Repair and DNA Polymerase Bypass of Clickable Pyrimidine Nucleotides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Enzymes and Oligonucleotides
2.2. DNA Glycosylase Assays
2.3. Singe-Turnover Kinetics of MBD4 or SMUG1
2.4. DNA Polymerase Assays
2.5. Steady-State DNA Polymerase Kinetics
2.6. Reporter Plasmid Experiments
3. Results
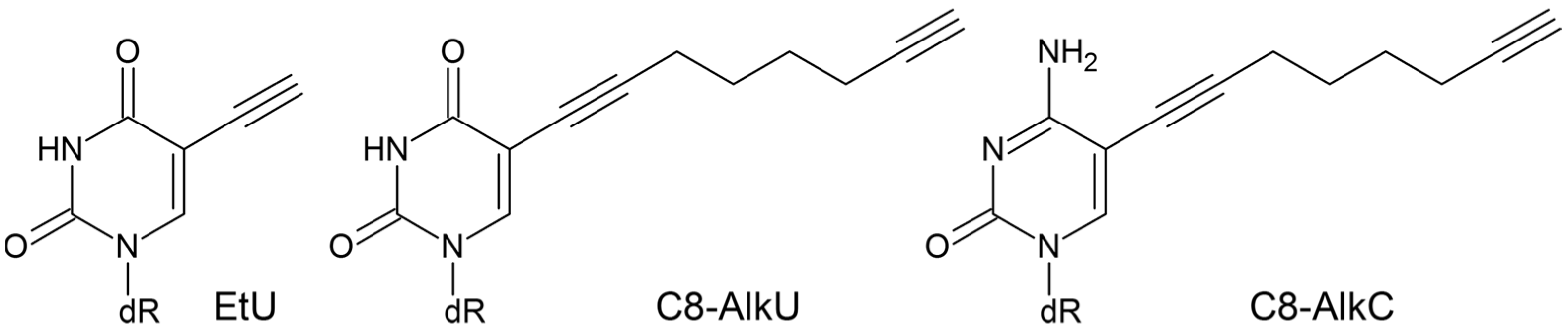
3.1. SMUG1 and MBD4 DNA Glycosylases Can Excise Clickable Pyrimidine Bases
3.2. Normal Coding and Miscoding by Clickable Pyrimidine Bases
3.3. Impaired Repair of Clickable Pyrimidine Bases in Human Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scinto, S.L.; Bilodeau, D.A.; Hincapie, R.; Lee, W.; Nguyen, S.S.; Xu, M.; am Ende, C.W.; Finn, M.G.; Lang, K.; Lin, Q.; et al. Bioorthogonal chemistry. Nat. Rev. Methods Primers 2021, 1, 30. [Google Scholar] [CrossRef] [PubMed]
- Smeenk, M.L.W.J.; Agramunt, J.; Bonger, K.M. Recent developments in bioorthogonal chemistry and the orthogonality within. Curr. Opin. Chem. Biol. 2021, 60, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-S.; Berdis, A.J. Visualizing nucleic acid metabolism using non-natural nucleosides and nucleotide analogs. Biochim. Biophys. Acta 2016, 1864, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Zuin Fantoni, N.; El-Sagheer, A.H.; Brown, T. A hitchhiker’s guide to click-chemistry with nucleic acids. Chem. Rev. 2021, 121, 7122–7154. [Google Scholar] [CrossRef] [PubMed]
- Solius, G.M.; Maltsev, D.I.; Belousov, V.V.; Podgorny, O.V. Recent advances in nucleotide analogue-based techniques for tracking dividing stem cells: An overview. J. Biol. Chem. 2021, 297, 101345. [Google Scholar] [CrossRef] [PubMed]
- Gramlich, P.M.E.; Wirges, C.T.; Manetto, A.; Carell, T. Postsynthetic DNA modification through the copper-catalyzed azide–alkyne cycloaddition reaction. Angew. Chem. Int. Ed. 2008, 47, 8350–8358. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, E.C.; Walker, G.C.; Siede, W.; Wood, R.D.; Schultz, R.A.; Ellenberger, T. DNA Repair and Mutagenesis; ASM Press: Washington, DC, USA, 2006; 1118p. [Google Scholar]
- Raiber, E.-A.; Hardisty, R.; van Delft, P.; Balasubramanian, S. Mapping and elucidating the function of modified bases in DNA. Nat. Rev. Chem. 2017, 1, 0069. [Google Scholar] [CrossRef]
- Christmann, M.; Tomicic, M.T.; Roos, W.P.; Kaina, B. Mechanisms of human DNA repair: An update. Toxicology 2003, 193, 3–34. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, E.C. DNA damage and repair. Nature 2003, 421, 436–440. [Google Scholar] [CrossRef]
- Neddermann, P.; Jiricny, J. Efficient removal of uracil from G•U mispairs by the mismatch-specific thymine DNA glycosylase from HeLa cells. Proc. Natl Acad. Sci. USA 1994, 91, 1642–1646. [Google Scholar] [CrossRef]
