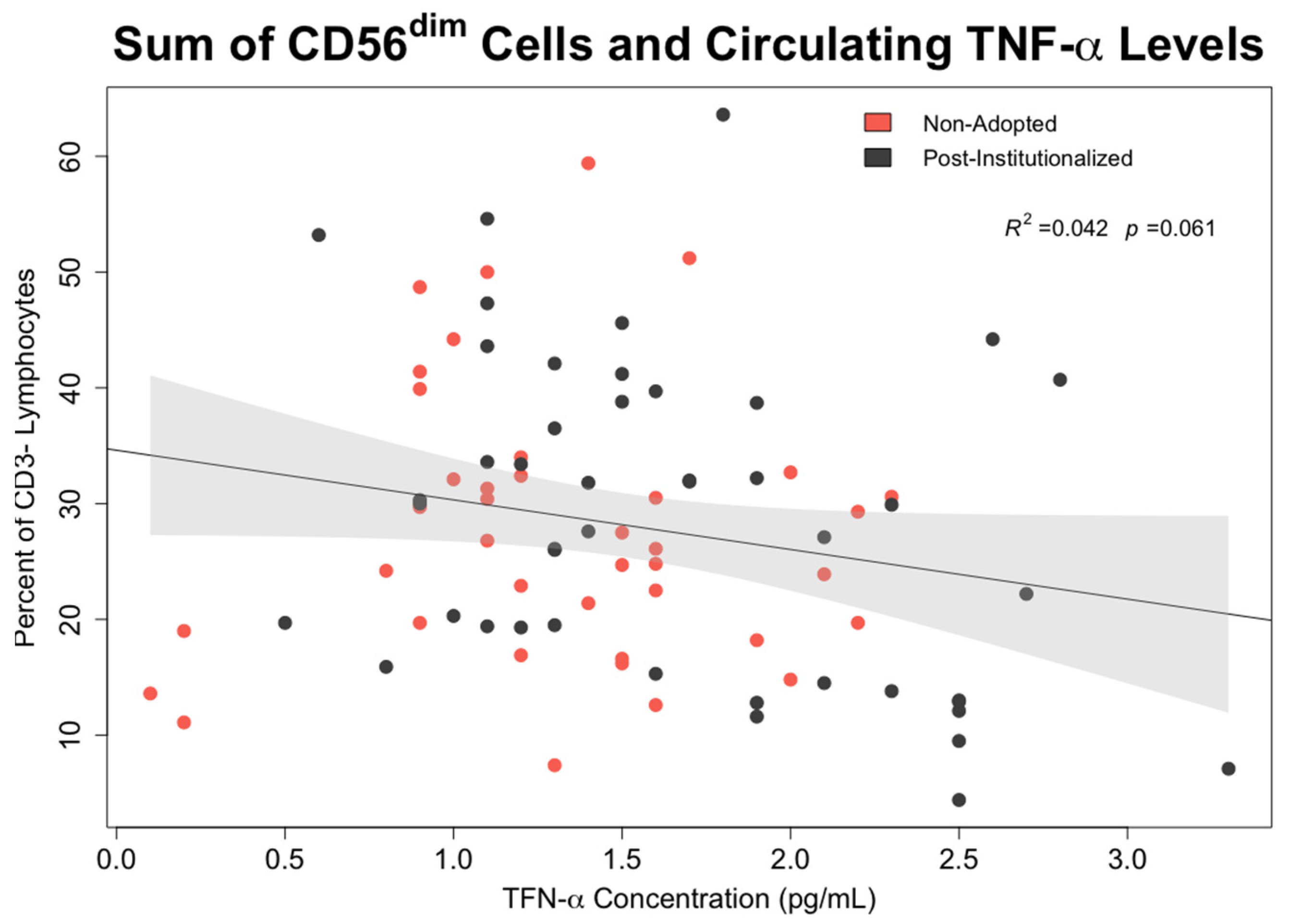
- Associations between early rearing, NK cells, and cytokine levels: The effects of early rearing on basal levels of TNF-α and IFN-γ levels as well as after in vitro stimulation with PMA/Io were then examined. To interrogate the strength of specific associations with NK measures, bivariate correlations between the two cytokines, the 5 primary NK cell subsets, and CMV antibody titer were also examined using Pearson’s test.
3. Results
3.1. Demographics
3.2. General Health
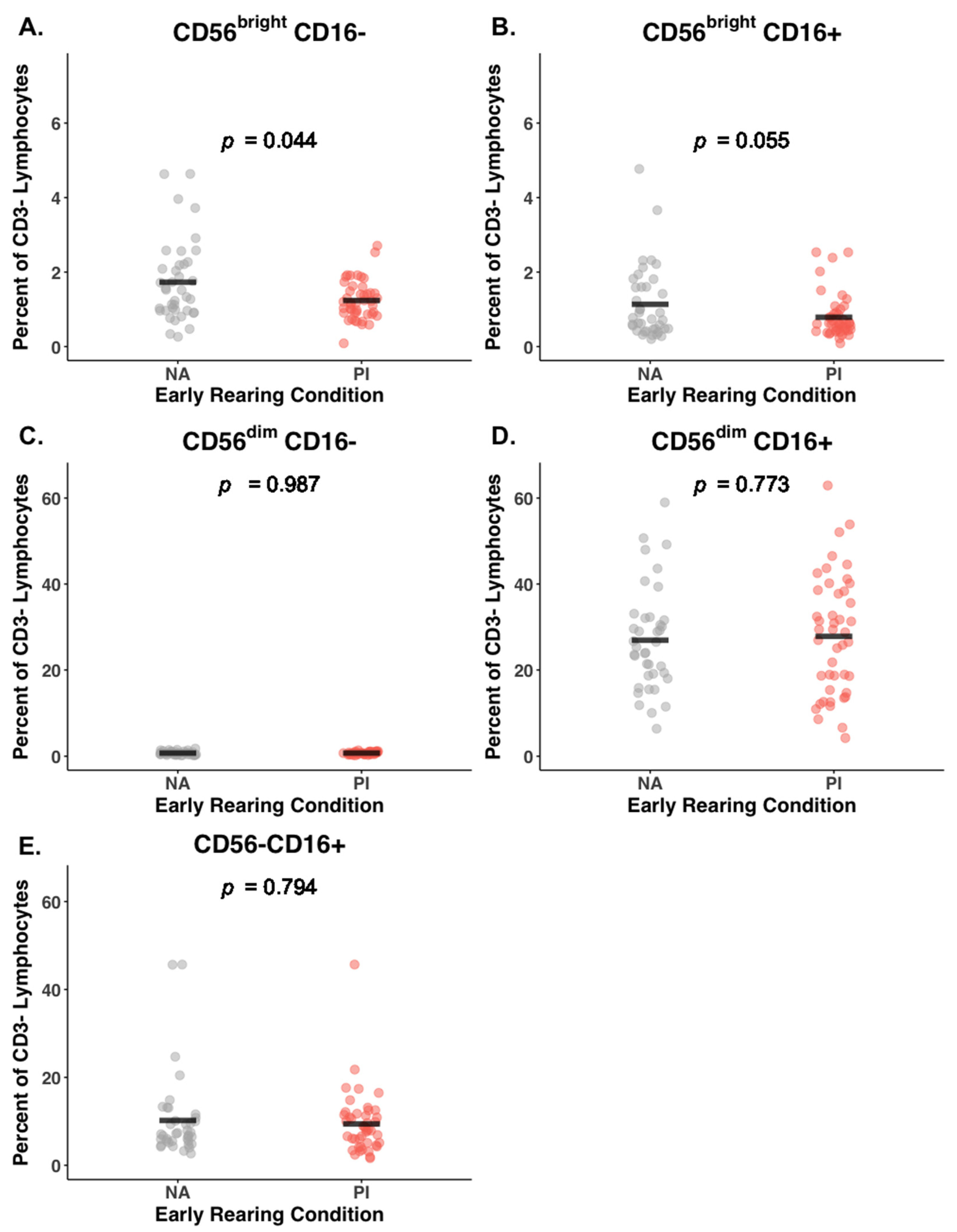
3.3. Early Rearing and NK Subsets
3.4. Effect of Early Rearing on Co-Expression of NKG2C and CD57
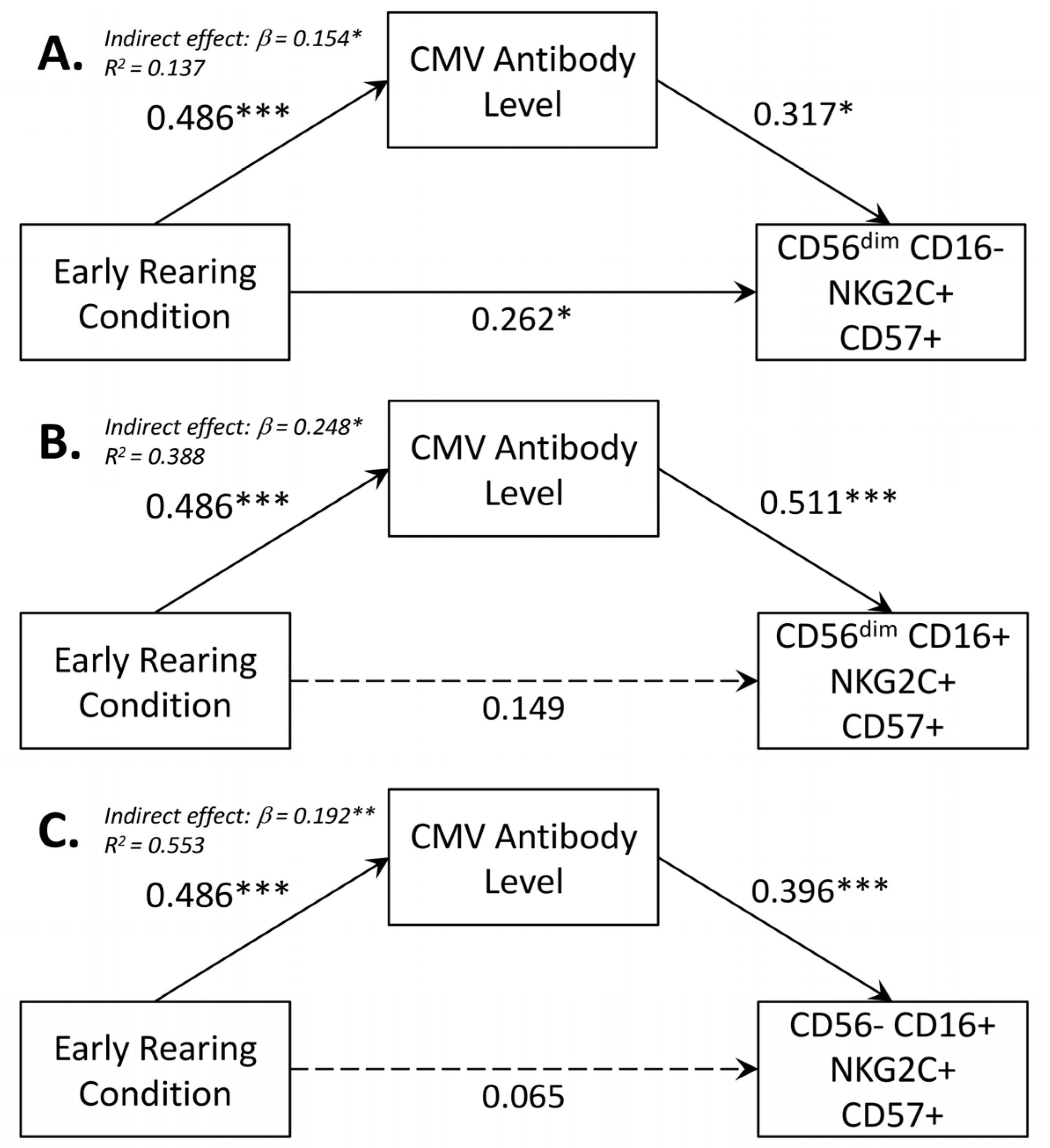
3.5. Mediating Effect of CMV Seropositivity on CD56dimCD16+ NK Cells
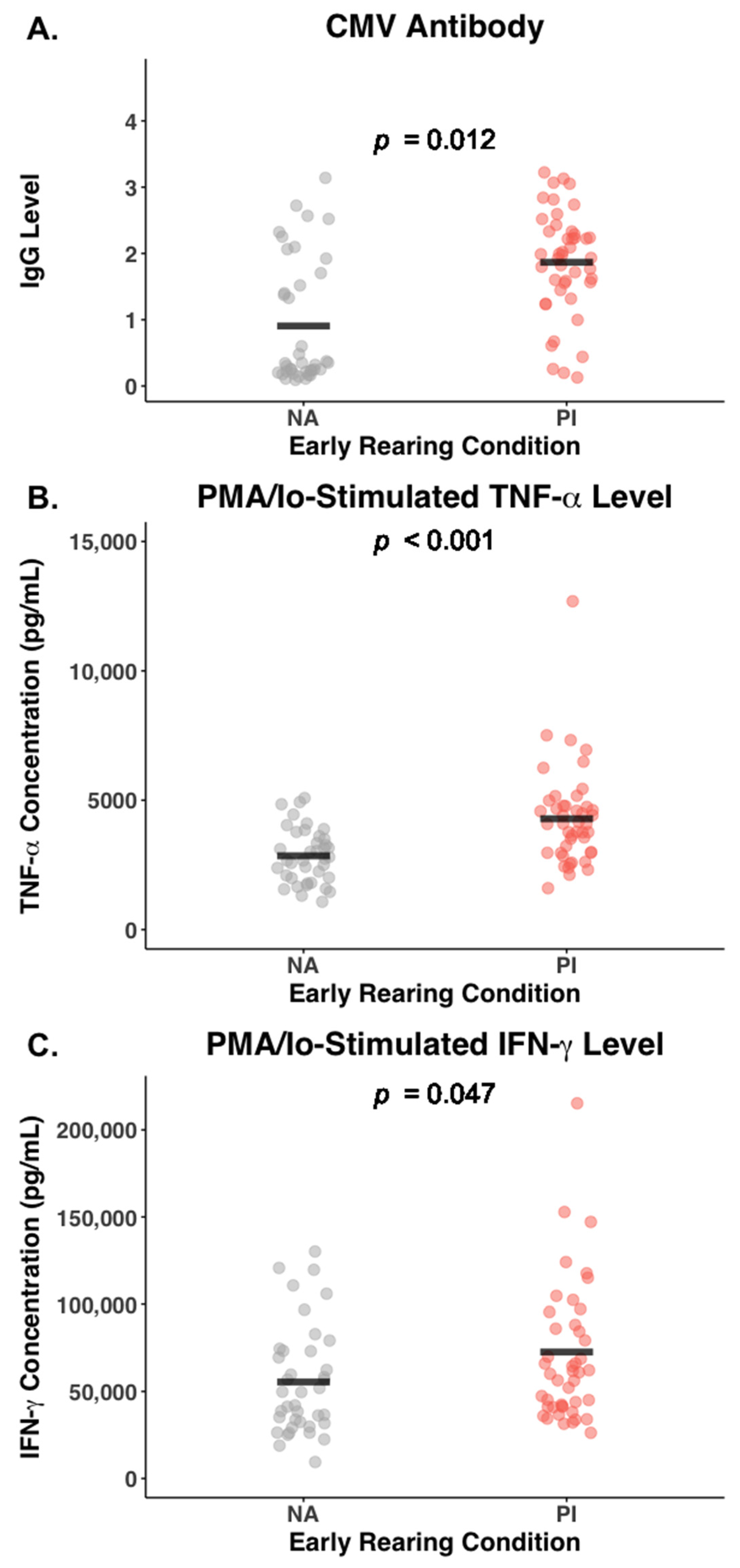
3.6. Effect of Early Rearing and CMV Status on Inflammatory Cytokines
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
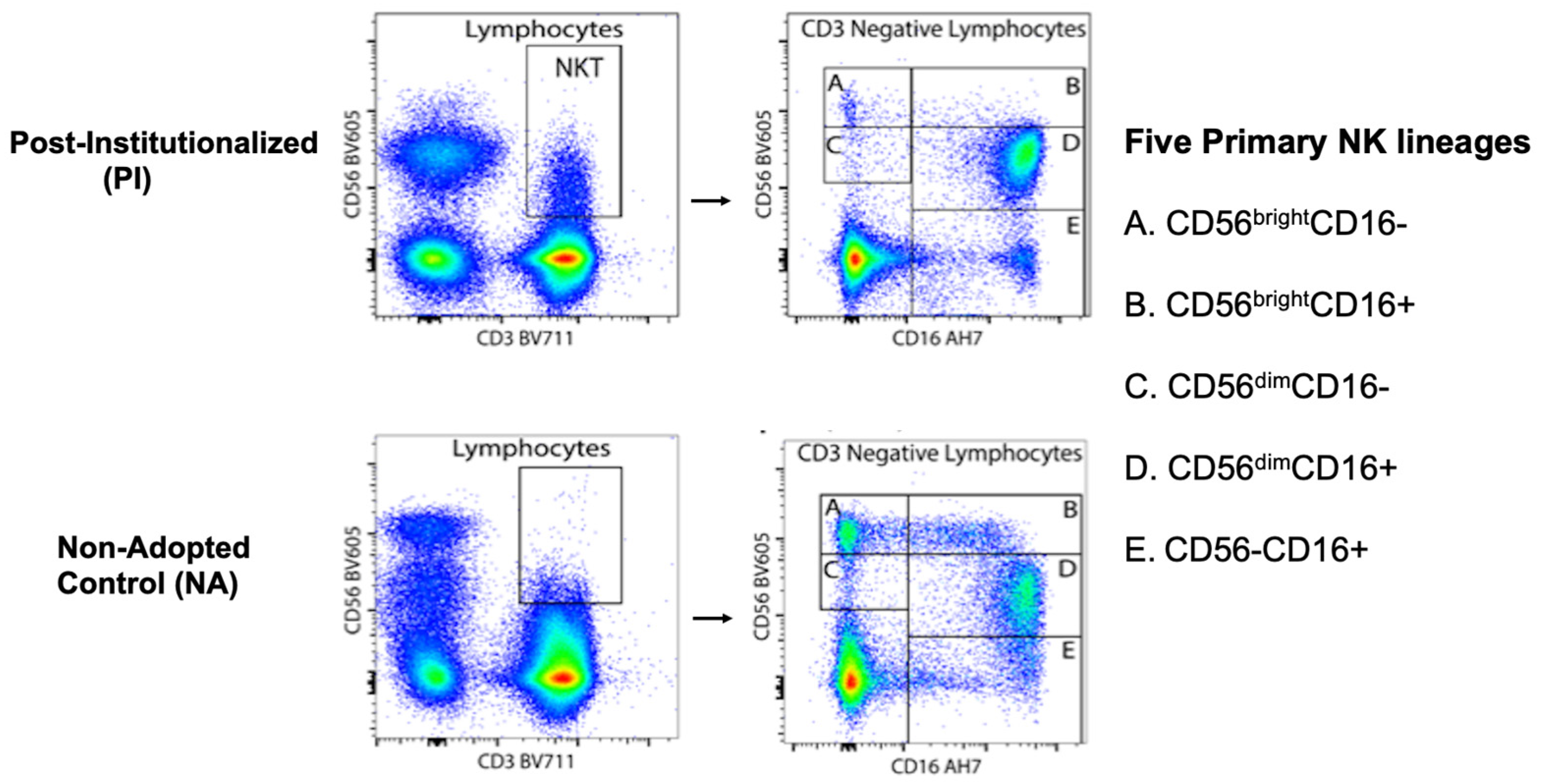

| Percent of NK Cell Subsets | ||||
|---|---|---|---|---|
| Female | Male | Sex | Sex X Rearing Interaction | |
| M (SD) | M (SD) | p | p | |
| Sum CD56 Proportion | 91.50 (4.84) | 90.85 (5.41) | 0.37 | 0.09 |
| Sum CD56bright | 2.35 (1.48) | 2.56 (1.68) | 0.36 | 0.21 |
| Sum CD56dim | 27.70 (13.12) | 28.69 (12.84) | 0.83 | 0.48 |
| CD56brightCD16− | 1.48 (0.87) | 1.45 (0.86) | 0.99 | 0.48 |
| NKG2C−CD57− | 95.02 (5.87) | 92.29 (4.61) | 0.06 | 0.80 |
| NKG2C−CD57+ | 1.18 (3.56) | 2.05 (4.57) | 0.31 | 0.67 |
| NKG2C+CD57− | 3.32 (2.39) | 4.68 (3.07) | 0.04 | 0.61 |
| NKG2C+CD57+ | 0.47 (1.36) | 0.89 (1.84) | 0.26 | 0.32 |
| CD56brightCD16+ | 0.85 (0.67) | 1.07 (0.94) | 0.14 | 0.15 |
| NKG2C−CD57− | 83.97 (11.28) | 77.73 (12.36) | 0.02 | 0.37 |
| NKG2C−CD57+ | 5.31 (5.88) | 6.79 (6.47) | 0.27 | 0.64 |
| NKG2C+CD57− | 7.83 (5.62) | 11.37 (7.32) | 0.02 | 0.95 |
| NKG2C+CD57+ | 2.86 (4.53) | 4.83 (7.71) | 0.20 | 0.06 |
| CD56dimCD16− | 0.76 (0.34) | 0.69 (0.34) | 0.44 | 0.96 |
| NKG2C−CD57− | 83.15 (11.96) | 79.22 (12.80) | 0.15 | 0.17 |
| NKG2C−CD57+ | 9.73 (9.60) | 12.16 (10.71) | 0.23 | 0.99 |
| NKG2C+CD57− | 4.18 (3.59) | 5.57 (4.22) | 0.16 | 0.11 |
| NKG2C+CD57+ | 2.43 (4.79) | 2.92 (4.49) | 0.70 | 0.11 |
| CD56dimCD16+ | 26.94 (13.10) | 28.00 (12.85) | 0.81 | 0.48 |
| NKG2C−CD57− | 39.95 (16.77) | 36.13 (15.71) | 0.35 | 0.27 |
| NKG2C−CD57+ | 47.91 (17.91) | 47.31 (14.85) | 0.93 | 0.41 |
| NKG2C+CD57− | 2.16 (2.25) | 3.02 (2.74) | 0.13 | 0.70 |
| NKG2C+CD57+ | 9.82 (16.37) | 13.54 (17.77) | 0.43 | 0.07 |
| CD56−CD16+ | 9.24 (8.74) | 10.44 (7.72) | 0.50 | 0.67 |
| NKG2C−CD57− | 79.06 (12.10) | 71.01 (13.08) | 0.007 | 0.63 |
| NKG2C−D57+ | 11.32 (9.76) | 12.19 (11.83) | 0.75 | 0.46 |
| NKG2C+CD57− | 7.28 (6.46) | 13.39 (11.55) | 0.004 | 0.24 |
| NKG2C+CD57+ | 2.30 (5.09) | 2.99 (4.76) | 0.63 | 0.53 |
| M (SD) | M (SD) | p | p | |
| WBC (103 per µL) | 6.16 (1.49) | 5.65 (1.73) | 0.06 | 0.40 |
| Lymphocyte (%) | 31.34 (8.27) | 36.14 (8.19) | 0.01 | 0.16 |
| Neutrophil (%) | 59.64 (8.65) | 51.70 (9.34) | 0.002 | 0.15 |
| NK (% of CD3−) | 14.63 (12.10) | 16.75 (8.89) | 0.40 | 0.73 |
| CMV titer | 1.41 (1.01) | 1.43 (0.97) | 0.97 | 0.97 |
| HSV titer | 0.76 (1.25) | 0.80 (1.27) | 0.98 | 0.11 |
| Blood TNF-α (pg/mL) | 1.33 (0.56) | 1.75 (0.62) | 0.005 | 0.37 |
| Blood IFN-γ (pg/mL) | 5.64 (17.67) | 4.45 (5.52) | 0.47 | 0.41 |
| PMA/Io TNF-α (ng/mL) | 3.06 (1.06) | 4.31 (2.11) | 0.001 | 0.09 |
| PMA/Io IFN-γ (ng/mL) | 52.18 (23.00) | 80.48 (50.04) | 0.02 | 0.05 |
| CRP (mg/L) | 1.72 (2.91) | 2.40 (4.42) | 0.37 | 0.39 |
References
- Cassidy, J.; Jones, J.D.; Shaver, P.R. Contributions of attachment theory and research: A framework for future research, translation and policy. Dev. Psychopathol. 2013, 25, 1415–1434. [Google Scholar] [CrossRef]
- Carlson, M.; Earls, F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Ann. N. Y. Acad. Sci. 1997, 807, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.A.; Fox, N.A.; Zeanah, C.H. Romania’s abandoned children: The effects of early profound psychosocial deprivation on the course of human development. Curr. Dir. Psych. Sci. 2023, 32, 515–521. [Google Scholar] [CrossRef]
- Miller, G.E.; Chen, E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychol. Sci. 2010, 21, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Measelle, J.R.; David, J.; Ablow, J.C. Increased levels of inflammation among infants with disorganized histories of attachment. Behav. Brain Res. 2017, 325, 260–267. [Google Scholar] [CrossRef]
- Chiang, J.J.; Bower, J.E.; Irwin, M.R.; Taylor, S.E.; Fuligni, A.J. Adiposity moderates links from early adversity and depressive symptoms to inflammatory reactivity to acute stress during late adolescence. Brain Behav. Immun. 2017, 66, 146–155. [Google Scholar] [CrossRef]
