Abstract
Soybean [Glycine max (L.) Merr.] is a short-day (SD) plant that is sensitive to photoperiod, which influences flowering, maturity, and even adaptation. TEOSINTE-BRANCHED1/CYCLOIDEA/PROLIFERATING CELL FACTOR (TCP) transcription factors have been shown to regulate photoperiodic flowering. However, the roles of TCPs in SD plants such as soybean, rice, and maize remain largely unknown. In this study, we cloned the GmTCP40 gene from soybean and investigated its expression pattern and function. Compared with wild-type (WT) plants, GmTCP40-overexpression plants flowered earlier under long-day (LD) conditions but not under SD conditions. Consistent with this, the overexpression lines showed upregulation of the flowering-related genes GmFT2a, GmFT2b, GmFT5a, GmFT6, GmAP1a, GmAP1b, GmAP1c, GmSOC1a, GmSOC1b, GmFULa, and GmAG under LD conditions. Further investigation revealed that GmTCP40 binds to the GmAP1a promoter and promotes its expression. Analysis of the GmTCP40 haplotypes and phenotypes of soybean accessions demonstrated that one GmTCP40 haplotype (Hap6) may contribute to delayed flowering at low latitudes. Taken together, our findings provide preliminary insights into the regulation of flowering time by GmTCP40 while laying a foundation for future research on other members of the GmTCP family and for efforts to enhance soybean adaptability.
1. Introduction
Soybean [Glycine max (L.) Merr.] is a short-day (SD) plant that is sensitive to photoperiod. Originally temperate plants, soybean plants have been grown over a wide range of latitudes worldwide, from 53° N to 35° S [1]. Importantly, the photoperiod sensitivity of a soybean variety determines where it can be planted. Because photoperiod sensitivity determines the timing of flowering and maturity, if a soybean variety is planted in a region with inappropriate day length, the plants will fail to mature. Therefore, it is important to elucidate the genetic networks of photoperiodic flowering to improve the adaptability of soybean to different regions.
A basic model for the regulation of flowering time in soybean has been established in which E3/E4-E1-GmFLOWERING LOCUS Ts (FTs) control flowering under different photoperiods. E1 is an inhibitor of the soybean flowering pathway, regulating the expression of GmFTs [2,3,4], and E3 (phytochrome A3 [PHYA3]) and E4 (PHYA2) directly bind to and stabilize E1 and its homologs [5]. FT proteins integrate signals and are transported from the leaves to the shoot apex to regulate flowering [6]. In soybean, GmFTs regulate flowering by indirectly influencing the expression of floral meristem identity genes such as GmAPETALA1a (AP1a), GmAP1b, GmSUPPRESSOR OF OVEREXPRESSOR OF CONSTANS 1a (SOC1a), and GmSOC1b.
The plant-specific TEOSINTE-BRANCHED1/CYCLOIDEA/PROLIFERATING CELL FACTOR (TCP) transcription factor, which is a key regulator of plant growth and development [7], also regulates photoperiodic flowering by directly or indirectly regulating the expression of floral integrators such as FT, CONSTANS (CO), SOC1, and AP1. The TCP transcription factor family was named based on the first characterized members, namely TEOSINTE BRANCHED1 (TB1) in maize (Zea mays), CYCLOIDEA (CYC) in snapdragon (Antirrhinum majus), and PROLIFERATING CELL FACTOR 1 (PCF1) and PCF2 in rice (Oryza sativa), which each contain a noncanonical basic helix–loop–helix motif referred to as the TCP domain [8,9,10]. The TCP family consists of two classes (Class I and Class II) that are distinguishable by specific amino acids in the TCP domain [11,12]. The Class II TCP members are divided into two subclasses: CINCINNATA (CIN) and CYC/TB1 [12,13].
In Arabidopsis, the Class I member TCP7 interacts with nuclear factor Y proteins and CO to promote flowering by regulating the expression of SOC1 [14]; TCP15 promotes flowering by regulating SOC1 expression [15]; and TCP8 and TCP23 contribute to flowering time determination by regulating FT and SOC1 expression [16]. The Class II TCP members TCP2, TCP3, TCP4, TCP10, and TCP24 are miR319-target genes that promote flowering by positively regulating CO expression [17]. TCP4 physically interacts with the flowering promoter GIGANTEA (GI) and promotes CO expression in a GI-dependent manner [18]. TCP5, TCP13, and TCP17 are integrated into the FT-FD complex and regulate flowering by directly binding to the promoter of AP1 [19]. TCP20 and TCP22 interact with LIGHT-REGULATED WD1 (LWD1) and activate the expression of the circadian clock pathway gene CIRCADIAN CLOCK ASSOCIATED1 (CCA1) to inhibit flowering [20]. TCPs have also been shown to play roles in flowering time regulation in soybean. GmTCP13, a member of the CIN subclass, has been identified as a candidate gene for QNE1 (QTL near E1) and functions as a positive regulator of flowering by enhancing the expression of GmFT2a and GmFT5a [21]. In addition, GmMRF2 promotes flowering by interacting with another member of the CIN subclass, GmTCP15, to induce the expression of GmSOC1b [22]. However, limited research has been conducted on the regulatory roles of GmTCPs in flowering, particularly of Class I GmTCPs.
In the present study, we cloned the GmTCP40 gene, analyzed its expression pattern, and identified its function in regulating flowering time. We also analyzed its flowering time regulatory mechanism and performed haplotype analysis. We found that GmTCP40 induces flowering by upregulating the expression of the downstream flowering-related gene GmAP1a under long-day (LD) conditions but not under SD conditions. Association analysis of haplotypes with flowering time across 175 resequenced soybean varieties revealed that one GmTCP40 haplotype, GmTCP40-Hap6, may contribute to later flowering at low latitudes. Taken together, our results indicate that GmTCP40 plays an important role in soybean flowering and may contribute to soybean adaptability.
2. Materials and Methods
2.1. Phylogenetic Analyses
The sequences of the TCP proteins from Arabidopsis thaliana, rice (Oryza sativa), maize (Zea mays), and soybean for phylogenetic analyses were downloaded from the Phytozome13 database (https://phytozome-next.jgi.doe.gov/, accessed on 8 July 2021). Phylogenetic trees for the TCP protein family were constructed using the neighbor-joining method, with a bootstrap value of 1000, in MEGA 7 software (https://www.megasoftware.net/, accessed on 15 July 2021) [23]. Then, the phylogenetic tree was visualized using the online tool Evolview (https://www.evolgenius.info/evolview/, accessed on 20 October 2023).
2.2. Plant Materials and Growth Conditions
The soybean varieties “Zigongdongdongdou (ZGDD)” and “Heihe27 (HH27)” were planted in controlled culture rooms at 26 °C under SD (12 h light/12 h dark) and LD (16 h light/8 h dark) conditions for analysis of the expression pattern of GmTCP40 (Glyma16G004300). ZGDD and HH27 have been used as model genotypes in the study of photoperiod responses. The photoperiod-sensitive variety ZGDD belongs to maturity group (MG) VIII, with genotype E1/E2/E3/E4. The photoperiod-insensitive variety HH27 belongs to MG 0, with genotype e1-as/e2-ns/e3-tr/E4 [24]. The soybean variety “Jack” was used to examine tissue-specific expression profiles of GmTCP40. Cotyledons and shoot apical meristems (SAMs) were collected 15 days after the emergence of soybean cotyledons from the soil surface (VE) [25]. After the plants had flowered, different organs (roots, leaves, stems, and flowers) were sampled for RNA extraction.
The soybean variety “Jack” was used for plant transformation. All the transgenic and wild-type (WT) plants were grown under SD and LD conditions for phenotypic measurements. There were five pots for each of the three transgenic lines and WT plants, with each pot containing four seeds. The planting substrate consisted of a mixture of soil and vermiculite in a ratio of 1:2. We collected leaves from the transgenic and WT plants for RNA extraction.
A diverse panel of 175 soybean varieties, namely 88 varieties from Northeast China, 49 varieties from Huang-Huai-Hai, and 38 varieties from South China, was used for GmTCP40 haplotype analysis (Supplementary Table S1). The panel was planted at seven different locations during 2016 and 2017: Sanya (18°18′ N, 112°39′ E) in 2016, Xiangtan (27°40′ N, 112°39′ E) in 2016, Jining (35°26′ N, 116°35′ E) in 2016, Xinxiang (35°08′ N, 113°45′ E) in 2016 and 2017, Beijing (40°13′ N, 116°33′ E) in 2016 and 2017, Changchun (43°50′ N, 124°82′ E) in 2017, and Heihe (50°24′ N, 127°49′ E) in 2017. These nine environments were named SY2016, JN2016, XX2016, BJ2016, XT2016, XX2017, BJ2017, CC2017, and HH2017, respectively (Supplementary Table S2). All the materials were arranged in randomized complete blocks with two replications. The flowering time across these nine environments for the 175 soybean varieties was recorded as the days from VE to the beginning of blooming (R1), defined as the appearance of the first flower at any node on the main stem [25]. The average flowering time of two replicates was used for analysis.