- Brandon, M.L.; Mi, L.-J.; Chaung, W.; Teebor, G.; Boorstein, R.J. 5-Chloro-2′-deoxyuridine cytotoxicity results from base excision repair of uracil subsequent to thymidylate synthase inhibition. Mutat. Res. 2000, 459, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.T.; Rodgers, M.T.; Hebert, A.S.; Ruslander, L.E.; Eisele, L.; Drohat, A.C. Specificity of human thymine DNA glycosylase depends on N-glycosidic bond stability. J. Am. Chem. Soc. 2006, 128, 12510–12519. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.P.; Cortellino, S.; Schupp, J.E.; Caretti, E.; Loh, T.; Kinsella, T.J.; Bellacosa, A. The DNA N-glycosylase MED1 exhibits preference for halogenated pyrimidines and is involved in the cytotoxicity of 5-iododeoxyuridine. Cancer Res. 2006, 66, 7686–7693. [Google Scholar] [CrossRef] [PubMed]
- Darwanto, A.; Theruvathu, J.A.; Sowers, J.L.; Rogstad, D.K.; Pascal, T.; Goddard, W.; Sowers, L.C. Mechanisms of base selection by human single-stranded selective monofunctional uracil-DNA glycosylase. J. Biol. Chem. 2009, 284, 15835–15846. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Zhang, X.; Cheng, X. Excision of thymine and 5-hydroxymethyluracil by the MBD4 DNA glycosylase domain: Structural basis and implications for active DNA demethylation. Nucleic Acids Res. 2012, 40, 8276–8284. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Hong, S.; Bhagwat, A.S.; Zhang, X.; Cheng, X. Excision of 5-hydroxymethyluracil and 5-carboxylcytosine by the thymine DNA glycosylase domain: Its structural basis and implications for active DNA demethylation. Nucleic Acids Res. 2012, 40, 10203–10214. [Google Scholar] [CrossRef] [PubMed]
- Moréra, S.; Grin, I.; Vigouroux, A.; Couvé, S.; Henriot, V.; Saparbaev, M.; Ishchenko, A.A. Biochemical and structural characterization of the glycosylase domain of MBD4 bound to thymine and 5-hydroxymethyuracil-containing DNA. Nucleic Acids Res. 2012, 40, 9917–9926. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, E.C. Suffering in silence: The tolerance of DNA damage. Nat. Rev. Mol. Cell Biol. 2005, 6, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Sale, J.E.; Lehmann, A.R.; Woodgate, R. Y-family DNA polymerases and their role in tolerance of cellular DNA damage. Nat. Rev. Mol. Cell Biol. 2012, 13, 141–152. [Google Scholar] [CrossRef]
- Wang, L.; Cao, X.; Yang, Y.; Kose, C.; Kawara, H.; Lindsey-Boltz, L.A.; Selby, C.P.; Sancar, A. Nucleotide excision repair removes thymidine analog 5-ethynyl-2′-deoxyuridine from the mammalian genome. Proc. Natl Acad. Sci. USA 2022, 119, e2210176119. [Google Scholar] [CrossRef]
- Gierlich, J.; Burley, G.A.; Gramlich, P.M.E.; Hammond, D.M.; Carell, T. Click chemistry as a reliable method for the high-density postsynthetic functionalization of alkyne-modified DNA. Org. Lett. 2006, 8, 3639–3642. [Google Scholar] [CrossRef] [PubMed]
