Hereditary Transthyretin Amyloidosis (hATTR) with Polyneuropathy Clusters Are Located in Ancient Mining Districts: A Possible Geochemical Origin of the Disease
Abstract
:1. Introduction
2. Materials and Methods
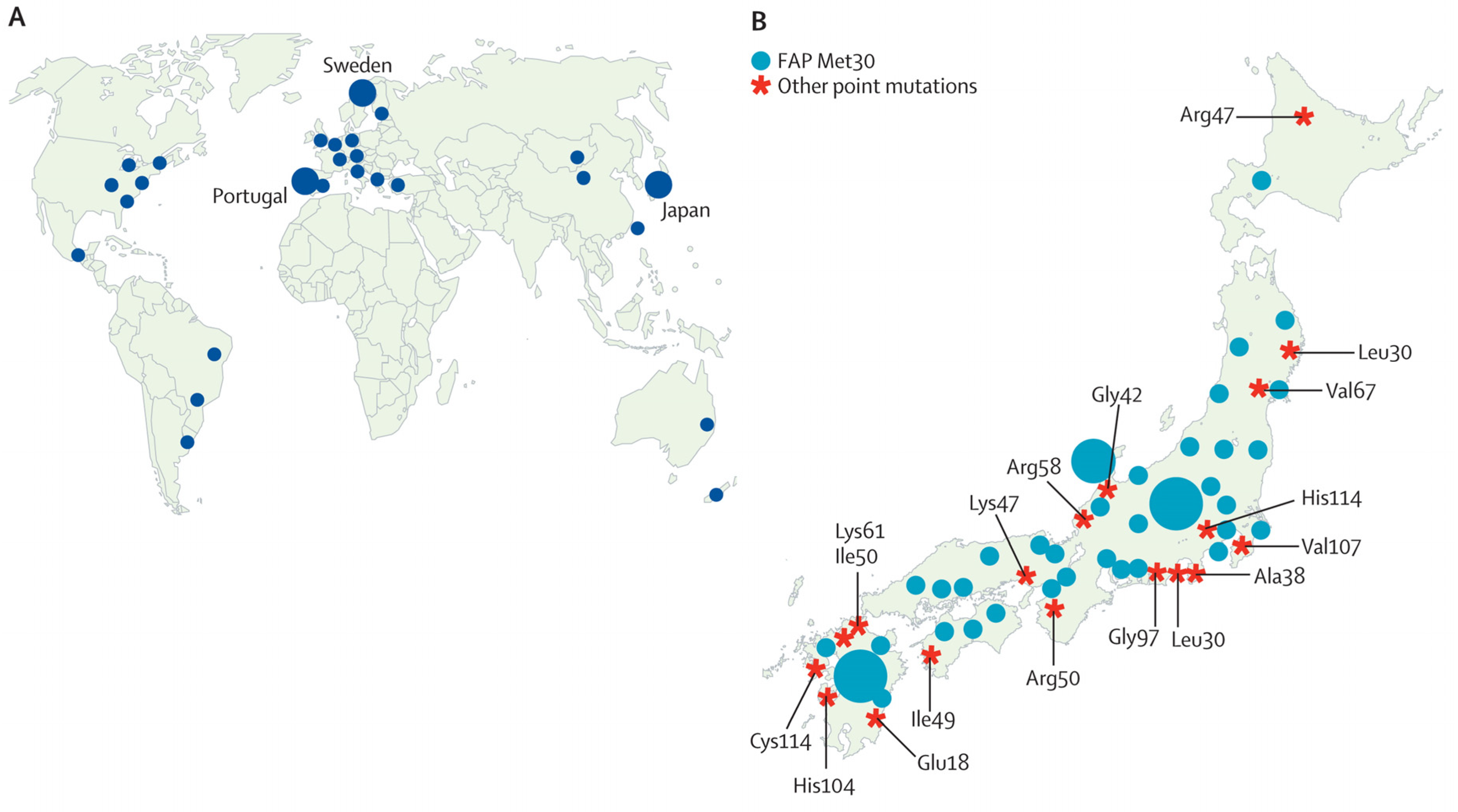
3. Results
3.1. Japan
3.2. Portugal
3.3. Sweden
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Plante-Bordeneuve, V.; Said, G. Familial amyloid polyneuropathy. Lancet Neurol. 2011, 10, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.A.; Coelho, T.; Barros, A.; Sousa, M. Corino de Andrade disease: Mechanisms and impact on reproduction. JBRA Assist. Reprod. 2017, 21, 105–114. [Google Scholar] [CrossRef]
- Benson, M.D. Liver transplantation and transthyretin amyloidosis. Muscle Nerve 2013, 47, 157–162. [Google Scholar] [CrossRef]
- Yokoyama, T.; Mizuguchi, M. Transthyretin Amyloidogenesis Inhibitors: From Discovery to Current Developments. J. Med. Chem. 2020, 63, 14228–14242. [Google Scholar] [CrossRef]
- Bulawa, C.E.; Connelly, S.; DeVit, M.; Wang, L.; Weigel, C.; Fleming, J.A.; Packman, J.; Powers, E.T.; Wiseman, R.L.; Foss, T.R.; et al. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc. Natl. Acad. Sci. USA 2012, 109, 9629–9634. [Google Scholar] [CrossRef]
- Adams, D.; Polydefkis, M.; González-Duarte, A.; Wixner, J.; Kristen, A.V.; Schmidt, H.H.; Berk, J.L.; López, I.A.L.; Dispenzieri, A.; Quan, D.; et al. Long-term safety and efficacy of patisiran for hereditary transthyretin-mediated amyloidosis with polyneuropathy: 12-month results of an open-label extension study. Lancet Neurol. 2021, 20, 49–59. [Google Scholar] [CrossRef]
- Brannagan, T.H.; Coelho, T.; Wang, A.K.; Polydefkis, M.J.; Dyck, P.J.; Berk, J.L.; Drachman, B.; Gorevic, P.; Whelan, C.; Conceição, I.; et al. Long-term efficacy and safety of inotersen for hereditary transthyretin amyloidosis: NEURO-TTR open-label extension 3-year update. J. Neurol. 2022, 269, 6416–6427. [Google Scholar] [CrossRef] [PubMed]
- Araki, S.; Ando, Y. Transthyretin-related familial amyloidotic polyneuropathy-Progress in Kumamoto, Japan (1967–2010). Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Sekijima, Y. Hereditary Transthyretin Amyloidosis, in GeneReviews® [Internet]; Adam, M.E.A., Ed.; University of Washington: Seattle, WA, USA, 2001. [Google Scholar]
- González-Duarte, A.; Soto, K.C.; Martínez-Baños, D.; Arteaga-Vazquez, J.; Barrera, F.; Berenguer-Sanchez, M.; Cantu-Brito, C.; García-Ramos, G.; Vidal, B.E. Familial amyloidosis with polyneuropathy associated with TTR Ser50Arg mutation. Amyloid 2012, 19, 171–176. [Google Scholar] [CrossRef]
- Iorio, A.; De Lillo, A.; De Angelis, F.; Di Girolamo, M.; Luigetti, M.; Sabatelli, M.; Pradotto, L.; Mauro, A.; Mazzeo, A.; Stancanelli, C.; et al. Non-coding variants contribute to the clinical heterogeneity of TTR amyloidosis. European journal of human genetics. Eur. J. Hum. Genet. 2017, 25, 1055–1060. [Google Scholar] [CrossRef]