2.3. RNA Extraction and qPCR
Total RNA was extracted using the RNA Easy Fast Plant Tissue Kit (TIANGEN Biotech [Beijing] Co., Ltd., China, Cat#DP452). cDNAs were synthesized with the FastKing RT kit (with gDNA) (TIANGEN Biotech [Beijing] Co., Ltd., Beijing, China, Cat#KR116). Quantitative PCR (qPCR) was performed using Taq Pro Universal SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd., Nanjing, China, Cat#Q712-02). Three replicates were performed for each sample. The 2−ΔΔCt method was used for the calculation of relative expression levels [26]. The primers used for qPCR are listed in Supplementary Table S3.
2.4. Plasmid Construction and Plant Transformation
The coding sequence (CDS) of GmTCP40 was cloned from “Jack” to construct the overexpression vector. The amplified cDNA was inserted into an XbaI-digested pTF101 vector [27] using the ClonExpress® Ultra One Step Cloning Kit (Vazyme Biotech Co., Ltd., Nanjing, China, Cat#C115). Expression of the GmTCP40-GFP fusion protein was driven by the CaMV 35S promoter (Supplementary Figure S4A). The recombinant vector pTF101-GmTCP40 was subsequently introduced into Agrobacterium tumefaciens strain EHA101 (Beijing Zoman Biotech Co., Ltd., Beijing, China, Cat#ZC1405) as described in Supplementary Methods. pTF101-GmTCP40 was subsequently transformed into the soybean variety “Jack” according to a previous protocol [28]. Transgenic plants were identified using Liberty Link strips with PAT proteins (Aojin Biotech [Tianjin] Co., Ltd., Tianjin, China, Cat#AG-002-SLF) and PCR. The primers used for plasmid construction are listed in Supplementary Table S3.
2.5. Subcellular Localization of GmTCP40
The Agrobacterium tumefaciens strain GV3101 carrying pTF101-GmTCP40 was injected into the leaves of Nicotiana benthamiana as described by Kubota et al. [18]. The fluorescence signals of GmTCP40-GFP were observed and imaged using an FV3000 confocal microscope (Olympus Corp., Shinjuku-ku, Japan).
2.6. Phenotyping and Statistical Analysis
The flowering times of the transgenic plants and WT plants were recorded as the number of days from VE to R1 [25]. All the statistical analyses (Student’s t-tests) were performed using GraphPad 8.
2.7. Haplotype Analysis of GmTCP40
To investigate the natural variation in GmTCP40, we analyzed the resequencing data of 175 varieties which were downloaded from the National Center for Biotechnology Information (NCBI) database under the Short Read Archive (SRA) accession numbers SRP062560 and PRJNA589345 [29]. Using Tassel 5 software, we changed the format to intuitively observe the changes in nucleotides compared with the reference genome Wm82.a2.v1 (https://phytozome-next.jgi.doe.gov/info/Gmax_Wm82_a2_v1, accessed on 13 September 2023). We conducted an association analysis of GmTCP40 haplotypes with flowering time using SPSS 21.0 via Duncan’s multiple range test using (* p < 0.05). GraphPad Prism 8 and Excel 2019 were used for visual mapping.
2.8. Yeast One-Hybrid System
The EGY48-LacZ system was employed for yeast one-hybrid (Y1H) assays (Clontech Laboratories, Inc., Mountain View, CA, USA). For the construction of the pB42AD-GmTCP40 vector, the pB42AD vector was digested with the EcoRI and XhoI restriction enzymes, followed by seamless ligation of the purified GmTCP40 CDS fragment into the linearized pB42AD vector using the ClonExpress® Ultra One Step Cloning Kit (Vazyme Biotech Co., Ltd., Nanjing, China, Cat#C115). A 3000 bp fragment of the GmAP1a promoter was amplified from the soybean variety “Jack”. The amplified fragment was subcloned into the pLacZ2u vector digested with the KpnI and XhoI restriction enzymes using the ClonExpress® Ultra One Step Cloning Kit (Vazyme Biotech Co., Ltd., Nanjing, China, Cat#C115). Three fragments (967 bp, 2955 bp, and 5541 bp) of the GmSOC1a promoter were amplified and inserted into different pLacZ2u vectors (Supplementary Figure S3A). Meanwhile, 1243 bp, 2906 bp, and 4943 bp fragments of the GmSOC1b promoter were cloned into different pLacZ2u vectors (Supplementary Figure S3B). Appropriate pairs of constructs were subsequently transformed into the yeast strain EGY48, as described in the Supplementary Methods. The yeast clones were grown on SD/-Trp/-Ura media (Beijing Coolabi Technology Co., Ltd., Beijing, China, Cat#PM2262), which was selected for yeast clones containing the desired plasmids, at 30 °C for 3 days. Then, the yeast clones were spotted onto SD media (lacking Trp and Ura) supplemented with X-gal, Galactose (Gal), and Raffinose (Raf) to detect interactions. The primers used for plasmid construction are listed in Supplementary Table S3.
2.9. Transient Luciferase Reporter Assays
To generate effector constructs, the CDS of GmTCP40 (with KpnI and PstI restriction sites on the primers to amplify the CDS) was inserted into the pGreenII 62-SK vector using the ClonExpress® Ultra One Step Cloning Kit (Vazyme Biotech Co., Ltd., Nanjing, China, Cat#C115). The amplified promoter of GmAP1a (with KpnI and PstI restriction sites on the primers to amplify the promoter) was subcloned into the pGreenII 0800-LUC vector using the ClonExpress® Ultra One Step Cloning Kit (Vazyme Biotech Co., Ltd., Nanjing, China, Cat#C115). The Renilla luciferase (REN) was used as an internal control. The relevant constructs were introduced into Agrobacterium strain EHA105 (pSoup) (Beijing Zoman Biotech Co., Ltd., Beijing, China, Cat#ZC1048) and subsequently co-infiltrated into tobacco leaves, as described by Kubota et al. [18]. Two days after infiltration, the injected leaves were sprayed with 2.5 mM beetle luciferin potassium salt (Promega Corp., Madison, USA, Cat#E1601) and then incubated in darkness for 5 min before being transferred to a Tannon5200 (Shanghai, China) for capturing the luciferase signals. Then, we collected leaves and performed extraction followed by the addition of substrates for REN and LUC separately to evaluate their activities with the Dual-Luciferase Reporter Gene Assay Kit (Yeasen Biotech Co., Ltd., Shanghai, China, Cat#11402ES60). The primers used for plasmid construction are listed in Supplementary Table S3.
3. Results
3.1. Cloning of GmTCP40 and Determination of Its Expression Patterns
A phylogenetic tree of the TCP family was constructed using TCP proteins from Arabidopsis, rice, maize, and soybean (Supplementary Figure S1). In soybean, 54 TCPs were identified and classified into two classes: Class I (26 GmTCPs) and Class II (28 GmTCPs) [6]. Class II was divided into two subclasses: CIN (19 GmTCPs) and CYC/TB (9 GmTCPs). The flowering regulators GmTCP13 and GmTCP15 were assigned to the CIN subclass, and their Arabidopsis homologs are associated with flowering time regulation (Supplementary Figure S1). The regulatory roles of numerous Class I genes such as AtTCP7, AtTCP15, AtTCP22, and AtTCP23 in photoperiodic flowering have been extensively investigated in Arabidopsis [14,15,16,20,30]. However, the precise regulatory roles of these TCPs in soybean remain to be fully elucidated. Here, we studied the soybean gene GmTCP40, a gene closely related to Arabidopsis Class I TCPs (Supplementary Figure S1).
