TRPA1 Covalent Ligand JT010 Modifies T Lymphocyte Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice, Mononuclear Cell Isolation
2.2. Immunfluorescent Staining
2.3. RNAscope In Situ Hybridization
2.4. Confocal Microscopy, Semi-Quantitative Image Segmentation, and Co-Localization Analysis
2.5. Measurement of Intracellular Calcium Signaling by Flow Cytometry
2.6. Monitoring Cell Death and Plasma Membrane Damage through Measuring Phosphatidylserine (PS) Exposure of the Cells by Annexin V–Binding Assay
2.7. Statistical Methods
3. Results
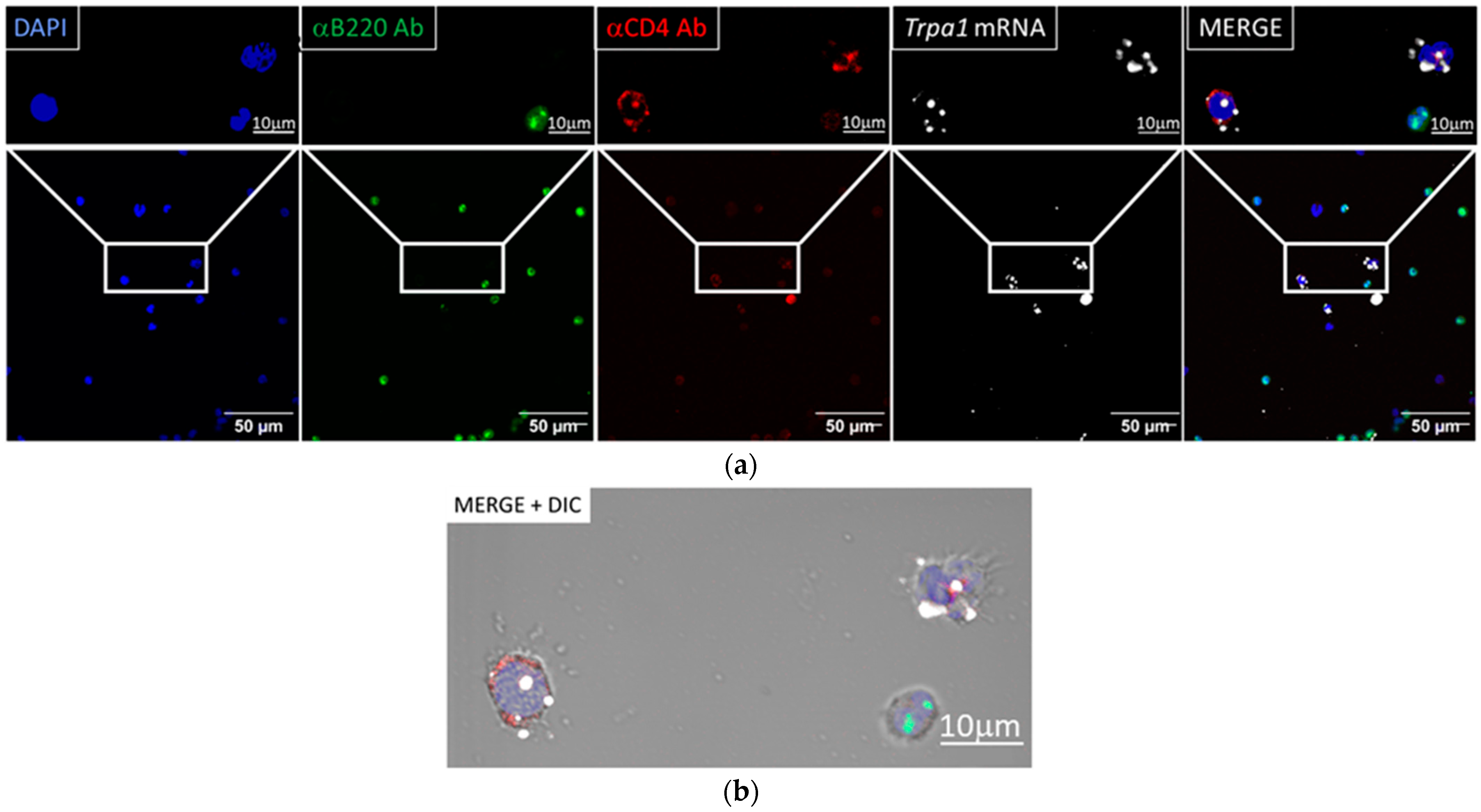
3.1. Trpa1 Transcripts Were Detected in CD4+ and CD14+ Cells by RNA Scope In Situ Hybridization
3.1.1. Trpa1 mRNA Was Detected by RNAscope ISH in CD4+ Cells
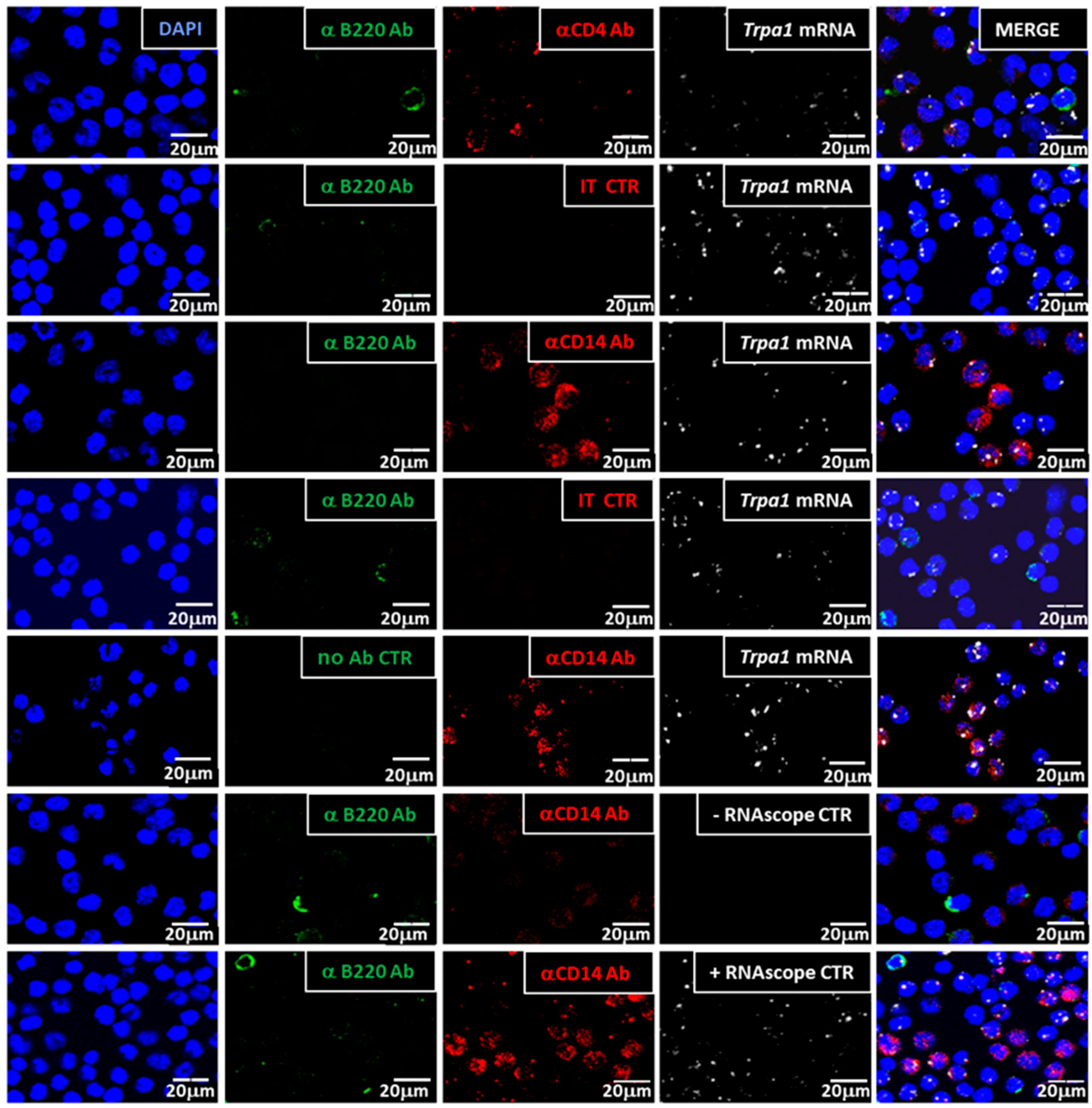
3.1.2. Trpa1 Transcripts Were Detected in CD14+, CD4+ Cells but Not in B Cells
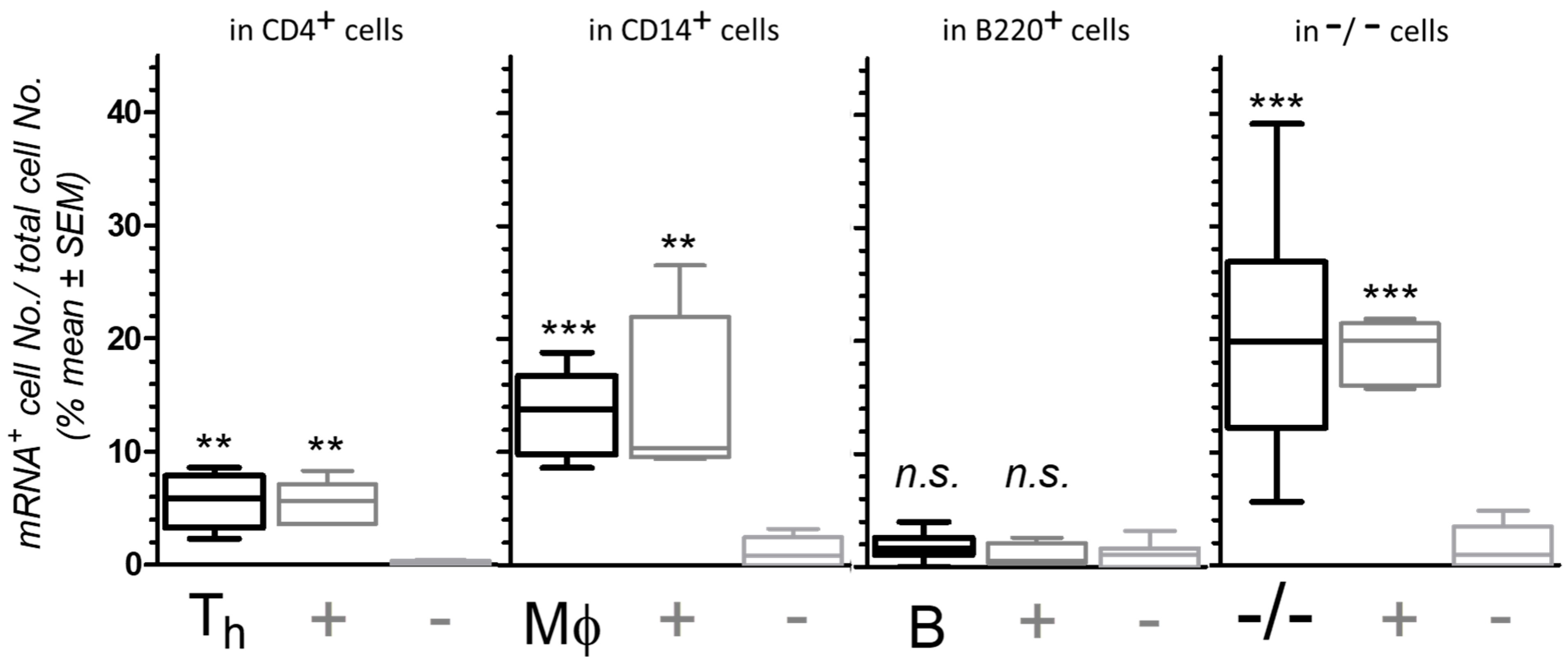
3.1.3. Semi-Quantification of mRNA Expression and CD Marker Co-Localization
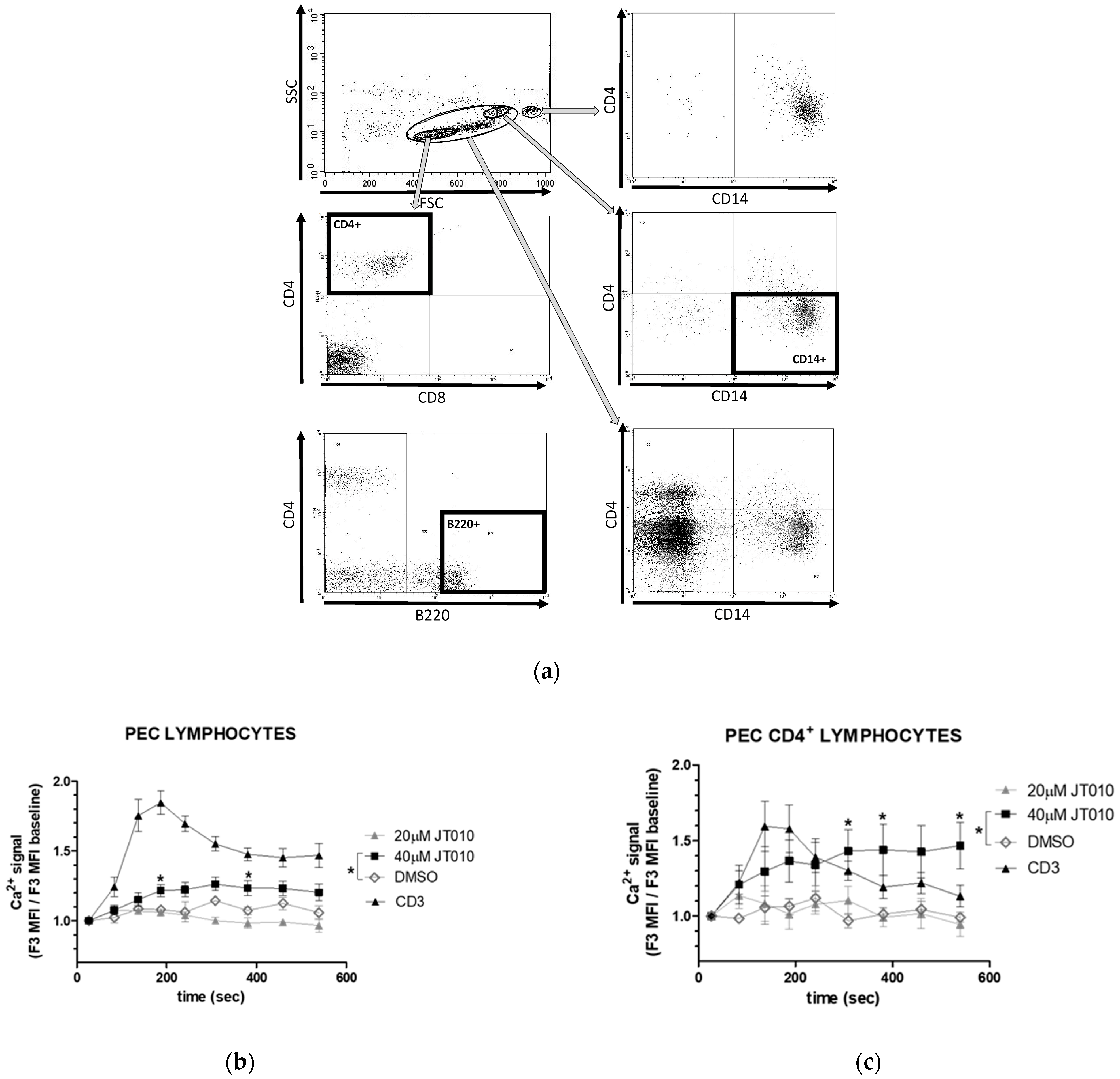
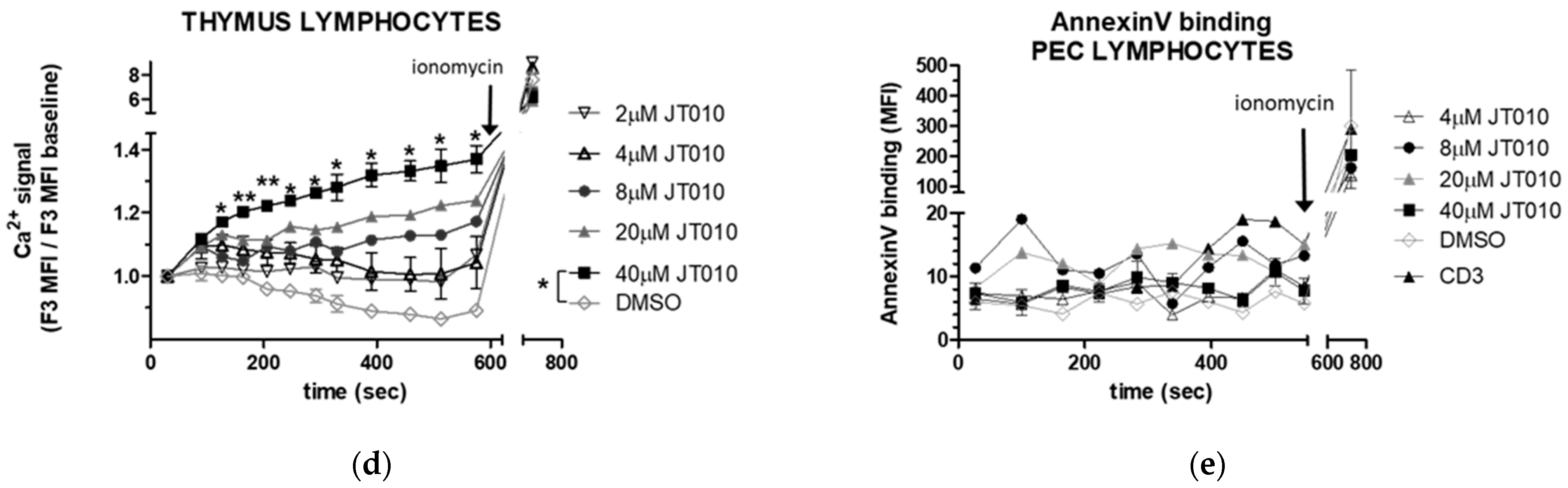
3.2. JT010 Stimulated Significant Elevation of Intracellular Ca2+ Level of Peritoneal and Thymus Cells Only at High Concentration
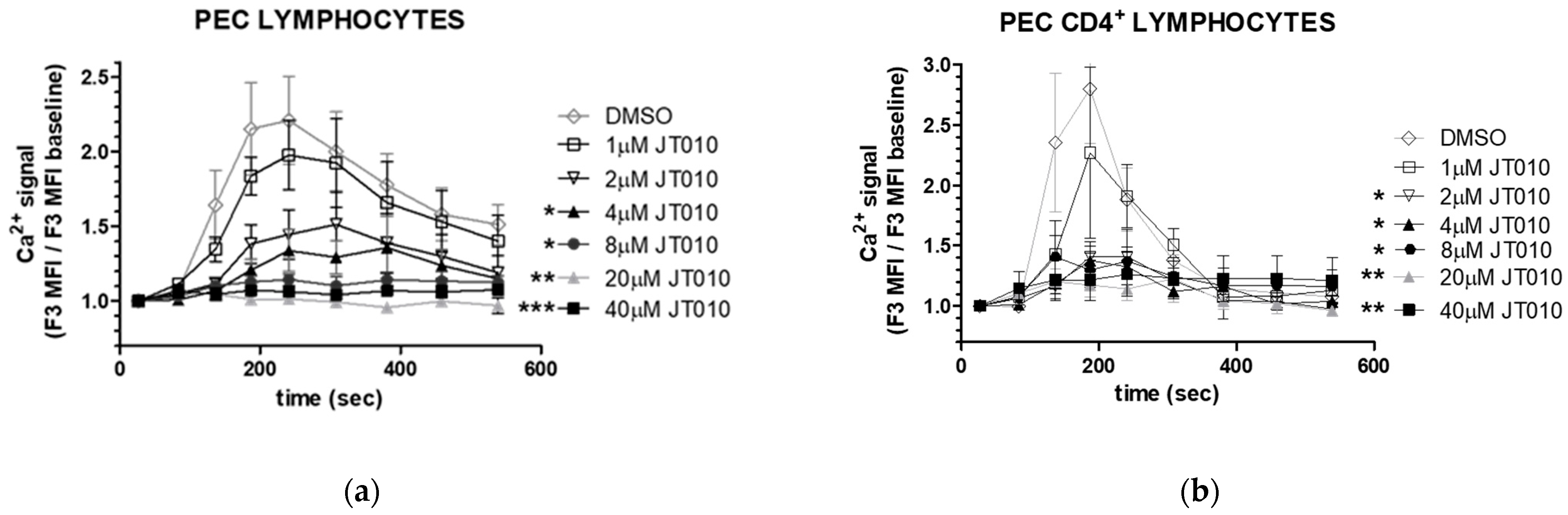
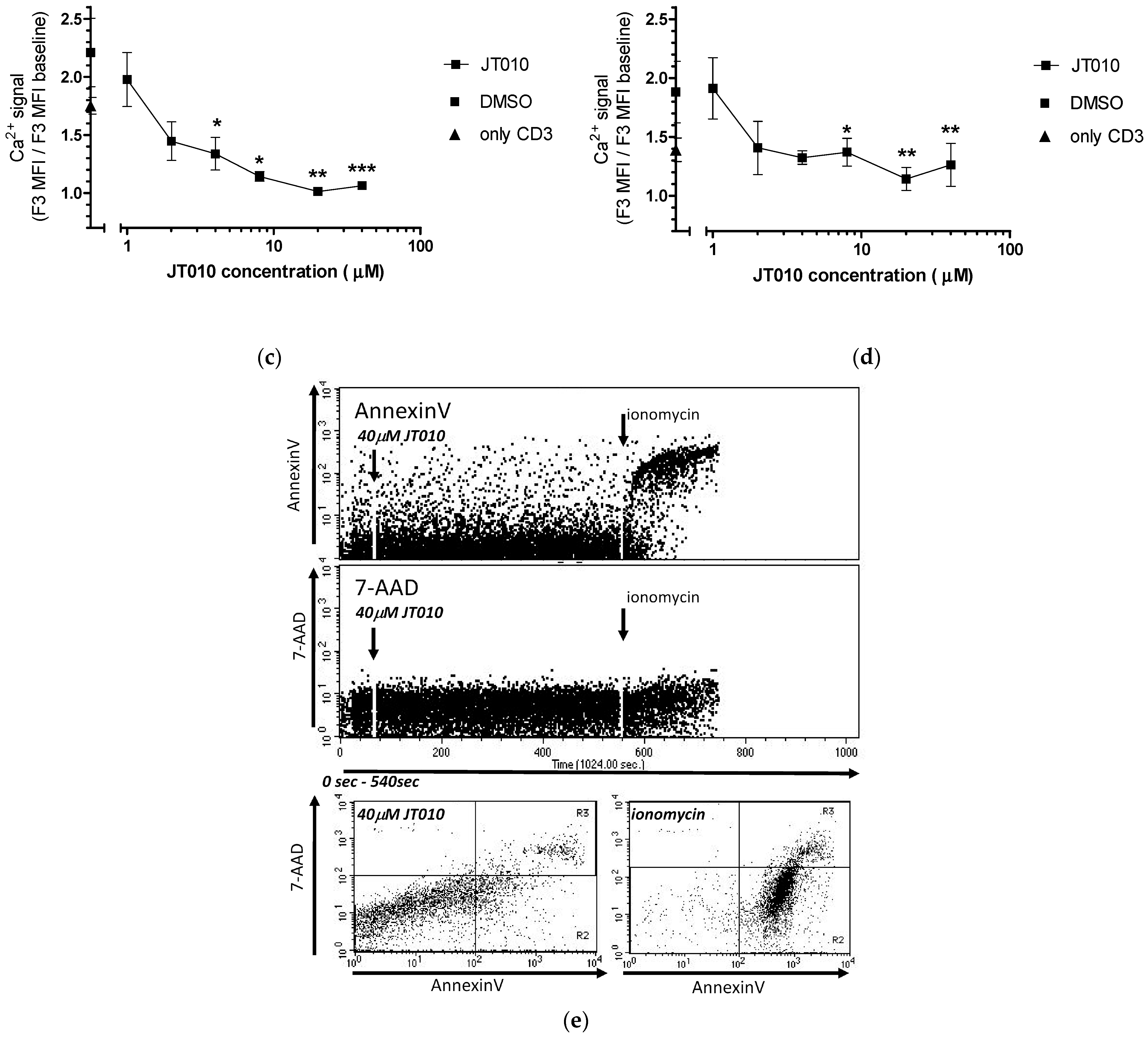
3.3. JT010 Inhibited TcR-Induced the Ca2+ Signal of Lymphocytes and CD4+ Lymphocytes a Concentration-Dependent Manner
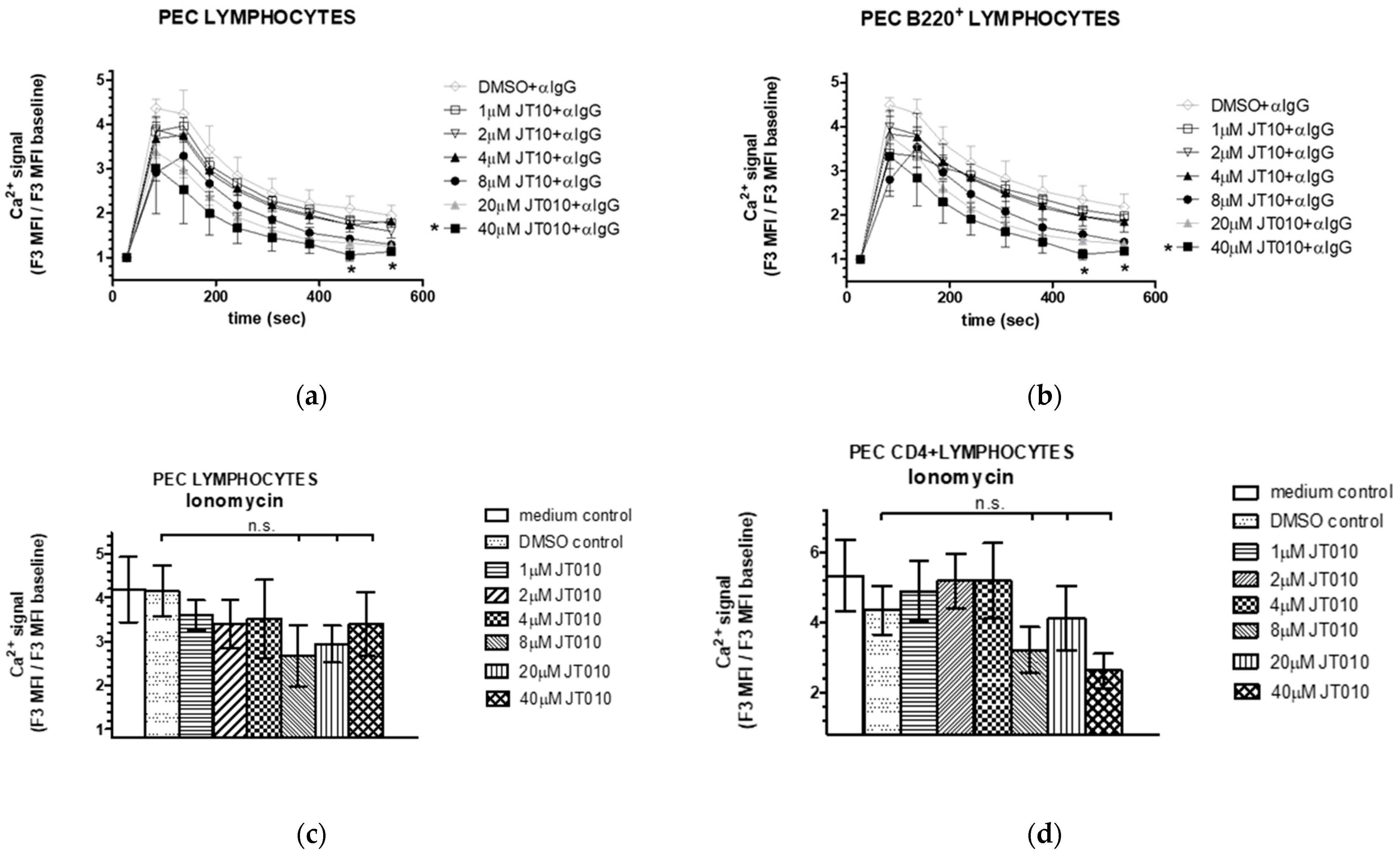
3.4. JT010 Neither Modified Peritoneal B Cell Activation nor Ionophore Ionomycin-Stimulated Elevation of Intracellular Ca2+ Level of Cells Isolated from Peritoneal Cavity of Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Talavera, K.; Startek, J.B.; Alvarez-Collazo, J.; Boonen, B.; Alpizar, Y.A.; Sanchez, A.; Naert, R.; Nilius, B. Mammalian Transient Receptor Potential TRPA1 Channels: From Structure to Disease. Physiol. Rev. 2020, 100, 725–803. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.S.; Fernandes, M.A.; Keeble, J.E. The functions of TRPA1 and TRPV1: Moving away from sensory nerves. Br. J. Pharmacol. 2012, 166, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.S.; Baxter, M.; Dubuis, E.; Birrell, M.A.; Belvisi, M.G. Transient receptor potential (TRP) channels in the airway: Role in airway disease. Br. J. Pharmacol. 2014, 171, 2593–2607. [Google Scholar] [CrossRef] [PubMed]
- Gouin, O.; L’Herondelle, K.; Lebonvallet, N.; Le Gall-Ianotto, C.; Sakka, M.; Buhé, V.; Plée-Gautier, E.; Carré, J.L.; Lefeuvre, L.; Misery, L.; et al. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: Pro-inflammatory response induced by their activation and their sensitization. Protein Cell 2017, 8, 644–661. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP channel drug discovery: From target validation to clinical studies. Nat. Rev. Drug Discov. 2022, 21, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Haruta, M.; Tomita, Y.; Matsumura, K.; Ikeda, T.; Yuno, A.; Hirayama, M.; Nakayama, H.; Mizuta, H.; Nishimura, Y.; et al. Generation of Large Numbers of Antigen-Expressing Human Dendritic Cells Using CD14-ML Technology. PLoS ONE 2016, 11, e0152384. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, R.J.; Lloyd, C.M. Regulation of immune responses by the airway epithelial cell landscape. Nat. Rev. Immunol. 2021, 21, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Brazzini, B.; Ghersetich, I.; Hercogova, J.; Lotti, T. The neuro-immuno-cutaneous-endocrine network: Relationship between mind and skin. Dermatol. Ther. 2003, 16, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Benhadou, F.; Mintoff, D.; Del Marmol, V. Psoriasis: Keratinocytes or Immune Cel–s—Which Is the Trigger? Dermatology 2019, 235, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Maglie, R.; Souza Monteiro de Araujo, D.; Antiga, E.; Geppetti, P.; Nassini, R.; De Logu, F. The Role of TRPA1 in Skin Physiology and Pathology. Int. J. Mol. Sci. 2021, 22, 3065. [Google Scholar] [CrossRef] [PubMed]