- Benson, M.D.; Kincaid, J.C. The molecular biology and clinical features of amyloid neuropathy. Muscle Nerve 2007, 36, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.; Huang, H.; Li, C.; Chang, C.; Chan, W.P.; Lin, K.; Wu, C. Natural history and survival rate of familial amyloidosis with polyneuropathy: A nationwide databank. Ann. Clin. Transl. Neurol. 2023, 10, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Koski, L.; Ronnevi, C.; Berntsson, E.; Wärmländer, S.K.T.S.; Roos, P.M. Metals in ALS TDP-43 Pathology. Int. J. Mol. Sci. 2021, 22, 12193. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, A.; Mei, Y.; Zhao, J.; Zhou, Q.; Li, Y.; Yang, M.; Xu, Q. Trace elements and Alzheimer dementia in population-based studies: A bibliometric and meta-analysis. Environ. Pollut. 2023, 318, 120782. [Google Scholar] [CrossRef] [PubMed]
- Koski, L.; Tshoni, U.A.; Olowoyo, J.O.; Kobyana, A.S.; Lion, N.G.; Mugivhisa, L.L.; Warmlander, S.K.; Roos, P.M. Occupational lead exposure in gasoline station forecourt attendants and other occupations in relation to ALS (amyotrophic lateral sclerosis) risk. medRxiv 2023. [Google Scholar] [CrossRef]
- Warmlander, S.K.T.S.; Osterlund, N.; Wallin, C.; Wu, J.; Luo, J.; Tiiman, A.; Jarvet, J.; Graslund, A. Metal binding to the amyloid-beta peptides in the presence of biomembranes: Potential mechanisms of cell toxicity. J. Biol. Inorg. Chem. 2019, 24, 1189–1196. [Google Scholar] [CrossRef]
- Vasta, R.; Callegaro, S.; Sgambetterra, S.; Cabras, S.; Di Pede, F.; De Mattei, F.; Matteoni, E.; Grassano, M.; Bombaci, A.; De Marco, G.; et al. Presymptomatic geographical distribution of ALS patients suggests the involvement of environmental factors in the disease pathogenesis. J. Neurol. 2023, 270, 5475–5482. [Google Scholar] [CrossRef]
- Åström, M.E.; Roos, P.M. Geochemistry of multiple sclerosis in Finland. Sci. Total Environ. 2022, 841, 156672. [Google Scholar] [CrossRef]
- Hellman, U.; Suhr, O. Regional differences and similarities of FAP in Sweden. Amyloid 2012, 19, 53–54. [Google Scholar] [CrossRef]
- Andrade, C. A peculiar form of peripheral neuropathy; familiar atypical generalized amyloidosis with special involvement of the peripheral nerves. Brain 1952, 75, 408–427. [Google Scholar] [CrossRef]
- Hamada, M.; Kobayashi, W.; Hiramatsu, Y.; Hasebe, N. Mineralogy, chronology and formation process of the epithermal gold–silver vein deposits in the historical Togi mine, Noto Peninsula, Japan. Resour. Geol. 2022, 72, e12294. [Google Scholar] [CrossRef]
- Kopp, B.; Zalko, D.; Audebert, M. Genotoxicity of 11 heavy metals detected as food contaminants in two human cell lines. Environ. Mol. Mutagen. 2018, 59, 202–210. [Google Scholar] [CrossRef]
- Li, H.; Xu, W.; Dai, M.; Wang, Z.; Dong, X.; Fang, T. Assessing heavy metal pollution in paddy soil from coal mining area, Anhui, China. Environ. Monit. Assess. 2019, 191, 518. [Google Scholar] [CrossRef] [PubMed]
- Lauer, N.; Vengosh, A.; Dai, S. Naturally Occurring Radioactive Materials in Uranium-Rich Coals and Associated Coal Combustion Residues from China. Environ. Sci. Technol. 2017, 51, 13487–13493. [Google Scholar] [CrossRef] [PubMed]
