Unraveling the Immune Regulatory Functions of USP5: Implications for Disease Therapy
Abstract
:1. Introduction
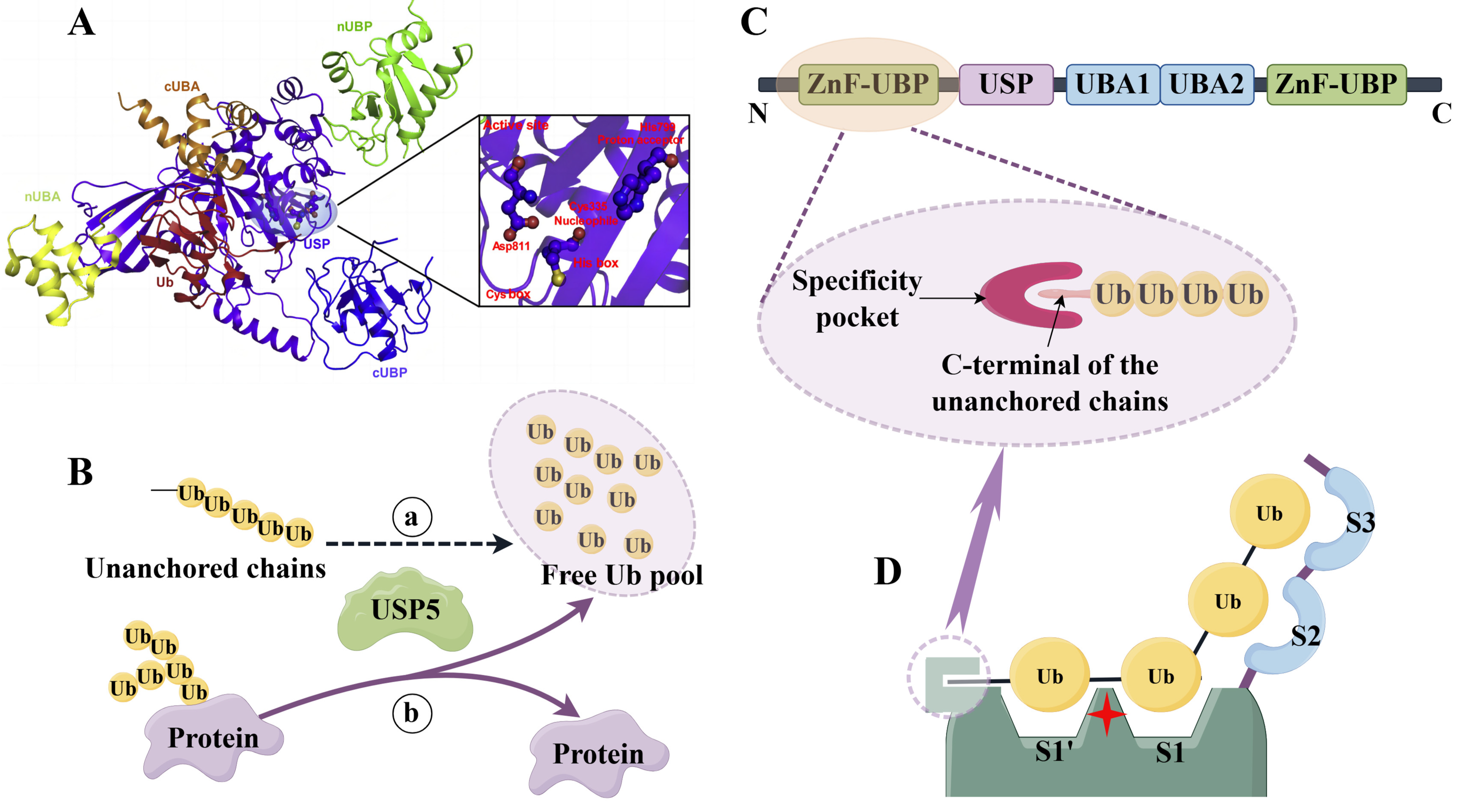
2. Structure and Function of USP5
2.1. Overview of the USP Family
2.2. Structure and Catalytic Activity of USP5
2.3. Role of USP5 in Maintaining Protein Stability and Signaling
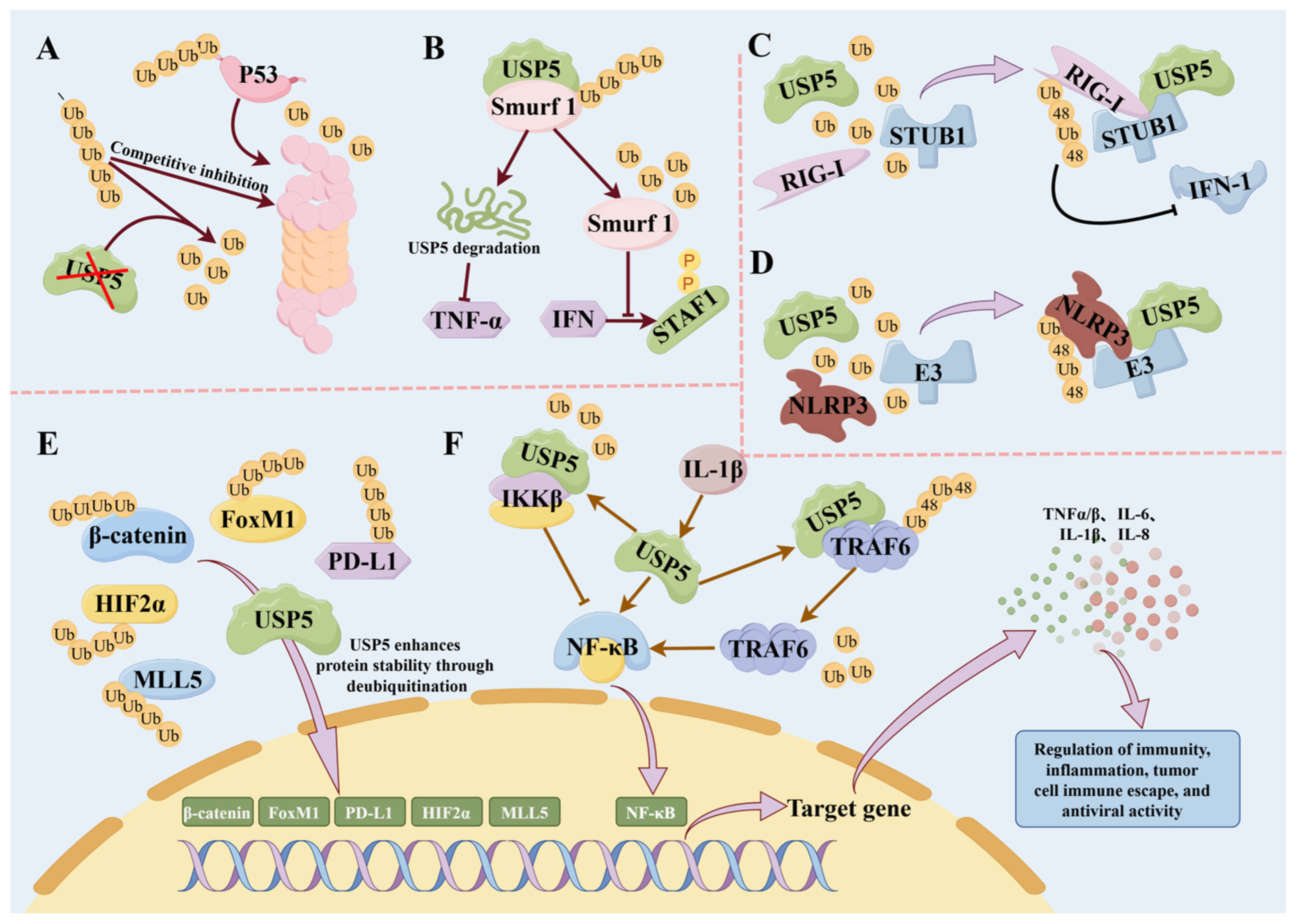
3. Role of USP5 in Immune Regulation
3.1. USP5 Regulates Immune Cell Activation, Differentiation, and Function
3.2. USP5 Regulates the Immune Response
4. USP5 Regulates Immune Signaling Pathways to Affect the Development of Disease
4.1. Role of USP5 in Autoimmune Diseases
4.2. Role of USP5 in Inflammatory Immunity
4.3. Role of USP5 in Antiviral Immunity
4.4. Role of USP5 in Tumor Immune Responses
5. Therapeutic Developments for Targeting USP5
6. Discussion and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilkinson, K.D.; Tashayev, V.L.; O’Connor, L.B.; Larsen, C.N.; Kasperek, E.; Pickart, C.M. Metabolism of the polyubiquitin degradation signal: Structure, mechanism, and role of isopeptidase T. Biochemistry 1995, 34, 14535–14546. [Google Scholar] [CrossRef] [PubMed]
- Ning, F.; Xin, H.; Liu, J.; Lv, C.; Xu, X.; Wang, M.; Wang, Y.; Zhang, W.; Zhang, X. Structure and function of USP5: Insight into physiological and pathophysiological roles. Pharmacol. Res. 2020, 157, 104557. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.L.; Chen, Z.; Melandri, F. Kinetic studies of isopeptidase T: Modulation of peptidase activity by ubiquitin. Biochemistry 1995, 34, 12616–12623. [Google Scholar] [CrossRef] [PubMed]
- Ansari-Lari, M.A.; Muzny, D.M.; Lu, J.; Lu, F.; Lilley, C.E.; Spanos, S.; Malley, T.; Gibbs, R.A. A gene-rich cluster between the CD4 and triosephosphate isomerase genes at human chromosome 12p13. Genome Res. 1996, 6, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Clague, M.J.; Urbé, S.; Komander, D. Breaking the chains: Deubiquitylating enzyme specificity begets function. Nat. Rev. Mol. Cell Biol. 2019, 20, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Nostramo, R.; Varia, S.N.; Zhang, B.; Emerson, M.M.; Herman, P.K. The catalytic activity of the Ubp3 deubiquitinating protease is required for efficient stress granule assembly in Saccharomyces cerevisiae. Mol. Cell. Biol. 2016, 36, 173–183. [Google Scholar] [CrossRef]
- Nakajima, S.; Lan, L.; Wei, L.; Hsieh, C.-L.; Rapić-Otrin, V.; Yasui, A.; Levine, A.S. Ubiquitin-Specific Protease 5 Is Required for the Efficient Repair of DNA Double-Strand Breaks. PLoS ONE 2014, 9, e84899. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, Y.; Qin, Y.; Hu, J.; Xie, W.; Qin, F.X.-F.; Cui, J. Broad and diverse mechanisms used by deubiquitinase family members in regulating the type I interferon signaling pathway during antiviral responses. Sci. Adv. 2018, 4, 2824. [Google Scholar] [CrossRef]
- Xie, X.; Matsumoto, S.; Endo, A.; Fukushima, T.; Kawahara, H.; Saeki, Y.; Komada, M. Deubiquitinases USP5 and USP13 are recruited to and regulate heat-induced stress granules by deubiquitinating activities. J. Cell Sci. 2018, 131, 210856. [Google Scholar] [CrossRef]
- Gao, S.-T.; Xin, X.; Wang, Z.-Y.; Hu, Y.-Y.; Feng, Q. USP5: Comprehensive insights into structure, function, biological and disease-related implications, and emerging therapeutic opportunities. Mol. Cell. Probes 2024, 73, 101944. [Google Scholar] [CrossRef]