- Grin, I.R.; Mechetin, G.V.; Kasymov, R.D.; Diatlova, E.A.; Yudkina, A.V.; Shchelkunov, S.N.; Gileva, I.P.; Denisova, A.A.; Stepanov, G.A.; Chilov, G.G.; et al. A new class of uracil–DNA glycosylase inhibitors active against human and vaccinia virus enzyme. Molecules 2021, 26, 6668. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, A.A.; Iakovlev, D.A.; Misovets, I.V.; Ishchenko, A.A.; Saparbaev, M.K.; Kuznetsov, N.A.; Fedorova, O.S. Pre-steady-state kinetic analysis of damage recognition by human single-strand selective monofunctional uracil-DNA glycosylase SMUG1. Mol. Biosyst. 2017, 13, 2638–2649. [Google Scholar] [CrossRef] [PubMed]
- Katafuchi, A.; Nakano, T.; Masaoka, A.; Terato, H.; Iwai, S.; Hanaoka, F.; Ide, H. Differential specificity of human and Escherichia coli endonuclease III and VIII homologues for oxidative base lesions. J. Biol. Chem. 2004, 279, 14464–14471. [Google Scholar] [CrossRef]
- Kakhkharova, Z.I.; Zharkov, D.O.; Grin, I.R. A low-activity polymorphic variant of human NEIL2 DNA glycosylase. Int. J. Mol. Sci. 2022, 23, 2212. [Google Scholar] [CrossRef]
- Kuznetsov, N.A.; Koval, V.V.; Zharkov, D.O.; Nevinsky, G.A.; Douglas, K.T.; Fedorova, O.S. Kinetics of substrate recognition and cleavage by human 8-oxoguanine-DNA glycosylase. Nucleic Acids Res. 2005, 33, 3919–3931. [Google Scholar] [CrossRef]
- Endutkin, A.V.; Yudkina, A.V.; Zharkov, T.D.; Kim, D.V.; Zharkov, D.O. Recognition of a clickable abasic site analog by DNA polymerases and DNA repair enzymes. Int. J. Mol. Sci. 2022, 23, 13353. [Google Scholar] [CrossRef]
- Ohashi, E.; Ogi, T.; Kusumoto, R.; Iwai, S.; Masutani, C.; Hanaoka, F.; Ohmori, H. Error-prone bypass of certain DNA lesions by the human DNA polymerase κ. Genes Dev. 2000, 14, 1589–1594. [Google Scholar] [CrossRef]
- Garcia-Diaz, M.; Bebenek, K.; Krahn, J.M.; Blanco, L.; Kunkel, T.A.; Pedersen, L.C. A structural solution for the DNA polymerase λ-dependent repair of DNA gaps with minimal homology. Mol. Cell 2004, 13, 561–572. [Google Scholar] [CrossRef]
- Miller, H.; Grollman, A.P. Kinetics of DNA polymerase I (Klenow fragment exo–) activity on damaged DNA templates: Effect of proximal and distal template damage on DNA synthesis. Biochemistry 1997, 36, 15336–15342. [Google Scholar] [CrossRef]
- Freisinger, E.; Grollman, A.P.; Miller, H.; Kisker, C. Lesion (in)tolerance reveals insights into DNA replication fidelity. EMBO J. 2004, 23, 1494–1505. [Google Scholar] [CrossRef]
- Lühnsdorf, B.; Kitsera, N.; Warken, D.; Lingg, T.; Epe, B.; Khobta, A. Generation of reporter plasmids containing defined base modifications in the DNA strand of choice. Anal. Biochem. 2012, 425, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.V.; Diatlova, E.A.; Zharkov, T.D.; Melentyev, V.S.; Yudkina, A.V.; Endutkin, A.V.; Zharkov, D.O. Back-up base excision DNA repair in human cells deficient in the major AP endonuclease, APE1. Int. J. Mol. Sci. 2024, 25, 64. [Google Scholar] [CrossRef] [PubMed]
