Short-Term Panax Ginseng Extract Supplementation Reduces Fasting Blood Triacylglycerides and Oxygen Consumption during Sub-Maximal Aerobic Exercise in Male Recreational Athletes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
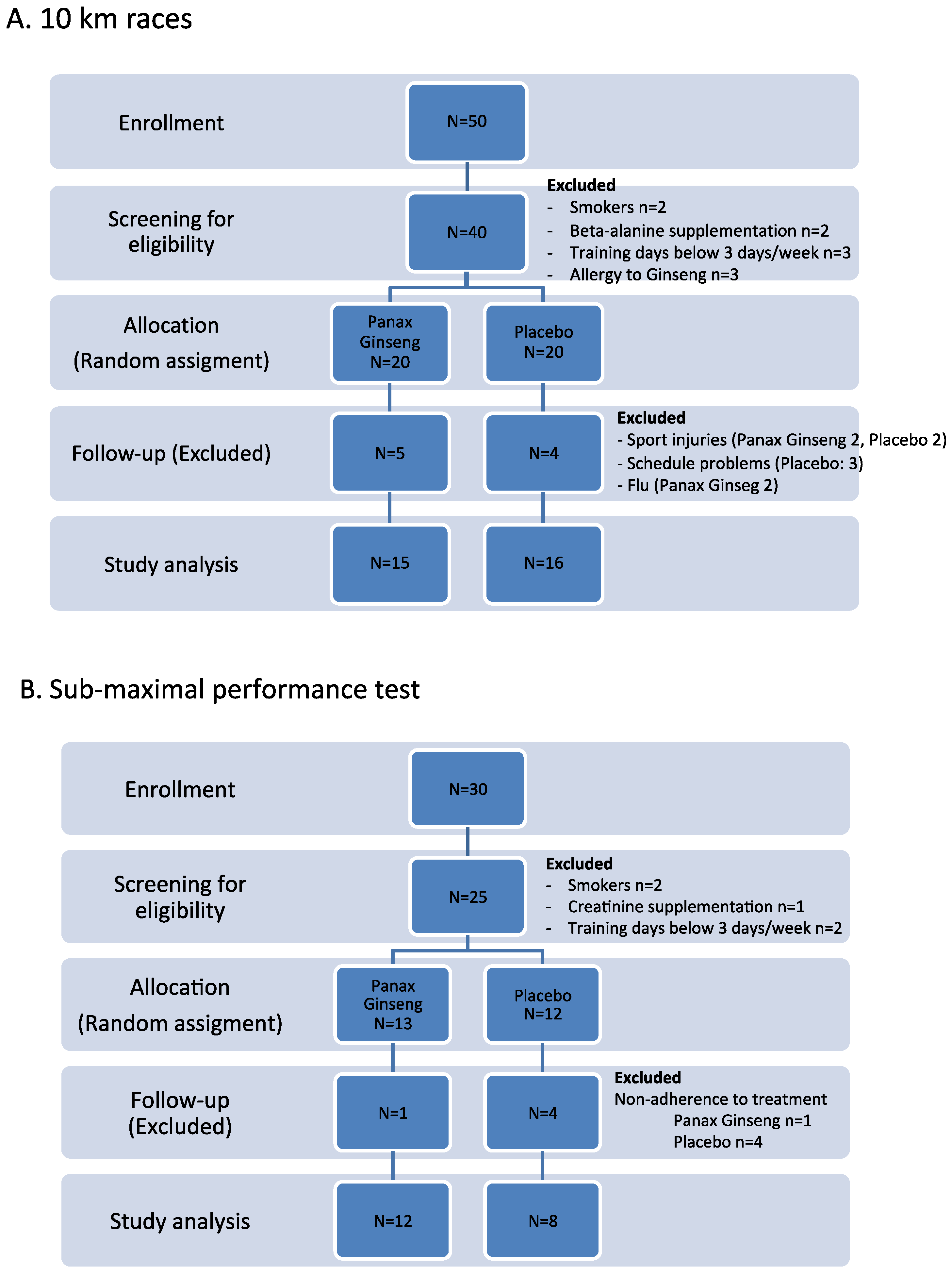
2.1.1. First Experiment: 10 km Races
2.1.2. Second Experiment: Sub-Maximal Aerobic Test
2.2. Participants
2.3. Interventions
2.4. Measurements
2.4.1. Energy Cost
2.4.2. Blood Biochemical Analysis
2.4.3. Ex Vivo Cellular Respiratory Capacity
2.5. Nutrition and Exercise Training Parameters
2.6. Statistical Analysis
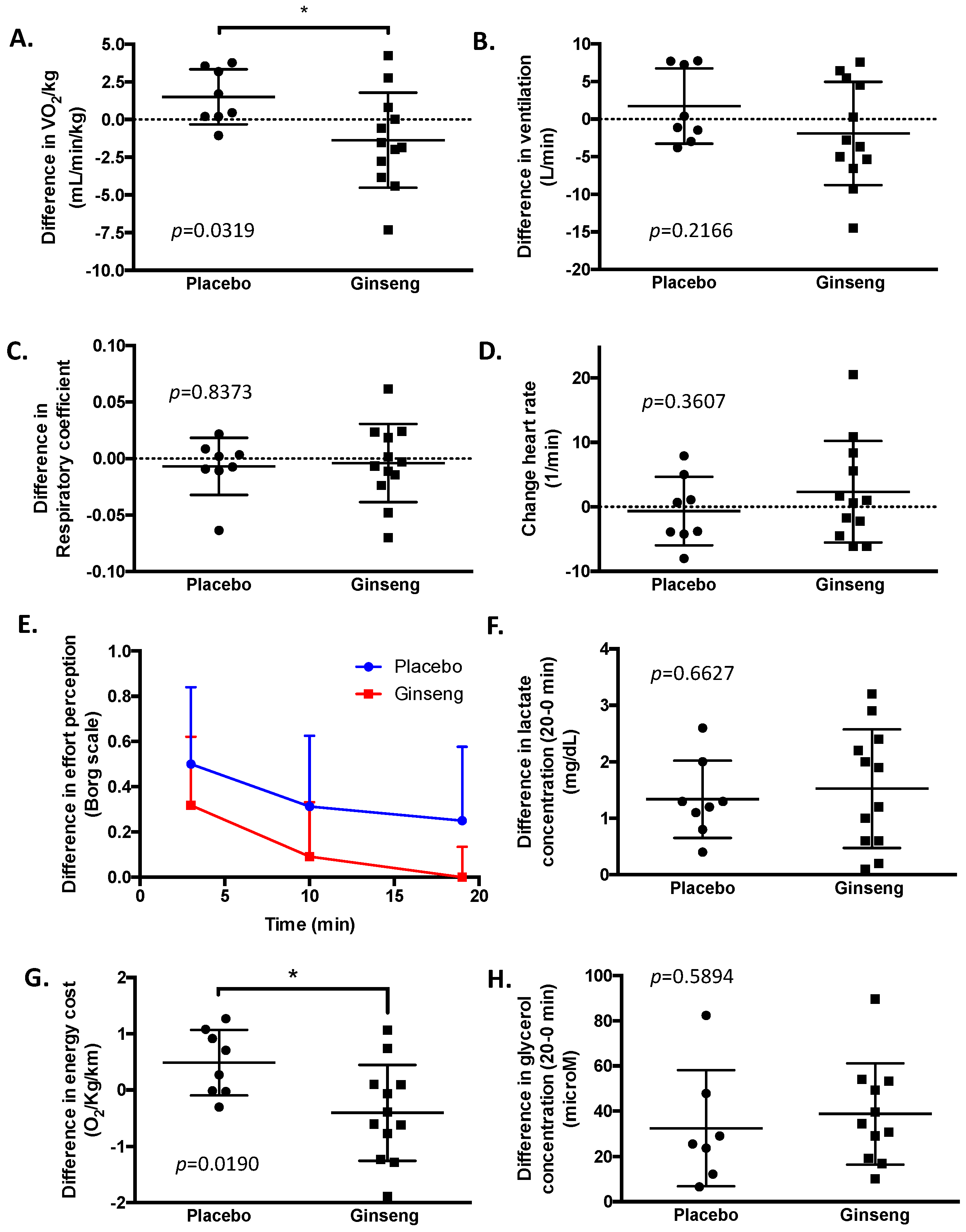
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, W.; Choi, H.-K.; Huang, L. State of Panax Ginseng Research: A Global Analysis. Molecules 2017, 22, 1518. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Santangelo, R. Panax Ginseng and Panax Quinquefolius: From Pharmacology to Toxicology. Food Chem. Toxicol. 2017, 107, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Bahrke, M.S.; Morgan, W.P. Evaluation of the Ergogenic Properties of Ginseng. Sports Med. 2000, 29, 113–133. [Google Scholar] [CrossRef]
- Petroczi, A.; Naughton, D.P. The Age-Gender-Status Profile of High Performing Athletes in the UK Taking Nutritional Supplements: Lessons for the Future. J. Int. Soc. Sports Nutr. 2008, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Petróczi, A.; Naughton, D.P.; Pearce, G.; Bailey, R.; Bloodworth, A.; McNamee, M. Nutritional Supplement Use by Elite Young UK Athletes: Fallacies of Advice Regarding Efficacy. J. Int. Soc. Sports Nutr. 2008, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Sundgot-Borgen, J.; Berglund, B.; Torstveit, M.K. Nutritional Supplements in Norwegian Elite Athletes–Impact of International Ranking and Advisors. Scand. J. Med. Sci. Sports 2003, 13, 138–144. [Google Scholar] [CrossRef]