- Froghi, S.; Grant, C.R.; Tandon, R.; Quaglia, A.; Davidson, B.; Fuller, B. New Insights on the Role of TRP Channels in Calcium Signalling and Immunomodulation: Review of Pathways and Implications for Clinical Practice. Clin. Rev. Allergy Immunol. 2021, 60, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Dou, B.; Zhang, Y.; Chen, Z.; Li, Y.; Fan, Z.; Ma, Y.; Du, S.; Wang, J.; Xu, Z.; et al. Inflammation-the role of TRPA1 channel. Front. Physiol. 2023, 14, 1093925. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.; Jalava, N.; Bratty, R.; Pertovaara, A. TRPA1 Antagonists for Pain Relief. Pharmaceuticals 2018, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Tai, Y.; He, D.; Liu, B.; Wang, C.; Shao, X.; Jordt, S.E.; Liu, B. ETAR and protein kinase A pathway mediate ET-1 sensitization of TRPA1 channel: A molecular mechanism of ET-1-induced mechanical hyperalgesia. Mol. Pain. 2019, 15, 1744806919842473. [Google Scholar] [CrossRef] [PubMed]
- Kun, J.; Szitter, I.; Kemény, A.; Perkecz, A.; Kereskai, L.; Pohóczky, K.; Vincze, A.; Gódi, S.; Szabó, I.; Szolcsányi, J.; et al. Upregulation of the transient receptor potential ankyrin 1 ion channel in the inflamed human and mouse colon and its protective roles. PLoS ONE 2014, 9, e108164. [Google Scholar] [CrossRef] [PubMed]
- Nassini, R.; Pedretti, P.; Moretto, N.; Fusi, C.; Carnini, C.; Facchinetti, F.; Viscomi, A.R.; Pisano, A.R.; Stokesberry, S.; Brunmark, C.; et al. Transient receptor potential ankyrin 1 channel localized to non-neuronal airway cells promotes non-neurogenic inflammation. PLoS ONE 2012, 7, e42454. [Google Scholar] [CrossRef] [PubMed]
- Hajna, Z.; Csekő, K.; Kemény, Á.; Kereskai, L.; Kiss, T.; Perkecz, A.; Szitter, I.; Kocsis, B.; Pintér, E.; Helyes, Z. Complex Regulatory Role of the TRPA1 Receptor in Acute and Chronic Airway Inflammation Mouse Models. Int. J. Mol. Sci. 2020, 21, 4109. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B. TRP channels in disease. Biochim. Biophys. Acta 2007, 1772, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Pereira, I.; Mendes, S.J.; Pereira, D.M.; Muniz, T.F.; Colares, V.L.; Monteiro, C.R.; Martins, M.M.; Grisotto, M.A.; Monteiro-Neto, V.; Monteiro, S.G.; et al. Transient Receptor Potential Ankyrin 1 Channel Expression on Peripheral Blood Leukocytes from Rheumatoid Arthritic Patients and Correlation with Pain and Disability. Front. Pharmacol. 2017, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.D.; Al-Haseni, A.; Rosenbaum, M.T.; Goldberg, L.J. Hyperkeratotic and hypertrophic lichen nitidus. Dermatol. Online J. 2017, 23, 15. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.H.; Oh, S.Y.; Lu, J.; Lou, H.; Myers, A.C.; Zhu, Z.; Zheng, T. TRPA1-dependent pruritus in IL-13-induced chronic atopic dermatitis. J. Immunol. 2013, 191, 5371–5382. [Google Scholar] [CrossRef] [PubMed]