- Danese, A.; Moffitt, T.; Harrington, H.; Milne, B.J.; Polanczyk, G.; Pariante, C.M.; Poulton, R. Caspi, Adverse childhood experiences and adult risk factors for age-related disease: Depression, inflammation, and clustering of metabolic risk markers. Arch. Pediatr. Adolesc. Med. 2009, 163, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Gunnar, M.R., IV. Growth failure in institutionalized children. Mongr. Soc. Res. Child Dev. 2011, 76, 92–126. [Google Scholar] [CrossRef]
- Danese, A.; Pariante, C.M.; Caspi, A.; Taylor, A.; Poulton, R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc. Natl. Acad. Sci. USA 2007, 104, 1319–1324. [Google Scholar] [CrossRef]
- Chen, E.; Miller, G.E.; Kobor, M.S.; Cole, S.W. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Mol. Psychiatr. 2011, 16, 729–737. [Google Scholar] [CrossRef]
- Lippold, M.; Davis, K.D.; McHale, S.M.; Buxton, O.; Almeida, D.M. Daily stressor reactivity during adolescence: The buffering role of parental warmth. Health Psych. 2016, 35, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; Cohen, S.; Russo, S.J.; Dinan, T.G. Resilience and immunity. Brain Behav. Immun. 2018, 74, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Masten, A.S.; Cicchetti, D. Resilience in development: Progress and Transformation. In Developmental Psychopathology, 3rd ed.; Cicchetti, D., Ed.; Wiley: New York, NY, USA, 2016; Volume 4, pp. 271–333. [Google Scholar]
- Ungar, M. Designing resilience research: Using multiple methods to investigate risk exposure, promotive and protective processes, and contextually relevant outcomes for children and youth. Child Abus. Negl. 2019, 96, 104098. [Google Scholar] [CrossRef] [PubMed]
- Gunnar, M.R.; DePasquale, C.E.; Reid, B.M.; Donzella, B.; Miller, B.S. Pubertal stress recalibration reverses the effects of early life stress in post-institutionalized children. Proc. Natl. Acad. Sci. USA 2019, 16, 48. [Google Scholar] [CrossRef]
- Mposhi, A.; Turner, J.D. How can early life adversity still exert an effect decades later? A question of timing, tissues and mechanisms. Front. Immunol. 2023, 12, 125533. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Slopen, N.; Nelson, C.A.; Zeanah, C.H.; Georgieff, M.K.; Fox, N. Catch-up growth, metabolic and cardiovascular risk in post-institutionalized Romanian adolescents. Pediatr. Res. 2018, 84, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.; Danese, A. Challenges in researching the immune pathways between early life adversity and psychopathology. Dev. Psychopathol. 2020, 32, 1597–1624. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.K.; Manoharan, M.S.; Lee, G.C.; McKinnon, L.R.; Meunier, J.A.; Steri, M.; Harper, N.; Fiorillo, E.; Smith, A.M.; Restrepo, M.I.; et al. Immune resilience despite inflammatory stress promotes longevity and favorable health outcomes including resistance to infection. Nat. Commun. 2023, 14, 3286. [Google Scholar] [CrossRef] [PubMed]
- Gabbay, V.; Klein, R.G.; Alonso, C.M.; Babb, J.S.; Nishawala, M.; de Jesus, G.; Hirsch, G.S.; Hottinger-Blanc, P.M.Z.; Gonzalez, C.J. Immune system dysregulation in adolescent major depressive disorder. J. Affect. Disord. 2009, 115, 177–182. [Google Scholar] [CrossRef]
- Entringer, S.; de Punder, K.; Overfeld, J.; Karaboycheva, G.; Dittrich, K.; Buss, C.; Winter, S.M.; Binder, E.B.; Heim, C. Immediate and longitudinal effects of maltreatment on systemic inflammation in young children. Dev. Psychopathol. 2020, 32, 1725–1731. [Google Scholar] [CrossRef]
- Elwenspoek, M.M.C.; Hengesch, X.; Leenen, F.A.D.; Schritz, A.; Sias, K.; Schaan, V.K.; Meriaux, S.B.; Schmitz, S.; Bonnemberger, F.; Schachinger, H.; et al. Proinflammatory T cell status associated with early life adversity. J. Immunol. 2017, 199, 4046–4055. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, D.; Akhtar, R.; Ciufolini, S.; Pariante, C.M.; Mondelli, V. Childhood trauma and adulthood inflammation: A meta-analysis of peripheral C- reactive protein, interleukin-6 and tumour necrosis factor-alpha. Mol. Psychiatr. 2016, 21, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Kuhlman, K.R.; Horn, S.R.; Chiang, J.J.; Bower, J.E. Early life adversity exposure and circulating markers of inflammation in children and adolescents: A systematic review and meta-analysis. Brain Behav. Immun. 2019, 86, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Cicchetti, D.; Handley, E.D.; Rogosch, F.A. Child maltreatment, inflammation, and internalizing symptoms: Investigating the roles of C-reactive protein, gene variation, and neuroendocrine regulation. Dev. Psychopathol. 2015, 27, 553–566. [Google Scholar] [CrossRef]