- Wrzesinski, S.H.; Wan, Y.Y.; Flavell, R.A. Transforming Growth Factor-β and the Immune Response: Implications for Anticancer Therapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13 Pt 1, 5262–5270. [Google Scholar] [CrossRef] [PubMed]
- Kareva, I. Immune Suppression in Pregnancy and Cancer: Parallels and Insights. Transl. Oncol. 2020, 13, 100759. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, D.A.; Fahmy, T.M.; Piccirillo, C.A.; La Cava, A. Rebalancing Immune Homeostasis to Treat Autoimmune Diseases. Trends Immunol. 2019, 40, 888–908. [Google Scholar] [CrossRef] [PubMed]
- Murakami, N.; Riella, L.V. Co-Inhibitory Pathways and Their Importance in Immune Regulation. Transplantation 2014, 98, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Gibson, S.A.; Buckley, J.A.; Qin, H.; Benveniste, E.N. Role of the JAK/STAT signaling pathway in regulation of innate immunity in neuroinflammatory diseases. Clin. Immunol. 2018, 189, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Smith, S.; Hu, X. Role of Notch signaling in regulating innate immunity and inflammation in health and disease. Protein Cell 2016, 7, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Hammond, T.R.; Marsh, S.E.; Stevens, B. Immune Signaling in Neurodegeneration. Immunity 2019, 50, 955–974. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Chen, G.-C.; Chien, C.-T. The deubiquitinase Leon/USP5 regulates ubiquitin homeostasis during Drosophila development. Biochem. Biophys. Res. Commun. 2014, 452, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, W.; Zhang, M.; Wang, P.; Zhao, K.; Zhao, X.; Yang, S.; Gao, C. USP4 Positively Regulates RIG-I-Mediated Antiviral Response through Deubiquitination and Stabilization of RIG-I. J. Virol. 2013, 87, 4507–4515. [Google Scholar] [CrossRef]
- Zhong, B.; Liu, X.; Wang, X.; Liu, X.; Li, H.; Darnay, B.G.; Lin, X.; Sun, S.-C.; Dong, C. Ubiquitin-Specific Protease 25 Regulates TLR4-Dependent Innate Immune Responses Through Deubiquitination of the Adaptor Protein TRAF3. Sci. Signal. 2013, 6, ra35. [Google Scholar] [CrossRef]
- Shi, J.; Liu, Y.; Xu, X.; Zhang, W.; Yu, T.; Jia, J.; Liu, C. Deubiquitinase USP47/UBP64E Regulates β-Catenin Ubiquitination and Degradation and Plays a Positive Role in Wnt Signaling. Mol. Cell. Biol. 2015, 35, 3301–3311. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, P.; Zhao, J.; Tan, Y.; Sheng, J.; He, S.; Du, X.; Huang, Y.; Yang, Y.; Li, J.; et al. USP12 promotes CD4+ T cell responses through deubiquitinating and stabilizing BCL10. Cell Death Differ. 2021, 28, 2857–2870. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Rush, J.S.; Graham, D.B.; Cao, Z.; Xavier, R.J. USP15 Deubiquitinates CARD9 to Downregulate C-Type Lectin Receptor–Mediated Signaling. ImmunoHorizons 2020, 4, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.W.; Varshavsky, A. Cloning and functional analysis of the ubiquitin-specific protease gene UBP1 of Saccharomyces cerevisiae. J. Biol. Chem. 1991, 266, 12021–12028. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cui, S.; Chen, Y.; Guo, S.; Chen, D. Ubiquitin specific peptidases and prostate cancer. PeerJ 2023, 11, e14799. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Zhu, F.; Liang, R.; Li, D.; Li, B. Regulation of T cell differentiation and function by ubiquitin-specific proteases. Cell. Immunol. 2019, 340, 103922. [Google Scholar] [CrossRef] [PubMed]
- Hariri, H.; St-Arnaud, R. Expression and Role of Ubiquitin-Specific Peptidases in Osteoblasts. Int. J. Mol. Sci. 2021, 22, 7746. [Google Scholar] [CrossRef]
