Modulation of Adverse Health Effects of Environmental Cadmium Exposure by Zinc and Its Transporters
Abstract
1. Introduction
2. Zinc versus Cadmium
2.1. Recommended Dietary Allowance for Zinc versus Tolerable Level for Cadmium
2.1.1. Zinc and Its RDA Values
2.1.2. Tolerable Levels of Cadmium
2.1.3. Loss of GFR as a Sensitive Toxic Endpoint
2.1.4. Summary of “Tolerable” Level of Cadmium
2.2. Absorption of Metal Nutrients versus Contaminant Cadmium: Overview
2.2.1. Dietary Zinc Absorption
2.2.2. High Absorption Rate of Cadmium: Role of Multiple Transporters
3. Zinc and the Cytotoxicity of Cadmium
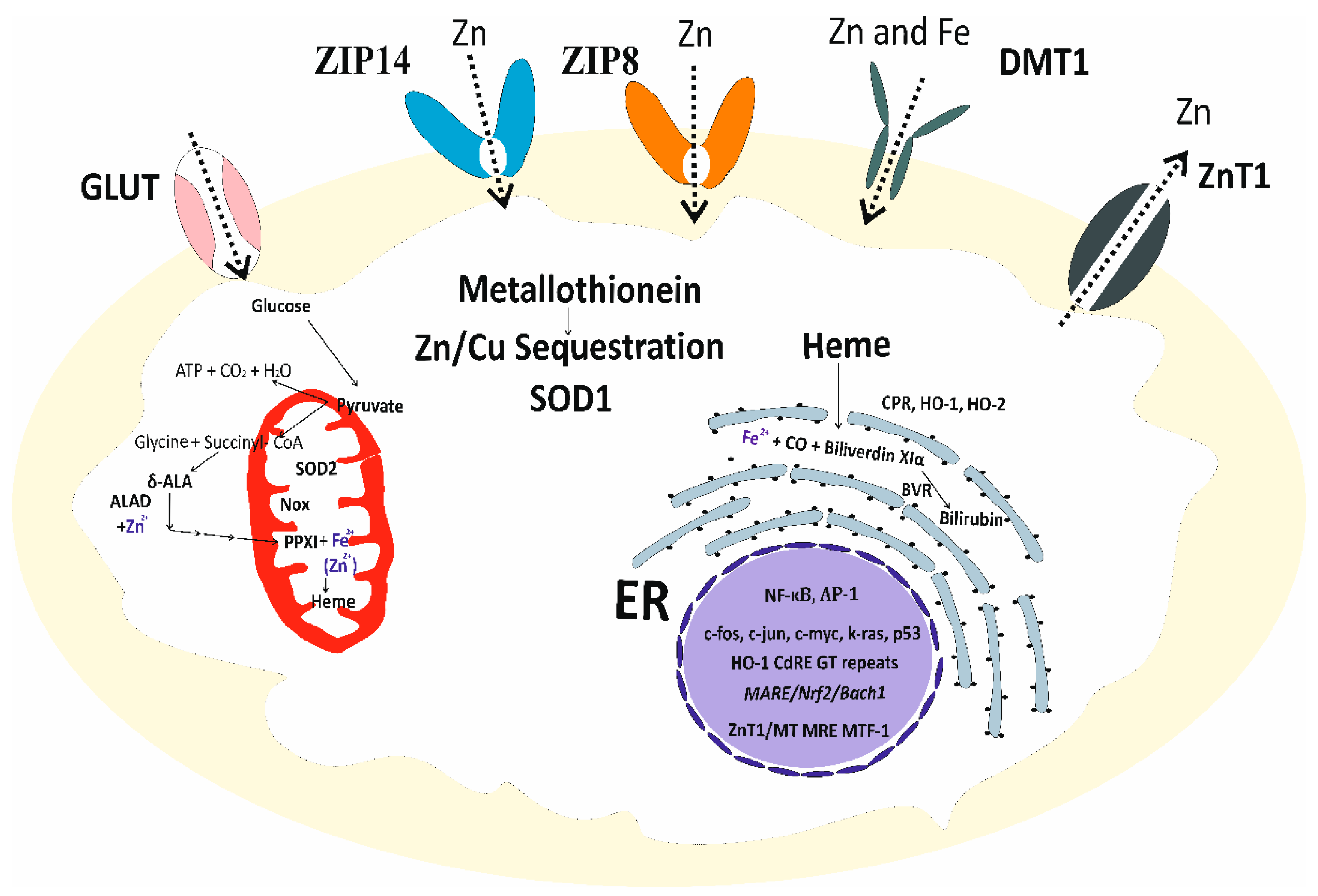
3.1. Zinc Homeostasis and Its Antioxidative Function
3.2. Cadmium-Induced Disruption of Cellular Zinc Homeostasis and Redox State
| Transporters | Transcripts Per 1000 β-Actin | ||||
|---|---|---|---|---|---|
| Batch I, 0 µM Cd2+ | Batch II, 0 µM Cd2+ | 1 µM Cd2+ | 2 µM Cd2+ | 4 µM Cd2+ | |
| SLC30A | |||||
| ZnT1 | 181 ± 23 | 365 ± 38 | 3007 ± 465 | 1434 ± 146 | 1216 ± 153 *** |
| ZnT2 | 0.01 ± 0.001 | 0.06 ± 0.01 | 73 ± 15 | 16 ± 1.9 | 11 ± 1.5 *** |
| ZnT3 | 0.03 ± 0.007 | 0.15 ± 0.01 | 0.24 ± 0.05 | 0.10 ± 0.02 | 0.10 ± 0.02 * |
| ZnT4 | 1.6 ± 0.26 | 11.4 ± 0.8 | 10 ± 1 | 8.7 ± 1 | 6.2 ± 0.5 ** |
| ZnT5 | 150 ± 19 | 510 ± 30 | 1038 ± 132 | 495 ± 54 | 568 ± 91 ** |
| ZnT6 | 4.5 ± 0.15 | 65 ± 8 | 77 ± 6 | 63 ± 13 | 57 ± 12 |
| ZnT7 | 734 ± 28 | 758 ± 76 | 1007 ± 136 | 706 ± 44 | 488 ± 63 * |
| ZnT10 | 0.04 ± 0.005 | 1.1 ± 0.2 | 2.4 ± 0.2 | 1.7 ± 0.2 | 1.1 ± 0.1 *** |
| SLC39A | |||||
| ZIP1 | 19.5 ± 2.0 | 82 ± 9 | 99 ± 15 | 55 ± 10 | 59 ± 12 * |
| ZIP2 | 0.02 ± 0.004 | 1.2 ± 0.1 | 0.8 ± 0.2 | 0.4 ± 0.1 | 0.2 ± 0.03 *** |
| ZIP3A | 9.1 ± 0.4 | 19 ± 1 | 23 ± 2.3 | 17 ± 1.7 | 14 ± 1.3 * |
| ZIP3B | 0.48 ± 0.05 | 4.1 ± 0.2 | 6.2 ± 0.7 | 4.4 ± 0.2 | 4.2 ± 0.4 * |
| ZIP4 | 0.18 ± 0.04 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.04 ± 0.01 | 0.06 ± 0.01 |
| ZIP5 | 0.01 ± 0.003 | 0.01 ± 0.001 | 0.01 ± 0.003 | 0.01 ± 0.002 | 0.01 ± 0.002 |
| ZIP6 | 18.3 ± 2.6 | 92 ± 8 | 133 ± 12 | 80 ± 9 | 75 ± 10 ** |
| ZIP7 | 121 ± 9.5 | 204 ± 25 | 342 ± 69 | 149 ± 32 | 94 ± 21 *** |
| ZIP8 | 0.09 ± 0.01 | 2.1 ± 0.2 | 2.6 ± 0.3 | 2.0 ± 0.2 | 2.7 ± 0.4 |
| ZIP10 | 5 ± 0.3 | 54 ± 4 | 30 ± 8 | 14 ± 3 | 14 ± 3 *** |
| ZIP14 | 83.4 ± 10.5 | 146 ± 19 | 218 ± 24 | 158 ± 26 | 128 ± 19 * |
4. Global Health Threat of Environmental Cadmium
4.1. Cadmium and the World’s Leading Causes of Death
4.2. Public Health Significance of Environmental Cadmium
4.3. Evidence for Mitigative Effects of Zinc
4.3.1. Cancer, COPD, and CKD
4.3.2. Cadmium, Macular Degeneration, and High-Dose Zinc Supplementation
4.4. Cadmium, Zinc, and Bone Resorption
4.4.1. Human Studies
4.4.2. Experimental Studies
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006, 5, 196–201. [Google Scholar] [CrossRef] [PubMed]
- King, J.C.; Shames, D.M.; Woodhouse, L.R. Zinc homeostasis in humans. J. Nutr. 2000, 130, 1360S–1366S. [Google Scholar] [CrossRef]
- King, J.C. Zinc: An essential but elusive nutrient. Am. J. Clin. Nutr. 2011, 94, 679S–684S. [Google Scholar] [CrossRef]
- Stiles, L.I.; Ferrao, K.; Mehta, K.J. Role of zinc in health and disease. Clin. Exp. Med. 2024, 24, 38. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.G.; King, J.C. The molecular basis for zinc bioavailability. Int. J. Mol. Sci. 2023, 24, 6561. [Google Scholar] [CrossRef]
- Andrew, D.; Gail, R.; Morag, B.; Kishor, R. Recommended reference intervals for copper and zinc in serum using the US National Health and Nutrition Examination surveys (NHANES) data. Clin. Chim. Acta 2023, 546, 117397. [Google Scholar] [CrossRef]
- Barman, N.; Salwa, M.; Ghosh, D.; Rahman, M.W.; Uddin, M.N.; Haque, M.A. Reference value for serum zinc level of adult population in Bangladesh. EJIFCC 2020, 31, 117–124. [Google Scholar]
- IOM Institute of Medicine. The National Academy of Sciences, Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; Institute of Medicine. Food and Nutrition Board, NRC. National Academies Press: Washington, DC, USA, 2001; pp. 442–501. [Google Scholar] [CrossRef]
- King, J.C. Does zinc absorption reflect zinc status? Int. J. Vitam. Nutr. Res. 2010, 80, 300–306. [Google Scholar] [CrossRef]
- King, J.C. Yet again, serum zinc concentrations are unrelated to zinc intakes. J. Nutr. 2018, 148, 1399–1401. [Google Scholar] [CrossRef]
- McDonald, C.M.; Suchdev, P.S.; Krebs, N.F.; Hess, S.Y.; Wessells, K.R.; Ismaily, S.; Rahman, S.; Wieringa, F.T.; Williams, A.M.; Brown, K.H.; et al. Adjusting plasma or serum zinc concentrations for inflammation: Biomarkers reflecting inflammation and nutritional determinants of anemia (BRINDA) project. Am. J. Clin. Nutr. 2020, 111, 927–937. [Google Scholar] [CrossRef]
- Molenda, M.; Kolmas, J. The role of zinc in bone tissue health and regeneration—A review. Biol. Trace Elem. Res. 2023, 201, 5640–5651. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K. The interaction of zinc with the multi-functional plasma thyroid hormone distributor protein, transthyretin: Evolutionary and cross-species comparative aspects. Biometals 2021, 34, 423–437. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Sadykhov, N.K.; Kartuesov, A.G.; Borisov, E.E.; Sukhorukov, V.N.; Orekhov, A.N. Interplay between Zn2+ homeostasis and mitochondrial functions in cardiovascular diseases and heart ageing. Int. J. Mol. Sci. 2022, 23, 6890. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a gatekeeper of immune function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Quan, Z.; Ni, J.; Li, H.; Qing, H. The many faces of the zinc finger protein 335 in brain development and immune system. Biomed. Pharmacother. 2023, 165, 115257. [Google Scholar] [CrossRef] [PubMed]