- Nattkemper, L.A.; Tey, H.L.; Valdes-Rodriguez, R.; Lee, H.; Mollanazar, N.K.; Albornoz, C.; Sanders, K.M.; Yosipovitch, G. The Genetics of Chronic Itch: Gene Expression in the Skin of Patients with Atopic Dermatitis and Psoriasis with Severe Itch. J. Investig. Dermatol. 2018, 138, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Chen, C.; Zhou, W.; Ma, X.; Pu, X.; Zeng, Y.; Zhou, W.; Lv, F. TRPA1 deficiency alleviates inflammation of atopic dermatitis by reducing macrophage infiltration. Life Sci. 2021, 266, 118906. [Google Scholar] [CrossRef] [PubMed]
- Sághy, É.; Sipos, É.; Ács, P.; Bölcskei, K.; Pohóczky, K.; Kemény, Á.; Sándor, Z.; Szőke, É.; Sétáló, G., Jr.; Komoly, S.; et al. TRPA1 deficiency is protective in cuprizone-induced demyelination-A new target against oligodendrocyte apoptosis. Glia 2016, 64, 2166–2180. [Google Scholar] [CrossRef] [PubMed]
- Bölcskei, K.; Kriszta, G.; Sághy, É.; Payrits, M.; Sipos, É.; Vranesics, A.; Berente, Z.; Ábrahám, H.; Ács, P.; Komoly, S.; et al. Behavioural alterations and morphological changes are attenuated by the lack of TRPA1 receptors in the cuprizone-induced demyelination model in mice. J. Neuroimmunol. 2018, 320, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kriszta, G.; Nemes, B.; Sándor, Z.; Ács, P.; Komoly, S.; Berente, Z.; Bölcskei, K.; Pintér, E. Investigation of Cuprizone-Induced Demyelination in mGFAP-Driven Conditional Transient Receptor Potential Ankyrin 1 (TRPA1) Receptor Knockout Mice. Cells 2019, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Dalenogare, D.P.; Ritter, C.; Bellinaso, F.R.A.; Kudsi, S.Q.; Pereira, G.C.; Fialho, M.F.P.; Lückemeyer, D.D.; Antoniazzi, C.T.D.; Landini, L.; Ferreira, J.; et al. Periorbital Nociception in a Progressive Multiple Sclerosis Mouse Model Is Dependent on TRPA1 Channel Activation. Pharmaceuticals 2021, 14, 831. [Google Scholar] [CrossRef] [PubMed]
- Peres, D.S.; Theisen, M.C.; Fialho, M.F.P.; Dalenogare, D.P.; Rodrigues, P.; Kudsi, S.Q.; Bernardes, L.B.; Ruviaro da Silva, N.A.; Lückemeyer, D.D.; Sampaio, T.B.; et al. TRPA1 involvement in depression- and anxiety-like behaviors in a progressive multiple sclerosis model in mice. Brain Res. Bull. 2021, 175, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mihai, D.P.; Ungurianu, A.; Ciotu, C.I.; Fischer, M.J.M.; Olaru, O.T.; Nitulescu, G.M.; Andrei, C.; Zbarcea, C.E.; Zanfirescu, A.; Seremet, O.C.; et al. Effects of Venlafaxine, Risperidone and Febuxostat on Cuprizone-Induced Demyelination, Behavioral Deficits and Oxidative Stress. Int. J. Mol. Sci. 2021, 22, 7183. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, I.E.; Schatzker, M.; Small, D.M. Rethinking Food Reward. Annu. Rev. Psychol. 2020, 71, 139–164. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhang, Y.; Yu, W.; Li, J.; Yao, J.; Zhang, J.; Wang, J.; Wang, C. Transient receptor potential ankyrin 1 (TRPA1) modulators: Recent update and future perspective. Eur. J. Med. Chem. 2023, 257, 115392. [Google Scholar] [CrossRef] [PubMed]
- Naert, R.; López-Requena, A.; Talavera, K. TRPA1 Expression and Pathophysiology in Immune Cells. Int. J. Mol. Sci. 2021, 22, 11460. [Google Scholar] [CrossRef] [PubMed]
- Inada, H.; Iida, T.; Tominaga, M. Different expression patterns of TRP genes in murine B and T lymphocytes. Biochem. Biophys. Res. Commun. 2006, 350, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Bertin, S.; Aoki-Nonaka, Y.; Lee, J.; de Jong, P.R.; Kim, P.; Han, T.; Yu, T.; To, K.; Takahashi, N.; Boland, B.S.; et al. The TRPA1 ion channel is expressed in CD4+ T cells and restrains T-cell-mediated colitis through inhibition of TRPV1. Gut 2017, 66, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.S.; Majhi, R.K.; Tiwari, A.; Acharya, T.; Kumar, P.S.; Saha, S.; Kumar, A.; Goswami, C.; Chattopadhyay, S. Transient receptor potential ankyrin1 channel is endogenously expressed in T cells and is involved in immune functions. Biosci. Rep. 2019, 39, BSR20191437. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Li, Y.; Fischer, M.J.M.; Steinhoff, M.; Chen, W.; Wang, J. Th2 Modulation of Transient Receptor Potential Channels: An Unmet Therapeutic Intervention for Atopic Dermatitis. Front. Immunol. 2021, 12, 696784. [Google Scholar] [CrossRef] [PubMed]
