Application of Cas12j for Streptomyces Editing
Abstract
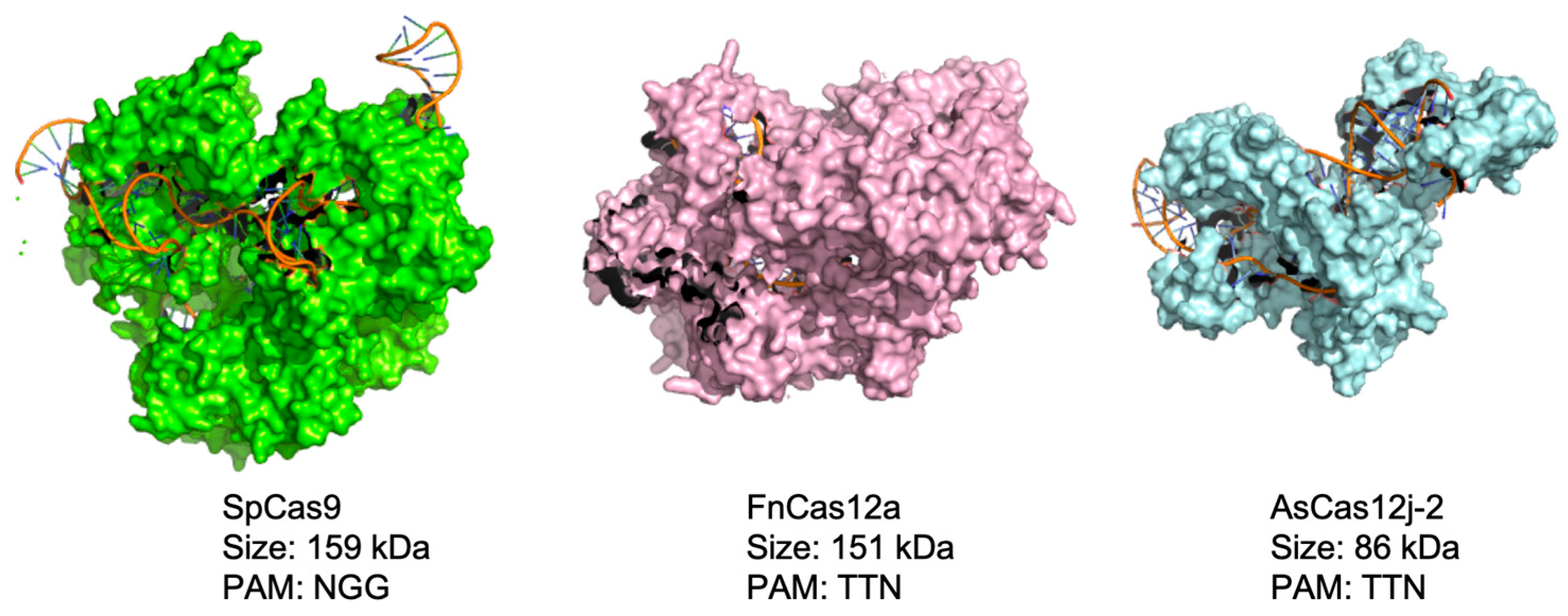
1. Introduction
2. Materials and Methods
2.1. Construction of Genome-Editing Plasmids
2.2. Target Site Prediction
2.3. Conjugation and Screening of Strains
2.4. Fermentation and Analysis
3. Results
3.1. Design and Characterization of an AsCas12j-2 Functional Vector
3.2. Editing in a Previously Inaccessible Strain
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Genilloud, O. Actinomycetes: Still a Source of Novel Antibiotics. Nat. Prod. Rep. 2017, 34, 1203–1232. [Google Scholar] [CrossRef]
- Onaka, H.; Mori, Y.; Igarashi, Y.; Furumai, T. Mycolic Acid-Containing Bacteria Induce Natural-Product Biosynthesis in Streptomyces Species. Appl. Environ. Microbiol. 2011, 77, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Bentley, S.D.; Chater, K.F.; Cerdeño-Tárraga, A.M.; Challis, G.L.; Thomson, N.R.; James, K.D.; Harris, D.E.; Quail, M.A.; Kieser, H.; Harper, D.; et al. Complete Genome Sequence of the Model Actinomycete Streptomyces Coelicolor A3(2). Nature 2002, 417, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Challis, G.L.; Ravel, J. Coelichelin, a New Peptide Siderophore Encoded by the Streptomyces Coelicolor Genome: Structure Prediction from the Sequence of Its Non-Ribosomal Peptide Synthetase. FEMS Microbiol. Lett. 2000, 187, 111–114. [Google Scholar] [CrossRef]
- Challis, G.L. Genome Mining for Novel Natural Product Discovery. J. Med. Chem. 2008, 51, 2618–2628. [Google Scholar] [CrossRef]
- Scherlach, K.; Hertweck, C. Triggering Cryptic Natural Product Biosynthesis in Microorganisms. Org. Biomol. Chem. 2009, 7, 1753–1760. [Google Scholar] [CrossRef]
- Xu, Z.; Ji, L.; Tang, W.; Guo, L.; Gao, C.; Chen, X.; Liu, J.; Hu, G.; Liu, L. Metabolic Engineering of Streptomyces to Enhance the Synthesis of Valuable Natural Products. Eng. Microbiol. 2022, 2, 100022. [Google Scholar] [CrossRef]
- Tay, D.W.P.; Tan, L.L.; Heng, E.; Zulkarnain, N.; Ching, K.C.; Wibowo, M.; Chin, E.J.; Tan, Z.Y.Q.; Leong, C.Y.; Ng, V.W.P.; et al. Exploring a General Multi-Pronged Activation Strategy for Natural Product Discovery in Actinomycetes. Commun. Biol. 2024, 7, 50. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Y.; Huang, C.; Luo, Y. Recent Advances in Silent Gene Cluster Activation in Streptomyces. Front. Bioeng. Biotechnol. 2021, 9, 632230. [Google Scholar] [CrossRef]
