Elevated Serum Xanthine Oxidase and Its Correlation with Antioxidant Status in Patients with Parkinson’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Reagents
2.3. Patients and Samples Collection
2.4. Biochemical Analysis
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Patients with PD and Those with ONDs
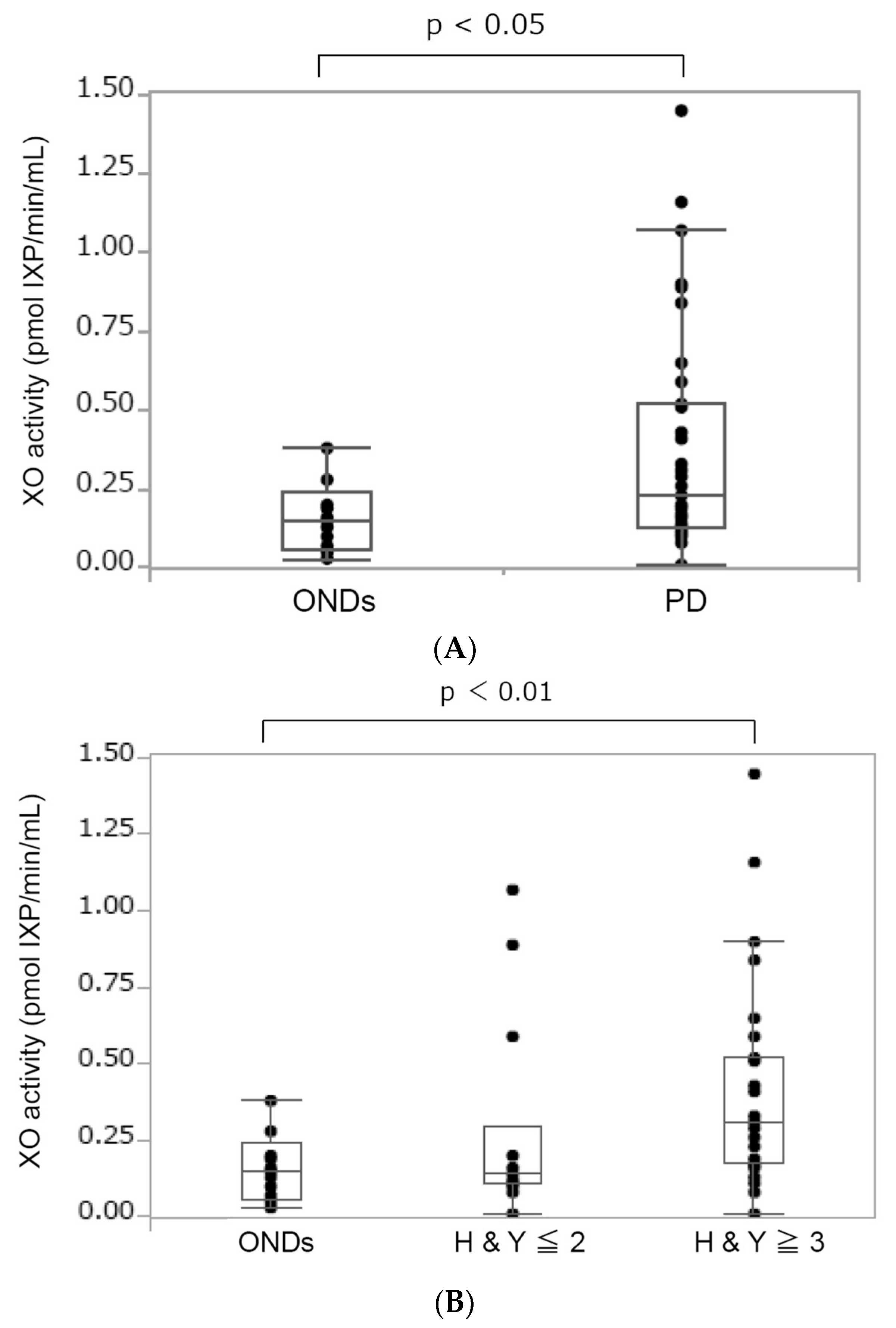
3.2. Increased XO Activity in Patients with PD
3.3. Antioxidant Status in Patients with PD
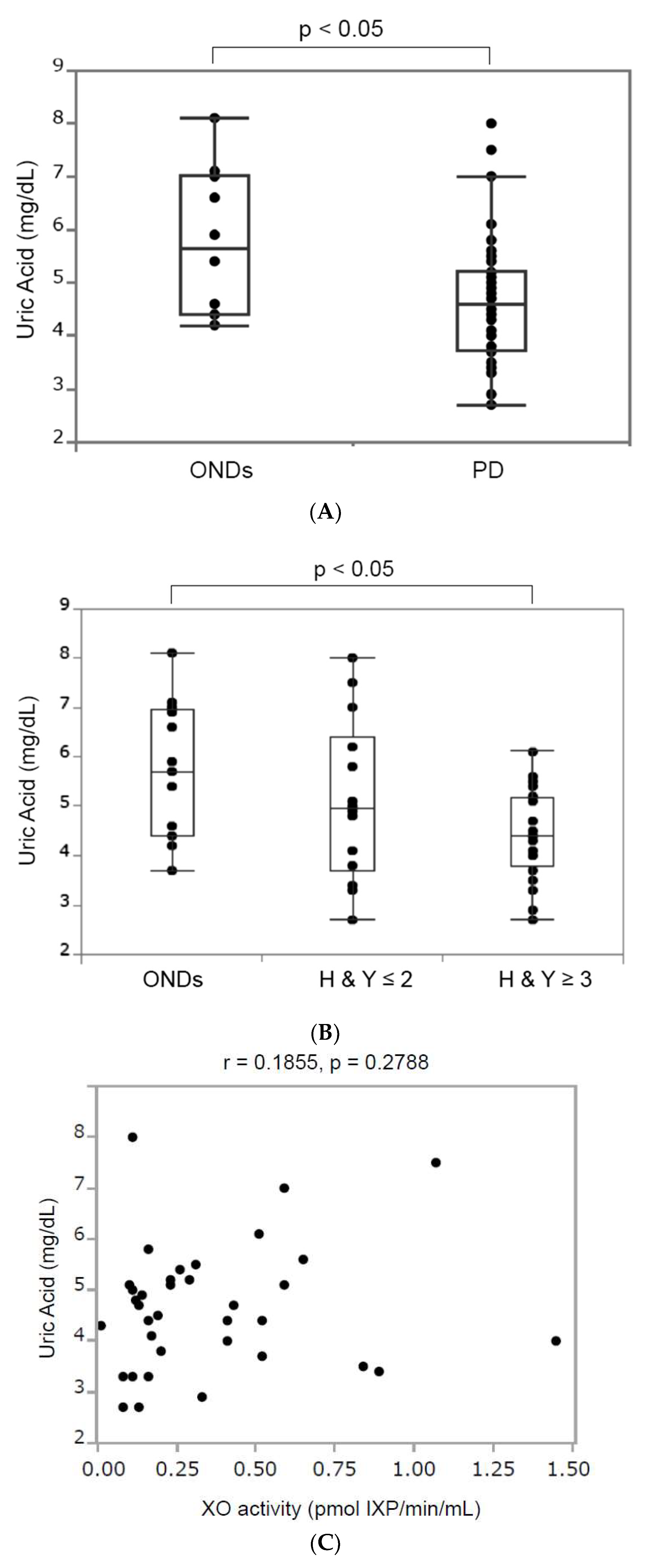
3.3.1. Serum Uric Acid
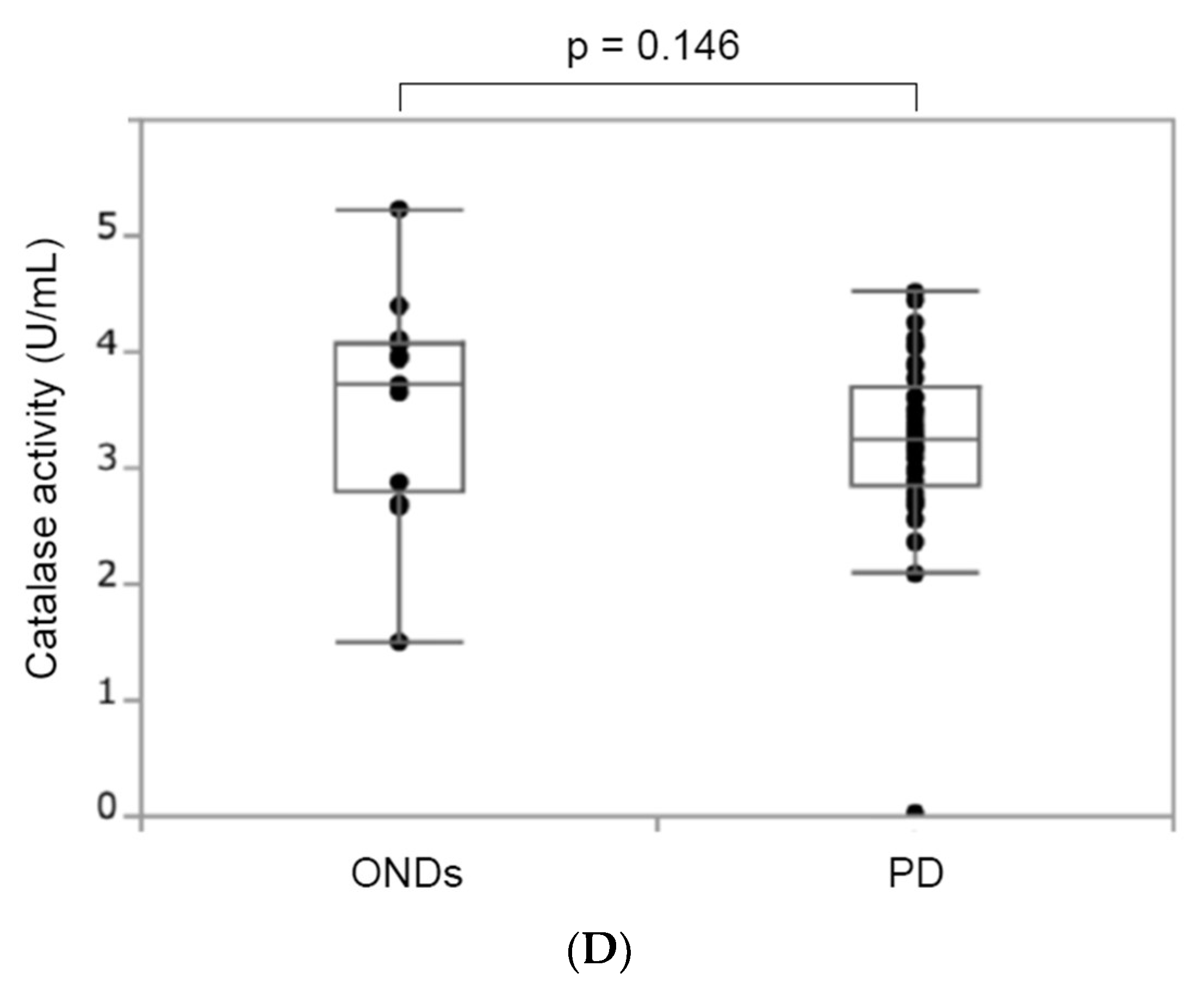
3.3.2. Catalase Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.W. Parkinson’s disease and parkinsonism: Neuropathology. Cold Spring Harb. Perspect. Med. 2012, 2, a009258. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.D.; Zhao, X.; Li, Y.; Li, G.R.; Liu, X.L. Damage to dopaminergic neurons by oxidative stress in Parkinson’s disease (Review). Int. J. Mol. Med. 2018, 41, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R.; Abramov, A.Y. Role of mitochondrial ROS in the brain: From physiology to neurodegeneration. FEBS Lett. 2018, 592, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Popa-Wagner, A.; Mitran, S.; Sivanesan, S.; Chang, E.; Buga, A.M. ROS and brain diseases: The good, the bad, and the ugly. Oxid. Med. Cell Longev. 2013, 2013, 963520. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Zhou, B.; Chen, Y.H.; Ma, Z.L.; Gou, Y.; Zhang, C.L.; Yu, W.F.; Jiao, L. Serum uric acid levels in patients with Parkinson’s disease: A meta-analysis. PLoS ONE 2017, 12, e0173731. [Google Scholar] [CrossRef] [PubMed]
