Effect of Acute Nutritional Ketosis on Circulating Levels of Growth Differentiation Factor 15: Findings from a Cross-Over Randomised Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Study Protocol
2.3. Laboratory Measurements
2.4. Anthropometric Measurements
2.5. Assessment of Eating Behaviour
2.6. Power Calculation
2.7. Statistical Analysis
3. Results
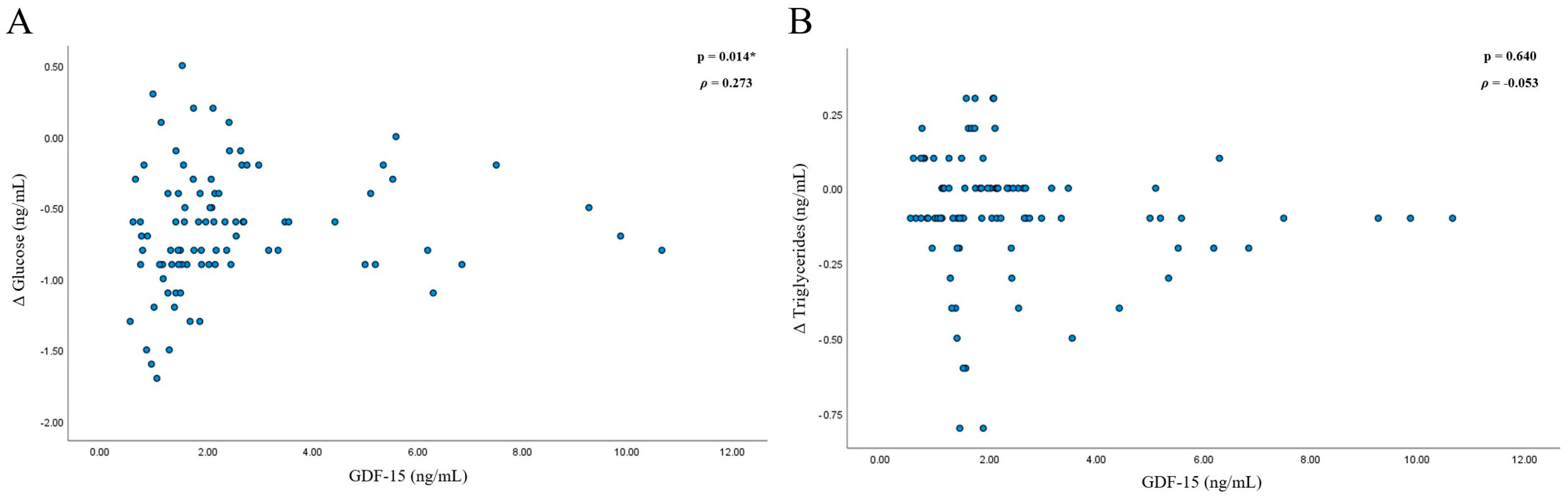
3.1. GDF-15 in the Study Population
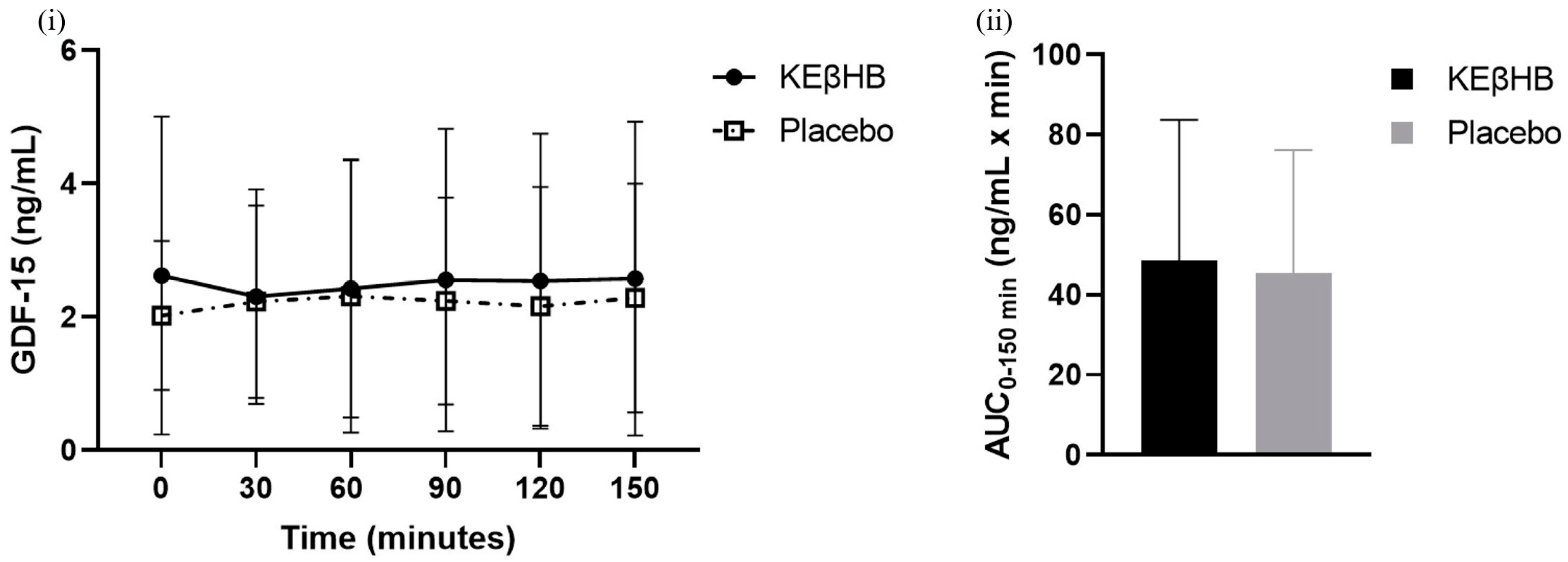
3.2. Effect of Acute Nutritional Ketosis on Levels of GDF-15
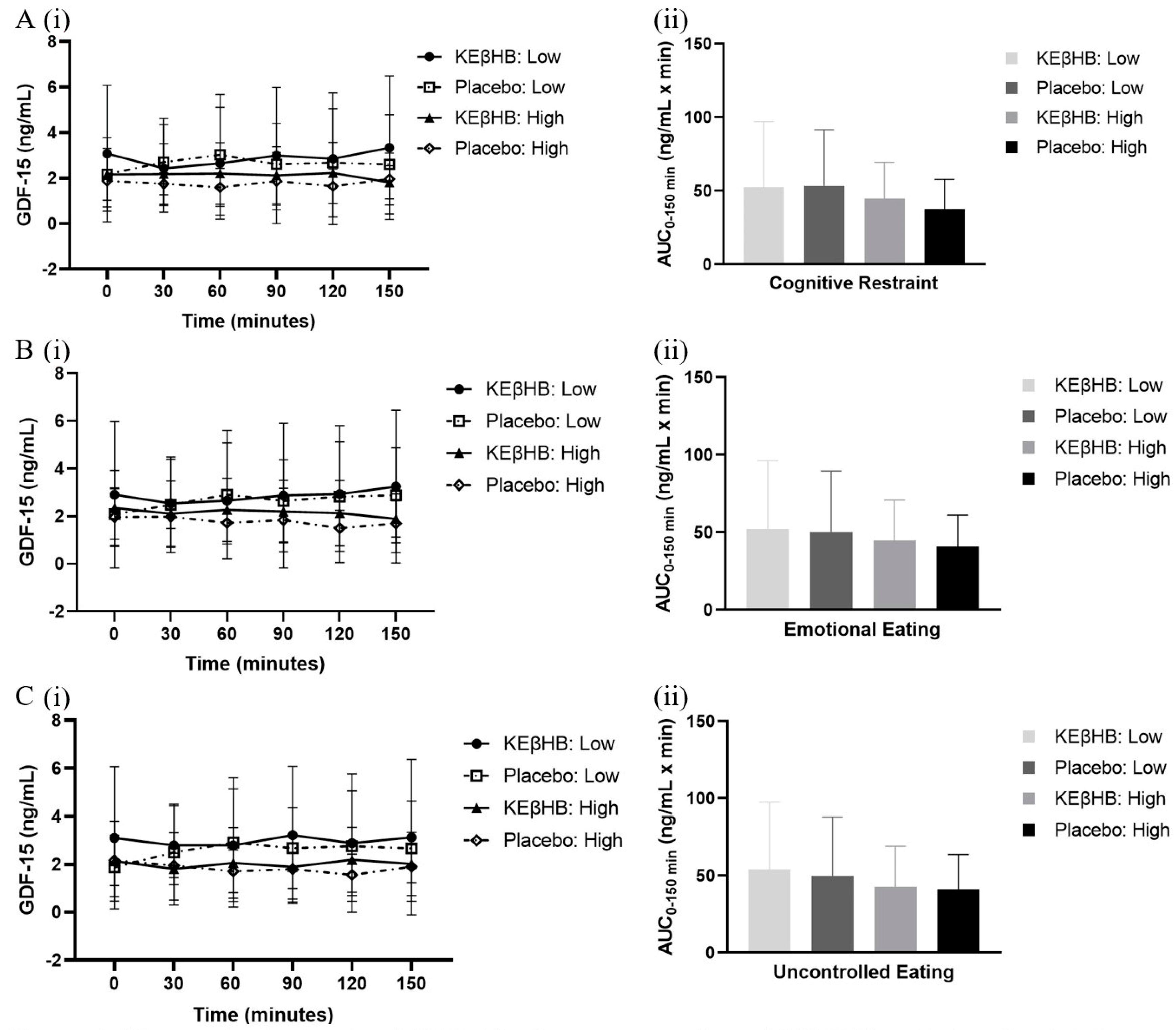
3.3. Role of Eating Behaviour
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loos, R.J.; Yeo, G.S. The genetics of obesity: From discovery to biology. Nat. Rev. Genet. 2022, 23, 120–133. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Amin, T.; Mercer, J.G. Hunger and satiety mechanisms and their potential exploitation in the regulation of food intake. Curr. Obes. Rep. 2016, 5, 106–112. [Google Scholar] [CrossRef]
- Price, C.J.; Hoyda, T.D.; Ferguson, A.V. The area postrema: A brain monitor and integrator of systemic autonomic state. Neuroscientist 2008, 14, 182–194. [Google Scholar] [CrossRef]
- Yang, L.; Chang, C.C.; Sun, Z.; Madsen, D.; Zhu, H.; Padkjær, S.B.; Wu, X.; Huang, T.; Hultman, K.; Paulsen, S.J.; et al. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat. Med. 2017, 23, 1158–1166. [Google Scholar] [CrossRef]
- Hale, C.; Véniant, M.M. Growth differentiation factor 15 as a potential therapeutic for treating obesity. Mol. Metab. 2021, 46, 101117. [Google Scholar] [CrossRef]
- Tsai, V.W.; Manandhar, R.; Jrøgensen, S.B.; Lee-Ng, K.K.; Zhang, H.P.; Marquis, C.P.; Jiang, L.; Husaini, Y.; Lin, S.; Sainsbury, A.; et al. The anorectic actions of the TGFβ cytokine MIC-1/GDF15 require an intact brainstem area postrema and nucleus of the solitary tract. PLoS ONE 2014, 9, e100370. [Google Scholar] [CrossRef]
