In Silico Analysis of the Ga3+/Fe3+ Competition for Binding the Iron-Scavenging Siderophores of P. aeruginosa—Implementation of Three Gallium-Based Complexes in the “Trojan Horse” Antibacterial Strategy
Abstract
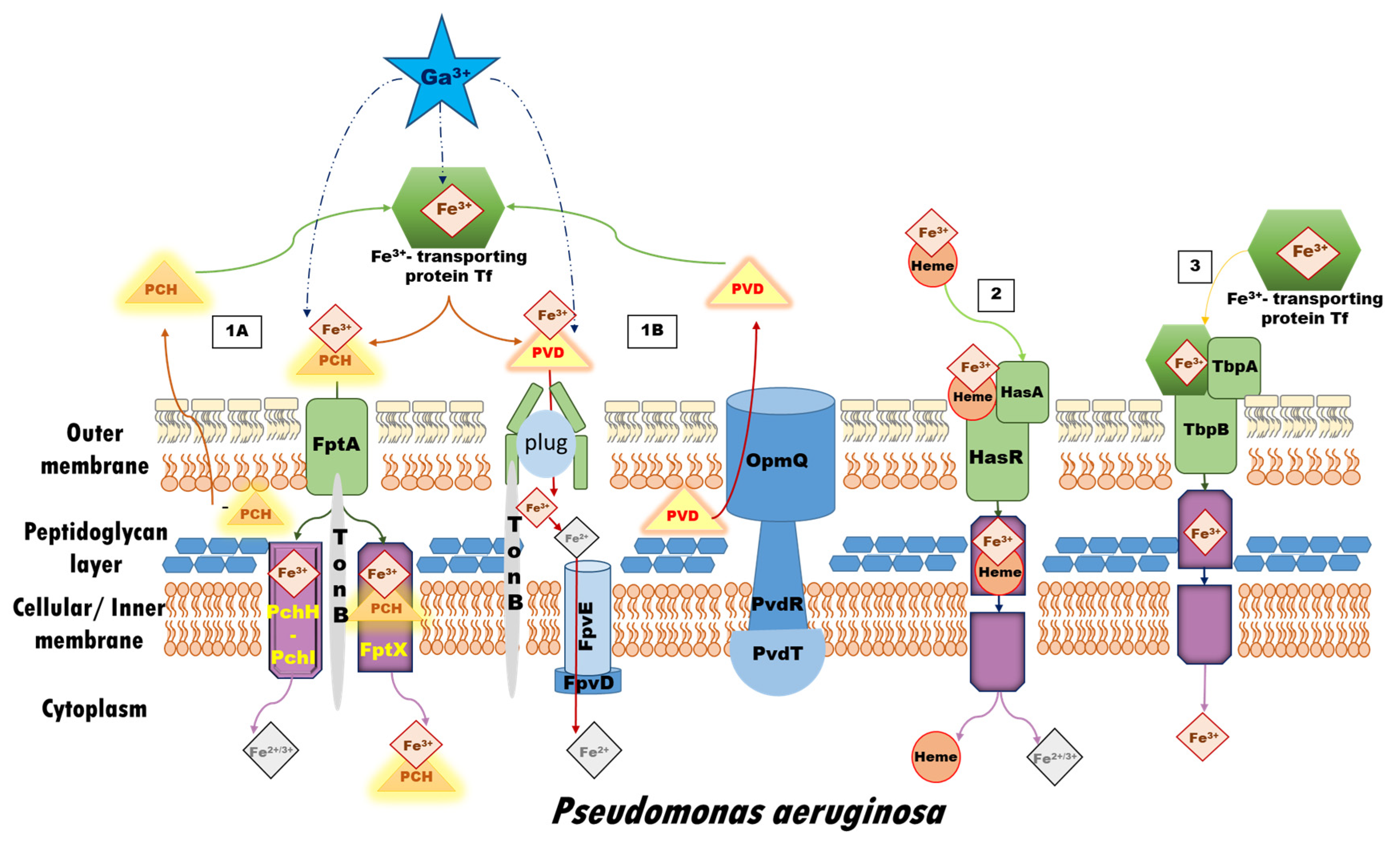
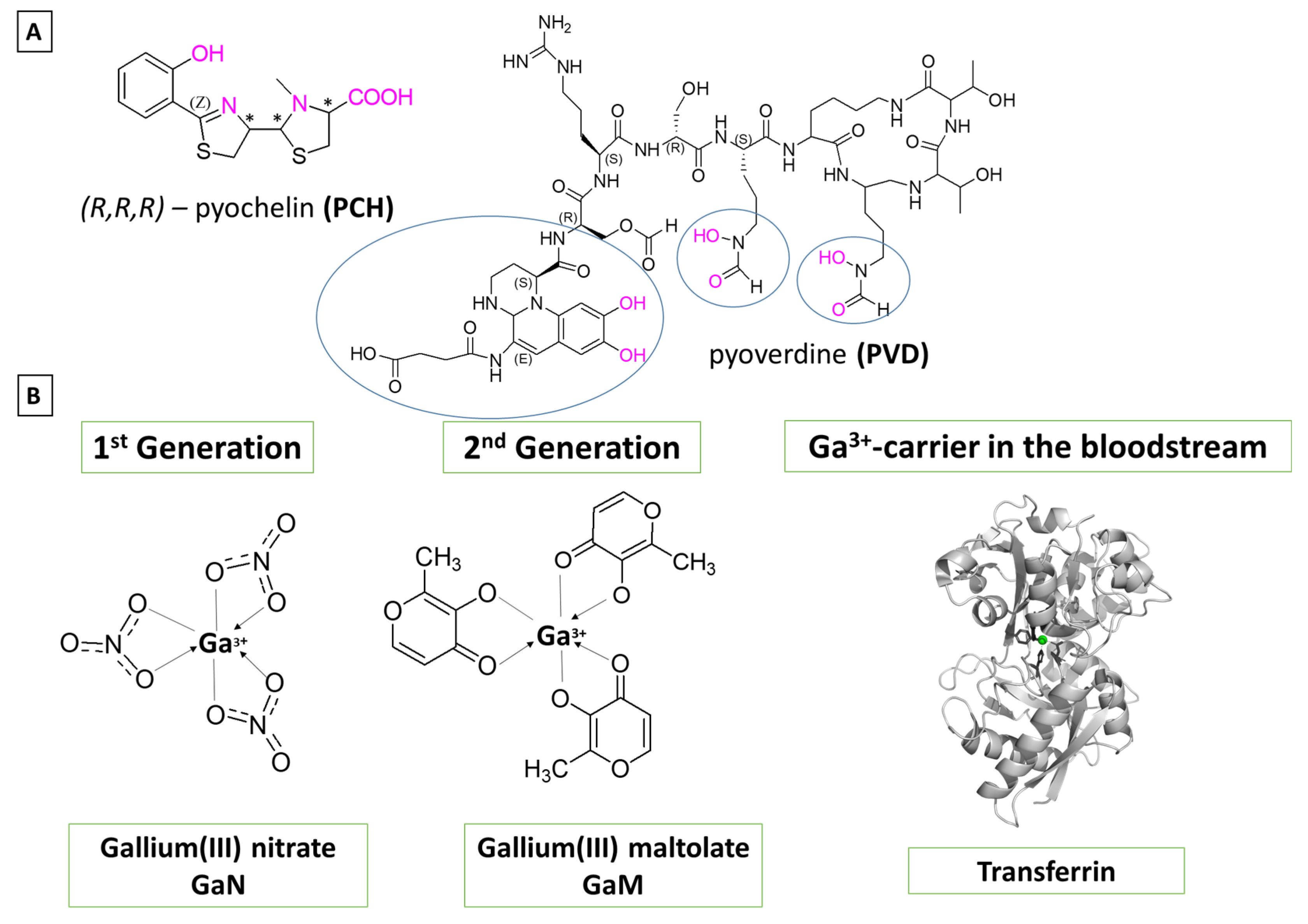
1. Introduction
2. Materials and Methods
2.1. Reactions Modeled
2.2. Computational Protocol
2.3. Calibration and Validation of the applied DFT Procedure
3. Results and Discussion
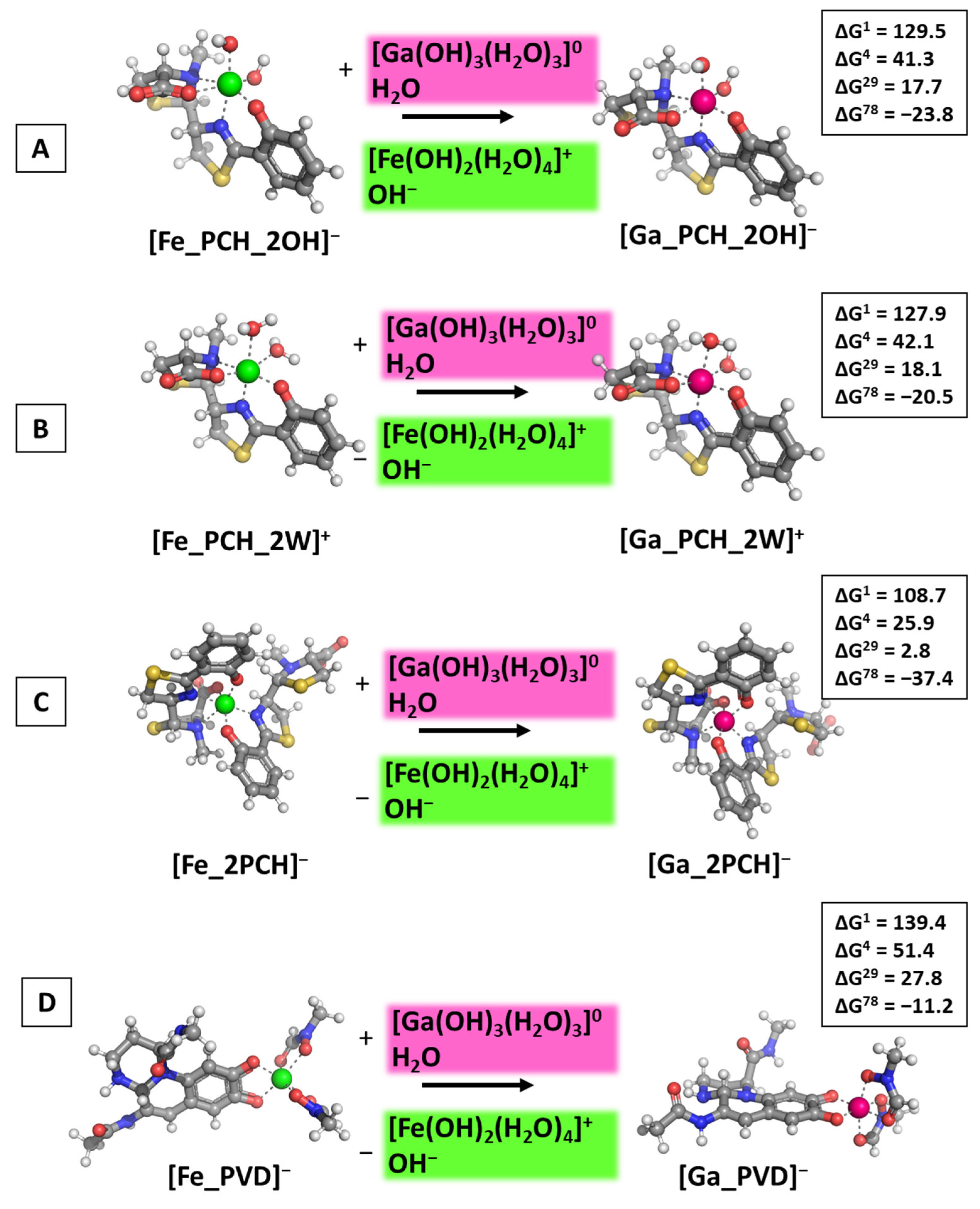
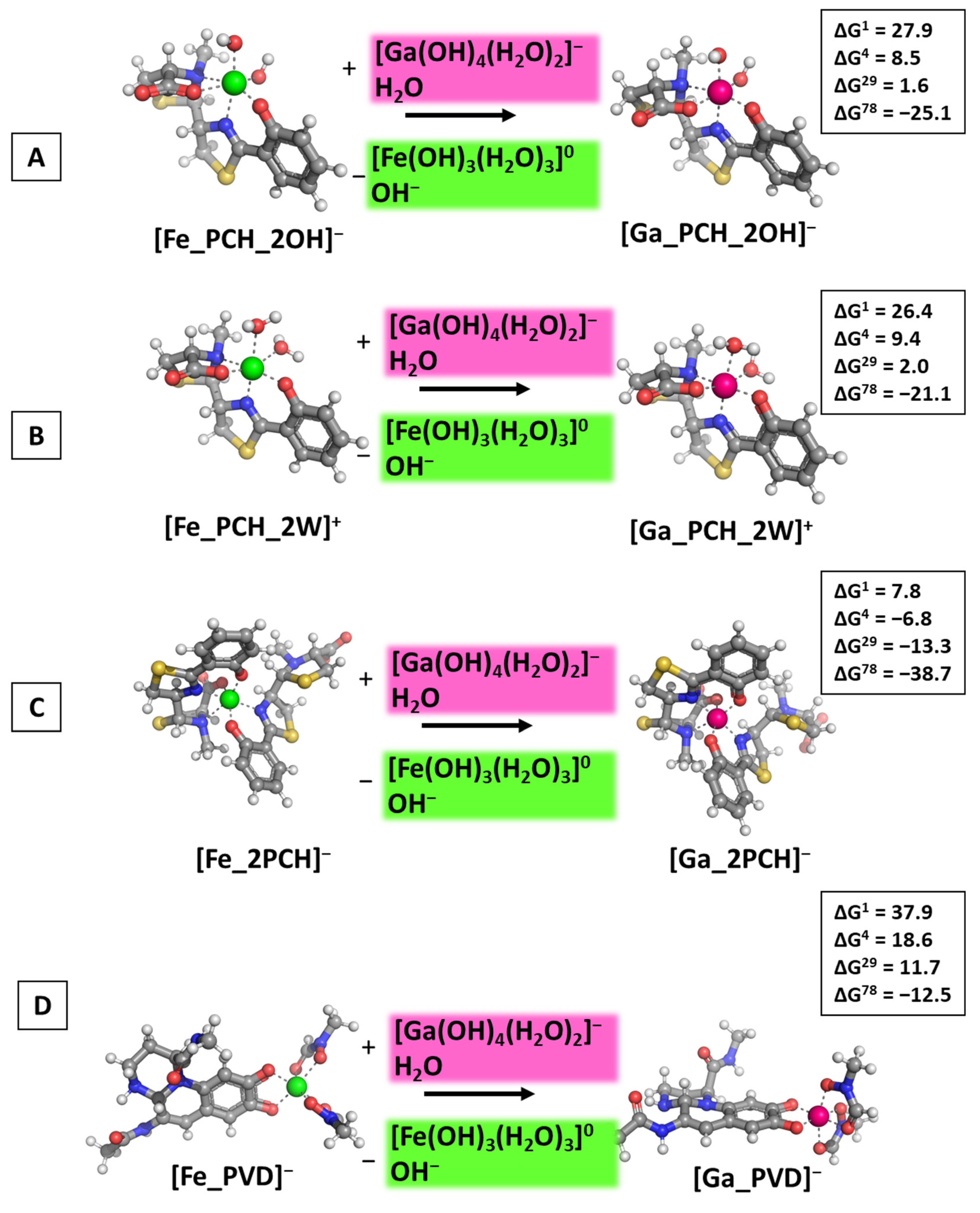
3.1. Reactions of Competition with Gallium Nitrate
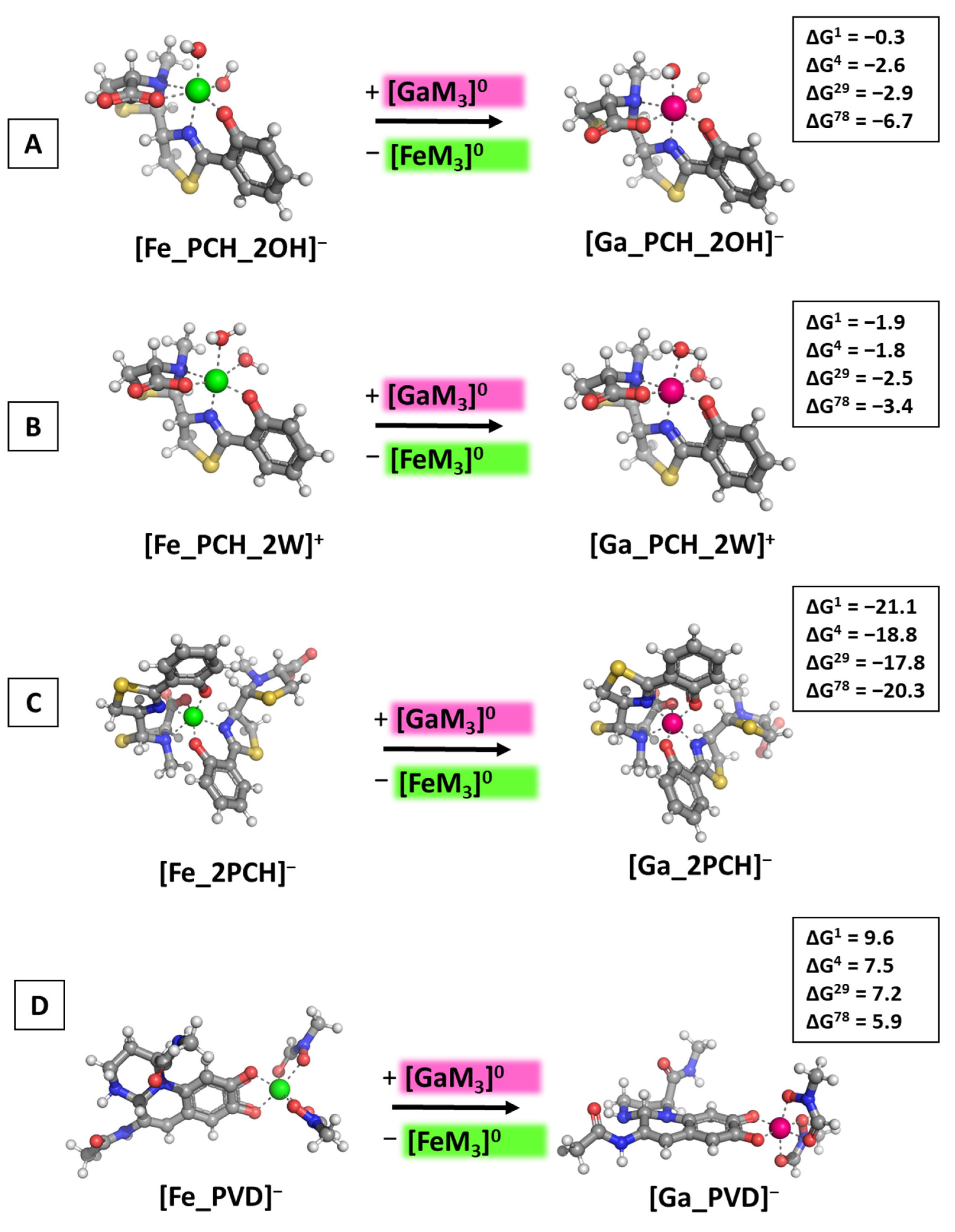
3.2. Reactions of Competition with Gallium Maltolate
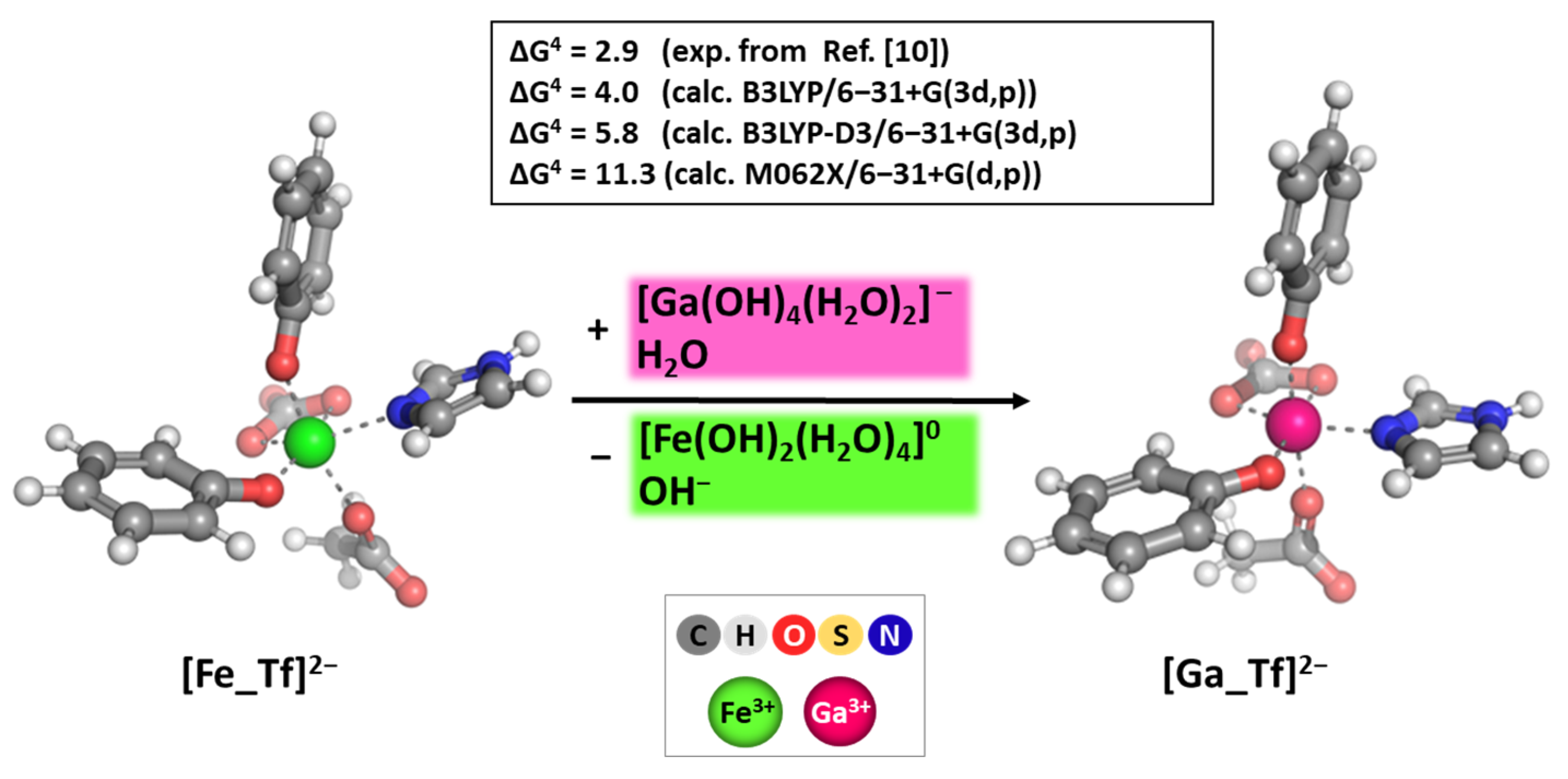
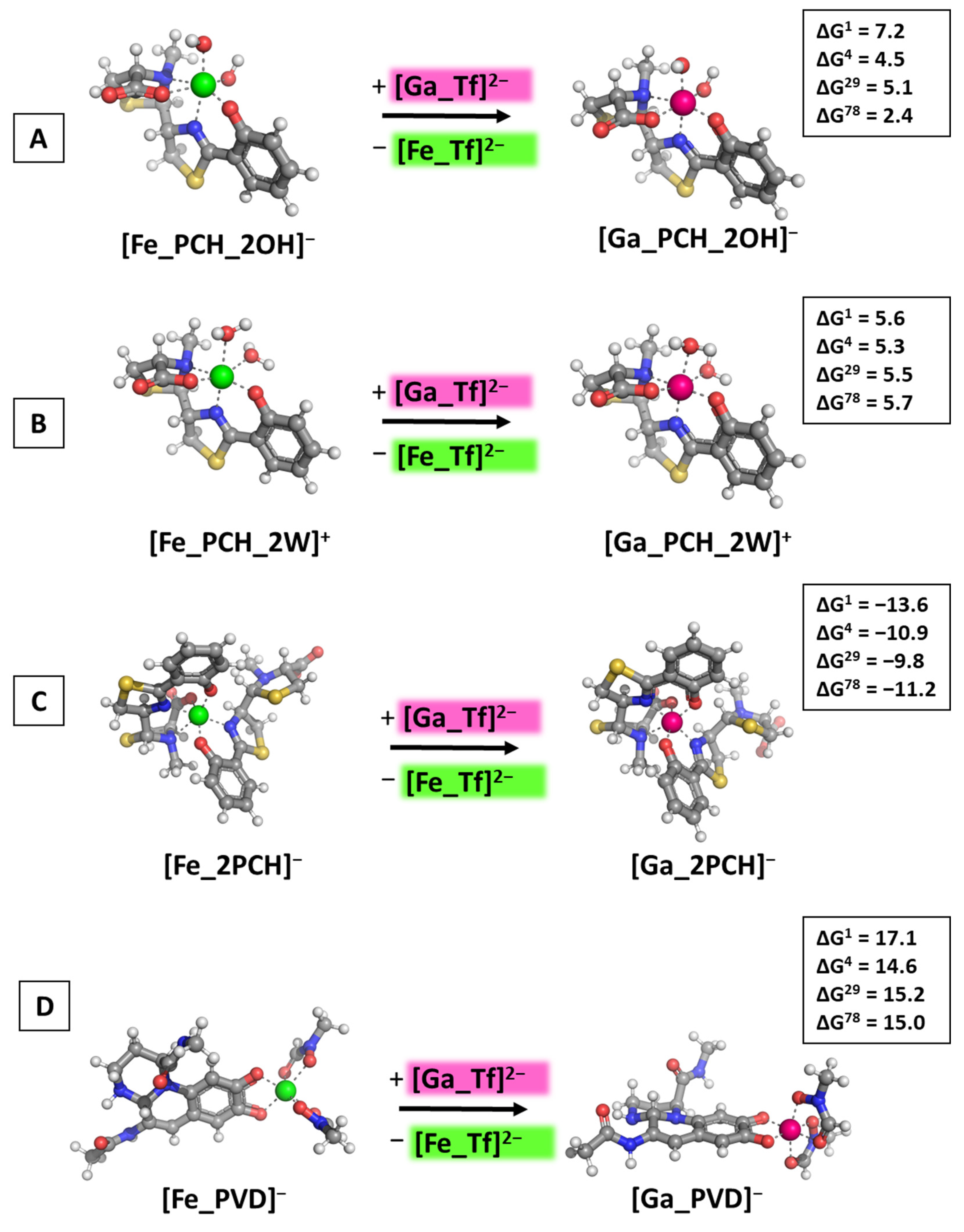
3.3. Reactions of Competition with Transferrin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ventola, C.L. The Antibiotic Resistance Crisis Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Levy, S.B.; Bonnie, M. Antibacterial Resistance Worldwide: Causes, Challenges and Responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef]
- Falagas, M.E.; Bliziotis, I.A. Pandrug-Resistant Gram-Negative Bacteria: The Dawn of the Post-Antibiotic Era? Int. J. Antimicrob. Agents 2007, 29, 630–636. [Google Scholar] [CrossRef]
- World Health Organization List of Antibiotic Resistant Priority Pathogens. Available online: http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/e (accessed on 23 January 2024).
- Rice, L.B. Federal Funding for the Study of Antimicrobial Resistance in Nosocomial Pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Zilberberg, M.D.; Shorr, A.F. Prevalence of Multidrug-Resistant Pseudomonas aeruginosa and Carbapenem-Resistant Enterobacteriaceae among Specimens from Hospitalized Patients with Pneumonia and Bloodstream Infections in the United States from 2000 to 2009. J. Hosp. Med. 2013, 8, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Castanheira, M.; Mendes, R.E.; Flamm, R.K. Frequency and Antimicrobial Susceptibility of Gram-Negative Bacteria Isolated from Patients with Pneumonia Hospitalized in ICUs of US Medical Centres (2015–2017). J. Antimicrob. Chemother. 2018, 73, 3053–3059. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef]
- Church, N.A.; McKillip, J.L. Antibiotic Resistance Crisis: Challenges and Imperatives. Biologia 2021, 76, 1535–1550. [Google Scholar] [CrossRef]
- Ratledge, C.; Dover, L.G. Iron Metabolism in Pathogenic Bacteria. Annu. Rev. Microbiol. 2000, 54, 881–941. [Google Scholar] [CrossRef]