- Khobta, A.; Kitsera, N.; Speckmann, B.; Epe, B. 8-Oxoguanine DNA glycosylase (Ogg1) causes a transcriptional inactivation of damaged DNA in the absence of functional Cockayne syndrome B (Csb) protein. DNA Repair 2009, 8, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Khobta, A.; Lingg, T.; Schulz, I.; Warken, D.; Kitsera, N.; Epe, B. Mouse CSB protein is important for gene expression in the presence of a single-strand break in the non-transcribed DNA strand. DNA Repair 2010, 9, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Kitsera, N.; Stathis, D.; Lühnsdorf, B.; Müller, H.; Carell, T.; Epe, B.; Khobta, A. 8-Oxo-7,8-dihydroguanine in DNA does not constitute a barrier to transcription, but is converted into transcription-blocking damage by OGG1. Nucleic Acids Res. 2011, 39, 5926–5934. [Google Scholar] [CrossRef] [PubMed]
- David, S.S.; O’Shea, V.L.; Kundu, S. Base-excision repair of oxidative DNA damage. Nature 2007, 447, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Zharkov, D.O. Base excision DNA repair. Cell. Mol. Life Sci. 2008, 65, 1544–1565. [Google Scholar] [CrossRef]
- Mullins, E.A.; Rodriguez, A.A.; Bradley, N.P.; Eichman, B.F. Emerging roles of DNA glycosylases and the base excision repair pathway. Trends Biochem. Sci. 2019, 44, 765–781. [Google Scholar] [CrossRef]
- Sjolund, A.B.; Senejani, A.G.; Sweasy, J.B. MBD4 and TDG: Multifaceted DNA glycosylases with ever expanding biological roles. Mutat. Res. 2013, 743–744, 12–25. [Google Scholar] [CrossRef]
- Kavli, B.; Slupphaug, G.; Krokan, H.E. Genomic uracil in biology, immunity and cancer. In DNA Damage, DNA Repair and Disease; Dizdaroglu, M., Lloyd, R.S., Eds.; Royal Society of Chemistry: London, UK, 2021; Volume 1, pp. 220–248. [Google Scholar]
- Fleming, A.M.; Burrows, C.J. Formation and processing of DNA damage substrates for the hNEIL enzymes. Free Radic. Biol. Med. 2017, 107, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Boiteux, S.; Coste, F.; Castaing, B. Repair of 8-oxo-7,8-dihydroguanine in prokaryotic and eukaryotic cells: Properties and biological roles of the Fpg and OGG1 DNA N-glycosylases. Free Radic. Biol. Med. 2017, 107, 179–201. [Google Scholar] [CrossRef] [PubMed]
- Petronzelli, F.; Riccio, A.; Markham, G.D.; Seeholzer, S.H.; Stoerker, J.; Genuardi, M.; Yeung, A.T.; Matsumoto, Y.; Bellacosa, A. Biphasic kinetics of the human DNA repair protein MED1 (MBD4), a mismatch-specific DNA N-glycosylase. J. Biol. Chem. 2000, 275, 32422–32429. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, H.; Haushalter, K.A.; Robins, P.; Barnes, D.E.; Verdine, G.L.; Lindahl, T. Excision of deaminated cytosine from the vertebrate genome: Role of the SMUG1 uracil–DNA glycosylase. EMBO J. 2001, 20, 4278–4286. [Google Scholar] [CrossRef] [PubMed]