- Pereira, S.M.P.; Merkin, S.S.; Seeman, T.; Power, C. Understanding associations of early life adversities with mid-life inflammatory profiles: Evidence from UK and USA. Brain Behav. Immun. 2019, 78, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Faugendes, C.P.; Glaser, R.; Kiecolt-Glaser, J.K. Stressful life experiences and immune dysregulation across the life span. Brain Behav. Immun. 2013, 27C, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Reiche, E.M.; Morimoto, H.K.; Nunes, S.M. Stress and depression-induced immune dysfunction: Implications for the development and progression of cancer. Int. Rev. Psychiatr. 2005, 17, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Wyman, P.A.; Moynihan, J.; Eberly, S.; Cox, C.; Cross, W.; Jin, X.; Caserta, M.T. Association of family stress with natural killer cell activity and the frequency of illness in children. Arch. Pediatr. Adolesc. Med. 2007, 161, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.M.; Coe, C.L.; Doyle, C.M.; Sheerar, D.S.; Slukvina, A.; Donzella, B.; Gunnar, M.R. Persistent skewing of the T-cell profile in adolescents adopted international from institutional care. Brain Behav. Immun. 2019, 77, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Elwenspoek, M.M.C.; Kuehn, A.; Muller, C.P.; Turner, J.D. The effects of early life adversity on the immune system. Psychoneuroendocrinology 2017, 82, 140–154. [Google Scholar] [CrossRef]
- Elwenspoek, M.M.C.; Sias, K.; Hengesch, X.; Schaan, V.K.; Leenen, F.A.D.; Adams, P.; Meriaux, S.B.; Schmitz, S.; Bonnemberger, F.; Ewen, A.; et al. T cell immunosenescence after early life adversity: Association with cytomegalovirus infection. Front. Immunol. 2017, 8, 1263. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.A.; LeRoy, A.S.; Majd, M.; Chen, J.Y.; Brown, R.L.; Christian, L.M.; Fagundes, C.P. Immune and epigenetic pathways linking childhood adversity and health across the lifespan. Front. Psych. 2021, 12, 788351. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.A.; Jones, M.J.; Doom, J.R.; MacIsaac, J.L.; Gunnar, M.R.; Kobor, M.S. Differential DNA methylation in peripheral blood mononuclear cells in adolescents exposed to significant early but not later childhood adversity. Dev. Psychopath. 2016, 28, 1385–1399. [Google Scholar] [CrossRef] [PubMed]
- Copeland, W.E.; Shanahan, L.; McGinnis, E.W.; Aberg, K.A.; van den Oord, E.J.G. Early adversities accelerate epigenetic aging into adulthood: A 10-year, within-subject analysis. J. Child Psych. Psychiatr. 2022, 63, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.B.; Patil, N.D.; Meriaux, S.B.; Theresine, M.; Leenen, F.A.D.; Elwenspoek, M.M.C.; Zimmer, J.; Turner, J.D. Unbiased screening identifies functional differences in NK cells after early life psycho-social stress. Front. Immunol. 2021, 12, 674532. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.P.; Shane, A.L. Infections associated with group childcare. In Principles and Practice of Pediatric Infectious Diseases, 5th ed.; Long, S.S., Prober, C.G., Fischer, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 24–32.e3. [Google Scholar] [CrossRef]
- Turner, J.E.; Campbell, J.P.; Edwards, K.M.; Howarth, L.J.; Pawelec, G.; Aldred, S.; Moss, P.; Drayson, M.T.; Burns, V.E.; Bosch, J.A. Rudimentary signs of immunosenescence in cytomegalovirus-seropositive healthy young adults. Age 2014, 36, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.E.; Redeker, A.; Arens, R.; van Baarle, D.; van den Berg, S.P.H.; Benedict, C.A.; Čičin-Šain, L.; Hill, A.B.; Wills, M.R. CMV immune evasion and manipulation of the immune system with aging. Geroscience 2017, 39, 273–291. [Google Scholar] [CrossRef]
- Chidrawar, S.; Khan, N.; Wei, W.; McLarnon, A.; Smith, N.; Nayak, L.; Moss, P. Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals. Clin. Exp. Immunol. 2009, 155, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, H.R.; Provine, N.M.; Bowyer, G.S.; Moreira Folegatti, P.; Belij-Rammerstorfer, S.; Flaxman, A.; Makinson, R.; Hill, A.V.; Ewer, K.J.; Pollard, A.J.; et al. CMV-associated T cell and NK cell terminal differentiation does not affect immunogenicity of ChAdOx1 vaccination. JCI Insight 2022, 7, e154187. [Google Scholar] [CrossRef]
- Wolf, N.K.; Kissiov, D.U.; Raulet, D.H. Roles of natural killer cells in immunity to cancer and applications to immunotherapy. Nat. Rev. Immunol. 2023, 23, 90–105. [Google Scholar] [CrossRef]
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural killer cells: Development, maturation, and clinical utilization. Front. Immunol. 2018, 9, 1869. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Jiang, J. Balancing act: The complex role of NK cells in immune regulation. Front. Immunol. 2023, 14, 1275028. [Google Scholar] [CrossRef]
