The Cerebrospinal Fluid Free-Glycans Hex1 and HexNAc1Hex1Neu5Ac1 as Potential Biomarkers of Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Patient Population
2.3. Sample Collection and Characterization
2.4. Isolation and Purification of Free-Glycans
2.5. N-Glycan Release and Purification
2.6. O-Glycan Release and Purification
2.7. Permethylation
2.8. Mass Spectrometry
2.9. Statistical Analysis
3. Results
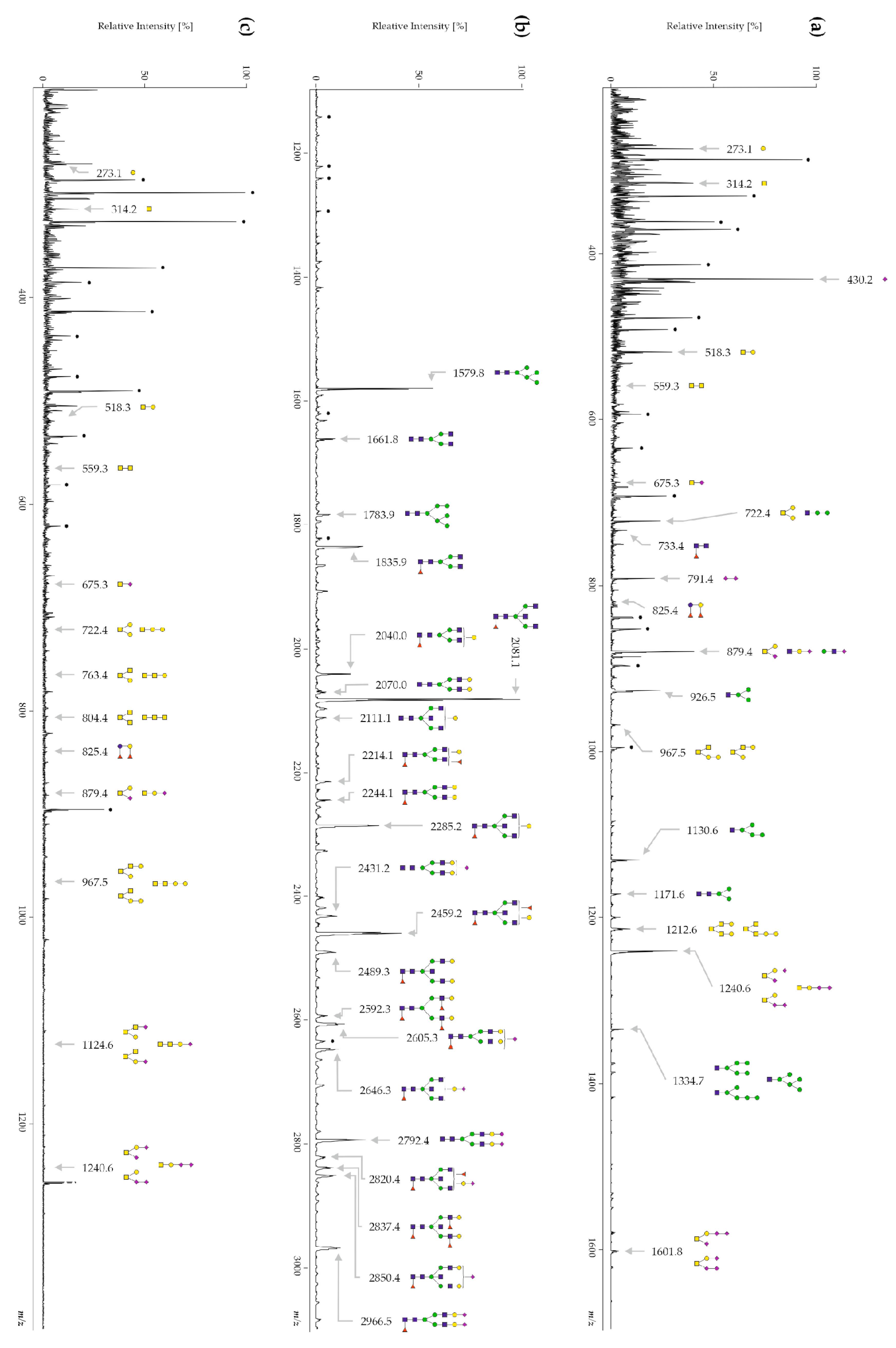
3.1. Human CSF Contains Free-, N- and O-Glycans
3.2. Inter-Day Reproducibility of Free-, N- and O-Glycans
3.3. The Glycan Profiles of Patients with AD Differ Significantly from Healthy Controls and Other Types of Dementia
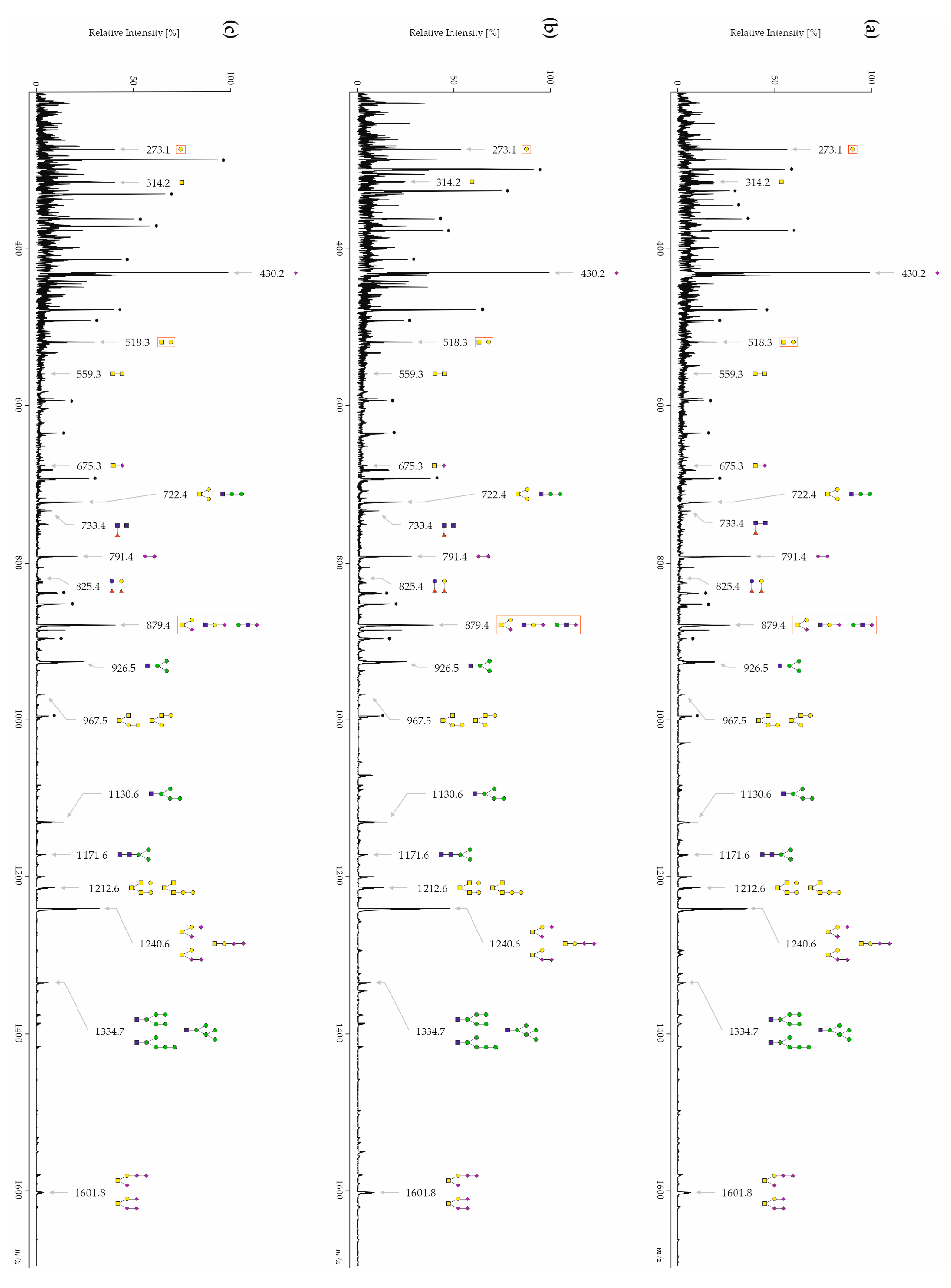
3.3.1. CSF Free-Glycans
3.3.2. CSF N-Glycans
3.3.3. CSF O-Glycans
3.4. The Sialic Acid Content Is Significantly Decreased in Free-Glycan Fragments and O-Glycans from Patients with AD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilbert, B.J. The role of amyloid beta in the pathogenesis of Alzheimer’s disease. J. Clin. Pathol. 2013, 66, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.alzint.org/resource/world-alzheimer-report-2023/ (accessed on 10 May 2023).
- Winblad, B.; Amouyel, P.; Andrieu, S.; Ballard, C.; Brayne, C.; Brodaty, H.; Cedazo-Minguez, A.; Dubois, B.; Edvardsson, D.; Feldman, H.; et al. Defeating Alzheimer’s disease and other dementias: A priority for European science and society. Lancet Neurol. 2016, 15, 455–532. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, D.; Jimenez-Diaz, L.; Navarro-Lopez, J.D. Past, present and future of therapeutic strategies against amyloid-beta peptides in Alzheimer’s disease: A systematic review. Ageing Res. Rev. 2021, 72, 101496. [Google Scholar] [CrossRef] [PubMed]
- McLaurin, J.; Yang, D.; Yip, C.M.; Fraser, P.E. Review: Modulating factors in amyloid-beta fibril formation. J. Struct. Biol. 2000, 130, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Yuzwa, S.A.; Vocadlo, D.J. O-GlcNAc modification and the tauopathies: Insights from chemical biology. Curr. Alzheimer Res. 2009, 6, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Balana, A.T.; Pratt, M.R. Mechanistic roles for altered O-GlcNAcylation in neurodegenerative disorders. Biochem. J. 2021, 478, 2733–2758. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, T.; Ferreira, S.; Dupont-Wallois, L.; Bussiere, T.; Dupire, M.J.; Delacourte, A.; Michalski, J.C.; Caillet-Boudin, M.L. Evidence of a balance between phosphorylation and O-GlcNAc glycosylation of Tau proteins—A role in nuclear localization. Biochim. Et Biophys. Acta 2003, 1619, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Iqbal, K.; Grundke-Iqbal, I.; Hart, G.W.; Gong, C.X. O-GlcNAcylation regulates phosphorylation of tau: A mechanism involved in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 10804–10809. [Google Scholar] [CrossRef]
- Alonso, A.D.; Grundke-Iqbal, I.; Barra, H.S.; Iqbal, K. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: Sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc. Natl. Acad. Sci. USA 1997, 94, 298–303. [Google Scholar] [CrossRef]
- Iqbal, K.; Alonso Adel, C.; Chen, S.; Chohan, M.O.; El-Akkad, E.; Gong, C.X.; Khatoon, S.; Li, B.; Liu, F.; Rahman, A.; et al. Tau pathology in Alzheimer disease and other tauopathies. Biochim. Biophys. Acta 2005, 1739, 198–210. [Google Scholar] [CrossRef]