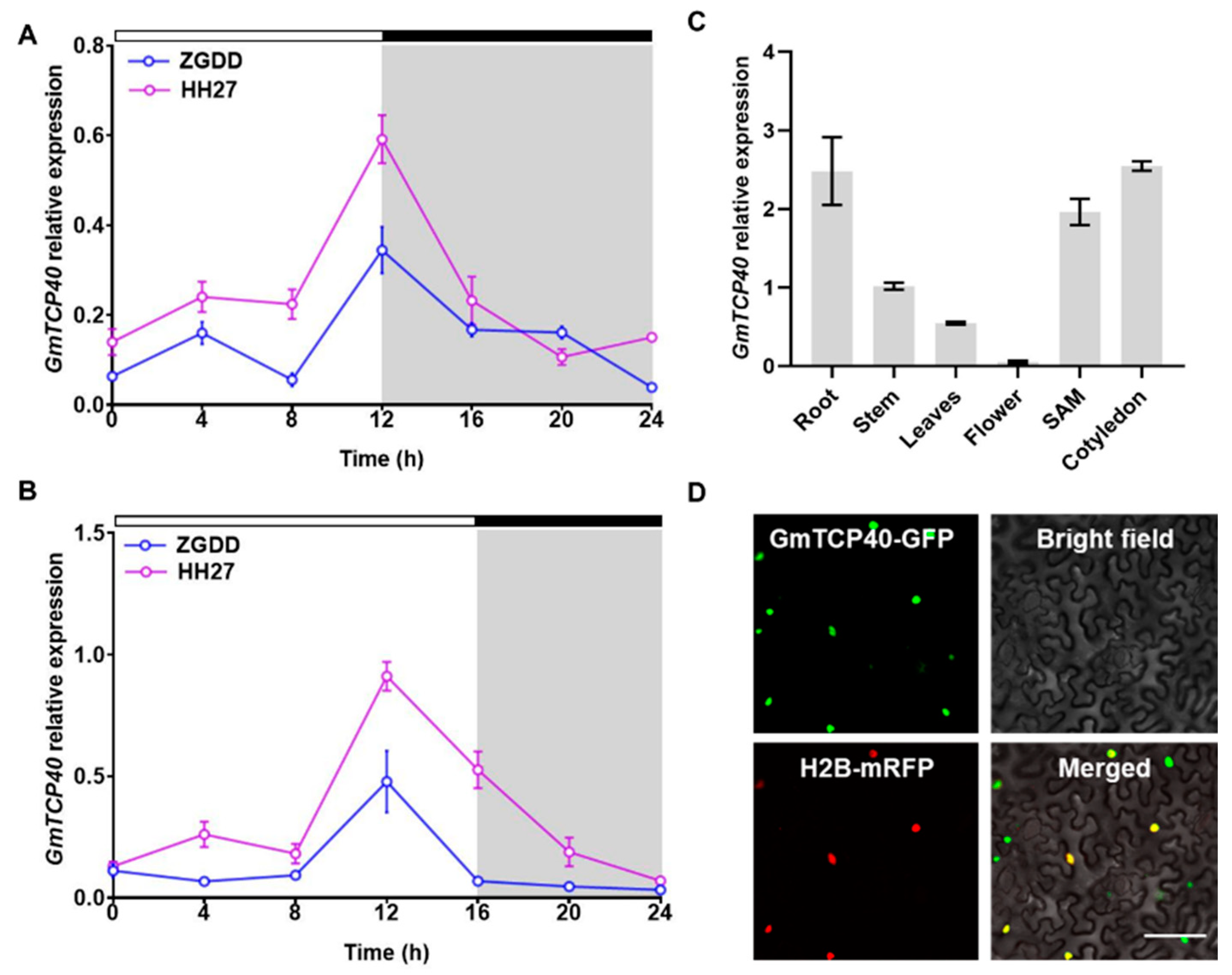
The expression patterns of GmTCP40 were initially investigated in the early-maturity soybean variety “HH27” and late-maturity soybean variety “ZGDD” under SD and LD conditions. HH27 flowered at 28.1 ± 1.7 days under SD condition and 27.6 ± 1.8 days under LD condition [31], while ZGDD flowered at 32.0 ± 2.5 days under SD conditions and maintained vegetative growth under LD conditions [31]. In both the HH27 and ZGDD plants, the expression of GmTCP40 peaked at 12 h after dawn under both SD and LD conditions (Figure 1A,B). The expression level of GmTCP40 was higher in HH27 than in ZGDD under SD and LD conditions.
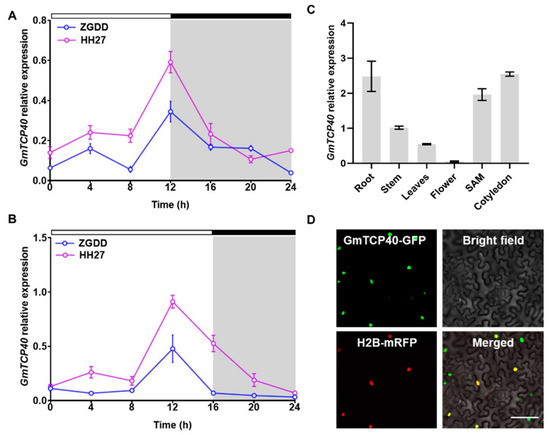
Figure 1.
Expression pattern analysis of GmTCP40. (A,B) Expression levels of GmTCP40 in the leaves of soybean varieties “Heihe27 (HH27)” and “Zigongdongdou (ZGDD)” under short-day (SD) (12 h light/12 h dark) (A) and long-day (LD) (16 h light/8 h dark) (B) conditions. The white and gray bars represent light and dark periods, respectively. (C) Expression levels of GmTCP40 in different organs of the variety “Jack”. (D) Subcellular localization of the GmTCP40-GFP fusion protein in Nicotiana benthamiana leaves. Scale bar, 20 μm. The data are presented as the mean ± SD of three biological replicates.
We subsequently examined the expression levels of GmTCP40 in various organs of the “Jack” variety under SD conditions. Notably, GmTCP40 was expressed predominantly in the cotyledons, roots, and SAM (Figure 1C), while lower expression levels were detected in the stem, leaf, and flower tissues.
To investigate the subcellular localization of the GmTCP40 protein, we generated a construct expressing a GmTCP40-GFP fusion protein and introduced it into N. benthamiana leaves through infiltration. Fluorescence signals from GmTCP40-GFP were observed within the nuclei of epidermal cells in N. benthamiana leaves (Figure 1D).
3.2. Overexpression of GmTCP40 Promotes Soybean Flowering under LD Conditions
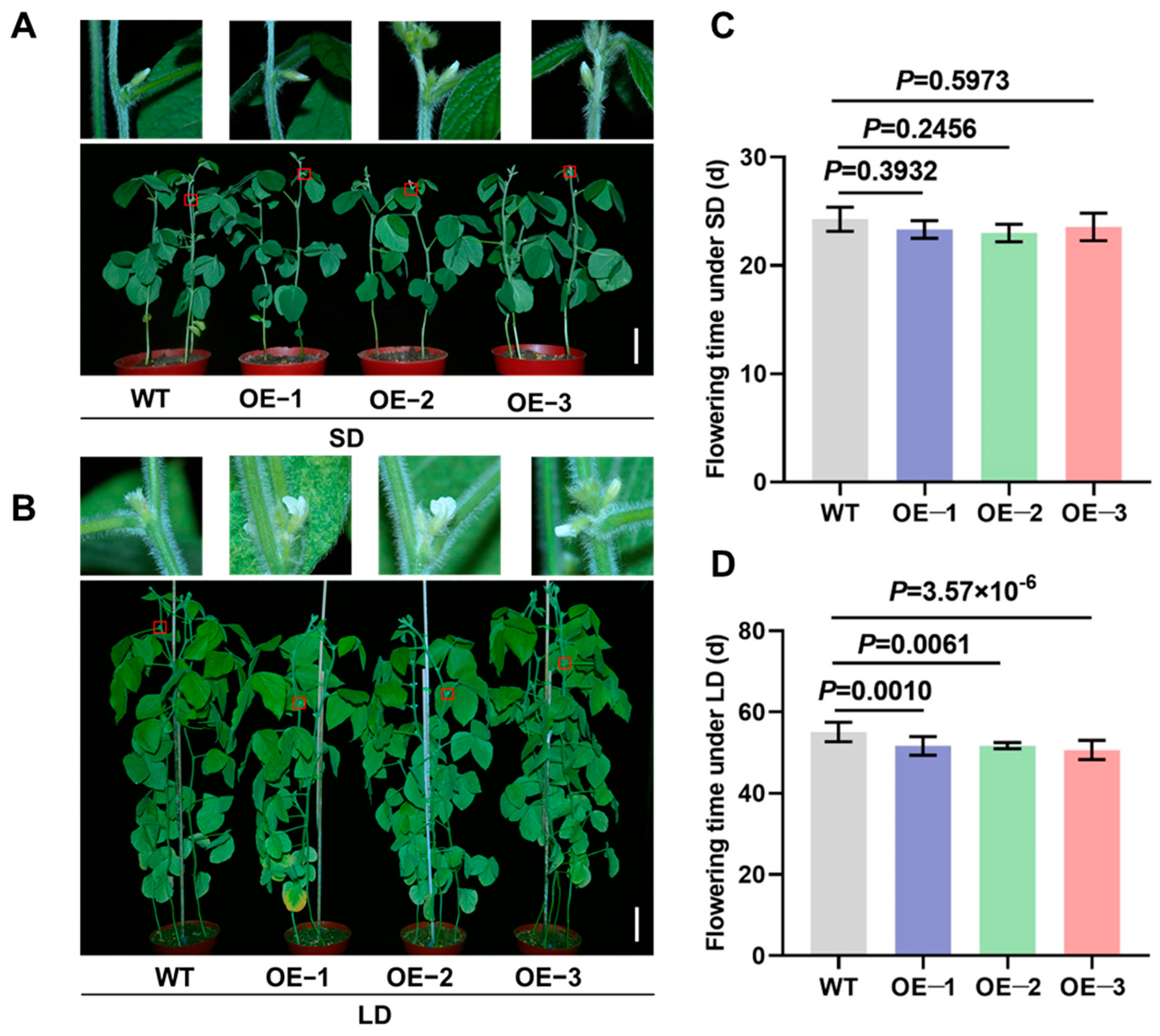
To further elucidate the regulatory role of GmTCP40 in flowering time, we cloned the CDS of GmTCP40 from the soybean variety “Jack” and generated transgenic plants overexpressing GmTCP40 under the control of the CaMV 35S promoter in the “Jack” variety. The transgenic plants were confirmed by the PAT protein test and PCR and qPCR analyses. Three transgenic lines were selected for phenotypic characterization. Under SD conditions, the three GmTCP40-overexpressing (OE) lines, OE-1 (23.33 days), OE-2 (23.00 days), and OE-3 (23.57 days), flowered slightly earlier than WT plants, which flowered at 24.29 days, but these differences were not significant (Figure 2A,C). However, under LD conditions, OE-1 (51.67 days), OE-2 (51.71 days), and OE-3 (50.64 days) plants flowered significantly earlier than the WT plants (55.08 days), a difference of 3.36–4.43 days (Figure 2B,D). These results indicate that GmTCP40 acts as a positive regulator of flowering.