- Ji, C.; Juárez-Hernández, R.E.; Miller, M.J. Exploiting Bacterial Iron Acquisition: Siderophore Conjugates. Future Med. Chem. 2012, 4, 297–313. [Google Scholar] [CrossRef]
- Sheldon, J.R.; Laakso, H.A.; Heinrichs, D.E. Iron Acquisition Strategies of Bacterial Pathogens. Microbiol. Spectr. 2016, 4, 43–85. [Google Scholar] [CrossRef] [PubMed]
- Hood, M.I.; Skaar, E.P. Nutritional Immunity: Transition Metals at the Pathogen-Host Interface. Nat. Rev. Microbiol. 2012, 10, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Sarkar, S.; Sarkar, B.; Sharma, B.K.; Bhattacharjee, S.; Tribedi, P. Microbial Siderophores and Their Potential Applications: A Review. Environ. Sci. Pollut. Res. 2016, 23, 3984–3999. [Google Scholar] [CrossRef] [PubMed]
- Hider, R.C.; Kong, X. Chemistry and Biology of Siderophores. Nat. Prod. Rep. 2010, 27, 637–657. [Google Scholar] [CrossRef] [PubMed]
- Cezard, C.; Farvacques, N.; Sonnet, P. Chemistry and Biology of Pyoverdines, Pseudomonas Primary Siderophores. Curr. Med. Chem. 2014, 22, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Miethke, M.; Marahiel, M.A. Siderophore-Based Iron Acquisition and Pathogen Control. Microbiol. Mol. Biol. Rev. 2007, 71, 413–451. [Google Scholar] [CrossRef]
- Mular, A.; Piasta, K.; Jedyńczuk, A.; Kamińska, K.; Olshvang, E.; Nolte, N.M.; Wojaczyńska, E.; Kozłowski, H.; Gumienna-Kontecka, E. The Diversity and Utility of Arylthiazoline and Aryloxazoline Siderophores: Challenges of Coordination Chemistry, Biological Activity and Selected Applications. Coord. Chem. Rev. 2024, 501, 215551. [Google Scholar] [CrossRef]
- Gumienna-Kontecka, E.; Carver, P.L. Building a Trojan Horse: Siderophore-Drug Conjugates for the Treatment of Infectious Diseases. In Essential Metals in Medicine: Therapeutic Use and Toxicity of Metal Ions in the Clinic; Metal Ions in Life Sciences; De Gruyter: Berlin, Germany; Boston, MA, USA, 2019; Volume 19, pp. 181–202. [Google Scholar] [CrossRef]
- Möllmann, U.; Heinisch, L.; Bauernfeind, A.; Köhler, T.; Ankel-Fuchs, D. Siderophores as Drug Delivery Agents: Application of the “Trojan Horse” Strategy. BioMetals 2009, 22, 615–624. [Google Scholar] [CrossRef]
- Wilson, B.R.; Bogdan, A.R.; Miyazawa, M.; Hashimoto, K.; Tsuji, Y. Siderophores in Iron Metabolism: From Mechanism to Therapy Potential. Trends Mol. Med. 2016, 22, 1077–1090. [Google Scholar] [CrossRef]
- Evans, A.; Kavanagh, K.A. Evaluation of Metal-Based Antimicrobial Compounds for the Treatment of Bacterial Pathogens. J. Med. Microbiol. 2021, 70, 001363. [Google Scholar] [CrossRef] [PubMed]