- de Oliveira, A.C.C.; Perez, A.C.; Prieto, J.G.; Duarte, I.D.G.; Alvarez, A.I. Protection of Panax Ginseng in Injured Muscles after Eccentric Exercise. J. Ethnopharmacol. 2005, 97, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, A.; Vila, L.; Voces, J.A.; Cabral, A.C.; Alvarez, A.I.; Prieto, J.G. Effects of a Standardized Panax Ginseng Extract on the Skeletal Muscle of the Rat: A Comparative Study in Animals at Rest and Under Exercise. Planta Med. 1999, 65, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Kim, J.; Park, J.; Yun, H.; Cheon, W.-K.; Kim, B.; Lee, C.-H.; Suh, H.; Lim, K. Red Ginseng Treatment for Two Weeks Promotes Fat Metabolism during Exercise in Mice. Nutrients 2014, 6, 1874–1885. [Google Scholar] [CrossRef]
- Yoon, S.-J.; Kim, K.-H.; Kim, C.-J.; Park, H.-C.; Kang, K.-H.; Kim, M.-J.; Kang, S.-M.; Heon Kwak, U.; Kim, H.-J. Effects of Red Ginseng Supplementation on Aerobic.Anaerobic Performance, Central and Peripheral Fatigue. J. Ginseng Res. 2008, 32, 210–219. [Google Scholar] [CrossRef]
- Lee, E.S.; Yang, Y.J.; Lee, J.H.; Yoon, Y.S. Effect of High-Dose Ginsenoside Complex (UG0712) Supplementation on Physical Performance of Healthy Adults during a 12-Week Supervised Exercise Program: A Randomized Placebo-Controlled Clinical Trial. J. Ginseng Res. 2018, 42, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Liu, Y.; Shi, A.; Wang, Z.; Aa, J.; Huang, X.; Liu, Y. Investigation of the Antifatigue Effects of Korean Ginseng on Professional Athletes by Gas Chromatography-Time-of-Flight-Mass Spectrometry-Based Metabolomics. J. AOAC Int. 2018, 101, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; McLung, J.; Nelson, A.G.; Welsch, M. Ginseng Supplementation Does Not Enhance Healthy Young Adults’ Peak Aerobic Exercise Performance. J. Am. Coll. Nutr. 1998, 17, 462–466. [Google Scholar] [CrossRef]
- Kulaputana, O.; Thanakomsirichot, S.; Anomasiri, W. Ginseng Supplementation Does Not Change Lactate Threshold and Physical Performances in Physically Active Thai Men. J. Med. Assoc. Thai 2007, 90, 1172–1179. [Google Scholar]
- Elia, M.; Livesey, G. Energy Expenditure and Fuel Selection in Biological Systems: The Theory and Practice of Calculations Based on Indirect Calorimetry and Tracer Methods. Metab. Control. Eat. Energy Expend. Bioenerg. Obes. 1992, 70, 68–131. [Google Scholar]
- Lee, N.H.; Son, C.G. Systematic Review of Randomized Controlled Trials Evaluating the Efficacy and Safety of Ginseng. J. Acupunct. Meridian Stud. 2011, 4, 85–97. [Google Scholar] [CrossRef] [PubMed]