- Celsi, F.; Peri, F.; Cavasin, J.; Zupin, L.; Cozzi, G.; Barbi, E.; Crovella, S. Transient Receptor Potential Ankyrin 1 (TRPA1) Methylation and Chronic Pain: A Systematic Review. Genes 2023, 14, 411. [Google Scholar] [CrossRef] [PubMed]
- Fila, M.; Pawlowska, E.; Szczepanska, J.; Blasiak, J. Epigenetic Connections of the TRPA1 Ion Channel in Pain Transmission and Neurogenic Inflammation—A Therapeutic Perspective in Migraine? Mol. Neurobiol. 2023, 60, 5578–5591. [Google Scholar] [CrossRef] [PubMed]
- Szabó, K.; Kemény, Á.; Balázs, N.; Khanfar, E.; Sándor, Z.; Boldizsár, F.; Gyulai, R.; Najbauer, J.; Pintér, E.; Berki, T. Presence of TRPA1 Modifies CD4+/CD8+ T Lymphocyte Ratio and Activation. Pharmaceuticals 2022, 15, 57. [Google Scholar] [CrossRef] [PubMed]
- Billeter, A.T.; Galbraith, N.; Walker, S.; Lawson, C.; Gardner, S.A.; Sarojini, H.; Galandiuk, S.; Polk, H.C., Jr. TRPA1 mediates the effects of hypothermia on the monocyte inflammatory response. Surgery 2015, 158, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Groot-Kormelink, P.J.; Fawcett, L.; Wright, P.D.; Gosling, M.; Kent, T.C. Quantitative GPCR and ion channel transcriptomics in primary alveolar macrophages and macrophage surrogates. BMC Immunol. 2012, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Szöllősi, A.G.; Oláh, A.; Tóth, I.B.; Papp, F.; Czifra, G.; Panyi, G.; Bíró, T. Transient receptor potential vanilloid-2 mediates the effects of transient heat shock on endocytosis of human monocyte-derived dendritic cells. FEBS Lett. 2013, 587, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Takaya, J.; Mio, K.; Shiraishi, T.; Kurokawa, T.; Otsuka, S.; Mori, Y.; Uesugi, M. A Potent and Site-Selective Agonist of TRPA1. J. Am. Chem. Soc. 2015, 137, 15859–15864. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, C.E.; Armache, J.P.; Gao, Y.; Cheng, Y.; Julius, D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 2015, 520, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, M.; Muraki, Y.; Hatano, N.; Suzuki, H.; Muraki, K. Potent Activation of Human but Not Mouse TRPA1 by JT010. Int. J. Mol. Sci. 2022, 23, 14297. [Google Scholar] [CrossRef] [PubMed]
- Bahia, P.K.; Parks, T.A.; Stanford, K.R.; Mitchell, D.A.; Varma, S.; Stevens, S.M., Jr.; Taylor-Clark, T.E. The exceptionally high reactivity of Cys 621 is critical for electrophilic activation of the sensory nerve ion channel TRPA1. J. Gen. Physiol. 2016, 147, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lin King, J.V.; Paulsen, C.E.; Cheng, Y.; Julius, D. Irritant-evoked activation and calcium modulation of the TRPA1 receptor. Nature 2020, 585, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Suo, Y.; Wang, Z.; Zubcevic, L.; Hsu, A.L.; He, Q.; Borgnia, M.J.; Ji, R.R.; Lee, S.Y. Structural Insights into Electrophile Irritant Sensing by the Human TRPA1 Channel. Neuron 2020, 105, 882–894.e5. [Google Scholar] [CrossRef] [PubMed]
- Grabinski, T.M.; Kneynsberg, A.; Manfredsson, F.P.; Kanaan, N.M. A method for combining RNAscope in situ hybridization with immunohistochemistry in thick free-floating brain sections and primary neuronal cultures. PLoS ONE 2015, 10, e0120120. [Google Scholar] [CrossRef] [PubMed]