- Marmol, F. Lithium: Bipolar Disorder and Neurodegenerative Diseases Possible Cellular Mechanisms of the Therapeutic Effects of Lithium. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Waszczykowska, A.; Żyro, D.; Ochocki, J.; Jurowski, P. Clinical Application and Efficacy of Silver Drug in Ophthalmology: A Literature Review and New Formulation of Eye Drops with Drug Silver (I) Complex of Metronidazole with Improved Dosage Form. Biomedicines 2021, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Diepenhorst, N.A.; Leach, K.; Keller, A.N.; Rueda, P.; Cook, A.E.; Tracie, L.; Nowell, C.; Pastoureau, P.; Sabatini, M.; Summers, R.J.; et al. Divergent Effects of Strontium and Calcium-Sensing Receptor Positive Allosteric Modulators (Calcimimetics) on Human Osteoclast Activity. Br. J. Pharmacol. 2018, 175, 4095–4108. [Google Scholar] [CrossRef] [PubMed]
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.; Pan, Z.; Fu, Z.; Jiang, X.; Cao, Z.; Li, J.; Wang, H. Gallium-Enabled Bactericidal Medicine. Mater. Today 2023, 67, 548–565. [Google Scholar] [CrossRef]
- Chitambar, C.R. Gallium Complexes as Anticancer Drugs. In Metallo-Drugs: Development and Action of Anticancer Agents; Metal Ions in Life Sciences; De Gruyter: Berlin, Germany; Boston, MA, USA, 2018; Volume 18, pp. 281–301. [Google Scholar] [CrossRef]
- Marmion, C.J. Metallo-Drugs: Development and Action of Anti-Cancer Agents. Coord. Chem. Rev. 2018, 371, 96–98. [Google Scholar] [CrossRef]
- Chitambar, C.R. Gallium Nitrate, Apoptotic Effects. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer Science: New York, NY, USA, 2013; pp. 807–821. [Google Scholar]
- Bernstein, L.R. Gallium, Therapeutic Effects. In Encyclopedia of Metallopoteins; Uversky, V.N., Kretsinger, R.H., Permyakov, E.A., Eds.; Springer Science: New York, NY, USA, 2013; pp. 823–835. [Google Scholar]
- Auger, C.; Lemire, J.; Appanna, V.; Appanna, V.D. Gallium in Bacteria, Metabolic and Medical Implications. In Encyclopedia of Metallopoteins; Springer Science: New York, NY, USA, 2013; pp. 800–807. [Google Scholar]
- Wang, F.; Wang, X.; Xie, E.; Wang, F.; Gan, Q.; Ping, S.; Wei, J.; Li, F.; Wang, Z. Simultaneous Incorporation of Gallium Oxide and Tantalum Microparticles into Micro-Arc Oxidation Coating of Titanium Possessing Antibacterial Effect and Stimulating Cellular Response. Biomater. Adv. 2022, 212736. [Google Scholar] [CrossRef]
