Neurosteroid Modulation of Synaptic and Extrasynaptic GABAA Receptors of the Mouse Nucleus Accumbens
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Electrophysiology Brain Slice Preparation
2.3. Electrophysiology Recording
2.4. Drugs
2.5. Electrophysiology Data Analysis
2.6. Electrophysiology Statistical Analysis
2.7. Immunohistochemistry Studies
2.8. Immunohistochemistry Image Acquisition
3. Results
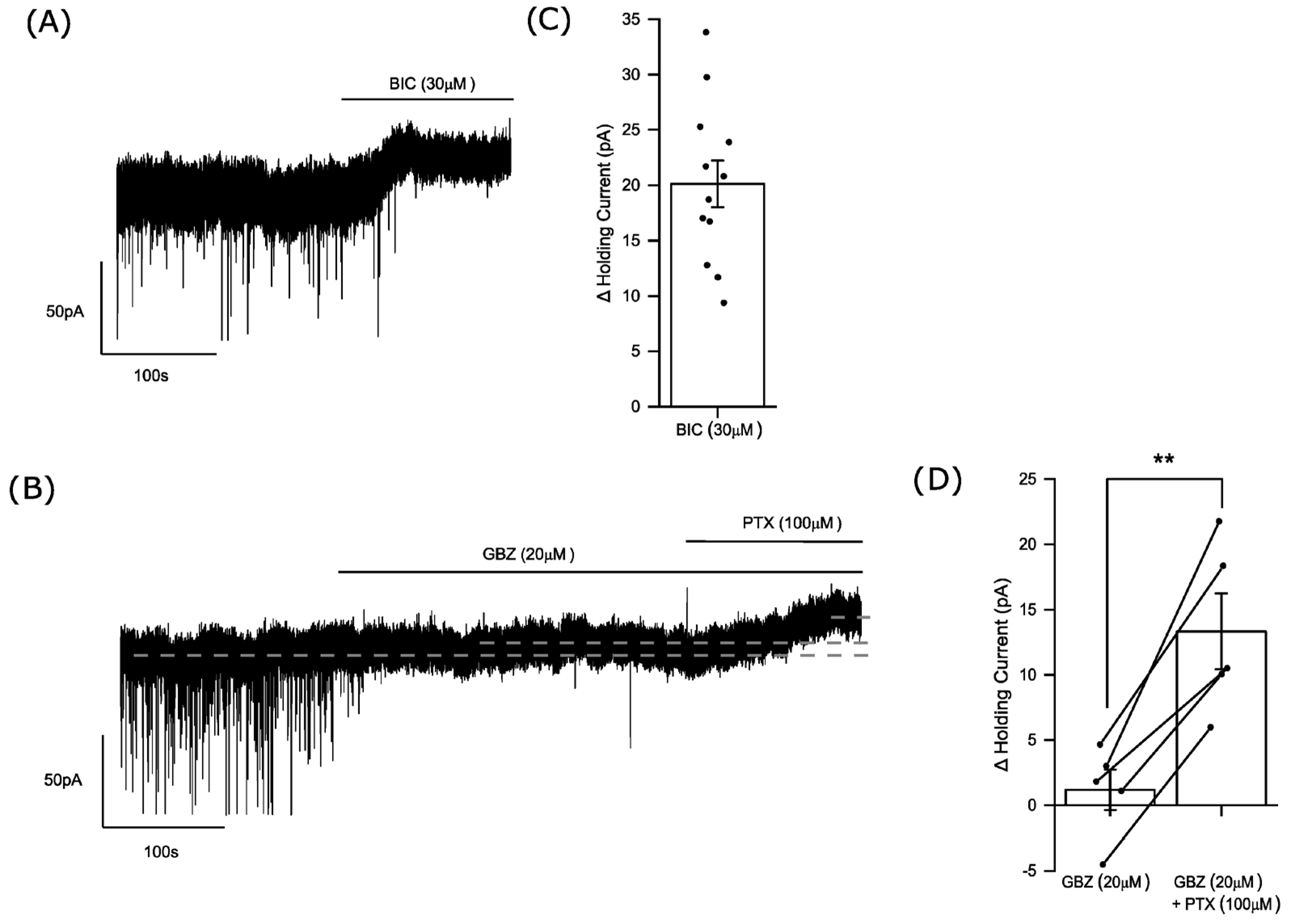
3.1. Phasic and Tonic Inhibition of Medium Spiny Neurons of the Nucleus Accumbens
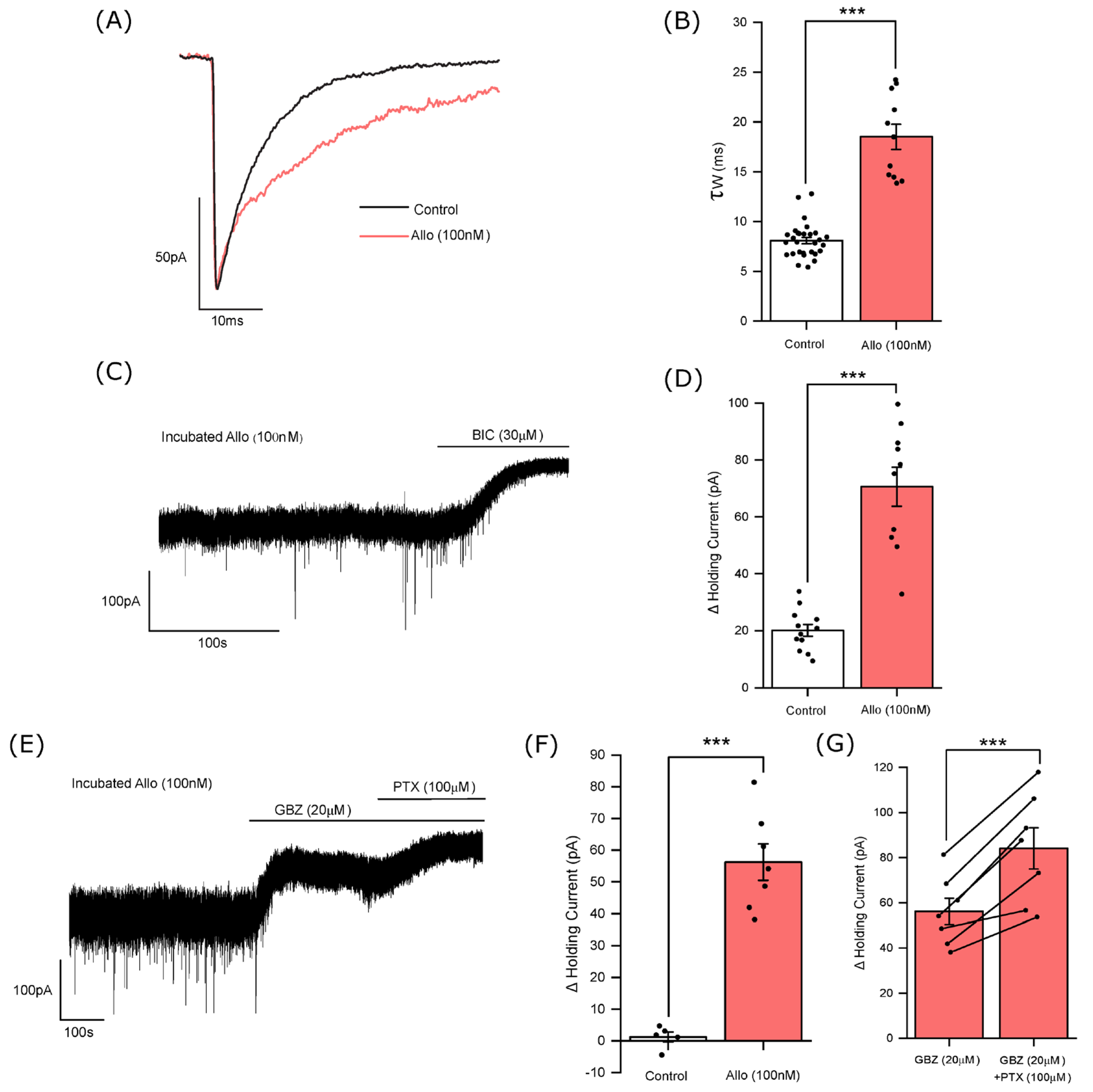
3.2. The Influence of Allopregnanolone on Phasic and Tonic Inhibition
3.3. Allopregnanolone Increases the Tonic Current in a GABA-Dependent Manner
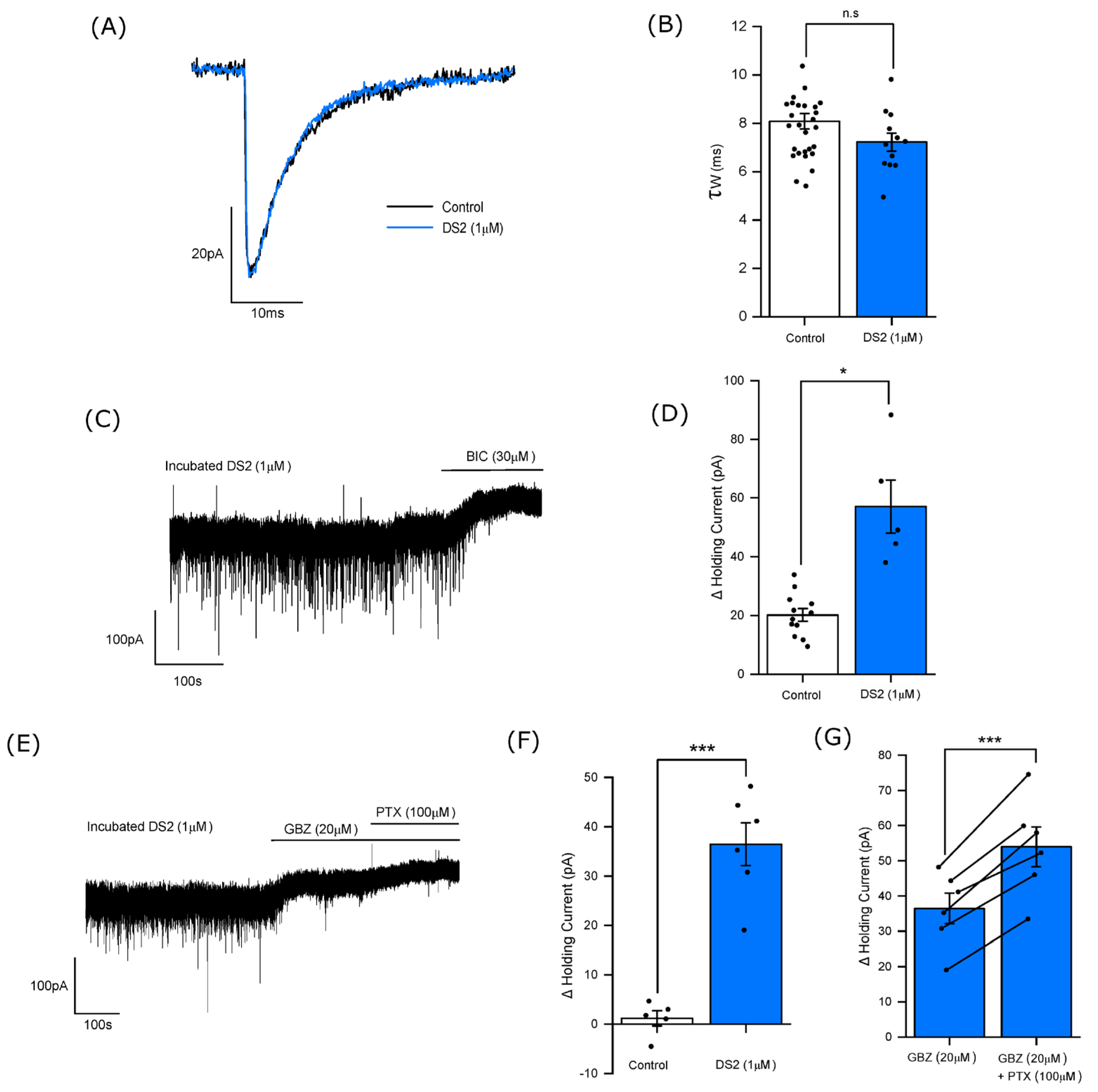
3.4. DS2 Selectively Enhances the Tonic Current
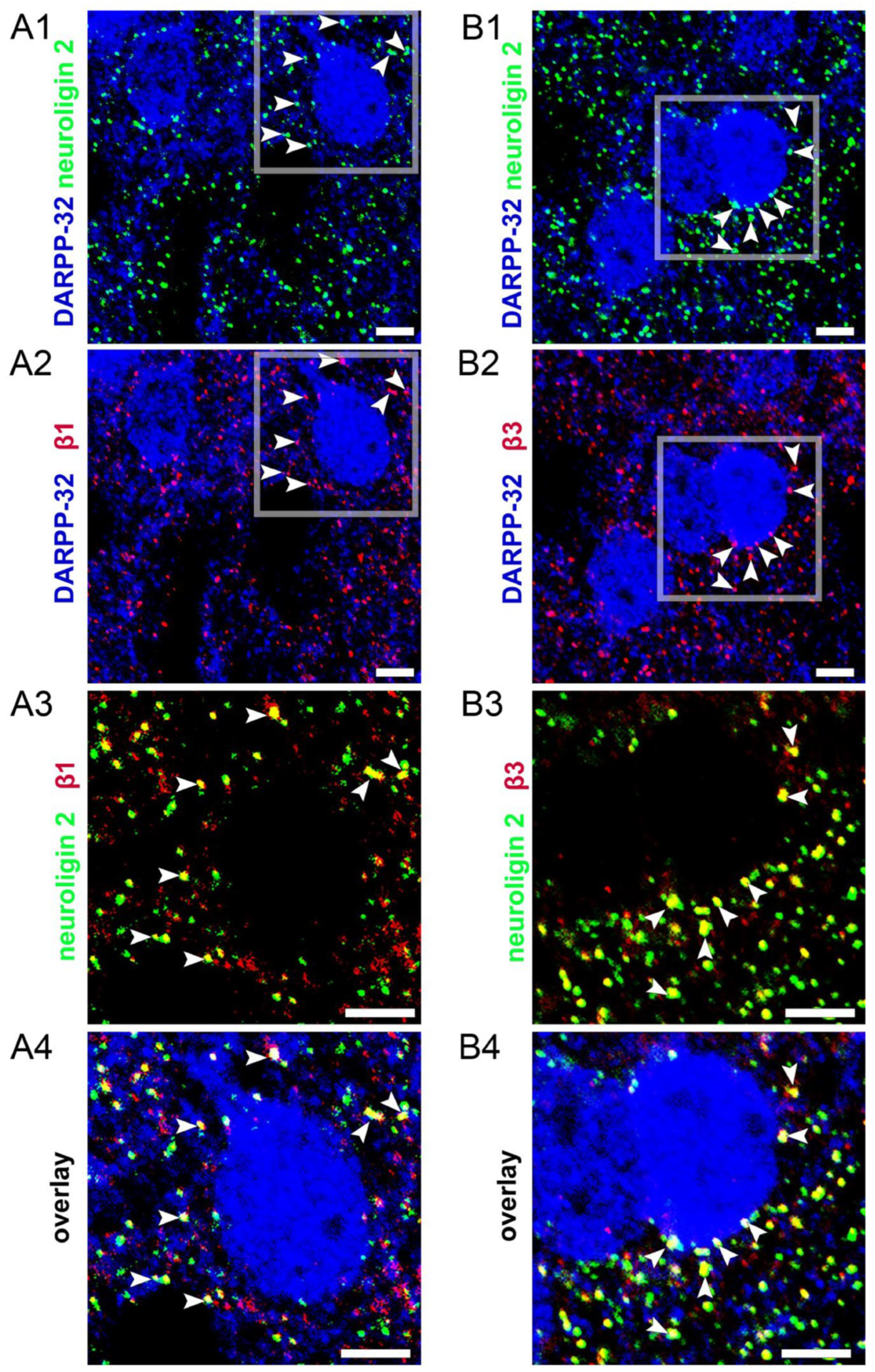
3.5. Endogenous Neurosteroids and Their Local Synthesis Influence Synaptic GABA-Ergic Neurotransmission of Accumbal Medium Spiny Neurons
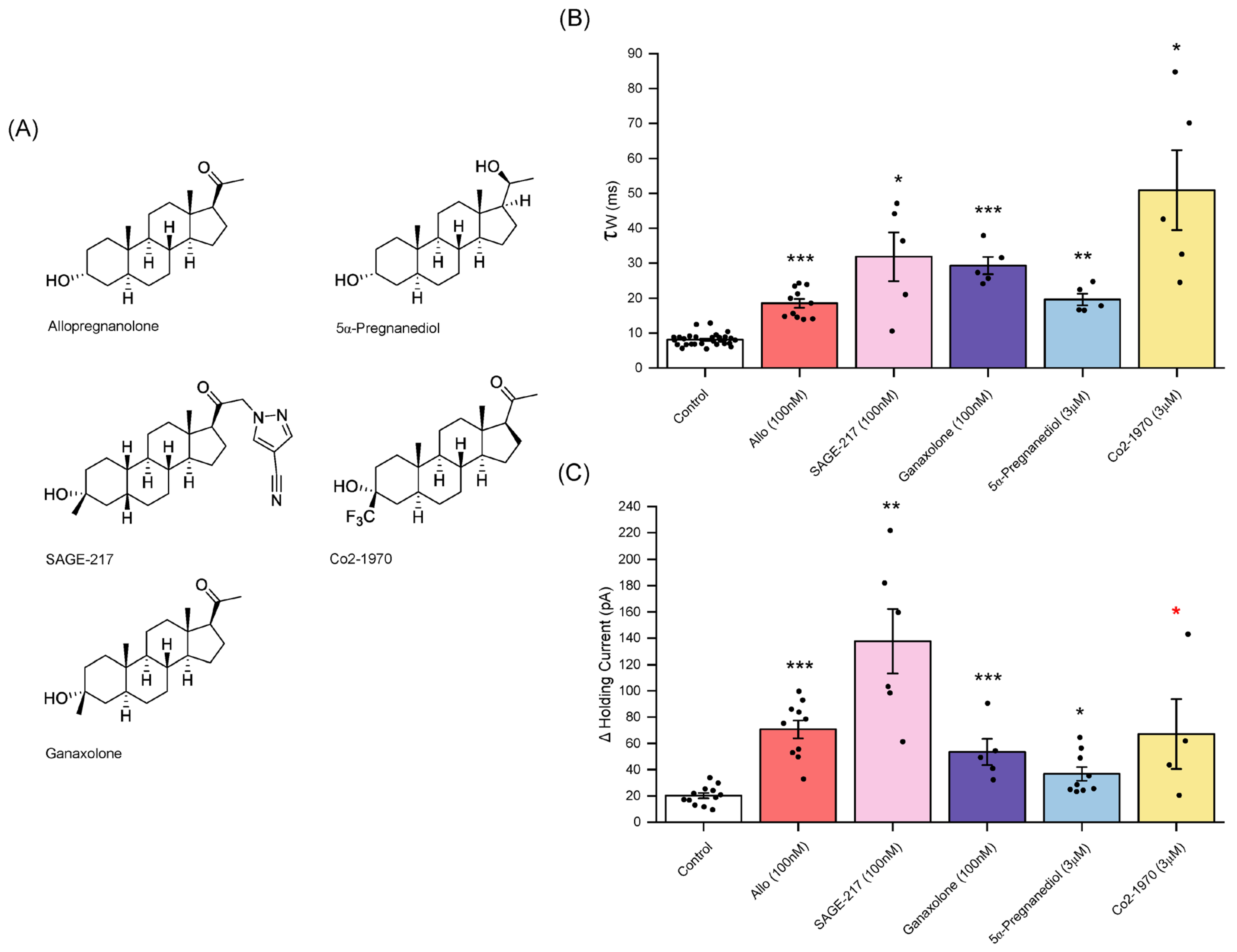
3.6. Sage-217 (Zuranolone)
3.7. Ganaxolone
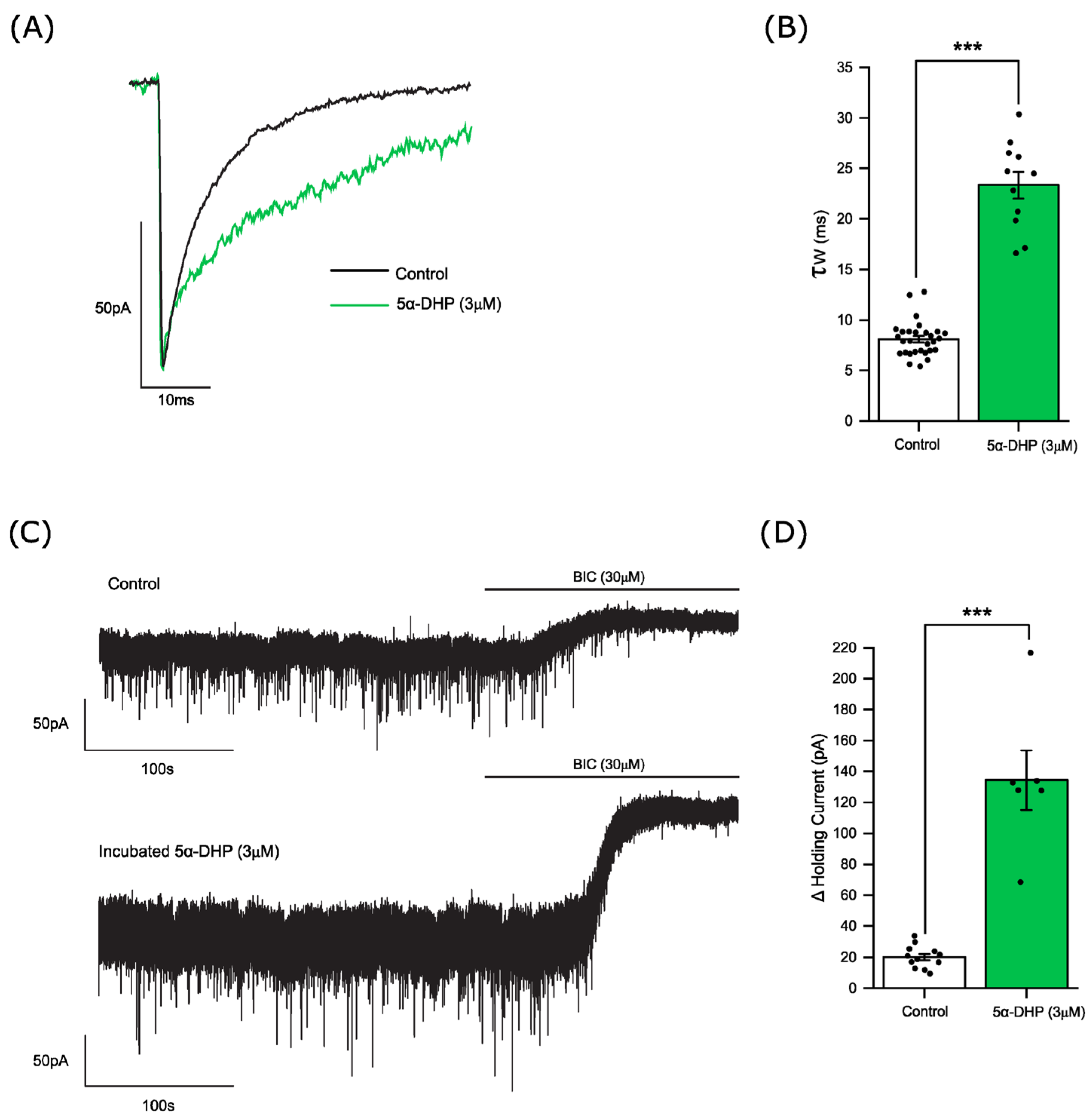
3.8. 5α-pregnan-3α,20α-diol
3.9. Co2-1970 (3α-Hydroxy-3β-Trifluromethyl-5α-Pregnan-20-One)
4. Discussion
4.1. The Tonic Current Is Mediated by Spontaneously Open GABAARs
4.2. The Influence of Allopregnanolone on Phasic and Tonic Inhibition
4.3. Evidence for an Endogenous Neurosteroid Tone and Local Neurosteroid Synthesis in the Nucleus Accumbens
4.4. The Profile of Neurosteroids and Synthetic Neuroactive Steroids of Potential Therapeutic Interest
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belelli, D.; Lambert, J.J. Neurosteroids: Endogenous regulators of the GABAA receptor. Nat. Rev. Neurosci. 2005, 6, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Meltzer-Brody, S.; Kanes, S.J. Allopregnanolone in postpartum depression: Role in pathophysiology and treatment. Neurobiol. Stress 2020, 12, 100212. [Google Scholar] [CrossRef] [PubMed]
- Zorumski, C.F.; Paul, S.M.; Covey, D.F.; Mennerick, S. Neurosteroids as novel antidepressants and anxiolytics: GABA-A receptors and beyond. Neurobiol. Stress 2019, 11, 100196–100206. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.; Balan, I.; Morrow, A.L.; Meltzer-Brody, S. Novel neurosteroid therapeutics for post-partum depression: Perspectives on clinical trials, program development, active research, and future directions. Neuropsychopharmacology 2023, 49, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Cutler, A.J.; Mattingly, G.W.; Maletic, V. Understanding the