- Dikshit, A.; Zong, H.; Anderson, C.; Zhang, B.; Ma, X.J. Simultaneous Visualization of RNA and Protein Expression in Tissue Using a Combined RNAscope™ In Situ Hybridization and Immunofluorescence Protocol. Methods Mol. Biol. 2020, 2148, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.H.; Huang, L.Y.; Weaver, S.; Archila, L.D.; McAfee, M.S.; Hirayama, A.V.; Chapuis, A.G.; Bleakley, M.; Rongvaux, A.; Turtle, C.J.; et al. Detection of engineered T cells in FFPE tissue by multiplex in situ hybridization and immunohistochemistry. J. Immunol. Methods. 2021, 492, 112955. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Leskinen, K.; Gritti, T.; Groma, V.; Arola, J.; Lepisto, A.; Sipponen, T.; Saavalainen, P.; Soderlund-Venermo, M. Prevalence, cell tropism, and clinical impact of human parvovirus persistence in adenomatous, cancerous, inflamed, and healthy intestinal mucosa. Front. Microbiol. 2022, 24, 914181. [Google Scholar] [CrossRef]
- D’Agostino, M.; Di Cecco, M.; Marani, C.; Vigili, M.G.; Sileno, S.; Volpi, C.C.; Gloghini, A.; Avitabile, D.; Magenta, A.; Rahimi, S. Positive Linear Relationship between Nucleophosmin Protein Expression and the Viral Load in HPV-Associated Oropharyngeal Squamous Cell Carcinoma: A Possible Tool for Stratification of Patients. Int. J. Mol. Sci. 2023, 24, 3482. [Google Scholar] [CrossRef] [PubMed]
- Kormos, V.; Kecskés, A.; Farkas, J.; Gaszner, T.; Csernus, V.; Alomari, A.; Hegedüs, D.; Renner, É.; Palkovits, M.; Zelena, D.; et al. Peptidergic neurons of the Edinger-Westphal nucleus express TRPA1 ion channel that is downregulated both upon chronic variable mild stress in male mice and in humans who died by suicide. J. Psychiatry Neurosci. 2022, 47, E162–E175. [Google Scholar] [CrossRef] [PubMed]
- Kiss, F.; Kormos, V.; Szőke, É.; Kecskés, A.; Tóth, N.; Steib, A.; Szállási, Á.; Scheich, B.; Gaszner, B.; Kun, J.; et al. Functional Transient Receptor Potential Ankyrin 1 and Vanilloid 1 Ion Channels Are Overexpressed in Human Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 1921. [Google Scholar] [CrossRef] [PubMed]
- Al-Omari, A.; Kecskés, M.; Gaszner, B.; Biró-Sütő, T.; Fazekas, B.; Berta, G.; Kuzma, M.; Pintér, E.; Kormos, V. Functionally active TRPA1 ion channel is downregulated in peptidergic neurons of the Edinger-Westphal nucleus upon acute alcohol exposure. Front. Cell Dev. Biol. 2023, 10, 1046559. [Google Scholar] [CrossRef] [PubMed]
- Konkoly, J.; Kormos, V.; Gaszner, B.; Correia, P.; Berta, G.; Biró-Sütő, T.; Zelena, D.; Pintér, E. Transient receptor potential ankyrin 1 ion channel expressed by the Edinger-Westphal nucleus contributes to stress adaptation in murine model of posttraumatic stress disorder. Front. Cell Dev. Biol. 2022, 10, 1059073. [Google Scholar] [CrossRef] [PubMed]
- Milicic, M.; Gaszner, B.; Berta, G.; Pintér, E.; Kormos, V. The Lack of TRPA1 Ion Channel Does Not Affect the Chronic Stress-Induced Activation of the Locus Ceruleus. Int. J. Mol. Sci. 2024, 25, 1765. [Google Scholar] [CrossRef] [PubMed]