- Smith, W.D.; Bardin, E.; Cameron, L.; Edmondson, C.L.; Farrant, K.V.; Martin, I.; Murphy, R.A.; Soren, O.; Turnbull, A.R.; Wierre-Gore, N.; et al. Current and Future Therapies for Pseudomonas aeruginosa Infection in Patients with Cystic Fibrosis. FEMS Microbiol. Lett. 2017, 364, fnx121. [Google Scholar] [CrossRef]
- Hijazi, S.; Visaggio, D.; Pirolo, M.; Frangipani, E.; Bernstein, L.; Visca, P. Antimicrobial Activity of Gallium Compounds on ESKAPE Pathogens. Front. Cell. Infect. Microbiol. 2018, 8, 316. [Google Scholar] [CrossRef] [PubMed]
- Goss, C.H.; Kaneko, Y.; Khuu, L.; Anderson, G.D.; Ravishankar, S.; Aitken, M.L.; Lechtzin, N.; Zhou, G.; Czyz, D.M.; McLean, K.; et al. Gallium Disrupts Bacterial Iron Metabolism and Has Therapeutic Effects in Mice and Humans with Lung Infections. Sci. Transl. Med. 2018, 10, eaat7520. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.R.; Britigan, B.E.; Narayanasamy, P. Iron/Heme Metabolism-Targeted Gallium(III) Nanoparticles Are Active against Extracellular and Intracellular Pseudomonas aeruginosa and Acinetobacter baumannii. Antimicrob. Agents Chemother. 2019, 63, e02643-18. [Google Scholar] [CrossRef]
- Choi, S.; Britigan, B.E.; Narayanasamy, P. Treatment of Virulent Mycobacterium Tuberculosis and HIV Coinfected Macrophages with Gallium Nanoparticles Inhibits Pathogen Growth and Modulates Macrophage Cytokine Production. mSphere 2019, 4, e00443-19. [Google Scholar] [CrossRef] [PubMed]
- Olakanmi, O.; Kesavalu, B.; Pasula, R.; Abdalla, M.Y.; Schlesinger, L.S.; Britigan, B.E. Gallium Nitrate Is Efficacious in Murine Models of Tuberculosis and Inhibits Key Bacterial Fe-Dependent Enzymes. Antimicrob. Agents Chemother. 2013, 57, 6074–6080. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- DeLeon, K.; Balldin, F.; Watters, C.; Hamood, A.; Griswold, J.; Sreedharan, S.; Rumbaugh, K.P. Gallium Maltolate Treatment Eradicates Pseudomonas aeruginosa Infection in Thermally Injured Mice. Antimicrob. Agents Chemother. 2009, 53, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Levinson, W. Review of Medical Microbiology and Immunology; McGraw-Hill Medical: New York, NY, USA, 2006; ISBN 9788578110796. [Google Scholar]
- Schalk, I.J.; Perraud, Q. Pseudomonas aeruginosa and Its Multiple Strategies to Access Iron. Environ. Microbiol. 2023, 25, 811–831. [Google Scholar] [CrossRef]
- Harris, W.R.; Messori, L. A Comparative Study of Aluminum(III), Gallium(III), Indium(III), and Thallium(III) Binding to Human Serum Transferrin. Coord. Chem. Rev. 2002, 228, 237–262. [Google Scholar] [CrossRef]
