The Expression of Serglycin Is Required for Active Transforming Growth Factor β Receptor I Tumorigenic Signaling in Glioblastoma Cells and Paracrine Activation of Stromal Fibroblasts via CXCR-2
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures and Reagents
2.2. Generation of Culture Media
2.3. RNA Isolation, cDNA Synthesis and Real-Time qPCR Analysis
2.4. Immunoblotting
2.5. Cell Cycle Analysis
2.6. Cell Proliferation Assay
2.7. Wound Healing Assay
2.8. ELISA
2.9. Bioinformatic Analysis
2.10. Statistical Analysis
3. Results
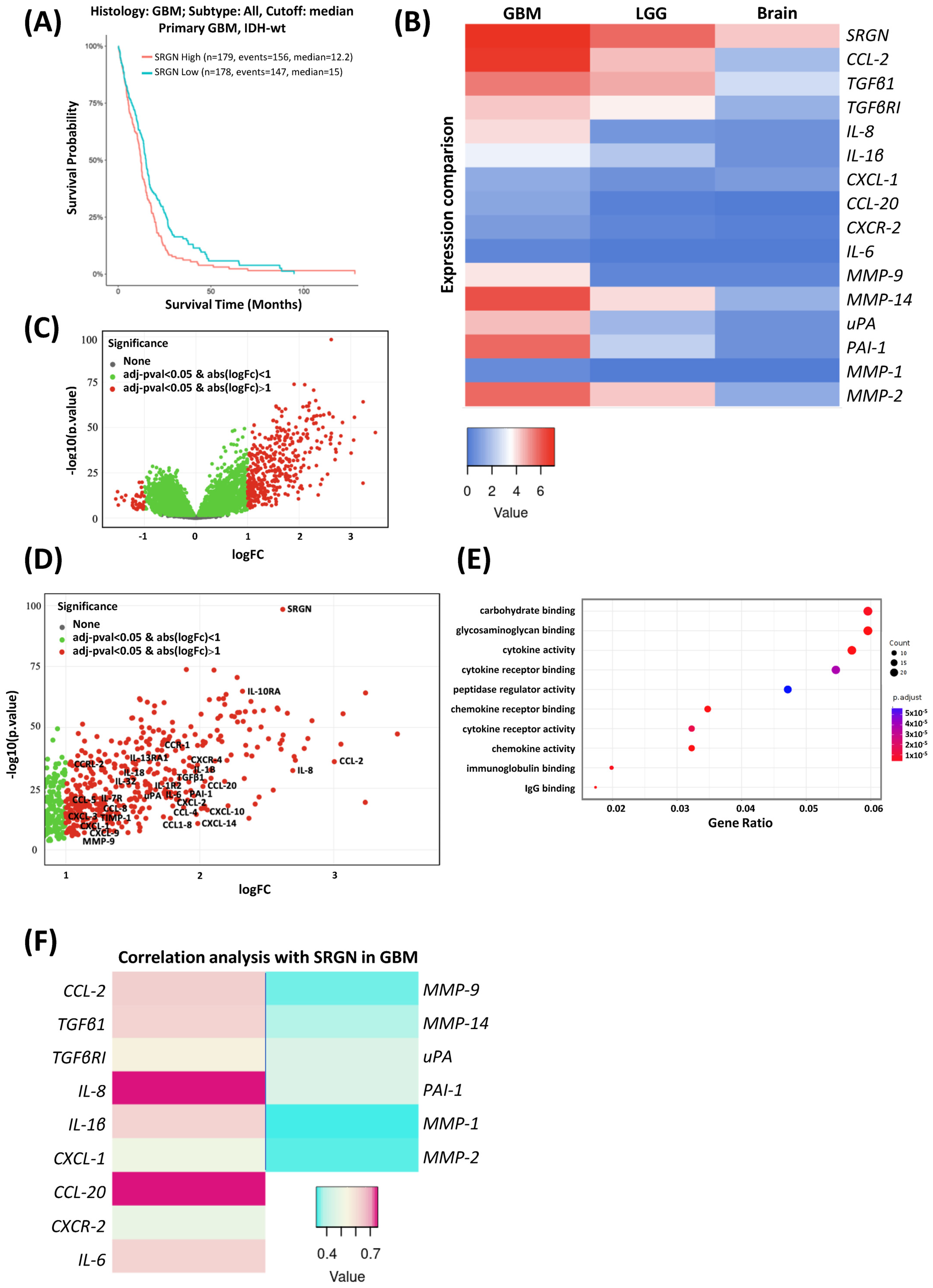
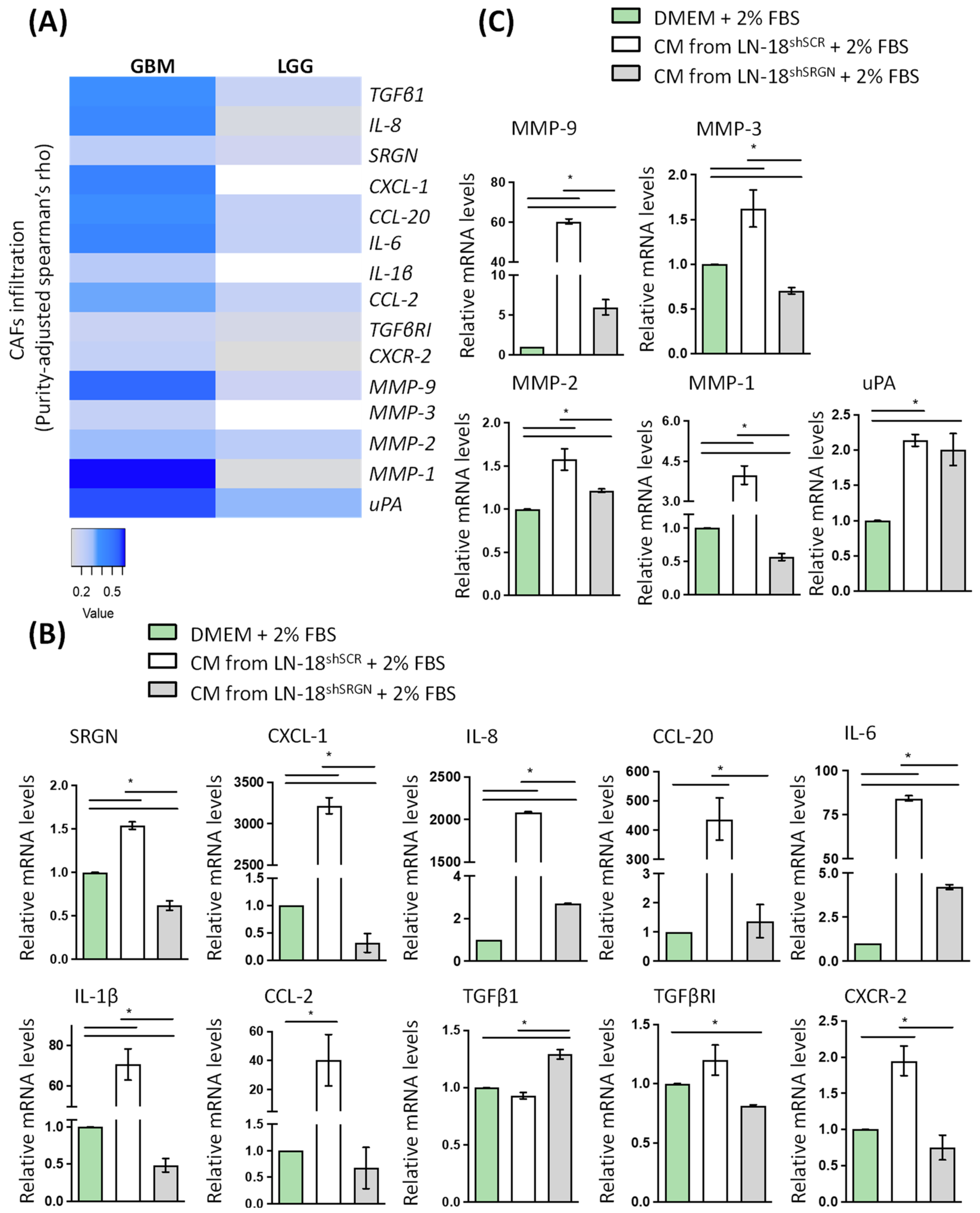
3.1. Serglycin Expression Is Associated with Low Survival and an Inflammatory Milieu in Glioblastoma
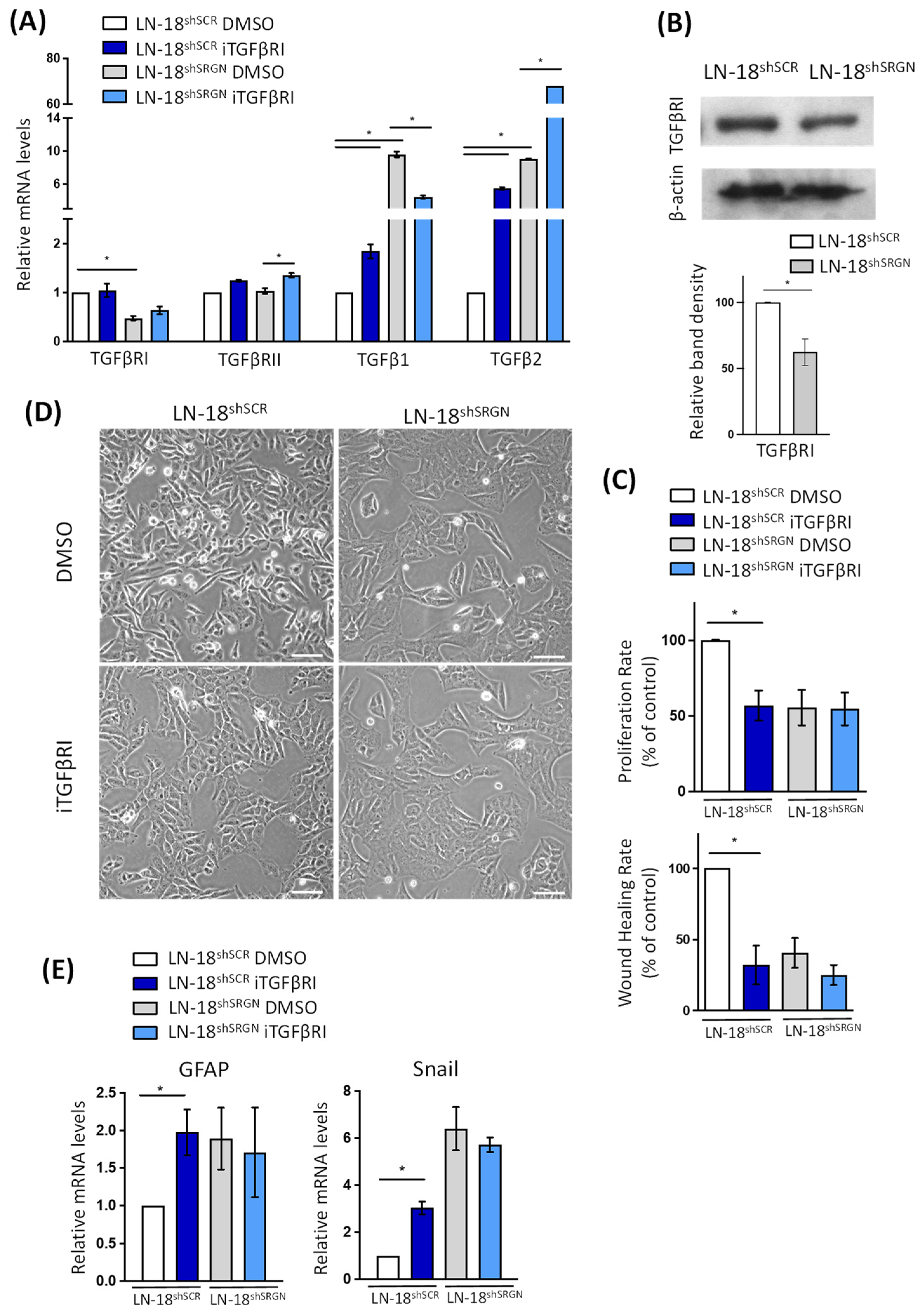
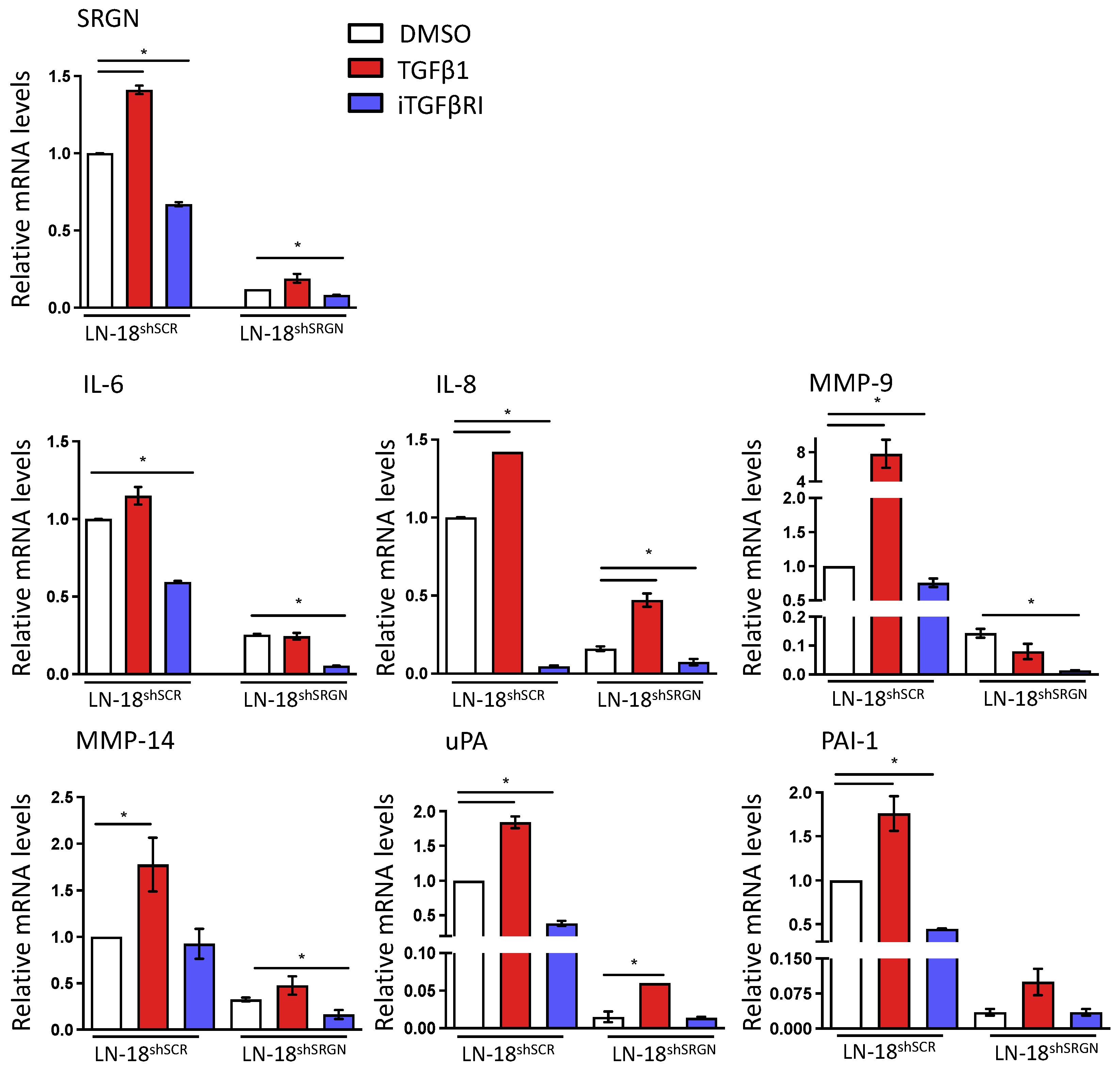
3.2. Serglycin Suppression Perturbs TGFβRI Pro-Tumorigenic Signaling in Glioblastoma Cells
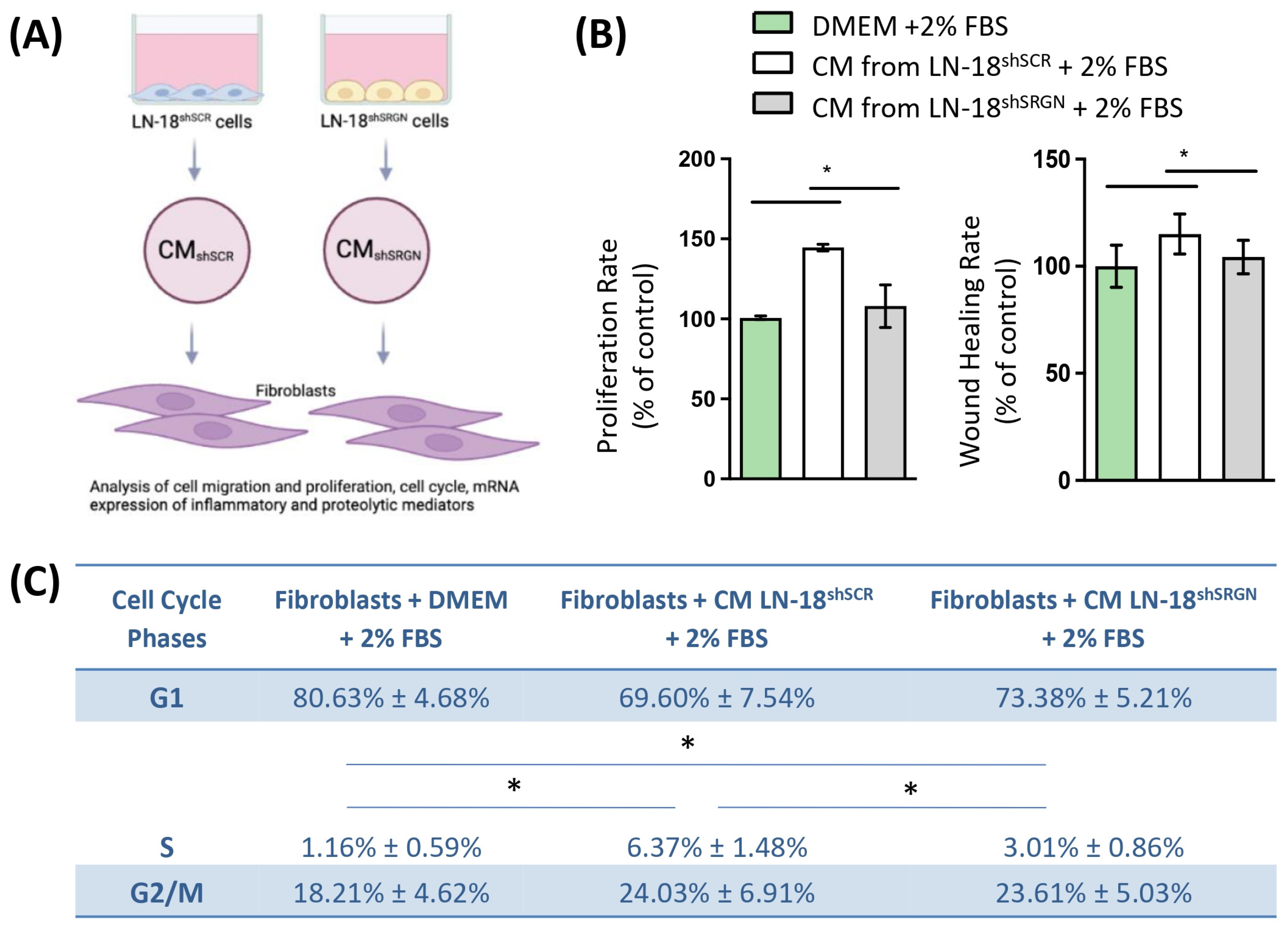
3.3. Serglycin Is Involved in the Paracrine Activation of Fibroblasts by Glioblastoma Cells
3.4. Active CXCR-2 Signaling Is Essential for Glioblastoma Cells–Fibroblasts Crosstalk and Activation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Patel, A.P.; Tirosh, I.; Trombetta, J.J.; Shalek, A.K.; Gillespie, S.M.; Wakimoto, H.; Cahill, D.P.; Nahed, B.V.; Curry, W.T.; Martuza, R.L.; et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014, 344, 1396–1401. [Google Scholar] [CrossRef]
- D’Alessio, A.; Proietti, G.; Sica, G.; Scicchitano, B.M. Pathological and Molecular Features of Glioblastoma and Its Peritumoral Tissue. Cancers 2019, 11, 469. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- Manou, D.; Caon, I.; Bouris, P.; Triantaphyllidou, I.E.; Giaroni, C.; Passi, A.; Karamanos, N.K.; Vigetti, D.; Theocharis, A.D. The Complex Interplay Between Extracellular Matrix and Cells in Tissues. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2019; Volume 1952, pp. 1–20. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Manou, D.; Karamanos, N.K. The extracellular matrix as a multitasking player in disease. FEBS J. 2019, 286, 2830–2869. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Neill, T.; Iozzo, R.V. Matrix modeling and remodeling: A biological interplay regulating tissue homeostasis and diseases. Matrix Biol. 2019, 75–76, 1–11. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Karamanos, N.K. Proteoglycans remodeling in cancer: Underlying molecular mechanisms. Matrix Biol. 2019, 75–76, 220–259. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Piperigkou, Z.; Theocharis, A.D.; Watanabe, H.; Franchi, M.; Baud, S.; Brézillon, S.; Götte, M.; Passi, A.; Vigetti, D.; et al. Proteoglycan Chemical Diversity Drives Multifunctional Cell Regulation and Therapeutics. Chem. Rev. 2018, 118, 9152–9232. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, S. Proteoglycans as Therapeutic Targets in Brain Cancer. Front. Oncol. 2020, 10, 1358. [Google Scholar] [CrossRef]