- Vickram, S.; Rohini, K.; Srinivasan, S.; Nancy Veenakumari, D.; Archana, K.; Anbarasu, K.; Jeyanthi, P.; Thanigaivel, S.; Gulothungan, G.; Rajendiran, N.; et al. Role of zinc (Zn) in human reproduction: A journey from initial spermatogenesis to childbirth. Int. J. Mol. Sci. 2021, 22, 2188. [Google Scholar] [CrossRef] [PubMed]
- King, J.C.; Brown, K.H.; Gibson, R.S.; Krebs, N.F.; Lowe, N.M.; Siekmann, J.H.; Raiten, D.J. Biomarkers of nutrition for development (BOND)-zinc review. J. Nutr. 2015, 146, 858S–885S. [Google Scholar] [CrossRef] [PubMed]
- Trame, S.; Wessels, I.; Haase, H.; Rink, L. A short 18 items food frequency questionnaire biochemically validated to estimate zinc status in humans. J. Trace Elem. Med. Biol. 2018, 49, 285–295. [Google Scholar] [CrossRef]
- Lowe, N.M.; Hall, A.G.; Broadley, M.R.; Foley, J.; Boy, E.; Bhutta, Z.A. Preventing and controlling zinc deficiency across the life course: A call to action. Adv. Nutr. 2024, 15, 100181. [Google Scholar] [CrossRef]
- Satarug, S.; Phelps, K.R. Chapter 14: Cadmium Exposure and Toxicity. In Metal Toxicology Handbook; Bagchi, D., Bagchi, M., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 219–274. [Google Scholar]
- Satarug, S.; Vesey, D.A.; Gobe, G.C.; Phelps, K.R. Estimation of health risks associated with dietary cadmium exposure. Arch. Toxicol. 2023, 97, 329–358. [Google Scholar] [CrossRef]
- Satarug, S.; Baker, J.R.; Reilly, P.E.; Moore, M.R.; Williams, D.J. Changes in zinc and copper homeostasis in human livers and kidneys associated with exposure to environmental cadmium. Hum. Exp. Toxicol. 2001, 20, 205–213. [Google Scholar] [CrossRef]
- Boon, P.E.; Pustjens, A.M.; Te Biesebeek, J.D.; Brust, G.M.H.; Castenmiller, J.J.M. Dietary intake and risk assessment of elements for 1- and 2-year-old children in the Netherlands. Food Chem. Toxicol. 2022, 161, 112810. [Google Scholar] [CrossRef] [PubMed]
- Fechner, C.; Hackethal, C.; Höpfner, T.; Dietrich, J.; Bloch, D.; Lindtner, O.; Sarvan, I. Results of the BfR MEAL Study: In Germany, mercury is mostly contained in fish and seafood while cadmium, lead, and nickel are present in a broad spectrum of foods. Food Chem. X 2022, 14, 100326. [Google Scholar] [CrossRef]
- Watanabe, T.; Kataoka, Y.; Hayashi, K.; Matsuda, R.; Uneyama, C. Dietary exposure of the Japanese general population to elements: Total diet study 2013–2018. Food Saf. 2022, 10, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Almerud, P.; Zamaratskaia, G.; Lindroos, A.K.; Bjermo, H.; Andersson, E.M.; Lundh, T.; Ankarberg, E.H.; Lignell, S. Cadmium, total mercury, and lead in blood and associations with diet, sociodemographic factors, and smoking in Swedish adolescents. Environ. Res. 2021, 197, 110991. [Google Scholar] [CrossRef]
- Pappas, R.S.; Fresquez, M.R.; Watson, C.H. Cigarette smoke cadmium breakthrough from traditional filters: Implications for exposure. J. Anal. Toxicol. 2015, 39, 45–51. [Google Scholar] [CrossRef]
- Kim, J.; Song, H.; Lee, J.; Kim, Y.J.; Chung, H.S.; Yu, J.M.; Jang, G.; Park, R.; Chung, W.; Oh, C.M.; et al. Smoking and passive smoking increases mortality through mediation effect of cadmium exposure in the United States. Sci. Rep. 2023, 13, 3878. [Google Scholar] [CrossRef]
- Świetlik, R.; Trojanowska, M. Chemical fractionation in environmental studies of potentially toxic particulate-bound elements in urban air: A critical review. Toxics 2022, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.T.; Jandev, V.; Petroni, M.; Atallah-Yunes, N.; Bendinskas, K.; Brann, L.S.; Heffernan, K.; Larsen, D.A.; MacKenzie, J.A.; Palmer, C.D.; et al. Airborne levels of cadmium are correlated with urinary cadmium concentrations among young children living in the New York state city of Syracuse, USA. Environ. Res. 2023, 223, 115450. [Google Scholar] [CrossRef]
- Willers, S.; Gerhardsson, L.; Lundh, T. Environmental tobacco smoke (ETS) exposure in children with asthma-relation between lead and cadmium, and cotinine concentrations in urine. Respir. Med. 2005, 99, 1521–1527. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Jung, C.R.; Lin, C.Y.; Hwang, B.F. Combined exposure to heavy metals in PM2.5 and pediatric asthma. J. Allergy Clin. Immunol. 2021, 147, 2171–2180.e13. [Google Scholar] [CrossRef]
- Madrigal, J.M.; Persky, V.; Jackson, B.P.; Bain, A.; Siemer, M.; Pappalardo, A.A.; Argos, M. Assessment of metal concentrations and associations with pulmonary function among children with asthma in Chicago, Illinois. Int. J. Environ. Res. Public Health 2021, 18, 7279. [Google Scholar] [CrossRef]
- Xue, M.; Wang, Q.; Pang, B.; Zhang, X.; Zhang, Y.; Deng, X.; Zhang, Z.; Niu, W. Association Between Circulating Zinc and Risk for Childhood Asthma and Wheezing: A Meta-analysis on 21 Articles and 2205 Children. Biol. Trace Elem. Res. 2024, 202, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Li, C.; Zhao, D.; Huang, L. Associations of micronutrients exposure with cadmium body burden among population: A systematic review. Ecotoxicol. Environ. Saf. 2023, 256, 114878. [Google Scholar] [CrossRef] [PubMed]
- Rentschler, G.; Kippler, M.; Axmon, A.; Raqib, R.; Skerfving, S.; Vahter, M.; Broberg, K. Cadmium concentrations in human blood and urine are associated with polymorphisms in zinc transporter genes. Metallomics 2014, 6, 885–891. [Google Scholar] [CrossRef] [PubMed]
- JECFA. Summary and Conclusions. In Proceedings of the Joint FAO/WHO Expert Committee on Food Additives and Contaminants, Seventy-Third Meeting, Geneva, Switzerland, 8–17 June 2010; JECFA/73/SC. Food and Agriculture Organization of the United Nations/World Health Organization: Geneva, Switzerland, 2011. Available online: https://apps.who.int/iris/handle/10665/44521 (accessed on 17 April 2024).