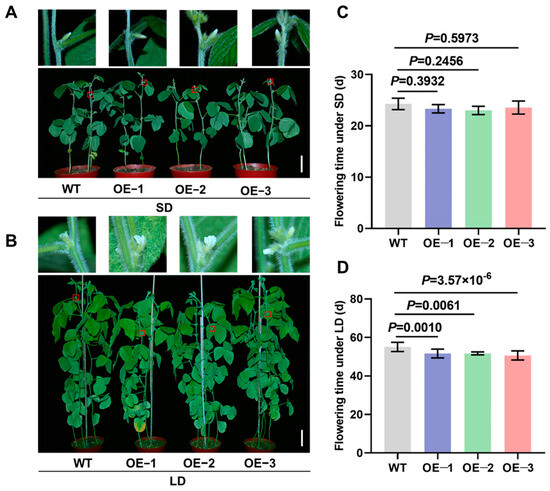
Figure 2.
Overexpression of GmTCP40 promotes flowering in soybean. (A,B) Flowering phenotypes of the three GmTCP40-overexpression lines (OE-1, OE-2, and OE-3) and wild-type (WT) plants under short-day (SD) (12 h light/12 h dark) (A) and long-day (LD) (16 h light/8 h dark) conditions (B). (C,D) Flowering times of OE-1, OE-2, and OE-3 lines and the WT plants under SD conditions (C) and LD conditions (D). Scale bar, 10 cm.
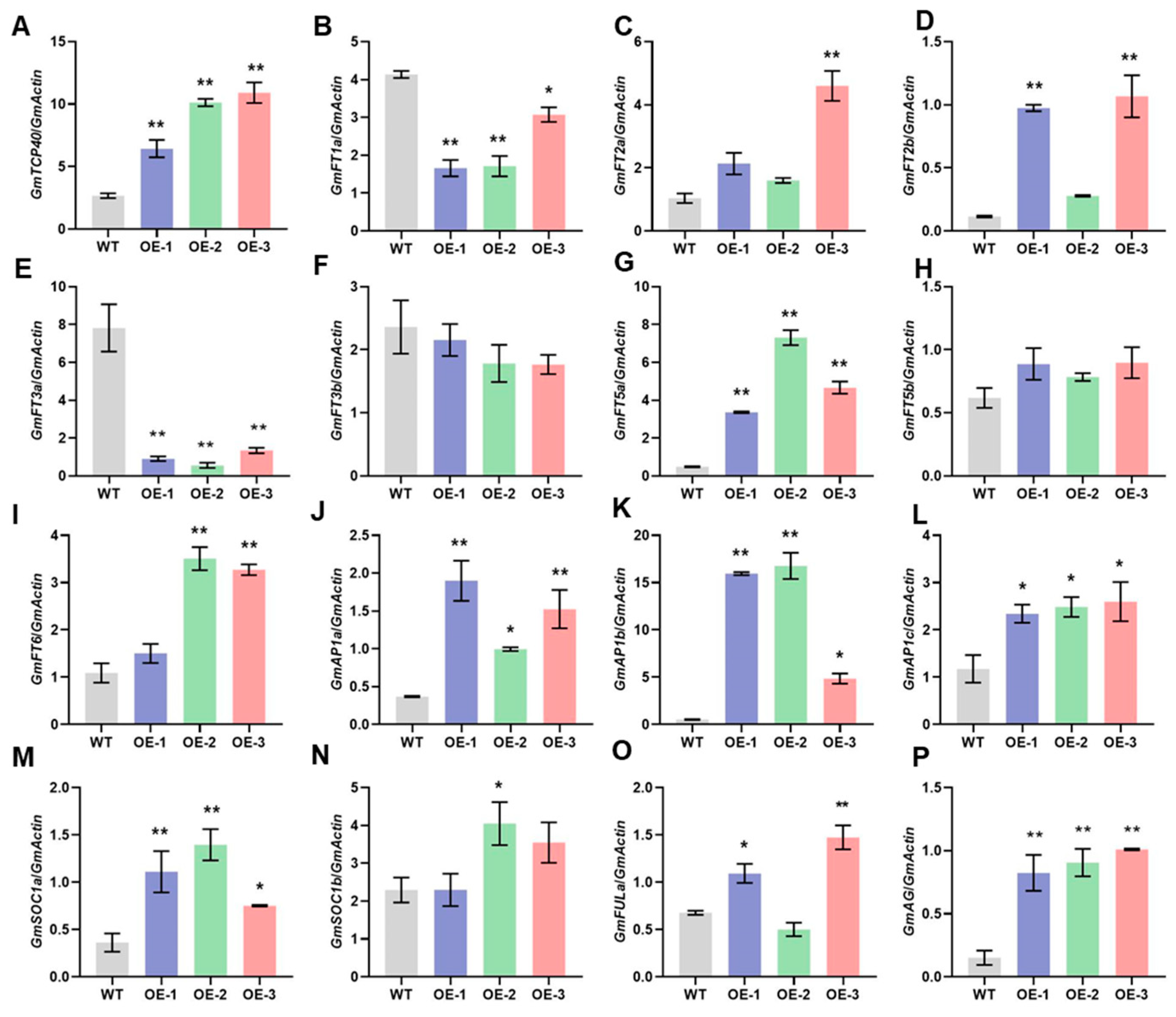
To elucidate the mechanisms by which GmtTCP40 regulates flowering time, we analyzed the expression levels of GmFTs (GmFT1a, GmFT2a, GmFT2b, GmFT3a, GmFT3b, GmFT5a, GmFT5b, and GmFT6), which are integrative factors in the flowering pathway. In addition, we examined the expression profiles of several downstream genes (GmAP1a, GmAP1b, GmAP1c, GmSOC1a, GmSOC1b, GmFRUITFULa [FULa], and GmAGAMOUS [AG]) in the WT and GmTCP40-OE plants. Under LD conditions, the GmTCP40-OE lines exhibited significantly higher expression levels of GmFT2a, GmFT2b, GmFT5a, GmFT6, GmAP1a, GmAP1b, GmAP1c, GmSOC1a, GmSOC1b, GmFULa, and GmAG than the WT plants; however, the expression levels of GmFT1a and GmFT3a were significantly lower in the OE-GmTCP40 plants than in the WT plants (Figure 3). There were no significant differences in the expression levels of GmFT3b or GmFT5b between the GmTCP40-OE and WT plants (Figure 3). Under SD conditions, no significant changes were observed in the expression of any of the genes in either the WT or GmTCP40-OE plants (Supplementary Figure S2). These results suggest that GmTCP40 promotes flowering by regulating the expression of downstream flowering-related genes.
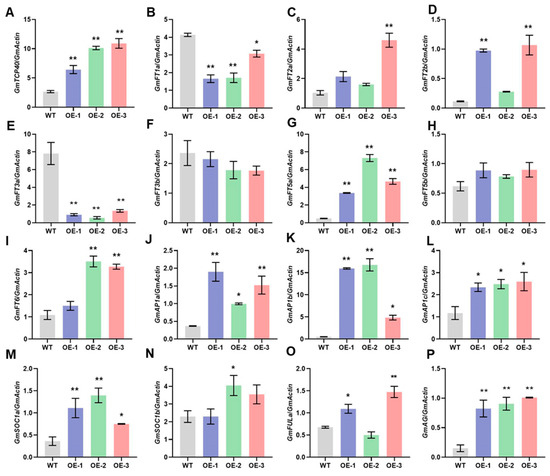
Figure 3.
Expression levels of flowering-related genes in WT plants and GmTCP40-overexpressing transgenic lines under long-day conditions. (A–P) Leaves from three GmTCP40-overexpression lines (OE-1, OE-2, and OE-3) and wild-type (WT) plants under long-day (LD) (16 h light/8 h dark) conditions were harvested for qRT-PCR analysis of the expression levels of GmTCP40 (A), GmFT1a (B), GmFT2a (C), GmFT2b (D), GmFT3a (E), GmFT3b (F), GmFT5a (G), GmFT5b (H), GmFT6 (I), GmAP1a (J), GmAP1b (K), GmAP1c (L), GmSOC1a (M), GmSOC1b (N), GmFULa (O), and GmAG (P). The relative expression level was normalized to that of GmActin. The data are presented as the means ± SDs of three replicates (** p < 0.01; * p < 0.05).