- Wang, D.; Day, E.A.; Townsend, L.K.; Djordjevic, D.; Jørgensen, S.B.; Steinberg, G.R. GDF15: Emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat. Rev. Endocrinol. 2021, 17, 592–607. [Google Scholar] [CrossRef]
- Xiong, Y.; Walker, K.; Min, X.; Hale, C.; Tran, T.; Komorowski, R.; Yang, J.; Davda, J.; Nuanmanee, N.; Kemp, D.; et al. Long-acting MIC-1/GDF15 molecules to treat obesity: Evidence from mice to monkeys. Sci. Transl. Med. 2017, 9, 412. [Google Scholar] [CrossRef] [PubMed]
- Mullican, S.E.; Lin-Schmidt, X.; Chin, C.N.; Chavez, J.A.; Furman, J.L.; Armstrong, A.A.; Beck, S.C.; South, V.J.; Dinh, T.Q.; Cash-Mason, T.D.; et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat. Med. 2017, 23, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.H.; Wang, A.; Hilliard, B.K.; Carvalho, F.; Rosen, C.E.; Ahasic, A.M.; Herzog, E.L.; Kang, I.; Pisani, M.A.; Yu, S.; et al. GDF15 is an inflammation-induced central mediator of tissue tolerance. Cell 2019, 178, 1231–1244. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, W.; Qian, J.; Tang, Y. Fasting exacerbates hepatic growth differentiation factor 15 to promote fatty acid β-oxidation and ketogenesis via activating XBP1 signaling in liver. Redox Biol. 2018, 16, 87–96. [Google Scholar] [CrossRef]
- Roekenes, J.; Martins, C. Ketogenic diets and appetite regulation. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 359–363. [Google Scholar] [CrossRef]
- Moriconi, E.; Camajani, E.; Fabbri, A.; Lenzi, A.; Massimiliano, C. Very-low-calorie ketogenic diet as a safe and valuable tool for long-term glycemic management in patients with obesity and type 2 diabetes. Nutrients 2021, 13, 758. [Google Scholar] [CrossRef]
- Bharmal, S.H.; Cho, J.; CAlarcon Ramos, G.; Ko, J.; Cameron-Smith, D.; Petrov, M.S. Acute nutritional ketosis and its implications for plasma glucose and glucoregulatory peptides in adults with prediabetes: A crossover placebo-controlled randomized trial. J. Nutr. 2021, 151, 921–929. [Google Scholar] [CrossRef]