- Verbeeck, M.; Salaets, P.; Smolders, E. Trace element concentrations in mineral phosphate fertilizers used in Europe: A balanced survey. Sci. Total Environ. 2020, 712, 136419. [Google Scholar] [CrossRef] [PubMed]
- Zarcinas, B.A.; Pongsakul, P.; McLaughlin, M.J.; Cozens, G. Heavy metals in soils and crops in Southeast Asia. 2. Thailand. Environ. Geochem. Health 2004, 26, 359–371. [Google Scholar] [CrossRef]
- Zou, M.; Zhou, S.; Zhou, Y.; Jia, Z.; Guo, T.; Wang, J. Cadmium pollution of soil-rice ecosystems in rice cultivation dominated regions in China: A review. Environ. Pollut. 2021, 280, 116965. [Google Scholar] [CrossRef] [PubMed]
- McDowell, R.W.; Gray, C.W. Do soil cadmium concentrations decline after phosphate fertiliser application is stopped: A comparison of long-term pasture trials in New Zealand? Sci. Total Environ. 2022, 804, 150047. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Xiao, W.; Chen, D.; Hu, J.; Gao, N.; Huang, M.; Ye, X. Comparison of transcriptome differences between two rice cultivars differing in cadmium translocation from spike-neck to grain. Int. J. Mol. Sci. 2024, 25, 3592. [Google Scholar] [CrossRef]
- Scavo, S.; Oliveri, V. Zinc ionophores: Chemistry and biological applications. J. Inorg. Biochem. 2022, 228, 111691. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Đorđević, A.B.; Yimthiang, S.; Vesey, D.A.; Gobe, G.C. The NOAEL equivalent of environmental cadmium exposure associated with GFR reduction and chronic kidney disease. Toxics 2022, 10, 614. [Google Scholar] [CrossRef]
- EFSA European Food Safety Agency. Statement on tolerable weekly intake for cadmium. EFSA J. 2011, 9, 1975. [Google Scholar]
- Qing, Y.; Yang, J.; Zhu, Y.; Li, Y.; Zheng, W.; Wu, M.; He, G. Dose-response evaluation of urinary cadmium and kidney injury biomarkers in Chinese residents and dietary limit standards. Environ. Health 2021, 20, 75. [Google Scholar] [CrossRef] [PubMed]
- Leconte, S.; Rousselle, C.; Bodin, L.; Clinard, F.; Carne, G. Refinement of health-based guidance values for cadmium in the French population based on modelling. Toxicol. Lett. 2021, 340, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, H.R.; Flannery, B.M.; Crosby, L.M.; Pouillot, R.; Farakos, S.M.S.; Van Doren, J.M.; Dennis, S.; Fitzpatrick, S.; Middleton, K. Reassessment of the cadmium toxicological reference value for use in human health assessments of foods. Regul. Toxicol. Pharmacol. 2023, 144, 105487. [Google Scholar] [CrossRef] [PubMed]
- Nishijo, M.; Nogawa, K.; Suwazono, Y.; Kido, T.; Sakurai, M.; Nakagawa, H. Lifetime cadmium exposure and mortality for renal diseases in residents of the cadmium-polluted Kakehashi River Basin in Japan. Toxics 2020, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Kippler, M.; Lönnerdal, B.; Goessler, W.; Ekström, E.C.; Arifeen, S.E.; Vahter, M. Cadmium interacts with the transport of essential micronutrients in the mammary gland—A study in rural Bangladeshi women. Toxicology 2009, 257, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.; Harari, F.; Llanos, M.; Vahter, M.; Ronco, A.M. Maternal-child transfer of essential and toxic elements through breast milk in a mine-waste polluted area. Am. J. Perinatol. 2014, 31, 993–1002. [Google Scholar] [CrossRef]
- Satarug, S. Is chronic kidney disease due to cadmium exposure inevitable and can it be reversed? Biomedicines 2024, 12, 718. [Google Scholar] [CrossRef]
- Tsai, K.F.; Hsu, P.C.; Lee, C.T.; Kung, C.T.; Chang, Y.C.; Fu, L.M.; Ou, Y.C.; Lan, K.C.; Yen, T.H.; Lee, W.C. Association between enzyme-linked immunosorbent assay-measured kidney injury markers and urinary cadmium levels in chronic kidney disease. J. Clin. Med. 2021, 11, 156. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Vesey, D.A.; Ruangyuttikarn, W.; Nishijo, M.; Gobe, G.C.; Phelps, K.R. The source and pathophysiologic significance of excreted cadmium. Toxics 2019, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Moffett, D.B.; Mumtaz, M.M.; Sullivan, D.W., Jr.; Whittaker, M.H. Chapter 13, General Considerations of Dose-Effect and Dose-Response Relationships. In Handbook on the Toxicology of Metals, 5th ed.; Nordberg, G., Costa, M., Eds.; General Considerations; Academic Press: Cambridge, MA, USA, 2022; Volume I, pp. 299–317. [Google Scholar]
- Satarug, S.; Gobe, G.C.; Vesey, D.A.; Phelps, K.R. Cadmium and lead exposure, nephrotoxicity, and mortality. Toxics 2020, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.M.; Schiffl, H. Smoking status, cadmium, and chronic kidney disease. Ren. Replace. Ther. 2024, 10, 17. [Google Scholar] [CrossRef]
- Satarug, S.; Vesey, D.A.; Khamphaya, T.; Pouyfung, P.; Gobe, G.C.; Yimthiang, S. Estimation of the cadmium nephrotoxicity threshold from loss of glomerular filtration rate and albuminuria. Toxics 2023, 11, 755. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Taylor, A.W.; Riley, M.; Byles, J.; Liu, J.; Noakes, M. Association between dietary patterns, cadmium intake and chronic kidney disease among adults. Clin. Nutr. 2018, 37, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Chou, X.; Li, X.; Min, Z.; Ding, F.; Ma, K.; Shen, Y.; Sun, D.; Wu, Q. Sirtuin-1 attenuates cadmium-induced renal cell senescence through p53 deacetylation. Ecotoxicol. Environ. Saf. 2022, 245, 114098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, M.; Xie, R. Associations between cadmium exposure and whole-body aging: Mediation analysis in the NHANES. BMC Public Health 2023, 23, 1675. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, Y.; Liu, F.; Chen, H.; Tan, T.; Yao, P.; Tang, Y. Biological aging mediates the associations between urinary metals and osteoarthritis among U.S. adults. BMC Med. 2022, 20, 207. [Google Scholar] [CrossRef]
- Wiuf, A.; Steffen, J.H.; Becares, E.R.; Gronberg, C.; Mahato, D.R.; Rasmussen, S.G.F.; Andersson, M.; Croll, T.; Gotfryd, K.; Gourdon, P. The two-domain elevator-type mechanism of zinc-transporting ZIP proteins. Sci. Adv. 2022, 8, eabn4331. [Google Scholar] [CrossRef]
- Kuliyev, E.; Zhang, C.; Sui, D.; Hu, J. Zinc transporter mutations linked to acrodermatitis enteropathica disrupt function and cause mistrafficking. J. Biol. Chem. 2021, 296, 100269. [Google Scholar] [CrossRef] [PubMed]
- Valentine, R.A.; Jackson, K.A.; Christie, G.R.; Mathers, J.C.; Taylor, P.M.; Ford, D. ZnT5 variant B is a bidirectional zinc transporter and mediates zinc uptake in human intestinal Caco-2 cells. J. Biol. Chem. 2007, 282, 14389–14393. [Google Scholar] [CrossRef] [PubMed]