- Nijman, S.M.B.; Luna-Vargas, M.P.A.; Velds, A.; Brummelkamp, T.R.; Dirac, A.M.G.; Sixma, T.K.; Bernards, R. A genomic and functional inventory of deubiquitinating enzymes. Cell 2005, 123, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Y.; Zhou, H. Advances in the Development Ubiquitin-Specific Peptidase (USP) Inhibitors. Int. J. Mol. Sci. 2021, 22, 4546. [Google Scholar] [CrossRef]
- Ming-Jer, Y.; Kai-Cheng, H.; Tony, E.L.; Wen-Chang, C.; Jan-Jong, H. The Role of Ubiquitin-Specific Peptidases in Cancer Progression. J. Biomed. Sci. 2019, 26, 42. [Google Scholar]
- Ye, Y.; Scheel, H.; Hofmann, K.; Komander, D. Dissection of USP catalytic domains reveals five common insertion points. Mol. Biosyst. 2009, 5, 1797–1808. [Google Scholar] [CrossRef] [PubMed]
- Komander, D.; Clague, M.J.; Urbe, S. Breaking the chains: Structure and function of the deubiquitinases. Nat. Rev. Mol. Cell Biol. 2009, 10, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Fraile, J.M.; Quesada, V.; Rodríguez, D.; Freije, J.M.P.; López-Otín, C. Deubiquitinases in cancer: New functions and therapeutic options. Oncogene 2012, 31, 2373–2388. [Google Scholar] [CrossRef] [PubMed]
- Quesada, V.; Díaz-Perales, A.; Gutiérrez-Fernández, A.; Garabaya, C.; Cal, S.; López-Otín, C. Cloning and enzymatic analysis of 22 novel human ubiquitin-specific proteases. Biochem. Biophys. Res. Commun. 2004, 314, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Ronau, J.A.; Beckmann, J.F.; Hochstrasser, M. Substrate specificity of the ubiquitin and Ubl proteases. Cell Res. 2016, 26, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Buchberger, A. From UBA to UBX: New words in the ubiquitin vocabulary. Trends Cell Biol. 2002, 12, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Song, A.-X.; Yang, H.; Gao, Y.-G.; Zhou, C.-J.; Zhang, Y.-H.; Hu, H.-Y. A Ubiquitin Shuttle DC-UbP/UBTD2 Reconciles Protein Ubiquitination and Deubiquitination via Linking UbE1 and USP5 Enzymes. PLoS ONE 2014, 9, e107509. [Google Scholar] [CrossRef] [PubMed]
- Avvakumov, G.V.; Walker, J.R.; Xue, S.; Allali-Hassani, A.; Asinas, A.; Nair, U.B.; Fang, X.; Zuo, X.; Wang, Y.-X.; Wilkinson, K.D.; et al. Two ZnF-UBP Domains in Isopeptidase T (USP5). Biochemistry 2012, 51, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ding, Y.; Zhou, C.; Wen, Y.; Zhang, N. Structural and functional studies of USP20 ZnF-UBP domain by NMR. Protein Sci. Publ. Protein Soc. 2019, 28, 1606–1619. [Google Scholar] [CrossRef]
- Mann, M.K.; Zepeda-Velázquez, C.A.; González-Álvarez, H.; Dong, A.; Kiyota, T.; Aman, A.M.; Loppnau, P.; Li, Y.; Wilson, B.; Arrowsmith, C.H.; et al. Structure–Activity Relationship of USP5 Inhibitors. J. Med. Chem. 2021, 64, 15017–15036. [Google Scholar] [CrossRef]
- Passos, C.d.S.; Simões-Pires, C.A.; Carrupt, P.-A.; Nurisso, A. Molecular dynamics of zinc-finger ubiquitin binding domains: A comparative study of histone deacetylase 6 and ubiquitin-specific protease 5. J. Biomol. Struct. Dyn. 2016, 34, 1–18. [Google Scholar] [CrossRef]
- Wang, S.; Juan, J.; Zhang, Z.; Du, Y.; Xu, Y.; Tong, J.; Cao, B.; Moran, M.F.; Zeng, Y.; Mao, X. Inhibition of the deubiquitinase USP5 leads to c-Maf protein degradation and myeloma cell apoptosis. Cell Death Dis. 2017, 8, e3058. [Google Scholar] [CrossRef] [PubMed]