- di Prampero, P. The Energy Cost of Human Locomotion on Land and in Water*. Int. J. Sports Med. 1986, 07, 55–72. [Google Scholar] [CrossRef]
- De Villiers, S.; Van Der Walt, J.G.; Procos, J. An Accurate, Sensitive and Reproducible Method for the Colorimetric Estimation of Free Fatty Acids in Plasma. Onderstepoort J. Vet. Res. 1977, 44, 169–172. [Google Scholar]
- Long, Q.; Huang, L.; Huang, K.; Yang, Q. Assessing Mitochondrial Bioenergetics in Isolated Mitochondria from Mouse Heart Tissues Using Oroboros 2k-Oxygraph. Methods Mol. Biol. 2019, 1966, 237. [Google Scholar] [CrossRef]
- Lee, N.H.; Jung, H.C.; Lee, S. Red Ginseng as an Ergogenic Aid: A Systematic Review of Clinical Trials. J. Exerc. Nutr. Biochem. 2016, 20, 13. [Google Scholar] [CrossRef]
- Hernández-García, D.; Granado-Serrano, A.B.; Martín-Gari, M.; Naudí, A.; Serrano, J.C.E. Efficacy of Panax Ginseng Supplementation on Blood Lipid Profile. A Meta-Analysis and Systematic Review of Clinical Randomized Trials. J. Ethnopharmacol. 2019, 243, 112090. [Google Scholar] [CrossRef] [PubMed]
- van Loon, L.J.C.; Greenhaff, P.L.; Constantin-Teodosiu, D.; Saris, W.H.M.; Wagenmakers, A.J.M. The Effects of Increasing Exercise Intensity on Muscle Fuel Utilisation in Humans. J. Physiol. 2001, 536, 295. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Tsekouras, Y.E.; Prentzas, K.I.; Basioukas, K.N.; Matsama, S.G.; Yanni, A.E.; Kavouras, S.A.; Sidossis, L.S. Acute Exercise-Induced Changes in Basal VLDL-Triglyceride Kinetics Leading to Hypotriglyceridemia Manifest More Readily after Resistance than Endurance Exercise. J. Appl. Physiol. 2008, 105, 1228–1236. [Google Scholar] [CrossRef]
- Shin, E.J.; Jo, S.; Choi, S.; Cho, C.W.; Lim, W.C.; Hong, H.D.; Lim, T.G.; Jang, Y.J.; Jang, M.; Byun, S.; et al. Red Ginseng Improves Exercise Endurance by Promoting Mitochondrial Biogenesis and Myoblast Differentiation. Molecules 2020, 25, 865. [Google Scholar] [CrossRef] [PubMed]
- Phung, H.M.; Jang, D.; Trinh, T.A.; Lee, D.; Nguyen, Q.N.; Kim, C.E.; Kang, K.S. Regulation of Appetite-Related Neuropeptides by Panax Ginseng: A Novel Approach for Obesity Treatment. J. Ginseng Res. 2022, 46, 609. [Google Scholar] [CrossRef]