- Sun, Y.; Ramirez, J.; Woski, S.A.; Vincent, J.B. The Binding of Trivalent Chromium to Low-Molecular-Weight Chromium-Binding Substance (LMWCr) and the Transfer of Chromium from Transferrin and Chromium Picolinate to LMWCr. J. Biol. Inorg. Chem. 2000, 5, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Kelson, A.B.; Carnevali, M.; Truong-Le, V. Gallium-Based Anti-Infectives: Targeting Microbial Iron-Uptake Mechanisms. Curr. Opin. Pharmacol. 2013, 13, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Oroujeni, M.; Garousi, J.; Andersson, K.; Löfblom, J.; Mitran, B.; Orlova, A.; Tolmachev, V. Preclinical Evaluation of [68Ga]Ga-DFO-ZEGFR:2377: A Promising Affibody-Based Probe for Noninvasive PET Imaging of EGFR Expression in Tumors. Cells 2018, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Porcaro, F.; Bonchi, C.; Ugolini, A.; Frangipani, E.; Polzonetti, G.; Visca, P.; Meneghini, C.; Battocchio, C. Understanding the Biomimetic Properties of Gallium in Pseudomonas aeruginosa: An XAS and XPS Study. Dalton Trans. 2017, 46, 7082–7091. [Google Scholar] [CrossRef] [PubMed]
- Nicolafrancesco, C.; Porcaro, F.; Pis, I.; Nappini, S.; Simonelli, L.; Marini, C.; Frangipani, E.; Visaggio, D.; Visca, P.; Mobilio, S.; et al. Gallium- and Iron-Pyoverdine Coordination Compounds Investigated by X-ray Photoelectron Spectroscopy and X-ray Absorption Spectroscopy. Inorg. Chem. 2019, 58, 4935–4944. [Google Scholar] [CrossRef] [PubMed]
- Kircheva, N.; Dudev, T. Gallium as an Antibacterial Agent: A DFT/SMD Study of the Ga3+/Fe3+ Competition for Binding Bacterial Siderophores. Inorg. Chem. 2020, 59, 6242–6254. [Google Scholar] [CrossRef]
- Kircheva, N.; Dudev, T. Competition between Abiogenic and Biogenic Metal Cations in Biological Systems: Mechanisms of Gallium‘s Anticancer and Antibacterial Effect. J. Inorg. Biochem. 2021, 214, 111309. [Google Scholar] [CrossRef] [PubMed]
- Chitambar, C.R.; Al-Gizawiy, M.M.; Alhajala, H.S.; Pechman, K.R.; Wereley, J.P.; Wujek, R.; Clark, P.A.; Kuo, J.S.; Antholine, W.E.; Schmainda, K.M. Gallium Maltolate Disrupts Tumor Iron Metabolism and Retards the Growth of Glioblastoma by Inhibiting Mitochondrial Function and Ribonucleotide Reductase. Mol. Cancer Ther. 2018, 17, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Mikuš, P.; Melník, M.; Forgácsová, A.; Krajčiová, D.; Havránek, E. Gallium Compounds in Nuclear Medicine and Oncology. Main Group Met. Chem. 2014, 37, 53–65. [Google Scholar] [CrossRef]