- Liu, Y.; Bharmal, S.H.; Kimita, W.; Petrov, M.S. Effect of acute ketosis on lipid profile in prediabetes: Findings from a cross-over randomized controlled trial. Cardiovasc. Diabetol. 2022, 21, 138. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2020. Diabetes Care 2020, 43, S14–S31. [Google Scholar] [CrossRef]
- Bharmal, S.H.; Alarcon Ramos, G.C.; Ko, J.; Petrov, M.S. Abdominal fat distribution modulates the metabolic effects of exogenous ketones in individuals with new-onset prediabetes after acute pancreatitis: Results from a randomized placebo-controlled trial. Clin. Nutr. ESPEN 2021, 43, 117–129. [Google Scholar] [CrossRef]
- De Lauzon, B.; Romon, M.; Deschamps, V.; Lafay, L.; Borys, J.M.; Karlsson, J.; Ducimetière, P.; Charles, M.A.; Group, F.L. The Three-Factor Eating Questionnaire-R18 is able to distinguish among different eating patterns in a general population. J. Nutr. 2004, 134, 2372–2380. [Google Scholar] [CrossRef]
- Anglé, S.; Engblom, J.; Eriksson, T.; Kautiainen, S.; Saha, M.-T.; Lindfors, P.; Lehtinen, M.; Rimpelä, A. Three factor eating questionnaire-R18 as a measure of cognitive restraint, uncontrolled eating and emotional eating in a sample of young Finnish females. Int. J. Behav. Nutr. Phys. Act. 2009, 6, 41. [Google Scholar] [CrossRef]
- Mullins, G.; Hallam, C.L.; Broom, I. Ketosis, ketoacidosis and very-low-calorie diets: Putting the record straight. Nutr. Bull. 2011, 36, 397–402. [Google Scholar] [CrossRef]
- Nair, S.N.; Welle, S.L.; Halliday, D.; Campbell, R.G. Effect of B-hydroxybutyrate on whole body leucine kinetics and fractional mixed skeletal muscle protein synthesis in humans. Am. J. Physiol. Endocrinol. Metab. 1988, 254, 198–205. [Google Scholar] [CrossRef]
- Evans, M.; Cogan, K.E.; Egan, B. Metabolism of ketone bodies during exercise and training: Physiological basis for exogenous supplementation. J. Physiol. 2017, 595, 2857–2871. [Google Scholar] [CrossRef]