- Manou, D.; Bouris, P.; Kletsas, D.; Götte, M.; Greve, B.; Moustakas, A.; Karamanos, N.K.; Theocharis, A.D. Serglycin activates pro-tumorigenic signaling and controls glioblastoma cell stemness, differentiation and invasive potential. Matrix Biol. Plus 2020, 6–7, 100033. [Google Scholar] [CrossRef]
- Roy, A.; Attarha, S.; Weishaupt, H.; Edqvist, P.-H.; Swartling, F.J.; Bergqvist, M.; Siebzehnrubl, F.A.; Smits, A.; Pontén, F.; Tchougounova, E. Serglycin as a potential biomarker for glioma: Association of serglycin expression, extent of mast cell recruitment and glioblastoma progression. Oncotarget 2017, 8, 24815. [Google Scholar] [CrossRef]
- Korpetinou, A.; Skandalis, S.S.; Moustakas, A.; Happonen, K.E.; Tveit, H.; Prydz, K.; Labropoulou, V.T.; Giannopoulou, E.; Kalofonos, H.P.; Blom, A.M.; et al. Serglycin is implicated in the promotion of aggressive phenotype of breast cancer cells. PLoS ONE 2013, 8, e78157. [Google Scholar] [CrossRef]
- Bouris, P.; Manou, D.; Sopaki-Valalaki, A.; Kolokotroni, A.; Moustakas, A.; Kapoor, A.; Iozzo, R.V.; Karamanos, N.K.; Theocharis, A.D. Serglycin promotes breast cancer cell aggressiveness: Induction of epithelial to mesenchymal transition, proteolytic activity and IL-8 signaling. Matrix Biol. 2018, 74, 35–51. [Google Scholar] [CrossRef]
- Korpetinou, A.; Papachristou, D.J.; Lampropoulou, A.; Bouris, P.; Labropoulou, V.T.; Noulas, A.; Karamanos, N.K.; Theocharis, A.D. Increased Expression of Serglycin in Specific Carcinomas and Aggressive Cancer Cell Lines. BioMed Res. Int. 2015, 2015, 690721. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Seidel, C.; Borset, M.; Dobra, K.; Baykov, V.; Labropoulou, V.; Kanakis, I.; Dalas, E.; Karamanos, N.K.; Sundan, A.; et al. Serglycin constitutively secreted by myeloma plasma cells is a potent inhibitor of bone mineralization in vitro. J. Biol. Chem. 2006, 281, 35116–35128. [Google Scholar] [CrossRef]
- Purushothaman, A.; Toole, B.P. Serglycin Proteoglycan Is Required for Multiple Myeloma Cell Adhesion, in Vivo Growth, and Vascularization. J. Biol. Chem. 2014, 289, 5499–5509. [Google Scholar] [CrossRef]