3.3. GmTCP40 Binds to the GmAP1a Promoter
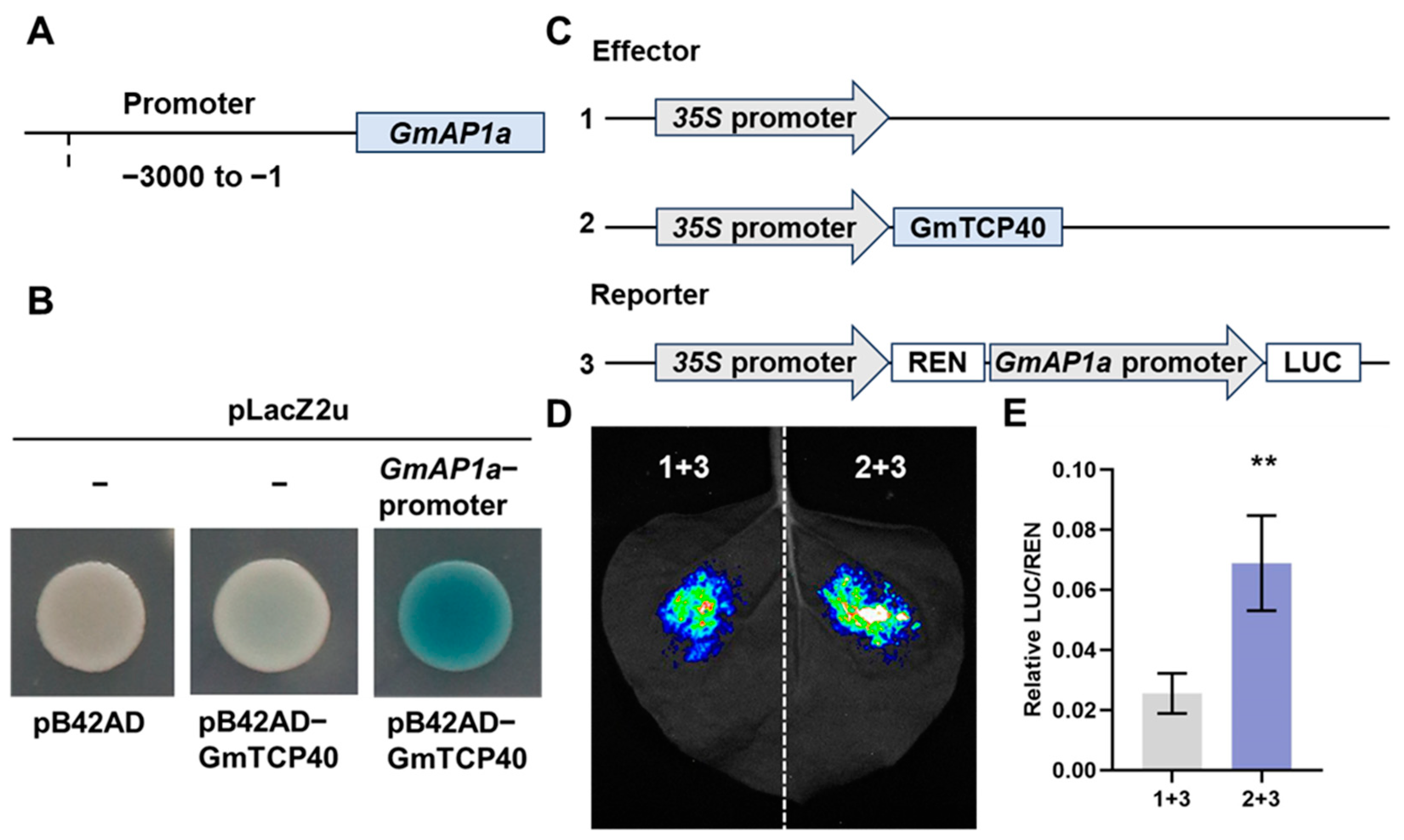
In Arabidopsis, TCP5, TCP13, and TCP17 have been shown to induce flowering by binding to the promoter of AP1 [19], and TCP7 and TCP15 bind to the SOC1 promoter to promote flowering [14,15]. In this study, we investigated whether GmTCP40 could bind to the promoters of GmAP1a, GmSOC1a, and GmSOC1b, which were upregulated in GmTCP40-OE plants. We cloned the promoters of the homologs of SOC1 (GmSOC1a and GmSOC1b) and AP1 (GmAP1a). Y1H assays showed that GmTCP40 bound to the promoter of GmAP1a but not to the promoter of GmSOC1a or GmSOC1b (Figure 4A,B and Figure S3), indicating that GmAP1a is a target gene of GmTCP40. Next, we performed transient luciferase reporter assays, which revealed that GmTCP40 activated transcription of the GmAP1a (Figure 4C–E).
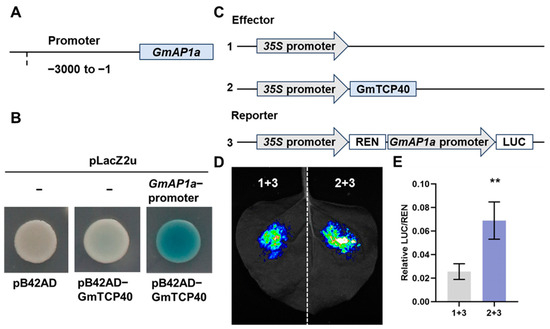
Figure 4.
GmTCP40 activates transcription of the GmAP1a. (A) The 3 kb promoter of GmAP1a was subcloned into the pLacZ2u vector. (B) The interaction between GmTCP40 and the GmAP1a promoter was examined by yeast one-hybrid (Y1H) assays. The transformants were assessed on SD/-Trp/-Ura media supplemented with 20 mM X-gal, Galactose (Gal), and Raffinose (Raf). Empty vectors served as the negative controls. (C) GmTCP40 was inserted into the effector construct pGreenII 62-SK, and the GmAP1a promoter was ligated into the reporter vector pGreenII 0800-LUC. Empty pGreenII 62-SK was used as the negative control. (D,E) The association of GmTCP40 with the GmAP1a promoter was investigated using transient luciferase reporter assays. The data are presented as the means ± SDs of three replicates (** p < 0.01).
3.4. Haplotype Analysis of GmTCP40 Reveals Its Roles in Flowering and Regional Adaptation
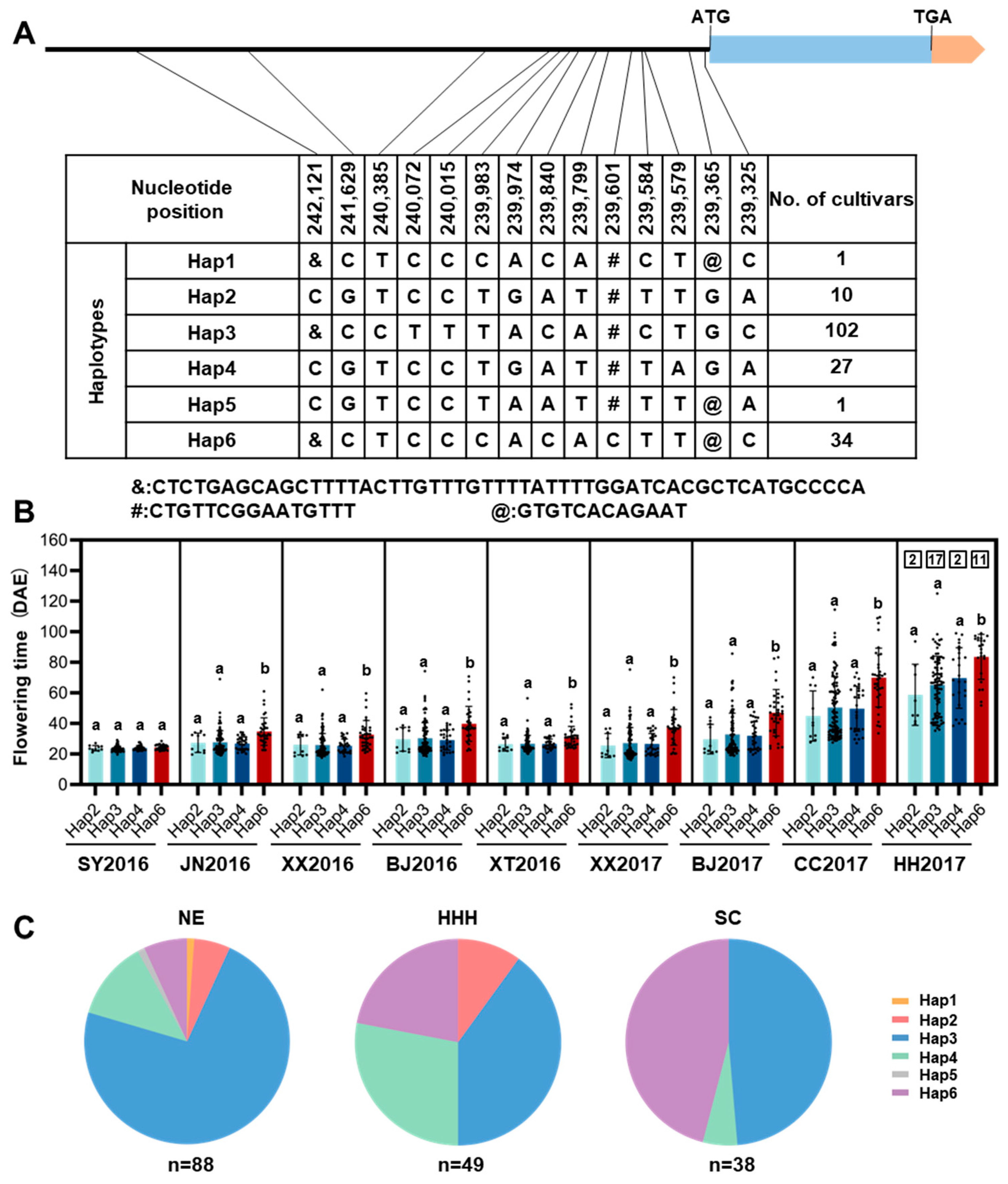
To evaluate the impact of natural variation in GmTCP40 on soybean adaptation, we examined the genotypes of GmTCP40 in 175 soybean varieties originating from diverse geographical regions across China (Supplementary Table S1). No frameshifts or amino acid substitutions were found in the CDS of GmTCP40. A total of 14 polymorphic loci, namely 11 single-nucleotide polymorphisms (SNPs) and three insertion-deletions (indels), were detected in the promoter region, and six haplotypes (Hap1–Hap6) were defined (Figure 5A). We analyzed the geographical distributions of the major haplotypes and their association with flowering time in nine environments across China: Sanya (18°18′ N, 112°39′ E) in 2016, Xiangtan (27°40′ N, 112°39′ E) in 2016, Jining (35°26′ N, 116°35′ E) in 2016, Xinxiang (35°08′ N, 113°45′ E) in 2016 and 2017, Beijing (40°13′ N, 116°33′E) in 2016 and 2017, Changchun (43°50′ N, 124°82′ E) in 2017, and Heihe (50°24′ N, 127°49′ E) in 2017 (Supplementary Table S2; Supplementary Table S4). GmTCP40-Hap3 was widely distributed across China and had flowering times similar to those of GmTCP40-Hap2 and GmTCP40-Hap4 (Figure 5B,C). GmTCP40-Hap6 exhibited a later flowering time in eight environments (Figure 5B), and the frequency of GmTCP40-Hap6 decreased with increasing latitude in China (Figure 5C). Analysis of the cis-elements in the GmTCP40 promoter region revealed two Box-4 and CAAT-box elements located between positions 239,601 and 239,616 (Supplementary Table S5). Box-4 is involved in the light response and the transcriptional regulation of many plant genes specifically expressed in flower organs. The CAAT-box is located in the promoter and enhancer regions of GmTCP40. A large sequence deletion from bp 239,601 to 239,616 in GmTCP40-Hap6 accessions may account for late flowering. These results indicated that naturally occurring mutations in GmTCP40 play an important role in soybean adaptation to a wide range of latitudes in China, and further characterization of GmTCP40-Hap6 might facilitate the genetic improvement of soybean.
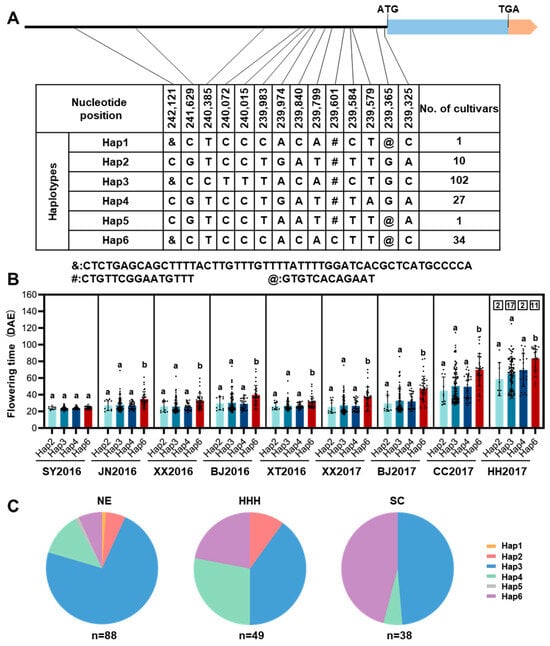
Figure 5.