- Harvey, C.J.B.; Tang, M.; Schlecht, U.; Horecka, J.; Fischer, C.R.; Lin, H.C.; Li, J.; Naughton, B.; Cherry, J.; Miranda, M.; et al. HEx: A Heterologous Expression Platform for the Discovery of Fungal Natural Products. Sci. Adv. 2018, 4, eaar5459. [Google Scholar] [CrossRef] [PubMed]
- Ameruoso, A.; Kcam, M.C.V.; Cohen, K.P.; Chappell, J. Activating Natural Product Synthesis Using CRISPR Interference and Activation Systems in Streptomyces. Nucleic Acids Res. 2022, 50, 7751–7760. [Google Scholar] [CrossRef] [PubMed]
- Yeo, W.L.; Heng, E.; Tan, L.L.; Lim, Y.W.; Lim, Y.H.; Hoon, S.; Zhao, H.; Zhang, M.M.; Wong, F.T. Characterization of Cas Proteins for CRISPR-Cas Editing in Streptomycetes. Biotechnol. Bioeng. 2019, 116, 2330–2338. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Charusanti, P.; Zhang, L.; Weber, T.; Lee, S.Y. CRISPR-Cas9 Based Engineering of Actinomycetal Genomes. ACS Synth. Biol. 2015, 4, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Wen, S.; Xu, W.; He, Z.; Zhai, G.; Liu, Y.; Deng, Z.; Sun, Y. Highly Efficient Editing of the Actinorhodin Polyketide Chain Length Factor Gene in Streptomyces Coelicolor M145 Using CRISPR/Cas9-CodA(Sm) Combined System. Appl. Microbiol. Biotechnol. 2015, 99, 10575–10585. [Google Scholar] [CrossRef] [PubMed]
- Goh, F.; Zhang, M.M.; Lim, T.R.; Low, K.N.; Nge, C.E.; Heng, E.; Yeo, W.L.; Sirota, F.L.; Crasta, S.; Tan, Z.; et al. Identification and Engineering of 32 Membered Antifungal Macrolactone Notonesomycins. Microb. Cell Fact. 2020, 19, 71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, D.; Zhu, J.; Liu, H.; Liang, S.; Luo, Y. Efficient Multiplex Genome Editing in Streptomyces via Engineered CRISPR-Cas12a Systems. Front. Bioeng. Biotechnol. 2020, 8, 726. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wei, K.; Zheng, G.; Liu, X.; Chen, S.; Jiang, W.; Lu, Y. CRISPR-Cpf1-Assisted Multiplex Genome Editing and Transcriptional Repression in Streptomyces. Appl. Environ. Microbiol. 2018, 84, e00827-18. [Google Scholar] [CrossRef] [PubMed]
- Pausch, P.; Al-Shayeb, B.; Bisom-Rapp, E.; Tsuchida, C.A.; Li, Z.; Cress, B.F.; Knott, G.J.; Jacobsen, S.E.; Banfield, J.F.; Doudna, J.A. Crispr-Casf from Huge Phages Is a Hypercompact Genome Editor. Science 2020, 369, 333–337. [Google Scholar] [CrossRef]
- Tan, L.L.; Heng, E.; Zulkarnain, N.; Hsiao, W.C.; Wong, F.T.; Zhang, M.M. CRISPR/Cas-Mediated Genome Editing of Streptomyces. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2022; Volume 2479. [Google Scholar]
- Rastogi, A.; Murik, O.; Bowler, C.; Tirichine, L. PhytoCRISP-Ex: A Web-Based and Stand-Alone Application to Find Specific Target Sequences for CRISPR/CAS Editing. BMC Bioinform. 2016, 17, 261. [Google Scholar] [CrossRef]