- Cragg, R.A.; Phillips, S.R.; Piper, J.M.; Varma, J.S.; Campbell, F.C.; Mathers, J.C.; Ford, D. Homeostatic regulation of zinc transporters in the human small intestine by dietary zinc supplementation. Gut 2005, 54, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Nishito, Y.; Kambe, T. Absorption mechanisms of iron, copper, and zinc: An overview. J. Nutr. Sci. Vitaminol. 2018, 64, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hoch, E.; Lin, W.; Chai, J.; Hershfinkel, M.; Fu, D.; Sekler, I. Histidine pairing at the metal transport site of mammalian ZnT transporters controls Zn2+ over Cd2+ selectivity. Proc. Natl. Acad. Sci. USA 2012, 109, 7202–7207. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, T.B.; Cousins, R.J. The multiple faces of the metal transporter ZIP14 (SLC39A14). J. Nutr. 2018, 148, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, G.J.; Aydemir, T.B.; Troche, C.; Martin, A.B.; Chang, S.M.; Cousins, R.J. Influence of ZIP14 (slc39A14) on intestinal zinc processing and barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G171–G178. [Google Scholar] [CrossRef] [PubMed]
- Park, J.D.; Cherrington, N.J.; Klaassen, C.D. Intestinal absorption of cadmium is associated with divalent metal transporter 1 in rats. Toxicol. Sci. 2002, 68, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Garrick, M.D.; Dolan, K.G.; Horbinski, C.; Ghio, A.J.; Higgins, D.; Porubcin, M.; Moore, E.G.; Hainsworth, L.N.; Umbreit, J.N.; Conrad, M.E.; et al. DMT1: A mammalian transporter for multiple metals. Biometals 2003, 16, 41–54. [Google Scholar] [CrossRef]
- Fujishiro, H.; Hamao, S.; Tanaka, R.; Kambe, T.; Himeno, S. Concentration-dependent roles of DMT1 and ZIP14 in cadmium absorption in Caco-2 cells. J. Toxicol. Sci. 2017, 42, 559–567. [Google Scholar] [CrossRef]
- Okazaki, Y. Iron from the gut: The role of divalent metal transporter 1. J. Clin. Biochem. Nutr. 2024, 74, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.J.; Shawki, A.; Ganz, T.; Nemeth, E.; Mackenzie, B. Functional properties of human ferroportin, a cellular iron exporter reactive also with cobalt and zinc. Am. J. Physiol. Cell Physiol. 2014, 306, C450–C459. [Google Scholar] [CrossRef] [PubMed]
- Kondaiah, P.; Yaduvanshi, P.S.; Sharp, P.A.; Pullakhandam, R. Iron and zinc homeostasis and interactions: Does enteric zinc excretion cross-talk with intestinal iron absorption? Nutrients 2019, 11, 1885. [Google Scholar] [CrossRef] [PubMed]
- Nose, Y.; Kim, B.E.; Thiele, D.J. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab. 2006, 4, 235–244. [Google Scholar] [CrossRef]
- Ohta, H.; Ohba, K. Involvement of metal transporters in the intestinal uptake of cadmium. J. Toxicol. Sci. 2020, 45, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Diaz de Barboza, G.; Guizzardi, S.; Tolosa de Talamoni, N. Molecular aspects of intestinal calcium absorption. World J. Gastroenterol. 2015, 21, 7142–7154. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.; Danko, T.; Bergeron, M.J.; Balazs, B.; Suzuki, Y.; Zsembery, A.; Hediger, M.A. Heavy metal cations permeate the TRPV6 epithelial cation channel. Cell Calcium 2011, 49, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.; Montalbetti, N.; Franz, M.C.; Graeter, S.; Simonin, A.; Hediger, M.A. Human TRPV5 and TRPV6: Key players in cadmium and zinc toxicity. Cell Calcium 2013, 54, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, R.H.; Chandler, J.S.; Meyer, S.A.; Smith, C.A.; Brindak, M.E.; Fullmer, C.S.; Penniston, J.T.; Kumar, R. Intestinal calcium transport and calcium extrusion processes at the basolateral membrane. J. Nutr. 1992, 122 (Suppl. S3), 662–671. [Google Scholar] [CrossRef]
- Wasserman, R.H. Vitamin D and the dual processes of intestinal calcium absorption. J. Nutr. 2004, 134, 3137–3139. [Google Scholar] [CrossRef]
- Bronner, F. Recent developments in intestinal calcium absorption. Nutr. Rev. 2009, 67, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Hoch, E.; Levy, M.; Hershfinkel, M.; Sekler, I. Elucidating the H+ Coupled Zn2+ Transport Mechanism of ZIP4; Implications in acrodermatitis enteropathica. Int. J. Mol. Sci. 2020, 21, 734. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhu, S.; Yang, Y.; Li, S.; Zhao, Z.; Wu, H. Caseinophosphopeptides overcome calcium phytate inhibition on zinc bioavailability by retaining zinc from coprecipitation as zinc/calcium phytate nanocomplexes. J. Agric. Food Chem. 2024, 72, 4757–4764. [Google Scholar] [CrossRef] [PubMed]
- Pérez de la Lastra, J.M.; Andrés-Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Theoretical three-dimensional zinc complexes with glutathione, amino acids and flavonoids. Stresses 2021, 1, 123–141. [Google Scholar] [CrossRef]
- Knez, M.; Graham, R.D.; Welch, R.M.; Stangoulis, J.C. New perspectives on the regulation of iron absorption via cellular zinc concentrations in humans. Crit. Rev. Food Sci. Nutr. 2017, 57, 2128–2143. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study (AREDS) Research Group. A randomized, placebo controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, H.; Miyazaki, T.; Nodera, M.; Miyajima, Y.; Suzuki, T.; Kido, T.; Suka, M. Zinc-excess intake causes the deterioration of renal function accompanied by an elevation in systemic blood pressure primarily through superoxide radical-induced oxidative stress. Int. J. Toxicol. 2014, 33, 288–296. [Google Scholar] [CrossRef]
- Fujita, Y.; el Belbasi, H.I.; Min, K.S.; Onosaka, S.; Okada, Y.; Matsumoto, Y.; Mutoh, N.; Tanaka, K. Fate of cadmium bound to phytochelatin in rats. Res. Commun. Chem. Pathol. Pharmacol. 1993, 82, 357–365. [Google Scholar] [PubMed]
- Langelueddecke, C.; Roussa, E.; Fenton, R.A.; Thévenod, F. Expression and function of the lipocalin-2 (24p3/NGAL) receptor in rodent and human intestinal epithelia. PLoS ONE 2013, 8, e71586. [Google Scholar] [CrossRef]
- Langelueddecke, C.; Lee, W.K.; Thévenod, F. Differential transcytosis and toxicity of the hNGAL receptor ligands cadmium-metallothionein and cadmium-phytochelatin in colon-like Caco-2 cells: Implications for in vivo cadmium toxicity. Toxicol. Lett. 2014, 226, 228–235. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Nomiyama, T.; Kumagai, N.; Dekio, F.; Uemura, T.; Takebayashi, T.; Nishiwaki, Y.; Matsumoto, Y.; Sano, Y.; Hosoda, K.; et al. Uptake of cadmium in meals from the digestive tract of young non-smoking Japanese female volunteers. J. Occup. Health 2003, 45, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, H.; Oguma, E.; Sasaki, S.; Miyamoto, K.; Ikeda, Y.; Machida, M.; Kayama, F. Comprehensive study of the effects of age, iron deficiency, diabetes mellitus, and cadmium burden on dietary cadmium absorption in cadmium-exposed female Japanese farmers. Toxicol. Appl. Pharmacol. 2004, 196, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Thévenod, F.; Fels, J.; Lee, W.K.; Zarbock, R. Channels, transporters and receptors for cadmium and cadmium complexes in eukaryotic cells: Myths and facts. Biometals 2019, 32, 469–489. [Google Scholar] [CrossRef] [PubMed]
- Thévenod, F.; Lee, W.K. Cadmium transport by mammalian ATP-binding cassette transporters. Biometals 2024, 37, 697–719. [Google Scholar] [CrossRef] [PubMed]