Analysis of the haplotypes of GmTCP40 in 175 soybean varieties. (A) Natural variation in the nucleotide sequence of GmTCP40. Blue and orange represent the coding region and UTR, respectively. The black line represents the promoter. (B) Flowering time of soybean varieties harboring major GmTCP40 haplotypes. Each dot represents a soybean variety and the number within each box indicates the number of varieties that did not flower. SY2016, JN2016, XX2016, BJ2016, XT2016, XX2017, BJ2017, CC2017, and HH2017: Sanya (18°18′ N, 112°39′ E) in 2016, Xiangtan (27°40′ N, 112°39′ E) in 2016, Jining (35°26′ N, 116°35′ E) in 2016, Xinxiang (35°08′ N, 113°45′ E) in 2016 and 2017, Beijing (40°13′ N, 116°33′ E) in 2016 and 2017, Changchun (43°50′ N, 124°82′ E) in 2017, and Heihe (50°24′ N, 127°49′ E) in 2017, respectively. DAE (days after emergence). The data are presented as the means ± SDs, and a and b indicate significant differences determined by Duncan’s test at p < 0.05. (C) Geographical distribution of soybean varieties harboring different alleles of GmTCP40. NE: Northeast China, HHH: Huang-Huai-Hai, SC: South China.
4. Discussion
In Arabidopsis, TCP family members exhibit diverse functions in photoperiodic flowering regulation. Class I TCPs, such as TCP7, TCP8, TCP14, and TCP15, have been identified as promoters of flowering, while TCP20, TCP22, and TCP23 act as inhibitors [14,15,20,31,32]. In addition, Class II TCPs have been found to be positive regulators of the flowering pathway, such as TCP2 and CIN TCPs (TCP3, TCP4, TCP5, TCP10, TCP13, TCP17, TCP24) [17,18,19]. In soybeans, GmTCP13 and GmTCP15, which belong to the CIN subclass of Class II, have been shown to enhance flowering [21,22]. Interestingly, to date, no Class I GmTCP gene has been identified as a regulator of flowering. Our study revealed that the Class I TCP gene GmTCP40 is also a flowering activator, highlighting the need to investigate the potential roles of other Class I GmTCPs in photoperiodic regulation pathways.
In Arabidopsis, the flowering integrator FT and TERMINAL FLOWER1 (TFL1) play antagonistic roles in regulating flowering with differences in their surface charges resulting from conserved amino acid variations [33,34]. Class I TCP members are potential candidates mediating the differential activities of FT and TFL1 [34]. In soybean, 10 FT homologs play antagonistic roles in regulating flowering with differences in conserved amino acids [35,36,37]. The functional divergence of these homologs may parallel that of FT and TFL1 and could be elucidated through Class I GmTCPs. In our study, we revealed that GmTCP40, a Class I GmTCP, promotes flowering. However, further exploration of the regulatory functions of other Class I GmTCPs is crucial for gaining a comprehensive understanding of photoperiodic regulation pathways in soybeans.
The TCP genes that promote flowering are regulated mainly by controlling downstream genes to regulate flowering. For example, TCP7 promoted flowering by regulating the expression of SOC1 and FT [14]. TCP15 enhanced expression of SOC1 [15]. TCP5, TCP13, and TCP17 regulated the expression levels of both CO, FT, AP1, FUL, and LFY [19]. Similarly, GmTCP40 induced the expression of GmFT2a, GmFT5a, GmAP1s, GmSOC1a, and GmSOC1b to promote flowering (Figure 3). Previous studies have demonstrated that TCP genes promote flowering by controlling downstream genes. TCP7 and TCP15 promote flowering by binding to the promoter of SOC1 [14,15], and GmTCP15 binds to the promoter of GmSOC1b, a homolog of SOC1 [22]. In Arabidopsis, TCP5, TCP13, and TCP17 induce flowering by binding to the promoter of AP1 [19], a gene that plays an important role in floral organ development [38]. We found that similar to these Arabidopsis TCPs, GmTCP40 binds to the promoter region of GmAP1a and does not bind to the promoter region of GmSOC1a or GmSOC1b (Supplementary Figure S3). Soybean has four AP1 homologs, and the gmap1 quadruple mutant shows delayed flowering, whereas overexpression of GmAP1a leads to early flowering [39]. GmAP1a directly binds to the promoter of Dt1, a repressor of flowering, to repress its expression [40]. Thus, GmTCP40 may promote flowering in soybean by promoting the expression of GmAP1a and potentially inhibiting the expression of Dt1. These findings contribute to our understanding of the photoperiodic flowering pathway.