- Erdő-Bonyár, S.; Rapp, J.; Minier, T.; Ráth, G.; Najbauer, J.; Czirják, L.; Németh, P.; Berki, T.; Simon, D. Toll-Like Receptor Mediated Activation of Natural Autoantibody Producing B Cell Subpopulations in an Autoimmune Disease Model. Int. J. Mol. Sci. 2019, 20, 6152. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Bene, J.; Balázs, N.; Szabó, K.; Berta, G.; Herczeg, R.; Gyenesei, A.; Balogh, P. Age-Associated B Cell Features of the Murine High-Grade B Cell Lymphoma Bc.DLFL1 and Its Extranodal Expansion in Abdominal Adipose Tissues. J. Immunol. 2022, 208, 2866–2876. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.L.; Kárteszi, J.; Prohászka, Z.; Kalmár, T.; Késmárky, G.; Koltai, K.; Nagy, Z.; Sebők, J.; Vas, T.; Molnár, K.; et al. Hemizygous nonsense variant in the moesin gene (MSN) leads to a new autoimmune phenotype of Immunodeficiency 50. Front. Immunol. 2022, 13, 919411. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Flanagan, J.; Su, N.; Wang, L.-C.; Bui, S.; Nielson, A.; Wu, X.; Vo, H.-T.; Ma, X.-J.; Luo, Y. RNAscope: A novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J. Mol. Diagn. 2012, 14, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Kugyelka, R.; Prenek, L.; Olasz, K.; Kohl, Z.; Botz, B.; Glant, T.T.; Berki, T.; Boldizsár, F. ZAP-70 Regulates Autoimmune Arthritis via Alterations in T Cell Activation and Apoptosis. Cells 2019, 8, 504. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Stirling, D.R.; Swain-Bowden, M.J.; Lucas, A.M.; Carpenter, A.E.; Cimini, B.A.; Goodman, A. CellProfiler 4: Improvements in speed, utility and usability. BMC Bioinform. 2021, 22, 433. [Google Scholar] [CrossRef] [PubMed]
- Minta, A.; Kao, J.P.; Tsien, R.Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J. Biol. Chem. 1989, 264, 8171–8178. [Google Scholar] [CrossRef] [PubMed]
- Boldizsár, F.; Berki, T.; Miseta, A.; Németh, P. Effect of hyperglycemia on the basal cytosolic free calcium level, calcium signal and tyrosine-phosphorylation in human T-cells. Immunol. Lett. 2002, 82, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Khanfar, E.; Olasz, K.; Gábris, F.; Gajdócsi, E.; Botz, B.; Kiss, T.; Kugyelka, R.; Berki, T.; Balogh, P.; Boldizsár, F. Ameliorated Autoimmune Arthritis and Impaired B Cell Receptor-Mediated Ca2+ Influx in Nkx2-3 Knock-out Mice. Int. J. Mol. Sci. 2020, 21, 6162. [Google Scholar] [CrossRef] [PubMed]
- Heber, S.; Gold-Binder, M.; Ciotu, C.I.; Witek, M.; Ninidze, N.; Kress, H.G.; Fischer, M.J.M. A Human TRPA1-Specific Pain Model. J. Neurosci. 2019, 39, 3845–3855. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, C.; McVey, J.H.; Elliott, J.I.; Sardini, A.; Kasza, I.; Mumford, A.D.; Naoumova, R.P.; Tuddenham, E.G.; Szabo, K.; Higgins, C.F. A novel missense mutation in ABCA1 results in altered protein trafficking and reduced phosphatidylserine translocation in a patient with Scott syndrome. Blood 2005, 106, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Sato-Hashimoto, M.; Nozu, T.; Toriba, R.; Horikoshi, A.; Akaike, M.; Kawamoto, K.; Hirose, A.; Hayashi, Y.; Nagai, H.; Shimizu, W.; et al. Microglial SIRPα regulates the emergence of CD11c+ microglia and demyelination damage in white matter. Elife 2019, 8, e42025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Feng, T.; Zhou, X.; Sullivan, P.M.; Hu, F.; Lou, Y.; Yu, J.; Feng, J.; Liu, H.; Chen, Y. Inactivation of TMEM106A promotes lipopolysaccharide-induced inflammation via the MAPK and NF-κB signaling pathways in macrophages. Clin. Exp. Immunol. 2021, 203, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Feske, S.; Wulff, H.; Skolnik, E.Y. Ion channels in innate and adaptive immunity. Annu. Rev. Immunol. 2015, 33, 291–353. [Google Scholar] [CrossRef] [PubMed]
- Trebak, M.; Kinet, J.P. Calcium signalling in T cells. Nat. Rev. Immunol. 2019, 19, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Mahtani, T.; Treanor, B. Beyond the CRAC: Diversification of ion signaling in B cells. Immunol. Rev. 2019, 291, 104–122. [Google Scholar] [CrossRef] [PubMed]