mechanism of action and clinical effects of neuroactive steroids and GABAergic compounds in major depressive disorder. Transl. Psychiatry 2023, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Luscher, B.; Maguire, J.L.; Rudolph, U.; Sibille, E. GABAA receptors as targets for treating affective and cognitive symptoms of depression. Trends Pharmacol. Sci. 2023, 44, 586–600. [Google Scholar] [CrossRef] [PubMed]
- Maguire, J.L.; Mennerick, S. Neurosteroids: Mechanistic considerations and clinical prospects. Neuropsychopharmacology 2023, 49, 73–82. [Google Scholar] [CrossRef]
- Patatanian, E.; Nguyen, D.R. Brexanolone: A novel drug for the treatment of postpartum depression. J. Pharm. Pract. 2022, 35, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.S.; Mbilinyi, R.H.; Estes, E. Preclinical and clinical pharmacology of brexanolone (allopregnanolone) for postpartum depression: A landmark journey from concept to clinic in neurosteroid replacement therapy. Psychopharmacology 2023, 240, 1841–1863. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.S. Neurosteroids as novel anticonvulsants for refractory status epilepticus and medical countermeasures for nerve agents: A 15-year journey to bring ganaxolone from bench to clinic. J. Pharmacol. Exp. Ther. 2024, 388, 273–300. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.M.; Pinna, G.; Guidotti, A. Allopregnanolone: From molecular pathophysiology to therapeutics. A historical perspective. Neurobiol. Stress 2020, 12, 100215. [Google Scholar] [CrossRef]
- Russo, S.J.; Nestler, E.J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 2013, 14, 609–625. [Google Scholar] [CrossRef]
- Heshmati, M.; Russo, S.J. Anhedonia and the brain reward circuitry in depression. Curr. Behav. Neurosci. Rep. 2015, 2, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C. Editorial: Toward neurobiological-based treatments of depression and anxiety: A potential case for the nucleus accumbens. J. Am. Acad. Child Adolesc. Psychiatry 2022, 61, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Rincón-Cortés, M.; Grace, A.A. Dopamine downregulation in novel rodent models useful for the study of postpartum depression. Front. Behav. Neurosci. 2022, 16, 1065558. [Google Scholar] [CrossRef] [PubMed]