- Rogaev, E.I.; Sherrington, R.; Rogaeva, E.A.; Levesque, G.; Ikeda, M.; Liang, Y.; Chi, H.; Lin, C.; Holman, K.; Tsuda, T.; et al. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature 1995, 376, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, R.; Rogaev, E.I.; Liang, Y.; Rogaeva, E.A.; Levesque, G.; Ikeda, M.; Chi, H.; Lin, C.; Li, G.; Holman, K.; et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 1995, 375, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, R.; Brás, J.; Hardy, J. SnapShot: Genetics of Alzheimer’s Disease. Cell 2013, 155, 968–968.e961. [Google Scholar] [CrossRef]
- 2023 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2023, 17, 327–406. [CrossRef]
- Ryman, D.C.; Acosta-Baena, N.; Aisen, P.S.; Bird, T.; Danek, A.; Fox, N.C.; Goate, A.; Frommelt, P.; Ghetti, B.; Langbaum, J.B.S.; et al. Symptom onset in autosomal dominant Alzheimer disease: A systematic review and meta-analysis. Neurology 2014, 83, 253–260. [Google Scholar] [CrossRef]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Petersen, R.C.; Weiner, M.W.; Aisen, P.S.; Shaw, L.M.; Vemuri, P.; Wiste, H.J.; Weigand, S.D.; et al. Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013, 12, 207–216. [Google Scholar] [CrossRef]
- Hansson, O. Biomarkers for neurodegenerative diseases. Nat. Med. 2021, 27, 954–963. [Google Scholar] [CrossRef]
- Dunne, R.A.; Aarsland, D.; O’Brien, J.T.; Ballard, C.; Banerjee, S.; Fox, N.C.; Isaacs, J.D.; Underwood, B.R.; Perry, R.J.; Chan, D.; et al. Mild cognitive impairment: The Manchester consensus. Age Ageing 2021, 50, 72–80. [Google Scholar] [CrossRef]
- Caselli, R.J.; Beach, T.G.; Yaari, R.; Reiman, E.M. Alzheimer’s disease a century later. J. Clin. Psychiatry 2006, 67, 1784–1800. [Google Scholar] [CrossRef]
- Boeve, B.F.; Maraganore, D.M.; Parisi, J.E.; Ahlskog, J.E.; Graff-Radford, N.; Caselli, R.J.; Dickson, D.W.; Kokmen, E.; Petersen, R.C. Pathologic heterogeneity in clinically diagnosed corticobasal degeneration. Neurology 1999, 53, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Boeve, B.F.; Silber, M.H.; Ferman, T.J.; Lucas, J.A.; Parisi, J.E. Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov. Disord. 2001, 16, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Caselli, R.J.; Jack, C.R.; Petersen, R.C.; Wahner, H.W.; Yanagihara, T. Asymmetric cortical degenerative syndromes: Clinical and radiologic correlations. Neurology 1992, 42, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Caselli, R.J.; Stelmach, G.E.; Caviness, J.N.; Timmann, D.; Royer, T.; Boeve, B.F.; Parisi, J.E. A kinematic study of progressive apraxia with and without dementia. Mov. Disord. 1999, 14, 276–287. [Google Scholar] [CrossRef] [PubMed]
- McKeith, I. Dementia with Lewy bodies. Dialogues Clin. Neurosci. 2004, 6, 333–341. [Google Scholar] [CrossRef] [PubMed]
- McKeith, I.G.; Dickson, D.W.; Lowe, J.; Emre, M.; O’Brien, J.T.; Feldman, H.; Cummings, J.; Duda, J.E.; Lippa, C.; Perry, E.K.; et al. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology 2005, 65, 1863–1872. [Google Scholar] [CrossRef]
- Tang-Wai, D.F.; Graff-Radford, N.R.; Boeve, B.F.; Dickson, D.W.; Parisi, J.E.; Crook, R.; Caselli, R.J.; Knopman, D.S.; Petersen, R.C. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology 2004, 63, 1168–1174. [Google Scholar] [CrossRef]
- Johnson, J.K.; Head, E.; Kim, R.; Starr, A.; Cotman, C.W. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch. Neurol. 1999, 56, 1233–1239. [Google Scholar] [CrossRef]
- Heurling, K.; Leuzy, A.; Zimmer, E.R.; Lubberink, M.; Nordberg, A. Imaging beta-amyloid using [(18)F]flutemetamol positron emission tomography: From dosimetry to clinical diagnosis. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 362–373. [Google Scholar] [CrossRef]