- Choi, S.R.; Switzer, B.; Britigan, B.E.; Narayanasamy, P. Gallium Porphyrin and Gallium Nitrate Synergistically Inhibit Mycobacterial Species by Targeting Different Aspects of Iron/Heme Metabolism. ACS Infect. Dis. 2020, 6, 2582–2591. [Google Scholar] [CrossRef]
- Bonchi, C.; Imperi, F.; Minandri, F.; Visca, P.; Frangipani, E. Repurposing of Gallium-Based Drugs for Antibacterial Therapy. BioFactors 2014, 40, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Scott, Z.W.; Choi, S.R.; Talmon, G.A.; Britigan, B.E.; Narayanasamy, P. Combining Gallium Protoporphyrin and Gallium Nitrate Enhances In Vitro and In Vivo Efficacy against Pseudomonas aeruginosa: Role of Inhibition of Bacterial Antioxidant Enzymes and Resultant Increase in Cytotoxic Reactive Oxygen Species. ACS Infect. Dis. 2022, 8, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Stojiljkovic, I.; Kumar, V.; Srinivasan, N. Non-Iron Metalloporphyrins: Potent Antibacterial Compounds That Exploit Haem/Hb Uptake Systems of Pathogenic Bacteria. Mol. Microbiol. 1999, 31, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Q.; Qi, X.; Li, Y.; Ma, H.; Grinholc, M.; Nakonieczna, J.; Yu, B.; Wang, X.; Zhang, L. Iron-Blocking Antibacterial Therapy with Cationic Heme-Mimetic Gallium Porphyrin Photosensitizer for Combating Antibiotic Resistance and Enhancing Photodynamic Antibacterial Activity. Chem. Eng. J. 2023, 451, 138261–138277. [Google Scholar] [CrossRef]
- Sun, W.; Qi, M.; Cheng, S.; Li, C.; Dong, B.; Wang, L. Gallium and Gallium Compounds: New Insights into the “Trojan Horse” Strategy in Medical Applications. Mater. Des. 2023, 227, 111704. [Google Scholar] [CrossRef]
- Ghssein, G.; Ezzeddine, Z. A Review of Pseudomonas aeruginosa Metallophores: Pyoverdine, Pyochelin and Pseudopaline. Biology 2022, 11, 1711. [Google Scholar] [CrossRef]
- Ghssein, G.; Matar, S.F. Chelating Mechanisms of Transition Metals by Bacterial Metallophores “Pseudopaline and Staphylopine”: A Quantum Chemical Assessment. Computation 2018, 6, 56. [Google Scholar] [CrossRef]
- Kircheva, N.; Dobrev, S.; Nikolova, V.; Angelova, S.; Dudev, T. Zinc and Its Critical Role in Retinitis pigmentosa: Insights from DFT/SMD Calculations. Inorg. Chem. 2020, 59, 17347–17355. [Google Scholar] [CrossRef]
- Vologzhannikova, A.A.; Shevelyova, M.P.; Kazakov, A.S.; Sokolov, A.S.; Borisova, N.I.; Permyakov, E.A.; Kircheva, N.; Nikolova, V.; Dudev, T.; Permyakov, S.E. Strontium Binding to α-Parvalbumin, a Canonical Calcium-Binding Protein of the “EF-Hand” Family. Biomolecules 2021, 11, 1158. [Google Scholar] [CrossRef]