- Villamil, M.A.; Chen, J.; Liang, Q.; Zhuang, Z. A Noncanonical Cysteine Protease USP1 Is Activated through Active Site Modulation by USP1-Associated Factor 1. Biochemistry 2012, 51, 2829–2839. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Zhao, J.; Zhang, Y.; Liu, Y.; Ma, C.; Yi, F.; Zheng, Y.; Zhang, L.; Cheng, T.; Liu, H.; et al. USP5 attenuates NLRP3 inflammasome activation by promoting autophagic degradation of NLRP3. Autophagy 2021, 18, 990–1004. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-W.; Ryu, K.-Y. Cellular ubiquitin pool dynamics and homeostasis. BMB Rep. 2014, 47, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Huang, Q.; Ye, X.; Lin, Y.; Chen, Y.; Lin, X.; Qu, J. Drosophila USP5 Controls the Activation of Apoptosis and the Jun N-Terminal Kinase Pathway during Eye Development. PLoS ONE 2014, 9, e92250. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Jiang, W.; Zhou, P.; Liu, L.; Wan, X.; Yuan, X.; Wang, X.; Chen, M.; Chen, J.; Yang, J.; et al. Mixed Lineage Leukemia 5 (MLL5) Protein Stability Is Cooperatively Regulated by O-GlcNac Transferase (OGT) and Ubiquitin Specific Protease 7 (USP7). PLoS ONE 2015, 10, e0145023. [Google Scholar] [CrossRef] [PubMed]
- Dayal, S.; Sparks, A.; Jacob, J.; Allende-Vega, N.; Lane, D.; Saville, M.K. Suppression of the Deubiquitinating Enzyme USP5 Causes the Accumulation of Unanchored Polyubiquitin and the Activation of p53. J. Biol. Chem. 2009, 284, 5030–5041. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Qi, W.; Pan, H.; Yang, F.; Deng, J. Overexpression of USP5 contributes to tumorigenesis in non-small cell lung cancer via the stabilization of β-catenin protein. Am. J. Cancer Res. 2018, 8, 2284–2295. [Google Scholar]
- Chen, Y.; Li, Y.; Xue, J.; Gong, A.; Yu, G.; Zhou, A.; Lin, K.; Zhang, S.; Zhang, N.; Gottardi, C.J.; et al. Wnt-induced deubiquitination FoxM1 ensures nucleus β-catenin transactivation. EMBO J. 2016, 35, 668–684. [Google Scholar] [CrossRef]
- Ling, X.; Huang, Q.; Xu, Y.; Jin, Y.; Feng, Y.; Shi, W.; Ye, X.; Lin, Y.; Hou, L.; Lin, X. The deubiquitinating enzyme Usp5 regulates Notch and RTK signaling during Drosophila eye development. FEBS Lett. 2017, 591, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Shlevkov, E.; Morata, G. A dp53/JNK-dependant feedback amplification loop is essential for the apoptotic response to stress in Drosophila. Cell Death Differ. 2012, 19, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Katsuki, S.; Chen, M.; Decano, J.L.; Halu, A.; Lee, L.H.; Pestana, D.V.S.; Kum, A.S.T.; Kuromoto, R.K.; Golden, W.S.; et al. Uremic Toxin Indoxyl Sulfate Promotes Proinflammatory Macrophage Activation Via the Interplay of OATP2B1 and Dll4-Notch Signaling. Circulation 2019, 139, 78–96. [Google Scholar] [CrossRef] [PubMed]
- Kummari, E.; Alugubelly, N.; Hsu, C.-Y.; Dong, B.; Nanduri, B.; Edelmann, M.J. Correction: Activity-Based Proteomic Profiling of Deubiquitinating Enzymes in Salmonella-Infected Macrophages Leads to Identification of Putative Function of UCH-L5 in Inflammasome Regulation. PLoS ONE 2015, 10, e0138635. [Google Scholar] [CrossRef] [PubMed]
- Omilusik, K.D.; Nadjsombati, M.S.; Yoshida, T.M.; Shaw, L.A.; Goulding, J.; Goldrath, A.W. Ubiquitin Specific Protease 1 Expression and Function in T Cell Immunity. J. Immunol. 2021, 207, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Dufner, A.; Kisser, A.; Niendorf, S.; Basters, A.; Reissig, S.; Schönle, A.; Aichem, A.; Kurz, T.; Schlosser, A.; Yablonski, D.; et al. The ubiquitin-specific protease USP8 is critical for the development and homeostasis of T cells. Nat. Immunol. 2015, 16, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Caamaño, J.; Hunter, C.A. NF-κB Family of Transcription Factors: Central Regulators of Innate and Adaptive Immune Functions. Clin. Microbiol. Rev. 2002, 15, 414–429. [Google Scholar] [CrossRef] [PubMed]