- Pfleger, J.; He, M.; Abdellatif, M. Mitochondrial Complex II Is a Source of the Reserve Respiratory Capacity That Is Regulated by Metabolic Sensors and Promotes Cell Survival. Cell Death Dis. 2015, 6, e1835. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Kwan, K.K.L.; Leung, K.W.; Yao, P.; Wang, H.; Dong, T.T.; Tsim, K.W.K. Ginseng Extracts Modulate Mitochondrial Bioenergetics of Live Cardiomyoblasts: A Functional Comparison of Different Extraction Solvents. J. Ginseng Res. 2019, 43, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Xie, W.; Sun, Y.; Dai, Z.; Li, G.; Sun, G.; Sun, X. Ginsenoside Rb1 and Mitochondria: A Short Review of the Literature. Mol. Cell Probes 2019, 43, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.D.; Chiu, C.H.; Hsu, Y.J.; Hou, C.W.; Chen, Y.M.; Huang, C.C. Changbai Mountain Ginseng (Panax Ginseng C.A. Mey) Extract Supplementation Improves Exercise Performance and Energy Utilization and Decreases Fatigue-Associated Parameters in Mice. Molecules 2017, 22, 237. [Google Scholar] [CrossRef]
- Wu, J.; Saovieng, S.; Cheng, I.-S.; Liu, T.; Hong, S.; Lin, C.-Y.; Su, I.-C.; Huang, C.-Y.; Kuo, C.-H. Ginsenoside Rg1 Supplementation Clears Senescence-Associated β-Galactosidase in Exercising Human Skeletal Muscle. J. Ginseng Res. 2019, 43, 580. [Google Scholar] [CrossRef]
- SH, Y.; CY, H.; SD, L.; MF, H.; RY, W.; CL, K.; CH, K. Decreased Eccentric Exercise-Induced Macrophage Infiltration in Skeletal Muscle after Supplementation with a Class of Ginseng-Derived Steroids. PLoS ONE 2014, 9, e0114649. [Google Scholar] [CrossRef] [PubMed]

| Placebo Group | Panax Ginseng Group | |||
|---|---|---|---|---|
| Basal | Two Weeks | Basal | Two Weeks | |
| A. 10 km races | ||||
| Volunteers | n = 16 | n = 15 | ||
| Age | 37.8 (32.9–42.7) | 34.3 (29.8–38.8) | ||
| Body weight (kg) | 75.7 (71.6–79.8) | 74.6 (70.0–79.3) | 74.5 (70.1–78.9) | 74.6 (70.0–79.3) |
| Training parameters | ||||
| Km/week | 21.7 (12.8–23.8) | 34.8 (25.4–44.2) | 26.5 (19.5–33.4) | 34.8 (28.4–41.1) |
| Min/week | 113 (66–124) | 192 (144–239) | 135 (108–162) | 183 (147–219) |
| Nutritional parameters before race | ||||