- Cantu-Medellin, N.; Kelley, E.E. Xanthine oxidoreductase-catalyzed reactive species generation: A process in critical need of reevaluation. Redox Biol. 2013, 1, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Gökçe Çokal, B.; Yurtdaş, M.; Keskin Güler, S.; Güneş, H.N.; Ataç Uçar, C.; Aytaç, B.; Durak, Z.E.; Yoldaş, T.K.; Durak, İ.; Çubukçu, H.C. Serum glutathione peroxidase, xanthine oxidase, and superoxide dismutase activities and malondialdehyde levels in patients with Parkinson’s disease. Neurol. Sci. 2017, 38, 425–431. [Google Scholar] [CrossRef]
- Chung, H.Y.; Baek, B.S.; Song, S.H.; Kim, M.S.; Huh, J.I.; Shim, K.H.; Kim, K.W.; Lee, K.H. Xanthine dehydrogenase/xanthine oxidase and oxidative stress. Age 1997, 20, 127–140. [Google Scholar] [CrossRef]
- Li, J.; O, W.; Li, W.; Jiang, Z.G.; Ghanbari, H.A. Oxidative stress and neurodegenerative disorders. Int. J. Mol. Sci. 2013, 14, 24438–24475. [Google Scholar] [CrossRef]
- Murphy, M.P. Nitric oxide and cell death. Biochim. Biophys. Acta (BBA)-Bioenerg. 1999, 1411, 401–414. [Google Scholar] [CrossRef]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lü, J.M.; Yao, Q. Hyperuricemia-Related Diseases and Xanthine Oxidoreductase (XOR) Inhibitors: An Overview. Med. Sci. Monit. 2016, 22, 2501–2512. [Google Scholar] [CrossRef] [PubMed]