- Hoitsma, N.M.; Whitaker, A.M.; Schaich, M.A.; Smith, M.R.; Fairlamb, M.S.; Freudenthal, B.D. Structure and function relationships in mammalian DNA polymerases. Cell. Mol. Life Sci. 2020, 77, 35–59. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Konigsberg, W.H. RB69 DNA polymerase structure, kinetics, and fidelity. Biochemistry 2014, 53, 2752–2767. [Google Scholar] [CrossRef] [PubMed]
- Moon, A.F.; Garcia-Diaz, M.; Batra, V.K.; Beard, W.A.; Bebenek, K.; Kunkel, T.A.; Wilson, S.H.; Pedersen, L.C. The X family portrait: Structural insights into biological functions of X family polymerases. DNA Repair 2007, 6, 1709–1725. [Google Scholar] [CrossRef] [PubMed]
- Beard, W.A.; Wilson, S.H. Structure and mechanism of DNA polymerase β. Biochemistry 2014, 53, 2768–2780. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Gao, Y. Translesion and repair DNA polymerases: Diverse structure and mechanism. Annu. Rev. Biochem. 2018, 87, 239–261. [Google Scholar] [CrossRef]
- Stern, H.R.; Sefcikova, J.; Chaparro, V.E.; Beuning, P.J. Mammalian DNA polymerase kappa activity and specificity. Molecules 2019, 24, 2805. [Google Scholar] [CrossRef]
- Shibutani, S.; Grollman, A.P. On the mechanism of frameshift (deletion) mutagenesis in vitro. J. Biol. Chem. 1993, 268, 11703–11710. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, T.A.; Bebenek, K. DNA replication fidelity. Annu. Rev. Biochem. 2000, 69, 497–529. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.Y.; Pannunzio, N.R.; Adachi, N.; Lieber, M.R. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat. Rev. Mol. Cell Biol. 2017, 18, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, A.M.; Chiruvella, K.K.; Ramsden, D.A.; Bebenek, K.; Kunkel, T.A.; Pedersen, L.C. Analysis of diverse double-strand break synapsis with Polλ reveals basis for unique substrate specificity in nonhomologous end-joining. Nat. Commun. 2022, 13, 3806. [Google Scholar] [CrossRef] [PubMed]
- Vande Berg, B.J.; Beard, W.A.; Wilson, S.H. DNA structure and aspartate 276 influence nucleotide binding to human DNA polymerase β: Implication for the identity of the rate-limiting conformational change. J. Biol. Chem. 2001, 276, 3408–3416. [Google Scholar] [CrossRef] [PubMed]
- Kitsera, N.; Rodriguez-Alvarez, M.; Emmert, S.; Carell, T.; Khobta, A. Nucleotide excision repair of abasic DNA lesions. Nucleic Acids Res. 2019, 47, 8537–8547. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Alvarez, M.; Kim, D.; Khobta, A. EGFP reporters for direct and sensitive detection of mutagenic bypass of DNA lesions. Biomolecules 2020, 10, 902. [Google Scholar] [CrossRef]
- Raindlová, V.; Janoušková, M.; Slavíčková, M.; Perlíková, P.; Boháčová, S.; Milisavljevič, N.; Šanderová, H.; Benda, M.; Barvík, I.; Krásný, L.; et al. Influence of major-groove chemical modifications of DNA on transcription by bacterial RNA polymerases. Nucleic Acids Res. 2016, 44, 3000–3012. [Google Scholar] [CrossRef]