- Kircheva, N.; Toshev, N.; Dudev, T. Holo-Chromodulin: Competition between the Native Cr3+ and Other Biogenic Cations (Fe3+, Fe2+, Mg2+, and Zn2+) for the Binding Sites. Metallomics 2022, 14, mfac082. [Google Scholar] [CrossRef]
- Angelova, S.E.; Kircheva, N.; Nikolova, V.; Dobrev, S.; Dudev, T. Electrostatic Interactions—Key Determinants of the Metal Selectivity in La3+ and Ca2+ Binding Proteins. Phys. Chem. Chem. Phys. 2023, 25, 18149–18157. [Google Scholar] [CrossRef]
- Kircheva, N.; Angelova, S.; Dobrev, S.; Petkova, V.; Nikolova, V.; Dudev, T. Cu+/Ag+ Competition in Type I Copper Proteins (T1Cu). Biomolecules 2023, 13, 681. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, V.; Kircheva, N.; Dobrev, S.; Angelova, S. Lanthanides as Calcium Mimetic Species in Calcium-Signaling/Buffering Proteins: The Effect of Lanthanide Type on the Ca2+/Ln3+ Competition. Int. J. Mol. Sci. 2023, 24, 6297. [Google Scholar] [CrossRef] [PubMed]
- Kircheva, N.; Dobrev, S.; Petkova, V.; Bakalova, S.; Kaneti, J.; Angelova, S. Theoretical Assessment of the Ligand/Metal/Quadruplex Recognition in the Non-Canonical Nucleic Acids Structures. Molecules 2023, 28, 6109. [Google Scholar] [CrossRef] [PubMed]
- Dabrowiak, J.C. Metals in Medicine; Wiley: Chichester, UK, 2009. [Google Scholar]
- Lin, Q.; Pilewski, J.M.; Di, Y.P. Acidic Microenvironment Determines Antibiotic Susceptibility and Biofilm Formation of Pseudomonas aeruginosa. Front. Microbiol. 2021, 12, 747834. [Google Scholar] [CrossRef]
- Brandel, J.; Humbert, N.; Elhabiri, M.; Schalk, I.J.; Mislin, G.L.A.; Albrecht-Gary, A.M. Pyochelin, a Siderophore of Pseudomonas aeruginosa: Physicochemical Characterization of the Iron(III), Copper(II) and Zinc(II) Complexes. Dalton Trans. 2012, 41, 2820–2834. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision D. 01; Gaussian Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- McQuire, D.A.; Simon, J.D. Physical Chemistry: A Molecular Approach; University Science Books: Sausalito, CA, USA, 1997. [Google Scholar]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Schrödinger, L.; DeLano, W. PyMOL. 2020. Available online: http://www.pymol.org/pymol (accessed on 1 February 2024).
- Nikolova, V.; Angelova, S.; Markova, N.; Dudev, T. Gallium as a Therapeutic Agent: A Thermodynamic Evaluation of the Competition between Ga3+ and Fe3+ Ions in Metalloproteins. J. Phys. Chem. B 2016, 120, 2241–2248. [Google Scholar] [CrossRef]
- Sham, T.K.; Hastings, J.B.; Perlman, M.L. Structure and Dynamic Behavior of Transition-Metal Ions in Aqueous Solution: An EXAFS Study of Electron-Exchange Reactions. J. Am. Chem. Soc. 1980, 102, 5904–5906. [Google Scholar] [CrossRef]