- VanItallie, T.B.; Nufert, T.H. Ketones: Metabolism’s ugly duckling. Nutr. Rev. 2003, 61, 327–341. [Google Scholar] [CrossRef]
- Schönfeld, P.; Reiser, G. Why does brain metabolism not favor burning of fatty acids to provide energy-Reflections on disadvantages of the use of free fatty acids as fuel for brain. J. Cereb. Blood Flow. Metab. 2013, 33, 1493–1499. [Google Scholar] [CrossRef]
- Poffé, C.; Ramaekers, M.; Van Thienen, R.; Hespel, P. Ketone ester supplementation blunts overreaching symptoms during endurance training overload. J. Physiol. 2019, 597, 3009–3027. [Google Scholar] [CrossRef]
- Hiroux, C.; Schouten, M.; de Glisezinski, I.; Simon, C.; Crampes, F.; Hespel, P.; Koppo, K. Effect of increased protein intake and exogenous ketosis on body composition, energy expenditure and exercise capacity during a hypocaloric diet in recreational female athletes. Front. Physiol. 2023, 13, 1063956. [Google Scholar] [CrossRef]
- Charles, S.; Liu, Y.; Kimita, W.; Ko, J.; Bharmal, S.H.; Petrov, M.S. Effect of D-β-hydroxybutyrate-(R)-1,3 butanediol on plasma levels of asprosin and leptin: Results from a randomised controlled trial. Food Funct. 2023, 14, 759–768. [Google Scholar] [CrossRef]
- Liu, Y.; Bharmal, S.H.; Kimita, W.; Petrov, M.S. Effect of d-β-hydroxybutyrate-(R)-1,3 butanediol on appetite regulation in people with prediabetes. Mol. Nutr. Food Res. 2023, 67, e2200615. [Google Scholar] [CrossRef]
- Stubbs, B.J.; Cox, P.J.; Evans, R.D.; Santer, P.; Miller, J.J.; Faull, O.K.; Magor-Elliott, S.; Hiyama, S.; Stirling, M.; Clarke, K. On the Metabolism of Exogenous Ketones in Humans. Front. Physiol. 2017, 8, 848. [Google Scholar] [CrossRef]
- Vestergaard, E.T.; Zubanovic, N.B.; Rittig, N.; Møller, N.; Kuhre, R.E.; Holst, J.J.; Rehfeld, J.F.; Thomsen, H.H. Acute ketosis inhibits appetite and decreases plasma concentrations of acyl ghrelin in healthy young men. Diabetes Obes. Metab. 2021, 23, 1834–1842. [Google Scholar] [CrossRef]