- Slavíčková, M.; Janoušková, M.; Šimonová, A.; Cahová, H.; Kambová, M.; Šanderová, H.; Krásný, L.; Hocek, M. Turning off transcription with bacterial RNA polymerase through CuAAC click reactions of DNA containing 5-ethynyluracil. Chemistry 2018, 24, 8311–8314. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M., III; Takeshita, M.; Grollman, A.P.; Demple, B. Incision activity of human apurinic endonuclease (Ape) at abasic site analogs in DNA. J. Biol. Chem. 1995, 270, 16002–16007. [Google Scholar] [CrossRef]
- Mundle, S.T.; Delaney, J.C.; Essigmann, J.M.; Strauss, P.R. Enzymatic mechanism of human apurinic/apyrimidinic endonuclease against a THF AP site model substrate. Biochemistry 2009, 48, 19–26. [Google Scholar] [CrossRef]
- Allgayer, J.; Kitsera, N.; Bartelt, S.; Epe, B.; Khobta, A. Widespread transcriptional gene inactivation initiated by a repair intermediate of 8-oxoguanine. Nucleic Acids Res. 2016, 44, 7267–7280. [Google Scholar] [CrossRef] [PubMed]
- Haskins, J.S.; Su, C.; Maeda, J.; Walsh, K.D.; Haskins, A.H.; Allum, A.J.; Froning, C.E.; Kato, T.A. Evaluating the genotoxic and cytotoxic effects of thymidine analogs, 5-ethynyl-2′-deoxyuridine and 5-bromo-2′-deoxyuridine to mammalian cells. Int. J. Mol. Sci. 2020, 21, 6631. [Google Scholar] [CrossRef]
- Branum, M.E.; Reardon, J.T.; Sancar, A. DNA repair excision nuclease attacks undamaged DNA: A potential source of spontaneous mutations. J. Biol. Chem. 2001, 276, 25421–25426. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, M.; Nagashima, J.-I.; Hasegawa, M.; Tamura, T.; Kitagata, R.; Hanawa, K.; Hososhima, S.-I.; Kasamatsu, T.; Ozaki, H.; Sawai, H. Systematic characterization of 2′-deoxynucleoside-5′-triphosphate analogs as substrates for DNA polymerases by polymerase chain reaction and kinetic studies on enzymatic production of modified DNA. Nucleic Acids Res. 2006, 34, 5383–5394. [Google Scholar] [CrossRef]
- Gierlich, J.; Gutsmiedl, K.; Gramlich, P.M.E.; Schmidt, A.; Burley, G.A.; Carell, T. Synthesis of highly modified DNA by a combination of PCR with alkyne-bearing triphosphates and click chemistry. Chemistry 2007, 13, 9486–9494. [Google Scholar] [CrossRef]
- Boorstein, R.J.; Cummings, A., Jr.; Marenstein, D.R.; Chan, M.K.; Ma, Y.; Neubert, T.A.; Brown, S.M.; Teebor, G.W. Definitive identification of mammalian 5-hydroxymethyluracil DNA N-glycosylase activity as SMUG1. J. Biol. Chem. 2001, 276, 41991–41997. [Google Scholar] [CrossRef] [PubMed]
- Kavli, B.; Sundheim, O.; Akbari, M.; Otterlei, M.; Nilsen, H.; Skorpen, F.; Aas, P.A.; Hagen, L.; Krokan, H.E.; Slupphaug, G. hUNG2 is the major repair enzyme for removal of uracil from U:A matches, U:G mismatches, and U in single-stranded DNA, with hSMUG1 as a broad specificity backup. J. Biol. Chem. 2002, 277, 39926–39936. [Google Scholar] [CrossRef]