- Li, X.-J.; Ong, C.K.; Cao, Y.; Xiang, Y.-Q.; Shao, J.-Y.; Ooi, A.; Peng, L.-X.; Lu, W.-H.; Zhang, Z.; Petillo, D.; et al. Serglycin Is a Theranostic Target in Nasopharyngeal Carcinoma that Promotes Metastasis. Cancer Res. 2011, 71, 3162–3172. [Google Scholar] [CrossRef]
- Korpetinou, A.; Skandalis, S.S.; Labropoulou, V.T.; Smirlaki, G.; Noulas, A.; Karamanos, N.K.; Theocharis, A.D. Serglycin: At the crossroad of inflammation and malignancy. Front. Oncol. 2014, 3, 327. [Google Scholar] [CrossRef]
- Manou, D.; Karamanos, N.K.; Theocharis, A.D. Tumorigenic functions of serglycin: Regulatory roles in epithelial to mesenchymal transition and oncogenic signaling. Semin. Cancer Biol. 2020, 62, 108–115. [Google Scholar] [CrossRef]
- Skliris, A.; Happonen, K.E.; Terpos, E.; Labropoulou, V.; Borset, M.; Heinegard, D.; Blom, A.M.; Theocharis, A.D. Serglycin inhibits the classical and lectin pathways of complement via its glycosaminoglycan chains: Implications for multiple myeloma. Eur. J. Immunol. 2011, 41, 437–449. [Google Scholar] [CrossRef]
- Kolset, S.O.; Pejler, G. Serglycin: A structural and functional chameleon with wide impact on immune cells. J. Immunol. 2011, 187, 4927–4933. [Google Scholar] [CrossRef]
- Malla, N.; Berg, E.; Theocharis, A.D.; Svineng, G.; Uhlin-Hansen, L.; Winberg, J.O. In vitro reconstitution of complexes between pro-matrix metalloproteinase-9 and the proteoglycans serglycin and versican. FEBS J. 2013, 280, 2870–2887. [Google Scholar] [CrossRef]
- Skliris, A.; Labropoulou, V.T.; Papachristou, D.J.; Aletras, A.; Karamanos, N.K.; Theocharis, A.D. Cell-surface serglycin promotes adhesion of myeloma cells to collagen type I and affects the expression of matrix metalloproteinases. FEBS J. 2013, 280, 2342–2352. [Google Scholar] [CrossRef]
- D’Ascola, A.; Scuruchi, M.; Avenoso, A.; Bruschetta, G.; Campo, S.; Mandraffino, G.; Campo, G.M. Serglycin is involved in inflammatory response in articular mouse chondrocytes. Biochem. Biophys. Res. Commun. 2018, 499, 506–512. [Google Scholar] [CrossRef]
- Scuruchi, M.; D’Ascola, A.; Avenoso, A.; Mandraffino, G.G.; Campo, S.S.; Campo, G.M. Serglycin as part of IL-1β induced inflammation in human chondrocytes. Arch. Biochem. Biophys. 2019, 669, 80–86. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, Y.; Zheng, G.; Jia, X.; Xiong, Y.; Luo, K.; Qiu, Q.; Qiu, N.; Yin, J.; Lu, M.; et al. SRGN-TGFβ2 regulatory loop confers invasion and metastasis in triple-negative breast cancer. Oncogenesis 2017, 6, e360. [Google Scholar] [CrossRef]
- Bruna, A.; Darken, R.S.; Rojo, F.; Ocaña, A.; Peñuelas, S.; Arias, A.; Paris, R.; Tortosa, A.; Mora, J.; Baselga, J.; et al. High TGFβ-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell 2007, 11, 147–160. [Google Scholar] [CrossRef]