- Berntsson, E.; Vosough, F.; Noormagi, A.; Padari, K.; Asplund, F.; Gielnik, M.; Paul, S.; Jarvet, J.; Tõugu, V.; Roos, P.M.; et al. Characterization of uranyl (UO22+) ion binding to amyloid beta (Aβ) peptides: Effects on Aβ structure and aggregation. ACS Chem. Neurosci. 2023, 14, 2618–2633. [Google Scholar] [PubMed]
- Duarte, J.C.; Rosas, F.M.; Terrinha, P.; Schellart, W.P.; Boutelier, D.; Gutscher, M.A.; Ribeiro, A. Are subduction zones invading the Atlantic? Evidence from the southwest Iberia margin. Geology 2013, 41, 839–842. [Google Scholar] [CrossRef]
- Meunier, E.; Dias, F.; Fonte, J.; Lima, A.; Rodrigues, A.; Bottaini, C.; Silva, R.J.C.; Veiga, J.P.; Pereira, M.F.C.; Figueiredo, E. Later prehistoric tin mining in the Ervedosa mine (Vinhais, Portugal): Evidence and context. Archaeol. Anthropol. Sci. 2023, 15, 43. [Google Scholar] [CrossRef]
- Comendador Rey, B.; Meunier, E.; Figueiredo, E.; Lackinger, A.; Fonte, J.; Fernández, C.F.; Lima, A.; Mirão, J.; Silva, R.J. Northwestern Iberian Tin Mining from Bronze Age to Modern Times: An overview. In The Tinworking Landscape of Dartmoor in a European Context; Newman, P., Ed.; Dartmoor Tinworking Research Group: Exeton, UK, 2017; pp. 133–153. [Google Scholar]
- Emslie, S.D.; Silva, A.M.; Valera, A.; Vila, E.V.; Melo, L.; Curate, F.; Fidalgo, D.; Inácio, N.; Moreno, M.M.; Cambra-Moo, O.; et al. The use and abuse of cinnabar in Late Neolithic and Copper Age Iberia. Int. J. Osteoarchaeol. 2022, 32, 202–214. [Google Scholar]
- Nolan, J.; Weber, K.A. Natural uranium contamination in major US aquifers linked to nitrate. Environ. Sci. Technol. Lett. 2015, 2, 215–220. [Google Scholar] [CrossRef]
- Carvalho, F.P.; Oliveira, J.M.; Lopes, I.; Batista, A. Radionuclides from past uranium mining in rivers of Portugal. J. Environ. Radioact. 2007, 98, 298–314. [Google Scholar] [CrossRef]
- Soares, H.; Boaventura, R.; Machado, A.; da Silva, J.E. Sediments as monitors of heavy metal contamination in the Ave river basin (Portugal): Multivariate analysis of data. Environ. Pollut. 1999, 105, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.W. Regional differences and similarities of familial amyloidotic polyneuropathy (FAP) presentation in Brazil. Amyloid 2012, 19 (Suppl. S1), 65–67. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, P.L.; Couto, C.A.; Clemente, C.; Farias, A.Q.; Palacios, S.A.; Mies, S.; Goldberg, A.C. Phenotypic expression of familial amyloid polyneuropathy in Brazil. Eur. J. Neurol. 2005, 12, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Buades-Reinés, J.; Raya-Cruz, M.; Gallego-Lezaún, C.; Ripoll-Vera, T.; Usón-Martín, M.; Andreu-Serra, H.; Cisneros-Barroso, E. Transthyretin familial amyloid polyneuropathy (TTR-FAP) in Mallorca: A comparison between late- and early-onset disease. J. Peripher. Nerv. Syst. 2016, 21, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Munar-Qués, M.; Saraiva, M.J.; Viader-Farré, C.; Zabay-Becerril, J.M.; Mulet-Ferrer, J. Genetic epidemiology of familial amyloid polyneuropathy in the Balearic Islands (Spain). Amyloid 2005, 12, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.A.H.; Estarellas, B.L.; Mateo, L.P.; Simonet, B.S. Aprovechamiento de recursos cupríferos en la edad del bronce de menorca: La mina de sa mitja lluna (Illa den Colom). Cuad. Prehist. Arqueol. Univ. Granada 2014, 24, 85–109. [Google Scholar]