- Moses-Kolko, E.L.; Fraser, D.; Wisner, K.L.; James, J.A.; Saul, A.T.; Fiez, J.A.; Phillips, M.L. Rapid habituation of ventral striatal response to reward receipt in postpartum depression. Biol. Psychiatry 2011, 70, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Nin, M.S.; Ferri, M.K.; Couto-Pereira, N.S.; Souza, M.F.; Azeredo, L.A.; Agnes, G.; Gomez, R.; Barros, H.M.T. The effect of intra-nucleus accumbens administration of allopregnanolone on δ and γ2 GABAA receptor subunit mRNA expression in the hippocampus and on depressive-like and grooming behaviors in rats. Pharmacol. Biochem. Behav. 2012, 103, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Finn, D.A.; Phillips, T.J.; Okorn, D.M.; Chester, J.A.; Cunningham, C.L. Rewarding effect of the neuroactive steroid 3α-hydroxy-5α-pregnan-20-one in mice. Pharmacol. Biochem. Behav. 1997, 56, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, M.H.; Ormerod, B.K.; Jhamandas, K.; Boegman, R.J.; Beninger, R.J. Neurosteroids and reward: Allopregnanolone produces a conditioned place aversion in rats. Pharmacol. Biochem. Behav. 2000, 67, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Vashchinkina, E.; Manner, A.K.; Vekovischeva, O.; Hollander, B.D.; Uusi-Oukari, M.; Aitta-Aho, T.; Korpi, E.R. Neurosteroid agonist at GABAA receptor induces persistent neuroplasticity in VTA dopamine neurons. Neuropsychopharmacology 2014, 39, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Rouge-Pont, F.; Mayo, W.; Marinelli, M.; Gingras, M.; Le Moal, M.; Piazza, P.V. The neurosteroid allopregnanolone increases dopamine release and dopaminergic response to morphine in the rat nucleus accumbens. Eur. J. Neurosci. 2002, 16, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Motzo, C.; Porceddu, M.L.; Flore, G.; Concas, A.; Dazzi, L.; Biggio, G. Inhibition of Basal and Stress-Induced Dopamine Release in the Cerebral Cortex and Nucleus Accumbens of Freely Moving Rats by the Neurosteroid Allopregnanolone. J. Psychopharmacol. 1996, 10, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Dornellas, A.P.S.; Macedo, G.C.; McFarland, M.H.; Gómez-A, A.; O’Buckley, T.K.; Da Cunha, C.; Morrow, A.L.; Robinson, D.L. Allopregnanolone decreases evoked dopamine release differently in rats by sex and estrous stage. Front. Pharmacol. 2021, 11, 608887. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, J.; Liu, Y.; Chen, K.H.; Baraban, J.M.; Qiu, Z. Ventral tegmental area astrocytes modulate cocaine reward by tonically releasing GABA. Neuron 2023, 111, 1104–1117.e6. [Google Scholar] [CrossRef] [PubMed]