- Smith, S.L.; Kennedy, P.R.; Stacey, K.B.; Worboys, J.D.; Yarwood, A.; Seo, S.; Solloa, E.H.; Mistretta, B.; Chatterjee, S.S.; Gunaratne, P.; et al. Diversity of peripheral blood human NK cells identified by single-cell RNA sequencing. Blood Adv. 2020, 4, 1388–1406. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Munoz, L.; Perna-Barrull, D.; Villalba, A.; Rodriguez-Fernandez, S.; Ampudia, R.-M.; Tenienete-Serra, A.; Vazquez, F.; Murillo, M.; Perez, J.; Corripio, R.; et al. NK cell subsets change in partial remission and early stages of pediatric Type 1 diabetes. Front. Immunol. 2021, 11, 611522. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.B.; Vasconcelos Cordoba, B.; Pavlovsky, C.; Moiraghi, B.; Varela, A.; Custidiano, R.; Fernandez, I.; Freitas, M.J.; Ventriglia, M.V.; Bendek, G.; et al. In-depth characterization of NK cell markers from CML patients who discontinued tyrosine kinase inhibitor therapy. Front. Immunol. 2023, 14, 1241600. [Google Scholar] [CrossRef]
- Michel, T.; Poli, A.; Cuapio, A.; Briquemont, B.; Iserentant, G.; Oilert, J.; Zimmer, J. Human CD56brightNK cells: An update. J. Immunol. 2016, 196, 2923–2931. [Google Scholar] [CrossRef]
- Romagnani, C.; Juelke, K.; Falco, M.; Morandi, B.; D’Agostino, A.; Costa, R.; Ratto, G.; Forte, G.; Carrega, P.; Lui, G.; et al. CD56brightCD16− killer Ig-like receptor− NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J. Immunol. 2007, 178, 4947–4955. [Google Scholar] [CrossRef]
- Biron, C.A.; Nguyen, K.G.; Pien, G.C.; Cousens, L.P.; Salazar-Mather, T.P. Natural killer cells in antiviral defense: Function and regulation by innate cytokines. Ann. Rev. Immunol. 1999, 17, 189–220. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Kelly, J.M.; Baxter, A.G.; Korner, H.; Sedvwick, J.D. An essential role for tumor necrosis factor in natural kill cell-mediated tumor rejection in the peritoneum. J. Exp. Med. 1998, 188, 1611–1619. [Google Scholar] [CrossRef]
- Wiedemann, G.M.; Santosa, E.K.; Grassmann, S.; Sheppard, S.; Le Luduec, J.-B.; Adams, N.M.; Dang, C.; Hsu, K.C.; Sun, J.C.; Lau, C.M. Deconvoluting global cytokine signaling networks in natural killer cells. Nat. Immunol. 2021, 22, 627–638. [Google Scholar] [CrossRef]
- Béziat, V.; Duffy, D.; Quoc, S.N.; Le Garff-Tavernier, M.; Decocq, J.; Combadière, B.; Debré, P.; Vieillard, V. CD56brightCD16+ NK cells: A functional intermediate stage of NK cell differentiation. J. Immunol. 2011, 186, 6753–6761. [Google Scholar] [CrossRef]
- Lopez-Verges, S.; Milush, J.M.; Pandy, S.; York, V.A.; Arakawa-Hoyt, J.; Pircher, H.; Norris, P.J.; Nixon, D.F.; Lanier, L.L. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood 2010, 116, 3865–3874. [Google Scholar] [CrossRef]
- White, M.J.; Nielsen, C.M.; McGregor, R.H.C.; Riley, E.M.; Goodier, M.R. Differential activation of CD57-defined natural killer cell subsets during recall responses to vaccine antigens. Immunology 2013, 142, 140–150. [Google Scholar] [CrossRef]
- Chiesa, M.D.; Falco, M.; Podesta, M.; Locatelli, F.; Moretta, L.; Frassoni, F.; Moretta, A. Phenotypic and functional heterogeneity of human NK cells developing after umbilical cord blood transplantation: A role for human cytomegalovirus. Blood 2012, 119, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Verges, S.; Milush, J.M.; Schwartz, B.S.; Pando, M.J.; Jarjoura, J.; York, V.A.; Houschins, J.P.; Miller, S.; Kang, S.-M.; Norris, P.J.; et al. Expansion of a unique CD57+NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 14725–14732. [Google Scholar] [CrossRef]
- Cui, A.; Huang, T.; Li, S.; Ma, A.; Perez, J.L.; Sander, C.; Keskin, D.B.; Wu, C.J.; Fraenkel, E.; Hacohen, N. Dictionary of immune responses to cytokines at single-cell resolution. Nature 2023, 625, 377–384. [Google Scholar] [CrossRef]
- Engel, M.L.; Coe, C.L.; Reid, B.M.; Donzella, B.; Gunnar, M.R. Selective inflammatory propensities in adopted adolescents. Psychoneuroendocrinology 2021, 124, 105065. [Google Scholar] [CrossRef]
- Managhan, J.H.; Robinson, J.O.; Dodge, J.A. The children’s life events inventory. J. Psychosomat. Res. 1979, 23, 63–68. [Google Scholar] [CrossRef]
- Puskar, K.R.; Grabiak, B.R.; Ren, D. The life events of adolescents: Implications for rural school nurses. J. Rural Nurs. Health Care 2011, 11, 8–16. [Google Scholar] [CrossRef]
- Boyce, W.T.; Essex, M.J.; Woodward, H.R.; Measelle, J.R.; Ablow, J.C.; Kupfer, D.J.; MacArthur Assessment Battery Working Group. The confluence of mental, physical, social, and academic difficulties in middle childhood. I: Exploring the “headwaters” of early life morbidities. J. Am. Acad. Child Adolesc. Psychiatr. 2002, 41, 580–587. [Google Scholar] [CrossRef]