| Energy (kcal) | 491 (380–602) | 429 (339–519) | 427 (318–535) | 537 (373–700) |
| Carbohydrates (g/kg body weight) | 0.81 (0.61–1.0) | 0.73 (0.62–0.84) | 0.75 (0.54–0.95) | 0.97 (0.76–1.19) a |
| Protein (g) | 18.8 (12.4–25.3) | 15.9 (11.5–20.4) | 16.5 (10.8–22.2) | 19.8 (12.7–26.9) |
| Lipids (g) | 15.2 (10.7–19.7) | 14.5 (9.2–19.9) | 12.7 (8.1–17.4) | 15.6 (5.2–25.9) |
| Hydration (mL) | 486 (344–628) | 481 (267–695) | 598 (404–792) | 532 (368–698) |
| B. Sub-maximal performance test | ||||
| Volunteers | n = 8 | n = 12 | ||
| Age | 37.4 (32.3–42.6) | 34.2 (27.6–40.7) | ||
| Body weight (kg) | 72.7 (68.0–77.4) | 72.8 (68.5–77.2) | 71.1 (66.4–75.8) | 71.6 (67.2–76.0) |
| Training parameters | ||||
| Km/week | 30.1 (16.1–44.2) | 24.1 (13.6–34.7) | 32.5 (21.5–43.6) | 33.7 (15.1–52.5) |
| Min/week | 201 (109–292) | 151 (94–208) | 167 (103–231) | 183 (102–264) |
| Nutritional parameters before test | ||||
| Energy (kcal) | 646 (422–869) | 674 (458–889) | 513 (402–624) | 669 (478–860) |
| Carbohydrates (g/kg body weight) | 1.18 (0.60–1.75) | 1.18 (0.80–1.56) | 0.96 (0.70–1.21) | 1.13 (0.79–1.47) a |
| Protein (g) | 23.9 (13.5–34.3) | 22.7 (16.6–28.9) | 17.7 (13.5–21.9) | 24.4 (16.9–31.8) a |
| Lipids (g) | 21.3 (13.2–29.5) | 23.9 (11.0–36.7) | 17.3 (12.3–22.3) | 25.4 (15.8–35.0) |
| Parameter | Placebo Group (n = 16) | Panax Ginseng Group (n = 15) | ||
|---|---|---|---|---|
| Pre-Race | Post-Race | Pre-Race | Post-Race | |
| First race | ||||
| Running time (min) | 47.4 (43.8–51.1) | 44.3 (41.4–47.1) | ||
| Borg’s perceived exertion (CR-10) | 6.4 (5.2–7.5) | 6.6 (5.5–7.8) | ||
| Blood lipid profile (mg/dL) | ||||
| Total lipids | 832 (783–880) | 919 (862–975) | 824 (755–894) | 933 (868–998) |
| Phospholipids | 144 (127–161) | 148 (134 161) | 168 (140–195) | 159 (130–188) |
| Total cholesterol | 170 (153–187) | 166 (146–187) | 162 (139–185) | 157 (138–175) |
| Triacylglycerides | 135 (108–163) | 164 (107–222) | 101 (72–130) | 108 (86–130) |
| Non-esterified fatty acids | 1.93 (1.37–2.50) | 1.70 (1.09–2.30) | 1.25 (1.01–2.27) | 1.26 (0.78–1.73) |
| Lactate (mmol/L) | 1.56 (1.16–1.96) | 3.15 (2.61–3.70) a | 1.50 (1.14–1.86) | 5.59 (4.12–7.06) a,b |