- Alcazar, J.; Frandsen, U.; Prokhorova, T.; Kamper, R.S.; Haddock, B.; Aagaard, P.; Suetta, C. Changes in systemic GDF15 across the adult lifespan and their impact on maximal muscle power: The Copenhagen Sarcopenia Study. J. Cachex-Sarcopenia Muscle 2021, 12, 1418–1427. [Google Scholar] [CrossRef]
- Soto-Mota, A.; Norwitz, N.G.; Evans, R.; Clarke, K.; Barber, T.M. Exogenous ketosis in patients with type 2 diabetes: Safety, tolerability and effect on glycaemic control. Endocrinol. Diabetes Metab. 2021, 4, e00264. [Google Scholar] [CrossRef]
- Soto-Mota, A.; Vansant, H.; Evans, R.D.; Clarke, K. Safety and tolerability of sustained exogenous ketosis using ketone monoester drinks for 28 days in healthy adults. Regul. Toxicol. Pharmacol. 2019, 109, 104506. [Google Scholar] [CrossRef]
- Walsh, J.J.; Neudorf, H.; Little, J.P. 14-Day Ketone Supplementation Lowers Glucose and Improves Vascular Function in Obesity: A Randomized Crossover Trial. J. Clin. Endocrinol. Metab. 2021, 106, 1738–1754. [Google Scholar] [CrossRef]
- Falkenhain, K.; Daraei, A.; Forbes, S.C.; Little, J.P. Effects of exogenous ketone supplemetation on blood glucose: A systematic review and meta-analysis. Adv. Nutr. 2022, 13, 1697–1714. [Google Scholar] [CrossRef]
| Characteristic | Participants (n = 18) 1 |
|---|---|
| Age, y | 55 ± 14 |
| Sex, n | |
| Men | 12 |
| Women | 6 |
| GDF-15, ng/mL | 2.1 (1.1–2.5) |
| Systolic blood pressure, mmHg | 135 ± 27 |
| Diastolic blood pressure, mmHg | 88 ± 13 |
| BMI, kg/m2 | 28.4 (24.5–30.9) |
| Hip circumference, cm | 108.5 (99.8–112.3) |
| Waist circumference, cm | 100.0 (90.3–107.5) |
| Molecule | KEβHB (n = 18) | Placebo (n = 18) | Mean Difference (95% CI) | d (Effect Size) | p-Value |
|---|---|---|---|---|---|
| GDF-15 (ng/mL × min) | 48.54 ± 35.21 | 45.50 ± 30.72 | −3.04 (−12.41, 6.33) | 0.09 | 0.503 |
| Domain | Time | KEβHB (n = 9) | Placebo (n = 9) | Mean Difference (95% CI) | d (Effect Size) | p-Value |
|---|---|---|---|---|---|---|
| Cognitive Restraint | ||||||