- Caja, L.; Tzavlaki, K.; Dadras, M.S.; Tan, E.J.; Hatem, G.; Maturi, N.P.; Morén, A.; Wik, L.; Watanabe, Y.; Savary, K.; et al. Snail regulates BMP and TGFβ pathways to control the differentiation status of glioma-initiating cells. Oncogene 2018, 37, 2515–2531. [Google Scholar] [CrossRef]
- Han, J.; Alvarez-Breckenridge, C.A.; Wang, Q.-E.; Yu, J. TGF-β signaling and its targeting for glioma treatment. Am. J. Cancer Res. 2015, 5, 945–955. [Google Scholar]
- Ikushima, H.; Todo, T.; Ino, Y.; Takahashi, M.; Miyazawa, K.; Miyazono, K. Autocrine TGF-β signaling maintains tumorigenicity of glioma-initiating cells through Sry-related HMG-box factors. Cell Stem Cell 2009, 5, 504–514. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Z.; Wu, R.; Yao, Q.; Gu, Z.; Liu, M. Correlation of C-X-C chemokine receptor 2 upregulation with poor prognosis and recurrence in human glioma. OncoTargets Ther. 2015, 8, 3203–3209. [Google Scholar] [CrossRef][Green Version]
- Brandenburg, S.; Müller, A.; Turkowski, K.; Radev, Y.T.; Rot, S.; Schmidt, C.; Bungert, A.D.; Acker, G.; Schorr, A.; Hippe, A.; et al. Resident microglia rather than peripheral macrophages promote vascularization in brain tumors and are source of alternative pro-angiogenic factors. Acta Neuropathol. 2016, 131, 365–378. [Google Scholar] [CrossRef]
- Urbantat, R.M.; Blank, A.; Kremenetskaia, I.; Vajkoczy, P.; Acker, G.; Brandenburg, S. The CXCL2/IL8/CXCR2 Pathway Is Relevant for Brain Tumor Malignancy and Endothelial Cell Function. Int. J. Mol. Sci. 2021, 22, 2634. [Google Scholar] [CrossRef]
- Jain, S.; Rick, J.W.; Joshi, R.S.; Beniwal, A.; Spatz, J.; Gill, S.; Chang, A.C.-C.; Choudhary, N.; Nguyen, A.T.; Sudhir, S.; et al. Single-cell RNA sequencing and spatial transcriptomics reveal cancer-associated fibroblasts in glioblastoma with protumoral effects. J. Clin. Investig. 2023, 133, e147087. [Google Scholar] [CrossRef]
- Chen, Z.; Zhuo, S.; He, G.; Tang, J.; Hao, W.; Gao, W.Q.; Yang, K.; Xu, H. Prognosis and Immunotherapy Significances of a Cancer-Associated Fibroblasts-Related Gene Signature in Gliomas. Front. Cell Dev. Biol. 2021, 9, 721897. [Google Scholar] [CrossRef]
- Madhugiri, V.S.; Moiyadi, A.V.; Shetty, P.; Gupta, T.; Epari, S.; Jalali, R.; Subeikshanan, V.; Dutt, A.; Sasidharan, G.M.; Roopesh Kumar, V.R.; et al. Analysis of Factors Associated with Long-Term Survival in Patients with Glioblastoma. World Neurosurg. 2021, 149, e758–e765. [Google Scholar] [CrossRef]