- Mussbacher, M.; Derler, M.; Basílio, J.; Schmid, J.A. NF-kappa B in monocytes and macrophages—An inflammatory master regulator in multitalented immune cells. Front. Immunol. 2023, 14, 1134661. [Google Scholar] [CrossRef]
- Huang, C.; Wang, W.; Huang, H.; Jiang, J.; Ding, Y.; Li, X.; Ma, J.; Hou, M.; Pu, X.; Qian, G.; et al. Kawasaki disease: Ubiquitin-specific protease 5 promotes endothelial inflammation via TNFα-mediated signaling. Pediatr. Res. 2022, 93, 1883–1890. [Google Scholar] [CrossRef]
- Luo, X.-B.; Xi, J.-C.; Liu, Z.; Long, Y.; Li, L.-T.; Luo, Z.-P.; Liu, D.-H. Proinflammatory Effects of Ubiquitin-Specific Protease 5 (USP5) in Rheumatoid Arthritis Fibroblast-Like Synoviocytes. Mediat. Inflamm. 2020, 2020, 8295149. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Ren, Y.; Zuo, Y.; Yuan, Y.; Zhao, P.; Wang, X.; Cheng, Q.; Liu, J.; Zhang, L.; Guo, T.; et al. Smurf1 represses TNF-α production through ubiquitination and destabilization of USP5. Biochem. Biophys. Res. Commun. 2016, 474, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, V.; Jakymiw, A.; Van Tubergen, E.; D’silva, N.; Kirkwood, K. Control of Cytokine mRNA Expression by RNA-binding Proteins and microRNAs. J. Dent. Res. 2012, 91, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Su, J.; Chen, X. Role of ubiquitin-specific protease 5 in the inflammatory response of chronic periodontitis. Oral Dis. 2023, 29, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Georges, A.; Coyaud, E.; Marcon, E.; Greenblatt, J.; Raught, B.; Frappier, L. USP7 Regulates Cytokinesis through FBXO38 and KIF20B. Sci. Rep. 2019, 9, 2724. [Google Scholar] [CrossRef] [PubMed]
- Chyuan, I.-T.; Tzeng, H.-T.; Chen, J.-Y. Signaling Pathways of Type I and Type III Interferons and Targeted Therapies in Systemic Lupus Erythematosus. Cells 2019, 8, 963. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Ye, Y.Q.; Taniguchi, K.; Yoshida, A.; Akiyama, T.; Yoshioka, Y.; Onose, J.-I.; Koshino, H.; Takahashi, S.; Yajima, A.; et al. Vialinin A is a ubiquitin-specific peptidase inhibitor. Bioorganic Med. Chem. Lett. 2013, 23, 4328–4331. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Zhang, W.; Xiang, Y.; Lu, X.; Liu, W.; Jia, K.; Yi, M. Ubiquitin-specific protease 5 was involved in the interferon response to RGNNV in sea perch (Lateolabrax japonicus). Fish Shellfish. Immunol. 2020, 103, 239–247. [Google Scholar] [CrossRef]
- Yao, J.; Li, C.; Shi, L.; Lu, Y.; Liu, X. Zebrafish ubiquitin-specific peptidase 5 (USP5) activates interferon resistance to the virus by increase the expression of RIG-I. Gene 2020, 751, 144761. [Google Scholar] [CrossRef]
- Qian, G.; Zhu, L.; Huang, C.; Liu, Y.; Ren, Y.; Ding, Y.; Qian, W.; Xu, Q.; Zheng, H.; Lv, H. Ubiquitin specific protease 5 negatively regulates the IFNs-mediated antiviral activity via targeting SMURF1. Int. Immunopharmacol. 2020, 87, 106763. [Google Scholar] [CrossRef]
- Hedl, M.; Yan, J.; Abraham, C. IRF5 and IRF5 Disease-Risk Variants Increase Glycolysis and Human M1 Macrophage Polarization by Regulating Proximal Signaling and Akt2 Activation. Cell Rep. 2016, 16, 2442–2455. [Google Scholar] [CrossRef] [PubMed]