- Maguire, E.P.; Macpherson, T.; Swinny, J.D.; Dixon, C.I.; Herd, M.B.; Belelli, D.; Stephens, D.N.; King, S.L.; Lambert, J.J. Tonic inhibition of accumbal spiny neurons by extrasynaptic α4βδ GABAA receptors modulates the actions of psychostimulants. J. Neurosci. 2014, 34, 823–838. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.J.; Maguire, E.P.; Cunningham, L.; Gunn, B.G.; Linke, M.; Zechner, U.; Dixon, C.I.; King, S.L.; Stephens, D.N.; Swinny, J.D.; et al. Early-life adversity selectively impairs α2-GABAA receptor expression in the mouse nucleus accumbens and influences the behavioral effects of cocaine. Neuropharmacology 2018, 141, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Knapp, S.; Maguire, E.P.; Hosie, A.M.; Thomas, P.; Mortensen, M.; Bhome, R.; Martinez, A.; Walker, S.E.; Dixon, C.I.; et al. Mutations in the Gabrb1 gene promote alcohol consumption through increased tonic inhibition. Nat. Commun. 2013, 4, 2816. [Google Scholar] [CrossRef] [PubMed]
- Walton, N.L.; Antonoudiou, P.; Barros, L.; Dargan, T.; DiLeo, A.; Evans-Strong, A.; Gabby, J.; Howard, S.; Paracha, R.; Sánchez, E.J.; et al. Impaired endogenous neurosteroid signaling contributes to behavioral deficits associated with chronic stress. Biol. Psychiatry 2023, 94, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Rewal, M.; Gill, T.M.; Ron, D.; Janak, P.H. Extrasynaptic delta-containing GABAA receptors in the nucleus accumbens dorsomedial shell contribute to alcohol intake. Proc. Natl. Acad. Sci. USA 2011, 108, 10–15. [Google Scholar] [CrossRef]
- Rewal, M.; Donahue, R.; Gill, T.M.; Nie, H.; Ron, D.; Janak, P.H. Alpha4 subunit-containing GABAA receptors in the accumbens shell contribute to the reinforcing effects of Alcohol. Addict. Biol. 2012, 17, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, T.; Dixon, C.I.; Robertson, J.; Sindarto, M.M.; Janak, P.H.; Belelli, D.; Lambert, J.J.; Stephens, D.N.; King, S.L. α4-containing GABAA receptors on drd2 neurons of the nucleus accumbens mediate instrumental responding for conditioned reinforcers and its potentiation by cocaine. eNeuro 2023, 10, ENEURO.0236-23.2023. [Google Scholar] [CrossRef]
- Watanabe, M.; Fukaya, M.; Sakimura, K.; Manabe, T.; Mishina, M.; Inoue, Y. Selective scarcity of NMDA receptor channel subunits in the stratum lucidum (mossy fibre-recipient layer) of the mouse hippocampal CA3 subfield. Eur. J. Neurosci. 1998, 10, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Ueno, S.; Bracamontes, J.; Zorumski, C.; Weiss, D.S.; Steinbach, J.H. Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABA(A) receptor. J. Neurosci. 1997, 17, 625–634. [Google Scholar] [CrossRef] [PubMed]