- Essex, M.J.; Boyce, W.T.; Goldstein, L.H.; Armstrong, J.M.; Kraemer, H.C.; Kupfer, D.J. The MacArthur Assessment Battery Working Group. The confluence of mental, physical, social, and academic difficulties in middle childhood. II: Developing the MacArthur Health and Behavior Questionnaire. J. Am. Acad. Child Adolesc. Psychiatr. 2002, 41, 588–603. [Google Scholar] [CrossRef] [PubMed]
- Wilk, K.L.; Loman, M.M.; Van Ryzin, M.J.; Armstrong, J.M.; Essex, M.J.; Pollak, S.D.; Gunnar, M.R. Behavioral and emotional symptoms of post-institutionalized children in middle childhood. J. Child Psychol. Psychiatry 2011, 52, 56–63. [Google Scholar] [CrossRef]
- Essex, M.J.; Kraemer, H.C.; Armstrong, J.M.; Boyce, W.T.; Goldsmith, H.H.; Klein, M.H.; Woodward, H.; Kupfer, D.J. Exploring risk factors for the emergence of children’s mental health. Arch. Gen Psych. 2006, 63, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Shirtcliff, E.A.; Essex, M.J. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Dev. Psychobiol. 2008, 50, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Moncunill, G.; Han, H.; Dobaño, C.; McElrath, M.J.; De Rosa, S.C. OMIP-024: Pan-leukocyte immunophenotypic characterization of PBMC subsets in human samples. Cytometry A 2014, 85, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, D. Trimming and winsorization. In Encyclopedia of Statistical Sciences; Kotz, S., Reid, C.B., Balakrishnan, N., Vidakovic, B., Johnson, N.L., Eds.; Wiley: Hoboken, NJ, USA, 2006; Volume 14, p. 8765. [Google Scholar]
- Aschbacher, K.; Hagan, M.; Steine, I.M.; Rivera, L.; Cole, S.; Baccarella, A.; Epel, E.S.; Lieberman, A.; Bush, N.R. Adversity in early life and pregnancy are immunologically distinct from total life adversity: Macrophage-associated phenotypes in women exposed to interpersonal violence. Translat. Psychiatr. 2021, 11, 391. [Google Scholar] [CrossRef] [PubMed]
- Schmeer, K.K.; Yoon, A. Socioeconomic status inequalities in low-grade inflammation during childhood. Arch. Dis. Child. 2016, 101, 1043–1047. [Google Scholar] [CrossRef]
- Ismail, N.I. Relative expression of receptors on uterine natural killer cells compared to peripheral blood natural killer cells. Front. Immunol. 2023, 14, 1166451. [Google Scholar] [CrossRef] [PubMed]
- Mortlock, R.D.; Wu, C.; Lake Potter, E.; Abraham, D.J.; Allan, D.S.J.; Gun Hong, S.; Roeder, M.; Dunbar, C.E. Tissue trafficking kinetics of rhesus macaque natural killer cells measured by serial intravascular staining. Front. Immunol. 2021, 12, 772332. [Google Scholar] [CrossRef]
- Ran, G.H.; Lin, Y.Q.; Tian, L.; Zhang, T.; Yan, D.M.; Yu, J.H.; Deng, Y.C. Natural killer cell homing and trafficking in tissues and tumors: From biology to application. Signal Transduct. Target. Ther. 2022, 7, 205. [Google Scholar] [CrossRef]
- Carrega, P.; Ferlazzo, G. Natural killer cell distribution and trafficking in human tissues. Front. Immunol. 2012, 3, 347. [Google Scholar] [CrossRef]
- Zhang, Y.; Wallace, D.L.; de Lara, C.M.; Ghattas, H.; Asquith, B.; Worth, A.; Griffin, G.E.; Taylor, G.P.; Tough, D.F.; Beverley, P.C.; et al. In vivo kinetics of human natural killer cells: The effects of ageing and acute and chronic viral infection. Immunology 2007, 121, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Huenecke, S.; Cappel, C.; Esser, R.; Pfirrmann, V.; Salzmann-Marique, E.; Betz, S.; Keitl, E.; Banisharif-Dehkordi, J.; Bakhtiar, S.; Konigs, C.; et al. Development of three different NK subpopulations during immune reconstitution after pediatric allogeneic hematopoietic stem cell transplantation: Prognostic markers in GvHD and viral infections. Front. Immunol. 2017, 8, 109. [Google Scholar] [CrossRef]
- Heath, J.; Newhook, N.; Comeau, E.; Gallant, M.; Fudge, N.; Grant, M. NKG2C(+)CD57(+) Natural killer cell expansion parallels cytomegalovirus-specific CD8(+) T cell evolution towards senescence. J. Immunol. Res. 2016, 7470124, 8. [Google Scholar] [CrossRef]
- Nielsen, C.M.; White, M.J.; Goodier, M.R.; Riley, E.M. Functional significance of CD57 expression on human NK cells and relevance to disease. Front. Immunol. 2013, 4, 422. [Google Scholar] [CrossRef]
- Kared, H.; Martelli, S.; Ng, T.P.; Pender, S.L.F.; Larbi, A. CD57 in human natural killer cells and T-lymphocytes. Cancer Immunol. Immunother. 2016, 65, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Judge, S.J.; Murphy, W.J.; Canter, R.J. Characterizing the dysfunctional NK cell: Assessing the clinical relevance of exhaustion, anergy, and senescence. Front. Cell Infect. Microbiol. 2020, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Kazubowska, L.; Foerster, J.; Schetz, D.; Kmiec, Z. CD56bright cells respond to stimulation until very advanced age revealing increased expression of cellular protective proteins SIRT1, HSP70 and SOD2. Immun. Aging 2018, 15, 31. [Google Scholar] [CrossRef]