| Low | Baseline | 0.32 ± 0.40 | 0.28 ± 0.25 | −0.04 (−0.24, 0.15) | 0.13 | 0.624 |
| Total AUC | 52.51 ± 44.50 | 53.48 ± 38.10 | 0.96 (14.82, 4.94) | 0.02 | 0.850 | |
| High | Baseline | 0.26 ± 0.26 | 0.20 ± 0.28 | −0.06 (0.36, 0.12) | 0.21 | 0.648 |
| Total AUC | 44.56 ± 24.87 | 37.52 ± 20.25 | −7.05 (−24.21, 10.12) | 0.31 | 0.372 | |
| Emotional Eating | ||||||
| Low | Baseline | 0.29 ± 0.39 | 0.28 ± 0.18 | −0.01 (−0.23, 0.22) | 0.02 | 0.941 |
| Total AUC | 52.31 ± 43.85 | 50.22 ± 39.29 | −2.09 (−12.03, 7.84) | 0.05 | 0.640 | |
| High | Baseline | 0.29 ± 0.29 | 0.20 ± 0.33 | −0.09 (−0.34, 0.15) | 0.30 | 0.410 |
| Total AUC | 44.76 ± 26.07 | 40.78 ± 20.27 | −3.99 (−22.59, 14.61) | 0.17 | 0.634 | |
| Uncontrolled Eating | ||||||
| Low | Baseline | 0.35 ± 0.35 | 0.19 ± 0.29 | −0.16 (−0.41, 0.10) | 0.48 | 0.197 |
| Total AUC | 54.20 ± 43.32 | 49.81 ± 38.19 | −4.39 (−12.75, 3.96) | 0.11 | 0.260 | |
| High | Baseline | 0.23 ± 0.31 | 0.28 ± 0.24 | 0.06 (−0.13, 0.24) | 0.20 | 0.512 |
| Total AUC | 42.87 ± 26.17 | 41.18 ± 22.47 | −1.69 (−21.02, 17.64) | 0.07 | 0.845 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charles, S.; Liu, Y.; Bharmal, S.H.; Kimita, W.; Petrov, M.S. Effect of Acute Nutritional Ketosis on Circulating Levels of Growth Differentiation Factor 15: Findings from a Cross-Over Randomised Controlled Trial. Biomolecules 2024, 14, 665. https://doi.org/10.3390/biom14060665
Charles S, Liu Y, Bharmal SH, Kimita W, Petrov MS. Effect of Acute Nutritional Ketosis on Circulating Levels of Growth Differentiation Factor 15: Findings from a Cross-Over Randomised Controlled Trial. Biomolecules. 2024; 14(6):665. https://doi.org/10.3390/biom14060665
Chicago/Turabian StyleCharles, Sanjali, Yutong Liu, Sakina H. Bharmal, Wandia Kimita, and Maxim S. Petrov. 2024. "Effect of Acute Nutritional Ketosis on Circulating Levels of Growth Differentiation Factor 15: Findings from a Cross-Over Randomised Controlled Trial" Biomolecules 14, no. 6: 665. https://doi.org/10.3390/biom14060665