- Lindqvist-Reis, P.; Munoz-Paez, A.; Diaz-Moreno, S.; Pattanaik, S.; Persson, I.; Sandström, M. The Structure of the Hydrated Gallium(III), Indium(III), and Chromium(III) Ions in Aqueous Solution. A Large Angle X-ray Scattering and EXAFS Study. Inorg. Chem. 1998, 37, 6675–6683. [Google Scholar] [CrossRef]
- Frangipani, E.; Bonchi, C.; Minandri, F.; Imperi, F.; Visca, P. Pyochelin Potentiates the Inhibitory Activity of Gallium on Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 5572–5575. [Google Scholar] [CrossRef] [PubMed]
| Fe3+ | Ga3+ | Difference R(Fe3+) − R(Ga3+) | ||||
|---|---|---|---|---|---|---|
| complex | calc | exp | calc | exp | calc | exp |
| Hexaaqua | 2.05 | 2.00 a | 1.96 | 1.96 b | 0.09 | 0.04 |
| Transferrin | 2.07 c | 2.03 d | 1.98 c | 1.98 e | 0.09 | 0.05 |
| Pyochelin | 2.18 | 2.18 f | 2.05 | 1.97 g | 0.13 | 0.21 |
| Pyoverdine | 2.07 h | 2.11 i | 1.98 h | 1.95 j | 0.09 | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kircheva, N.; Dobrev, S.; Petkova, V.; Yocheva, L.; Angelova, S.; Dudev, T. In Silico Analysis of the Ga3+/Fe3+ Competition for Binding the Iron-Scavenging Siderophores of P. aeruginosa—Implementation of Three Gallium-Based Complexes in the “Trojan Horse” Antibacterial Strategy. Biomolecules 2024, 14, 487. https://doi.org/10.3390/biom14040487
Kircheva N, Dobrev S, Petkova V, Yocheva L, Angelova S, Dudev T. In Silico Analysis of the Ga3+/Fe3+ Competition for Binding the Iron-Scavenging Siderophores of P. aeruginosa—Implementation of Three Gallium-Based Complexes in the “Trojan Horse” Antibacterial Strategy. Biomolecules. 2024; 14(4):487. https://doi.org/10.3390/biom14040487
Chicago/Turabian StyleKircheva, Nikoleta, Stefan Dobrev, Vladislava Petkova, Lyubima Yocheva, Silvia Angelova, and Todor Dudev. 2024. "In Silico Analysis of the Ga3+/Fe3+ Competition for Binding the Iron-Scavenging Siderophores of P. aeruginosa—Implementation of Three Gallium-Based Complexes in the “Trojan Horse” Antibacterial Strategy" Biomolecules 14, no. 4: 487. https://doi.org/10.3390/biom14040487
APA StyleKircheva, N., Dobrev, S., Petkova, V., Yocheva, L., Angelova, S., & Dudev, T. (2024). In Silico Analysis of the Ga3+/Fe3+ Competition for Binding the Iron-Scavenging Siderophores of P. aeruginosa—Implementation of Three Gallium-Based Complexes in the “Trojan Horse” Antibacterial Strategy. Biomolecules, 14(4), 487. https://doi.org/10.3390/biom14040487