- Masaoka, A.; Matsubara, M.; Hasegawa, R.; Tanaka, T.; Kurisu, S.; Terato, H.; Ohyama, Y.; Karino, N.; Matsuda, A.; Ide, H. Mammalian 5-formyluracil-DNA glycosylase. 2. Role of SMUG1 uracil-DNA glycosylase in repair of 5-formyluracil and other oxidized and deaminated base lesions. Biochemistry 2003, 42, 5003–5012. [Google Scholar] [CrossRef]
- Matsubara, M.; Tanaka, T.; Terato, H.; Ohmae, E.; Izumi, S.; Katayanagi, K.; Ide, H. Mutational analysis of the damage-recognition and catalytic mechanism of human SMUG1 DNA glycosylase. Nucleic Acids Res. 2004, 32, 5291–5302. [Google Scholar] [CrossRef]
- Knævelsrud, I.; Slupphaug, G.; Leiros, I.; Matsuda, A.; Ruoff, P.; Bjelland, S. Opposite-base dependent excision of 5-formyluracil from DNA by hSMUG1. Int. J. Radiat. Biol. 2009, 85, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Mi, R.; Dong, L.; Kaulgud, T.; Hackett, K.W.; Dominy, B.N.; Cao, W. Insights from xanthine and uracil DNA glycosylase activities of bacterial and human SMUG1: Switching SMUG1 to UDG. J. Mol. Biol. 2009, 385, 761–778. [Google Scholar] [CrossRef]
- Wibley, J.E.A.; Waters, T.R.; Haushalter, K.; Verdine, G.L.; Pearl, L.H. Structure and specificity of the vertebrate anti-mutator uracil-DNA glycosylase SMUG1. Mol. Cell 2003, 11, 1647–1659. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Burdzy, A.; Sowers, L.C. Repair of the mutagenic DNA oxidation product, 5-formyluracil. DNA Repair 2003, 2, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Liu, Y.; Upadhyay, A.K.; Chang, Y.; Howerton, S.B.; Vertino, P.M.; Zhang, X.; Cheng, X. Recognition and potential mechanisms for replication and erasure of cytosine hydroxymethylation. Nucleic Acids Res. 2012, 40, 4841–4849. [Google Scholar] [CrossRef] [PubMed]
- Manvilla, B.A.; Maiti, A.; Begley, M.C.; Toth, E.A.; Drohat, A.C. Crystal structure of human methyl-binding domain IV glycosylase bound to abasic DNA. J. Mol. Biol. 2012, 420, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Quintana, V.G.; Sweasy, J.B. NTHL1 in genomic integrity, aging and cancer. DNA Repair 2020, 93, 102920. [Google Scholar] [CrossRef] [PubMed]
- Oswalt, L.E.; Eichman, B.F. NEIL3: A unique DNA glycosylase involved in interstrand DNA crosslink repair. DNA Repair 2024, 139, 103680. [Google Scholar] [CrossRef] [PubMed]
- Crouse, G.F. Non-canonical actions of mismatch repair. DNA Repair 2016, 38, 102–109. [Google Scholar] [CrossRef]
- Ramachandran, S.; Henikoff, S. MINCE-Seq: Mapping in vivo nascent chromatin with EdU and sequencing. Methods Mol. Biol. 2018, 1832, 159–168. [Google Scholar] [CrossRef]
- Ming, X.; Zhu, B.; Zhang, Z. Simultaneously measuring the methylation of parent and daughter strands of replicated DNA at the single-molecule level by Hammer-seq. Nat. Protoc. 2021, 16, 2131–2157. [Google Scholar] [CrossRef] [PubMed]