- Yenson, V.; Baumgarth, N. Purification and immune phenotyping of B-1 cells from body cavities of mice. Methods Mol. Biol. 2014, 1190, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Baumgarth, N. A two-phase model of B-cell activation. Immunol. Rev. 2000, 176, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Baumgarth, N. B-cell immunophenotyping. Methods Cell Biol. 2004, 75, 643–662. [Google Scholar] [CrossRef] [PubMed]
- Lábadi, A.; Balogh, P. Differential preferences in serosal homing and distribution of peritoneal B-cell subsets revealed by in situ CFSE labeling. Int. Immunol. 2009, 21, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lin, J.X.; Li, P.; Skinner, J.; Leonard, W.J.; Morse, H.C., 3rd. New insights into heterogeneity of peritoneal B-1a cells. Ann. N. Y Acad. Sci. 2015, 1362, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Silva-Sanchez, A.; Randall, T.D.; Meza-Perez, S. Specialized immune responses in the peritoneal cavity and omentum. J. Leukoc. Biol. 2021, 109, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Rathod, S. T cells in the peritoneum. Int. Rev. Cell Mol. Biol. 2022, 371, 15–41. [Google Scholar] [CrossRef] [PubMed]
- Romano, B.; Borrelli, F.; Fasolino, I.; Capasso, R.; Piscitelli, F.; Cascio, M.; Pertwee, R.; Coppola, D.; Vassallo, L.; Orlando, P.; et al. The cannabinoid TRPA1 agonist cannabichromene inhibits nitric oxide production in macrophages and ameliorates murine colitis. Br. J. Pharmacol. 2013, 169, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Romano, B.; Pagano, E.; Orlando, P.; Capasso, R.; Cascio, M.G.; Pertwee, R.; Marzo, V.D.; Izzo, A.A.; Borrelli, F. Pure Δ9-tetrahydrocannabivarin and a Cannabis sativa extract with high content in Δ9-tetrahydrocannabivarin inhibit nitrite production in murine peritoneal macrophages. Pharmacol. Res. 2016, 113(Pt A), 199–208. [Google Scholar] [CrossRef] [PubMed]
- De Logu, F.; Nassini, R.; Materazzi, S.; Carvalho Gonçalves, M.; Nosi, D.; Rossi Degl’Innocenti, D.; Marone, I.M.; Ferreira, J.; Li Puma, S.; Benemei, S.; et al. Schwann cell TRPA1 mediates neuroinflammation that sustains macrophage-dependent neuropathic pain in mice. Nat. Commun. 2017, 8, 1887. [Google Scholar] [CrossRef] [PubMed]
- Vig, M.; Kinet, J.P. Calcium signaling in immune cells. Nat. Immunol. 2009, 10, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Launay, P.; Fleig, A.; Perraud, A.L.; Scharenberg, A.M.; Penner, R.; Kinet, J.P. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 2002, 109, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Launay, P.; Cheng, H.; Srivatsan, S.; Penner, R.; Fleig, A.; Kinet, J.P. TRPM4 regulates calcium oscillations after T cell activation. Science 2004, 306, 1374–1377. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, M.; Wasim, L.; Buhari, F.H.M.; Zhao, T.; Mahtani, T.; Ho, J.; Kang, S.; Deason-Towne, F.; Perraud, A.L.; Schmitz, C.; et al. The channel-kinase TRPM7 regulates antigen gathering and internalization in B cells. Sci. Signal 2018, 11, eaah6692. [Google Scholar] [CrossRef] [PubMed]
- Wenning, A.S.; Neblung, K.; Strauss, B.; Wolfs, M.J.; Sappok, A.; Hoth, M.; Schwarz, E.C. TRP expression pattern and the functional importance of TRPC3 in primary human T-cells. Biochim. Biophys. Acta 2011, 1813, 412–423. [Google Scholar] [CrossRef] [PubMed]