- Leclerc, B.; Abulrob, A. Perspectives in molecular imaging using staging biomarkers and immunotherapies in Alzheimer’s disease. Sci. World J. 2013, 2013, 589308. [Google Scholar] [CrossRef]
- Sabri, O.; Seibyl, J.; Rowe, C.; Barthel, H. Beta-amyloid imaging with florbetaben. Clin. Transl. Imaging 2015, 3, 13–26. [Google Scholar] [CrossRef]
- Barthel, H.; Gertz, H.J.; Dresel, S.; Peters, O.; Bartenstein, P.; Buerger, K.; Hiemeyer, F.; Wittemer-Rump, S.M.; Seibyl, J.; Reininger, C.; et al. Cerebral amyloid-beta PET with florbetaben (18F) in patients with Alzheimer’s disease and healthy controls: A multicentre phase 2 diagnostic study. Lancet Neurol. 2011, 10, 424–435. [Google Scholar] [CrossRef]
- Herholz, K.; Ebmeier, K. Clinical amyloid imaging in Alzheimer’s disease. Lancet Neurol. 2011, 10, 667–670. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Blom, E.S.; Giedraitis, V.; Zetterberg, H.; Fukumoto, H.; Blennow, K.; Hyman, B.T.; Irizarry, M.C.; Wahlund, L.O.; Lannfelt, L.; Ingelsson, M. Rapid progression from mild cognitive impairment to Alzheimer’s disease in subjects with elevated levels of tau in cerebrospinal fluid and the APOE epsilon4/epsilon4 genotype. Dement. Geriatr. Cogn. Disord. 2009, 27, 458–464. [Google Scholar] [CrossRef]
- Samgard, K.; Zetterberg, H.; Blennow, K.; Hansson, O.; Minthon, L.; Londos, E. Cerebrospinal fluid total tau as a marker of Alzheimer’s disease intensity. Int. J. Geriatr. Psychiatry 2010, 25, 403–410. [Google Scholar] [CrossRef]
- Seubert, P.; Vigo-Pelfrey, C.; Esch, F.; Lee, M.; Dovey, H.; Davis, D.; Sinha, S.; Schlossmacher, M.; Whaley, J.; Swindlehurst, C.; et al. Isolation and quantification of soluble Alzheimer’s beta-peptide from biological fluids. Nature 1992, 359, 325–327. [Google Scholar] [CrossRef]
- Sadigh-Eteghad, S.; Sabermarouf, B.; Majdi, A.; Talebi, M.; Farhoudi, M.; Mahmoudi, J. Amyloid-beta: A crucial factor in Alzheimer’s disease. Med. Princ. Pract. 2015, 24, 1–10. [Google Scholar] [CrossRef]
- Jarrett, J.T.; Berger, E.P.; Lansbury, P.T., Jr. The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: Implications for the pathogenesis of Alzheimer’s disease. Biochemistry 1993, 32, 4693–4697. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.M.; Vanderstichele, H.; Knapik-Czajka, M.; Clark, C.M.; Aisen, P.S.; Petersen, R.C.; Blennow, K.; Soares, H.; Simon, A.; Lewczuk, P.; et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann. Neurol. 2009, 65, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Welge, V.; Fiege, O.; Lewczuk, P.; Mollenhauer, B.; Esselmann, H.; Klafki, H.W.; Wolf, S.; Trenkwalder, C.; Otto, M.; Kornhuber, J.; et al. Combined CSF tau, p-tau181 and amyloid-beta 38/40/42 for diagnosing Alzheimer’s disease. J. Neural Transm. 2009, 116, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.A.; Arvanitakis, Z.; Leurgans, S.E.; Bennett, D.A. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann. Neurol. 2009, 66, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; Zetterberg, H.; Fagan, A.M. Fluid biomarkers in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006221. [Google Scholar] [CrossRef] [PubMed]
- Peracaula, R.; Royle, L.; Tabarés, G.; Mallorquí-Fernández, G.; Barrabés, S.; Harvey, D.J.; Dwek, R.A.; Rudd, P.M.; de Llorens, R. Glycosylation of human pancreatic ribonuclease: Differences between normal and tumor states. Glycobiology 2003, 13, 227–244. [Google Scholar] [CrossRef] [PubMed]