- Gieryng, A.; Pszczolkowska, D.; Walentynowicz, K.A.; Rajan, W.D.; Kaminska, B. Immune microenvironment of gliomas. Lab. Investig. 2017, 97, 498–518. [Google Scholar] [CrossRef]
- Razavi, S.-M.; Lee, K.E.; Jin, B.E.; Aujla, P.S.; Gholamin, S.; Li, G. Immune evasion strategies of glioblastoma. Front. Surg. 2016, 3, 11. [Google Scholar] [CrossRef]
- Ha, E.T.; Antonios, J.P.; Soto, H.; Prins, R.M.; Yang, I.; Kasahara, N.; Liau, L.M.; Kruse, C.A. Chronic inflammation drives glioma growth: Cellular and molecular factors responsible for an immunosuppressive microenvironment. Neuroimmunol. Neuroinflamm. 2014, 1, 66–76. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Piperigkou, Z.; Passi, A.; Götte, M.; Rousselle, P.; Vlodavsky, I. Extracellular matrix-based cancer targeting. Trends Mol. Med. 2021, 27, 1000–1013. [Google Scholar] [CrossRef]
- Caja, L.; Bellomo, C.; Moustakas, A. Transforming growth factor β and bone morphogenetic protein actions in brain tumors. FEBS Lett. 2015, 589, 1588–1597. [Google Scholar] [CrossRef]
- Peñuelas, S.; Anido, J.; Prieto-Sánchez, R.M.; Folch, G.; Barba, I.; Cuartas, I.; García-Dorado, D.; Poca, M.A.; Sahuquillo, J.; Baselga, J.; et al. TGF-β increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell 2009, 15, 315–327. [Google Scholar] [CrossRef]
- Savary, K.; Caglayan, D.; Caja, L.; Tzavlaki, K.; Bin Nayeem, S.; Bergström, T.; Jiang, Y.; Uhrbom, L.; Forsberg-Nilsson, K.; Westermark, B.; et al. Snail depletes the tumorigenic potential of glioblastoma. Oncogene 2013, 32, 5409–5420. [Google Scholar] [CrossRef]
- Doyle, K.P.; Cekanaviciute, E.; Mamer, L.E.; Buckwalter, M.S. TGFβ signaling in the brain increases with aging and signals to astrocytes and innate immune cells in the weeks after stroke. J. Neuroinflamm. 2010, 7, 62. [Google Scholar] [CrossRef]
- Schachtrup, C.; Ryu, J.K.; Helmrick, M.J.; Vagena, E.; Galanakis, D.K.; Degen, J.L.; Margolis, R.U.; Akassoglou, K. Fibrinogen triggers astrocyte scar formation by promoting the availability of active TGF-β after vascular damage. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 5843–5854. [Google Scholar] [CrossRef]
- Arsura, M.; Panta, G.R.; Bilyeu, J.D.; Cavin, L.G.; Sovak, M.A.; Oliver, A.A.; Factor, V.; Heuchel, R.; Mercurio, F.; Thorgeirsson, S.S.; et al. Transient activation of NF-κB through a TAK1/IKK kinase pathway by TGF-β1 inhibits AP-1/SMAD signaling and apoptosis: Implications in liver tumor formation. Oncogene 2003, 22, 412–425. [Google Scholar] [CrossRef]
- Safina, A.; Vandette, E.; Bakin, A.V. ALK5 promotes tumor angiogenesis by upregulating matrix metalloproteinase-9 in tumor cells. Oncogene 2007, 26, 2407–2422. [Google Scholar] [CrossRef]