In soybean, FDc interacts with GmFT5a to directly co-induce GmAP1a expression [40]. Soybean FD homologs, such as GmFDL12 and GmFDL19, interact with both GmFT2a and GmFT5a, while others, such as GmFDL06, interact exclusively with GmFT5a, and GmFDL15 interacts specifically with GmFT5b [41,42]. The interaction of GmTCP13 with GmFT2a and GmFT5a was verified by bimolecular fluorescence complementation assays [21]. In Arabidopsis, TCP5, TCP13, and TCP17 interact with the FT-FD complex to bind to the AP1 promoter [19]. This indicates that GmTCP40 may interact with the GmFT5a-FDc complex to regulate the expression of GmAP1a. This mechanism could play an important role in soybeans, especially in regulating flowering time. Further research on the interaction between GmTCP40 and the GmFT5a-FDc complex would help refine the soybean photoperiodic flowering regulatory network.
In a previous study of soybean, two nonsynonymous SNPs were identified in the CDS of GmTCP13 [21]. In addition, a nonsynonymous CDS SNP and seven additional upstream SNPs were observed in the TCP homolog BRC1 (Glyma.06G210600), which has been proposed as a candidate gene involved in regulating branch development [43]. In contrast, SNPs in GmTCP40 were exclusively detected in the promoter region (Figure 5A). Therefore, the TCP protein is relatively conserved in soybeans without any instances of premature termination. A previous study showed that, compared with varieties carrying other haplotypes, GmTCP13-Hap3 varieties displayed a significantly delayed flowering [21]. Similarly, GmTCP40-Hap6 varieties also exhibited a later flowering time and were distributed mainly in South China (SC) (Figure 5B,C).
Deciphering the genetic mechanisms underlying soybean flowering time and regional adaptability is a crucial objective for breeders because of the extensive distribution of soybean varieties resulting from abundant natural variation and diverse combinations of genes and QTLs regulating flowering time. A natural variant of GmELF3 provides soybean plants with an extended juvenile phase that enhances their adaptability in tropical regions [44,45]. In addition, natural variants of GmPRR3a and GmPRR37/3b contribute to soybean adaptation in high-latitude regions [46,47]. The variations in the promoter likely endow GmTCP40-Hap6 with a moderate but appropriate level of activity, leading to late flowering and adaption to low latitudes. Developing kompetitive allele specific PCR (KASP) markers based on these variations and utilizing the markers for genotyping could facilitate efforts to breed soybean varieties with the optimal flowering time for the target planting area, which may facilitate the introduction of soybeans varieties in China.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biom14040465/s1, Supplementary Methods; Supplementary Figure S1: Phylogenetic analysis of TCP homologs from four different plant species; Supplementary Figure S2: Expression levels of flowering-related genes in WT plants and GmTCP40-OE transgenic lines under SD conditions; Supplementary Figure S3: GmTCP40 does not bind to the promoters of GmSOC1a and GmSOC1b; Supplementary Figure S4: Diagrams of pTF101-GmTCP40 and pB42AD-GmTCP40 vector. Supplementary Table S1: Source information of 175 resequenced soybean varieties; Supplementary Table S2: Flowering time of major GmTCP40 haplotypes across 9 environments with sowing time and day length information; Supplementary Table S3: The nucleotide sequences of the primers used in this study; Supplementary Table S4: The flowering time of the 175 soybean varieties across different environments; Supplementary Table S5: The functional predictions of cis-elements identified in the GmTCP40 promoter.
Author Contributions
Conceptualization, F.C. and L.Z.; methodology, L.W., L.Z. and P.W.; software, L.W. and P.W.; validation, L.W. and L.Z.; investigation, L.W. and P.W.; resources, T.W., S.Y., B.J., S.S. and T.H.; writing—original draft preparation, L.Z.; writing—review and editing, X.X., L.W., H.J., M.W. and T.H.; visualization, L.Z.; funding acquisition, T.H. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by three grants as follows: the first funding: the National Key R&D Program of China (2021YFF1001203); the second funding: China Agriculture Research System (CARS-04); the third funding: CAAS Agricultural Science and Technology Innovation Project.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hou, Z.; Fang, C.; Liu, B.; Yang, H.; Kong, F. Origin, Variation, and Selection of Natural Alleles Controlling Flowering and Adaptation in Wild and Cultivated Soybean. Mol. Breed. 2023, 43, 36. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Watanabe, S.; Yamada, T.; Tsubokura, Y.; Nakashima, H.; Zhai, H.; Anai, T.; Sato, S.; Yamazaki, T.; Lü, S.; et al. Positional Cloning and Characterization Reveal the Molecular Basis for Soybean Maturity Locus E1 that Regulates Photoperiodic Flowering. Proc. Natl. Acad. Sci. USA 2012, 109, E2155–E2164. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yamagishi, N.; Zhao, C.; Takeshima, R.; Kasai, M.; Watanabe, S.; Kanazawa, A.; Yoshikawa, N.; Liu, B.; Yamada, T.; et al. The Soybean-specific Maturity Gene E1 Family of Floral Repressors Controls Night-break Responses through Down-regulation of FLOWERING LOCUS T Orthologs. Plant Physiol. 2015, 168, 1735–1746. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Takeshima, R.; Harigai, K.; Xu, M.; Kong, F.; Liu, B.; Kanazawa, A.; Yamada, T.; Abe, J. Loss of Function of the E1-like-b Gene Associates with Early Flowering under Long-day Conditions in Soybean. Front. Plant Sci. 2019, 9, 1867. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Dong, L.; Tang, Y.; Li, H.; Cheng, Q.; Li, H.; Zhang, T.; Ma, L.; Xiang, H.; Chen, L.; et al. Novel and multifaceted regulations of photoperiodic flowering by phytochrome A in soybean. Proc. Natl. Acad. Sci. USA 2022, 119, e2208708119. [Google Scholar] [CrossRef]
- Corbesier, L.; Vincent, C.; Jang, S.; Fornara, F.; Fan, Q.; Searle, I.; Giakountis, A.; Farrona, S.; Gissot, L.; Turnbull, C.; et al. FT Protein Movement Contributes to Long-distance Signaling in Floral Induction of Arabidopsis. Science 2007, 316, 1030–1033. [Google Scholar] [CrossRef]
- Viola, I.L.; Gonzalez, D.H. TCP TranscriptionFactors in Plant Reproductive Development: Juggling Multiple Roles. Biomolecules 2023, 13, 750. [Google Scholar] [CrossRef] [PubMed]
- Cubas, P.N.; Doebley, J.; Coen, E. The TCP Domain: A Motif Found in Proteins Regulating Plant Growth and Development. Plant J. 1999, 18, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, S.; Ohashi, Y. PCF1 and PCF2 Specifically Bind to Cis-elements in the Rice Proliferating Cell Nuclear Antigen Gene. Plant Cell 1997, 9, 1607–1619. [Google Scholar]
- Navaud, O.; Dabos, P.; Carnus, E.; Tremousaygue, D.; Hervé, C. TCP Transcription Factors Predate the Emergence of Land Plants. J. Mol. Evol. 2007, 65, 23–33. [Google Scholar] [CrossRef]
- Martín-Trillo, M.; Cubas, P. TCP Genes: A Family Snapshot Ten Years Later. Trends Plant Sci. 2010, 15, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Xu, S.; Liu, N.; Zhang, G.; Hu, Q.; Gong, Y. Soybean TCP Transcription Factors: Evolution, Classification, Protein Interaction and Stress and Hormone Responsiveness. Plant Physiol. Biochem. 2018, 127, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; Pabón-Mora, N.; Theuß, V.S.; Busch, A.; Zachgo, S. Analysis of the CYC/TB1 Class of TCP Transcription Factors in Basal Angiosperms and Magnoliids. Plant J. 2015, 81, 559–571. [Google Scholar] [CrossRef]
- Li, X.; Zhang, G.; Liang, Y.; Hu, L.; Zhu, B.; Qi, D.; Cui, S.; Zhao, H. TCP7 Interacts with Nuclear Factor-Ys to Promote Flowering by Directly Regulating SOC1 in Arabidopsis. Plant J. 2021, 108, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Lucero, L.E.; Manavella, P.A.; Gras, D.E.; Ariel, F.D.; Gonzalez, D.H. Class I and Class II TCP Transcription Factors Modulate SOC1-dependent Flowering at Multiple Levels. Mol. Plant 2017, 10, 1571–1574. [Google Scholar] [CrossRef] [PubMed]