- Patel, M. Targeting Oxidative Stress in Central Nervous System Disorders. Trends Pharmacol. Sci. 2016, 37, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am. J. Physiol. 1996, 271 Pt 1, C1424–C1437. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Xu, H.; Sun, Q.; Yu, X.; Chen, W.; Wei, H.; Jiang, J.; Xu, Y.; Lu, W. The Role of Oxidative Stress in Hyperuricemia and Xanthine Oxidoreductase (XOR) Inhibitors. Oxid. Med. Cell Longev. 2021, 2021, 1470380. [Google Scholar] [CrossRef] [PubMed]
- Battelli, M.G.; Bortolotti, M.; Bolognesi, A.; Polito, L. Pro-Aging Effects of Xanthine Oxidoreductase Products. Antioxidants 2020, 9, 839. [Google Scholar] [CrossRef] [PubMed]
- Vida, C.; Kobayashi, H.; Garrido, A.; Martínez de Toda, I.; Carro, E.; Molina, J.A.; De la Fuente, M. Lymphoproliferation Impairment and Oxidative Stress in Blood Cells from Early Parkinson’s Disease Patients. Int. J. Mol. Sci. 2019, 20, 771. [Google Scholar] [CrossRef] [PubMed]
- Sakuta, H.; Suzuki, K.; Miyamoto, T.; Miyamoto, M.; Numao, A.; Fujita, H.; Watanabe, Y.; Hirata, K. Serum uric acid levels in Parkinson’s disease and related disorders. Brain Behav. 2017, 7, e00598. [Google Scholar] [CrossRef]
- Annanmaki, T.; Muuronen, A.; Murros, K. Low plasma uric acid level in Parkinson’s disease. Mov. Disord. 2007, 22, 1133–1137. [Google Scholar] [CrossRef]
- de Lau, L.M.; Koudstaal, P.J.; Hofman, A.; Breteler, M.M. Serum uric acid levels and the risk of Parkinson disease. Ann. Neurol. 2005, 58, 797–800. [Google Scholar] [CrossRef]