- Beyersmann, D.; Haase, H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals 2001, 14, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Krezel, A.; Hao, Q.; Maret, W. The zinc/thiolate redox biochemistry of metallothionein and the control of zinc ion fluctuations in cell signaling. Arch. Biochem. Biophys. 2007, 463, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Hübner, C.; Haase, H. Interactions of zinc- and redox-signaling pathways. Redox Biol. 2021, 41, 101916. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Smith, M.J.; Siow, R.C.M.; Aarsland, D.; Maret, W.; Mann, G.E. Interactions between zinc and NRF2 in vascular redox signalling. Biochem. Soc. Trans. 2024, 52, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef]
- Hara, T.; Takeda, T.A.; Takagishi, T.; Fukue, K.; Kambe, T.; Fukada, T. Physiological roles of zinc transporters: Molecular and genetic importance in zinc homeostasis. J. Physiol. Sci. 2017, 67, 283–301. [Google Scholar] [CrossRef]
- Hara, T.; Yoshigai, E.; Ohashi, T.; Fukada, T. Zinc transporters as potential therapeutic targets: An updated review. J. Pharmacol. Sci. 2022, 148, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Nishito, Y.; Kambe, T. Zinc transporter 1 (ZNT1) expression on the cell surface is elaborately controlled by cellular zinc levels. J. Biol. Chem. 2019, 294, 15686–15697. [Google Scholar] [CrossRef]
- Laity, J.H.; Andrews, G.K. Understanding the mechanisms of zinc-sensing by metal-response element binding transcription factor-1 (MTF-1). Arch. Biochem. Biophys. 2007, 463, 201–210. [Google Scholar] [CrossRef]
- Mor, M.; Beharier, O.; Cook, D.I.; Campbell, C.R.; Gheber, L.A.; Katz, A.; Moran, A.; Etzion, Y. ZnT1 induces a crosstalk between T-type and L-type calcium channels through interactions with Raf-1 kinase and the calcium channel beta2 subunit. Metallomics 2023, 15, mfad031. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; He, B.; Gao, Y.; Wang, X.; Liu, X.; Sun, L. Structural insights into the calcium-coupled zinc export of human ZnT1. Sci. Adv. 2024, 10, eadk5128. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuča, K.; Musílek, K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef]
- Harraz, M.M.; Marden, J.J.; Zhou, W.; Zhang, Y.; Williams, A.; Sharov, V.S.; Nelson, K.; Luo, M.; Paulson, H.; Schoneich, C.; et al. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J. Clin. Investig. 2008, 118, 659–670. [Google Scholar] [CrossRef]
- Li, M.S.; Adesina, S.E.; Ellis, C.L.; Gooch, J.L.; Hoover, R.S.; Williams, C.R. NADPH oxidase-2 mediates zinc deficiency-induced oxidative stress and kidney damage. Am. J. Physiol. Cell Physiol. 2017, 312, C47–C55. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Ryu, M.S. Cellular zinc deficiency impairs heme biosynthesis in developing erythroid progenitors. Nutrients 2023, 15, 281. [Google Scholar] [CrossRef]
- Petering, D.H. Reactions of the Zn proteome with Cd2+ and other xenobiotics: Trafficking and toxicity. Chem. Res. Toxicol. 2016, 30, 189–202. [Google Scholar] [CrossRef]
- Kumagai, A.; Ando, R.; Miyatake, H.; Greimel, P.; Kobayashi, T.; Hirabayashi, Y.; Shimogori, T.; Miyawaki, A. A bilirubin-inducible fluorescent protein from eel muscle. Cell 2013, 153, 1602–1611. [Google Scholar] [CrossRef]
- Takeda, T.A.; Mu, A.; Tai, T.T.; Kitajima, S.; Taketani, S. Continuous de novo biosynthesis of haem and its rapid turnover to bilirubin are necessary for cytoprotection against cell damage. Sci. Rep. 2015, 5, 10488. [Google Scholar] [CrossRef] [PubMed]
- Vitek, L.; Hinds, T.D., Jr.; Stec, D.E.; Tiribelli, C. The physiology of bilirubin: Health and disease equilibrium. Trends Mol. Med. 2023, 29, 315–328. [Google Scholar] [CrossRef]
- Xue, J.; Wang, S.; Wu, J.; Hannafon, B.N.; Ding, W.Q. Zinc at sub-cytotoxic concentrations induces heme oxygenase-1 expression in human cancer cells. Cell Physiol. Biochem. 2013, 32, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Moulis, J.M. Cellular mechanisms of cadmium toxicity related to the homeostasis of essential metals. Biometals 2010, 23, 877–896. [Google Scholar] [CrossRef] [PubMed]
- Nzengue, Y.; Candéias, S.M.; Sauvaigo, S.; Douki, T.; Favier, A.; Rachidi, W.; Guiraud, P. The toxicity redox mechanisms of cadmium alone or together with copper and zinc homeostasis alteration: Its redox biomarkers. J. Trace Elem. Med. Biol. 2011, 25, 171–180. [Google Scholar] [CrossRef]
- Moulis, J.M.; Bourguinon, J.; Catty, P. Chapter 23 Cadmium. In Binding, Transport and Storage of Metal Ions in Biological Cells; Wolfgang, M., Anthony, W., Eds.; RSC Metallobiology Series No. 2; The Royal Society of Chemistry: London, UK, 2014; pp. 695–746. [Google Scholar]
- Kusak, R.; Nasiadek, M.; Stragierowicz, J.; Hanke, W.; Kilanowicz, A. Changes in endogenous essential metal homeostasis in the liver and kidneys during a six-month follow-up period after Subchronic Cadmium Exposure. Int. J. Mol. Sci. 2024, 25, 3829. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Garrett, S.H.; Somji, S.; Sens, M.A.; Sens, D.A. Zinc, zinc transporters, and cadmium cytotoxicity in a cell culture model of human urothelium. Toxics 2021, 9, 94. [Google Scholar] [CrossRef]
- Kessels, J.E.; Wessels, I.; Haase, H.; Rink, L.; Uciechowski, P. Influence of DNA-methylation on zinc homeostasis in myeloid cells: Regulation of zinc transporters and zinc binding proteins. J. Trace Elem. Med. Biol. 2016, 37, 125–133. [Google Scholar] [CrossRef]
- Nagamatsu, S.; Nishito, Y.; Yuasa, H.; Yamamoto, N.; Komori, T.; Suzuki, T.; Yasui, H.; Kambe, T. Sophisticated expression responses of ZNT1 and MT in response to changes in the expression of ZIPs. Sci. Rep. 2022, 12, 7334. [Google Scholar] [CrossRef]
- Verzelloni, P.; Urbano, T.; Wise, L.A.; Vinceti, M.; Filippini, T. Cadmium exposure and cardiovascular disease risk: A systematic review and dose-response meta-analysis. Environ. Pollut. 2024, 345, 123462. [Google Scholar] [CrossRef]
- Chen, S.; Shen, R.; Shen, J.; Lyu, L.; Wei, T. Association of blood cadmium with all-cause and cause-specific mortality in patients with hypertension. Front. Public Health 2023, 11, 1106732. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, D.; Shi, F.; Wang, F.; Liu, X.; Wen, H.; Mubarik, S.; Yu, C. Association of serum 25(OH)D, cadmium, CRP with all-cause, cause-specific mortality: A prospective cohort study. Front. Nutr. 2022, 9, 803985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Ma, Z.; Dang, Y.; Yang, Y.; Cao, S.; Ouyang, C.; Shi, X.; Pan, J.; Hu, X. Associations of urinary and blood cadmium concentrations with all-cause mortality in US adults with chronic kidney disease: A prospective cohort study. Environ. Sci. Pollut. Res. Int. 2023, 30, 61659–61671. [Google Scholar] [CrossRef] [PubMed]