- Hostetter, M.K.; Iverson, S.; Dole, K.; Johnson, D. Unsuspected infectious diseases and other medical diagnoses in the evaluation of internationally adopted children. Pediatrics 1989, 83, 559–564. [Google Scholar] [CrossRef]
- Coe, C.L.; Lubach, G.R. Critical periods of special health relevance for psychoneuroimmunology. Brain. Beh. Immun. 2003, 17, 3–12. [Google Scholar] [CrossRef]
- Schleifer, S.J.; Bartlett, J.A.; Keller, S.E.; Eckholdt, H.M.; Shiflett, S.C.; Delaney, B.R. Immunity in adolescents with major depression. J. Am. Acad. Child Adolesc. Psychiatr. 2002, 41, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.A.; Schleifer, S.J.; Demetrikopoulos, M.K.; Keller, S.E. Immune differences in children with and without depression. Biol. Psychiatr. 1995, 38, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Shain, B.N.; Kronfol, Z.; Naylor, M.; Goel, K.; Evans, T.; Schaefer, S. Natural killer cell activity in adolescents with major depression. Biol. Psychiatr. 1992, 29, 481–484. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chiang, J.J.; Lam, P.H.; Chen, E.; Miller, G.E. Psychological stress during childhood and adolescence and its association with inflammation across the lifespan: A critical review and meta-analysis. Psych. Bull. 2022, 148, 27–66. [Google Scholar] [CrossRef]
- Slopen, N.; Loucks, E.B.; Appleton, A.A.; Kawachi, I.; Kubzansky, L.D.; Non, A.L.; Buka, S.; Gilman, S.E. Early origins of inflammation: An examination of prenatal and childhood social adversity in a prospective cohort study. Psychoneuroendocrinology 2015, 51, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Amand, M.; Iserentant, G.; Poli, A.; Sleiman, M.; Fievez, V.; Sanchez, I.P.; Sauvageot, N.; Michel, T.; Aouali, N.; Janji, B.; et al. Human CD56dimCD16dim cells as an individualized natural killer cell subset. Front. Immunol. 2017, 8, 699. [Google Scholar] [CrossRef]
- Forconi, C.S.; Oduor, C.I.; Oluoch, P.O.; Ong-echa, J.M.; Munz, C.; Bailey, J.A.; Moormann, A.M. A new hope for CD56negCD16pos NK cells as unconventional cytotoxic mediators: An adaptation to chronic diseases. Front. Cell Infect. Microbio. 2020, 10, 162. [Google Scholar] [CrossRef]
- Shirtcliff, E.; Coe, C.L.; Pollack, S.D. Early childhood stress is associated with elevated antibody levels to Herpes simplex virus type 1. Proc. Natl. Acad. Sci. USA 2009, 106, 2962–2967. [Google Scholar] [CrossRef]
- Glaser, R.; Kiecolt-Glaser, J.K. Chronic stress modulates the virus-specific immune response to latent herpes simplex virus Type 1. Ann. Behav Med. 1997, 19, 78–82. [Google Scholar] [CrossRef]
- Nelson, C.A.; Zeanah, C.H.; Fox, N.A. How early experience shapes human development: The case of psychosocial deprivation. Neural Plast. 2019, 2019, 1676285. [Google Scholar] [CrossRef]
- Herzberg, M.P.; Gunnar, M.R. Early life stress and brain function: Activity and connectivity associated with processing emotion and reward. Neuroimage 2020, 209, 116493. [Google Scholar] [CrossRef] [PubMed]
- Takatsuru, Y.; Miyagawa, K. Editorial: Chronic effects on brain development induced by early life stress. Front. Neurosci. 2023, 17, 1293325. [Google Scholar] [CrossRef] [PubMed]
- do Prado, C.H.; Grassi-Oliveira, R.; Daruy-Filho, L.; Wieck, A.; Bauer, M.E. Evidence for immune activation and resistance to glucocorticoids following childhood maltreatment in adolescents without psychopathology. Neuropsychopharmacology 2017, 42, 2272–2282. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Liu, Y.; Guo, D.; Lin, W.J.; Tang, Y. Natural killer cells in the central nervous system. Cell Commun. Signal 2023, 21, 341. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.-D.; Ransohoff, R.M. Natural killer cells in the central nervous system. In Natural Killer Cells; Lotze, M.T., Thomson, A.W., Eds.; Academic Press: Cambridge, MA, USA, 2010; Chapter 28; pp. 373–383. [Google Scholar] [CrossRef]
- Rodriguez-Martin, E.; Picon, C.; Costa-Frossard, L.; Alenda, R.; Saniz de la Maza, S.; Roldan, E.; Espino, M.; Villar, L.M.; Alvarez-Cermeno, J.C. Natural killer cell subsets in cerebrospinal fluid of patients with multiple sclerosis. Clin. Exp. Immunol. 2014, 180, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Shearer, W.T.; Rosenblatt, H.M.; Gelman, R.S.; Oyomopito, R.; Plaeger, S.; Stiehm, E.R.; Warra, D.W.; Douglas, S.D.; Luzuriaga, K.; McFarland, E.J.; et al. Lymphocyte subsets in healthy children from birth through 18 years of age: The Pediatric AIDS Clinical Trials Group P1009 study. J. Allerg. Clin. Immunol. 2003, 112, 973–980. [Google Scholar] [CrossRef]