- van der Wouden, P.E.; Zwinderman, M.R.H.; Chen, D.; Borghesan, M.; Dekker, F.J. Protocol of the double-click seq method to evaluate the deposition bias of chromatin proteins. Curr. Protoc. 2023, 3, e805. [Google Scholar] [CrossRef] [PubMed]

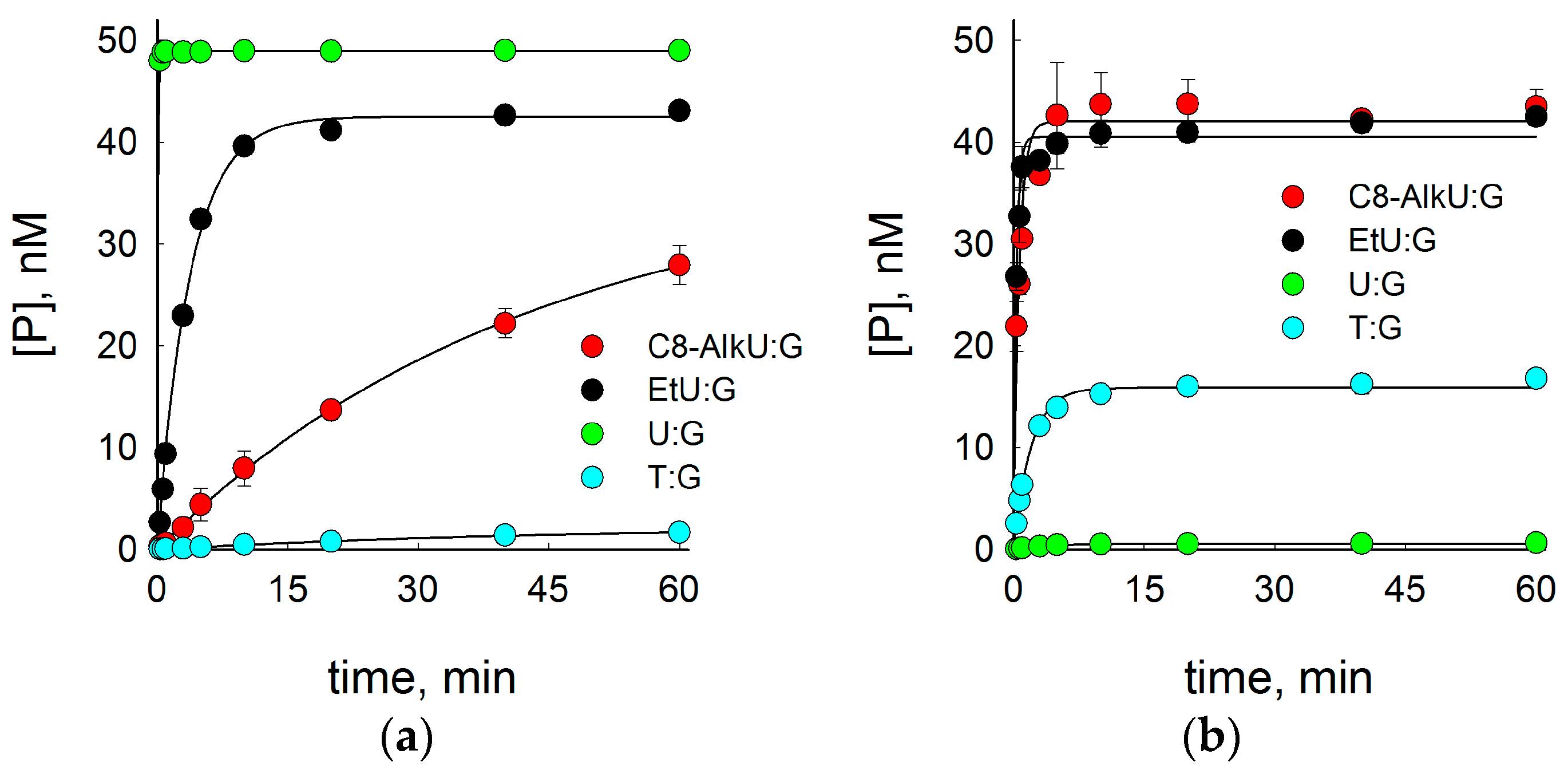
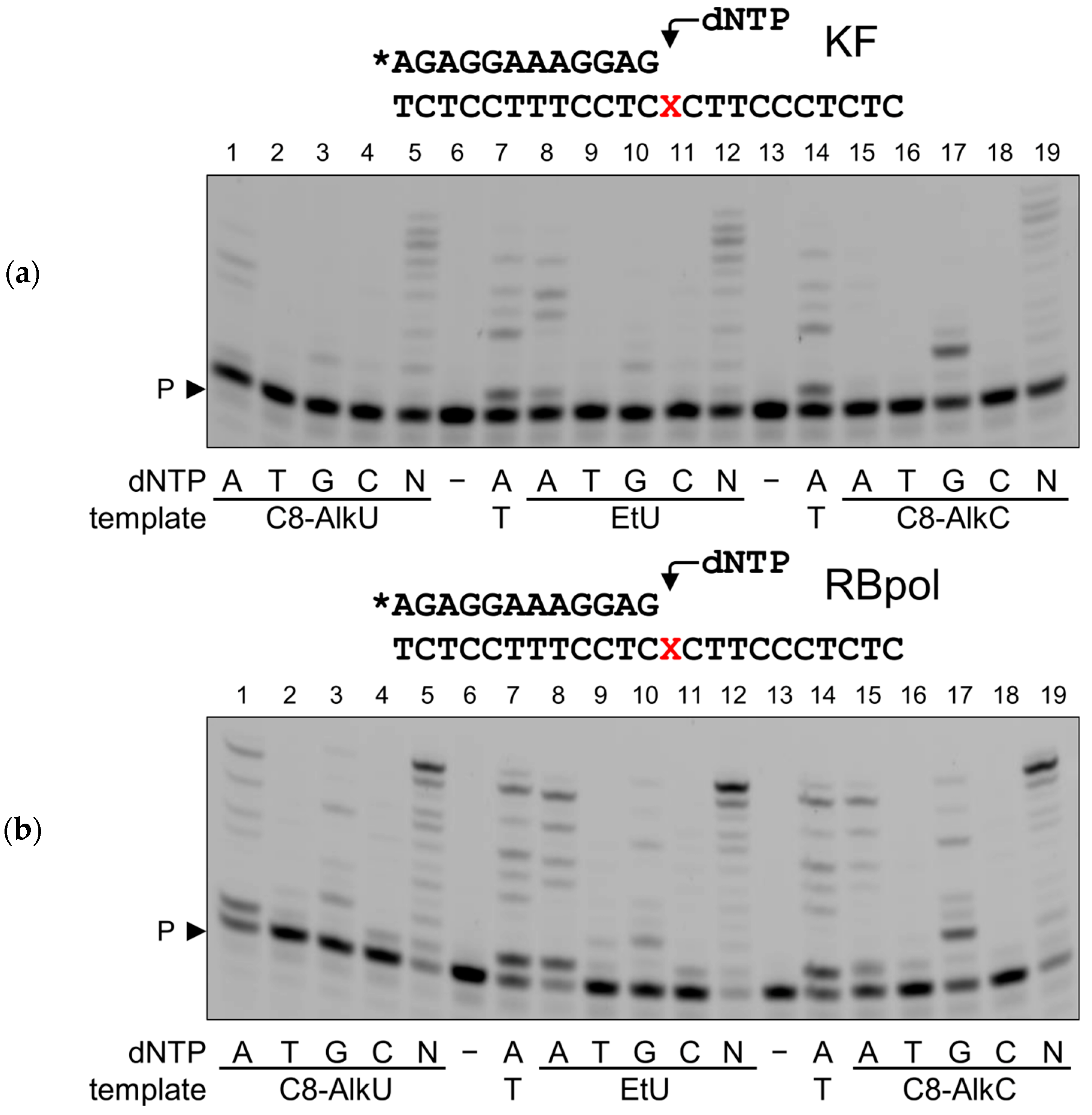
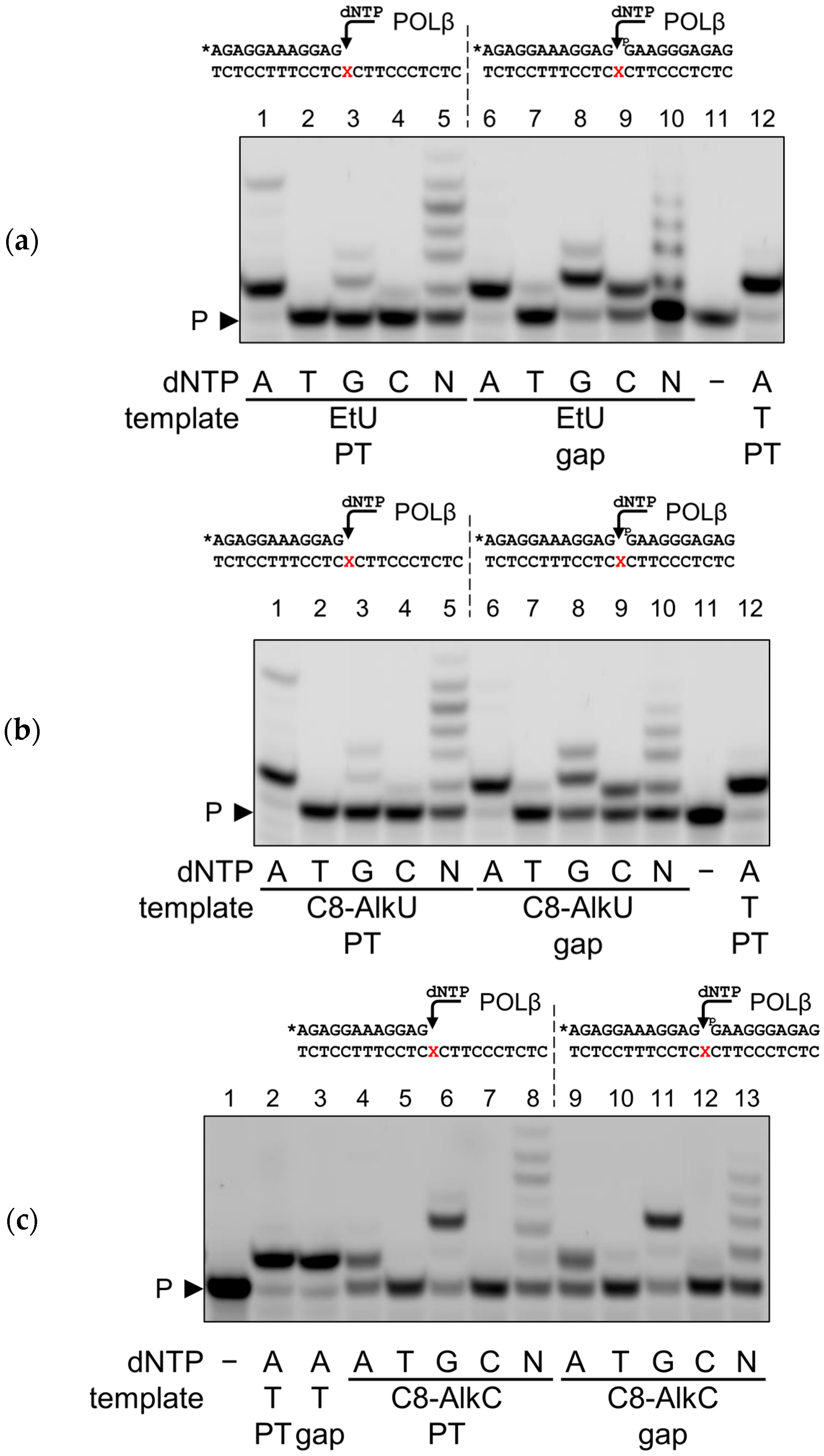


| Enzyme | Substrate | |||
|---|---|---|---|---|
| U:G | T:G | EtU:G | C8-AlkU:G | |
| k2 app, min−1 | ||||
| SMUG1 | >12 | (2.3 ± 0.5) × 10−2 | (2.6 ± 0.1) × 10−1 | (2.3 ± 0.3) × 10−2 |
| MBD4 | (2.7 ± 0.4) × 10−1 | (5.0 ± 0.3) × 10−1 | 2.9 ± 0.2 | 1.6 ± 0.2 |
| Enzyme | Template | Incoming | KM, µM | kcat, s−1 | kcat/KM, µM−1s−1 | ferr b | gap/PT c |
|---|---|---|---|---|---|---|---|
| RBpol | EtU | dATP | 5.1 ± 1.1 | 0.027 ± 0.001 | (5.2 ± 1.1) × 10−3 | ||
| C8-AlkU | dATP | n/s d | n/s | (1.8 ± 0.1) × 10−5 | |||
| C8-AlkC | dGTP | 1.2 ± 0.3 | 0.036 ± 0.001 | (3.0 ± 0.8) × 10−2 | |||
| POLβ | EtU PT | dATP | 120 ± 30 | 0.47 ± 0.04 | (3.9 ± 1.1) × 10−3 | ||
| EtU gap | dATP | 5.5 ± 2.5 | 0.17 ± 0.01 | (3.2 ± 1.4) × 10−2 | 8.1 | ||
| EtU gap | dGTP | 28 ± 6 | 0.049 ± 0.002 | (1.8 ± 0.4) × 10−3 | 0.056 | ||
| EtU gap | dCTP | 56 ± 12 | 0.025 ± 0.001 | (4.5 ± 1.0) × 10−4 | 0.014 | ||
| C8-AlkU PT | dATP | 95 ± 32 | 0.51 ± 0.05 | (5.4 ± 1.9) × 10−3 | |||
| C8-AlkU gap | dATP | 9.0 ± 2.7 | 0.28 ± 0.01 | (3.0 ± 0.9) × 10−2 | 5.6 | ||
| C8-AlkU gap | dGTP | 27 ± 6 | 0.022 ± 0.001 | (8.1 ± 1.7) × 10−4 | 0.026 | ||
| C8-AlkU gap | dCTP | 20 ± 6 | 0.018 ± 0.001 | (9.3 ± 2.6) × 10−4 | 0.030 | ||
| C8-AlkC PT | dGTP | 66 ± 19 | 0.38 ± 0.03 | (5.8 ± 1.7) × 10−3 | |||
| C8-AlkC gap | dGTP | 7.6 ± 1.4 | 0.24 ± 0.01 | (3.2 ± 0.6) × 10−2 | 5.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Endutkin, A.V.; Yudkina, A.V.; Zharkov, T.D.; Barmatov, A.E.; Petrova, D.V.; Kim, D.V.; Zharkov, D.O. Repair and DNA Polymerase Bypass of Clickable Pyrimidine Nucleotides. Biomolecules 2024, 14, 681. https://doi.org/10.3390/biom14060681
Endutkin AV, Yudkina AV, Zharkov TD, Barmatov AE, Petrova DV, Kim DV, Zharkov DO. Repair and DNA Polymerase Bypass of Clickable Pyrimidine Nucleotides. Biomolecules. 2024; 14(6):681. https://doi.org/10.3390/biom14060681
Chicago/Turabian StyleEndutkin, Anton V., Anna V. Yudkina, Timofey D. Zharkov, Alexander E. Barmatov, Daria V. Petrova, Daria V. Kim, and Dmitry O. Zharkov. 2024. "Repair and DNA Polymerase Bypass of Clickable Pyrimidine Nucleotides" Biomolecules 14, no. 6: 681. https://doi.org/10.3390/biom14060681
APA StyleEndutkin, A. V., Yudkina, A. V., Zharkov, T. D., Barmatov, A. E., Petrova, D. V., Kim, D. V., & Zharkov, D. O. (2024). Repair and DNA Polymerase Bypass of Clickable Pyrimidine Nucleotides. Biomolecules, 14(6), 681. https://doi.org/10.3390/biom14060681