- Safina, A.; Ren, M.Q.; Vandette, E.; Bakin, A.V. TAK1 is required for TGF-β 1-mediated regulation of matrix metalloproteinase-9 and metastasis. Oncogene 2008, 27, 1198–1207. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, L.; Zhang, B.; Li, J.; Dou, X.; Zhao, R.C. TGF-β1-induced PI3K/Akt/NF-kappaB/MMP9 signalling pathway is activated in Philadelphia chromosome-positive chronic myeloid leukaemia hemangioblasts. J. Biochem. 2011, 149, 405–414. [Google Scholar] [CrossRef]
- Seomun, Y.; Kim, J.T.; Joo, C.K. MMP-14 mediated MMP-9 expression is involved in TGF-β1-induced keratinocyte migration. J. Cell. Biochem. 2008, 104, 934–941. [Google Scholar] [CrossRef]
- Santibáñez, J.F.; Iglesias, M.; Frontelo, P.; Martínez, J.; Quintanilla, M. Involvement of the Ras/MAPK signaling pathway in the modulation of urokinase production and cellular invasiveness by transforming growth factor-β1 in transformed keratinocytes. Biochem. Biophys. Res. Commun. 2000, 273, 521–527. [Google Scholar] [CrossRef]
- Kocic, J.; Bugarski, D.; Santibanez, J.F. SMAD3 is essential for transforming growth factor-β1-induced urokinase type plasminogen activator expression and migration in transformed keratinocytes. Eur. J. Cancer 2012, 48, 1550–1557. [Google Scholar] [CrossRef]
- Tobar, N.; Villar, V.; Santibanez, J.F. ROS-NFkappaB mediates TGF-β1-induced expression of urokinase-type plasminogen activator, matrix metalloproteinase-9 and cell invasion. Mol. Cell. Biochem. 2010, 340, 195–202. [Google Scholar] [CrossRef]
- Nagamine, Y.; Medcalf, R.L.; Muñoz-Cánoves, P. Transcriptional and posttranscriptional regulation of the plasminogen activator system. Thromb. Haemost. 2005, 93, 661–675. [Google Scholar]
- Santibanez, J.F. Transforming growth factor-β and urokinase-type plasminogen activator: Dangerous partners in tumorigenesis—Implications in skin cancer. ISRN Dermatol. 2013, 2013, 597927. [Google Scholar] [CrossRef]
- Hu, J.; Jo, M.; Eastman, B.M.; Gilder, A.S.; Bui, J.D.; Gonias, S.L. uPAR Induces Expression of Transforming Growth Factor β and Interleukin-4 in Cancer Cells to Promote Tumor-Permissive Conditioning of Macrophages. Am. J. Pathol. 2014, 184, 3384–3393. [Google Scholar] [CrossRef]
- Annes, J.P.; Munger, J.S.; Rifkin, D.B. Making sense of latent TGFβ activation. J. Cell Sci. 2003, 116, 217–224. [Google Scholar] [CrossRef]
- Jenkins, G. The role of proteases in transforming growth factor-β activation. Int. J. Biochem. Cell Biol. 2008, 40, 1068–1078. [Google Scholar] [CrossRef]