- Investigators TPSGS-P. Inosine to Increase Serum and Cerebrospinal Fluid Urate in Parkinson Disease: A Randomized Clinical Trial. JAMA Neurol. 2014, 71, 141–150. [Google Scholar] [CrossRef]
- Kachroo, A.; Schwarzschild, M.A. Allopurinol reduces levels of urate and dopamine but not dopaminergic neurons in a dual pesticide model of Parkinson’s disease. Brain Res. 2014, 1563, 103–109. [Google Scholar] [CrossRef]
- Lai, S.-W.; Lin, C.-L.; Liao, K.-F. Association between allopurinol use and Parkinson’s disease in older adults. Eur. Geriatr. Med. 2018, 9, 377–381. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid. Med. Cell Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Sautin, Y.Y.; Johnson, R.J. Uric acid: The oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 2008, 27, 608–619. [Google Scholar] [CrossRef]

| ONDs (n = 13) | PD (n = 41) | p Value | |
|---|---|---|---|
| Male | 7 (53.9) | 22 (53.7) | 1 |
| Age (years) | 72.7 ± 9.0 | 70.5 ± 9.9 | 0.62 |
| Age of onset (years) | 63.1 ± 10.4 | ||
| Disease duration (years) | 7.0 ± 5.5 | ||
| Hoehn and Yahr stages | 3 (2–3.5) | ||
| LEDD (mg) | 459 ± 305 |
| ONDs (n = 13) | HY ≤ 2 (n = 14) | HY ≥ 3 (n = 27) | p Value | |
|---|---|---|---|---|
| Male | 7 (53.9) | 8 (57.1) | 14 (51.9) | 0.95 |
| Age (years) | 72.7 ± 9.0 | 67.0 ± 12.2 | 72.3 ± 8.1 | 0.52 |