- Tägt, J.; Helte, E.; Donat-Vargas, C.; Larsson, S.C.; Michaëlsson, K.; Wolk, A.; Vahter, M.; Kippler, M.; Åkesson, A. Long-term cadmium exposure and fractures, cardiovascular disease, and mortality in a prospective cohort of women. Environ. Int. 2022, 161, 107114. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Wang, C.W.; Wu, D.W.; Lee, W.H.; Chen, Y.C.; Li, C.H.; Tsai, C.C.; Lin, W.Y.; Chen, S.C.; Hung, C.H.; et al. Association of heavy metals with overall mortality in a Taiwanese population. Nutrients 2021, 13, 2070. [Google Scholar] [CrossRef]
- Chiu, L.C.; Lee, C.S.; Hsu, P.C.; Li, H.H.; Chan, T.M.; Hsiao, C.C.; Kuo, S.C.; Ko, H.W.; Lin, S.M.; Wang, C.H.; et al. Urinary cadmium concentration is associated with the severity and clinical outcomes of COVID-19: A bicenter observational cohort study. Environ. Health 2024, 23, 29. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Deng, Y.P.; Xu, J.; Zhu, F.M.; He, Q.Y.; Tang, M.M.; Liu, Y.; Yang, J.; Liu, H.Y.; Fu, L.; et al. Association of blood cadmium concentration with chronic obstructive pulmonary disease progression: A prospective cohort study. Respir. Res. 2024, 25, 91. [Google Scholar] [CrossRef] [PubMed]
- Nogawa, K.; Yamada, Y.; Honda, R.; Tsuritani, I.; Kobayashi, E.; Ishizaki, M. Copper and zinc levels in serum and urine of cadmium-exposed people with special reference to renal tubular damage. Environ. Res. 1984, 33, 29–38. [Google Scholar] [CrossRef]
- Nakajima, M.; Kobayashi, E.; Suwazono, Y.; Uetani, M.; Oishi, M.; Inaba, T.; Kido, T.; Shaikh, Z.A.; Nogawa, K. Excretion of urinary cadmium, copper, and zinc in cadmium-exposed and non-exposed subjects, with special reference to urinary excretion of beta2-microglobulin and metallothionein. Biol. Trace Elem. Res. 2005, 108, 17–31. [Google Scholar] [CrossRef]
- Satarug, S.; Nishijo, M.; Ujjin, P.; Moore, M.R. Chronic exposure to low-level cadmium induced zinc-copper dysregulation. J. Trace Elem. Med. Biol. 2018, 46, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.Y.; Yim, D.H.; Huang, M.; Park, C.H.; Kim, G.B.; Yu, S.D.; Choi, B.S.; Park, J.D.; Kim, Y.D.; Kim, H. Copper-zinc imbalance induces kidney tubule damage and oxidative stress in a population exposed to chronic environmental cadmium. Int. Arch. Occup. Environ. Health 2020, 93, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Romero, M.; Rojas-Lima, E.; Rubio-Gutiérrez, J.C.; Aztatzi-Aguilar, O.G.; Narváez-Morales, J.; Esparza-García, M.; Barrera-Hernández, Á.; Mejia, M.Á.; Mendez-Hernández, P.; Medeiros, M.; et al. Associations among environmental exposure to trace elements and biomarkers of early kidney damage in the pediatric population. Biometals 2024, 37, 721–737. [Google Scholar] [CrossRef]
- Satarug, S.; Ujjin, P.; Vanavanitkun, Y.; Baker, J.R.; Moore, M.R. Influence of body iron store status and cigarette smoking on cadmium body burden of healthy Thai women and men. Toxicol. Lett. 2004, 148, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, M.E.; Wong, L.Y.; Osterloh, J.D. Smoking status and urine cadmium above levels associated with subclinical renal effects in U.S. adults without chronic kidney disease. Int. J. Hyg. Environ. Health 2011, 214, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, S.M.; Adams, S.V.; Shafer, M.; Meliker, J.R.; Li, W.; Luo, J.; Neuhouser, M.L.; Newcomb, P.A. Urinary cadmium and estimated dietary cadmium in the Women’s Health Initiative. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Caffrey, J.L.; Lin, J.W.; Bayliss, D.; Faramawi, M.F.; Bateson, T.F.; Sonawane, B. Increased risk of cancer mortality associated with cadmium exposures in older Americans with low zinc intake. J. Toxicol. Environ. Health A 2013, 76, 1–15. [Google Scholar] [CrossRef]
- Lin, Y.S.; Caffrey, J.L.; Chang, M.H.; Dowling, N.; Lin, J.W. Cigarette smoking, cadmium exposure, and zinc intake on obstructive lung disorder. Respir. Res. 2010, 11, 53. [Google Scholar] [CrossRef]
- Lin, Y.S.; Ho, W.C.; Caffrey, J.L.; Sonawane, B. Low serum zinc is associated with elevated risk of cadmium nephrotoxicity. Environ. Res. 2014, 134, 33–38. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, H.; Yang, Y.; Cao, Y.; Liang, D. Association between dietary zinc intake and increased renal function in US adults. Biol. Trace Elem. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, M.; Schmitz-Valckenberg, S.; Chakravarthy, U. Age-related macular degeneration: A review. JAMA 2024, 331, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Harju, N. Regulation of oxidative stress and inflammatory responses in human retinal pigment epithelial cells. Acta Ophthalmol. 2022, 100 (Suppl. S273), 3–59. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Sinha, D.; Blasiak, J.; Kauppinen, A.; Veréb, Z.; Salminen, A.; Boulton, M.E.; Petrovski, G. Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy 2013, 9, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.R.; Ferrington, D.A. Perspective on AMD pathobiology: A bioenergetic crisis in the RPE. Investig. Ophthalmol. Vis. Sci. 2018, 59, AMD41–AMD47. [Google Scholar] [CrossRef] [PubMed]
- Erie, J.C.; Good, J.A.; Butz, J.A.; Pulido, J.S. Reduced zinc and copper in the retinal pigment epithelium and choroid in age-related macular degeneration. Am. J. Ophthalmol. 2009, 147, 276–282.e1. [Google Scholar] [CrossRef]
- Leung, K.W.; Liu, M.; Xu, X.; Seiler, M.J.; Barnstable, C.J.; Tombran-Tink, J. Expression of ZnT and ZIP zinc transporters in the human RPE and their regulation by neurotrophic factors. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Twarog, M.; Li, N.; Banks, A.; Schustak, J.; Bao, Y.; Huang, Q.; Medley, Q.G. Zinc transport from the endoplasmic reticulum to the cytoplasm via Zip7 is necessary for barrier dysfunction mediated by inflammatory signaling in RPE cells. PLoS ONE 2022, 17, e0271656. [Google Scholar] [CrossRef] [PubMed]
- Wills, N.K.; Ramanujam, V.M.; Chang, J.; Kalariya, N.; Lewis, J.R.; Weng, T.X.; van Kuijk, F.J. Cadmium accumulation in the human retina: Effects of age, gender, and cellular toxicity. Exp. Eye Res. 2008, 86, 41–51. [Google Scholar] [CrossRef]
- Wills, N.K.; Ramanujam, V.M.; Kalariya, N.; Lewis, J.R.; van Kuijk, F.J. Copper and zinc distribution in the human retina: Relationship to cadmium accumulation, age, and gender. Exp. Eye Res. 2008, 87, 80–88. [Google Scholar] [CrossRef]