- Dallas, S.L.; Rosser, J.L.; Mundy, G.R.; Bonewald, L.F. Proteolysis of latent transforming growth factor-β (TGF-β)-binding protein-1 by osteoclasts. A cellular mechanism for release of TGF-β from bone matrix. J. Biol. Chem. 2002, 277, 21352–21360. [Google Scholar] [CrossRef]
- Tatti, O.; Vehviläinen, P.; Lehti, K.; Keski-Oja, J. MT1-MMP releases latent TGF-β1 from endothelial cell extracellular matrix via proteolytic processing of LTBP-1. Exp. Cell Res. 2008, 314, 2501–2514. [Google Scholar] [CrossRef]
- Santibanez, J.F.; Obradović, H.; Kukolj, T.; Krstić, J. Transforming growth factor-β, matrix metalloproteinases, and urokinase-type plasminogen activator interaction in the cancer epithelial to mesenchymal transition. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2018, 247, 382–395. [Google Scholar] [CrossRef]
- Yamada, D.; Kobayashi, S.; Wada, H.; Kawamoto, K.; Marubashi, S.; Eguchi, H.; Ishii, H.; Nagano, H.; Doki, Y.; Mori, M. Role of crosstalk between interleukin-6 and transforming growth factor-β 1 in epithelial–mesenchymal transition and chemoresistance in biliary tract cancer. Eur. J. Cancer 2013, 49, 1725–1740. [Google Scholar] [CrossRef]
- Lu, S.; Dong, Z. Characterization of TGF-β-regulated interleukin-8 expression in human prostate cancer cells. Prostate 2006, 66, 996–1004. [Google Scholar] [CrossRef]
- Bhola, N.E.; Balko, J.M.; Dugger, T.C.; Kuba, M.G.; Sánchez, V.; Sanders, M.; Stanford, J.; Cook, R.S.; Arteaga, C.L. TGF-β inhibition enhances chemotherapy action against triple-negative breast cancer. J. Clin. Investig. 2013, 123, 1348–1358. [Google Scholar] [CrossRef]
- Tellez-Gabriel, M.; Tekpli, X.; Reine, T.M.; Hegge, B.; Nielsen, S.R.; Chen, M.; Moi, L.; Normann, L.S.; Busund, L.R.; Calin, G.A.; et al. Serglycin Is Involved in TGF-β Induced Epithelial-Mesenchymal Transition and Is Highly Expressed by Immune Cells in Breast Cancer Tissue. Front. Oncol. 2022, 12, 868868. [Google Scholar] [CrossRef]
- Tyan, S.-W.; Hsu, C.-H.; Peng, K.-L.; Chen, C.-C.; Kuo, W.-H.; Eva, Y.-H.L.; Shew, J.-Y.; Chang, K.-J.; Juan, L.-J.; Lee, W.-H. Breast cancer cells induce stromal fibroblasts to secrete ADAMTS1 for cancer invasion through an epigenetic change. PLoS ONE 2012, 7, e35128. [Google Scholar] [CrossRef]
- Yan, D.; Cui, D.; Zhu, Y.; Chan, C.K.W.; Choi, C.H.J.; Liu, T.; Tsao, S.W.; Ma, S.; Cheung, A.L.M. Serglycin-induced interleukin-1β from oesophageal cancer cells upregulate hepatocyte growth factor in fibroblasts to promote tumour angiogenesis and growth. Clin. Transl. Med. 2022, 12, e1031. [Google Scholar] [CrossRef]