| Cytokines (pg/mL) | ||||
| IL-1Ra | 211 (114–309) | 249 (170–239) | 340 (0–690) | 367 (44–690) |
| IL-6 | 13.6 (4.4–22.8) | 13.8 (4.5–23.0) | 3.7 (0–8.8) | 8.9 (1.7–16.1) |
| IL-8 | 10.2 (7.3–13.0) | 13.3 (10.6–16.0) | 8.1 (4.0–12.2) | 10.7 (6.5–14.9) |
| IL-10 | 16.3 (0–37.5) | 83.3 (39.3–127.3) | 9.2 (0.7–17.6) | 43.1 (26.3–60.0) a |
| TNFα | 15.6 (11.0–20.2) | 18.1 (13.7–22.4) | 13.2 (10.2–16.2) | 15.6 (12.1–19.1) |
| Second race | ||||
| Running time (min) | 45.5 (42.0–49.1) | 42.8 (39.8–45.8) | ||
| Borg’s perceived exertion (CR-10) | 6.9 (6.0–7.7) | 7.4 (6.7–8.2) | ||
| Blood lipid profile (mg/dL) | ||||
| Total lipids | 874 (813–935) | 943 (881–1006) | 774 (711–837) a | 959 (867–1051) |
| Phospholipids | 153 (137–170) | 152 (121–183) | 150 (121–178) | 152 (118–187) |
| Total cholesterol | 180 (159–200) | 172 (151–193) | 155 (133–176) | 162 (145–179) |
| Triacylglycerides | 138 (103–174) | 126 (103–148) | 93 (72–114) a,b | 95 (75–115) |
| Non-esterified fatty acids | 1.02 (0.94–1.56) | 1.70 (1.24–2.14) | 0.51 (0.73–1.81) | 1.45 (1.04–1.86) |
| Lactate (mmol/L) | 1.33 (1.12–1.53) | 3.04 (2.37–3.71) a | 1.26 (1.07–1.45) | 4.85 (3.03–6.67) a,b |
| Cytokines (pg/mL) | ||||
| IL-1Ra | 275 (106–443) | 271 (134–409) | 231 (73–389) | 333 (0–682) |
| IL-6 | 7.4 (3.7–11.0) | 10.0 (5.1–14.9) | 5.1 (0–10.4) | 7.2 (3.5–10.9) a |
| IL-8 | 12.4 (7.8–17.0) | 13.9 (11.0–16.8) | 8.7 (4.4–13.1) | 12.9 (9.0–16.8) a |
| IL-10 | 11.6 (2.9–20.3) | 86.7 (39.7–133.6) a | 8.2 (2.6–13.9) | 76.2 (37.6–114.8) a,b |
| TNFα | 16.9 (13.4–20.4) | 18.8 (14.9–22.8) | 13.5 (11.1–15.9) | 16.4 (13.5–19.4) |
| Parameter | Placebo Group (n = 8) | Panax Ginseng Group (n = 12) | ||
|---|---|---|---|---|
| Pre-Test | Post-Test | Pre-Test | Post-Test | |
| First test | ||||
| VO2 (mL/min/kg) | 36.0 (33.5–38.5) | 41.2 (35.4–47.1) | ||
| VCO2 (mL/min/kg) | 34.1 (31.6–36.6) | 39.0 (33.6–44.3) | ||
| RER | 0.95 (0.93–0.96) | 0.95 (0.93–0.95) | ||
| VE (L/min) | 29.1 (26.3–31.9) | 29.2 (27.5–30.9) | ||
| VE/VO2 | 27.7 (25.0–30.3) | 27.7 (26.1–29.4) | ||
| Heart rate (bpm) | 154 (148–161) | 152 (143–161) | ||
| Borg’s perceived exertion (CR-10) | 4.4 (2.9–5.9) | 3.9 (3.2–4.6) | ||