- McCartney, M.R.; Deeb, T.Z.; Henderson, T.N.; Hales, T.G. Tonically active GABAA receptors in hippocampal pyramidal neurons exhibit constitutive GABA-independent gating. Mol. Pharmacol. 2007, 71, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Sexton, C.A.; Penzinger, R.; Mortensen, M.; Bright, D.P.; Smart, T.G. Structural determinants and regulation of spontaneous activity in GABAA receptors. Nat. Commun. 2021, 12, 5457. [Google Scholar] [CrossRef] [PubMed]
- Dalby, N.O.; Falk-Petersen, C.B.; Leurs, U.; Scholze, P.; Krall, J.; Frølund, B.; Wellendorph, P. Silencing of spontaneous activity at α4β1/3δ GABAA receptors in hippocampal granule cells reveals different ligand pharmacology. Br. J. Pharmacol. 2020, 177, 3975–3990. [Google Scholar] [CrossRef] [PubMed]
- Wlodarczyk, A.I.; Sylantyev, S.; Herd, M.B.; Kersanté, F.; Lambert, J.J.; Rusakov, D.A.; Linthorst, A.C.E.; Semyanov, A.; Belelli, D.; Pavlov, I.; et al. GABA-independent GABAA receptor openings maintain tonic currents. J. Neurosci. 2013, 33, 3905–3914. [Google Scholar] [CrossRef] [PubMed]
- Varoqueaux, F.; Jamain, S.; Brose, N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur. J. Cell Biol. 2004, 83, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Gredell, J.A.; Turnquist, P.A.; MacIver, M.B.; Pearce, R.A. Determination of diffusion and partition coefficients of propofol in rat brain tissue: Implications for studies of drug action in vitro. Br. J. Anaesth. 2004, 93, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Benkwitz, C.; Liao, M.; Laster, M.J.; Sonner, J.M.; Eger, E.I.; Pearce, R.A. Determination of the EC50 amnesic concentration of etomidate and its diffusion profile in brain tissue: Implications for in vitro studies. Anesthesiology 2007, 106, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.L.; Wafford, K.A.; Brown, A.R.; Belelli, D.; Lambert, J.J.; Mirza, N.R. A study of subunit selectivity, mechanism and site of action of the delta selective compound 2 (DS2) at human recombinant and rodent native GABA(A) receptors. Br. J. Pharmacol. 2013, 168, 1118–1132. [Google Scholar] [CrossRef] [PubMed]
- Belelli, D.; Casula, A.; Ling, A.; Lambert, J.J. The Influence of Subunit Composition on the Interaction of Neurosteroids with GABAA Receptors. Neuropharmacology 2002, 43, 651–661. [Google Scholar] [CrossRef]
- Sun, C.; Zhu, H.; Clark, S.; Gouaux, E. Cryo-EM structures reveal native GABAA receptor assemblies and pharmacology. Nature 2023, 622, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Durkin, E.J.; Muessig, L.; Herlt, T.; Lumb, M.J.; Patel, R.; Thomas, P.; Bright, D.P.; Jurd, R.; Moss, S.J.; Dickenson, A.H.; et al. Brain neurosteroids are natural anxiolytics targeting α2 subunit γ-aminobutyric acid type-A receptors. bioRxiv 2018. [Google Scholar] [CrossRef]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.