- Peracaula, R.; Tabarés, G.; Royle, L.; Harvey, D.J.; Dwek, R.A.; Rudd, P.M.; de Llorens, R. Altered glycosylation pattern allows the distinction between prostate-specific antigen (PSA) from normal and tumor origins. Glycobiology 2003, 13, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Opdenakker, G.; Rudd, P.M.; Ponting, C.P.; Dwek, R.A. Concepts and principles of glycobiology. FASEB J. 1993, 7, 1330–1337. [Google Scholar] [CrossRef]
- Fukuda, M.N.; Sasaki, H.; Lopez, L.; Fukuda, M. Survival of recombinant erythropoietin in the circulation: The role of carbohydrates. Blood 1989, 73, 84–89. [Google Scholar] [CrossRef]
- Gu, J.; Isaji, T.; Xu, Q.; Kariya, Y.; Gu, W.; Fukuda, T.; Du, Y. Potential roles of N-glycosylation in cell adhesion. Glycoconj. J. 2012, 29, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Biskup, K.; Braicu, E.I.; Sehouli, J.; Fotopoulou, C.; Tauber, R.; Berger, M.; Blanchard, V. Serum glycome profiling: A biomarker for diagnosis of ovarian cancer. J. Proteome Res. 2013, 12, 4056–4063. [Google Scholar] [CrossRef]
- Gornik, O.; Royle, L.; Harvey, D.J.; Radcliffe, C.M.; Saldova, R.; Dwek, R.A.; Rudd, P.; Lauc, G. Changes of serum glycans during sepsis and acute pancreatitis. Glycobiology 2007, 17, 1321–1332. [Google Scholar] [CrossRef]
- Abd Hamid, U.M.; Royle, L.; Saldova, R.; Radcliffe, C.M.; Harvey, D.J.; Storr, S.J.; Pardo, M.; Antrobus, R.; Chapman, C.J.; Zitzmann, N.; et al. A strategy to reveal potential glycan markers from serum glycoproteins associated with breast cancer progression. Glycobiology 2008, 18, 1105–1118. [Google Scholar] [CrossRef]
- Akasaka-Manya, K.; Manya, H.; Sakurai, Y.; Wojczyk, B.S.; Kozutsumi, Y.; Saito, Y.; Taniguchi, N.; Murayama, S.; Spitalnik, S.L.; Endo, T. Protective effect of N -glycan bisecting GlcNAc residues on β-amyloid production in Alzheimer’s disease. Glycobiology 2010, 20, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Akasaka-Manya, K.; Manya, H.; Sakurai, Y.; Wojczyk, B.S.; Spitalnik, S.L.; Endo, T. Increased bisecting and core-fucosylated N-glycans on mutant human amyloid precursor proteins. Glycoconj. J. 2008, 25, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Kitazume, S.; Nakagawa, K.; Oka, R.; Tachida, Y.; Ogawa, K.; Luo, Y.; Citron, M.; Shitara, H.; Taya, C.; Yonekawa, H.; et al. In vivo cleavage of alpha2,6-sialyltransferase by Alzheimer beta-secretase. J. Biol. Chem. 2005, 280, 8589–8595. [Google Scholar] [CrossRef] [PubMed]
- Maguire, T.M.; Breen, K.C. A decrease in neural sialyltransferase activity in Alzheimer’s disease. Dementia 1995, 6, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Maguire, T.M.; Gillian, A.M.; O’Mahony, D.; Coughlan, C.M.; Dennihan, A.; Breen, K.C. A decrease in serum sialyltransferase levels in Alzheimer’s disease. Neurobiol. Aging 1994, 15, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Kitazume, S.; Tachida, Y.; Oka, R.; Shirotani, K.; Saido, T.C.; Hashimoto, Y. Alzheimer’s β-secretase, β-site amyloid precursor protein-cleaving enzyme, is responsible for cleavage secretion of a Golgi-resident sialyltransferase. Proc. Natl. Acad. Sci. USA 2001, 98, 13554–13559. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.G.; Veillon, L.; Mechref, Y. N-Glycan Profile of Cerebrospinal Fluids from Alzheimer’s Disease Patients Using Liquid Chromatography with Mass Spectrometry. J. Proteome Res. 2019, 18, 3770–3779. [Google Scholar] [CrossRef] [PubMed]
- Goyallon, A.; Cholet, S.; Chapelle, M.; Junot, C.; Fenaille, F. Evaluation of a combined glycomics and glycoproteomics approach for studying the major glycoproteins present in biofluids: Application to cerebrospinal fluid. Rapid Commun. Mass. Spectrom. 2015, 29, 461–473. [Google Scholar] [CrossRef]