- Rudy, B.J.; Wilson, C.M.; Durako, S.; Moscicki, A.-B.; Muenz, L.; Douglas, S.D. Peripheral blood lymphocyte subsets in adolescents: A longitudinal analysis from the Reach project. Clin. Diagn. Lab. Immunol. 2002, 9, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, E.G.; Meriaux, S.B.; Guebels, P.; Muller, C.P.; Leenen, F.A.D.; Elwenspoek, M.M.C.; Thiele, I.; Hertel, J.; Turner, J.D. Early-life adversity leaves its imprint on the oral microbiome for than 20 years and associated with long-term immune changes. Int. J. Mol. Sci. 2021, 22, 12682. [Google Scholar] [CrossRef] [PubMed]
- Mondelli, V.; Vernon, A.C. From early adversities to immune activation in psychiatric disorders: The role of the sympathetic nervous system. Clin. Exp. Immunol. 2019, 197, 319–328. [Google Scholar] [CrossRef]
- Gunnar, M.R.; Bowen, M. What was learned from studying the effects of early institutional deprivation. Pharmacol. Biochem. Behav. 2021, 210, 173272. [Google Scholar] [CrossRef]
- Semmes, E.C.; Chen, J.-L.; Goswami, R.; Burt, T.D.; Permar, S.R.; Fouda, G.G. Understanding early-life adaptive immunity to guide interventions for pediatric health. Front. Immunol. 2021, 11, 595297. [Google Scholar] [CrossRef] [PubMed]
- Carolyn, M.; Nielsen, M.; White, J.; Bottomley, C.; Lusa, C.; Rodríguez-Galán, A.; Turner, S.E.G.; Goodier, M.R.; Riley, E.M. Impaired NK cell responses to pertussis and H1N1 influenza vaccine antigens in human cytomegalovirus-infected individuals. J. Immunol 2015, 194, 4657–4667. [Google Scholar] [CrossRef]
- Yovel, G.; Khakhar, K.; Ben-Eliyahu, S. The effects of sex, menstrual cycle and oral contraceptives on the number and activity of natural killer cells. Gynecol. Oncol. 2001, 81, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.; Arjona, A.; Sarkar, D.K. Role of sympathetic nervous system in the entrainment of circadian natural killer cell function. Brain Beh. Immun. 2011, 25, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Eddy, J.L.; Krukovwski, K.; Janusek, L.; Mathews, H.L. Glucocorticoids regulate natural kill cell function epigenetically. Cellular Immunol. 2014, 290, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Tosato, F.; Bucciol, G.; Pantano, G.; Putti, M.C.; Sanzari, M.C.; Basso, G.; Piebani, M. Lymphocyte subsets reference values in childhood. Cytometry 2015, 87A, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Yabuhara, A.; Kawai, H.; Komiyama, A. Development of natural killer cell cytotoxicity during childhood: Marked increase in number of natural killer cells with adequate cytotoxic abilities during infancy to early childhood. Pediatr. Res. 1990, 28, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.Y.; Cornstock, S.S.; Shunk, J.M.; Monaco, M.H.; Donovan, S.M. Natural killer cell populations and cytotoxic activity in pigs fed mother’s milk, formula, or formula supplemented with bovine lactoferrin. Pediatr. Res. 2013, 74, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Coe, C.L.; Lubach, G.R.; Schneider, M.L.; Dierschke, D.J.; Ershler, W.B. Early rearing conditions alter immune responses in the developing infant primate. Pediatrics 1992, 90, 505–509. [Google Scholar] [CrossRef]
- Reid, B.M.; Desjardins, C.; Thyagarajan, B.; Linden, M.A.; Gunnar, M. Early life stress is associated with alterations in lymphocyte subsets independent of increased inflammation in adolescents. Biomolecules 2024, 14, 262. [Google Scholar] [CrossRef]

| PI (n = 45) | NA (n = 39) | p-Value 2 | |
|---|---|---|---|
| Mean age (yr) | 16.6 (2.2) | 16.0 (1.7) | 0.15 |
| Female, n (%) 1 | 25 (55.6%) | 22 (56.4%) | 0.94 |
| LEC score | 5.07 (2.95) | 5.08 (3.17) | 0.99 |
| HBQ-C score | 2.32 (0.68) | 2.17 (0.68) | 0.30 |
| Height (cm) | 163.6 (9.2) | 170.6 (10.1) | 0.001 |
| Weight (kg) | 60.5 (13.3) | 64.8 (16.7) | 0.20 |
| WBC (103 per µL) | 6.16 (1.66) | 5.87 (1.59) | 0.39 |
| Lymphocyte (%) | 34.80 (9.49) | 31.91 (7.07) | 0.07 |
| Neutrophil (%) | 54.98 (10.84) | 57.48 (8.23) | 0.14 |
| CD3− (% of Lymph) | 36.48 (12.29) | 36.03 (13.40) | 0.79 |
| NK (% of Lymph) | 15.64 (10.98) | 15.48 (10.72) | 0.92 |
| CD56bright (% of CD3−) | 2.03 (0.96) | 2.93 (1.96) | 0.008 |
| CD56dim (% of CD3−) | 28.56 (13.92) | 27.65 (11.85) | 0.75 |
| CMV titer | 1.87 (0.79) | 0.91 (0.94) | 0.001 |
| HSV titer | 0.99 (1.34) | 0.53 (1.10) | 0.06 |
| Blood TNF-α (pg/mL) | 1.75 (0.62) | 1.33 (0.56) | 0.009 |
| Blood IFN-γ (pg/mL) | 3.95 (3.73) | 6.46 (3.15) | 0.66 |
| PMA/Io TNF-α (pg/mL) | 4269.3 (1895.1) | 2847.5 (1067.5) | 0.001 |
| PMA/Io IFN-γ (pg/mL) | 72,631.1 (44,700.3) | 55,430.8 (31,183.1) | 0.047 |
| CRP (mg/L) | 2.24 (4.07) | 1.75 (3.06) | 0.64 |
| Cell Type | PI (n = 45) % | NA (n = 39) % | Rearing Coeff. 1 (SE) | 95% CI | p | Corr. p 2 | Cohen’s 3 (F2) |
|---|---|---|---|---|---|---|---|
| Sum NK (% CD3−) | |||||||
| CD56+proportion | 92.20 (0.41) | 90.00 (0.58) | 0.24 (0.10) | (0.05, 0.43) | 0.01 | 0.03 | 0.07 |