- Wu, E.W.; Schaumberg, D.A.; Park, S.K. Environmental cadmium and lead exposures and age-related macular degeneration in U.S. adults: The National Health and Nutrition Examination Survey 2005 to 2008. Environ. Res. 2014, 133, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.C.; Cho, E.; Jee, D. Association between blood cadmium level and age-related macular degeneration in a representative Korean population. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5702–5710. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Zhao, D.; Cho, J.; Guallar, E. Cadmium exposure and age-related macular degeneration. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Kikuchi, M.; Wisedpanichkij, R.; Li, B.; Takeda, K.; Na-Bangchang, K.; Moore, M.R.; Hirayama, K.; Shibahara, S. Prevention of cadmium accumulation in retinal pigment epithelium with manganese and zinc. Exp. Eye Res. 2008, 87, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y.; Clemons, T.E.; Agrón, E.; Sperduto, R.D.; Sangiovanni, J.P.; Kurinij, N.; Davis, M.D.; Age-Related Eye Disease Study Research Group. Long-term effects of vitamins C and E, beta-carotene, and zinc on age-related macular degeneration: AREDS report no. 35. Ophthalmology 2013, 120, 1604–1611.e4. [Google Scholar] [CrossRef] [PubMed]
- Aoshima, K. Epidemiology of renal tubular dysfunction in the inhabitants of a cadmium-polluted area in the Jinzu river basin in Toyama Prefecture. Tohoku J. Exp. Med. 1987, 152, 151–172. [Google Scholar] [CrossRef]
- Nambunmee, K.; Honda, R.; Nishijo, M.; Swaddiwudhipong, W.; Nakagawa, H.; Ruangyuttikarn, W. Bone resorption acceleration and calcium reabsorption impairment in a Thai population with high cadmium exposure. Toxicol. Mech. Methods 2010, 20, 7–13. [Google Scholar] [CrossRef]
- Nishijo, M.; Nambunmee, K.; Suvagandha, D.; Swaddiwudhipong, W.; Ruangyuttikarn, W.; Nishino, Y. Gender-specific impact of cadmium exposure on bone metabolism in older people living in a cadmium-polluted area in Thailand. Int. J. Environ. Res. Public Health 2017, 14, 401. [Google Scholar] [CrossRef] [PubMed]
- Nambunmee, K.; Nishijo, M.; Swaddiwudhipong, W.; Ruangyuttikarn, W. Bone fracture risk and renal dysfunction in a highly cadmium exposed Thai population. J. Res. Health Sci. 2018, 18, e00419. [Google Scholar]
- Ellis, J.K.; Athersuch, T.J.; Thomas, L.D.; Teichert, F.; Pérez-Trujillo, M.; Svendsen, C.; Spurgeon, D.J.; Singh, R.; Järup, L.; Bundy, J.G.; et al. Metabolic profiling detects early effects of environmental and lifestyle exposure to cadmium in a human population. BMC Med. 2012, 10, 61. [Google Scholar] [CrossRef]
- Suvagandha, D.; Nishijo, M.; Swaddiwudhipong, W.; Honda, R.; Ohse, M.; Kuhara, T.; Nakagawa, H.; Ruangyuttikarn, W. A biomarker found in cadmium exposed residents of Thailand by metabolome analysis. Int. J. Environ. Res. Public Health 2014, 11, 3661–3677. [Google Scholar] [CrossRef] [PubMed]
- Thévenod, F.; Lee, W.K.; Garrick, M.D. Iron and cadmium entry into renal mitochondria: Physiological and toxicological implications. Front. Cell Dev. Biol. 2020, 8, 848. [Google Scholar] [CrossRef] [PubMed]
- Ran, D.; Zhou, D.; Liu, G.; Ma, Y.; Ali, W.; Yu, R.; Wang, Q.; Zhao, H.; Zhu, J.; Zou, H.; et al. Reactive oxygen species control osteoblast apoptosis through SIRT1/PGC-1α/P53Lys382 signaling, mediating the onset of Cd-induced osteoporosis. J. Agric. Food Chem. 2023, 71, 5991–6002. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, T.B.; Troche, C.; Kim, J.; Kim, M.H.; Teran, O.Y.; Leeuwenburgh, C.; Cousins, R.J. Aging amplifies multiple phenotypic defects in mice with zinc transporter Zip14 (Slc39a14) deletion. Exp. Gerontol. 2016, 85, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Tsukamoto, M.; Saito, M.; Hojyo, S.; Fukada, T.; Takami, M.; Furuichi, T. Disruption of the mouse Slc39a14 gene encoding zinc transporter ZIP14 is associated with decreased bone mass, likely caused by enhanced bone resorption. FEBS Open Bio 2018, 8, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Wang, H.; Wu, Z.; Wang, P.; Yang, L.; Li, X.; Sun, K.; Zhu, X.; Zhang, R. Effects of cadmium on osteoblast cell line: Exportin 1 accumulation, p-JNK activation, DNA damage and cell apoptosis. Ecotoxicol. Environ. Saf. 2021, 208, 111668. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhuo, L.; Ran, D.; Ma, Y.; Luo, T.; Zhao, H.; Song, R.; Zou, H.; Zhu, J.; Gu, J.; et al. Cadmium induces apoptosis via generating reactive oxygen species to activate mitochondrial p53 pathway in primary rat osteoblasts. Toxicology 2020, 446, 152611. [Google Scholar] [CrossRef]
- Seo, H.J.; Cho, Y.E.; Kim, T.; Shin, H.I.; Kwun, I.S. Zinc may increase bone formation through stimulating cell proliferation, alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells. Nutr. Res. Pract. 2010, 4, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Li, Z.; Wang, L.; Qiu, D.; Zhang, X.; Xin, X.; Cai, Z.; Lei, B. Metabolic signatures for safety assessment of low-level cadmium exposure on human osteoblast-like cells. Ecotoxicol. Environ. Saf. 2021, 207, 111257. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, G.; Gu, S.; Jin, T.; Shao, C. Effects of cadmium on osteoblasts and osteoclasts in vitro. Environ. Toxicol. Pharmacol. 2009, 28, 232–236. [Google Scholar] [CrossRef]
- Amin, N.; Clark, C.C.T.; Taghizadeh, M.; Djafarnejad, S. Zinc supplements and bone health: The role of the RANKL-RANK axis as a therapeutic target. J. Trace Elem. Med. Biol. 2020, 57, 126417. [Google Scholar] [CrossRef]
- Liu, W.; Dai, N.; Wang, Y.; Xu, C.; Zhao, H.; Xia, P.; Gu, J.; Liu, X.; Bian, J.; Yuan, Y.; et al. Role of autophagy in cadmium-induced apoptosis of primary rat osteoblasts. Sci. Rep. 2016, 6, 20404. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Shen, H.; Zhu, J.; Zhu, Y.; He, Y.; Li, Z.; Lu, H. Geniposide attenuates cadmium-induced oxidative stress injury via Nrf2 signaling in osteoblasts. Mol. Med. Rep. 2019, 20, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Hadley, K.B.; Newman, S.M.; Hunt, J.R. Dietary zinc reduces osteoclast resorption activities and increases markers of osteoblast differentiation, matrix maturation, and mineralization in the long bones of growing rats. J. Nutr. Biochem. 2010, 21, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Lönnerdal, B. Role of zinc in cellular zinc trafficking and mineralization in a murine osteoblast-like cell line. J. Nutr. Biochem. 2011, 22, 172–178. [Google Scholar] [CrossRef]
- Suzuki, T.; Katsumata, S.; Matsuzaki, H.; Suzuki, K. Dietary zinc deficiency induces oxidative stress and promotes tumor necrosis factor-α- and interleukin-1β-induced RANKL expression in rat bone. J. Clin. Biochem. Nutr. 2016, 58, 122–129. [Google Scholar] [CrossRef]


| Nutrient/ Contaminant | Recommended Zinc Intake Level versus Tolerable Cadmium Level | Reference |
|---|---|---|
| Zinc (atomic weight 65.4) | An adult female (60 kg): 8 mg/day, or 0.13 mg per kg of body weight per day. An adult male (70 kg): 11 mg/day, or 0.16 mg per kg of body weight per day. Infants: 2–3 mg/day. Children: 5–9 mg/day. Pregnant women: 11–12 mg/day. Lactating women: 12–13 mg/day. | The U.S. National Academy of Sciences Institute of Medicine (IOM) [8] |
| Cadmium (atomic weight 112.4) | A tolerable lifetime intake of 2 g or an intake of 0.83 μg per kg body weight per day (58 µg per day for a 70 kg person). a Assumption: Absorption rate at 3–7%; urinary excretion of β2-microglobulin (β2M) levels ≥ 300 µg/g creatinine indicated kidney toxicity with a threshold level of 5.24 μg Cd/g creatinine. | JECFA [38] |