- Strieter, R.M.; Burdick, M.D.; Mestas, J.; Gomperts, B.; Keane, M.P.; Belperio, J.A. Cancer CXC chemokine networks and tumour angiogenesis. Eur. J. Cancer 2006, 42, 768–778. [Google Scholar] [CrossRef]
- Bizzarri, C.; Beccari, A.R.; Bertini, R.; Cavicchia, M.R.; Giorgini, S.; Allegretti, M. ELR+ CXC chemokines and their receptors (CXC chemokine receptor 1 and CXC chemokine receptor 2) as new therapeutic targets. Pharmacol. Ther. 2006, 112, 139–149. [Google Scholar] [CrossRef]
- Cheng, Y.; Ma, X.-L.; Wei, Y.-Q.; Wei, X.-W. Potential roles and targeted therapy of the CXCLs/CXCR2 axis in cancer and inflammatory diseases. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2019, 1871, 289–312. [Google Scholar] [CrossRef]
- Liu, Q.; Li, A.; Tian, Y.; Wu, J.D.; Liu, Y.; Li, T.; Chen, Y.; Han, X.; Wu, K. The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev. 2016, 31, 61–71. [Google Scholar] [CrossRef]
- Veenstra, M.; Ransohoff, R.M. Chemokine receptor CXCR2: Physiology regulator and neuroinflammation controller? J. Neuroimmunol. 2012, 246, 1–9. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manou, D.; Golfinopoulou, M.-A.; Alharbi, S.N.D.; Alghamdi, H.A.; Alzahrani, F.M.; Theocharis, A.D. The Expression of Serglycin Is Required for Active Transforming Growth Factor β Receptor I Tumorigenic Signaling in Glioblastoma Cells and Paracrine Activation of Stromal Fibroblasts via CXCR-2. Biomolecules 2024, 14, 461. https://doi.org/10.3390/biom14040461
Manou D, Golfinopoulou M-A, Alharbi SND, Alghamdi HA, Alzahrani FM, Theocharis AD. The Expression of Serglycin Is Required for Active Transforming Growth Factor β Receptor I Tumorigenic Signaling in Glioblastoma Cells and Paracrine Activation of Stromal Fibroblasts via CXCR-2. Biomolecules. 2024; 14(4):461. https://doi.org/10.3390/biom14040461
Chicago/Turabian StyleManou, Dimitra, Maria-Angeliki Golfinopoulou, Sara Naif D. Alharbi, Hind A. Alghamdi, Fatimah Mohammed Alzahrani, and Achilleas D. Theocharis. 2024. "The Expression of Serglycin Is Required for Active Transforming Growth Factor β Receptor I Tumorigenic Signaling in Glioblastoma Cells and Paracrine Activation of Stromal Fibroblasts via CXCR-2" Biomolecules 14, no. 4: 461. https://doi.org/10.3390/biom14040461
APA StyleManou, D., Golfinopoulou, M.-A., Alharbi, S. N. D., Alghamdi, H. A., Alzahrani, F. M., & Theocharis, A. D. (2024). The Expression of Serglycin Is Required for Active Transforming Growth Factor β Receptor I Tumorigenic Signaling in Glioblastoma Cells and Paracrine Activation of Stromal Fibroblasts via CXCR-2. Biomolecules, 14(4), 461. https://doi.org/10.3390/biom14040461