| Sum CD56bright | 2.03 (0.96) | 2.93 (1.96) | −0.28 (0.10) | (−0.46, −0.09) | 0.004 | 0.02 | 0.08 |
| Sum CD56dim | 28.56 (13.92) | 27.65 (11.85) | −0.00 (0.11) | (−0.21, 0.21) | 0.97 | 0.99 | 0.00 |
| CD56brightCD16− | 1.24 (0.51) | 1.73 (1.09) | −0.23 (0.11) | (−0.43, −0.03) | 0.02 | 0.04 | 0.06 |
| NKG2C−CD57− | 92.78 (7.82) | 95.01 (3.85) | −0.19 (0.90) | (−0.35, −0.03) | 0.02 | 0.04 | 0.04 |
| NKG2C−CD57+ | 2.12 (4.99) | 0.91 (2.43) | 0.20 (0.11) | (−0.02, 0.41) | 0.07 | 0.10 | 0.04 |
| NKG2C+CD57− | 4.01 (2.74) | 3.82 (2.85) | 0.18 (0.10) | (−0.00, 0.36) | 0.05 | 0.08 | 0.03 |
| NKG2C+CD57+ | 0.98 (2.00) | 0.27 (0.79) | 0.30 (0.10) | (0.10, 0.50) | 0.003 | 0.01 | 0.11 |
| CD56brightCD16+ | 0.79 (0.58) | 1.14 (0.98) | −0.22 (0.10) | (−0.42, −0.02) | 0.03 | 0.06 | 0.05 |
| NKG2C−CD57− | 77.97 (12.46) | 84.96 (10.06) | −0.29 (0.10) | (−0.47, −0.10) | 0.002 | 0.01 | 0.10 |
| NKG2C−CD57+ | 7.62 (7.01) | 4.04 (4.33) | 0.24 (0.09) | (0.06, 0.41) | 0.008 | 0.02 | 0.06 |
| NKG2C+CD57− | 9.46 (5.76) | 9.31 (7.58) | 0.22 (0.90) | (0.09, 0.37) | 0.006 | 0.02 | 0.05 |
| NKG2C+CD57+ | 5.52 (7.52) | 1.66 (3.09) | 0.38 (0.10) | (0.19, 0.56) | 0.000 | 0.001 | 0.17 |
| CD56dimCD16− | 0.73 (0.28) | 0.73 (0.40) | 0.05 (0.11) | (−0.16, 0.26) | 0.64 | 0.99 | 0.00 |
| NKG2C−CD57− | 78.43 (13.28) | 84.87 (10.48) | −0.23 (0.11) | (−0.44, −0.02) | 0.03 | 0.06 | 0.06 |
| NKG2C−CD57+ | 10.80 (10.12) | 8.29 (7.72) | 0.24 (0.11) | (0.04, 0.44) | 0.02 | 0.04 | 0.06 |
| NKG2C+CD57− | 5.30 (4.17) | 4.21 (3.57) | 0.26 (0.08) | (0.11, 0.40) | 0.000 | 0.001 | 0.08 |
| NKG2C+CD57+ | 3.19 (4.44) | 2.01 (4.85) | 0.42 (0.09) | (0.25, 0.59) | 0.000 | 0.001 | 0.21 |
| CD56dimCD16+ | 27.82 (13.82) | 26.92 (11.97) | 0.00 (0.11) | (−0.21, 0.21) | 0.99 | 0.77 | 0.00 |
| NKG2C−CD57− | 33.61 (14.06) | 43.64 (17.26) | −0.30 (0.10) | (−0.49, −0.11) | 0.002 | 0.01 | 0.10 |
| NKG2C−CD57+ | 48.08 (16.04) | 47.14 (17.29) | 0.03 (0.12) | (−0.20, 0.25) | 0.83 | 0.92 | 0.00 |
| NKG2C+CD57− | 3.06 (2.80) | 1.95 (1.96) | 0.25 (0.10) | (0.05, 0.45) | 0.02 | 0.04 | 0.07 |
| NKG2C+CD57+ | 15.10 (18.20) | 7.27 (14.62) | 0.40 (0.10) | (0.21, 0.59) | 0.000 | 0.003 | 0.20 |
| CD56−CD16+ | 9.40 (7.17) | 10.19 (9.49) | −0.05 (0.11) | (−0.26, 0.16) | 0.63 | 0.79 | 0.00 |
| NKG2C−CD57− | 74.10 (13.12) | 77.14 (13.06) | −0.12 (0.11) | (−0.33, 0.09) | 0.26 | 0.36 | 0.02 |
| NKG2C−CD57+ | 11.36 (10.46) | 12.101 (1.011) | 0.08 (0.11) | (−0.20, 0.23) | 0.88 | 0.94 | 0.00 |
| NKG2C+CD57− | 10.52 (10.44) | 9.34 (8.38) | −0.04 (0.11) | (−0.25, 0.18) | 0.75 | 0.87 | 0.00 |
| NKG2C+CD57+ | 3.54 (5.72) | 1.53 (3.59) | 0.26 (0.10) | (0.07, 0.45) | 0.008 | 0.02 | 0.04 |
| CMV-IgG Level on Early Rearing | NK Cell Subsets on CMV-Specific IgG | NK Cell Subset on Early Rearing 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NK Cell | β (SE) 2 | 95% CI 3 | β (SE) | 95% CI | Total Effect | 95% CI | Direct Effect | 95% CI 3 | Indirect Effect | 95% CI |
| CD56dim CD16− NKG2C+ CD57+ | 0.486 (0.092) | (0.301, 0.660) | 0.317 (0.131) | (0.067, 0.580) | 0.416 (0.091) | (0.223, 0.580) | 0.262 (0.031) | (0.004, 0.477) | 0.154 (0.079) | (0.028, 0.337) |
| CD56dim CD16+ NKG2C+ CD57+ | 0.486 (0.092) | (0.301, 0.660) | 0.511 (0.086) | (0.347, 0.683) | 0.398 (0.099) | (0.196, 0.581) | 0.149 (0.112) | (−0.077, 0.361) | 0.248 (0.069) | (0.132, 0.398) |
| CD56−CD16+ NKG2C+ CD57+ | 0.486 (0.092) | (0.301, 0.660) | 0.396 (0.114) | (0.172, 0.621) | 0.258 (0.101) | (0.055 0.450) | 0.065 (0.127) | (−0.193, 0.313) | 0.192 (0.072) | (0.072, 0.356) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wood, E.K.; Reid, B.M.; Sheerar, D.S.; Donzella, B.; Gunnar, M.R.; Coe, C.L. Lingering Effects of Early Institutional Rearing and Cytomegalovirus Infection on the Natural Killer Cell Repertoire of Adopted Adolescents. Biomolecules 2024, 14, 456. https://doi.org/10.3390/biom14040456
Wood EK, Reid BM, Sheerar DS, Donzella B, Gunnar MR, Coe CL. Lingering Effects of Early Institutional Rearing and Cytomegalovirus Infection on the Natural Killer Cell Repertoire of Adopted Adolescents. Biomolecules. 2024; 14(4):456. https://doi.org/10.3390/biom14040456
Chicago/Turabian StyleWood, Elizabeth K., Brie M. Reid, Dagna S. Sheerar, Bonny Donzella, Megan R. Gunnar, and Christopher L. Coe. 2024. "Lingering Effects of Early Institutional Rearing and Cytomegalovirus Infection on the Natural Killer Cell Repertoire of Adopted Adolescents" Biomolecules 14, no. 4: 456. https://doi.org/10.3390/biom14040456
APA StyleWood, E. K., Reid, B. M., Sheerar, D. S., Donzella, B., Gunnar, M. R., & Coe, C. L. (2024). Lingering Effects of Early Institutional Rearing and Cytomegalovirus Infection on the Natural Killer Cell Repertoire of Adopted Adolescents. Biomolecules, 14(4), 456. https://doi.org/10.3390/biom14040456