- Ban, T.; Sato, G.R.; Nishiyama, A.; Akiyama, A.; Takasuna, M.; Umehara, M.; Suzuki, S.; Ichino, M.; Matsunaga, S.; Kimura, A.; et al. Lyn Kinase Suppresses the Transcriptional Activity of IRF5 in the TLR-MyD88 Pathway to Restrain the Development of Autoimmunity. Immunity 2016, 45, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Su, Z.; Wang, Z.; Xu, H. USP7 is associated with greater disease activity in systemic lupus erythematosus via stabilization of the IFNα receptor. Mol. Med. Rep. 2017, 16, 2274–2280. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Ye, Y.Q.; Okada, K.; Taniguchi, K.; Yoshida, A.; Sugaya, K.; Onose, J.-I.; Koshino, H.; Takahashi, S.; Yajima, A.; et al. Ubiquitin-Specific Peptidase 5, a Target Molecule of Vialinin A, Is a Key Molecule of TNF-α Production in RBL-2H3 Cells. PLoS ONE 2013, 8, e80931. [Google Scholar] [CrossRef]
- Xiao, K.; He, W.; Guan, W.; Hou, F.; Yan, P.; Xu, J.; Zhou, T.; Liu, Y.; Xie, L. Mesenchymal stem cells reverse EMT process through blocking the activation of NF-κB and Hedgehog pathways in LPS-induced acute lung injury. Cell Death Dis. 2020, 11, 863. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, Y.; Zhang, P.; Li, Y.; Yang, Y.; Yang, Y.; Zhu, J.; Song, X.; Jiang, G.; Fan, J. Hemorrhagic shock primes for lung vascular endothelial cell pyroptosis: Role in pulmonary inflammation following LPS. Cell Death Dis. 2016, 7, e2363. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.; Feng, Y.; Du, Y.; Xing, C.; Chu, J.; Ou, J.; Liu, X.; Zhu, M.; Qian, C.; Yin, B.; et al. USP3 plays a critical role in the induction of innate immune tolerance. Embo Rep. 2023, 24, e57828. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-H.; Zhao, X.-B.; Hu, W.-W.; Chen, W.-L. Research progress on ubiquitin-specific protease in antiviral immunity. Zhejiang Da Xue Xue Bao Yi Xue Ban = J. Zhejiang Univ. Med. Sci. 2015, 44, 578–583. [Google Scholar] [CrossRef]
- Engel, E.; Viargues, P.; Mortier, M.; Taillebourg, E.; Couté, Y.; Thevenon, D.; Fauvarque, M.-O. Identifying USPs regulating immune signals in Drosophila: USP2 deubiquitinates Imd and promotes its degradation by interacting with the proteasome. Cell Commun. Signal. 2014, 12, 41. [Google Scholar] [CrossRef]
- Collins, S.E.; Mossman, K.L. Danger, diversity and priming in innate antiviral immunity. Cytokine Growth Factor Rev. 2014, 25, 525–531. [Google Scholar] [CrossRef]
- Qian, G.; Hu, X.; Li, G.; Ding, Y.; Zhu, L.; Zheng, H.; Li, M.; Li, Z.; Pan, J.; Li, Y.; et al. Smurf1 restricts the antiviral function mediated by USP25 through promoting its ubiquitination and degradation. Biochem. Biophys. Res. Commun. 2018, 498, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Zhang, M.; Zhang, M.-X.; Ren, Y.; Jin, J.; Zhao, Q.; Pan, Z.; Wu, M.; Shu, H.-B.; Dong, C.; et al. Induction of USP25 by viral infection promotes innate antiviral responses by mediating the stabilization of TRAF3 and TRAF6. Proc. Natl. Acad. Sci. USA 2015, 112, 11324–11329. [Google Scholar] [CrossRef] [PubMed]
- Potu, H.; Peterson, L.F.; Pal, A.; Verhaegen, M.; Cao, J.; Talpaz, M.; Donato, N.J. Usp5 links suppression of p53 and FAS levels in melanoma to the BRAF pathway. Oncotarget 2014, 5, 5559–5569. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y.; Wu, H.-Y.; Mao, X.-F.; Jiang, L.-X.; Wang, Y.-X. USP5 promotes tumorigenesis and progression of pancreatic cancer by stabilizing FoxM1 protein. Biochem. Biophys. Res. Commun. 2017, 492, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, W.-M.; Lu, Y.-F.; Feng, L.; Li, L.; Pan, M.-Z.; Sun, Y.; Suen, C.-W.; Guo, W.; Pang, J.