- Cobb, R.E.; Wang, Y.; Zhao, H. High-Efficiency Multiplex Genome Editing of Streptomyces Species Using an Engineered CRISPR/Cas System. ACS Synth. Biol. 2015, 4, 723–728. [Google Scholar] [CrossRef]
- Ng, S.B.; Kanagasundaram, Y.; Fan, H.; Arumugam, P.; Eisenhaber, B.; Eisenhaber, F. The 160K Natural Organism Library, a Unique Resource for Natural Products Research. Nat. Biotechnol. 2018, 36, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Medema, M.H.; Blin, K.; Cimermancic, P.; De Jager, V.; Zakrzewski, P.; Fischbach, M.A.; Weber, T.; Takano, E.; Breitling, R. AntiSMASH: Rapid Identification, Annotation and Analysis of Secondary Metabolite Biosynthesis Gene Clusters in Bacterial and Fungal Genome Sequences. Nucleic Acids Res. 2011, 39, W339–W346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Wong, F.T.; Wang, Y.; Luo, S.; Lim, Y.H.; Heng, E.; Yeo, W.L.; Cobb, R.E.; Enghiad, B.; Ang, E.L.; et al. CRISPR–Cas9 Strategy for Activation of Silent Streptomyces Biosynthetic Gene Clusters. Nat. Chem. Biol. 2017, 13, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, X.; Wang, J.; Xiang, S.; Feng, X.; Yang, K. An Engineered Strong Promoter for Streptomycetes. Appl. Environ. Microbiol. 2013, 79, 4484–4492. [Google Scholar] [CrossRef] [PubMed]
- Heng, E.; Tan, L.L.; Zhang, M.M.; Wong, F.T. CRISPR-Cas Strategies for Natural Product Discovery and Engineering in Actinomycetes. Process Biochem. 2021, 102, 261–268. [Google Scholar] [CrossRef]
- Braesel, J.; Lee, J.H.; Arnould, B.; Murphy, B.T.; Eustáquio, A.S. Diazaquinomycin Biosynthetic Gene Clusters from Marine and Freshwater Actinomycetes. J. Nat. Prod. 2019, 82, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Chai, B.; Ding, Y.; He, M.; Zheng, L.; Teng, Y.; Deng, Z.; Yu, Y.; Liu, T. Overproduction of Gentamicin B in Industrial Strain Micromonospora Echinospora CCTCC M 2018898 by Cloning of the Missing Genes GenR and GenS. Metab. Eng. Commun. 2019, 9, e00096. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.R.; Townsend, C.A. A Dual Role for a Polyketide Synthase in Dynemicin Enediyne and Anthraquinone Biosynthesis. Nat. Chem. 2018, 10, 231–236. [Google Scholar] [CrossRef]
- Schaffert, L.; Schneiker-Bekel, S.; Gierhake, J.; Droste, J.; Persicke, M.; Rosen, W.; Pühler, A.; Kalinowski, J. Absence of the Highly Expressed Small Carbohydrate-Binding Protein Cgt Improves the Acarbose Formation in Actinoplanes sp. SE50/110. Appl. Microbiol. Biotechnol. 2020, 104, 5395–5408. [Google Scholar] [CrossRef]