- Fogli, A.; Merle, C.; Roussel, V.; Schiffmann, R.; Ughetto, S.; Theisen, M.; Boespflug-Tanguy, O. CSF N-glycan profiles to investigate biomarkers in brain developmental disorders: Application to leukodystrophies related to eIF2B mutations. PLoS ONE 2012, 7, e42688. [Google Scholar] [CrossRef]
- Furukawa, J.-I.; Hanamatsu, H.; Yokota, I.; Hirayama, M.; Ando, T.; Kobayashi, H.; Ohnishi, S.; Miura, N.; Okada, K.; Sakai, S.; et al. Comprehensive Glycomic Approach Reveals Novel Low-Molecular-Weight Blood Group-Specific Glycans in Serum and Cerebrospinal Fluid. J. Proteome Res. 2021, 20, 2812–2822. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Seino, J.; Fujihira, H.; Sato, K.; Fujinawa, R.; Sumer-Bayraktar, Z.; Ishii, N.; Matsuo, I.; Nakaya, S.; Suzuki, T. Occurrence of free N-glycans with a single GlcNAc at the reducing termini in animal sera. Glycobiology 2022, 32, 314–332. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuka, K.; Watanabe, S.; Kinoshita, M.; Kamisue, K.; Yamada, K.; Hayakawa, T.; Suzuki, T.; Kakehi, K. Free glycans derived from glycoproteins present in human sera. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 928, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Hanzawa, K.; Tanaka-Okamoto, M.; Murakami, H.; Mukai, M.; Takahashi, H.; Omori, T.; Ikezawa, K.; Ohkawa, K.; Ohue, M.; Miyamoto, Y. Investigation of acidic free-glycans in urine and their alteration in cancer. Glycobiology 2021, 31, 391–409. [Google Scholar] [CrossRef] [PubMed]
- Hanzawa, K.; Tanaka-Okamoto, M.; Murakami, H.; Suzuki, N.; Mukai, M.; Takahashi, H.; Omori, T.; Ikezawa, K.; Ohkawa, K.; Ohue, M.; et al. Increased levels of acidic free-N-glycans, including multi-antennary and fucosylated structures, in the urine of cancer patients. PLoS ONE 2022, 17, e0266927. [Google Scholar] [CrossRef] [PubMed]
- Alonzi, D.S.; Su, Y.H.; Butters, T.D. Urinary glycan markers for disease. Biochem. Soc. Trans. 2011, 39, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Chalabi, S.; Easton, R.L.; Patankar, M.S.; Lattanzio, F.A.; Morrison, J.C.; Panico, M.; Morris, H.R.; Dell, A.; Clark, G.F. The expression of free oligosaccharides in human seminal plasma. J. Biol. Chem. 2002, 277, 32562–32570. [Google Scholar] [CrossRef] [PubMed]
- Boeddrich, A.; Haenig, C.; Neuendorf, N.; Blanc, E.; Ivanov, A.; Kirchner, M.; Schleumann, P.; Bayraktaroglu, I.; Richter, M.; Molenda, C.M.; et al. A proteomics analysis of 5xFAD mouse brain regions reveals the lysosome-associated protein Arl8b as a candidate biomarker for Alzheimer’s disease. Genome Med. 2023, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Reinke, S.O.; Bayer, M.; Berger, M.; Hinderlich, S.; Blanchard, V. The analysis of N-glycans of cell membrane proteins from human hematopoietic cell lines reveals distinctions in their pattern. Biol. Chem. 2012, 393, 731–747. [Google Scholar] [CrossRef]
- Ciucanu, I.; Kerek, F. Rapid and simultaneous methylation of fatty and hydroxy fatty acids for gas—Liquid chromatographic analysis. J. Chromatogr. A 1984, 284, 179–185. [Google Scholar] [CrossRef]
- Wedepohl, S.; Kaup, M.; Riese, S.B.; Berger, M.; Dernedde, J.; Tauber, R.; Blanchard, V. N-glycan analysis of recombinant L-Selectin reveals sulfated GalNAc and GalNAc-GalNAc motifs. J. Proteome Res. 2010, 9, 3403–3411. [Google Scholar] [CrossRef] [PubMed]