-X.; et al. Usp5 functions as an oncogene for stimulating tumorigenesis in hepatocellular carcinoma. Oncotarget 2017, 8, 50655–50664. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Wu, W.; Wang, Z.; Lu, G.; Sun, J.; Jin, X.; Xie, L.; Wang, X.; Tan, C.; Wang, Z.; et al. USP5 Promotes Metastasis in Non-Small Cell Lung Cancer by Inducing Epithelial-Mesenchymal Transition via Wnt/β-Catenin Pathway. Front. Pharmacol. 2020, 11, 668. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Qiao, Y.; Chen, C.; Zang, H.; Zhang, X.; Qi, F.; Chang, C.; Yang, F.; Sun, M.; Lin, S.; et al. USP5 facilitates non-small cell lung cancer progression through stabilization of PD-L1. Cell Death Dis. 2021, 12, 1051. [Google Scholar] [CrossRef]
- Cao, L.; Liu, H.; Huang, C.; Guo, C.; Zhao, L.; Gao, C.; Xu, Y.; Wang, G.; Liang, N.; Li, S. USP5 knockdown alleviates lung cancer progression via activating PARP1-mediated mTOR signaling pathway. Biol. Direct 2023, 18, 16. [Google Scholar] [CrossRef]
- Huang, W.; Liu, X.; Zhang, Y.; Deng, M.; Li, G.; Chen, G.; Yu, L.; Jin, L.; Liu, T.; Wang, Y.; et al. USP5 promotes breast cancer cell proliferation and metastasis by stabilizing HIF2α. J. Cell. Physiol. 2022, 237, 2211–2219. [Google Scholar] [CrossRef]
- Harrigan, J.A.; Jacq, X.; Martin, N.M.; Jackson, S.P. Deubiquitylating enzymes and drug discovery: Emerging opportunities. Nat. Rev. Drug Discov. 2018, 17, 57–77. [Google Scholar] [CrossRef]
- Kim, S.; Woo, S.M.; Min, K.-J.; Seo, S.U.; Lee, T.-J.; Kubatka, P.; Kim, D.E.; Kwon, T.K. WP1130 Enhances TRAIL-Induced Apoptosis through USP9X-Dependent miR-708-Mediated Downregulation of c-FLIP. Cancers 2019, 11, 344. [Google Scholar] [CrossRef] [PubMed]
- Kapuria, V.; Peterson, L.F.; Fang, D.; Bornmann, W.G.; Talpaz, M.; Donato, N.J. Deubiquitinase Inhibition by Small-Molecule WP1130 Triggers Aggresome Formation and Tumor Cell Apoptosis. Cancer Res. 2010, 70, 9265–9276. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, H.; Zhu, W.; Zhou, B.; Ying, J.; Wu, J.; Zhang, H.; Sun, H.; Gao, S. Deubiquitinase inhibitor degrasyn suppresses metastasis by targeting USP5-WT1-E-cadherin signalling pathway in pancreatic ductal adenocarcinoma. J. Cell. Mol. Med. 2020, 24, 1370–1382. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, L.; Niu, X.; Guo, Y.; Zhao, J.; Li, L.; Zhao, J. EOAI, a ubiquitin-specific peptidase 5 inhibitor, prevents non-small cell lung cancer progression by inducing DNA damage. BMC Cancer 2023, 23, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cui, Z.; Xie, Z.; Li, C.; Xu, C.; Guo, X.; Yu, J.; Chen, T.; Facchinetti, F.; Bohnenberger, H.; et al. Deubiquitinase USP5 promotes non-small cell lung cancer cell proliferation by stabilizing cyclin D1. Transl. Lung Cancer Res. 2021, 10, 3995–4011. [Google Scholar] [CrossRef] [PubMed]
- Kapuria, V.; Peterson, L.F.; Showalter, H.H.; Kirchhoff, P.D.; Talpaz, M.; Donato, N.J. Protein cross-linking as a novel mechanism of action of a ubiquitin-activating enzyme inhibitor with anti-tumor activity. Biochem. Pharmacol. 2011, 82, 341–349. [Google Scholar] [CrossRef]