| Lactate (mmol/L) | 2.1 (1.8–2.5) | 4.0 (3.3–4.7) a | 2.3 (2.7–3.9) | 3.6 (2.9–4.3) a |
| Blood lipid profile (mg/dL) | ||||
| Total lipids | 599 (556–642) | 665 (557–772) | 671 (593–749) | 679 (605–754) |
| Phospholipids | 184 (163–204) | 210 (178–242) | 175 (156–194) | 202 (173–232) |
| Total cholesterol | 183 (134–231) | 182 (133–231) | 165 (138–191) | 173 (148–197) |
| Triacylglycerides | 63 (48–79) | 71 (58–84) | 85 (56–114) | 93 (67–120) |
| Non-esterified fatty acids | 0.96 (0–2.32) | 1.03 (0.46–1.60) | 0.67 (0.29–1.06) | 1.36 (0.62–2.10) |
| Cytokines (pg/mL) | ||||
| IL-1Ra | 168 (31–305) | 214 (84–344) | 114 (42–186) | 132 (76–189) |
| IL-6 | 4.1 (1.2–7.1) | 6.7 (2.5–10.9) | 16.8 (0.0–42.8) | 23.1 (0.0–63.3) |
| IL-8 | 8.5 (4.2–12.8) | 12.2 (4.6–19.8) | 7.0 (4.2–9.8) | 7.9 (4.9–11.0) |
| IL-10 | 4.2 (2.1–6.4) | 6.9 (3.5–10.4) a | 8.0 (3.8–12.1) | 9.6 (3.7–15.5) |
| TNF-α | 14.3 (11.6–17.0) | 21.8 (15.2–28.5) a | 13.5 (9.4–17.7) | 14.5 (10.2–18.8) |
| Second test | ||||
| VO2 (mL/min/kg) | 37.8 (34.5–41.1) | 39.8 (34.1–45.5) | ||
| VCO2 (mL/min/kg) | 35.6 (32.7–38.5) | 38.2 (33.1–43.3) | ||
| RER | 0.94 (0.92–0.96) | 0.95 (0.92–0.96) | ||
| VE (L/min) | 29.1 (26.8–31.3) | 29.7 (28.1–31.4) | ||
| VE/VO2 | 27.4 (25.1–29.7) | 28.1 (26.3–30.0) | ||
| Heart rate (bpm) | 154 (148–161) | 155 (146–164) | ||
| Borg’s perceived exertion (CR-10) | 4.4 (3.2–5.8) | 4.0 (3.1–5.0) | ||
| Lactate (mmol/L) | 2.0 (1.5–2.5) | 3.3 (2.7–4.0) a | 2.1 (1.7–2.5) | 3.6 (2.9–4.3) a |
| Blood lipid profile (mg/dL) | ||||
| Total lipids | 660 (586–735) | 688 (650–727) | 592 (519–665) a | 670 (599–741) |
| Phospholipids | 184 (165–204) | 215 (189–241) | 174 (151–197) | 204 (175–233) |
| Total cholesterol | 189 (143–234) | 217 (164–270) | 165 (143–186) | 181 (153–209) |
| Triacylglycerides | 65 (57–74) | 69 (55–83) | 71 (52–90) a | 86 (60–113) |
| Non-esterified fatty acids | 1.57 (0–3.58) | 0.97 (0.07–1.87) | 0.65 (0.45–0.84) | 1.46 (0.88–2.05) |
| Cytokines (pg/mL) | ||||
| IL-1Ra | 167 (95–239) | 255 (88–422) | 130 (35–226) | 175 (47–303) a,b |
| IL-6 | 4.0 (1.5–6.4) | 5.2 (0.3–10.1) | 2.6 (0.4–5.7) | 2.6 (0.3–4.9) |
| IL-8 | 8.5 (2.9–14.1) | 10.7 (5.5–15.9) | 6.7 (4.5–8.8) | 8.0 (5.2–10.8) |