- López, J.J.; Salido, G.M.; Pariente, J.A.; Rosado, J.A. Interaction of STIM1 with endogenously expressed human canonical TRP1 upon depletion of intracellular Ca2+ stores. J. Biol. Chem. 2006, 281, 28254–28264. [Google Scholar] [CrossRef] [PubMed]
- Jardín, I.; Redondo, P.C.; Salido, G.M.; Rosado, J.A. Phosphatidylinositol 4,5-bisphosphate enhances store-operated calcium entry through hTRPC6 channel in human platelets. Biochim. Biophys. Acta 2008, 1783, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Jardin, I.; Gómez, L.J.; Salido, G.M.; Rosado, J.A. Dynamic interaction of hTRPC6 with the Orai1-STIM1 complex or hTRPC3 mediates its role in capacitative or non-capacitative Ca2+ entry pathways. Biochem. J. 2009, 420, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Albarrán, L.; Lopez, J.J.; Dionisio, N.; Smani, T.; Salido, G.M.; Rosado, J.A. Transient receptor potential ankyrin-1 (TRPA1) modulates store-operated Ca2+ entry by regulation of STIM1-Orai1 association. Biochim. Biophys. Acta 2013, 1833, 3025–3034. [Google Scholar] [CrossRef] [PubMed]
- Akopian, A.N. Regulation of nociceptive transmission at the periphery via TRPA1-TRPV1 interactions. Curr. Pharm. Biotechnol. 2011, 12, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Sadofsky, L.R.; Sreekrishna, K.T.; Lin, Y.; Schinaman, R.; Gorka, K.; Mantri, Y.; Haught, J.C.; Huggins, T.G.; Isfort, R.J.; Bascom, C.C.; et al. Unique Responses are Observed in Transient Receptor Potential Ankyrin 1 and Vanilloid 1 (TRPA1 and TRPV1) Co-Expressing Cells. Cells 2014, 3, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.M.; Hargreaves, K.M.; Akopian, A.N. TRPA1-mediated responses in trigeminal sensory neurons: Interaction between TRPA1 and TRPV1. Eur. J. Neurosci. 2009, 29, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.J.; Balasuriya, D.; Jeggle, P.; Goetze, T.A.; McNaughton, P.A.; Reeh, P.W.; Edwardson, J.M. Direct evidence for functional TRPV1/TRPA1 heteromers. Pflug. Arch. 2014, 466, 2229–2241. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.J.; Jeske, N.A.; Akopian, A.N. Transient receptor potential V1 regulates activation and modulation of transient receptor potential A1 by Ca2+. Neuroscience 2010, 171, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.J.; Patel, K.N.; Jeske, N.A.; Bierbower, S.M.; Zou, W.; Tiwari, V.; Zheng, Q.; Tang, Z.; Mo, G.C.; Wang, Y.; et al. Tmem100 Is a Regulator of TRPA1-TRPV1 Complex and Contributes to Persistent Pain. Neuron 2015, 85, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Weyer, A.D.; Stucky, C.L. Loosening pain’s grip by tightening TRPV1-TRPA1 interactions. Neuron 2015, 85, 661–663. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, K.; Makkai, G.; Konkoly, J.; Kormos, V.; Gaszner, B.; Berki, T.; Pintér, E. TRPA1 Covalent Ligand JT010 Modifies T Lymphocyte Activation. Biomolecules 2024, 14, 632. https://doi.org/10.3390/biom14060632
Szabó K, Makkai G, Konkoly J, Kormos V, Gaszner B, Berki T, Pintér E. TRPA1 Covalent Ligand JT010 Modifies T Lymphocyte Activation. Biomolecules. 2024; 14(6):632. https://doi.org/10.3390/biom14060632
Chicago/Turabian StyleSzabó, Katalin, Géza Makkai, János Konkoly, Viktória Kormos, Balázs Gaszner, Tímea Berki, and Erika Pintér. 2024. "TRPA1 Covalent Ligand JT010 Modifies T Lymphocyte Activation" Biomolecules 14, no. 6: 632. https://doi.org/10.3390/biom14060632
APA StyleSzabó, K., Makkai, G., Konkoly, J., Kormos, V., Gaszner, B., Berki, T., & Pintér, E. (2024). TRPA1 Covalent Ligand JT010 Modifies T Lymphocyte Activation. Biomolecules, 14(6), 632. https://doi.org/10.3390/biom14060632