| Age of onset (years) | 62.5 ± 13.1 | 63.6 ± 9.4 | 0.93 | |
| Disease duration (years) | 3.9 ± 3.0 | 8.7 ± 5.8 | <0.05 * | |
| LEDD (mg) | 332 ± 266 | 524 ± 308 | 0.05 | |
| HT | 10 (76.9) | 5 (35.7) | 8 (29.6) | <0.05 * |
| DM | 2 (15.4) | 2 (14.3) | 4 (14.8) | 1 |
| CKD | 3 (23.1) | 4 (28.6) | 6 (22.2) | 0.9 |
| Current smoking | 2 (15.4) | 0 (0) | 0 (0) | <0.05 * |
| Thyroid disease | 3 (23.1) | 0 (0) | 2 (7.4) | 0.11 |
| Ischemic heart disease | 0 (0) | 0 (0) | 1 (3.7) | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haryuni, R.D.; Nukui, T.; Piao, J.-L.; Shirakura, T.; Matsui, C.; Sugimoto, T.; Baba, K.; Nakane, S.; Nakatsuji, Y. Elevated Serum Xanthine Oxidase and Its Correlation with Antioxidant Status in Patients with Parkinson’s Disease. Biomolecules 2024, 14, 490. https://doi.org/10.3390/biom14040490
Haryuni RD, Nukui T, Piao J-L, Shirakura T, Matsui C, Sugimoto T, Baba K, Nakane S, Nakatsuji Y. Elevated Serum Xanthine Oxidase and Its Correlation with Antioxidant Status in Patients with Parkinson’s Disease. Biomolecules. 2024; 14(4):490. https://doi.org/10.3390/biom14040490
Chicago/Turabian StyleHaryuni, Ratna Dini, Takamasa Nukui, Jin-Lan Piao, Takashi Shirakura, Chieko Matsui, Tomoyuki Sugimoto, Kousuke Baba, Shunya Nakane, and Yuji Nakatsuji. 2024. "Elevated Serum Xanthine Oxidase and Its Correlation with Antioxidant Status in Patients with Parkinson’s Disease" Biomolecules 14, no. 4: 490. https://doi.org/10.3390/biom14040490