- Wolf, T.; Gren, T.; Thieme, E.; Wibberg, D.; Zemke, T.; Pühler, A.; Kalinowski, J. Targeted Genome Editing in the Rare Actinomycete Actinoplanes sp. SE50/110 by Using the CRIPSR/Cas9 System. J. Biotechnol. 2016, 231, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Heng, E.; Lim, Y.W.; Leong, C.Y.; Ng, V.W.P.; Ng, S.B.; Lim, Y.H.; Wong, F.T. Enhancing armeniaspirols production through multi-level engineering of a native Streptomyces producer. Microb. Cell Fact. 2023, 22, 84. [Google Scholar] [CrossRef] [PubMed]

| Cas Constructs a | Editing Efficiency b | # Exconjugants c | References |
|---|---|---|---|
| Streptomyces albus J1074 | |||
| AsCas12j-2 (template 2) | 12.5% (1/8) | >400 | This study |
| FnCas12a (template 2) | 87% (7/8) | >400 | [13] |
| SpCas9 (template 1) | 100% (8/8) | >400 | [13] |
| SaCas9 (template 1) | 87% (7/8) | >400 | [13] |
| Sth1Cas9 (template 2) | 100% (8/8) | >400 | [13] |
| Streptomyces sp. NRRL S-244 | |||
| AsCas12j-2 (template 1) | 62.5% (5/8) | >400 | This study |
| FnCas12a (template 1) | 100% (8/8) | 38 | [13] |
| Cluster | Cas Constructs | Insertion (kb) | Editing Efficiency a | # Exconjugants b |
|---|---|---|---|---|
| 5 | AsCas12j-2 | 0.1 | 50% (3/6) | >50 |
| FnCas12a | 0% (0/8) | >50 | ||
| 74 | AsCas12j-2 | 0.1 | 75% (3/4) | 4 |
| FnCas12a | 0% (0/6) | 6 | ||
| 52 c | AsCas12j-2 | 1 | 75% (3/4) | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, L.L.; Heng, E.; Leong, C.Y.; Ng, V.; Yang, L.K.; Seow, D.C.S.; Koduru, L.; Kanagasundaram, Y.; Ng, S.B.; Peh, G.; et al. Application of Cas12j for Streptomyces Editing. Biomolecules 2024, 14, 486. https://doi.org/10.3390/biom14040486
Tan LL, Heng E, Leong CY, Ng V, Yang LK, Seow DCS, Koduru L, Kanagasundaram Y, Ng SB, Peh G, et al. Application of Cas12j for Streptomyces Editing. Biomolecules. 2024; 14(4):486. https://doi.org/10.3390/biom14040486
Chicago/Turabian StyleTan, Lee Ling, Elena Heng, Chung Yan Leong, Veronica Ng, Lay Kien Yang, Deborah Chwee San Seow, Lokanand Koduru, Yoganathan Kanagasundaram, Siew Bee Ng, Guangrong Peh, and et al. 2024. "Application of Cas12j for Streptomyces Editing" Biomolecules 14, no. 4: 486. https://doi.org/10.3390/biom14040486
APA StyleTan, L. L., Heng, E., Leong, C. Y., Ng, V., Yang, L. K., Seow, D. C. S., Koduru, L., Kanagasundaram, Y., Ng, S. B., Peh, G., Lim, Y. H., & Wong, F. T. (2024). Application of Cas12j for Streptomyces Editing. Biomolecules, 14(4), 486. https://doi.org/10.3390/biom14040486