- Hermanns, M.H. Avances en el estudio histórico de la mina de galena de Bunyola (isla de Mallorca). Sagvntvm 2014, 46, 189–200. [Google Scholar]
- Vicens, D.; Ginard, A.; Crespí, D.; Bover, P.; Gràcia, F. The endocarst and mines of the serra de na Burguesa (Mallorca, Balearic Islands). 1. Current knowledge speleogenetic, topographic, mining and speleothems forms. Bolleti Soc. d’Hist. Nat. Balear. 2011, 54, 117–132. [Google Scholar]
- Yoshioka, K.; Furuya, H.; Sasaki, H.; Saraiva, M.J.M.; Costa, P.P.; Sakaki, Y. Haplotype analysis of familial amyloidotic polyneuropathy. Evidence for multiple origins of the Val----Met mutation most common to the disease. Hum. Genet. 1989, 82, 9–13. [Google Scholar] [CrossRef]
- Holmgren, G.; Wikström, L.; Lundgren, H.; Suhr, O.B. Discordant penetrance of the trait for familial amyloidotic polyneuropathy in two pairs of monozygotic twins. J. Intern. Med. 2004, 256, 453–456. [Google Scholar] [CrossRef]
- Weihed, P.; Bergman, J.; Bergström, U. Métallogeny and tectonic evolution of the early proterozoic skellefte district, Northern Sweden. Precambrian Res. 1992, 58, 143–167. [Google Scholar] [CrossRef]
- Hellman, U.; Alarcon, F.; Lundgren, H.-E.; Suhr, O.B.; Bonaiti-Pellié, C.; Planté-Bordeneuve, V. Heterogeneity of penetrance in familial amyloid polyneuropathy, ATTR Val30Met, in the Swedish population. Amyloid 2008, 15, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Jonasson, J.; Cederquist, K.; Suhr, O.B. Frequency of the transthyretin Val30Met mutation in the northern Swedish population. Amyloid 2014, 21, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, G.; Costa, M. Handbook on the Toxicology of Metals, 5th ed.; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Saporta, M.A.d.C.; Plante-Bordeneuve, V.; Misrahi, M.; Cruz, M.W. Discordant expression of familial amyloid polyneuropathy in monozygotic Brazilian twins. Amyloid 2009, 16, 38–41. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roos, P.M.; Wärmländer, S.K.T.S. Hereditary Transthyretin Amyloidosis (hATTR) with Polyneuropathy Clusters Are Located in Ancient Mining Districts: A Possible Geochemical Origin of the Disease. Biomolecules 2024, 14, 652. https://doi.org/10.3390/biom14060652
Roos PM, Wärmländer SKTS. Hereditary Transthyretin Amyloidosis (hATTR) with Polyneuropathy Clusters Are Located in Ancient Mining Districts: A Possible Geochemical Origin of the Disease. Biomolecules. 2024; 14(6):652. https://doi.org/10.3390/biom14060652
Chicago/Turabian StyleRoos, Per M., and Sebastian K. T. S. Wärmländer. 2024. "Hereditary Transthyretin Amyloidosis (hATTR) with Polyneuropathy Clusters Are Located in Ancient Mining Districts: A Possible Geochemical Origin of the Disease" Biomolecules 14, no. 6: 652. https://doi.org/10.3390/biom14060652