| IL-10 | 5.9 (2.2–9.8) | 8.6 (4.2–13.0) | 7.7 (2.2–13.2) | 13.6 (0–28.2) a |
| TNF-α | 13.8 (9.4–18.3) | 18.3 (10.3–26.3) | 14.4 (10.9–17.9) | 19.2 (9.1–29.2) |
| Parameter | Placebo Group | Panax Ginseng Group | ||||
|---|---|---|---|---|---|---|
| Pre-Treatment | Post-Treatment | p-Value | Pre-Treatment | Post-Treatment | p-Value | |
| Routine respiration | 292 ± 53 | 304 ± 23 | 0.8435 | 383 ± 51 | 232 ± 31 | 0.0639 |
| Uncoupled respiration | 525 ± 110 | 651 ± 113 | 0.4725 | 585 ± 66 | 607 ± 50 | 0.8098 |
| Spare respiratory capacity | 234 ± 57 | 348 ± 95 | 0.3627 | 202 ± 63 | 374 ± 20 | 0.0592 |
| Lactate (mg/dL) | 2.04 ± 0.22 | 2.84 ± 0.26 * | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-García, D.; Granado-Serrano, A.B.; Martín-Gari, M.; Ensenyat, A.; Naudí, A.; Serrano, J.C.E. Short-Term Panax Ginseng Extract Supplementation Reduces Fasting Blood Triacylglycerides and Oxygen Consumption during Sub-Maximal Aerobic Exercise in Male Recreational Athletes. Biomolecules 2024, 14, 533. https://doi.org/10.3390/biom14050533
Hernández-García D, Granado-Serrano AB, Martín-Gari M, Ensenyat A, Naudí A, Serrano JCE. Short-Term Panax Ginseng Extract Supplementation Reduces Fasting Blood Triacylglycerides and Oxygen Consumption during Sub-Maximal Aerobic Exercise in Male Recreational Athletes. Biomolecules. 2024; 14(5):533. https://doi.org/10.3390/biom14050533
Chicago/Turabian StyleHernández-García, Didier, Ana Belén Granado-Serrano, Meritxell Martín-Gari, Assumpta Ensenyat, Alba Naudí, and Jose C. E. Serrano. 2024. "Short-Term Panax Ginseng Extract Supplementation Reduces Fasting Blood Triacylglycerides and Oxygen Consumption during Sub-Maximal Aerobic Exercise in Male Recreational Athletes" Biomolecules 14, no. 5: 533. https://doi.org/10.3390/biom14050533
APA StyleHernández-García, D., Granado-Serrano, A. B., Martín-Gari, M., Ensenyat, A., Naudí, A., & Serrano, J. C. E. (2024). Short-Term Panax Ginseng Extract Supplementation Reduces Fasting Blood Triacylglycerides and Oxygen Consumption during Sub-Maximal Aerobic Exercise in Male Recreational Athletes. Biomolecules, 14(5), 533. https://doi.org/10.3390/biom14050533