-J.; Zeng, C.-M.; Wang, C.; Covey, D.F.; Zorumski, C.F.; Mennerick, S. Cyclodextrins sequester neuroactive steroids and differentiate mechanisms that rate limit steroid actions. Br. J. Pharmacol. 2007, 150, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.R.; Herd, M.B.; Belelli, D.; Lambert, J.J. Developmentally regulated neurosteroid synthesis enhances GABAergic neurotransmission in mouse thalamocortical neurones. J. Physiol. 2015, 593, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.R.; Mitchell, S.J.; Peden, D.R.; Herd, M.B.; Seifi, M.; Swinny, J.D.; Belelli, D.; Lambert, J.J. During postnatal development endogenous neurosteroids influence GABA-ergic neurotransmission of mouse cortical neurons. Neuropharmacology 2016, 103, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Gee, K.W.; Bolger, M.B.; Brinton, R.E.; Coirini, H.; McEwen, B.S. Steroid modulation of the chloride ionophore in rat brain: Structure-activity requirements, regional dependence and mechanism of action. J. Pharmacol. Exp. Ther. 1988, 246, 803–812. [Google Scholar] [PubMed]
- Gee, K.W.; McCauley, L.D.; Lan, N.C. A putative receptor for neurosteroids on the GABAA receptor complex: The pharmacological properties and therapeutic potential of epalons. Crit. Rev. Neurobiol. 1995, 9, 207–227. [Google Scholar] [PubMed]
- Belelli, D.; Lambert, J.J.; Peters, J.A.; Gee, K.W.; Lan, N.C. Modulation of human recombinant GABAA receptors by pregnanediols. Neuropharmacology 1996, 35, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Carboni, E.; Gee, K.W.; Wieland, S.; Lan, N.C. Anxiolytic properties of endogenously occurring pregnanediols in two rodent models of anxiety. Psychopharmacology 1996, 126, 173–178. [Google Scholar] [CrossRef] [PubMed]
- McCauley, L.D.; Liu, V.; Chen, J.S.; Hawkinson, J.E.; Lan, N.C.; Gee, K.W. Selective actions of certain neuroactive pregnanediols at the γ-aminobutyric acid type a receptor complex in rat brain. Mol. Pharmacol. 1995, 47, 354–362. [Google Scholar] [PubMed]
- Hawkinson, J.E.; Drewe, J.A.; Kimbrough, C.L.; Chen, J.S.; Hogenkamp, D.J.; Lan, N.C.; Gee, K.W.; Shen, K.Z.; Whittemore, E.R.; Woodward, R.M. 3α-hydroxy-3β-trifluoromethyl-5α-pregnan-20-one (Co 2-1970): A partial agonist at the neuroactive steroid site of the γ-aminobutyric acidA receptor. Mol. Pharmacol. 1996, 49, 897–906. [Google Scholar] [PubMed]