- Maass, K.; Ranzinger, R.; Geyer, H.; von der Lieth, C.W.; Geyer, R. “Glyco-peakfinder”—De novo composition analysis of glycoconjugates. Proteomics 2007, 7, 4435–4444. [Google Scholar] [CrossRef] [PubMed]
- Ceroni, A.; Maass, K.; Geyer, H.; Geyer, R.; Dell, A.; Haslam, S.M. GlycoWorkbench: A Tool for the Computer-Assisted Annotation of Mass Spectra of Glycans. J. Proteome Res. 2008, 7, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Damerell, D.; Ceroni, A.; Maass, K.; Ranzinger, R.; Dell, A.; Haslam, S.M. The GlycanBuilder and GlycoWorkbench glycoinformatics tools: Updates and new developments. Biol. Chem. 2012, 393, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Nimtz, M.; Getzlaff, R.; Conradt, H.S. ‘Brain-type’ N-glycosylation of asialo-transferrin from human cerebrospinal fluid. FEBS Lett. 1995, 359, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Palmigiano, A.; Barone, R.; Sturiale, L.; Sanfilippo, C.; Bua, R.O.; Romeo, D.A.; Messina, A.; Capuana, M.L.; Maci, T.; Le Pira, F.; et al. CSF N-glycoproteomics for early diagnosis in Alzheimer’s disease. J. Proteom. 2016, 131, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Stanta, J.L.; Saldova, R.; Struwe, W.B.; Byrne, J.C.; Leweke, F.M.; Rothermund, M.; Rahmoune, H.; Levin, Y.; Guest, P.C.; Bahn, S.; et al. Identification of N-glycosylation changes in the CSF and serum in patients with schizophrenia. J. Proteome Res. 2010, 9, 4476–4489. [Google Scholar] [CrossRef]
- Barone, R.; Sturiale, L.; Fiumara, A.; Palmigiano, A.; Bua, R.O.; Rizzo, R.; Zappia, M.; Garozzo, D. CSF N-glycan profile reveals sialylation deficiency in a patient with GM2 gangliosidosis presenting as childhood disintegrative disorder. Autism Res. 2016, 9, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, M.; Tillack, L.; de Carvalho, M.; Pinto, S.; Conradt, H.S.; Costa, J. Phosphoneurofilament heavy chain and N-glycomics from the cerebrospinal fluid in amyotrophic lateral sclerosis. Clin. Chim. Acta 2015, 438, 342–349. [Google Scholar] [CrossRef]
- Harada, Y.; Hirayama, H.; Suzuki, T. Generation and degradation of free asparagine-linked glycans. Cell Mol. Life Sci. 2015, 72, 2509–2533. [Google Scholar] [CrossRef]
- Moore, S.E. Transport of free polymannose-type oligosaccharides from the endoplasmic reticulum into the cytosol is inhibited by mannosides and requires a thapsigargin-sensitive calcium store. Glycobiology 1998, 8, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. The cytoplasmic peptide:N-glycanase (Ngly1)-basic science encounters a human genetic disorder. J. Biochem. 2015, 157, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Catabolism of N-glycoproteins in mammalian cells: Molecular mechanisms and genetic disorders related to the processes. Mol. Asp. Med. 2016, 51, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Suttapitugsakul, S.; Stavenhagen, K.; Donskaya, S.; Bennett, D.A.; Mealer, R.G.; Seyfried, N.T.; Cummings, R.D. Glycoproteomics Landscape of Asymptomatic and Symptomatic Human Alzheimer’s Disease Brain. Mol. Cell Proteom. 2022, 21, 100433. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Saito, K.; Ito, H.; Hashimoto, Y. Transferrin isoforms in cerebrospinal fluid and their relation to neurological diseases. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2019, 95, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Nakakita, S.; Natsuka, S.; Ikenaka, K.; Hase, S. Development-dependent expression of complex-type sugar chains specific to mouse brain. J. Biochem. 1998, 123, 1164–1168. [Google Scholar] [CrossRef]