- Meng, J.; Ai, X.; Lei, Y.; Zhong, W.; Qian, B.; Qiao, K.; Wang, X.; Zhou, B.; Wang, H.; Huai, L.; et al. USP5 promotes epithelial-mesenchymal transition by stabilizing SLUG in hepatocellular carcinoma. Theranostics 2019, 9, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-W.; Ji, K.; Zhu, X.-Y.; Wu, X.-Y.; Lin, R.-T.; Xie, C.-C.; Cai, Z.-L.; Chen, J.-J. Natural isoflavone formononetin inhibits IgE-mediated mast cell activation and allergic inflammation by increasing IgE receptor degradation. Food Funct. 2023, 14, 2857–2869. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Caballero, A.; Gadotti, V.M.; Ali, Y.; Bladen, C.; Gambeta, E.; Van Humbeck, J.F.; MacCallum, J.L.; Zamponi, G.W. A Synthetically Accessible Small-Molecule Inhibitor of USP5-Cav3.2 Calcium Channel Interactions with Analgesic Properties. ACS Chem. Neurosci. 2022, 13, 524–536. [Google Scholar] [CrossRef]
- Gadotti, V.M.; Caballero, A.G.; Berger, N.D.; Gladding, C.M.; Chen, L.; Pfeifer, T.A.; Zamponi, G.W. Small Organic Molecule Disruptors of Cav3.2—USP5 Interactions Reverse Inflammatory and Neuropathic Pain. Mol. Pain 2015, 11, 12. [Google Scholar] [CrossRef]
- Zhang, Z.; Tong, J.; Tang, X.; Juan, J.; Cao, B.; Hurren, R.; Chen, G.; Taylor, P.; Xu, X.; Shi, C.-X.; et al. The ubiquitin ligase HERC4 mediates c-Maf ubiquitination and delays the growth of multiple myeloma xenografts in nude mice. Blood 2016, 127, 1676–1686. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.F.; Sun, H.; Liu, Y.; Potu, H.; Kandarpa, M.; Ermann, M.; Courtney, S.M.; Young, M.; Showalter, H.D.; Sun, D.; et al. Targeting deubiquitinase activity with a novel small-molecule inhibitor as therapy for B-cell malignancies. Blood 2015, 125, 3588–3597. [Google Scholar] [CrossRef] [PubMed]
| Inhibitor | Target | Mechanism | Diseases | References |
|---|---|---|---|---|
| WP1130 | USP5/9X/14/24/37 | Inhibition of USP5 activity by inducing the upregulation of the proapoptotic protein p53 | Cancers | [29,91,92] |
| PYR-41 | USP5/9X | PYR-41 reduced USP5 protein levels in a dose-dependent manner by inducing protein cross-linking to form high molecular weight complexes | Lymphoma | [96] |
| Formononetin | USP5 | Formononetin directly acts on USP5 to destabilize SLUG and inhibit EMT in HCC | HCC | [97,98] |
| The inhibition of USP5 increases the degradation of FcεRI signaling and induces FcεRIγ ubiquitination, thereby inhibiting IgE-mediated mast cell activation | Allergic inflammation | [98] | ||
| II-1, Suramin, Gossypetin | USP5 | The inhibition of the biochemical interaction between USP5 and the Cav3.2 domain III-IV linker ultimately reduces pain-related information transmission | Inflammatory and neuropathic pain | [99,100] |
| Mebendazole | USP5 | The inhibition of USP5 expression and disruption of the interaction between USP5 and c-Maf results in increased c-Maf ubiquitination and subsequent c-Maf degradation | Multiple myeloma | [42,101] |
| EOAI | USP5/9X/14/24/ | EOAI is a pan-deubiquitinase inhibitor that targets USP5 | Pancreatic ductal adenocarcinoma, NSCLC | [10,29,94,102] |
| Vialinin A | USP4/5 | Vialinin A inhibits the enzymatic activity of USP5 and inhibits the ability of USP5 to hydrolyze Ub-AMC in a dose-dependent manner | Inflammation | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Chen, C.; He, P.; Du, Y.; Zhu, B. Unraveling the Immune Regulatory Functions of USP5: Implications for Disease Therapy. Biomolecules 2024, 14, 683. https://doi.org/10.3390/biom14060683
Gu J, Chen C, He P, Du Y, Zhu B. Unraveling the Immune Regulatory Functions of USP5: Implications for Disease Therapy. Biomolecules. 2024; 14(6):683. https://doi.org/10.3390/biom14060683
Chicago/Turabian StyleGu, Jinyi, Changshun Chen, Pu He, Yunjie Du, and Bingdong Zhu. 2024. "Unraveling the Immune Regulatory Functions of USP5: Implications for Disease Therapy" Biomolecules 14, no. 6: 683. https://doi.org/10.3390/biom14060683