- Brown, N.; Kerby, J.; Bonnert, T.P.; Whiting, P.J.; Wafford, K.A. Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br. J. Pharmacol. 2002, 136, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Vallée, M. Structure-activity relationship studies on neuroactive steroids in memory, alcohol and stress-related functions: A crucial benefit from endogenous level analysis. Psychopharmacology 2014, 231, 3243–3255. [Google Scholar] [CrossRef]
- Agís-Balboa, R.C.; Pinna, G.; Zhubi, A.; Maloku, E.; Veldic, M.; Costa, E.; Guidotti, A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 14602–14607. [Google Scholar] [CrossRef] [PubMed]
- Modgil, A.; Parakala, M.L.; Ackley, M.A.; Doherty, J.J.; Moss, S.J.; Davies, P.A. Endogenous and synthetic neuroactive steroids evoke sustained increases in the efficacy of GABAergic inhibition via a protein kinase C-dependent mechanism. Neuropharmacology 2017, 113, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Parakala, M.L.; Zhang, Y.; Modgil, A.; Chadchankar, J.; Vien, T.N.; Ackley, M.A.; Doherty, J.J.; Davies, P.A.; Moss, S.J. Metabotropic, but not allosteric, effects of neurosteroids on GABAergic inhibition depend on the phosphorylation of GABAA receptors. J. Biol. Chem. 2019, 294, 12220–12230. [Google Scholar] [CrossRef]
- Althaus, A.L.; Ackley, M.A.; Belfort, G.M.; Gee, S.M.; Dai, J.; Nguyen, D.P.; Kazdoba, T.M.; Modgil, A.; Davies, P.A.; Moss, S.J.; et al. Preclinical characterization of zuranolone (SAGE-217), a selective neuroactive steroid GABAA receptor positive allosteric modulator. Neuropharmacology 2020, 181, 108333. [Google Scholar] [CrossRef] [PubMed]
- Belelli, D.; Peters, J.A.; Phillips, G.D.; Lambert, J.J. The immediate and maintained effects of neurosteroids on GABAA receptors. Curr. Opin. Endocr. Metab. Res. 2022, 24, 100333. [Google Scholar] [CrossRef]
- Vien, T.N.; Ackley, M.A.; Doherty, J.J.; Moss, S.J.; Davies, P.A. Preventing phosphorylation of the GABAAR β3 subunit compromises the behavioral effects of neuroactive steroids. Front. Mol. Neurosci. 2022, 15, 817996. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Dong, J.; Thomas, P. Characterization, neurosteroid binding and brain distribution of human membrane progesterone receptors δ and ε (mPRδ and mPRε) and mPRδ involvement in neurosteroid inhibition of apoptosis. Endocrinology 2013, 154, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. Ganaxolone: First approval. Drugs 2022, 82, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Jayakar, S.S.; Chiara, D.C.; Zhou, X.; Wu, B.; Bruzik, K.S.; Miller, K.W.; Cohen, J.B. Photoaffinity labeling identifies an intersubunit steroid-binding site in heteromeric GABA type A (GABAA) receptors. J. Biol. Chem. 2020, 295, 11495–11512. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.; Frishman, H.B.; Levin, S.; Schwartz, S. The value of urinary pregnanediol estimation for monitoring early pregnancies. Fertil. Steril. 1978, 29, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Rosciszewska, D.; Buntner, B.; Guz, I.; Zawisza, L. Ovarian hormones, anticonvulsant drugs, and seizures during the menstrual cycle in women with epilepsy. J. Neurol. Neurosurg. Psychiatry 1986, 49, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.I.; Zorumski, C.F. Rapid and novel treatments in psychiatry: The future is now. Neuropsychopharmacology 2024, 49, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Maguire, J.; Mody, I. GABAAR plasticity during pregnancy: Relevance to postpartum depression. Neuron 2008, 59, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Campagna, J.J.; Jagodzinska, B.; Wi, D.; Cohn, W.; Lee, J.; Zhu, C.; Huang, C.S.; Molnar, L.; Houser, C.R.; et al. A therapeutic small molecule lead enhances γ-oscillations and improves cognition/memory in Alzheimer’s disease model mice. bioRxiv 2023. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitchell, S.J.; Phillips, G.D.; Tench, B.; Li, Y.; Belelli, D.; Martin, S.J.; Swinny, J.D.; Kelly, L.; Atack, J.R.; Paradowski, M.; et al. Neurosteroid Modulation of Synaptic and Extrasynaptic GABAA Receptors of the Mouse Nucleus Accumbens. Biomolecules 2024, 14, 460. https://doi.org/10.3390/biom14040460
Mitchell SJ, Phillips GD, Tench B, Li Y, Belelli D, Martin SJ, Swinny JD, Kelly L, Atack JR, Paradowski M, et al. Neurosteroid Modulation of Synaptic and Extrasynaptic GABAA Receptors of the Mouse Nucleus Accumbens. Biomolecules. 2024; 14(4):460. https://doi.org/10.3390/biom14040460
Chicago/Turabian StyleMitchell, Scott J., Grant D. Phillips, Becks Tench, Yunkai Li, Delia Belelli, Stephen J. Martin, Jerome D. Swinny, Louise Kelly, John R. Atack, Michael Paradowski, and et al. 2024. "Neurosteroid Modulation of Synaptic and Extrasynaptic GABAA Receptors of the Mouse Nucleus Accumbens" Biomolecules 14, no. 4: 460. https://doi.org/10.3390/biom14040460
APA StyleMitchell, S. J., Phillips, G. D., Tench, B., Li, Y., Belelli, D., Martin, S. J., Swinny, J. D., Kelly, L., Atack, J. R., Paradowski, M., & Lambert, J. J. (2024). Neurosteroid Modulation of Synaptic and Extrasynaptic GABAA Receptors of the Mouse Nucleus Accumbens. Biomolecules, 14(4), 460. https://doi.org/10.3390/biom14040460