| Cadmium | BMDL or the NOAEL equivalent value of Cd exposure was 0.0104 µg/L of filtrate (≈0.01−0.02 µg/g creatinine). Assumption: Cd accumulation level producing a 5% decline in the eGFR represented the upper limit of a permissible Cd exposure level. | Satarug et al., 2022 [45] |
| Cadmium | A tolerable intake of 0.36 μg/kg body weight per day (25.2 µg per day for a 70 kg person) Assumption: Urinary β2M excretion levels ≥ 300 µg/g creatinine indicated kidney toxicity with a threshold level of 1 μg Cd/g creatinine | EFSA [46] |
| Cadmium | A tolerable intake level of 0.28 μg per kg body weight per day (16.8 µg per day for a 60 kg person). Assumption: Urinary β2M excretion levels ≥ 300 µg/g creatinine to indicate kidney toxicity with a threshold level of 3.07 μg Cd/g creatinine. | Qing et al., 2021 [47] |
| Cadmium | A tolerable intake level of 0.35 μg Cd per kg body weight per day, assuming a bone toxicity threshold level of 0.5 μg/g creatinine. | Leconte et al., 2021 [48] |
| Cadmium | By the reverse dosimetry PBPK model, tolerable intake levels range between 0.21 and 0.36 μg per kg body weight per day, assuming a similar bone and kidney toxicity threshold level of 0.5 μg/g creatinine. | Schaefer et al., 2023 [49] |
| Metal | Transporter | Localization | Description |
|---|---|---|---|
| Zn | SLC39A4 (ZIP4) | Apical membrane | Two zinc-binding sites in histidine-rich motif [64]. Transport dietary Zn into enterocytes. Functional loss due to mutation causes Zn deficiency [65]. |
| Zn | SLC30A5 (ZnT5) variant B | Apical membrane | Transport Zn into enterocytes [66]. Zn supplementation depresses ZIP4 and ZnT5B protein levels in the ileum [67]. |
| Zn | SLC30A1 (ZnT1) | Basolateral membrane | Transport Zn from cytoplasm to extracellular medium or intracellular vesicles [68,69]. Histidine-rich motif confers Zn selectivity over Cd. This Cd discrimination is unique to mammalian ZnT1 [69]. |
| Fe, Mn, Cd | SLC39A14 (ZIP14) | Basolateral membrane | Trafficking Zn to tight junctions, especially in jejunum for maintenance of intestinal barrier [70,71]. |
| Fe, Cd Cu, Zn | SLC11A2 (DMT1) | Apical membrane | Same high affinity for Cd as it has for Fe (Km 0.5~1 μM); high abundance in duodenum [72,73,74,75,76]. |
| Fe, Zn, Co | SLC40A1 (FPN1) | Basolateral membrane | Transport Fe, Zn, and Co but not Cu, Cd, or Mn from basolateral membrane into portal blood [77,78]. |
| Cu, Fe, Zn | SLC31A1 (CTR1) | Apical membrane, Early endosome | Transport dietary Cu into enterocytes [68,79]. |
| Cu, Cd | ATP7A | Trans-Golgi network, Cytosol Basolateral membrane | Transport Cu into portal blood, and ATP7A mutations are associated with Menkes disease [68]. May contribute to Cd absorption [80]. |
| Ca, Cd | TRPV5, TRPV6 | Apical membrane | Transport Ca2+ into enterocytes [81] and may provide Cd entry routes into enterocytes [82,83]. |
| Ca | Calbindin-D9k | Cytoplasm | Transport Ca to basolateral membrane and extrusion of Ca into portal blood [83,84,85]. Expression in ileum is induced by 1,25-dihydroxycholecalciferol [85]. |
| Study Population | Effects Observed and Cadmium Exposure Levels | Reference |
|---|---|---|
| United States NHANES, 1999–2012, n = 12,208 with hypertension Mean follow-up 10.8 years | Among those with hypertension, respective HRs (95% CI) for all-cause, CVD, and Alzheimer’s disease mortality were 1.85 (1.59, 2.14), 1.76 (1.33, 2.34), and 3.41 (1.54, 7.51), comparing blood Cd levels ≥ 0.80 versus ≤0.25 μg/L. HR (95% CI) for CVD mortality among non-smokers who had hypertension was 2.12 (1.36, 3.30) | Chen et al., 2023 [127] |
| United States NHANES, 2001–2010, n = 2945 with diabetes Mean follow-up period, 9.1 years | Among those with diabetes, HR (95% CI) for all-cause mortality was 1.49 (1.06, 2.09) at urinary Cd levels > 0.60 μg/L. HR (95% CI) for all-cause mortality was 1.65 (1.24, 2.19) at serum CRP levels > 0.49 mg/dL. HR (95% CI) for cancer mortality was 3.25 (1.82, 5.80) at serum CRP levels > 0.49 mg/dL. | Liu et al., 2022 [128] |
| United States NHANES, 1999–2014, n = 1825 with CKD Mean follow-up period, 6.8 years | Among those with CKD, HR (95% CI) for all-cause mortality was 1.75 (1.28, 2.39) at urinary Cd levels ≥ 0.60 μg/g creatinine. HR (95%CI) for death from any causes was 1.59 (1.17, 2.15) at blood Cd levels ≥ 0.70 μg/L. | Zhang et al., 2023 [129] |
| Sweden n = 4024 women, aged 56–85 years Mean follow-up 10.5 years | Respective HRs (95% CI) for all-cause mortality and any fracture were 1.38 (1.10, 1.74) and 1.20 (1.01,1.43), comparing top tertile of urinary Cd (median 0.54 µg/g creatinine) with bottom tertial (median urinary Cd of 0.20 µg/g creatinine). | Tägt et al., 2022 [130] |
| Taiwan n = 2497 (1001 males, 1496 females), Median follow-up 3.5 years. | HR (95%CI) for all-cause mortality was 1.35 (1.09, 1.68) per 1 μg/L increment of urinary Cd. HR (95%CI) for all-cause mortality was 1.35 (1.15, 1.58) per 1 μg/dL increment of urinary copper. | Liu et al., 2021 [131] |
| Taiwan n = 252 with severe COVID-19, n = 322 with non-severe COVID-19 June 2022–July 2023 | Among subjects with COVID-19, OR (95%CI) for severe symptoms was 5.35 (1.12, 25.6) at urinary Cd levels > 2.05 μg/g creatinine. | Chiu et al., 2024 [132] |
| China n = 196 COPD patients Enrolment: September 2020–June 2022, Follow-up 2 years | Per every 1 µg/L increase in blood Cd, respective ORs (95% CI) for acute exacerbation, hospitalization, and mortality were 2.24 (1.17, 4.29), 1.28 (1.13, 1.56), and 1.69 (1.06, 2.70). After adjustment for potential confounders, respective ORs (95%CI) for acute exacerbation and mortality were 2.26 (1.03, 4.24) and 2.12 (1.13, 7.11) per 1 µg/L increment of blood Cd. | Sun et al., 2024 [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirovic, A.; Cirovic, A.; Yimthiang, S.; Vesey, D.A.; Satarug, S. Modulation of Adverse Health Effects of Environmental Cadmium Exposure by Zinc and Its Transporters. Biomolecules 2024, 14, 650. https://doi.org/10.3390/biom14060650
Cirovic A, Cirovic A, Yimthiang S, Vesey DA, Satarug S. Modulation of Adverse Health Effects of Environmental Cadmium Exposure by Zinc and Its Transporters. Biomolecules. 2024; 14(6):650. https://doi.org/10.3390/biom14060650
Chicago/Turabian StyleCirovic, Ana, Aleksandar Cirovic, Supabhorn Yimthiang, David A. Vesey, and Soisungwan Satarug. 2024. "Modulation of Adverse Health Effects of Environmental Cadmium Exposure by Zinc and Its Transporters" Biomolecules 14, no. 6: 650. https://doi.org/10.3390/biom14060650
APA StyleCirovic, A., Cirovic, A., Yimthiang, S., Vesey, D. A., & Satarug, S. (2024). Modulation of Adverse Health Effects of Environmental Cadmium Exposure by Zinc and Its Transporters. Biomolecules, 14(6), 650. https://doi.org/10.3390/biom14060650