- Camoirano, A.; Alem, A.L.; Gonzalez, D.H.; Viola, I.L. The N-terminal Region Located Upstream of the TCP Domain is Responsible for the Antagonistic Action of the Arabidopsis thaliana TCP8 and TCP23 transcription factors on flowering time. Plant Sci. 2023, 328, 111571. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cheng, X.; Liu, P.; Li, D.; Chen, T.; Gu, X.; Sun, J. MicroRNA319-regulated TCPs Interact with FBHs and PFT1 to Activate CO Transcription and Control Flowering Time in Arabidopsis. PLoS Genet. 2017, 13, e1006833. [Google Scholar] [CrossRef]
- Kubota, A.; Ito, S.; Shim, J.S.; Johnson, R.S.; Song, Y.H.; Breton, G.; Goralogia, G.S.; Kwon, M.S.; Laboy Cintrón, D.; Koyama, T.; et al. TCP4-dependent Induction of CONSTANS Transcription Requires GIGANTEA in Photoperiodic Flowering in Arabidopsis. PLoS Genet. 2017, 13, e1006856. [Google Scholar] [CrossRef]
- Li, D.; Zhang, H.; Mou, M.; Chen, Y.; Xiang, S.; Chen, L.; Yu, D. Arabidopsis class II TCP Transcription Factors Integrate with the FT-FD Module to Control Flowering. Plant Physiol. 2019, 181, 97–111. [Google Scholar] [CrossRef]
- Wu, J.; Tsai, H.L.; Joanito, I.; Wu, Y.; Chang, C.W.; Li, Y.; Wang, Y.; Hong, J.; Chu, J.; Hsu, C.; et al. LWD-TCP Complex Activates the Morning Gene CCA1 in Arabidopsis. Nat. Commun. 2016, 7, 13181. [Google Scholar] [CrossRef]
- Xia, Z.; Zhai, H.; Zhang, Y.; Wang, Y.; Wang, L.; Xu, K.; Wu, H.; Zhu, J.; Jiao, S.; Wan, Z.; et al. QNE1 is A Key Flowering Regulator Determining the Length of the Vegetative Period in Soybean Cultivars. Sci. China Life Sci. 2022, 65, 2472–2490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, L.; Cai, Y.; Su, Q.; Chen, Y.; Li, M.; Hou, W. A Novel MORN-motif Type Gene GmMRF2 Controls Flowering Time and Plant Height of Soybean. Int. J. Biol. Macromol. 2023, 245, 125464. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, L.; Cao, X.; Liu, L.; Jiang, B.; Zhang, C.; Jia, H.; Lyu, X.; Su, Y.; Cai, Y.; et al. Cotyledons Facilitate the Adaptation of Early-Maturing Soybean Varieties to High-Latitude Long-Day Environments. Plant Cell Environ. 2021, 44, 2551–2564. [Google Scholar] [CrossRef] [PubMed]
- Fehr, W.; Caviness, C.; Burmood, D.; Pennington, J. Stage of Development Descriptions for Soybeans, Glycine Max (L.) Merrill. Crop Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Paz, M.M.; Martinez, J.C.; Kalvig, A.B.; Fonger, T.M.; Wang, K. Improved cotyledonary node method using an alternative explant derived from mature seed for efficient Agrobacterium-mediated soybean transformation. Plant Cell Rep. 2006, 25, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cai, Y.; Liu, X.; Yao, W.; Guo, C.; Sun, S.; Wu, C.; Jiang, B.; Han, T.; Hou, W. Improvement of soybean agrobacterium-mediated transformation efficiency by adding glutamine and asparagine into the culture media. Int. J. Mol. Sci. 2018, 19, 3039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, T.; Wang, L.; Jiang, B.; Zhen, C.; Yuan, S.; Hou, W.; Wu, C.; Han, T.; Sun, S. A Combined Linkage and GWAS Analysis Identifies QTLs Linked to Soybean Seed Protein and Oil Content. Int. J. Mol. Sci. 2019, 20, 5915. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, X.; Chen, F.; Yuan, S.; Wu, T.; Jiang, B.; Sapey, E.; Wu, C.; Sun, S.; Guo, C.; et al. Establishment of a Novel Experimental System for Studying the Photoperiodic Response of Short-Day Dicots Using Soybean ‘Cotyledon-Only Plant’ as Material. Front. Plant Sci. 2023, 13, 1101715. [Google Scholar] [CrossRef]
- Balsemão-Pires, E.; Andrade, L.R.; Sachetto-Martins, G. Functional Study of TCP23 in Arabidopsis thaliana during Plant Development. Plant Physiol. Biochem. 2013, 67, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, X.; Mo, X.; Zhong, L.; Zhang, J.; Mo, B.; Kuai, B. Overexpression of TCP8 Delays Arabidopsis Flowering through a FLOWERING LOCUS C-dependent Pathway. BMC Plant Biol. 2019, 19, 534. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Kaya, H.; Goto, K.; Iwabuchi, M.; Araki, T. A Pair of Related Genes with Antagonistic Roles in Mediating Flowering Signals. Science 1999, 286, 1960–1962. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.W.; Weigel, D. Structural Features Determining Flower-promoting Activity of Arabidopsis FLOWERING LOCUS T. Plant Cell 2014, 26, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Liu, B.; Xia, Z.; Sato, S.; Kim, B.M.; Watanabe, S.; Yamada, T.; Tabata, S.; Kanazawa, A.; Harada, K.; et al. Two Coordinately Regulated Homologs of FLOWERING LOCUS T Are Involved in the Control of Photoperiodic Flowering in Soybean. Plant Physiol. 2010, 154, 1220–1231. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Lü, S.; Liang, S.; Wu, H.; Zhang, X.; Liu, B.; Kong, F.; Yuan, X.; Li, J.; Xia, Z. GmFT4, a Homolog of FLOWERING LOCUS T, is Positively Regulated by E1 and Functions as a Flowering Repressor in Soybean. PLoS ONE 2014, 9, e89030. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fang, C.; Yang, Y.; Lv, T.; Su, T.; Chen, L.; Nan, H.; Li, S.; Zhao, X.; Lu, S.; et al. Overcoming the Genetic Compensation Response of Soybean Florigens to Improve Adaptation and Yield at Low Latitudes. Curr. Biol. 2021, 31, 755–3767. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.L.; Alvarez, J.; Weigel, D.; Meyerowitz, E.M.; Smyth, D.R. Control of Flower Development in Arabidopsis thaliana by APETALA1 and Interacting Genes. Development 1993, 119, 721. [Google Scholar] [CrossRef]
- Chen, L.; Nan, H.; Kong, L.; Yue, L.; Yang, H.; Zhao, Q.; Fang, C.; Li, H.; Cheng, Q.; Lu, S.; et al. Soybean AP1 Homologs Control Flowering Time and Plant Height. J. Integr. Plant Biol. 2020, 62, 1868–1879. [Google Scholar] [CrossRef]
- Yue, L.; Li, X.; Fang, C.; Chen, L.; Yang, H.; Yang, J.; Chen, Z.; Nan, H.; Chen, L.; Zhang, Y.; et al. FT5a Interferes with the Dt1-AP1 Feedback Loop to Control Flowering Time and Shoot Determinacy in Soybean. J. Integr. Plant Biol. 2021, 63, 1004–1020. [Google Scholar] [CrossRef]
- Takeshima, R.; Nan, H.; Harigai, K.; Dong, L.; Zhu, J.; Lu, S.; Xu, M.; Yamagishi, N.; Yoshikawa, N.; Liu, B.; et al. Functional Divergence between Soybean FLOWERING LOCUS T Orthologues FT2a and FT5a in Post-Flowering Stem Growth. J. Exp. Bot. 2019, 70, 3941–3953. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Chen, L.; Cai, Y.; Wang, L.; Chen, Y.; Zhang, J.; Liu, L.; Zhang, Y.; Yuan, S.; Gao, Y.; et al. The FLOWERING LOCUS T 5b Positively Regulates Photoperiodic Flowering and Improves the Geographical Adaptation of Soybean. Plant Cell Environ. 2023, 47, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.; Ha, J.; Kim, M.Y.; Choi, M.S.; Kang, S.T.; Jeong, S.C.; Moon, J.K.; Lee, S.H. GmBRC1 Is a Candidate Gene for Branching in Soybean (Glycine max (L.) Merrill). Int. J. Mol. Sci. 2019, 20, 135. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhao, X.; Hu, Y.; Zhang, T.; Li, S.; Cheng, Q.; Kong, L.; Li, X.; Bu, T.; Li, H.; et al. Natural Variation at the Soybean J Locus Improves Adaptation to the Tropics and Enhances Yield. Nat. Genet. 2017, 49, 773–779. [Google Scholar] [CrossRef]
- Fang, C.; Liu, J.; Zhang, T.; Su, T.; Li, S.; Cheng, Q.; Kong, L.; Li, X.; Bu, T.; Li, H.; et al. A Recent Retrotransposon Insertion of J Caused E6 Locus Facilitating Soybean Adaptation into Low Latitude. J. Integr. Plant Biol. 2021, 63, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, Y.; Li, Y.; Lu, H.; Hong, H.; Tian, Y.; Li, H.; Zhao, T.; Zhou, X.; Liu, J.; et al. A Domestication-associated Gene GmPRR3b Regulates the Circadian Clock and Flowering Time in Soybean. Mol. Plant 2020, 13, 745–759. [Google Scholar] [CrossRef]
- Lu, S.; Dong, L.; Fang, C.; Liu, S.; Kong, L.; Cheng, Q.; Chen, L.; Su, T.; Nan, H.; Zhang, D.; et al. Stepwise Selection on Homologous PRR Genes Controlling Flowering and Maturity during Soybean Domestication. Nat. Genet. 2020, 52, 428–436. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).