- Kizuka, Y.; Kitazume, S.; Fujinawa, R.; Saito, T.; Iwata, N.; Saido, T.C.; Nakano, M.; Yamaguchi, Y.; Hashimoto, Y.; Staufenbiel, M.; et al. An aberrant sugar modification of BACE1 blocks its lysosomal targeting in Alzheimer’s disease. EMBO Mol. Med. 2015, 7, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Kizuka, Y.; Taniguchi, N. Neural functions of bisecting GlcNAc. Glycoconj. J. 2018, 35, 345–351. [Google Scholar] [CrossRef]
- Wang, W.; Gopal, S.; Pocock, R.; Xiao, Z. Glycan Mimetics from Natural Products: New Therapeutic Opportunities for Neurodegenerative Disease. Molecules 2019, 24, 4604. [Google Scholar] [CrossRef]
- Schedin-Weiss, S.; Gaunitz, S.; Sui, P.; Chen, Q.; Haslam, S.M.; Blennow, K.; Winblad, B.; Dell, A.; Tjernberg, L.O. Glycan biomarkers for Alzheimer disease correlate with T-tau and P-tau in cerebrospinal fluid in subjective cognitive impairment. FEBS J. 2020, 287, 3221–3234. [Google Scholar] [CrossRef]
- Liu, F.; Zaidi, T.; Iqbal, K.; Grundke-Iqbal, I.; Gong, C.X. Aberrant glycosylation modulates phosphorylation of tau by protein kinase A and dephosphorylation of tau by protein phosphatase 2A and 5. Neuroscience 2002, 115, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Grundke-Iqbal, I.; Iqbal, K. Glycosylation of microtubule-associated protein tau: An abnormal posttranslational modification in Alzheimer’s disease. Nat. Med. 1996, 2, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Kitazume, S.; Tachida, Y.; Oka, R.; Kotani, N.; Ogawa, K.; Suzuki, M.; Dohmae, N.; Takio, K.; Saido, T.C.; Hashimoto, Y. Characterization of α2,6-Sialyltransferase Cleavage by Alzheimer’s β-Secretase (BACE1)*. J. Biol. Chem. 2003, 278, 14865–14871. [Google Scholar] [CrossRef] [PubMed]

| Patients with AD | Disease Control Patients | Healthy Controls | |
|---|---|---|---|
| Number of patients (m/f) | 89 (43/46) | 86 (55/31) | 87 (48/39) |
| Age of the patient cohorts (years) | |||
| Mean (m/f) | 76 (75/76) | 73 (72/75) | 66 (67/65) |
| Median (m/f) | 77 (77/77) | 75 (72/77) | 66 (66/65) |
| SD | 6.7 | 8.3 | 8.5 |
| Range | 44–88 | 52–89 | 45–84 |
| MMSE | |||
| Mean (m/f) | 23.5 (23.6/23.5) | 23.6 (24.2/22.5) | 28.7 (28.8/28.7) |
| Median (m/f) | 24.0 (25.0/24.0) | 25.0 (25.0/23.5) | 29.0 (29.0/29.0) |
| SD | 3.8 | 4.6 | 1.1 |
| Range | 12.0–30.0 | 3.0–30.0 | 26.0–30.0 |
| Aβ ratio | |||
| Mean (m/f) | 0.051 (0.049/0.053) | 0.085 (0.085/0.085) | 0.088 (0.090/0.086) |
| Median (m/f) | 0.049 (0.048/0.051) | 0.095 (0.091/0.097) | 0.096 (0.097/0.094) |
| SD | 0.012 | 0.024 | 0.020 |
| Range | 0.025–0.103 | 0.027–0.120 | 0.034–0.115 |
| p-tau (pg/mL) | |||
| Mean (m/f) | 104.0 (112.0/96.4) | 53.1 (53.3/52.6) | 40.9 (41.2/40.6) |
| Median (m/f) | 98.1 (105.3/94.9) | 41.9 (42.0/40.6) | 37.2 (38.5/32.1) |
| SD | 42.9 | 37.4 | 14.4 |
| Range | 34.0–279.0 | 24.0–253.0 | 17.0–91.0 |
| t-tau (pg/mL) | |||
| Mean (m/f) | 674.6 (724.8/627.7) | 385.3 (381.5/391.9) | 282.8 (290.7/273.0) |
| Median (m/f) | 615.0 (625.0/612.5) | 308.0 (297.0/320.0) | 241.0 (267.5/237.0) |
| SD | 260.6 | 235.6 | 116.7 |
| Range | 231.0–1505.0 | 114.0–1349.0 | 96.0–583.0 |
| m/z | Composition | Patients with AD [%] | DC Patients [%] | HC Patients [%] | p Value AD/DC | p Value AD/HC | p Value DC/HC |
|---|---|---|---|---|---|---|---|
| 273.1 | Hex1 | 16.65 ± 5.27 | 11.46 ± 5.68 | 10.09 ± 3.95 | ≤0.01 | ≤0.01 | |
| 314.2 | HexNAc1 | 10.73 ± 2.40 | 11.61 ± 2.66 | 10.40 ± 2.68 | ≤0.05 | ≤0.01 | |
| 430.2 | Neu5Ac1 | 19.08 ± 3.32 | 18.60 ± 3.22 | 18.77 ± 4.24 | |||
| 518.3 | Hex1HexNAc1 | 5.62 ± 0.78 | 6.93 ± 1.04 | 6.68 ± 1.27 | ≤0.01 | ≤0.01 | |
| 559.3 | HexNAc2 | 1.19 ± 0.28 | 1.54 ± 0.49 | 1.31 ± 0.37 | ≤0.01 | ≤0.05 | ≤0.01 |
| 675.3 | Neu5Ac1HexNAc1 | 0.83 ± 0.14 | 1.12 ± 0.25 | 0.96 ± 0.18 | ≤0.01 | ≤0.01 | ≤0.01 |
| 722.4 | Hex2HexNAc1 | 3.98 ± 0.61 | 4.94 ± 0.83 | 4.39 ± 0.96 | ≤0.01 | ≤0.01 | ≤0.01 |
| 733.4 | Fuc1HexNAc2 | 1.73 ± 0.27 | 2.06 ± 0.49 | 2.07 ± 0.47 | ≤0.01 | ≤0.01 | |
| 791.4 | Neu5Ac2 | 3.71 ± 2.26 | 3.45 ± 2.07 | 4.46 ± 3.02 | |||
| 825.4 | Fuc2Hex2 | 0.95 ± 0.35 | 1.11 ± 0.49 | 1.05 ± 0.34 | ≤0.05 | ≤0.05 | |
| 879.4 | Neu5Ac1Hex1HexNAc1 | 6.49 ± 1.13 | 7.38 ± 1.28 | 7.69 ± 0.97 | ≤0.01 | ≤0.01 | ≤0.05 |
| 926.5 | Hex3HexNAc1 | 4.34 ± 0.76 | 5.17 ± 0.98 | 5.06 ± 1.15 | ≤0.01 | ≤0.01 | |
| 967.5 | Hex2HexNAc2 | 0.91 ± 0.15 | 1.04 ± 0.20 | 1.05 ± 0.26 | ≤0.01 | ≤0.01 | |
| 1130.6 | Hex4HexNAc1 | 2.46 ± 0.46 | 2.81 ± 0.60 | 2.89 ± 0.74 | ≤0.01 | ≤0.01 | |
| 1171.6 | Hex3HexNAc2 | 1.03 ± 0.20 | 1.08 ± 0.22 | 1.17 ± 0.31 | ≤0.01 | ||
| 1212.6 | Hex2HexNAc3 | 2.06 ± 0.53 | 2.24 ± 0.70 | 2.07 ± 0.52 | |||
| 1240.6 | Neu5Ac2Hex1HexNAc1 | 8.05 ± 2.16 | 8.17 ± 2.00 | 8.77 ± 1.87 | |||
| 1334.7 | Hex5HexNAc1 | 1.08 ± 0.26 | 1.17 ± 0.29 | 1.28 ± 0.39 | ≤0.01 | ||
| 1601.8 | Neu5Ac3Hex1HexNAc1 | 1.29 ± 0.52 | 0.95 ± 0.49 | 1.35 ± 0.55 | ≤0.01 | ≤0.01 | |
| Other | 7.81 | 7.18 | 8.51 |
| Male Patients | Female Patients | ||||||
|---|---|---|---|---|---|---|---|
| m/z | Composition | AD/DC | AD/HC | DC/HC | AD/DC | AD/HC | DC/HC |
| 1579.8 | Hex5HexNAc2 | ≤0.05 | ≤0.01 | ||||
| 1661.8 | Hex3HexNAc4 | ≤0.01 | |||||
| 1783.9 | Hex6HexNAc2 | ≤0.05 | |||||
| 1835.9 | Fuc1Hex3HexNAc4 | ≤0.01 | ≤0.05 | ||||
| 2040.0 | Fuc1Hex4HexNAc4 | ≤0.05 | |||||
| 2081.1 | Fuc1Hex3HexNAc5 | ≤0.01 | |||||
| 2111.1 | Hex4HexNAc5 | ≤0.01 | ≤0.05 | ||||
| 2214.1 | Fuc2Hex4HexNAc4 | ≤0.01 | ≤0.05 | ||||
| 2244.1 | Fuc1Hex5HexNAc4 | ≤0.05 | ≤0.01 | ||||
| 2431.2 | Neu5Ac1Hex5HexNAc4 | ≤0.05 | |||||
| 2459.2 | Fuc2Hex4HexNAc5 | ≤0.05 | ≤0.01 | ≤0.01 | ≤0.05 | ≤0.05 | ≤0.01 |
| 2489.3 | Fuc1Hex5HexNAc5 | ≤0.05 | |||||
| 2592.3 | Fuc3Hex5HexNAc4 | ≤0.05 | |||||
| 2605.3 | Neu5Ac1Fuc1Hex5HexNAc4 | ≤0.01 | ≤0.05 | ≤0.01 | ≤0.01 | ||
| 2646.3 | Neu5Ac1Fuc1Hex4HexNAc5 | ≤0.05 | ≤0.01 | ≤0.01 | ≤0.01 | ||
| 2837.4 | Fuc3Hex5HexNAc5 | ≤0.05 | ≤0.05 | ||||
| 2850.4 | Neu5Ac1Fuc1Hex5HexNAc5 | ≤0.05 | ≤0.05 | ≤0.01 | |||
| 2966.5 | Neu5Ac2Fuc1Hex5HexNAc4 | ≤0.01 | ≤0.01 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krüger, L.; Biskup, K.; Schipke, C.G.; Kochnowsky, B.; Schneider, L.-S.; Peters, O.; Blanchard, V. The Cerebrospinal Fluid Free-Glycans Hex1 and HexNAc1Hex1Neu5Ac1 as Potential Biomarkers of Alzheimer’s Disease. Biomolecules 2024, 14, 512. https://doi.org/10.3390/biom14050512
Krüger L, Biskup K, Schipke CG, Kochnowsky B, Schneider L-S, Peters O, Blanchard V. The Cerebrospinal Fluid Free-Glycans Hex1 and HexNAc1Hex1Neu5Ac1 as Potential Biomarkers of Alzheimer’s Disease. Biomolecules. 2024; 14(5):512. https://doi.org/10.3390/biom14050512
Chicago/Turabian StyleKrüger, Lynn, Karina Biskup, Carola G. Schipke, Bianca Kochnowsky, Luisa-Sophie Schneider, Oliver Peters, and Véronique Blanchard. 2024. "The Cerebrospinal Fluid Free-Glycans Hex1 and HexNAc1Hex1Neu5Ac1 as Potential Biomarkers of Alzheimer’s Disease" Biomolecules 14, no. 5: 512. https://doi.org/10.3390/biom14050512
APA StyleKrüger, L., Biskup, K., Schipke, C. G., Kochnowsky, B., Schneider, L.-S., Peters, O., & Blanchard, V. (2024). The Cerebrospinal Fluid Free-Glycans Hex1 and HexNAc1Hex1Neu5Ac1 as Potential Biomarkers of Alzheimer’s Disease. Biomolecules, 14(5), 512. https://doi.org/10.3390/biom14050512






